Abstract
Background
Phosphorus (P) is an essential element for plant growth and development but it is often a limiting nutrient in soils. Hence, P acquisition from soil by plant roots is a subject of considerable interest in agriculture, ecology and plant root biology. Root architecture, with its shape and structured development, can be considered as an evolutionary response to scarcity of resources.
Scope
This review discusses the significance of root architecture development in response to low P availability and its beneficial effects on alleviation of P stress. It also focuses on recent progress in unravelling cellular, physiological and molecular mechanisms in root developmental adaptation to P starvation. The progress in a more detailed understanding of these mechanisms might be used for developing strategies that build upon the observed explorative behaviour of plant roots.
Conclusions
The role of root architecture in alleviation of P stress is well documented. However, this paper describes how plants adjust their root architecture to low-P conditions through inhibition of primary root growth, promotion of lateral root growth, enhancement of root hair development and cluster root formation, which all promote P acquisition by plants. The mechanisms for activating alterations in root architecture in response to P deprivation depend on changes in the localized P concentration, and transport of or sensitivity to growth regulators such as sugars, auxins, ethylene, cytokinins, nitric oxide (NO), reactive oxygen species (ROS) and abscisic acid (ABA). In the process, many genes are activated, which in turn trigger changes in molecular, physiological and cellular processes. As a result, root architecture is modified, allowing plants to adapt effectively to the low-P environment. This review provides a framework for understanding how P deficiency alters root architecture, with a focus on integrated physiological and molecular signalling.
Keywords: Low phosphate, phosphorus acquisition, primary root, lateral root, root hair, cluster root, sugars, auxins, ethylene, cytokinins, nitric oxide, reactive oxygen species
INTRODUCTION
Nutrient stresses have increasingly curtailed crop production due to scarcity of resources. Phosphorus (P) is a critical macronutrient required for numerous functions in plant, including energy generation, nucleic acid synthesis, photosynthesis, glycolysis, respiration, membrane synthesis and stability, enzyme activation/inactivation, redox reactions, signalling, carbohydrate metabolism and nitrogen fixation (Abel et al., 2002; Vance et al., 2003). Meanwhile, P deficiency is considered as one of the greatest limitations in agricultural production (Schachtman et al., 1998; Lynch and Brown, 2008). It has been estimated that 5·7 billion hectares of land worldwide are deficient in P. Concentrations of phosphate in soil solutions are generally <10 µm, which are well below the critical level that is needed for the optimal performance of crops (Batjes, 1997). This problem of P deficiency might be mitigated by the application of concentrated fertilizers that provide soluble Pi for plants. The practice, however, is inherently inefficient due to chemical immobilization of P and agricultural run-off (Abel et al., 2002). To overcome low P availability, plants have evolved a complex array of tightly controlled adaptive mechanisms for maintaining P homeostasis. One of the main mechanisms is to maximize the ability of the root to absorb P from the soil. The alteration of root architecture is a powerful vehicle for the development of crop plants with an efficient P acquisition ability.
Root architecture, the spatial configuration of a root system in the soil, has been shown to be important for plant P acquisition (Lynch, 1995). Root architecture is highly plastic in its developmental response to low-P conditions. A number of research papers and reviews have shown that genotypic adaptations to P deficiency allow the changes in root architecture that facilitate P acquisition (Gahoonia et al., 2001; Lynch and Brown, 2001; Williamson et al., 2001; López-Bucio et al., 2002; Hermans et al., 2006; Liu et al., 2008; Lynch and Brown, 2008; Vance, 2008; Richardson et al., 2009; Wang et al., 2009; Ramaekers et al., 2010; Rouached et al., 2010; Chiou and Lin, 2011; Péret et al., 2011). The plant Arabidopsis thaliana, particularly the Columbia (Col-0) ecotype, has been shown to be a useful model to dissect the alteration of root morphology and architecture triggered by P deficiency (Williamson et al., 2001; Sánchez-Calderón et al., 2005). Low P availability in arabidopsis promotes the development of a highly branched root system to the detriment of the primary root, characterized by the stimulated formation and emergence of lateral roots and root hairs (Bates and Lynch, 1996; Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002; Pérez-Torres et al., 2008; Péret et al., 2011). In other native and cultivated plants, including maize (Zea mays), rice (Oryza sativa), common bean (Phaseolus vulgaris), white lupin (Lupinus albus), tomato (Solanum lycopersicum) and Brassica nigra, low P availability also modifies root architecture traits such as primary root length, root branching, number and length of lateral roots and enhancement of root hair and cluster root formation (Dinkelaker et al., 1995; Carswell et al., 1996; Borch et al., 1999; Kim et al., 2008; Lambers et al., 2011; Jin et al., 2012).
