Abstract
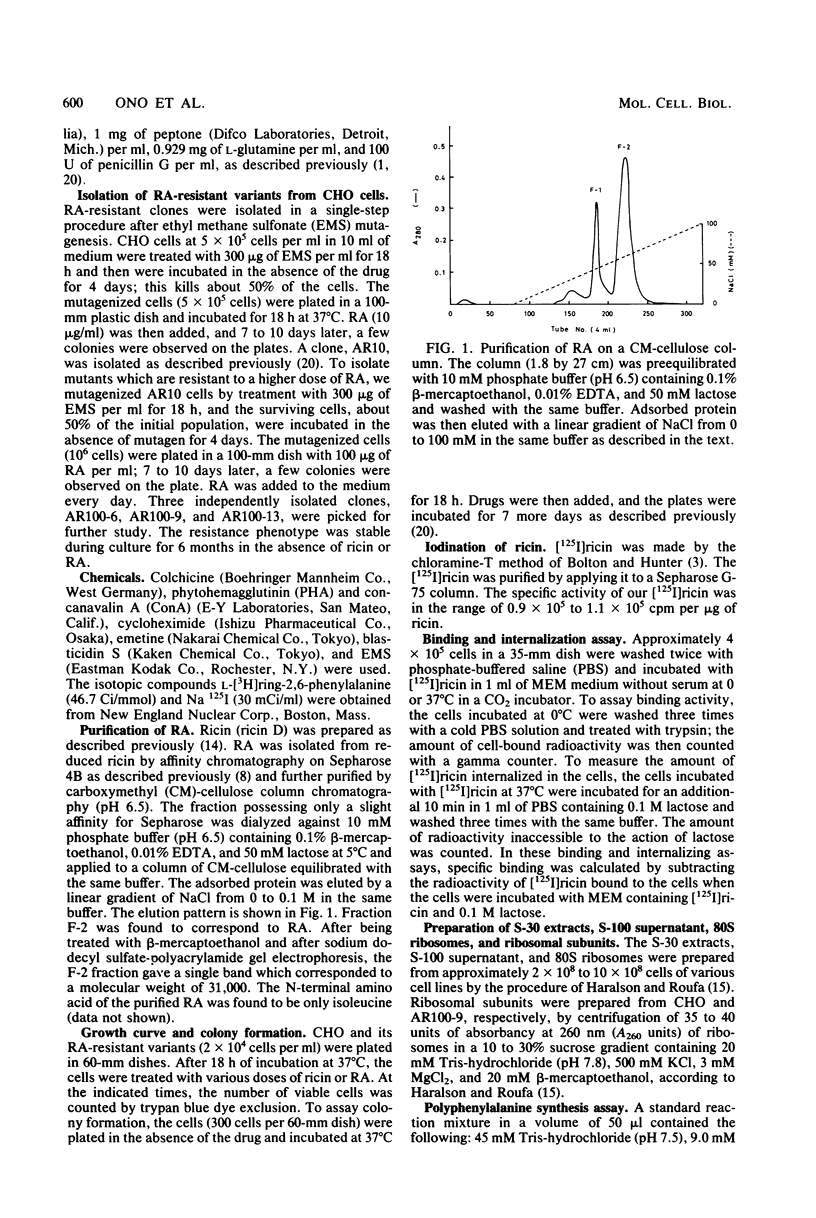
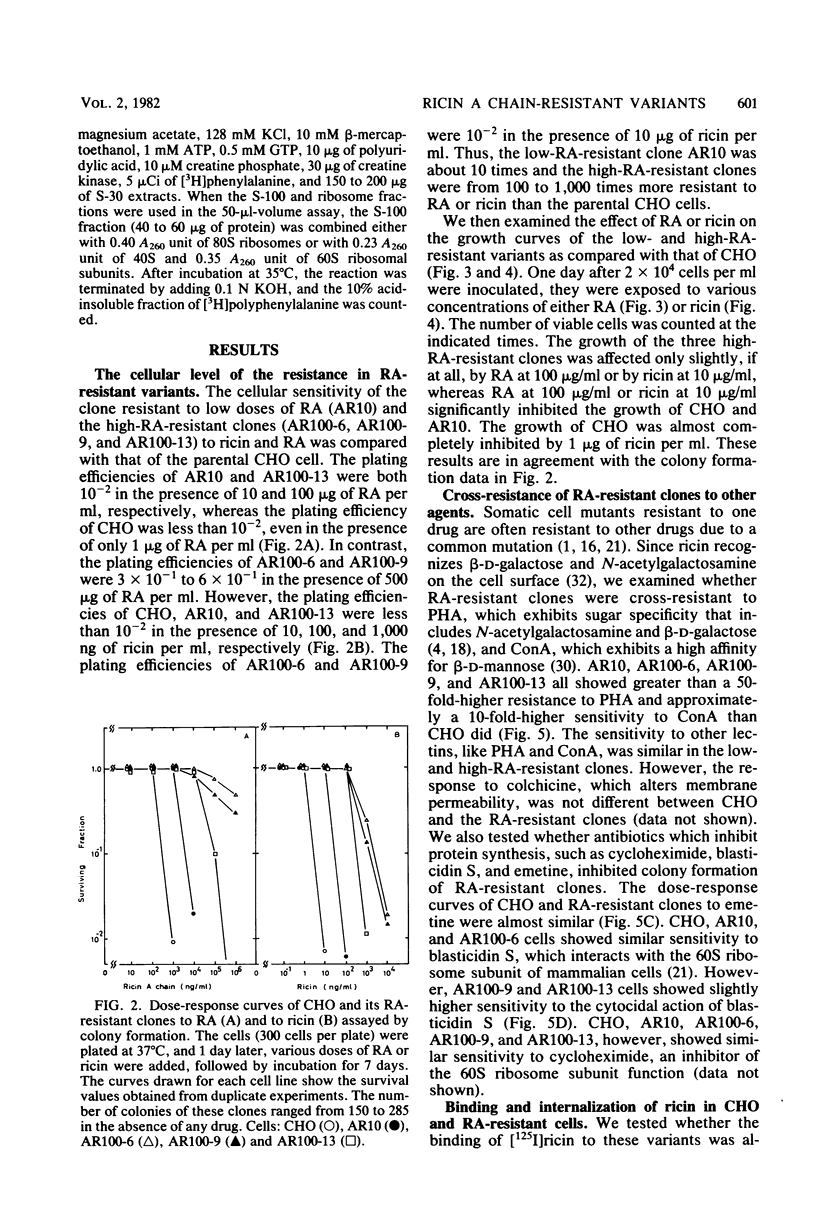
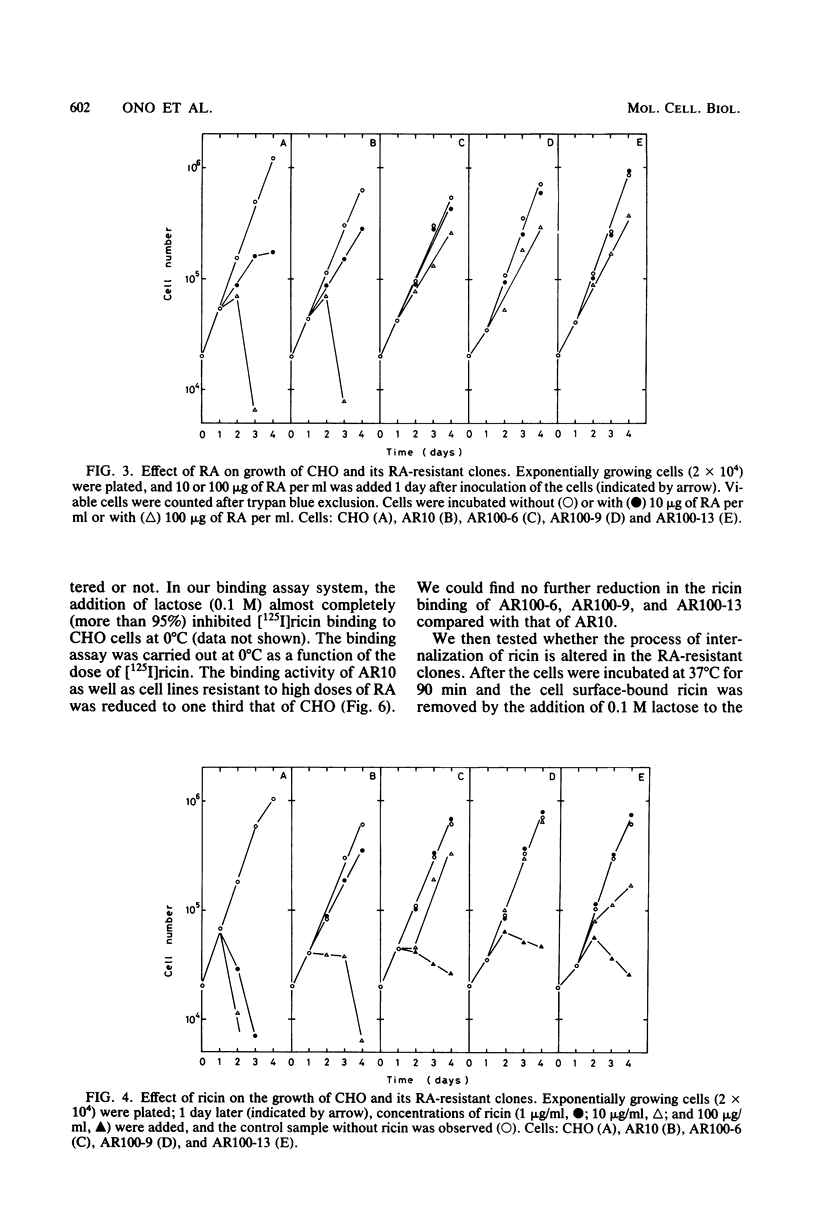
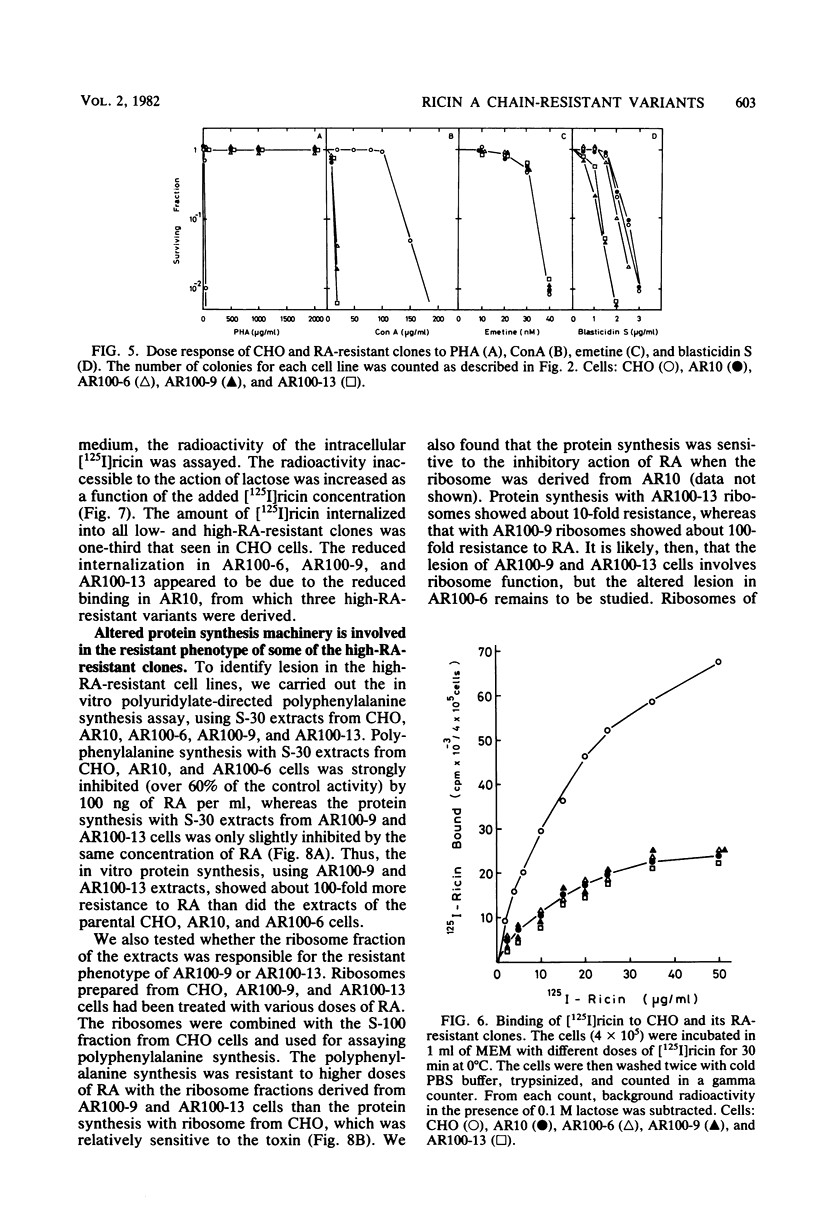
Ricin, a toxic lectin from Ricinus communis, is composed of two different polypeptide chains, A and B, and the ricin A chain (RA) blocks protein synthesis. We studied cell lines resistant to cytotoxic action of RA. One low-RA-resistant cell line, AR10, isolated from Chinese hamster ovary (CHO) cells, was resistant to a low dose of RA (1 μg/ml) and showed a 10-fold-higher resistance to RA and ricin than that of CHO. We further mutagenized AR10 to isolate high-RA-resistant cell lines AR100-6, AR100-9, and AR100-13, which were resistant to higher doses of RA and ricin (100- to 1,000-fold) than CHO was. The binding of [125I]ricin to AR10, AR100-6, AR100-9, and AR100-13 cells was decreased to about 30% of that of CHO. The internalization of [125I]ricin in AR10 cells and in the high-RA-resistant clones was the same. Polyuridylate-dependent polyphenylalanine synthesis, using S-30 extracts from either AR100-9 or AR100-13, was about 100-fold more resistant to the inhibitory action of RA than when CHO, AR10, and AR100-6 cells extracts were used. The protein synthesis with ribosomes (80S) from AR100-9 or AR100-13 was 10- to 100-fold more resistant to RA than it was with parental ribosomes when combined with the S-100 fraction of CHO cells. The polyphenylalanine synthesis assay using the ribosomes constituted from the 60S subunit of AR100-9 and the 40S subunit of CHO indicated that the resistant phenotype of AR100-9 cells is due to an alteration of the 60S ribosomal subunit.