Abstract
Functional organization of the brain can be fundamentally altered by auditory deprivation. Previous studies found that the superior temporal cortex in deaf people is reorganized to process non-auditory stimuli, as revealed by the extrinsic task-induced brain activities. However, it is unknown how the intrinsic activities of this region are impacted by deafness. This study explored this issue using resting-state functional magnetic resonance imaging. We examined 60 congenitally deaf (CD) individuals, 39 acquired deaf (AD) individuals, and 38 hearing controls (HC), and focused on the effect of deafness on the intra- and inter-regional synchronization of different parts of superior temporal sulcus (STS). We found that intra-regional synchronization or regional homogeneity (ReHo) of the middle STS (mSTS) was decreased in AD compared with HC or CD, while the CD had preserved ReHo in mSTS. Greater connectivity was observed between mSTS and posterior STS in CD and HC than in AD, while both CD and AD had weaker connectivity of mSTS with the anterior STS (aSTS) compared with HC. Moreover, the connectivity of mSTS–aSTS in CD and AD was associated with their language skills. These findings confirmed our hypothesis that the intrinsic function of different parts of STS is distinctly impacted by deafness.
Keywords: acquired deaf, congenitally deaf, functional connectivity, regional homogeneity, resting-state fMRI
Introduction
Sensory experience is critical for functional brain development (Bavelier and Neville 2002). Permanent sensory deprivation, for example, in case of deafness or blindness, will fundamentally alter the functional organization of the brain, specifically, the region(s) originally responsible for that sensory processing (Bavelier and Neville 2002; Merabet and Pascual-Leone 2010). Substantial research has addressed the issue of how auditory deprivation affects the functional development of the superior temporal cortex. Some evidence suggests that this region in deaf individuals is functionally organized to process non-auditory stimuli. For example, congenitally deaf (CD) individuals exhibited significant activation in the posterior superior temporal cortex when responding to visual (Finney et al. 2001, 2003) or tactile stimuli (Levanen et al. 1998; Auer et al. 2007). Moreover, irrespective of the age of onset of deafness, both early- and late-deaf individuals showed comparable activation in the posterior superior temporal cortex when processing sign language (Sadato et al. 2004). This evidence suggests that the function of the superior temporal cortex in deaf people may develop to process non-auditory inputs regardless of the amount of auditory experience.
Up to now, most available brain imaging evidence on the organization of superior temporal cortex in deaf people has examined task-related activity. A sole focus on task-related activity, however, ignores the alternative possibility that brain functions are mainly intrinsic (Raichle 2010). Exploring intrinsic brain activity is important for understanding functional brain development, because it may best capture the essence of brain function (Raichle 2010). Recent evidence from functional magnetic resonance imaging (fMRI) showed that brain intrinsic activity can predict both task-induced activity and behavioral performance (Mennes et al. 2010, 2011; Koyama et al. 2011). Some previous studies using positron emission tomography have explored glucose metabolism at rest in cochlear-implanted deaf individuals (Catalan-Ahumada et al. 1993; Deggouj et al. 1995; Hirano et al. 2000; Lee et al. 2001, 2003; Strelnikov et al. 2010). These results suggest that the function of auditory cortex in deaf people dynamically changes after cochlear implanting. Because these studies mainly focused on the adaptive mechanisms induced by the recovery of auditory system after cochlear implanting, they could not address the question of functionally adaptive processes simply corresponding to hearing loss, particularly the effects of early auditory deprivation. Deaf adults who do not undergo cochlear implanting surgery offer a unique opportunity to explore this cortical mechanism.
Anatomical studies have shown that the superior temporal cortex, particularly, the superior temporal sulcus (STS), is composed of several distinct unimodal and multimodal regions (Seltzer and Pandya 1978). Non-human studies have characterized 3 STS subregions (posterior STS [pSTS], middle STS [mSTS], and anterior STS [aSTS]) that differ in intercortical connectivity (Seltzer and Pandya 1989a, 1989b; Carmichael and Price 1995; Barbas 2000), cytoarchitectural properties (Seltzer and Pandya 1989a, 1989b), and chemoarchitectural attributes (Padberg et al. 2003). Growing evidence also suggests that different parts of the human STS may play varied roles. The pSTS appears to play an essential role in integrating auditory and visual information (Calvert et al. 2000; Beauchamp et al. 2004; van Atteveldt et al. 2004; Noesselt et al. 2007) as well as in integrating auditory and somatosensory information (Beauchamp et al. 2008). Non-human primate studies have also shown that some neurons in pSTS respond to both visual and auditory stimulation (Benevento et al. 1977). In line with these findings, tracer injection studies found that pSTS received projections from both the visual and auditory cortex (Seltzer and Pandya 1994). The mSTS appears to be specialized for human voice processing, regardless whether speech or non-speech (Belin et al. 2000; Fecteau et al. 2004). Evidence from non-human studies has also shown that a homogeneous area lying in the mSTS receives inputs only from auditory-related areas (Seltzer and Pandya 1978), suggesting that this is a unimodal area for voice processing. As to deaf people, one study found more prominent activation in this region in early- than in late-deaf individuals when processing sign language (Sadato et al. 2004), suggesting functional plasticity of the middle part of the superior temporal cortex is sensitive to the period when auditory deprivation occurs. The aSTS appears to be mainly involved in processing meaningful speech (Scott et al. 2000; Narain et al. 2003). Some argue that the anterior portion of temporal cortex (temporal pole) is involved in sentence-level processing (Vandenberghe et al. 2002; Humphries et al. 2005, 2006), suggesting that this region might involve combinatorial semantics (McClelland and Rogers 2003; Hickok and Poeppel 2007). As to deaf studies, similar activation has been found in deaf individuals and hearing controls (HC) when processing their primary language, but aSTS was not activated when deaf individuals were reading English sentences (Neville et al. 1998). This suggests that the function of aSTS in deaf people may develop to process sign language but not written language. Although research is not conclusive, it appears that auditory derivation distinctly affects the function of different parts of STS.
In the current study, we explored the intra-regional synchronization and inter-regional synchronization of the superior temporal cortex using resting-state fMRI to examine the role of auditory experience on the functional brain organization of the deaf individuals without cochlear implants. For intra-regional synchronization, we calculated brain regional homogeneity (ReHo) during resting state, which provides an indicator of local synchronization within a brain region (Zang et al. 2004). For inter-regional synchronization, we calculated functional connectivity among brain regions using a seed-based approach. We examined both CD and acquired deaf (AD) to directly determine the effect of auditory deprivation on intrinsic function of the superior temporal cortex. Specifically, we focused on the STS because different parts of STS may be distinctly impacted by the auditory deprivation. Although pSTS and aSTS may develop to process different stimuli in deaf individuals, these regions appear to develop normally. In contrast, the function of mSTS is sensitive to the period when auditory deprivation occurs and may not develop normally. Based on the previous literature, we hypothesized that, compared with pSTS and aSTS, the intra-regional synchronization of mSTS would be most impacted by the auditory deprivation. We also explored possible changes of inter-regional connectivity by examining functional connectivity with seed regions showing altered intra-regional synchronization. We further explored whether intra- and inter-regional synchronization of the superior temporal cortex in deaf people was related with their language skills.