Root architecture is a highly plastic trait, and varies among species, and its plasticity is strongly controlled by plant regulators and inherent genetic factors. It has been known that alteration in root architecture under low P is linked with changes in phytohormone composition and concentration, and involves expression of a number of genes. A suite of studies have implicated that the localized concentration, transport of and/or sensitivity to sugars and hormonal signals play a significant role in root development during low P supply (Nacry et al., 2005; Jiang et al., 2007; Karthikeyan et al., 2007; Zhou et al., 2008a; Rubio et al., 2009; Santos-Beneit et al., 2009; B.L. Wang et al., 2010; Chiou and Lin, 2011; Péret et al., 2011). In particular, sugars, auxin and ethylene have well-documented roles in modulating root architecture during P deficiency.
Genetic studies have identified a series of genes and a class of transcriptional regulators that mediate alteration of root architecture in response to low P in various plant species. There are increasing numbers of publications focusing on the need to improve plant P-uptake efficiency and crop yield, and for better management of P fertilizer use (e.g. Abel et al., 2002; Lynch, 2007; Rubio et al., 2009; Ramaekers et al., 2010; Chiou and Lin, 2011; Hammond and White, 2011; Péret et al., 2011; Jin et al., 2012; Veneklaas et al., 2012). However, these publications do not specifically consider the systemic strategies by which plants adjust their root structure and morphology in response to low-P conditions. This review provides a framework for understanding changes in root architecture in response to P limitation with a focus on integrated physiological and molecular signals.
THE INFLUENCE OF LOW P AVAILABILITY ON ROOT ARCHITECTURE
Root architecture includes the shape and structure of the root system (Hodge et al., 2009). The shape of the root system refers to rooting depth, elongation and density of lateral roots and root hairs, the spatial location of roots and the way in which the root system occupies the soil. Root structure defines the variety of the components constituting the root system and their relationship (Hodge et al., 2009). Two recent reviews on the root development and structure of monocots and dicots have been provided by Lynch (2007) and Ramaekers et al. (2010). The root architecture of monocots and dicots differs significantly, but the main adaptive root traits are common among all vascular plant species for the purpose of enhancing P acquisition. Therefore, in this review, a comprehensive ‘root architecture’ including root system shape and its structure has been used to summarize the multiplicity of root parameters. The majority of root architecture research in response to P has focused on the species Arabidopsis thaliana (arabidopsis) and white lupin; thus, these will henceforth be used as models for genetic purposes in this review.
Root distribution presented a strong positive relationship with P distribution that is most strongly influenced by soil tillage, rhizosphere pH, fertilizer management and cultivation time (Holanda et al., 1998; Andraski and Bundy, 2003; Vu et al., 2009). Although root architecture may play an important role in a no-till system, and P stratification was characterized in no-till systems as a result of a surface-applied fertilizer without incorporation, it is known that root architecture presented a strong positive relationship with P distribution. Meanwhile, the chemical nature of soil P is also affected by tillage practice, with P solubility being increased under conservation tillage (Zibilske and Bradford, 2003). Furthermore, tillage practices that mix the topsoil would also mix the previously applied P, which significantly affects building of the root architecture (Bolland and Brennan, 2006). Therefore, it appears that the effect of P distribution on the root architecture exists in both no-tillage and tillage systems. In addition, rhizosphere pH and fertilizer management also play an important role in root and P distribution in the soil profile. Jing et al. (2010) reported that localized supply of superphosphate plus ammonium sulfate stimulated root proliferation, acidified the rhizosphere, increased mobilization and uptake of P and enhanced maize growth in a calcareous soil in the field. Consequently, in the practice of spatial deployment of the root system response to P in soil, the following factors should be considered: the soil tillage, rhizosphere pH, water status, fertilizer management system and cultivation time, as all these strongly relate to root distribution.
