Abstract
Patients with schizophrenia exhibit cognitive and sensory impairment, and object recognition deficits have been linked to sensory deficits. The “frame and fill” model of object recognition posits that low spatial frequency (LSF) information rapidly reaches the prefrontal cortex (PFC) and creates a general shape of an object that feeds back to the ventral temporal cortex to assist object recognition. Visual dysfunction findings in schizophrenia suggest a preferential loss of LSF information. This study used functional magnetic resonance imaging (fMRI) and resting state functional connectivity (RSFC) to investigate the contribution of visual deficits to impaired object “framing” circuitry in schizophrenia. Participants were shown object stimuli that were intact or contained only LSF or high spatial frequency (HSF) information. For controls, fMRI revealed preferential activation to LSF information in precuneus, superior temporal, and medial and dorsolateral PFC areas, whereas patients showed a preference for HSF information or no preference. RSFC revealed a lack of connectivity between early visual areas and PFC for patients. These results demonstrate impaired processing of LSF information during object recognition in schizophrenia, with patients instead displaying increased processing of HSF information. This is consistent with findings of a preference for local over global visual information in schizophrenia.
Keywords: cortical circuitry, magnocellular, object recognition, schizophrenia, visual
Introduction
Cognitive dysfunction in schizophrenia is well documented (Carter et al. 2008) and is related to functional outcome (Green 2006). A growing literature including steady-state and transient event-related potential, psychophysical, and functional magnetic resonance imaging (fMRI) studies provides evidence that sensory impairment is also a core feature of schizophrenia (Slaghuis 1998; Foxe et al. 2001; Braus et al. 2002; Ardekani et al. 2003; Brenner et al. 2003; Butler et al. 2005; Kim et al. 2005; Schechter et al. 2005; Butler et al. 2006; Yeap et al. 2006; Butler et al. 2008; Martinez et al. 2008; Silverstein et al. 2010b; Chen 2011), and this impairment may propagate to higher cognitive processes such as motion processing (Kim et al. 2006), perceptual grouping and organization (Kurylo et al. 2007; Silverstein and Keane 2011), reading (Revheim et al. 2006), and emotion recognition (Leitman et al. 2005; Turetsky et al. 2007; Butler et al. 2009; Leitman et al. 2011). Further, recent findings indicate that the bottom-up propagation of deficits from sensory to higher level processes in schizophrenia occur even when top-down processes are intact (Dias et al. 2011). However, the specific contributions of visual deficits to higher cognitive dysfunction have yet to be fully elucidated. The present study utilized functional magnetic resonance imaging (fMRI) to investigate object recognition in schizophrenia, a task which links visual input to higher cognitive functioning.
Object recognition occurs in the ventral temporal cortex (VTC) (Hubel and Wiesel 1962; Tanaka 1993; Pasupathy and Connor 1999; Vogels et al. 2001) and depends on converging visual processing input. The VTC receives input from the magnocellular and parvocellular subcortical visual pathways (Kaplan and Shapley 1982, 1986; Wurtz and Kandel 2000). The magnocellular pathway responds rapidly and is biased toward responding to low spatial frequency (LSF) (i.e., low resolution) information, which it preferentially relays to cortical dorsal stream areas (Ungerleider and Mishkin 1982; Shapley 1990; Merigan and Maunsell 1993). The dorsal stream, in turn, projects to the prefrontal cortex (Wise et al. 1997; Endo et al. 1999; Petrides and Pandya 1999; Saron et al. 2001). One theory of object processing is that a general shape of an object stimulus is activated in the PFC, and this “frame” of the object is fed back to the VTC (Ullman 1995; Bar 2003; Bar et al. 2006; Kveraga et al. 2007; Sehatpour et al. 2010). The parvocellular pathway, on the other hand, responds more slowly and is biased toward responding to high spatial frequency (HSF) (i.e., fine detail) information, which it preferentially relays to cortical ventral stream areas including the VTC (Ungerleider and Mishkin 1982; Shapley 1990; Merigan and Maunsell 1993). The VTC thus receives both dorsal and ventral stream inputs, which interact during object recognition. Due to the different speeds of the 2 pathways, the input from the PFC arrives in the VTC in time to provide a frame of an object which is then “filled” by fine detail information arriving later from the parvocellular pathway (Ullman 1995; Schmolesky et al. 1998; Schroeder et al. 1998; Lamme and Roelfsema 2000; Bar 2003; Bar et al. 2006; Kveraga et al. 2007; Sehatpour et al. 2010; Tapia and Breitmeyer 2011). Under the frame and fill model, object recognition is achieved by gradually integrating fine details of an object into a coherent whole in the VTC (Tanaka 1993, 1996; Bar et al. 2001; Grill-Spector et al. 2001; Malach et al. 2002; Brincat and Connor 2006), and the PFC frame facilitates this process by constraining it to a limited number of possible objects (Bar 2003; Bar et al. 2006; Kveraga et al. 2007).
Other recent findings support the idea that low resolution global information is processed prior to fine detail information in object recognition (Chen 2005; Conci et al. 2011; de la Rosa et al. 2011). However, it is unclear which cortical areas process this information. Some evidence indicates that global information is represented as early as primary visual cortex and transmitted directly to the VTC (Altmann et al. 2003; Kourtzi et al. 2003; Ban et al. 2006; Mannion et al. 2010), while other findings suggest that primary visual cortex represents only fine detail information, and only higher areas process global information (Kourtzi and Huberle 2005; Swettenham et al. 2010). The frame and fill model suggests that the VTC receives global information from higher areas such as the PFC and fine detail information from primary visual cortex. Indeed, the laminar profile of the earliest responses of VTC neurons in the macaque monkey was consistent with initial input from the dorsal stream and/or higher cortical areas, rather than with initial afferent input from occipital visual areas (Chen et al. 2007). In addition, connections between the frontal cortex and temporal cortex in the macaque were found to be crucial for object recognition (Parker and Gaffan 1998).
Bar and colleagues have studied visual pathway contributions to human object recognition utilizing MEG and fMRI. They found that stimuli biased toward the magnocellular pathway by using LSF or low contrast increased PFC activity, whereas stimuli biased toward the parvocellular pathway by using HSF or isoluminant chromatic contrast produced less PFC activity and more ventral stream activity (Bar et al. 2006; Kveraga et al. 2007). In addition, PFC activity was related to a performance advantage for magnocellular-biased stimuli in an object recognition task (Kveraga et al. 2007). Effective connectivity analysis of MEG time courses revealed interactions between occipital visual areas and PFC, followed by later interactions between PFC and VTC. These interactions took place only for magnocellular-biased and -unbiased stimuli, and not for parvocellular-biased stimuli (Bar et al. 2006). Taken together, these results suggest that during object recognition, the PFC rapidly receives low-resolution magnocellular pathway information via the dorsal stream, which in turn feeds back to the VTC. This feedback provides the frame of the object, which is then filled by the fine detail parvocellular pathway information.
Object recognition deficits have been found in schizophrenia with behavioral studies in which patients failed to integrate fine details into whole object representations (Doniger et al. 2002; Sehatpour et al. 2010). Early-stage visual deficits in schizophrenia preferentially involving the magnocellular pathway (Butler et al. 2001; Schechter et al. 2003; Butler et al. 2005; Kéri et al. 2005; Kim et al. 2005; Schechter et al. 2005; Butler et al. 2008; Martinez et al. 2008; Butler et al. 2009; Green et al. 2009) suggest that the framing function of the PFC may be impaired, though parvocellular deficits (Slaghuis 1998; Brittain et al. 2010) and increased magnocellular responses (Green et al. 1994; Kiss et al. 2010) have also been found. The preferential magnocellular deficit in schizophrenia is thought to arise as a result of impaired nonlinear gain (i.e., signal amplification) (Butler et al. 2005; Kim et al. 2005; Butler et al. 2008; Green et al. 2009) mediated by N-Methyl-d-aspartate (NMDA) glutamate receptors as this mechanism is used by magnocellular neurons far more than by parvocellular neurons (Fox et al. 1990; Kwon et al. 1992; Daw et al. 1993; Zemon and Gordon 2006; Lisman et al. 2008) and appears to be impaired in schizophrenia (Goff and Coyle 2001; Javitt 2004; Krystal et al. 2005; Javitt 2009; Kantrowitz and Javitt 2010a, 2010b). Findings of parvocellular dysfunction may occur under conditions that drive that pathway to utilize this nonlinear gain mechanism normally favored by the magnocellular pathway (Butler et al. 2005). However, strong evidence in favor of a preferential magnocellular deficit in schizophrenia suggests a specific bottom-up deficit in the propagation of magnocellular-biased information to the dorsal stream and PFC during object recognition.