Materials and Methods
Participants
We recruited 142 right-handed participants with no history of neurological or psychiatric illness for our study, including CD, AD, and HC. Two AD were excluded because of poor image quality, 2 CD were excluded because of bad segmentation, and 1 AD was excluded because of excessive head motion for the resting-state data (see Image Preprocessing). After these exclusions, there were 60 CD (37 females, mean age 21.2 ± 2.24 years, range 17–28), 39 AD (19 females, mean age 21.6 ± 1.70 years, range 19–26), and 38 HC (21 females, mean age 21.8 ± 2.25 years, range 17–28) entering into the analysis. All CD (better ear: mean 98.7 ± 6.98 dB, range 91–120; left ear: mean 100.5 ± 7.63 dB, range 91–120; right ear: mean 101.7 ± 8.23 dB, range 91–125) and AD (better ear: mean 99.1 ± 7.63 dB, range 91–120; left ear: mean 101.3 ± 7.77 dB, range 92–120; right ear: mean 102.4 ± 9.66 dB, range 91–125) exhibited profound hearing loss. There were no significant differences in level of hearing in the better ear (t = 0.275, P = 0.784), left ear (t = 0.559, P = 0.577), or right ear (t = 0.368, P = 0.714) between CD and AD. Each deaf participant had normal intelligence quotient (IQ) scores as determined by Raven's Standard Progressive Matrices (Raven 1976). All deaf individuals had >50% on the appropriate norms. None of the deaf individuals wore hearing aids before 6 years old or in the past 3 years. The Chinese sign language was the primary language of all deaf individuals. The causes of deafness were genetic, pregnancy-related virus, meningitis, otitis media, ototoxic medications, complications at childbirth, or unknown. In AD, age ranges of the onset of deafness were from 6 months to 6 years (mean age 2.70 ± 1.72). There were no significant differences in age (F = 1.160, P = 0.317) and sex (χ2 = 1.629, P = 0.443) between the HC, AD, and CD. Institutional review board approval was obtained from the State Key Laboratory of Cognitive Neuroscience and Learning at Beijing Normal University. Informed written consent was obtained from each participant. Detailed characteristics of CD and AD are presented in Supplementary Tables S1 and S2.
Notable the comparisons between CD and AD may be confounded with different etiology, since some causes of deafness, such as maternal rubella in some individuals, may have additional effects on the central nervous system. We tried to minimize such an effect by only recruiting deaf participants who had normal IQ (tested by Raven's Progressive Matrices) and who did not show any significant brain abnormalities (as examined by an experienced neuroradiologist). However, we cannot exclude the possibility that there might be subtle effects on the central nervous system.
Language Skill Assessment
We administered 2 tests of written language to evaluate whether the synchronization at rest was associated with language skills. In the first test, deaf individuals (except 6 CD and 2 AD) were shown a series of 4 pictures and asked to write a story within 30 min. The primary criteria for scoring were 1) understanding of the pictures and 2) organization with good sentence structure and choice of vocabulary. The final score for each participant was calculated by averaging scores from 3 trained appraisers, using a 6-point (0–5) scale. If the highest and the lowest scores differed by more than 2 points on the scale, scores from 2 other appraisers were obtained. In this case, the final score was the average of 3 middle scores.
In the second test, 31 CD and 38 AD were assessed with the character recognition measure and assessment scale (CRMA; Wang and Tao 1993). CRMA is a widely used standardized test for Mandarin reading that requires writing a word when given a Chinese character. Scores for the test indicate character recognition and the understanding of the meaning of characters. We also tested 32 age- and sex-matched HC who did not receive the fMRI scanning.
Imaging Acquisition
Functional and structural images were acquired on a Siemens 3T Tim Trio scanner using a standard head coil. Head fixation with foam pads and ear protection with earplugs was used for each subject. During resting-state fMRI, participants were instructed to hold still, keep their eyes closed but not fall asleep or think of anything in particular. Imaging parameters of resting-state fMRI were 25 axial slices with echo-planar image pulse sequence, repetition time of 2000 ms, echo time of 30 ms, slice thickness of 5 mm, flip angle of 90°, and field of view (FOV) = 200 × 200 mm. Imaging parameters of T1-weighted anatomical image were sagittal acquisition with a 256 × 256 matrix, repetition time of 2530 ms, echo time of 3.45 ms, flip angle of 7°, number of excitations = 1, FOV = 256 × 256 mm, 1-mm slice thickness.
Imaging Preprocessing
We used SPM5 (http://www.fil.ion.ucl.ac.uk/spm) for preprocessing and General Linear Model statistical analysis. Data of the first 10 volumes were discarded to remove the effects of instability of initial magnetic resonance imaging signal and get the participants used to the environment. The remaining 230 images were preprocessed including slice timing, motion correction, co-registration to the structural data, spatial normalization to the MNI (Montreal Neurological Institute) template image, and spatial re-sampling (3 × 3 × 3 mm). Imaging data for participants with head motion >3 mm in transition or 3° in rotation for the fMRI were discarded. Then the linear trend was removed. A temporal band-pass filter (0.01–0.08 Hz) was applied to remove very low-frequency drifts and physiological high-frequency noise (Lowe et al. 1998; Greicius et al. 2003) by using resting-state fMRI data analysis toolkit (REST; Song et al. 2011, www.restfmri.net).
ReHo Analyses
Using SPM5 software (http://www.fil.ion.ucl.ac.uk/spm), intracranial voxels were created as a brain mask. Kendall coefficient of concordance (KCC) was used to measure ReHo or local synchronization of the ranked time series within a functional cluster (Zang et al. 2004). In our study, we measured ReHo of the nearest 27 neighboring voxels in a voxel-wise way. An individual KCC map was calculated for every participant. This procedure was implemented by using the REST V1.4 (Song et al. 2011; http://www.restfmri.net). For standardization purposes, individual ReHo maps were divided by its global average ReHo within the whole-brain mask. Then individual standard ReHo maps were spatially smoothed with 6-mm full width at half maximum (FWHM).
