Abstract
Introduction
The number of cases of osteoporotic vertebral compression fracture (OVCF) with intravertebral cleft (IVC) with delayed neurologic deficit (DND) is increasing as the population ages. However, the cause of DND is poorly understood, and no definitive treatment of the disease has been established. The purpose of this study was to clarify the radiographic parameters contributing to the occurrence of DND, and to evaluate the efficacy and safety of percutaneous vertebroplasty for this pathology.
Methods
Percutaneous vertebroplasty was prospectively performed for 244 patients with OVCF with IVC; 30 had DND and 214 did not. Radiographic parameters of local kyphotic angle, percent spinal canal compromise and intravertebral instability were investigated for correlations to DND. Procedural outcomes were evaluated using visual analog scale (VAS), Oswestry Disability Index (ODI), and modified Frankel grades.
Results
Before vertebroplasty, no substantial difference in local kyphotic angle was seen between OVCF with IVC with and without DND, but percent spinal canal compromise and intravertebral instability were greater in OVCF with IVC with DND (P < 0.001). After vertebroplasty, 25 of 30 cases (84 %) of OVCF with IVC with DND achieved clinically meaningful improvement (CMI), but 5 (17 %) did not. Patients with CMI showed substantial improvements in intravertebral instability (P < 0.001), and no change in local kyphotic angle or percent spinal canal compromise. In five patients without CMI, four showed an initial improvement, but subsequent vertebral fracture adjacent to the treated vertebra caused neurologic re-deterioration. One patient with percent spinal canal compromise 54.9 % and intravertebral instability 4° achieved no neurologic improvement following vertebroplasty. No serious complications or adverse events related to the procedure were encountered.
Conclusions
Intravertebral instability is the dominant cause of DND. Percutaneous vertebroplasty appears effective and safe in the treatment of OVCF with IVC with DND. Patients with less intravertebral instability and severe spinal canal compromise could be candidates for conventional surgical treatment.
Keywords: Percutaneous vertebroplasty, Osteoporotic vertebral fracture, Intravertebral cleft, Delayed neurologic deficit, Spine
Introduction
Osteoporotic vertebral compression fracture (OVCF) has been considered to show a benign natural history. However, rigorous follow-up studies have clarified that up to 30 % of OVCF does not respond adequately to standard conservative therapy [18, 22, 33]. Several reports have noted that the presence of Kümmell disease is associated with deteriorated prognosis for OVCF with intravertebral cleft (IVC). This disease could prolong back pain and predispose to vertebral collapse, which may result in delayed neurologic deficit (DND) [12, 14, 16, 17, 20, 21, 30]. The following factors have been suspected as causes of DND: (1) progression of kyphosis with vertebral collapse; (2) neural compression secondary to retropulsed bone fragments; and (3) intravertebral instability at the fracture site [1–3, 12, 13, 27, 32, 34]. The causes of DND with OVCF with IVC, however, remain poorly understood.
Surgical management has been performed for OVCF with IVC with DND. However, multiple medical comorbidities with advanced age and unreliable fixation in weakened osteoporotic bone have caused surgical treatments to result in significant morbidity and poor outcome [7, 9, 18]. On the other hand, the advent of vertebroplasty has marked a new era in the treatment of OVCF. This cement augmentation technique produces excellent outcomes in >85 % of patients treated [14]. However, percutaneous vertebroplasty for OVCF with IVC with DND may have been considered relatively contraindicated because of the possibility of neural complications induced by epidural cement leakage [4, 10, 14]. No definitive treatment of OVCF with IVC with DND has yet been established.
We have prospectively performed percutaneous vertebroplasty for OVCF with IVC with and without DND. The objectives of the current study were to clarify the radiographic parameters contributing to the occurrence of DND, and to evaluate the efficacy and safety of percutaneous vertebroplasty performed for OVCF with IVC with DND.
Materials and methods
Patients
A total of 321 consecutive patients with OVCF with IVC underwent percutaneous vertebroplasty at a tertiary referral center, JA Hiroshima General Hospital. For simple analysis of clinical data, 77 patients treated with vertebroplasty at more than a single level were excluded and the remaining 244 patients with single-level OVCF with IVC were enrolled in this study. The study protocol was approved by the ethics committee of our hospital, and written informed consent for this study design was obtained from all patients.
Radiographic diagnosis of OVCF with IVC
OVCF with IVC was radiographically diagnosed based on a positive finding of an intravertebral cleft described as an intravertebral transverse, linear radiolucent shadow on plain radiogram and/or CT, as a hypointense area on T2-weighted MR imaging similar to the signal intensity of gas, as a linear well-demarcated focus of T2 prolongation similar to the signal intensity of adjacent cerebrospinal fluid, and as a cleft-shaped non-contrast area within the vertebral body on fat-suppressed contrast-enhanced MR imaging, respectively (Fig. 1).
Fig. 1.