Evidence for this pattern of dysfunction has been found in studies of perceptual closure, a task in which participants are asked to recognize fragmented objects. For instance, impairments in perceptual closure, as well as impairments in event-related potential (ERP) components associated with perceptual closure and dorsal stream activity, were found in schizophrenia patients, while a ventral stream ERP component remained intact (Doniger et al. 2002). These ERP findings were recently replicated and, using fMRI an impaired network of dorsal stream, ventral stream, PFC, and hippocampal activity related to perceptual closure was found (Sehatpour et al. 2010). Path analysis suggested that dorsal stream deficits led to PFC deficits, which in turn led to ventral stream and hippocampal deficits. These studies support the findings of a dorsal stream–PFC–VTC circuit in object recognition, and its impairment in schizophrenia.
The current study sought to further clarify the link between early-stage visual deficits and impaired ability to frame objects in schizophrenia by utilizing the same fMRI object recognition paradigm as Bar et al. (2006) and extending it to schizophrenia. This paradigm biases object stimuli toward the magnocellular or parvocellular pathway by filtering them to contain LSF or HSF information, respectively. Using ERP and fMRI, Martinez et al. (2012) recently demonstrated that schizophrenia patients have impaired activity in extrastriate visual areas in response to LSF, but not to HSF, grating stimuli. This suggests that patients may display similar deficits to LSF object stimuli, and that these deficits may propagate to the PFC, resulting in impaired framing feedback to the VTC. In addition to this fMRI paradigm, this study also utilized resting state functional connectivity (RSFC) to examine the functional networks underlying object recognition in both schizophrenia patients and healthy control participants.
Methods
Participants
Twenty-four patients who met DSM-IV criteria for schizophrenia and 17 healthy volunteers participated. Patients were recruited through inpatient and outpatient facilities associated with the Nathan Kline Institute for Psychiatric Research. Diagnoses were obtained using the Structured Clinical Interview for DSM-IV (SCID) (First et al. 1997) and available clinical information. Controls were recruited through the Volunteer Recruitment Program at the Nathan Kline Institute. All participants provided informed consent and received cash compensation for their time. The study was approved by the Nathan Kline Institutional Review Board. Healthy volunteers with a history of SCID-defined Axis I psychiatric disorders were excluded. Patients and controls were excluded if they had any neurological or ophthalmological disorders, including glaucoma or cataracts, that might affect performance or if they met criteria for alcohol or substance dependence within the last 6 months or abuse within the last month. All participants had normal or corrected-to-normal visual acuity of 20/32 or better on the Logarithmic Visual Acuity Chart (Precision Vision). All patients were receiving antipsychotic medication at the time of testing, except for 1 patient who had refused to continue haloperidol decanoate. Chlorpromazine equivalents were calculated as previously described (Woods 2003, 2005, 2011). All data reported below are mean ± standard deviation.
Groups did not differ significantly in age (patients, 37.39 ± 9.67; controls, 36.41 ± 7.65; P = 0.73) or gender (patients, 19 males, 5 females; controls, 13 males, 4 females; P = 0.83). Patients had significantly lower socioeconomic status (SES) as measured by the 4-factor Hollingshead Scale (patients, 23.67 ± 6.68; controls, 41.88 ± 10.13; t(29) = − 5.86, P < 0.001), although parental SES did not significantly differ between groups (patients, 32.82 ± 11.21; controls, 42.75 ± 14.19; P = 0.06). Patients also had significantly reduced IQ (patients, 92.53 ± 7.33; controls, 98.56 ± 6.79; t(29) = − 2.38, P = 0.02) and education as measured by the highest grade achieved (patients, 11.25 ± 1.77; controls, 14.06 ± 1.53; t(30) = − 4.81, P < 0.001). Patients were ill for an average of 14.22 ± 7.48 years, had an average Global Assessment of Functioning (GAF) score of 38.52 ± 11.05, and were receiving antipsychotic doses equivalent to an average of 938.41 ± 672.63 mg of chlorpromazine per day. Although demographic data for some variables were unavailable for some patients, the overall sample characteristics were similar to those in recent publications from our group (Dias et al. 2011, Martinez et al. 2012).
Functional Magnetic Resonance Imaging
Apparatus
A 3T Siemens TIM Trio magnetic resonance scanner at the Nathan Kline Institute was used for all functional and structural scans. Functional scans contained 34 axial slices, with TR = 2000 ms, TE = 30 ms, and voxel size = 2.5 × 2.5 × 2.8 mm, with a 0.7 mm gap. High-resolution structural scans were performed with a 3D magnetization prepared rapid acquisition gradient echo (MPRAGE) sequence, having 192 sagittal slices with TR = 2500 ms, TE = 3.5 ms, FA = 8°, and voxel size = 1 × 1 × 1 mm. Slice time correction, motion correction, normalization to a value of 100, smoothing (8 mm FWHM Gaussian kernel), skull stripping, deconvolution of relevant time series, and first-order regression analyses were performed using AFNI (http://afni.nimh.nih.gov/; Cox 1996). Functional and structural scans were coregistered and transformed into a common Talairach space using the Automatic Registration Toolbox (Ardekani et al. 2004; Klein et al. 2009).
Stimuli and procedure
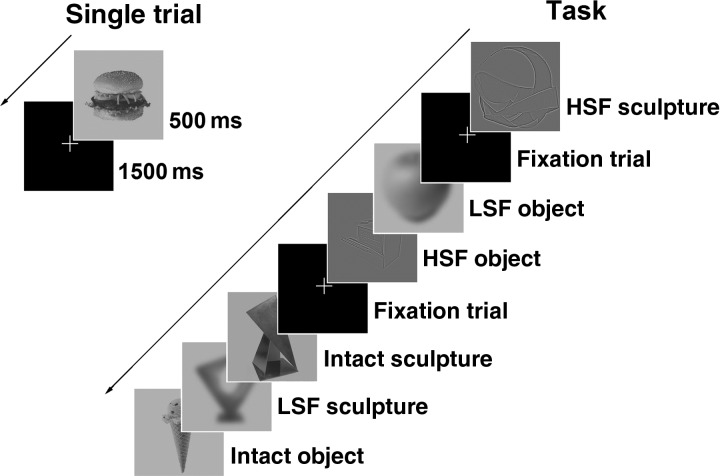
Grayscale images of ordinary objects and abstract sculptures were filtered to contain only LSF (≈6 cycles per image) or HSF (≈30 cycles per image) information, as reported by Bar et al. (2006). All LSF, HSF, and unfiltered (Intact) images had an identical root mean square contrast of 50.26%, which is often reported for complex visual stimuli (Ojanpää and Näsänen 2003). Two fMRI scanning blocks were performed, each consisting of 101 trials. In each block, 24 ordinary object images were each presented in Intact, LSF, and HSF form (Fig. 1). Five abstract sculpture stimuli were also presented in each block (Intact:LSF:HSF ratio of 1:2:2, 2:1:2, or 2:2:1 randomly determined for each block). Abstract sculpture stimuli were purposely chosen to not resemble ordinary objects. In addition, 24 fixation trials were presented in each block to create “jitter” in the image time series to better sample the hemodynamic response function. Each trial consisted of 500 ms for image presentation, followed by 1500 ms of fixation, except for fixation trials which consisted solely of 2000 ms of fixation. Object image trials, sculpture image trials, and fixation trials were presented in a random order. In a forced choice task, participants indicated by button press whether the stimulus depicted an ordinary object or an abstract sculpture, with response time limited to 2000 ms after stimulus onset. Responses and reaction times were recorded.