The volume of interest, that is, superior temporal cortex, was defined as the bilateral superior and middle temporal gyri (STG and MTG, and therefore includes STS) based on automated anatomical labeling (Tzourio-Mazoyer et al. 2002). We conducted 1-way analysis of variance (ANOVA) among CD, AD, and HC within the volume of interest. Multiple-comparison correction was performed with the program AlphaSim in REST, which estimates the overall significance level through Monte Carlo simulations (Song et al. 2011). In running AlphaSim, uncorrected P-value was set at 0.005 and FWHM was set at 6 mm. Bilateral superior temporal cortices were chosen as the brain mask. Cluster size >270 mm3 was considered as significant, which is associated with a corrected P-value (alpha) of 0.05. To determine which groups were driving this omnibus difference, we conducted post hoc Tukey pairwise comparisons with a threshold of 0.05 among groups for the mean of each cluster identified in the ANOVA analysis.
Although we focused on the effect with the superior temporal cortex bilaterally, we also performed a whole-brain analysis with a 1-way ANOVA to explore whether other brain regions outside of the superior temporal cortex showed significant differences among CD, AD, and HC and the following post hoc analysis. The results are presented in the Supplementary Materials.
Functional Connectivity Analysis
The seed region was defined as the cluster identified in the ANOVA analysis showing significant ReHo difference among the 3 groups in STS (see Fig. 1A for exact location). First, we calculated the functional connectivity between the time course of the seed region and the time courses of each voxel within the superior temporal cortex. Before the calculation, all images were smoothed (FWHM = 6 mm). After the calculation, we transformed individual r-maps to Z-maps using Fisher's Z test. A 1-way ANOVA was applied in each voxel to find significant functional connectivity differences among CD, AD, and HC (P< 0.05, AlphaSim corrected). In performing AlphaSim, the same uncorrected P-value and FWHM as that in ReHo analysis were used to estimate the significance, using the bilateral superior temporal cortex as a mask. We further conducted post hoc Tukey pairwise comparisons with a threshold of 0.05 among groups for each cluster identified in the ANOVA analysis.
Figure 1.
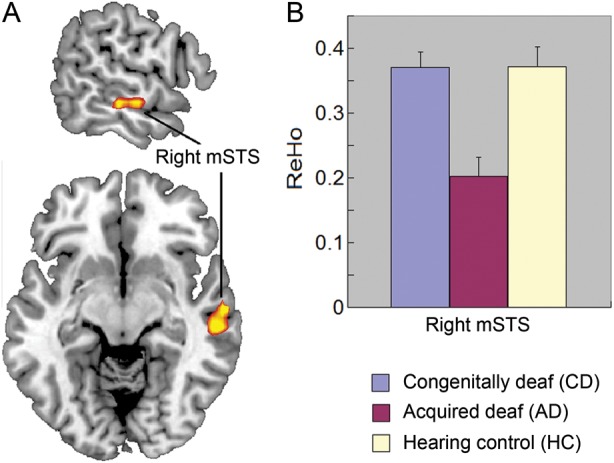
Comparison of ReHo among CD, AD, and HC. (A) The significant group difference of ReHo in the right mSTS revealed by ANOVA. (B) Reduced ReHo in the right mSTS in AD compared with HC and CD. The error bars present the standard errors in (B).
In addition, we calculated the functional connectivity between the seed region with other brain regions outside of the superior temporal cortex in a voxel-by-voxel way. Then we performed the ANOVA analysis to explore the group difference of the functional connectivity with these regions (Holmes et al. 2005). Cluster(s) >648 mm3 were considered significant, which is associated with a corrected P-value (alpha) of 0.05. We also conducted post hoc Tukey pairwise comparisons with a threshold of 0.05 among groups for each region identified in the ANOVA analysis.
Correlation Analyses on Intra-/Inter-Regional Synchronization and Language Skills
Given the importance of superior temporal cortex in language processing, we further explored whether the intra- and inter-regional synchronization in the superior temporal cortex in deaf individuals was correlated with their language skills. We calculated the correlation of either ReHo or functional connectivity values in each voxel of superior temporal cortex with either the scores of CRMA or that of the story writing separately for CD and AD groups. Again, a corrected P-value of <0.05 was set as the threshold, which is associated with a cluster size >270 mm3 combined with uncorrected P< 0.005.
Results
ReHo Results
The ANOVA within the anatomical mask of superior temporal cortex showed differences in the right mSTS (MNI coordinates [63 −12 −9], F = 9.53, cluster size = 1323 mm3) among CD, AD, and HC, with a threshold of P (corrected) <0.05 determined by AlphaSim (Fig. 1A). The post hoc pairwise tests further found that AD had lower ReHo when compared with CD and HC in the right mSTS (P< 0.001, Fig. 1B). There was no difference between the CD and HC.
Whole-brain analysis revealed significant differences in bilateral supplementary motor area, right precentral gyrus, left inferior frontal gyrus, left superior frontal gyrus, and left putamen (Supplementary Table S3 and Fig. S1). Detailed results of post hoc pairwise tests were presented in Supplementary Table S3.
Functional Connectivity Results
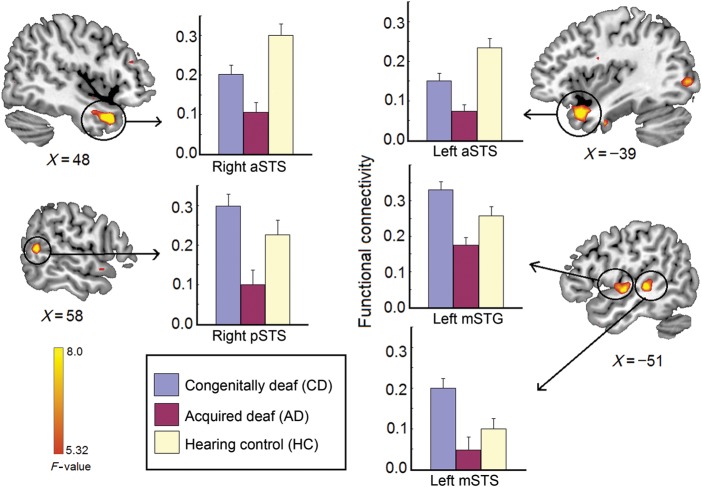
We first calculated the temporal correlation between the seed region (right mSTS, see Fig. 1A for the exact location) with every other voxel within the superior temporal cortex. Several brain regions within either the ipsilateral or contralateral superior temporal cortex showed a significant difference among the 3 groups in the ANOVA, including the right aSTS and pSTS, the left aSTS and mSTS, and left middle STG (mSTG; Fig. 2 and Table 1). Post hoc pairwise tests showed that for both left and right aSTS, both CD and AD had reduced functional connectivity compared with HC. But for the right pSTS and left mSTS/mSTG, only AD showed reduced functional connectivity compared with HC. Instead, CD had a trend of enhanced functional connectivity compared with HC, though only significant in the left mSTS (Fig. 2). For all of these regions, CD had enhanced functional connectivity compared with AD.