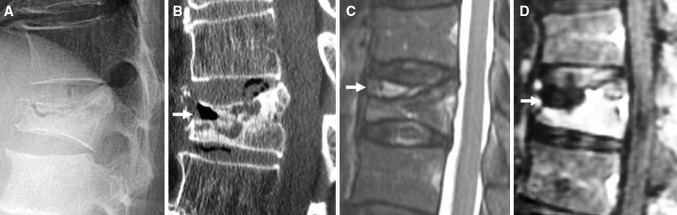
The following sequence of images illustrates a patient with osteoporotic vertebral compression fracture with intravertebral cleft. a The fracture compresses through the intravertebral cleft beneath the superior endplate, demonstrating the maximal degree of height loss on lateral radiography with the patient in a sitting position. b The fracture plane gaps open because the patient is undergoing reformatted CT in a supine position (arrow). c Sagittal T2-weighed MR image demonstrating an area of hyperintensity within a region of hypointensity (arrow). d Sagittal fat-suppressed contrast-enhanced MR image revealing an intravertebral cleft as a non-contrast area (arrow)
Clinical diagnosis of DND
Delayed neurologic deficit was clinically diagnosed based on the following criteria: (1) neurologically intact immediately after OVCF; (2) sensory and/or motor involvement appearing in an insidious and tardy process with progression of vertebral collapse; and (3) intractable pain of lower extremities in a sitting and/or standing position with improvement on lying down (postural leg pain) [5, 13, 25].
Inclusion and exclusion criteria
Selection criteria of percutaneous vertebroplasty for OVCF with IVC were: (1) sufficient back pain ≥4 (current rating for pain intensity on a scale from 0 to 10) refractory to standard medical treatment that consisted of bed rest, analgesics, and/or external back bracing for at least 3 months; and (2) radiographic OVCF with IVC with the presence of point tenderness on manual palpation. Exclusion criteria included: (1) spinal cancer, active infection, or uncorrectable bleeding diatheses; (2) inability to provide informed consent; and (3) a likelihood of noncompliance with direct follow-up.
Vertebroplasty technique
Experienced spine surgeons performed all procedures. After general anesthesia, patients were carefully positioned in a prone position with extended posture on a radiolucent four-poster spinal frame (Allen spinal system; Allen Medical Systems, Acton, MA). Next, 14-gauge bone needles (Ossiris; Hakko, Nagano, Japan) were inserted into an intravertebral cleft through a bilateral transpedicular approach with direct biplane observation using a couple of fluoroscopes (OEC 9900 Elite; GE Healthcare). The intravertebral cleft was a confluent reservoir and specifically targeted for polymethylmethacrylate (PMMA) (Osteobond; Zimmer, Warsaw, IN) injection. Before PMMA injection, cavitygram of the IVC was performed using nonionic contrast agent (Omnipaque 300; Nycomed, Princeton, NJ) to exclude needle placement within the basivertebral venous complex [6], and measured the capacity of the IVC. Persistent opacification could obscure visualization of the PMMA. The residual contrast material was washed out with normal saline to clear the IVC adequately to visualize PMMA. Barium-opacified PMMA of the same volume as the capacity of the IVC was gently injected using 2-ml syringes. PMMA was injected from the one-sided needle with low pressure and filled the IVC. PMMA assumed the shape of the IVC without evidence of extravasation into surrounding bone marrow space, paravertebral soft tissues or epidural space. The procedure was terminated when the cleft was filled with PMMA. On the next day after vertebroplasty, CT was performed to determine whether extravertebral PMMA leakage had occurred. A radiologist and orthopedic surgeon not involved in the procedure reviewed CT images independently and reached a consensus for each case.
Radiographic assessment
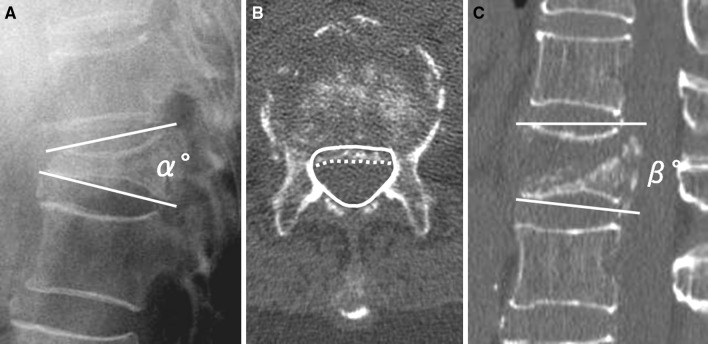
The following three radiographic parameters were assessed: (1) local kyphotic angle, measured as the angle between the lower and upper endplates of the uninvolved vertebrae adjusted cephalic and caudal to the fractured vertebra on lateral radiography with the patient in a sitting position; (2) percent spinal canal compromise, calculated by dividing the area of intrusion by total spinal canal area multiplied by 100; and (3) intravertebral instability of the affected vertebra, measured as the difference between local kyphotic angle on lateral radiography with the patient in a sitting position and that on sagittal reconstructed CT in a supine position (Fig. 2).
Fig. 2.