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S., Kuwano M. Isolation and preliminary characterization of bleomycin-resistant mutants from Chinese hamster ovary cells. J Cell Physiol. 1981 Apr;107(1):147–153. doi: 10.1002/jcp.1041070116. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb C., Kornfeld S. Isolation and characterization of two mouse L cell lines resistant to the toxic lectin ricin. J Biol Chem. 1976 Dec 25;251(24):7761–7768. [PubMed] [Google Scholar]
- Gottlieb C., Skinner A. M., Kornfeld S. Isolation of a clone of Chinese hamster ovary cells deficient in plant lectin-binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1078–1082. doi: 10.1073/pnas.71.4.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. The molecular basis of emetine resistance in Chinese hamster ovary cells: alteration in the 40S ribosomal subunit. Cell. 1977 Jan;10(1):61–66. doi: 10.1016/0092-8674(77)90140-4. [DOI] [PubMed] [Google Scholar]
- Haralson M. A., Roufa D. J. A temperature-sensitive mutation affecting the mammalian 60 S ribosome. J Biol Chem. 1975 Nov 25;250(22):8618–8623. [PubMed] [Google Scholar]
- Hidaka K., Akiyama S. I., Kuwano M. Amphotericin B resistance is recessive in Chinese hamster hybrid cells. J Cell Physiol. 1981 Jan;106(1):41–47. doi: 10.1002/jcp.1041060106. [DOI] [PubMed] [Google Scholar]