Figure 2.
Group differences of functional connectivity with right mSTS (the seed region) within the bilateral superior temporal cortex among CD, AD, and HC. Brain regions showing group differences include right aSTS, right pSTS, left aSTS, left mSTG, and left mSTS. The error bars present the standard errors.
Table 1.
Group differences of functional connectivity with right mSTS (the seed region) in resting state
| Location | Volume (mm3) | F-value | MNI coordinates (mm) |
Post hoc analysis (Tukey, P< 0.05) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Right anterior STS | 1566 | 12.98 | 48 | 12 | −30 | HC > CD > AD |
| Right posterior STS | 540 | 9.82 | 57 | −51 | 9 | HC and CD > AD |
| Left anterior STS | 1620 | 10.04 | −39 | 15 | −27 | HC > CD > AD |
| Left middle STS | 1107 | 9.11 | −51 | −30 | 0 | CD > AD |
| Left middle STG | 783 | 8.56 | −51 | −9 | −3 | CD > HC > AD |
Note: Location is the anatomical areas within the superior temporal cortex that showed group functional connectivity differences in ANOVA among CD, AD, and HC. Corrected P < 0.05. STG: superior temporal gyrus; STS: superior temporal sulcus; mSTS: middle superior temporal sulcus; MNI: Montreal Neurological Institute; HC: hearing control; CD: congenitally deaf individuals; AD: acquired deaf individuals. MNI coordinates are those of the corresponding maximum peak of each cluster.
Effects not listed for the post hoc analyses are not significant at P < 0.05.
Some brain regions outside of temporal cortex also showed group differences (Supplementary Table S3 and Fig. S2). Most brain regions actually showed a negative connectivity with the seed region (right mSTS) in the HC group, including the left precuneus, cerebellum (vermis), the left thalamus, left middle occipital gyrus, and right precentral gyrus. Among these regions, both CD and AD showed the reduced negative connectivity compared with HC in the left precuneus, cerebellum (vermis), and the left thalamus, whereas they showed an enhanced negative connectivity compared with HC in the left middle occipital gyrus and right precentral gyrus. In addition, both CD and AD showed an enhanced positive connectivity compared with HC in the anterior cingulate cortex, whereas they showed the reduced positive connectivity compared with the HC in the left medial superior frontal gyrus (Supplementary Fig. S2).
Behavioral and Correlation Results
For the scores of story writing, there was no significant difference between CD and AD (t = 0.899, P = 0.371; CD: 3.85 ± 0.09; AD: 3.97 ± 0.11). For the scores of CRMA, significant differences were found when comparing CD, AD, and HC (F2,98 = 34.507, P = 4.52 × 10−12; CD: 2859 ± 51; AD: 2984 ± 47; HC: 3426 ± 51). Post hoc tests further showed that CD and AD had reduced scores compared with HC (CD: P = 1.71 × 10−11; AD: P = 1.51 × 10−8), but no significant difference was found between CD and AD (P = 0.225).
The results of correlation analyses are shown in Table 2. For ReHo, significant correlation was only found for right STG in the CD group with story writing. For the functional connectivity (with the seed region of right mSTS), significant correlations were found at both the right aSTS/aMTG and the left mSTS with either CRMA or story writing. Specifically, CD group showed a significant correlation between functional connectivity of both left mSTS (MNI [−54, −33, 6]) and right aMTG (MNI [51, −3, −27]) with CRMA, and left mSTS/STG (MNI [−51, −39, 21]) with story writing. The AD group showed a significant correlation between functional connectivity of the left aSTS (MNI [−54, 0, −15]) with story writing (Fig. 3).
Table 2.
Correlation of intra- and inter-regional synchronization in the superior temporal cortex with the language skills in deaf individuals
| Brain location | Group | Test | Volume (mm3) | T-value | MNI coordinates (mm) |
||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Intra-regional synchronization (ReHo) | |||||||
| Right mSTG | CD | Story writing | 459 | 3.36 | 63 | −24 | 15 |
| Inter-regional synchronization (functional connectivity) | |||||||
| Left mSTS | CD | CRMA | 1026 | 3.98 | −54 | −33 | −6 |
| Right aMTG | CD | CRMA | 378 | 4.03 | 51 | −3 | −27 |
| Left mSTS/STG | CD | Story writing | 621 | 3.45 | −51 | −39 | 21 |
| Left aSTS | AD | Story writing | 459 | 4.40 | −54 | 0 | −15 |
Location is the anatomical areas in regions that showed group differences of correlation between either ReHo or functional connectivity with language performance in CD and AD. Corrected P < 0.05. mSTG: middle superior temporal gyrus; mSTS: middle superior temporal sulcus; CRMA: character recognition measure and assessment scale; MNI: Montreal Neurological Institute; ReHo: regional homogeneity; aMTG: anterior middle temporal gyrus; aSTS: anterior superior temporal sulcus; CD: congenitally deaf individuals; AD: acquired deaf individuals.
Figure 3.
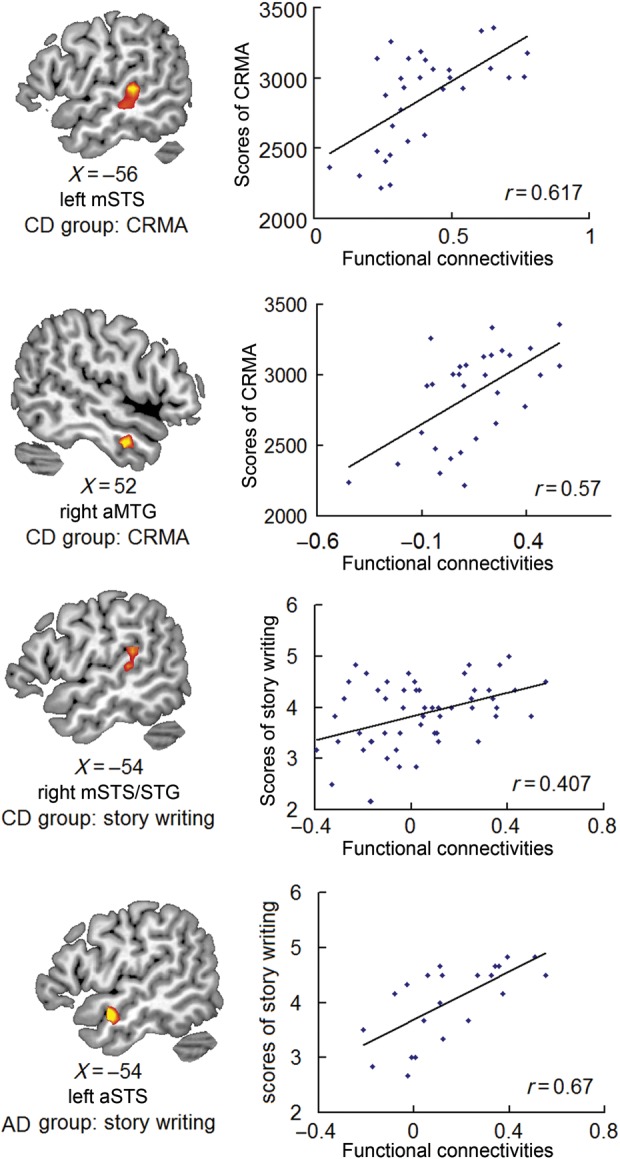
Significant correlations between functional connectivity and language skills in CD and AD. The left side shows the regions that exhibited significant correlation between functional connectivity with right mSTS and language skills. The right side shows the corresponding scatter diagrams.