Three radiographic parameters demonstrating a “local kyphotic angle” (α°) measured using Cobb’s method with the patient in a sitting position and b “percent spinal canal compromise” calculated by dividing the area of intrusion by the total spinal canal area multiplied by 100. The total canal area is outlined by the solid line; the area of the retropulsed vertebral wall is demarcated by the dotted line. Areas of the spinal canal and retropulsed posterior wall are calculated from the total number of pixels per cross-sectional area (pixel/mm2). a, c “Intravertebral instability”, defined as the difference between local kyphotic angle on lateral radiography with the patient in a sitting position (α°) and that on a sagittal reformatted CT scan in a supine position (β°); α°–β°
Outcome measures
Back pain and postural leg pain were measured using the VAS score of 0 to 10, with 0 indicating no pain, and 10 indicating the maximum imaginable pain [28]. Physical disability was measured on the ODI on a scale of 0 to 100 %, with higher scores indicating greater disability [8]. To measure various neurological symptoms at thoracolumbar junction, we used a modification of the Frankel neurologic grading system where ‘‘A’’ designated complete absence of motor and sensory function, and ‘‘E2’’ being the least neurologic impairment. We used the modified Frankel neurologic grading system because this system specifically focuses on the thoracolumbar neurologic functional impairment. For the quantitative analysis, we added a numeric numbering system to represent neurologic function on a scale of 1–7, with seven being complete deficit and one being the least affected (Table 1) [36]. Improvement rate was calculated using the following formula: (baseline score − postoperative score)/baseline score × 100 %. Clinically meaningful improvement (CMI) was defined as an improvement rate ≥30 % from baseline [24].
Table 1.
Numeric neurologic functional grades
| 7-A | Complete motor and sensory loss |
| 6-B | Sensation preserved. No voluntary motor function |
| 5-C | Motor function less than fair grade (nonfunctional for any useful purposes) |
| 4-D1 | Preserved motor at lowest functional grade; MMT 3+/5+ |
| 3-D2 | Preserved motor at midfunctional grade; MMT 3+ to 4+/5+ |
| 2-E1 | Preserved motor ad high functional grade; MMT 4+ to 5+/5+ |
| 1-E2 | Motor and sensory function normal. May still have abnormal reflexes |
| 0-F | Normal |
Modified Frankel neurologic functional grades are represented by the alphabet letter; a numeric designation precedes the letter
Patients were followed directly and periodically after vertebroplasty. In patients who died or could not return to our hospital, the latest complete neurologic examination and radiographs were used for evaluation. Orthopedic surgeons not involved in treatment performed the follow-up and clinical examinations to assess neurologic recovery and functional status. The questionnaire of the VAS score and ODI was self-administered to avoid interviewer bias.
Statistical analysis
To clarify causes of DND, we compared radiographic parameters between the DND group and the control group consisting of non-DND patients. This study was designed to have the number of patients in the DND group and the control group at a ratio of 1:2. The matching criteria were age in years (based on decade), sex and spinal level of OVCF with IVC. Plain radiogram and CT were mixed and observers were blinded to the clinical status of patients. Variability of intraobserver measurement was within 3 % for the entire study. In addition, pre- and post-procedural radiographic parameters were compared in patients with DND who achieved CMI. To evaluate the efficacy and safety of vertebroplasty, procedural outcomes and procedure-related complications were compared between patients with and without DND.
Clinical characteristics and radiographic parameters were analyzed using the Mann–Whitney U test or Chi-square test. Associations between radiographic parameters and DND were analyzed using multivariate logistic-regression models. Procedural outcomes were analyzed using the Wilcoxon signed-ranks test, Mann–Whitney U test, and/or the Bonferroni–Dunn post hoc test. Procedure-related complications were analyzed with the Mann–Whitney U test, Chi-square test, or Fisher’s exact test. Statistical significance was defined at the level of p < 0.05 for a two-sided hypothesis. Mean data are presented ± standard deviation. Analyses were performed using SPSS version 16.0 (SPSS, Chicago, IL).
Results
Baseline characteristics of the 244 patients
Of the 244 patients, 30 had DND and 214 did not (Table 2). OVCF with IVC clustered at the thoracolumbar junction (T11–L2), seen in 26 of 30 (87 %) OVCF with IVC cases with DND and 177 of 214 (83 %) OVCF with IVC cases without DND. No significant differences between groups were seen in age, sex, spinal level of OVCF with IVC, or duration from OVCF to vertebroplasty.
Table 2.
Baseline characteristics of the 244 patients
| Characteristic parameters | DND group (n = 30) | Non-DND group (n = 214) |
|---|---|---|
| Age (year) | 77.5 ± 8.2 | 77.0 ± 7.0 |
| Female sex—no. (%) | 24 (80) | 146 (68) |
| Spinal level of OVFC with IVC—no. (%) | ||
| T7–T10 | 2 (7) | 12 (6) |
| T11 | 1 (3) | 15 (7) |
| T12 | 11 (37) | 69 (32) |
| L1 | 9 (30) | 72 (34) |
| L2 | 5 (17) | 21 (10) |
| L3–L5 | 2 (7) | 25 (12) |
| Duration from OVFC to PVP (week) | 19.2 ± 11.2 | 28.7 ± 36.4 |
| Duration from OVFC to DND (week) | 9.1 ± 9.0 | – |
| VAS score for back pain | 8.1 ± 3.9 | 8.6 ± 1.4 |
| ODI† | 73.9 ± 13.4 % | 51.1 ± 21.9 |
| Numeric neurologic functional grade—no. (spine level of OVFC with IVC—no.) | ||
| 5-C | 12 (T10—2, T12—7, L1—1, L2—2) six patients with T12 OVFC with IVC had bilateral drop-foot | – |
| 4-D | 2 (T12—2) | – |
| 3-D2 | 4 (L1—2, L3—2) | – |
| 2-E1 | 12 (T11—1, T12—2, L1—6, L2—3) all patients had postural leg pain, VAS: 8.2 ± 1.8 | – |
| 0-F | – | 214 |
Plus-minus values are mean ± SD
DND delayed neurologic deficit, IVC intravertebral deft, PVP percutaneous vertebroplasty, VAS visual analog scale, ODI Oswestry disability index
†P < 0.001 for the comparison between the two groups
Patients with OVCF with IVC were unable to perform activities of daily living because of severe back pain. Physical ability was more greatly disturbed in OVCF with IVC with DND. Among 12 patients with a numeric neurologic functional grade of 5-C, six patients with T12 OVCF with IVC showed bilateral drop-foot without leg pain. All 12 patients with grade 2-E1 had severe postural leg pain involving bilateral thighs, which deteriorated in a sitting and/or standing position and compelled the patients to remain lying down or bedridden.