Primary root development in response to low P
The adaptive response of primary root growth to low P shows species and genotypic variations. A significant P deficiency-induced root response is a reduction in primary root growth in plants such as arabidopsis (Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002, 2003; Jain et al., 2007; Pérez-Torres et al., 2008; Tyburski et al., 2010, 2012). The length from the root tip to the first lateral root or root hair was found to be significantly shorter in plants grown under low-P than under high-P conditions (Williamson et al., 2001). Among 73 arabidopsis ecotypes, half showed reduced growth of primary roots at low P supply, and a quarter of the tested ecotypes were not responsive to P availability (Chevalier et al., 2003), suggesting that root growth inhibition is genetically determined rather than only metabolically controlled. For the last 25 % of accessions, low P was found to influence either the primary roots or lateral roots, indicating the occurrence of different adaptive strategies. Later, the identification of quantitative trait loci (QTLs) controlling the root growth response to low P further demonstrated the existence of such a control (Reymond et al., 2006). Unlike arabidopsis, many other plant species displayed longer roots under low-P conditions. Alterations in primary root elongation are typical phenomena in response to P deprivation in rice (O. sativa) (Wissuwa, 2003; Shimizu et al., 2004; Yi et al., 2005). Similarly, there is no reduction or a slight enhancement in root elongation of maize (Z. mays L.) under low-P conditions (Mollier and Pellerin, 1999). Further, a survey of 14 dicots and monocots in solution culture showed that all the species tested had the same degree of primary root elongation under low and high P supply (Narayanan and Reddy, 1982).
A primary root is the first root to emerge in both dicots and monocots, and is derived from the embryonically formed meristematic tissue. When a primary root encounters a low-P medium, its growth is restrained. It is shown that reduced growth of the primary roots of P-deficient plants correlates with the reduction of cell differentiation within the primary root meristem and the inhibition of cell proliferation in the root elongation zone (Ticconi et al., 2004; Sánchez-Calderón et al., 2005, 2006; Svistoonoff et al., 2007). It was observed that in low-P media, the number of cells in the elongation and meristematic regions of wild-type roots decreased >90 % when compared with the same regions in high-P plants (Sánchez-Calderón et al., 2006). Ma et al. (2003) showed that in the arabidopsis primary root, the length of meristems was not affected by P deficiency, but the length of the rapid elongation zone was decreased. However, a reduction in cell length in the root tip of plants grown in low-P media has been reported previously in arabidopsis (Williamson et al., 2001). This response is fast (within 2 d) and requires a physical contact between the root tip and the low-P medium (Linkohr et al., 2002; Svistoonoff et al., 2007); this is associated with exhaustion of the primary root meristem (Sánchez-Calderón et al., 2005). However, root meristem exhaustion under low P availability is not a direct consequence of P deficiency, but rather is under a genetic programme that controls root growth (Sánchez-Calderón et al., 2006). Kinetic studies of expression of the cell cycle marker CycB1, 1:: uidA and the quiescent centre (QC) identity marker QC46:GUS showed that the reduction in cell proliferation in the primary root was preceded by alterations in the QC under low P supply (Sánchez-Calderón et al., 2005). Overall, these results suggest that low P availability induced arabidopsis roots to enter a determinate developmental programme in which cell division is arrested and cell differentiation is promoted.
The reduced growth of the primary root results in a shallow root system, which exploits topsoil resources efficiently, and may be advantageous in low-P soils. However, this may inadvertently result in reduced water acquisition since the topsoil is often dried out and water availability typically increases with soil depth in a terminal drought environment. It was pointed out that plant genotypes selected for adaptation to low P soils might be more sensitive to drought (Ho et al., 2004; Lynch and Ho, 2005). The optimization model from Ho et al. (2004) indicated that the marginal rate of substitution of water for P is the primary determining factor for predicting the distribution of roots in the soil. However, Beebe et al. (2008) showed that drought-resistant lines had superior yields in a low-P environment; one line even outperformed the low-P-tolerant control by a 41 % yield increase. This indicates that yield potential under drought stress and under low-P conditions may not be mutually exclusive.
Development- and hormone-related genes have been reported in arabidopsis as mediators of changes in root architecture in response to P deficiency (Table 1). Arabidopsis mutants defective in LOW PHOSPHATE ROOT1 (LPR1) and its close paralogue LOW PHOSPHATE ROOT2 (LPR2) strongly weaken growth inhibition of the primary root under P-limited conditions (<50 µm) (Svistoonoff et al., 2007). The results of Svistoonoff et al. (2007) strongly indicated that the root cap is the site of the sensing and/or response to low concentrations of exogenous P. Furthermore, LPR1 and LPR2 encode multicopper oxidases (MCOs) that may play a role in plant root development in response to low-P conditions. A recent study revealed that PHOSPHORUS STARVATION-INSENSITIVE (PSI) was a new allele of LPR1 and that an AC-repeat element in the promoter played an important role in controlling the expression of LPR1 (X.M. Wang et al., 2010). It is known that LPR1 proteins in the root cap can influence the activity and distribution of a hormone-like compound (Svistoonoff et al., 2007). Likewise, the psi mutation shows less sensitivity to auxin than the wild type, and enhances the ability to maintain the auxin response in the root tip under low P supply (X.M. Wang et al., 2010). SIZ1 negatively regulates P starvation-induced inhibition of primary root elongation through the control of auxin patterning in arabidopsis (Miura et al., 2011). These results implied that auxin participates in the adaptation of primary root growth under low-P stress, but the mechanism underlying this process remains to be elucidated.