Due to some practical reasons, we only collected the scores of CRMA for 31 CD, while 60 CD received the resting fMRI scanning. To investigate whether including only 31 CD in the analysis biased the results, we compared the values of both the ReHo of right mSTS and the connectivity between mSTS and pSTS/aSTS for the 31 CD who received the CRMA versus the 29 CD who did not. We found that these 2 subgroups did not have significant differences in ReHo of the right mSTS (t = 0.093, P = 0.926) or connectivity between mSTS and pSTS (t = 0.32, P = 0.72) or between mSTS and aSTS (t = − 0.88, P = 0.38). These results suggest that including only 31 CD did not influence the results reported.
Discussion
We examined fMRI during resting in CD, AD, and HC. When compared with HC, AD had reduced ReHo in the right mSTS, whereas CD had preserved ReHo in this region. As to functional connectivity, CD had a greater connectivity between the seed region determined by ReHo (mSTS) and ipsilateral pSTS or contralateral mSTS/mSTG compared with AD. This may suggest greater cross-modal plasticity in CD than in AD due to connectivity with the pSTS believed to be involved in multisensory integration. It may also suggest that partial auditory experience in the AD reduced cross-modal plasticity. In addition, both AD and CD had reduced connectivity of bilateral aSTS with the seed region. Finally, the connectivity of bilateral aSTS/aMTG and the left mSTS/mSTG with the seed region was associated with their language performance, suggesting that the alteration of the inter-regional synchronization may impact language performance in deaf individuals. This discussion will highlight how these results provide a deeper understanding of the forces behind the organization of temporal cortex in deaf individuals.
Role of Cross-Modal Plasticity
We found that the individuals of the CD group who have no early hearing experience exhibited preserved ReHo in mSTS when compared with the HC group. We suggest that the possible reason is cross-modal plasticity caused by the deprivation of auditory experience. Previous studies have shown that mSTS receives projections predominantly from auditory cortex (Seltzer and Pandya 1978) and functions to process human speech information (Belin et al. 2000; Fecteau et al. 2004). Auditory deprivation might lead to cross-modal plasticity in the mSTS, shifting the role of this region to the processing of biological motion. Increased activity in the middle and posterior STG, but not the primary auditory cortex, has been revealed in CD individuals when processing sign language (Nishimura et al. 1999). Behavioral studies have also shown that CD have better visual performance (Bavelier et al. 2006), possibly indicating that they have recruited other regions of the brain for processing visual stimuli.
The reduced ReHo in mSTS in the AD group may suggest less cross-modal plasticity in this region compared with the CD group. This is consistent with previous findings showing that the age of the onset of deafness is critical in the cross-modal plasticity of deaf brain. For example, previous studies have found that deaf individuals usually have better visual skills in attentionally demanding tasks (Bavelier et al. 2006). However, the evidence for cross-modal plasticity has been mainly from congenitally (Neville and Lawson 1987; Bavelier et al. 2000) or early deaf individuals (Sladen et al. 2005). A brain imaging study found that early deaf individuals show stronger activation than late deaf individuals in mSTS (Sadato et al. 2004). In addition, deaf individuals with cochlear implanting showed that the onset age of deafness plays an important role in speech language development (Osberger et al. 1991; Fryauf-Bertschy et al. 1992) and brain activation during speech processing (Okazawa et al. 1996; Naito et al. 1997). This evidence suggests that the earlier the individuals' acquired deafness, the greater the cross-modal plasticity.
The suggestion that there is an enhanced cross-modal plasticity in mSTS in CD is also consistent with greater connectivity between mSTS and pSTS for CD compared with AD. Previous studies in HC have shown enhanced activity for multimodal stimuli compared with unimodal ones in pSTS (Calvert et al. 2000; Beauchamp et al. 2004; van Atteveldt et al. 2004; Noesselt et al. 2007; Beauchamp et al. 2008). As reported above, the mSTS appears to have a different function in HC, as it is sensitive to human speech (Belin et al. 2000; Fecteau et al. 2004). In contrast, studies on CD have found that both the mSTS and pSTS process visual objects (Petitto et al. 2000; Sadato et al. 2004). The enhanced functional connectivity found in our study between mSTS and pSTS for CD may be associated with the recruitment of the mSTS to process visual language. Alternately, both middle and posterior regions of the superior temporal cortex have been reported to be associated with motion (Allison et al. 2000; Bavelier et al. 2001; Grossman and Blake 2002; Beauchamp et al. 2003; Peuskens et al. 2005) and face processing in HC (Allison et al. 2000; Haxby et al. 2000; Ishai et al. 2005). Absent auditory information, CD individuals make use of these types of visual information to a greater degree to communicate with others, and this may be reflected in their greater recruitment of the middle and posterior STS (Bavelier et al. 2001; Finney et al. 2001, 2003). The possible structural mechanism of this cross-modal plasticity is that CD individuals have maintained redundant neuronal connections in the superior temporal cortex, allowing neural rewiring of auditory-related cortex to process visual stimuli (Li et al. 2012).
Implications for Language Processing
For both the AD and CD groups, we found reduced connectivity between the right mSTS and bilateral aSTS. In the correlation analysis, we also found that the functional connectivity of right mSTS and aSTS/MTG is positively correlated with language performance. For hearing people, the mSTS usually collaborates with anterior temporal cortex to process auditory language (Humphries et al. 2001; Rimol et al. 2005; Uppenkamp et al. 2006) and music (Peretz and Zatorre 2005). The dual stream model of speech processing has argued that the dorsal stream supports an interface of phonological information with the frontal articulatory system, whereas a ventral stream maps acoustic speech to conceptual and semantic representations (McClelland and Rogers 2003; Hickok and Poeppel 2007). The anterior STS/MTG, a part of ventral stream, has been suggested to be involved in linking speech with lexical/conceptual information (McClelland and Rogers 2003; Hickok and Poeppel 2007). The decreased connectivity between right mSTS and bilateral aSTS for both CD and AD groups may result from the absence of the links between word forms and lexical/conceptual representations.