Figure 1.
Object recognition task used during fMRI scanning. Ordinary object and abstract sculpture stimuli were shown either in their Intact state or filtered to contain only low spatial frequency (LSF) or high spatial frequency (HSF) information. A single 2000 ms stimulus trial consisted of stimulus presentation (500 ms) followed by fixation (1500 ms) (left-hand side of Fig. 1). In addition, there were 24 “fixation alone” trials consisting of 2000 ms of fixation that were randomly presented among stimulus trials (right-hand side of Fig. 1). Participants pressed buttons on stimulus trials to indicate whether the image depicted an ordinary object or an abstract sculpture.
Analysis
First-order regression analyses for each participant isolated fMRI activity related to correct Intact, LSF, and HSF ordinary object trials, resulting in beta maps. These results were then used for higher-order group analyses. A whole-brain mixed-effects 2-way ANOVA with group and condition as factors was performed on the beta maps to examine differences in activity between stimulus conditions and between groups. Regions of interest (ROIs) were determined based on the interaction of group and condition. Two a priori ROIs were calculated from the Talairach Tournoux atlas, for bilateral Brodmann area 17 (BA 17) and bilateral fusiform gyrus (FG). These areas represent basic visual processing and VTC areas, respectively. For each ROI, each participant's average beta value for each condition was calculated, and these were compared within and across groups.
Resting State Functional Connectivity
Apparatus
Scans were performed with the same scanner described above. Functional scans contained 34 axial slices, with TR = 2000 ms, TE = 30 ms, and voxel size = 2.5 × 2.5 × 2.7 mm with a 0.8 mm skip. Preprocessing was conducted using the 1000 Functional Connectomes Project (Biswal et al. 2010) scripts, available at http://fcon_1000.projects.nitrc.org/indi/pro/nki.html. The MPRAGE was segmented using FSL's (http://www.fmrib.ox.ac.uk/fsl/) FAST software to obtain the masks for white matter (WM) and cerebrospinal fluid (CSF). The WM and CSF time series were then spatially averaged for their respective compartments. These time series, as well as those for the 6 motion parameters, were used as covariates of no interest in a general linear model (GLM), and were regressed from the native-space EPI time series.
Stimuli and procedure
Participants were instructed to keep their eyes open and remain still and alert. A total of 180 functional scans were performed, the first 5 of which were discarded prior to preprocessing.
Analysis
Seven seed regions were defined as the 2 a priori ROIs and 5 functionally derived ROIs as defined above. For each participant, average time series data were extracted for each ROI and correlated with the time series of all other ROIs. These correlations were standardized using Fisher's r-to-z transformation. Correlation z-scores for each group were tested against zero in a 1-sample t-test to determine which connections between ROIs were significant.
Results
Behavioral Results
A main effect of stimulus condition (F(2,117) = 22.832, P < 0.001) showed that reaction time was faster for Intact stimuli (controls: M = 694.29, SD = 176.52; patients: M = 730.77, SD = 170.20) than LSF stimuli (controls: M = 772.32, SD = 190.38; patients: M = 818.14, SD = 177.57) (t(40) = − 7.961, P < 0.001) and HSF stimuli (controls: M = 793.78, SD = 238.37; patients: M = 827.32, SD = 187.94) (t(40) = − 4.898, P < 0.001). A main effect of stimulus condition (F 2,117 = 29.598, P < 0.001) also revealed that accuracy was higher for Intact stimuli (controls: M = 76.34%, SD = 25.29%; patients: M = 81.23%, SD = 17.26%) than LSF stimuli (controls: M = 55.53%, SD = 28.51%; patients: M = 58.04%, SD = 21.82%) (t(40) = 6.493, P < 0.001) and HSF stimuli (controls: M = 52.82%, SD = 32.15%; patients: M = 54.86%, SD = 26.46%) (t(40) = 7.383, P < 0.001). However, no between-group differences in reaction time or accuracy were found for any stimulus condition, and no interactions between group and condition were found. The lack of between-group differences indicates that the task was not more difficult for patients or controls for any stimulus condition. Only correct trials were used in the fMRI analyses.
Functional Magnetic Resonance Imaging
A whole-brain mixed-effects 2-way ANOVA revealed a significant interaction between group and condition in 5 regions (F = 4.09, cluster size = 48, corrected P = 0.001). These regions were then defined as ROIs (Table 1), and post-hoc t-tests were performed on average beta coefficients within these ROIs. Similar t-tests were performed for average beta coefficients for the 2 a priori ROIs (Table 2).
Table 1.
Functional ROIs based on the group-by-condition interaction
| ROI | Brodmann area | Center of mass | Number of voxels |
|---|---|---|---|
| pCun | |||
| Left | 7, 31 | 6.9, 49.6, 36.6 | 178 |
| Right | 7, 31 | −6.8, 59.0, 30.3 | 76 |
| STG (right) | 40 | −56.6, 46.7, 19.5 | 96 |
| CD (left) | – | 15.5, −16.2, −0.8 | 67 |
| MPFC (left) | 9 | 7.1, −47.9, 21.0 | 98 |
| DLPFC (left) | 46 | 41.7, −43.7, 5.6 | 88 |
Table 2.
A priori ROIs based on the Talairach Tournoux atlas
| ROI | Number of voxels |
|---|---|
| BA 17 | 313 |
| FG | |
| Left | 547 |
| Right | 546 |
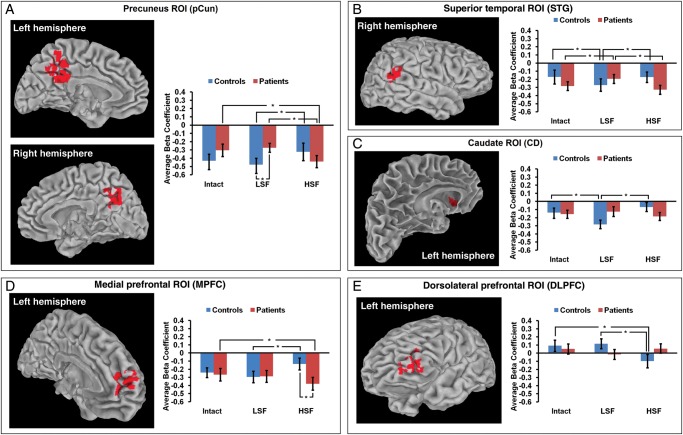
A bilateral precuneus ROI (pCun) demonstrated an interaction in which controls had more activity for LSF than HSF stimuli (t(16) = − 2.313, P < 0.05), while patients had more activity for HSF than LSF (t(23) = 3.233, P < 0.005) and Intact stimuli (t(23) = 2.198, P < 0.05). Controls also had significantly greater activity than patients for LSF stimuli (t(39) = −2.229, P < 0.05) (Fig. 2A).
Figure 2.
ROIs showing an interaction between group and stimulus condition. For all ROIs, F = 4.09, cluster size = 48, corrected P = 0.001. For all bar graphs, *P< 0.05.
The right superior temporal gyrus ROI (STG) showed a similar interaction pattern to the pCun ROI. Controls had more activity for LSF than HSF (t(16) = −2.311, P < 0.05) and Intact stimuli (t(16) = −2.621, P < 0.05), while patients had less activity for LSF than HSF (t(23) = 3.570, P < 0.005) and Intact stimuli (t(23) = 2.748, P < 0.05) (Fig. 2B).
The left caudate ROI (CD) again showed greater activity for LSF than HSF (t(16) = −4.707, P < 0.001) and Intact stimuli (t(16) = −2.506, P < 0.05) for controls, but no significant differences between conditions in patients (Fig. 2C).