Table 1.
Overview of genes and their functions, and related mutants with altered root architecture in response to low P
| Species | Gene name | Gene function during P starvation | Related phenotype grown in low P | References |
|---|---|---|---|---|
| Primary root | ||||
| Arabidopsis | LPI1, LPI2 | Pi starvation signalling | Longer primary root | Sánchez-Calderón et al. (2006) |
| Arabidopsis | PDR2 | Pi starvation signalling | Shorter primary root | Ticconi et al. (2004) |
| Arabidopsis | PLDζ1 | Pi starvation signalling | Retarded primary root growth | Li et al. (2006) |
| Arabidopsis | PLDζ2 | Phospholipases | Retarded primary root growth | Li et al. (2006) |
| Arabidopsis | over Pht1;5 | Pi starvation signalling | Shorter primary root | Nagarajan et al. (2011) |
| Arabidopsis | PHR1 | Transcription factor, Pi starvation signalling | Slight reduction in the root-to-shoot ratio | Rubio et al. (2001); Bustos et al. (2010) |
| Arabidopsis | LPR1, LPR2 | Hormone-like signalling | Primary root growth maintained | Svistoonoff et al. (2007) |
| Arabidopsis | PLT1/PLT2 | Auxin signalling, meristem maintenance | Shorter primary root in double mutant | Aida et al. (2004) |
| Arabidopsis | SIZ1 | SUMO E3 ligase; auxin signalling | Cessation of primary root growth | Miura et al. (2005, 2011) |
| Arabidopsis | PSI | Auxin signalling | Impaired inhibition of primary root | B.L. Wang et al. (2010) |
| Arabidopsis | VTC1 | Ascorbate signalling | Shorter primary root | Tyburski et al. (2012) |
| Arabidopsis | GIN2 | Sugar signalling | Shorter primary root | Karthikeyan et al. (2007) |
| Arabidopsis | FRY1 | Phytochrome, light signal | Decrease in primary root elongation growth | Chen and Xiong (2010); Hirsch et al. (2011) |
| Rice | over OsPHR2 | Pi starvation signalling | Enhanced induction rate of primary root | Zhou et al. (2008b) |
| Rice | OsMYB2P-1 | Pi starvation signalling | Longer primary root | Dai et al. (2012) |
| Rice | LTN1 | Pi starvation signalling | Enhanced elongation of primary root | Hu et al. (2011) |
| Lateral roots | ||||
| Arabidopsis | LPR1 | Hormone-like signalling | Reduced lateral root development | López-Bucio et al. (2005) |
| Arabidopsis | LPI | Pi starvation signalling | Reduced lateral root number | Sánchez-Calderón et al. (2006) |
| Arabidopsis | ARF7 | Auxin signalling | More lateral roots | Pérez-Torres et al. (2008) |
| Arabidopsis | ARF19 | Auxin signalling | More lateral roots | Pérez-Torres et al. (2008) |
| Arabidopsis | FRY1 | Phytochrome, light signal | Reduced lateral root initiation and elongation | Chen and Xiong (2010); Hirsch et al. (2011) |
| Arabidopsis | SIZ1 | SUMO E3 ligase; auxin signalling | Promoted lateral root growth | Miura et al. (2005, 2011) |
| Arabidopsis | PDR2 | Pi starvation signalling | Numerous, short, highly branched lateral roots | Ticconi et al. (2004) |
| Arabidopsis | PLDζ1/PLDζ2 | Phospholipases | Longer lateral roots | Li et al. (2006) |
| Arabidopsis | PLT1; 1PLT1; 4 | Phosphate transport | Faster lateral root growth | Shin et al. (2004) |
| Arabidopsis | PNP | Methylerythritol-dependent biosynthesis | Numerous, short, highly branched lateral roots | Marchive et al. (2009) |
| Arabidopsis | GIN2 | Sugar signalling | Reduction in the number of lateral roots | Karthikeyan et al. (2007) |
| Arabidopsis | WRKY 75RNAi | Transcription factor | Promoted formation of lateral roots | Devaiah et al. (2007a) |
| Rice | OsMYB2P-1 | Pi starvation signalling | Longer adventitious roots | Dai et al. (2012) |
| Rice | over OsPHR2 | Pi starvation signalling | Enhanced induction rate of adventitious roots | Zhou et al. (2008b) |
| Rice | LTN1 | Pi starvation signalling | Enhanced elongation of adventitious roots | Hu et al. (2011) |