Although studies have found bidirectional connections between the hemispheres during language processing, they have also shown a stronger influence of the right on the left hemisphere and stronger intra-hemispheric connections within the left hemisphere (Bitan et al. 2010). These results support a model of cooperation between hemispheres, but left hemisphere specialization when processing speech signals for comprehension. Moreover, the dual stream model of speech processing argues that the processing of combinatorial semantics is left hemisphere dominant (Hickok and Poeppel 2007). Sign language processing in the deaf is also left hemisphere dominant (Sakai et al. 2005). Studies have shown that left STG and MTG are involved in sign language in deaf individuals (Neville et al. 1998; Petitto et al. 2000; Emmorey et al. 2002, 2005; MacSweeney et al. 2002, 2006; Sakai et al. 2005). The role of processing sign language may facilitate intrafunctional synchronization within the left STS. A previous study also found that the fractional anisotropy of right STG, but not left STG, was altered in deaf individuals (Kim et al. 2009). Consistent with this, we only found changed ReHo in the right mSTS in AD. Finally, we found connectivity between right mSTS and left mSTS/mSTG for the CD compared with the other 2 groups. This is consistent with previous studies that congenitally native deaf signers displayed similar activation within classical language areas of the left hemisphere but greater activation in homologous areas of the right hemisphere when compared with HC (Neville et al. 1998), indicating that CD signers may rely on greater cooperation of bilateral temporal regions.
Interestingly, we also found that both CD and AD have greater functional connectivity than HC at several brain regions either positively (anterior cingulate cortex) or negatively (left middle occipital gyrus and right precentral gyrus). The anterior cingulate cortex is important for cognitive monitoring and conflict resolving (Carter et al. 1999; Abutalebi et al. 2011), left middle occipital gyrus has been implicated in human body motion or action perception (Astafiev et al. 2004; Corina and Knapp 2008), and right precentral gyrus has been shown to be involved in hand motion or finger movement (Baraldi et al. 1999; Joliot et al. 1999). Both human action perception and hand/figure movements are critical components of sign language processing. Given both CD and AD individuals use sign language while HC individuals use spoken language, it is possible that the enhanced functional connectivity in these regions is related with sign language processing. Future studies are required to address this issue.
Conclusion
In conclusion, we found that ReHo of the right mSTS was preserved in CD, but there was increased connectivity between mSTS and pSTS than AD. This suggests possible cross-modal plasticity caused by the deprivation of auditory experience. The AD had weaker ReHo in right mSTS and had reduced connectivity of this region with pSTS, possibly indicating that they did not benefit from cross-modal plasticity due to altered early auditory experience. Finally, the AD and CD had weaker connectivity of mSTS with aSTS/aMTG than HC. The alteration of mSTS–aSTS connectivity in both CD and AD appears to interfere with the normal linking of word forms with combinatorial semantics that give rise to meaning. These findings suggest that the intrinsic function in different parts of the superior temporal cortex is distinctly impacted by the auditory experience (×Fig. 3).
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by grants from National Nature Science Foundation of China (NSFC, 30870757, 31170969) to G.D. and Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT) to D.P. This work was also supported by grants from the National Institute of Child Health and Human Development (HD042049) to J.R.B.
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr287. doi:10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. 2000;4:267–278. doi: 10.1016/s1364-6613(00)01501-1. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci. 2004;7:542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- Auer ET, Jr, Bernstein LE, Sungkarat W, Singh M. Vibrotactile activation of the auditory cortices in deaf versus hearing adults. Neuroreport. 2007;18:645–648. doi: 10.1097/WNR.0b013e3280d943b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi P, Porro CA, Serafini M, Pagnoni G, Murari C, Corazza R, Nichelli P. Bilateral representation of sequential finger movements in human cortical areas. Neurosci Lett. 1999;269:95–98. doi: 10.1016/s0304-3940(99)00433-4. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Res Bull. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Brozinsky C, Tomann A, Mitchell T, Neville H, Liu G. Impact of early deafness and early exposure to sign language on the cerebral organization for motion processing. J Neurosci. 2001;21:8931–8942. doi: 10.1523/JNEUROSCI.21-22-08931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Dye MW, Hauser PC. Do deaf individuals see better? Trends Cogn Sci. 2006;10:512–518. doi: 10.1016/j.tics.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Neville HJ. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- Bavelier D, Tomann A, Hutton C, Mitchell T, Corina D, Liu G, Neville H. Visual attention to the periphery is enhanced in congenitally deaf individuals. J Neurosci. 2000;20:RC93. doi: 10.1523/JNEUROSCI.20-17-j0001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp MS, Argall BD, Bodurka J, Duyn JH, Martin A. Unraveling multisensory integration: patchy organization within human STS multisensory cortex. Nat Neurosci. 2004;7:1190–1192. doi: 10.1038/nn1333. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Lee KE, Haxby JV, Martin A. FMRI responses to video and point-light displays of moving humans and manipulable objects. J Cogn Neurosci. 2003;15:991–1001. doi: 10.1162/089892903770007380. [DOI] [PubMed] [Google Scholar]
- Beauchamp MS, Yasar NE, Frye RE, Ro T. Touch, sound and vision in human superior temporal sulcus. Neuroimage. 2008;41:1011–1020. doi: 10.1016/j.neuroimage.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Benevento LA, Fallon J, Davis BJ, Rezak M. Auditory–visual interaction in single cells in the cortex of the superior temporal sulcus and the orbital frontal cortex of the macaque monkey. Exp Neurol. 1977;57:849–872. doi: 10.1016/0014-4886(77)90112-1. [DOI] [PubMed] [Google Scholar]