The left medial prefrontal ROI (MPFC) also showed greater activity for LSF than HSF stimuli (t(16) = 3.381, P < 0.005) for controls, and greater activity for HSF than Intact stimuli (t(23) = −2.444, P < 0.05) for patients. In addition, patients had significantly greater activity than controls for the HSF condition (t(39) = −2.188, P < 0.05) (Fig. 2D).
The left dorsolateral prefrontal ROI (DLPFC) demonstrated differences in activity between conditions for controls, but not patients. For controls, activity for HSF stimuli differed significantly from activity for LSF (t(16) = −3.632, P < 0.005) and Intact stimuli (t(16) = −2.686, P < 0.05). In this case, HSF related activity was negative compared with fixation, while Intact and LSF-related activity was positive (Fig. 2E).
The a priori bilateral Brodmann area 17 ROI (BA 17) showed no differences between conditions for controls. Patients had significantly higher activity for Intact than LSF stimuli (t(23) = 2.517, P < 0.05) (Fig. 3A). The a priori bilateral fusiform gyrus ROI (FG) also did not have differences between conditions for controls. Patients had significantly higher activity for Intact than LSF (t(23) = 3.165, P < 0.005) and HSF stimuli (t(23) = 3.227, P < 0.005) (Fig. 3B). There were no significant between-group differences for A17 or FG.
Figure 3.
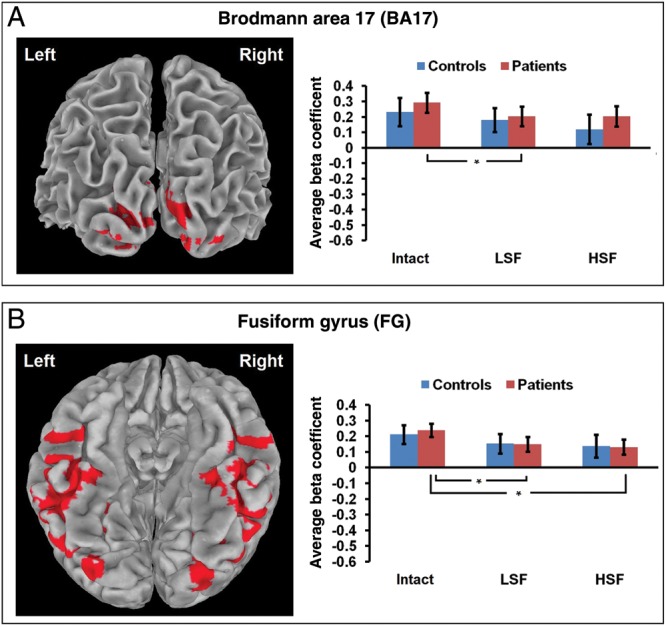
A priori ROIs defined by Talairach Tournoux atlas. For all bar graphs, *P< 0.05.
Resting State Functional Connectivity
Seeds for the RSFC data were defined as the fMRI ROIs described above. Significant correlations between seeds (P < 0.05) revealed differing functional networks between controls and patients (Fig. 4). Controls had 4 correlations that patients lacked, which were BA 17 and DLPFC, BA 17 and MPFC, DLPFC and MPFC, and BA 17 and STG. Patients had 3 correlations that controls lacked, which were BA 17 and CD, pCun and CD, and BA 17 and pCun. These resting state correlations established functional networks for controls and patients.
Figure 4.
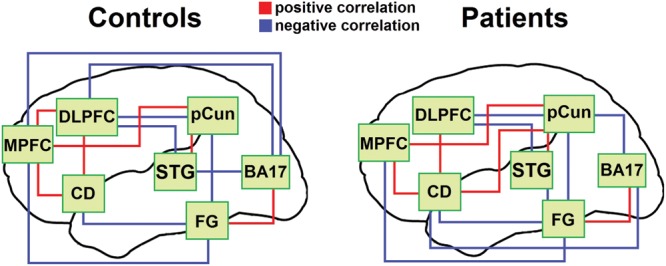
RSFC correlations. Seed regions consisted of the 5 functional and 2 a priori fMRI ROIs applied to a resting state fMRI scan. pCun = bilateral precuneus, STG = right superior temporal gyrus, CD = left caudate, MPFC = left medial prefrontal, DLPFC = left dorsolateral prefrontal, BA 17 = bilateral Brodmann area 17, FG = bilateral fusiform gyrus.
Discussion
This study used an object recognition fMRI task that Bar et al. (2006) had previously used to explore the theory that LSF information projecting to the PFC creates a low resolution frame of an object and that this information feeds back to the VTC to facilitate object recognition in normal individuals. Like the results of Bar et al. (2006), the present fMRI results for healthy controls showed that the PFC responded differently for stimuli containing LSF information than for stimuli containing only HSF information. The present study extended the paradigm to schizophrenia patients. Whereas previous studies have shown impairments in perceptual closure involving impaired PFC activity in schizophrenia (Doniger et al. 2002; Sehatpour et al. 2010), the current study used LSF and HSF stimuli to examine the specific contributions of each type of information to object recognition in patients.
Functional Magnetic Resonance Imaging
Reaction time for controls and patients did not differ significantly for the object recognition task, indicating that patients were able to perform the task as quickly as controls for all stimulus conditions. This was perhaps not surprising given that the task involved only a small number of “catch” trials, and that these trials used abstract sculpture stimuli that were purposely chosen not to resemble ordinary objects. The lack of group differences in behavioral data indicated that patients were able to recognize the objects in this task normally, whereas differences in fMRI results indicated that they accomplished recognition using different patterns of cortical activation, possibly reflecting a different strategy. Recent findings indicate that even under conditions of approximately normal behavioral performance for visual tasks, schizophrenia patients may show impaired patterns of cortical activation (Spencer et al. 2003; Spencer et al. 2004; Silverstein et al. 2010b). The current results extend this idea to object recognition, with patients showing normal reaction times but abnormal patterns of activity over a widespread cortical network.
The 2 a priori ROIs examined were bilateral occipital area 17 and fusiform gyrus. In area 17, patients had decreased activation for LSF compared with Intact stimuli, whereas controls showed no difference in activation between the different types of stimuli. Previous research has found alterations in occipital cortex anatomy (Selemon et al. 1995; Dorph-Petersen et al. 2007) in post-mortem studies of schizophrenia patients as well as thinning of occipital cortex in an MRI study of unmedicated first-episode patients (Narr et al. 2005). Decreased fMRI activation has also been found in primary visual cortex to LSF, but not HSF, grating stimuli in schizophrenia patients compared with controls (Martinez et al. 2008). The current result of an impaired pattern of response within the schizophrenia group supports the idea that preferential magnocellular dysfunction in schizophrenia may result in a reduced occipital cortical response to LSF information.
The fusiform gyrus was chosen as an a priori VTC ROI due to its well-documented involvement in object recognition (Gerlach et al. 2002; Simons et al. 2003; Hofer et al. 2007; Liu et al. 2008; Haist et al. 2010; Konen et al. 2011). While the lateral occipital complex (LOC) is also well known to be involved in object recognition (Grill-Spector et al. 2001; Lerner et al. 2002; Sehatpour et al. 2010), definitions of LOC boundaries often include posterior parts of the fusiform gyrus (Grill-Spector et al. 1999; Malach et al. 2002). Bar et al. (2001, 2006) have repeatedly observed activity throughout the fusiform gyrus related to object recognition, particularly in studies of the frame and fill model (Kveraga et al. 2007), and thus the current study, which utilized one of their paradigms, also utilized this area as an a priori ROI. In this fusiform gyrus ROI, patients had decreased activation for LSF and HSF compared with Intact stimuli, whereas controls showed no difference in activation between stimulus types. Martinez et al. (2012) recently found decreased fMRI activation in fusiform gyrus to LSF, but not HSF, simple grating stimuli in schizophrenia patients compared with controls, while Silverstein et al. (2010a) found increased fusiform activity to LSF and HSF filtered faces in schizophrenia. The current results, together with these other recent findings, suggest that the fusiform gyrus processes spatial frequencies abnormally in schizophrenia, and differently for various types of stimuli. The current results support findings of impaired transmission of LSF object information to the fusiform gyrus, and suggest that pure HSF processing of objects is also impaired in schizophrenia. While the fusiform gyrus receives direct input from primary visual areas, this project also sought to study dorsal stream and PFC contributions to object recognition in schizophrenia.