- Hyman R., Lacorbiere M., Stavarek S., Nicolson G. Derivation of lymphoma variants with reduced sensitivity to plant lectins. J Natl Cancer Inst. 1974 Mar;52(3):963–969. doi: 10.1093/jnci/52.3.963. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Stanley P. Altered cell surface glycoproteins in phytohemagglutinin-resistant mutants of Chinese hamster ovary cells. Biochim Biophys Acta. 1975 May 6;389(2):401–406. doi: 10.1016/0005-2736(75)90332-6. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Matsui K., Takenaka K., Akiyama S., Endo H. A mouse leukemia cell mutant resistant to blasticidin S. Int J Cancer. 1977 Aug 15;20(2):296–302. doi: 10.1002/ijc.2910200219. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Tabuki T., Akiyama S., Mifune K., Takatsuki A., Tamura G., Ikehara Y. Isolation and characterization of Chinese hamster ovary cell mutants with altered sensitivity to high doses of tunicamycin. Somatic Cell Genet. 1981 Sep;7(5):507–521. doi: 10.1007/BF01549655. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Takenaka K., Ono M. The cross-resistance of mouse blasticidin S-resistant cell lines to puromycin and sparsomycin, inhibitors of ribosome function. Biochim Biophys Acta. 1979 Jul 26;563(2):479–489. doi: 10.1016/0005-2787(79)90066-2. [DOI] [PubMed] [Google Scholar]
- Li E., Kornfeld S. Structure of the altered oligosaccharide present in glycoproteins from a clone of Chinese hamster ovary cells deficient in N-acetylglucosaminyltransferase activity. J Biol Chem. 1978 Sep 25;253(18):6426–6431. [PubMed] [Google Scholar]