- Bitan T, Lifshitz A, Breznitz Z, Booth JR. Bidirectional connectivity between hemispheres occurs at multiple levels in language processing but depends on sex. J Neurosci. 2010;30:11576–11585. doi: 10.1523/JNEUROSCI.1245-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert GA, Campbell R, Brammer MJ. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol. 2000;10:649–657. doi: 10.1016/s0960-9822(00)00513-3. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Carter CS, Botvinick MM, Cohen JD. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- Catalan-Ahumada M, Deggouj N, De Volder A, Melin J, Michel C, Veraart C. High metabolic activity demonstrated by positron emission tomography in human auditory cortex in case of deafness of early onset. Brain Res. 1993;623:287–292. doi: 10.1016/0006-8993(93)91439-y. [DOI] [PubMed] [Google Scholar]
- Corina DP, Knapp HP. Signed language and human action processing: evidence for functional constraints on the human mirror-neuron system. Ann N Y Acad Sci. 2008;1145:100–112. doi: 10.1196/annals.1416.023. [DOI] [PubMed] [Google Scholar]
- Deggouj N, Devolder A, Catalan M, Melin J, Michel C, Gersdorff M, Veraart C. Positron emission tomography in deaf patients at rest. Adv Otorhinolaryngol. 1995;50:31–37. doi: 10.1159/000424431. [DOI] [PubMed] [Google Scholar]
- Emmorey K, Damasio H, McCullough S, Grabowski T, Ponto LL, Hichwa RD, Bellugi U. Neural systems underlying spatial language in American Sign Language. Neuroimage. 2002;17:812–824. [PubMed] [Google Scholar]
- Emmorey K, Grabowski T, McCullough S, Ponto LL, Hichwa RD, Damasio H. The neural correlates of spatial language in English and American Sign Language: a PET study with hearing bilinguals. Neuroimage. 2005;24:832–840. doi: 10.1016/j.neuroimage.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Armony JL, Joanette Y, Belin P. Is voice processing species-specific in human auditory cortex? An fMRI study. Neuroimage. 2004;23:840–848. doi: 10.1016/j.neuroimage.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Finney EM, Clementz BA, Hickok G, Dobkins KR. Visual stimuli activate auditory cortex in deaf subjects: evidence from MEG. Neuroreport. 2003;14:1425–1427. doi: 10.1097/00001756-200308060-00004. [DOI] [PubMed] [Google Scholar]
- Finney EM, Fine I, Dobkins KR. Visual stimuli activate auditory cortex in the deaf. Nat Neurosci. 2001;4:1171–1173. doi: 10.1038/nn763. [DOI] [PubMed] [Google Scholar]
- Fryauf-Bertschy H, Tyler RS, Kelsay DM, Gantz BJ. Performance over time of congenitally deaf and postlingually deafened children using a multichannel cochlear implant. J Speech Hear Res. 1992;35:913–920. doi: 10.1044/jshr.3504.913. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain areas active during visual perception of biological motion. Neuron. 2002;35:1167–1175. doi: 10.1016/s0896-6273(02)00897-8. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends Cogn Sci. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci. 2007;8:393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- Hirano S, Naito Y, Kojima H, Honjo I, Inoue M, Shoji K, Tateya I, Fujiki N, Nishizawa S, Konishi J. Functional differentiation of the auditory association area in prelingually deaf subjects. Auris Nasus Larynx. 2000;27:303–310. doi: 10.1016/s0385-8146(00)00072-9. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, MacDonald A, 3rd, Carter CS, Barch DM, Andrew Stenger V, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci. 2006;18:665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Love T, Swinney D, Hickok G. Response of anterior temporal cortex to syntactic and prosodic manipulations during sentence processing. Hum Brain Mapp. 2005;26:128–138. doi: 10.1002/hbm.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C, Willard K, Buchsbaum B, Hickok G. Role of anterior temporal cortex in auditory sentence comprehension: an fMRI study. Neuroreport. 2001;12:1749–1752. doi: 10.1097/00001756-200106130-00046. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P. Face perception is mediated by a distributed cortical network. Brain Res Bull. 2005;67:87–93. doi: 10.1016/j.brainresbull.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Joliot M, Papathanassiou D, Mellet E, Quinton O, Mazoyer N, Courtheoux P, Mazoyer B. fMRI and PET of self-paced finger movement: comparison of intersubject stereotaxic averaged data. Neuroimage. 1999;10:430–447. doi: 10.1006/nimg.1999.0483. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Park SY, Kim J, Lee DH, Park HJ. Alterations of white matter diffusion anisotropy in early deafness. Neuroreport. 2009;20:1032–1036. doi: 10.1097/WNR.0b013e32832e0cdd. [DOI] [PubMed] [Google Scholar]
- Koyama MS, Di Martino A, Zuo XN, Kelly C, Mennes M, Jutagir DR, Castellanos FX, Milham MP. Resting-state functional connectivity indexes reading competence in children and adults. J Neurosci. 2011;31:8617–8624. doi: 10.1523/JNEUROSCI.4865-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Lee JS, Oh SH, Kim SK, Kim JW, Chung JK, Lee MC, Kim CS. Cross-modal plasticity and cochlear implants. Nature. 2001;409:149–150. doi: 10.1038/35051653. [DOI] [PubMed] [Google Scholar]
- Lee JS, Lee DS, Oh SH, Kim CS, Kim JW, Hwang CH, Koo J, Kang E, Chung JK, Lee MC. PET evidence of neuroplasticity in adult auditory cortex of postlingual deafness. J Nucl Med. 2003;44:1435–1439. [PubMed] [Google Scholar]
- Levanen S, Jousmaki V, Hari R. Vibration-induced auditory-cortex activation in a congenitally deaf adult. Curr Biol. 1998;8:869–872. doi: 10.1016/s0960-9822(07)00348-x. [DOI] [PubMed] [Google Scholar]
- Li Y, Ding G, Booth JR, Huang R, Lv Y, Zang Y, He Y, Peng D. Sensitive period for white-matter connectivity of superior temporal cortex in deaf people. Hum Brain Mapp. 2012;33:349–359. doi: 10.1002/hbm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Mock BJ, Sorenson JA. Functional connectivity in single and multislice echoplanar imaging using resting-state fluctuations. Neuroimage. 1998;7:119–132. doi: 10.1006/nimg.1997.0315. [DOI] [PubMed] [Google Scholar]
- MacSweeney M, Campbell R, Woll B, Brammer MJ, Giampietro V, David AS, Calvert GA, McGuire PK. Lexical and sentential processing in British Sign Language. Hum Brain Mapp. 2006;27:63–76. doi: 10.1002/hbm.20167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacSweeney M, Woll B, Campbell R, McGuire PK, David AS, Williams SC, Suckling J, Calvert GA, Brammer MJ. Neural systems underlying British Sign Language and audio-visual English processing in native users. Brain. 2002;125:1583–1593. doi: 10.1093/brain/awf153. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003;4:310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, Milham MP. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nat Rev Neurosci. 2010;11:44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y, Hirano S, Honjo I, Okazawa H, Ishizu K, Takahashi H, Fujiki N, Shiomi Y, Yonekura Y, Konishi J. Sound-induced activation of auditory cortices in cochlear implant users with post- and prelingual deafness demonstrated by positron emission tomography. Acta Otolaryngol. 1997;117:490–496. doi: 10.3109/00016489709113426. [DOI] [PubMed] [Google Scholar]