The 5 functionally derived ROIs show which cortical areas process the spatial frequency of objects differently for controls than patients. The precuneus and superior temporal gyrus ROIs show similar patterns of response, with controls having increased activity for LSF over HSF stimuli, and patients having the opposite pattern. In addition, patients showed significantly decreased responses to LSF stimuli compared with controls in the precuneus. The caudate ROI shows the same pattern of increased activity for LSF over HSF stimuli in controls as the precuneus and superior temporal gyrus, but no differential activations for stimulus type in patients. These results suggest that patients with schizophrenia have a deficit in these 3 areas for processing LSF information. The pattern of deficits in the parietal and superior temporal areas in particular supports ERP and fMRI findings of dorsal stream deficits in schizophrenia (Doniger et al. 2002; Sehatpour et al. 2010; Dias et al. 2011; Martinez et al. 2012), which are preferentially seen to LSF information (Butler et al. 2007; Martinez et al. 2012). In addition, the observed pattern of increased HSF over LSF activation for patients fits with recent findings showing an increase as well as persistence in sensory ERP components in response to HSF over LSF gratings in schizophrenia (Martinez et al. 2012). In the presence of deficits in processing LSF information, the precuneus and superior temporal gyrus may compensate with more active processing of HSF information in schizophrenia. This is consistent with a recent fMRI study that showed greater activity in early visual areas for HSF rather than LSF face stimuli in schizophrenia patients but the opposite pattern in controls (Silverstein et al. 2010a). The current result of increased response to HSF stimuli may also be related to recent findings of greater interference of local on global processing for patients with schizophrenia but the opposite pattern in controls (Coleman et al. 2009; Kemner et al. 2009).
With regard to frontal activity, the MPFC ROI showed increased activity to LSF over HSF stimuli for controls, but increased activity to HSF over Intact stimuli for patients, and a significantly greater response to HSF stimuli for patients over controls. For controls, this shows that the MPFC strongly activates to LSF information found in LSF and Intact stimuli, but activates much less to pure HSF information. This is consistent with previous findings that the PFC uses LSF information to create a low resolution frame for an object stimulus (Bar et al. 2006; Chen et al. 2007; Kveraga et al. 2007; Sehatpour et al. 2010). For patients, this area has normal activity to LSF and Intact stimuli, but significantly more activity to HSF stimuli. Together with the findings in dorsal stream areas, this result suggests that HSF information is transmitted by the dorsal stream to the PFC in schizophrenia, possibly to compensate for deficits in LSF information. Patients may preferentially utilize HSF information, rather than LSF information, when constructing a frame for an object stimulus.
For controls, the DLPFC ROI shows a striking pattern of positive activity for Intact and LSF stimuli and negative activity for purely HSF stimuli. Using the same fMRI paradigm, Bar et al. (2006) found small negative activations to Intact and LSF stimuli and a large negative activation to HSF stimuli in the orbitofrontal cortex, which they replicated with MEG. These previous findings, and the current results for both the DLPFC and MPFC frontal areas, illustrate the selectiveness of the PFC response to LSF information during object recognition. Both Intact and LSF stimuli contain LSF information, and have similar activations, whereas HSF stimuli that lack LSF information have significantly different activations. This is consistent with the PFC utilizing LSF information to rapidly create a frame for an object in controls. Patients, on the other hand, showed no differences in activation between stimulus conditions in the DLPFC, indicating that they are not preferentially processing any particular spatial frequency in this area. While patients may use the MPFC to attempt object framing with compensatory HSF information, the DLPFC may not be utilized for object recognition in schizophrenia.
These results extend and further clarify previous findings regarding object recognition in both healthy individuals and schizophrenia patients. This study used the same fMRI paradigm previously used by Bar et al. (2006) to investigate the cortical areas involved in object recognition. This previous study used an a priori orbitofrontal ROI to represent PFC based on anatomical evidence that orbitofrontal cortex has connections with areas known to be involved in object recognition. The current study, however, defined 2 PFC ROIs based on the functional interaction of spatial frequency and group. Thus, the PFC regions analyzed here show where spatial frequency processing differs between healthy controls and schizophrenia patients, and this knowledge extends the previous findings of Bar et al. (2006) by showing additional PFC regions involved in object recognition. Further, recent anatomical findings in humans have demonstrated relationships between primary visual cortex and both MPFC and DLPFC (Harvey et al. 2011; Song et al. 2011), as well as between dorsal stream parietal areas and DLPFC (Catani et al. 2002), suggesting direct involvement of these frontal regions in visual processing. WM fiber tracts from DLPFC to the fusiform gyrus and inferior occipital cortex have also been described in humans (see Catani et al. 2002 for a review), indicating an anatomical substrate for the feedback of framing information from the DLPFC to the VTC.
A recent study of object recognition in schizophrenia using a perceptual closure task revealed a network involving dorsal stream areas and PFC that supported the frame and fill model and its impairment in schizophrenia (Sehatpour et al. 2010). This study provided evidence that early-stage visual dysfunction in schizophrenia propagates through the dorsal stream to the PFC, leading to dysregulation of ventral object recognition areas. This suggests that PFC involvement in object recognition is directly tied to early-stage visual processing as well as VTC object recognition function, supporting the framing function of the PFC. By using spatial frequency filtered stimuli, the current study expands this model of impaired framing in schizophrenia by specifically demonstrating deficits in LSF information processing and compensation with HSF information. As LSF and HSF information are preferentially transmitted by the magnocellular and parvocellular pathways, respectively, this provides further support for the idea that preferential magnocellular deficits in schizophrenia underlie impaired object framing.
An alternative model of object recognition holds that the dorsal stream and PFC are not required to process global information. Previous findings have demonstrated that simple contour information in early visual areas is integrated in progressively anterior regions of the ventral stream to form general shape representations (Bar et al. 2001; Grill-Spector et al. 2001; Brincat and Connor 2006; Bell et al. 2011), thus indicating that the ventral stream may carry general shape information independent of dorsal stream or PFC input. Feedback from the PFC or other areas may provide a prediction signal that would either be consistent or inconsistent with visual input emerging from the occipital and temporal regions (Dima et al. 2009; Friston and Kiebel 2009; Silverstein et al. 2009). Under this model, the current findings of impaired PFC activity in schizophrenia may reflect a failure of ability to utilize predictions successfully. Further work is necessary to disentangle this hypothesis from the frame and fill hypothesis.
Resting State Functional Connectivity
In the RSFC analysis, controls had significant correlations between early visual area 17 and the 2 frontal areas, MPFC and DLPFC, whereas patients lacked this connectivity. For controls, MPFC and DLPFC were functionally connected, as might be expected from their mutual preference for LSF information in the fMRI results. Together, these results indicate that for controls, MPFC and DLPFC receive LSF information directly related to early visual processing in area 17, and work together to process this information. This pattern supports previous findings with effective connectivity (Bar et al. 2006; Sehatpour et al. 2010) and anatomical tracts (Catani et al. 2002; Song et al. 2011) that occipital visual and dorsal stream areas are connected to MPFC and DLPFC, and that these regions are in turn connected to the fusiform gyrus. This study extended these prior results by utilizing a separate resting state scan, together with functionally determined seed regions, to analyze functional connectivity in this object recognition circuit.