Radiographic assessment before vertebroplasty
Mean local kyphotic angle, percent spinal canal compromise, and intravertebral instability were 17.0 ± 8.8°, 35.4 ± 13.1 %, and 10.6 ± 5.9° in the DND group, and 15.6 ± 7.7°, 23.1 ± 11.1 %, and 5.3 ± 3.3° in the control group, respectively. No significant difference in local kyphotic angle was evident between groups. However, percent spinal canal compromise and intravertebral instability were greater in the DND group than in the control group (P < 0.001). Logistic regression analysis with multivariate models showed that local kyphotic angle had no substantial correlation to DND, while percent spinal canal compromise and intravertebral instability were associated with DND (Table 3).
Table 3.
Radiographic parameters before vertebroplasty, the DND group vs. the control group
| Radiographic parameters | DND group (n = 30) | Control group (n = 60) | Odds ratio |
|---|---|---|---|
| Local kyphotic angle (°) | 17.0 ± 8.8 | 15.6 ± 7.7 | [0.94; 95 % [CI] 0.88–1.04; P = 0.31] |
| Percent spinal canal compromise (%)† | 35.4 ± 13.1 | 23.1 ± 11.1 | [1.10; 95 % [CI] 1.05–1.17; P < 0.001] |
| Intravertebral instability (°)† | 10.6 ± 5.9 | 5.3 ± 3.3 | [1.43; 95 % [CI] 1.18–1.73; P < 0.001] |
Plus-minus values are mean ± SD. Logistic regression analysis with multivariate models shows independence and specific correlation between the radiographic parameters and DND [odds ratio; 95 % confidence interval (CI); P value]
DND delayed neurologic deficit
†P < 0.001 for the comparison by the Mann–whitney U test between the two groups
Outcomes of vertebroplasty
All 30 patients with DND (100 %) and all 214 patients without DND (100 %) were directly followed at 1 and 6 months; follow-up was maintained for 25 (83.3 %) and 176 (82.2 %) at 12 months, and 25 (83.3 %) and 162 (75.7 %) at 24 months after vertebroplasty, and the duration of follow-up was 18.6 ± 12.6 and 28.5 ± 10.1 months, respectively. General health problems associated with advanced age made it difficult for patients to attend our clinical examination.
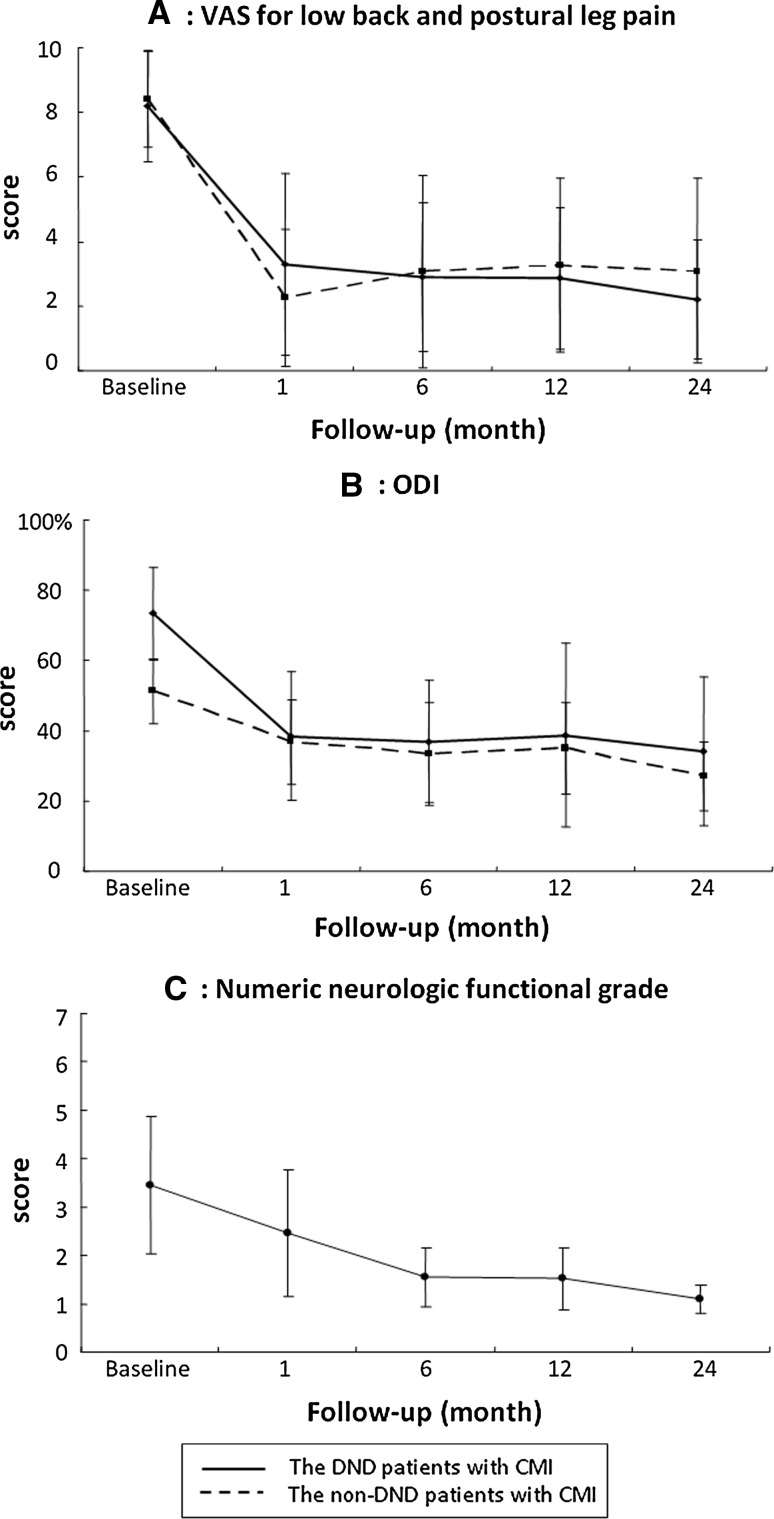
CMI was achieved in 25 patients with DND (83.3 %) and 161 without DND (75.2 %). VAS scores for back pain and postural leg pain as well as ODI were meaningfully improved immediately after vertebroplasty. Numeric neurologic functional grades gradually improved (Fig. 3). No significant differences in improvement rate of VAS score and ODI were seen between groups during the period of direct follow-up. Bilateral drop-foot in six patients recovered with manual muscle testing scores for tibialis anterior muscles from 1.7 ± 0.5 before vertebroplasty to 2.5 ± 1.3 at 1 month (P = 0.32), and 4.0 ± 1.4 at 3 months (P = 0.024) after the intervention.
Fig. 3.