- Meager A., Ungkitchanukit A., Hughes R. C. Variants of hamster fibroblasts resistant to Ricinus communis toxin (ricin). Biochem J. 1976 Jan 15;154(1):113–124. doi: 10.1042/bj1540113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson G. L., Smith J. R., Hyman R. Dynamics of toxin and lectin receptors on a lymphoma cell line and its toxin-resistant variant using ferritin-conjugated, 125I-labeled ligand. J Cell Biol. 1978 Aug;78(2):565–576. doi: 10.1083/jcb.78.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsnes S., Fernandez-Puentes C., Carrasco L., Vazquez D. Ribosome inactivation by the toxic lectins abrin and ricin. Kinetics of the enzymic activity of the toxin A-chains. Eur J Biochem. 1975 Dec 1;60(1):281–288. doi: 10.1111/j.1432-1033.1975.tb21001.x. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Saltvedt E., Pihl A. Isolation and comparison of galactose-binding lectins from Abrus precatorius and Ricinus communis. J Biol Chem. 1974 Feb 10;249(3):803–810. [PubMed] [Google Scholar]
- Poretz R. D., Goldstein I. J. An examination of the topography of the saccharide binding sites of concanavalin A and of the forces involved in complexation. Biochemistry. 1970 Jul 7;9(14):2890–2896. doi: 10.1021/bi00816a021. [DOI] [PubMed] [Google Scholar]