For patients, the PFC areas are functionally disconnected from each other and from area 17, and show either a preference for HSF information or no spatial frequency preference. Recent studies comparing patients with schizophrenia with controls have shown a lack of connectivity between prefrontal cortex and occipital visual areas during a visual attention task (Harvey et al. 2011), as well as reduced functional connectivity between DLPFC and both fusiform gyrus and primary occipital visual areas during a spatial working memory task (Kang et al. 2011). This study demonstrated that these areas lack functional connectivity even during the resting state, in the absence of a task.
The current functional connectivity results thus support the role of the PFC in the normal object recognition circuit, and the dysfunction of this PFC circuit in schizophrenia. In addition, both groups have correlations between MPFC and fusiform gyrus, as well as several indirect functional pathways between frontal areas and fusiform gyrus. This supports the feedback of PFC framing information to the VTC, and echoes previous functional connectivity (Bar et al. 2006; Sehatpour et al. 2010) and anatomical (Catani et al. 2002) findings linking PFC and VTC.
Conclusions
Healthy controls demonstrated strong selectivity for LSF information in both dorsal stream areas and frontal areas, consistent with the framing function of the PFC during object recognition (Schmolesky et al. 1998; Schroeder et al. 1998; Lamme and Roelfsema 2000; Bar 2003; Bar et al. 2006; Chen et al. 2007; Kveraga et al. 2007). The functional connections between PFC and VTC indicated that the low resolution frame derived in the PFC feeds back to object recognition processes in the VTC. Schizophrenia patients, however, activated less selectively to LSF information in basic visual, dorsal stream, frontal, and VTC areas. Instead, dorsal stream and MPFC ROIs showed a preference for HSF information, indicating that object framing in schizophrenia may rely on HSF information in the absence of strong LSF information. These results are consistent with findings that preferential magnocellular and dorsal stream deficits in schizophrenia lead to impaired framing feedback to the VTC during object recognition (Doniger et al. 2002; Sehatpour et al. 2010), and generally support the propagation of sensory deficits to higher cognitive functions in schizophrenia (Leitman et al. 2005; Kim et al. 2006; Revheim et al. 2006; Kurylo et al. 2007; Butler et al. 2009; Leitman et al. 2011). While top-down processing is certainly deficient in schizophrenia, future investigations of bottom-up dysfunction will further clarify the underlying causes of cognitive deficits in this disorder.
Notes
Conflict of Interest: The authors report the following conflicts of interest: Dr. Daniel Javitt holds intellectual property rights for use of NMDA agonists, including glycine, d-serine, and glycine transport inhibitors in treatment of schizophrenia. Dr. Javitt is a major shareholder in Glytech, Inc. and Amino Acids Solutions, Inc. Within the past year, Dr. Javitt has served as a paid consultant to Sepracor, AstraZeneca, Pfizer, Cypress, Merck, Sunovion, Eli Lilly, and BMS. Daniel Calderone, Matthew Hoptman, Antigona Martinez, Sangeeta Nair-Collins, Cristina Mauro, Moshe Bar, and Pamela Butler have no conflicts of interest in relation to the subject of this study.
Funding
This work was supported by the National Institutes of Health (RO1 MH084848 to P.D.B. and M.J.H., R21 MH084031 to M.J.H., and R37 MH049334 and P50 MH086385 to D.C.J.), and the scanner used in this study was funded in part by S10 RR022972 NCRR High End Instrumentation grant to Craig A. Branch, PhD) and the Graduate Center of the City University of New York (Doctoral Student Research Grant to D.J.C.
References
- Altmann CF, Bulthoff HH, Kourtzi Z. Perceptual organization of local elements into global shapes in the human visual cortex. Curr Biol. 2003;13:342–349. doi: 10.1016/s0960-9822(03)00052-6. [DOI] [PubMed] [Google Scholar]
- Ardekani BA, Bachman AH, Strother SC, Fujibayashi Y, Yonekura Y. Impact of inter-subject image registration on group analysis of fMRI data. Int Congr Ser. 2004;1265:49–59. [Google Scholar]
- Ardekani BA, Nierenberg J, Hoptman MJ, Javitt DC, Lim KO. MRI study of white matter diffusion anisotropy in schizophrenia. Neuroreport. 2003;14:2025–2029. doi: 10.1097/00001756-200311140-00004. [DOI] [PubMed] [Google Scholar]
- Ban H, Yamamoto H, Fukunaga M, Nakagoshi A, Umeda M, Tanaka C, Ejima Y. Toward a common circle: interhemispheric contextual modulation in human early visual areas. J Neurosci. 2006;26:8804–8809. doi: 10.1523/JNEUROSCI.1765-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15:600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Hämäläinen MS, Marinkovic K, Schacter DL, Rosen BR, et al. Top-down facilitation of visual recognition. Proc Natl Acad Sci USA. 2006;103:449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Tootell RBH, Schacter DL, Greve DN, Fischl B, Mendola JD, Rosen BR, Dale AM. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001;29:529–535. doi: 10.1016/s0896-6273(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Bell J, Gheorghiu E, Hess RF, Kingdom FA. Global shape processing involves a hierarchy of integration stages. Vision Res. 2011;51:1760–1766. doi: 10.1016/j.visres.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenia patients: A functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Brenner CA, Wilt MA, Lysaker PH, Koyfman A, O'Donnell BF. Psychometrically matched visual processing tasks in schizophrenia spectrum disorders. J Abnorm Psychol. 2003;112:28–37. [PubMed] [Google Scholar]
- Brincat SL, Connor CE. Dynamic shape synthesis in posterior inferotemporal cortex. Neuron. 2006;49:17–24. doi: 10.1016/j.neuron.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Brittain PJ, Surguladze S, McKendrick AM, Ffytche DH. Backward and forward visual masking in schizophrenia and its relation to global motion and global form perception. Schizophr Res. 2010;124:134–141. doi: 10.1016/j.schres.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Butler PD, Abeles IY, Weiskopf NG, Tambini A, Jalbrzikowski M, Legatt ME, Zemon V, Loughead J, Gur RC, Javitt DC. Sensory contributions to impaired emotion processing in schizophrenia. Schizophr Bull. 2009;35:1095–1107. doi: 10.1093/schbul/sbp109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Hoptman MJ, Nierenberg J, Foxe JJ, Javitt DC, Lim KO. Visual white matter integrity in schizophrenia. Am J Psychiatry. 2006;163:2011–2013. doi: 10.1176/appi.ajp.163.11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Martinez A, Foxe JJ, Kim D, Zemon V, Silipo G, Mahoney J, Shpaner M, Jalbrzikowski M, Javitt DC. Subcortical visual dysfunction in schizophrenia drives secondary cortical impairments. Brain. 2007;130:417–430. doi: 10.1093/brain/awl233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 2001;158:1126–1133. doi: 10.1176/appi.ajp.158.7.1126. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 2008;64:40–47. doi: 10.1016/j.biopsych.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, Barch DM, Buchanan RW, Bullmore E, Krystal JH, Cohen J, Geyer M, Green M, Nuechterlein KH, Robbins T, et al. Identifying cognitive mechanisms targeted for treatment development in schizophrenia: an overview of the first meeting of the Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia Initiative. Biol Psychiatry. 2008;64:4–10. doi: 10.1016/j.biopsych.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Chen CM, Lakatos P, Shah AS, Mehta AD, Givre SJ, Javitt DC, Schroeder CE. Functional anatomy and interaction of fast and slow visual pathways in macaque monkeys. Cereb Cortex. 2007;17:1561–1569. doi: 10.1093/cercor/bhl067. [DOI] [PubMed] [Google Scholar]
- Chen L. The topological approach to perceptual organization. Vision Cogn. 2005;12:553–637. [Google Scholar]