Post-procedural scores of VAS, ODI, and numeric neurologic functional grade in patients with CMI. VAS score for low back pain (a) and ODI (b) were improved immediately after vertebroplasty (P < 0.001). Numeric neurologic functional grade (c) gradually improved after the intervention (1 month: P = 0.005; 6–24 months: P < 0.001) for comparison with baseline scores. Results are expressed as mean ± standard deviation (SD). VAS visual analog scale, DND delayed neurologic deficit, CMI clinically meaningful improvement, ODI Oswestry disability index
Five patients with DND (17 %) and 53 without DND (25 %) failed to achieve CMI. Four patients with DND and 53 without DND showed an initial improvement following vertebroplasty, but subsequent vertebral fracture adjacent to the treated vertebra resulted in deteriorated outcomes. No significant difference in incidence of vertebral fracture adjacent to the augmented vertebra was seen between groups (P = 0.57). Only 1 patient with DND had percent spinal canal compromise of 54.9 % and intravertebral instability of 2°, with no neurologic improvement following vertebroplasty.
Radiographic parameters in patients with DND who achieved CMI
Table 4 shows pre- and post-procedural radiographic parameters in the 25 patients with DND who achieved CMI. No substantial improvements were seen in local kyphotic angle or percent spinal canal compromise, but intravertebral instability was significantly improved after vertebroplasty (P < 0.001).
Table 4.
Pre- and post-procedural radiographic parameters in the 25 patients with DND who achieved CMI
| Radiographic parameters | Pre-PVP | Post-PVP (1 month) | Final follow up (24 months) |
|---|---|---|---|
| Local kyphotic angle (°) | 16.4 ± 8.1 | 6.5 ± 9.3 | 13.8 ± 13.2 |
| Percent spinal canal compromise (%) | 32.4 ± 11.9 | 31.9 ± 10.5 | 35.5 ± 9.8 |
| Intravertebral instability (°)† | 9.6 ± 4.0 | 2.6 ± 2.2 | 3.7 ± 2.2 |
Plus-minus values are means ± 1SD. There were no significant differences in local kyphotic angle and percent spinal canal compromise between pre- and post-PVP (by the Student’s t test)
DND delayed neurologic deficit, CM clinically meaningful improvement, PVP percutaneous vertebroplasty
†P < 0.001 for comparison between pre- and post-PVP
Procedure-related complications
The cement volume injected was 6.3 ± 2.7 ml (range, 1.8–12 ml) and 4.0 ± 2.6 ml (range, 0.5–12 ml) in cases of OVCF with IVC with and without DND, respectively, with a substantially larger volume in OVCF with IVC with DND (P < 0.001). No significant difference in incidence of cement leakage was seen between OVCF with IVC with DND (3 of 30, 10 %) and without DND (53 of 214, 24.8 %) (P = 0.07). Leakage occurred into the epidural veins (0 of 30), perivertebral soft tissue (0 of 30), intervertebral disc space (3 of 30, 10 %) and lung (0 of 30) in OVCF with IVC with DND and into the epidural veins (3 of 214, 1.4 %), perivertebral soft tissue (11 of 214, 5.1 %), intervertebral disc space (39 of 214, 18.2 %) and lung (0 of 214) in OVCF with IVC without DND (Table 5). No patients in the current study presented with neurologic deterioration related to epidural cement leakage, or with systemic complications such as cement embolism that could be attributed to the procedure itself.