- Pöche H., Zakrzewski S., Nierhaus K. H. Resistance against cycloheximide in cell lines from Chinese hamster and human cells is conferred by the large subunit of cytoplasmic ribosomes. Mol Gen Genet. 1979 Sep;175(2):181–185. doi: 10.1007/BF00425534. [DOI] [PubMed] [Google Scholar]
- Ray B., Wu H. C. Internalization of ricin in Chinese hamster ovary cells. Mol Cell Biol. 1981 Jun;1(6):544–551. doi: 10.1128/mcb.1.6.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Robbins J. C., Hunter T. R., Nicolson G. L. Ricinus communis toxin-mediated inhibition of protein synthesis in cell-free extracts of a toxin-resistant variant mouse lymphoma cell line. J Supramol Struct. 1976;5(4):515(367)–520(372). doi: 10.1002/jss.400050408. [DOI] [PubMed] [Google Scholar]
- Rosen S. W., Hughes R. C. Effects of neuraminidase on lectin binding by wild-type and ricin-resistant strains of hamster fibroblasts. Biochemistry. 1977 Nov 1;16(22):4908–4915. doi: 10.1021/bi00641a026. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Binding, uptake and degradation of the toxic proteins abrin and ricin by toxin-resistant cell variants. Eur J Biochem. 1978 Jan 2;82(1):13–23. doi: 10.1111/j.1432-1033.1978.tb11992.x. [DOI] [PubMed] [Google Scholar]
- Stanley P., Caillibot V., Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975 Oct;6(2):121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Lewis W. H., Parfett C. L. Somatic cell genetics: a review of drug resistance, lectin resistance and gene transfer in mammalian cells in culture. Can J Genet Cytol. 1980;22(4):443–496. doi: 10.1139/g80-056. [DOI] [PubMed] [Google Scholar]