- Narain C, Scott SK, Wise RJ, Rosen S, Leff A, Iversen SD, Matthews PM. Defining a left-lateralized response specific to intelligible speech using fMRI. Cereb Cortex. 2003;13:1362–1368. doi: 10.1093/cercor/bhg083. [DOI] [PubMed] [Google Scholar]
- Neville HJ, Bavelier D, Corina D, Rauschecker J, Karni A, Lalwani A, Braun A, Clark V, Jezzard P, Turner R. Cerebral organization for language in deaf and hearing subjects: biological constraints and effects of experience. Proc Natl Acad Sci USA. 1998;95:922–929. doi: 10.1073/pnas.95.3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neville HJ, Lawson D. Attention to central and peripheral visual space in a movement detection task: an event-related potential and behavioral study. II. Congenitally deaf adults. Brain Res. 1987;405:268–283. doi: 10.1016/0006-8993(87)90296-4. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Hashikawa K, Doi K, Iwaki T, Watanabe Y, Kusuoka H, Nishimura T, Kubo T. Sign language ‘heard’ in the auditory cortex. Nature. 1999;397:116. doi: 10.1038/16376. [DOI] [PubMed] [Google Scholar]
- Noesselt T, Rieger JW, Schoenfeld MA, Kanowski M, Hinrichs H, Heinze HJ, Driver J. Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. J Neurosci. 2007;27:11431–11441. doi: 10.1523/JNEUROSCI.2252-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazawa H, Naito Y, Yonekura Y, Sadato N, Hirano S, Nishizawa S, Magata Y, Ishizu K, Tamaki N, Honjo I, et al. Cochlear implant efficiency in pre- and postlingually deaf subjects. A study with H2(15)O and PET. Brain. 1996;119(Pt 4):1297–1306. doi: 10.1093/brain/119.4.1297. [DOI] [PubMed] [Google Scholar]
- Osberger MJ, Todd SL, Berry SW, Robbins AM, Miyamoto RT. Effect of age at onset of deafness on children's speech perception abilities with a cochlear implant. Ann Otol Rhinol Laryngol. 1991;100:883–888. doi: 10.1177/000348949110001104. [DOI] [PubMed] [Google Scholar]
- Padberg J, Seltzer B, Cusick CG. Architectonics and cortical connections of the upper bank of the superior temporal sulcus in the rhesus monkey: an analysis in the tangential plane. J Comp Neurol. 2003;467:418–434. doi: 10.1002/cne.10932. [DOI] [PubMed] [Google Scholar]
- Peretz I, Zatorre RJ. Brain organization for music processing. Annu Rev Psychol. 2005;56:89–114. doi: 10.1146/annurev.psych.56.091103.070225. [DOI] [PubMed] [Google Scholar]
- Petitto LA, Zatorre RJ, Gauna K, Nikelski EJ, Dostie D, Evans AC. Speech-like cerebral activity in profoundly deaf people processing signed languages: implications for the neural basis of human language. Proc Natl Acad Sci USA. 2000;97:13961–13966. doi: 10.1073/pnas.97.25.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. Eur J Neurosci. 2005;21:2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- Raven JC. Standard progressive matrices: sets A, B, C, D & E. Oxford: Oxford Psychologists Press; 1976. [Google Scholar]
- Rimol LM, Specht K, Weis S, Savoy R, Hugdahl K. Processing of sub-syllabic speech units in the posterior temporal lobe: an fMRI study. Neuroimage. 2005;26:1059–1067. doi: 10.1016/j.neuroimage.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Sadato N, Yamada H, Okada T, Yoshida M, Hasegawa T, Matsuki K, Yonekura Y, Itoh H. Age-dependent plasticity in the superior temporal sulcus in deaf humans: a functional MRI study. BMC Neurosci. 2004;5:56. doi: 10.1186/1471-2202-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai KL, Tatsuno Y, Suzuki K, Kimura H, Ichida Y. Sign and speech: amodal commonality in left hemisphere dominance for comprehension of sentences. Brain. 2005;128:1407–1417. doi: 10.1093/brain/awh465. [DOI] [PubMed] [Google Scholar]
- Scott SK, Blank CC, Rosen S, Wise RJ. Identification of a pathway for intelligible speech in the left temporal lobe. Brain. 2000;123(Pt 12):2400–2406. doi: 10.1093/brain/123.12.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Res. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Frontal lobe connections of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989a;281:97–113. doi: 10.1002/cne.902810108. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Intrinsic connections and architectonics of the superior temporal sulcus in the rhesus monkey. J Comp Neurol. 1989b;290:451–471. doi: 10.1002/cne.902900402. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Parietal, temporal, and occipital projections to cortex of the superior temporal sulcus in the rhesus monkey: a retrograde tracer study. J Comp Neurol. 1994;343:445–463. doi: 10.1002/cne.903430308. [DOI] [PubMed] [Google Scholar]
- Sladen DP, Tharpe AM, Ashmead DH, Wesley Grantham D, Chun MM. Visual attention in deaf and normal hearing adults: effects of stimulus compatibility. J Speech Lang Hear Res. 2005;48:1529–1537. doi: 10.1044/1092-4388(2005/106). [DOI] [PubMed] [Google Scholar]
- Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelnikov K, Rouger J, Demonet JF, Lagleyre S, Fraysse B, Deguine O, Barone P. Does brain activity at rest reflect adaptive strategies? Evidence from speech processing after cochlear implantation. Cereb Cortex. 2010;20:1217–1222. doi: 10.1093/cercor/bhp183. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uppenkamp S, Johnsrude IS, Norris D, Marslen-Wilson W, Patterson RD. Locating the initial stages of speech-sound processing in human temporal cortex. Neuroimage. 2006;31:1284–1296. doi: 10.1016/j.neuroimage.2006.01.004. [DOI] [PubMed] [Google Scholar]
- van Atteveldt N, Formisano E, Goebel R, Blomert L. Integration of letters and speech sounds in the human brain. Neuron. 2004;43:271–282. doi: 10.1016/j.neuron.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Vandenberghe R, Nobre AC, Price CJ. The response of left temporal cortex to sentences. J Cogn Neurosci. 2002;14:550–560. doi: 10.1162/08989290260045800. [DOI] [PubMed] [Google Scholar]
- Wang X, Tao B. Chinese character recognition test battery and assessment scale for primary school children. Shanghai, China: Shanghai Education Press; 1993. [Google Scholar]
- Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. Neuroimage. 2004;22:394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.