Table 5.
Cement leakage outside vertebra
| Location | DND group (n = 30) | Non-DND group (n = 214) | P value‡ |
|---|---|---|---|
| Epidural vein—no. (%) | 0 (0) | 3 (1.4) | 0.67 |
| Perivertebral soft tissue—no. (%) | 0 (0) | 11 (5.1) | 0.23 |
| Intravertebral disk space—no. (%) | 3 (10) | 39 (18.2) | 0.49 |
| Lung—no. (%) | 0 (0) | 0 (0) | – |
| Total—no. (%) | 3 (10) | 53 (24.8) | 0.07 |
‡Between both groups, comparisons for occurrence rates of cement leakage were analysed with Fisher’s exact test and Chi-square test
Discussion
This study showed that intravertebral instability and spinal canal compromise could result in the development of DND. In particular, intravertebral instability is considered to be the predominant cause of DND. Percutaneous vertebroplasty is an effective and safe intervention providing clinically meaningful improvement. We have demonstrated that percutaneous vertebroplasty can be readily performed by injection of PMMA into the IVC with the assistance of a cavitygram.
In the literatures, the following factors have been suspected as causes of DND: (1) neural compression secondary to retropulsed bone fragments; (2) progression of kyphosis with vertebral collapse; and (3) intravertebral instability at the fracture site. In accordance with this theory, several types of surgical decompression and fusion through either an anterior and/or posterior approach have been performed [1, 12, 15, 19, 26, 31, 34, 37]. However, some authors have reported that conservative treatment could provide reliable neurologic improvement. Heggeness et al. [13] performed a retrospective study on nine cases of OVCF with IVC with DND. Three with profound and evolving neurologic deficits were treated surgically. Six with significant medical problems and/or relatively minor motor deficits were managed non-operatively. Patients treated surgically recovered full neurologic function, while patients treated non-operatively recovered objective neurologic function. Ataka et al. [2] performed posterior instrumented fusion without neural decompression as treatment for 14 consecutive patients with DND. These procedures provided neurologic improvement and relief of back pain without major complications. They hypothesized that intravertebral instability at the fracture site rather than neural compression is the major cause of DND. In our study, prior to vertebroplasty, we found no significant difference in local kyphotic angle between patients with and without DND. Percent spinal canal compromise and intravertebral instability were greater in the DND group than in the non-DND group. After vertebroplasty, patients with DND who achieved CMI showed substantial improvement of intravertebral instability, although no improvements were attained in local kyphotic angle or percent spinal canal compromise. Our results support the hypothesis that intravertebral instability represents the main cause of DND.
No definitive surgical options are available for the treatment of OVCF with IVC with DND. A combined anterior and posterior procedure may maximize the chances for successful fusion, particularly with multiple points of spinal fixation and occasionally with PMMA augmentation [18]. However, large surgical interventions are still challenging for patients of advanced age, with medical co-morbidities, or with poor fixation secondary to osteoporosis [29]. As the population ages, numbers of patients with OVCF with IVC with DND are rapidly increasing. Setting a guideline for the treatment of this pathology is an urgent issue needing to be tackled.
There are some reports about the incidence of cement leakage in vertebroplasty for OVCF with or without IVC. Nieuwenhuijse et al. [23] and Ha et al. [11] reported that there were higher rates of cement leakage in patients with IVC. On the other hand, Tanigawa et al. [35] reported that there was no statistically significant difference in the incidence of cement leakage between OVCF with cleft and without cleft. Although the differences of the results of these studies are unclear, there is a risk of cement leakage in vertebroplasty for OVCF. In our series, bone needles were inserted into an IVC through a bilateral transpedicular approach and IVC was specifically targeted for PMMA injection. Furthermore, cavitygram of the IVC was performed and measured the capacity of the intravertebral cleft in order to increase the risk of cement leakage. PMMA was injected from the one-sided needle with low pressure and filled the intravertebral cleft. In our series, the rate of incidence of cement leakage was observed in 56 of 244 (23.0 %). This rate of cement leakage seems to be low compared with other studies [11, 23, 35]. PMMA injection into the IVC after cavitygram might reduce the risk of cement leakage.
The current study identified intravertebral instability as the main cause of DND with OVCF with IVC and found that vertebroplasty could be readily performed with injection of the same amount of PMMA as the capacity of the cleft, to stabilize the affected vertebra. Based on our results, we believe that vertebroplasty could be an alternative method for the treatment of OVCF with IVC with DND, as an effective and safe intervention. However, vertebroplasty for patients with severe spinal canal compromise and less intravertebral instability could not achieve any neurologic improvement. Vertebroplasty is not indicated for OVCF with IVC with DND that is affected by static factors other than intravertebral instability. Our study could not statistically describe the cut-off value for spinal canal compromise or intravertebral instability between the CMI group and the non-CMI group because of the small number of patients who failed to achieve CMI. To establish the concrete algorithm for the treatment of this pathology, we should perform a comparative study between vertebroplasty and surgical treatment. However, the significant co-morbidities of our elderly patients with advanced osteoporosis made it difficult to set a randomized controlled trial.