- Chen Y. Abnormal visual motion processing in schizophrenia: a review of research progress. Schizophr Bull. 2011;37:709–715. doi: 10.1093/schbul/sbr020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman MJ, Cestnick L, Krastoshevsky O, Krause V, Huang Z, Mendell NR, Levy DL. Schizophrenia patients show deficits in shifts of attention to different levels of global-local stimuli: evidence for magnocellular dysfunction. Schizophr Bull. 2009;35:1108–1116. doi: 10.1093/schbul/sbp090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conci M, Tollner T, Leszczynski M, Muller HJ. The time-course of global and local attentional guidance in Kanizsa-figure detection. Neuropsychologia. 2011;49:2456–2464. doi: 10.1016/j.neuropsychologia.2011.04.023. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Daw NW, Stein PSG, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- de la Rosa S, Choudhery RN, Chatziastros A. Visual object detection, categorization, and identification tasks are associated with different time courses and sensitivities. J Exp Psychol Hum Percept Perform. 2011;37:38–47. doi: 10.1037/a0020553. [DOI] [PubMed] [Google Scholar]
- Dias EC, Butler PD, Hoptman MJ, Javitt DC. Early sensory contributions to contextual encoding deficits in schizophrenia. Arch Gen Psychiatry. 2011;68:654–664. doi: 10.1001/archgenpsychiatry.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima D, Roiser JP, Dietrich DE, Bonnemann C, Lanfermann H, Emrich HM, Dillo W. Understanding why patients with schizophrenia do not perceive the hollow-mask illusion using dynamic causal modelling. Neuroimage. 2009;46:1180–1186. doi: 10.1016/j.neuroimage.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 2007;501:290–301. doi: 10.1002/cne.21243. [DOI] [PubMed] [Google Scholar]
- Endo H, Kizuka T, Masuda T, Takeda T. Automatic activation in the human primary motor cortex synchronized with movement preparation. Cogn Brain Res. 1999;3:229–239. doi: 10.1016/s0926-6410(99)00024-5. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. New York: New York State Psychiatric Institute; 1997. [Google Scholar]
- Fox K, Sato H, Daw N. The effect of varying stimulus intensity on NMDA-receptor activity in cat visual cortex. J Neurophysiol. 1990;64:1413–1428. doi: 10.1152/jn.1990.64.5.1413. [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: Impaired P1 generation revealed by high-density electrical mapping. NeuroReport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- Friston K, Kiebel S. Predictive coding under the free-energy principle. Philos Trans R Soc Lond B Biol Sci. 2009;364:1211–1221. doi: 10.1098/rstb.2008.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach C, Aaside CT, Humphreys GW, Gade A, Paulson OB, Law I. Brain activity related to integrative processes in visual object recognition: bottom-up integration and the modulatory influence of stored knowledge. Neuropsychologia. 2002;40:1254–1267. doi: 10.1016/s0028-3932(01)00222-6. [DOI] [PubMed] [Google Scholar]
- Goff DC, Coyle JT. The emerging role of glutamate in the pathophysiology and treatment of schizophrenia. Am J Psychiatry. 2001;158:1367–1377. doi: 10.1176/appi.ajp.158.9.1367. [DOI] [PubMed] [Google Scholar]
- Green MF. Cognitive impairment and functional outcome in schizophrenia and bipolar disorder. J Clin Psychiatry. 2006;67:e12. [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. Perception measurement in clinical trials of schizophrenia: Promising paradigms from CNTRICS. Schizophr Bull. 2009;35:163–181. doi: 10.1093/schbul/sbn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch Gen Psychiatry. 1994;51:945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41:1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kushnir T, Edelman S, Avidan G, Itzchak Y, Malach R. Differential processing of objects under various viewing conditions in the human lateral occipital complex. Neuron. 1999;24:187–203. doi: 10.1016/s0896-6273(00)80832-6. [DOI] [PubMed] [Google Scholar]
- Haist F, Lee K, Stiles J. Individuating faces and common objects produces equal responses in putative face-processing areas Cin the ventral occipitotemporal cortex. Front Hum Neurosci. 2010;4:181. doi: 10.3389/fnhum.2010.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Lee J, Cohen MS, Engel SA, Glahn DC, Nuechterlein KH, Wynn JK, Green MF. Altered dynamic coupling of lateral occipital complex during visual perception in schizophrenia. Neuroimage. 2011;55:1219–1226. doi: 10.1016/j.neuroimage.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer A, Siedentopf CM, Ischebeck A, Rettenbacher MA, Widschwendter CG, Verius M, Golaszewski SM, Koppelstaetter F, Felber S, Wolfgang Fleischhacker W. The neural regions sustaining episodic encoding and recognition of objects. Brain Cogn. 2007;63:159–166. doi: 10.1016/j.bandc.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9:984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: Bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SS, Sponheim SR, Chafee MV, MacDonald AW. Disrupted functional connectivity for controlled visual processing as a basis for impaired spatial working memory in schizophrenia. Neuropsychologia. 2011;49:2836–2847. doi: 10.1016/j.neuropsychologia.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: The final common pathway on the road to schizophrenia? Brain Res Bull. 2010a;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Javitt DC. Thinking glutamatergically: Changing concepts of schizophrenia based upon changing neurochemical models. Clin Schizophr Related Psychoses. 2010b;4:189–200. doi: 10.3371/CSRP.4.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. The primate retina contains two types of ganglion cells, with high and low contrast sensitivity. Proc Natl Acad Sci USA. 1986;83:2755–2757. doi: 10.1073/pnas.83.8.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Shapley RM. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J Physiol. 1982;330:125–143. doi: 10.1113/jphysiol.1982.sp014333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemner C, Foxe JJ, Tankink JE, Kahn RS, Lamme VA. Abnormal timing of visual feedback processing in young adults with schizophrenia. Neuropsychologia. 2009;47:3105–3110. doi: 10.1016/j.neuropsychologia.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Kéri S, Kelemen O, Janka Z, Benedek G. Visual-perceptual dysfunctions are possible endophenotypes of schizophrenia: Evidence from the psychophysical investigation of magnocellular and parvocellular pathways. Neuropsychology. 2005;19:649–656. doi: 10.1037/0894-4105.19.5.649. [DOI] [PubMed] [Google Scholar]
- Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Zemon V, Saperstein A, Butler PD, Javitt DC. Dysfunction of early-stage visual processing in schizophrenia: Harmonic analysis. Schizophr Res. 2005;76:55–65. doi: 10.1016/j.schres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kiss I, Fabian A, Benedek G, Keri S. When doors of perception open: visual contrast sensitivity in never-medicated, first-episode schizophrenia. J Abnorm Psychol. 2010;119:586–593. doi: 10.1037/a0019610. [DOI] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, Christensen GE, Collins DL, Gee J, Hellier P, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Behrmann M, Nishimura M, Kastner S. The functional neuroanatomy of object agnosia: a case study. Neuron. 2011;71:49–60. doi: 10.1016/j.neuron.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtzi Z, Huberle E. Spatiotemporal characteristics of form analysis in the human visual cortex revealed by rapid event-related fMRI adaptation. Neuroimage. 2005;28:440–452. doi: 10.1016/j.neuroimage.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: monkey and human FMRI studies. Neuron. 2003;37:333–346. doi: 10.1016/s0896-6273(02)01174-1. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Perry EB, Gueorguieva R, Belger A, Madonick SH, Abi-Dargham A, Cooper TB, MacDougall L, Abi-Saab W, D'Souza C. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: Implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–995. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- Kurylo DD, Pasternak R, Silipo G, Javitt DC, Butler PD. Perceptual organization by proximity and similarity in schizophrenia. Schizophr Res. 2007;95:205–214. doi: 10.1016/j.schres.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kveraga K, Boshyan J, Bar M. Magnocellular projections as the trigger of top-down facilitation in recognition. J Neurosci. 2007;27:13232–13240. doi: 10.1523/JNEUROSCI.3481-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YH, Nelson SB, Toth LJ, Sur M. Effect of stimulus contrast and size on NMDA receptor activity in cat lateral geniculate nucleus. J Neurophysiol. 1992;68:182–196. doi: 10.1152/jn.1992.68.1.182. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Roelfsema PR. The distinct modes of vision offered by feedforward and recurrent processing. Trends Neurosci. 2000;23:571–579. doi: 10.1016/s0166-2236(00)01657-x. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Foxe JJ, Butler PD, Saperstein A, Revheim N, Javitt DC. Sensory contributions to impaired prosodic processing in schizophrenia. Biol Psychiatry. 2005;58:56–61. doi: 10.1016/j.biopsych.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Leitman DI, Wolf DH, Laukka P, Ragland JD, Valdez JN, Turetsky BI, Gur RE, Gur RC. Not pitch perfect: sensory contributions to affective communication impairment in schizophrenia. Biol Psychiatry. 2011;70:611–618. doi: 10.1016/j.biopsych.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Lerner Y, Hendler T, Malach R. Object-completion effects in the human lateral occipital complex. Cereb Cortex. 2002;12:163–177. doi: 10.1093/cercor/12.2.163. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, Grace AA. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Steinmetz NA, Farley AB, Smith CD, Joseph JE. Mid-fusiform activation during object discrimination reflects the process of differentiating structural descriptions. J Cogn Neurosci. 2008;20:1711–1726. doi: 10.1162/jocn.2008.20116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malach R, Levy I, Hasson U. The topography of high-order human object areas. Trends Cogn Sci. 2002;6:176–184. doi: 10.1016/s1364-6613(02)01870-3. [DOI] [PubMed] [Google Scholar]
- Mannion DJ, McDonald JS, Clifford CW. The influence of global form on local orientation anisotropies in human visual cortex. Neuroimage. 2010;52:600–605. doi: 10.1016/j.neuroimage.2010.04.248. [DOI] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Bickel S, Dias EC, Butler PD, Javitt DC. Consequences of Magnocellular Dysfunction on Processing Attended Information in Schizophrenia. Cereb Cortex. 2012;22:1282–1293. doi: 10.1093/cercor/bhr195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A, Hillyard SA, Dias EC, Hagler DJ, Butler PD, Guilfoyle DN, Jalbrzikowski M, Silipo G, Javitt DC. Magnocellular pathway impairment in schizophrenia: Evidence from functional magnetic resonance imaging. J Neurosci. 2008;28:7492–7500. doi: 10.1523/JNEUROSCI.1852-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merigan WH, Maunsell JHR. How parallel are the primate visual pathways? Annu Rev Neurosci. 1993;16:369–402. doi: 10.1146/annurev.ne.16.030193.002101. [DOI] [PubMed] [Google Scholar]
- Narr KL, Toga AW, Szeszko P, Thompson PM, Woods RP, Robinson D, Sevy S, Wang Y, Schrock K, Bilder RM. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. doi: 10.1016/j.biopsych.2005.03.043. [DOI] [PubMed] [Google Scholar]
- Ojanpää H, Näsänen R. Utilisation of spatial frequency information in face search. Vision Res. 2003;43:2505–2515. doi: 10.1016/s0042-6989(03)00459-0. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur J Neurosci. 1998;10:3044–3057. doi: 10.1046/j.1460-9568.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- Pasupathy A, Connor CE. Responses to contour features in macaque area V4. J Neurophysiol. 1999;82:2490–2502. doi: 10.1152/jn.1999.82.5.2490. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: Comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11:1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Revheim N, Butler PD, Schechter I, Jalbrzikowski M, Silipo G, Javitt DC. Reading impairment and visual processing deficits in schizophrenia. Schizophr Res. 2006;87:238–245. doi: 10.1016/j.schres.2006.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saron CD, Schroeder CE, Foxe JJ, Vaughan HG. Visual activation of frontal cortex: segregation from occipital activity. Cogn Brain Res. 2001;12:75–88. doi: 10.1016/s0926-6410(01)00036-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- Schechter I, Butler PD, Zemon VM, Revheim N, Saperstein AM, Jalbrzikowski M, Pasternak R, Silipo G, Javitt DC. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Hanes DP, Thompson KG, Leutgeb S, Schall JD, Leventhal AG. Signal timing across the macaque visual system. J Neurophysiol. 1998;79:3272–3278. doi: 10.1152/jn.1998.79.6.3272. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Mehta AD, Givre SJ. A spatiotemporal profile of visual system activation revealed by current source density analysis in the awake macaque. Cereb Cortex. 1998;8:575–592. doi: 10.1093/cercor/8.7.575. [DOI] [PubMed] [Google Scholar]
- Sehatpour P, Dias EC, Butler PD, Revheim N, Guilfoyle DN, Foxe JJ, Javitt DC. Impaired visual object processing across an occipital- frontal- hippocampal brain network in schizophrenia: An integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. discussion 819–820. [DOI] [PubMed] [Google Scholar]
- Shapley R. Visual sensitivity and parallel retinocortical channels. Annu Rev Psychol. 1990;41:635–658. doi: 10.1146/annurev.ps.41.020190.003223. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, All SD, Kasi R, Berten S, Essex B, Lathrop KL, Little DM. Increased fusiform area activation in schizophrenia during processing of spatial frequency-degraded faces, as revealed by fMRI. Psychol Med. 2010a;40:1159–1169. doi: 10.1017/S0033291709991735. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Berten S, Essex B, All SD, Kasi R, Little DM. Perceptual organization and visual search processes during target detection task performance in schizophrenia, as revealed by fMRI. Neuropsychologia. 2010b;48:2886–2893. doi: 10.1016/j.neuropsychologia.2010.05.030. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009;8:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 2011;37:690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Koutstaal W, Prince S, Wagner AD, Schacter DL. Neural mechanisms of visual object priming: evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–626. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- Song C, Schwarzkopf DS, Kanai R, Rees G. Reciprocal anatomical relationship between primary sensory and prefrontal cortices in the human brain. J Neurosci. 2011;31:9472–9480. doi: 10.1523/JNEUROSCI.0308-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, Shenton ME, McCarley RW. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci USA. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swettenham JB, Anderson SJ, Thai NJ. MEG responses to the perception of global structure within glass patterns. PLoS One. 2010;5:e13865. doi: 10.1371/journal.pone.0013865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annu Rev Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- Tanaka K. Neuronal mechanisms of object recognition. Science. 1993;262:685–688. doi: 10.1126/science.8235589. [DOI] [PubMed] [Google Scholar]
- Tapia E, Breitmeyer BG. Visual consciousness revisited: magnocellular and parvocellular contributions to conscious and nonconscious vision. Psychol Sci. 2011;22:934–942. doi: 10.1177/0956797611413471. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman S. Sequence seeking and counter streams: A computational model for bidirectional information flow in the visual cortex. Cereb Cortex. 1995;1:1–11. doi: 10.1093/cercor/5.1.1. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of visual behavior. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- Vogels R, Biederman I, Bar M, Lorincz A. Inferior temporal neurons show greater sensitivity to nonaccidental than to metric shape differences. J Cogn Neurosci. 2001;13:444–453. doi: 10.1162/08989290152001871. [DOI] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: Corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Woods SW. 2005. Calculation of CPZ Equivalents Retrieved May 7, 2012 from http://www.scottwilliamwoods.com/files/Equivtext.doc .
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- Woods SW. 2011. Chlorpromazine Equivalent Doses for the Newer Atypical Antipsychotics Retrieved May 7, 2012 from http://www.scottwilliamwoods.com/files/WoodsEquivUpdate.doc .
- Wurtz RH, Kandel ER. Central Visual Pathways. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. 4 ed. New York, NY: McGraw-Hill; 2000. pp. 523–545. [Google Scholar]
- Yeap S, Kelly SP, Sehatpour P, Magno E, Javitt DC, Garavan H, Thakore JH, Foxe JJ. Early visual sensory deficits as endophenotypes for schizophrenia: High-density electrical mapping in clinically unaffected first-degree relatives. Archives of General Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- Zemon V, Gordon J. Luminance-contrast mechanisms in humans: Visual evoked potentials and a nonlinear model. Vision Res. 2006;46:4163–4180. doi: 10.1016/j.visres.2006.07.007. [DOI] [PubMed] [Google Scholar]