Conclusions
Intravertebral instability and spinal canal compromise could result in the development of DND. In particular, intravertebral instability is considered to be the predominant cause of DND. Percutaneous vertebroplasty is an effective and safe intervention providing clinically meaningful improvement. We have demonstrated that percutaneous vertebroplasty can be readily performed by injection of PMMA into the IVC with the assistance of a cavitygram. It is not suggested that this procedure is applicable to the overall cases of OVCF with IVC with DND. Patients with less intravertebral instability and severe spinal canal compromise should be considered as candidates for spinal reconstructive surgery.
Acknowledgments
We thank Prof. John G. Heller (Department of Orthopaedic Surgery, Emory University Medical Center and the Spine Surgery Service, Emory Orthopaedic and Spine Center, Decatur, Georgia) for helpful comments. We are also indebted to Dr. Hiroto Kuwabara (Division of Nuclear Medicine, Department of Radiology, Johns Hopkins University, Baltimore, MD) for his helpful suggestions and excellent assistance in creating this article.
Conflict of interest
None.
References
- 1.Arciero RA, Leung KYK, Pierce JH. Spontaneous unstable burst fracture of the thoracolumbar spine in osteoporosis. Spine. 1989;14:114–117. doi: 10.1097/00007632-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 2.Ataka H, Tanno T, Yamazaki M. Posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. Eur Spine J. 2009;18:69–76. doi: 10.1007/s00586-008-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baba H, Maezawa Y, Kamitani K, Furusawa N, Imura S, Tomita K. Osteoporotic vertebral collapse with late neurological complications. Paraplegia. 1995;33:281–289. doi: 10.1038/sc.1995.64. [DOI] [PubMed] [Google Scholar]
- 4.Boszczyk BM, Bierschneider M, Schmid K, Grillhosl A, Robert B. Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J Neurosurg. 2004;100(1 Suppl):32–37. doi: 10.3171/spi.2004.100.1.0032. [DOI] [PubMed] [Google Scholar]
- 5.Chevalier X, Wrona N, Avouac B, Larget-Piet B. Thigh pain and multiple vertebral osteonecroses: value of magnetic resonance imaging. J Rheumatol. 1991;18:1627–1630. [PubMed] [Google Scholar]
- 6.Do HM, Jensen ME, Marx WF, Kallmes DF. Percutaneous vertebroplasty in vertebral osteonecrosis (Kummell’s spondylitis) Neurosurg Focus. 1999;7(1):e2. doi: 10.3171/foc.1999.7.1.4. [DOI] [PubMed] [Google Scholar]
- 7.Dvorak MF, Fisher CG. Revision spine surgery in the presence of osteoporosis. In: Margulies JY, Aebi M, Farcy JP, editors. Revision spine surgery. 1. St. Louis: Mosby; 1999. pp. 646–653. [Google Scholar]
- 8.Fairbank J, Davies J, Couper J, O’Brien JP. The oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 9.Faciszewski T, Winter RB, Lonstein JE, Denis F, Johnson L. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine. 1995;14:1592–1599. doi: 10.1097/00007632-199507150-00007. [DOI] [PubMed] [Google Scholar]
- 10.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ha KY, Lee JS, Kim KW, Chon JS. Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. JBJS-Br. 2006;88(5):629–633. doi: 10.1302/0301-620X.88B5.17345. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa K, Homma T, Uchiyama S, Takahashi H. Vertebral pseudarthrosis in the osteoporotic spine. Spine. 1998;23:2201–2206. doi: 10.1097/00007632-199810150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Heggeness MH. Spine fracture with neurological deficit in osteoporosis. Osteoporosis Int. 1993;3:215–221. doi: 10.1007/BF01623679. [DOI] [PubMed] [Google Scholar]
- 14.Jang JS, Kim DY, Lee SH. Efficacy of percutaneous vertebroplasty in the treatment of intravertebral pseudoarthrosis associated with noninfected avascular necrosis of the vertebral body. Spine. 2003;28:1588–1592. [PubMed] [Google Scholar]
- 15.Kaneda K, Asano S, Hashimoto T, Satoh S, Fujiya M. The treatment of osteoporoticposttraumatic vertebral collapse using the Kaneda device and a bioactive ceramic vertebral prosthesis. Spine. 1992;17:S295–S303. doi: 10.1097/00007632-199208001-00015. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan PA, Orton DF, Asleson RJ. Osteoporosis with vertebral compression fractures, retropulsed fragments and neurologic compromise. Radiology. 1987;165:533–535. doi: 10.1148/radiology.165.2.3659378. [DOI] [PubMed] [Google Scholar]
- 17.Kawaguchi S, Horigome K, Yajima H, Oda T, Kii Y, Ida K, et al. Symptomatic relevance of intravertebral cleft in patients with osteoporotic vertebral fracture. J Neurosurg Spine. 2010;13:267–275. doi: 10.3171/2010.3.SPINE09364. [DOI] [PubMed] [Google Scholar]
- 18.Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6:479–487. doi: 10.1016/j.spinee.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Kim KT, Suk KS, Kim JM, Lee SH. Delayed vertebral collapse with neurological deficits secondary to osteoporosis. Int Orthop. 2003;27:65–69. doi: 10.1007/s00264-002-0418-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lafforgue P. Pathophysiology and natural history of avascular necrosis of bone. Jt Bone Spine. 2006;73:500–507. doi: 10.1016/j.jbspin.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 21.McKiernan F, Faciszewski T. Intravertebral clefts in osteoporotic vertebral compression fractures. Arthritis Rheum. 2003;48:1414–1419. doi: 10.1002/art.10984. [DOI] [PubMed] [Google Scholar]
- 22.Melton LJ., III Epidemiology of vertebral fractures in women. Am J Epidemiol. 1989;129:1000–1011. doi: 10.1093/oxfordjournals.aje.a115204. [DOI] [PubMed] [Google Scholar]
- 23.Nieuwenhuijse MJ, van Rijswijk CS, van Erkel AR, Dijkstra SP. The intravertebral cleft in painful long-standing osteoporotic vertebral compression fractures treated with percutaneous vertebroplasty: diagnostic assessment and clinical significance. Spine. 2012;37(11):974–981. doi: 10.1097/BRS.0b013e318238bf22. [DOI] [PubMed] [Google Scholar]
- 24.Ostelo RW, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, Bouter LM, de Vet HC. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 25.Sailhan F, Funck-Brentano T, Dougados M, Anract P. Leg pain may reveal vertebral osteoporosis. J Rheumatol. 2009;36:2621–2622. doi: 10.3899/jrheum.090452. [DOI] [PubMed] [Google Scholar]
- 26.Saita K, Hoshino Y, Kikkawa I, Nakamura H. Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine. 2000;25:2832–2835. doi: 10.1097/00007632-200011010-00018. [DOI] [PubMed] [Google Scholar]
- 27.Saito F, Takahashi K, Tanaka S, Torio T, Iizuka H, Wei C, Oda H. Effects of vertebroplasty for delayed-onset paraplegia caused by vertebral pseudarthrosis. J Orthop Sci. 2011;16(6):673–681. doi: 10.1007/s00776-011-0155-y. [DOI] [PubMed] [Google Scholar]
- 28.Scott J, Huskisson EC. Graphic representation of pain. Pain. 1976;2:175–184. doi: 10.1016/0304-3959(76)90113-5. [DOI] [PubMed] [Google Scholar]
- 29.Singh K, Heller JG, Samartzis D, Price JS, An HS, Yoon ST, Rhee J, Ledlie JT, Phillips FM. Open vertebral cement augmentation combined with lumbar decompression for the operative management of thoracolumbar stenosis secondary to osteoporotic burst fractures. J Spinal Disord Tech. 2005;18:413–419. doi: 10.1097/01.bsd.0000173840.59099.06. [DOI] [PubMed] [Google Scholar]
- 30.Steel HH. Kümmell’s disease. Am J Surg. 1951;81:161–167. doi: 10.1016/0002-9610(51)90206-1. [DOI] [PubMed] [Google Scholar]
- 31.Suk SI, Kim JH, Lee SM, Chung ER, Lee JH. Anterior-posterior surgery versus posterior closing wedge osteotomy in posttraumatic kyphosis with neurologic compromised osteoporotic fracture. Spine. 2003;28:2170–2175. doi: 10.1097/01.BRS.0000090889.45158.5A. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland CJ, Miler F, Wang GJ. Early progressive kyphosis following compression fractures. Two cases reports from a series of “stable” thoracolumbar compression fractures. Clin Orthop Relat Res. 1983;173:216–220. [PubMed] [Google Scholar]
- 33.Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J. 2008;17(10):1380–1390. doi: 10.1007/s00586-008-0753-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka S, Kubota M, Fujimoto Y, Hayashi J, Nishikawa K. Conus medullaris syndrome secondary to an L1 burst fracture in osteoporosis. Spine. 1993;18:2131–2134. doi: 10.1097/00007632-199310001-00034. [DOI] [PubMed] [Google Scholar]
- 35.Tanigawa N, Kariya S, Komemushi A, Tokuda T, Nakatani M, Yagi R, Sawada S. Cement leakage in percutaneous vertebroplasty for osteoporotic compression fractures with or without intravertebral clefts. AJR Am J Roentgenol. 2009;193(5):442–445. doi: 10.2214/AJR.09.2774. [DOI] [PubMed] [Google Scholar]
- 36.Tsou PM, Wang J, Khoo L, Shamie AN, Holly L. A thoracic and lumbar spine injury severity classification based on neurologic function grade, spinal canal deformity, and spinal biomechanical stability. Spine J. 2006;6:636–647. doi: 10.1016/j.spinee.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Uchida K, Nakajima H, Yayama T, Miyazaki T, Hirai T, Kobayashi S, Chen K, Guerrero AR, Baba H. Vertebroplasty-augmented short-segment posterior fixation of osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine: comparisons with posterior surgery without vertebroplasty and anterior surgery. J Neurosurg Spine. 2010;13:612–621. doi: 10.3171/2010.5.SPINE09813. [DOI] [PubMed] [Google Scholar]