| Root hairs | ||||
| Arabidopsis | LPI1, LPI2 | Pi starvation signalling | Shorter root hair | Sánchez-Calderón et al. (2006) |
| Arabidopsis | bHLH32 | Transcription factor | Increased root hair formation | Chen et al. (2007) |
| Arabidopsis | over Pht1;5 | Pi starvation signalling | Increased root hair proliferation | Nagarajan et al. (2011) |
| Arabidopsis | SIZ1 | SUMO E3 ligase; auxin signalling | Promoted root hair growth | Miura et al. (2005) |
| Arabidopsis | FBX2 | Pi starvation signalling | Increased root hair formation | Chen et al. (2008) |
| Arabidopsis | IPK | Phytate synthesis | Constitutive production of root hairs | Stevenson-Paulik et al. (2005) |
| Arabidopsis | UBP14/PER1 | Ubiquitin protease | Inhibition of root hair growth | Li et al. (2010) |
| Arabidopsis | HSP2 | Ethylene signalling | Increased root hair formation | Lei et al. (2011b) |
| Arabidopsis | GA1,3 | Gibberellic acid signalling | Shorter root hair | Jiang et al. (2007) |
| Arabidopsis | RSI4 | bHLH transcription factor | Development of very short root hairs | Yi et al. (2010) |
| Arabidopsis | WRKY 75RNAi | Transcription factor | Constitutive production of root hairs | Devaiah et al. (2007a) |
| Arabidopsis | PHR1/PHL1 | Pi starvation signalling | Inhibition in root hair growth | Bustos et al. (2010) |
| Arabidopsis | PHR1 | Transcription factor, Pi starvation signalling | Increase in root hair length | Bustos et al. (2010) |
| Arabidopsis | PHL1 | Pi starvation signalling | Sustains root hair length | Bustos et al. (2010) |
| White lupin | GPX-PDE1RNAi | Glycerophosphodiester phosphodiesterases | Reduction in root hair development | Cheng et al. (2011) |
| Cluster roots | ||||
| White lupin | LaSAP1 | Secreted acid phosphatase | Upregulated in P-deficient cluster roots | Wasaki et al. (1999) |
| White lupin | LaPT1 | High-affinity Pi transporter | Upregulated in P-deficient cluster roots | Liu et al. (2001) |
| White lupin | LaMATE | Multidrug and toxin efflux | Upregulated in P-deficient cluster roots | Uhde-Stone et al. (2005) |
| White lupin | LaPEPC | Phosphoenolpyruvate | Upregulated in P-deficient cluster roots | Uhde-Stone et al. (2003a) |
| White lupin | LaGPX-PDE1 | Glycerophosphodiester phosphodiesterases | Highly expressed in P-deficient cluster roots | Cheng et al. (2011) |
| White lupin | LaGPX-PDE2 | Glycerophosphodiester phosphodiesterases | Highly expressed in P-deficient cluster roots | Cheng et al. (2011) |
| White lupin | CKX | Cytokinin signalling | Upregulated in P-deficient cluster roots | Vance et al. (2003) |
SCARECROW (SCR) and SHORT-ROOT (SHR) are members of the GRAS transcription factor (TF) family and key regulators of radial root patterning. PHOSPHATE DEFICIENCY RESPONSE 2 (PDR2) is required for maintaining nuclear SCR protein under low-P conditions, which would reduce root meristematic activity (Ticconi et al., 2004, 2009). The hypersensitivity to low P of the pdr2 mutant (Ticconi et al., 2004) appears as an exacerbation of such a response. In addition, the root meristem exhaustion probably perturbs auxin circulation in the root tip (Kramer and Bennett, 2006), and consequently impacts on branching in older parts of the root. Interestingly, PDR2 and LPR1 function in opposite ways in regulating the response of root growth to P deficiency in the endoplasmic reticulum (Ticconi et al., 2004). The siz1 mutant seedlings are also hypersensitive to low P, displaying a reduced growth of the primary root (Miura et al., 2005). However, SIZ1 may represent the element linking the low-P stress and reactive oxygen species (ROS) (Desnos, 2008). Whether SIZ1 has a role in regulating P-deficiency-induced primary root growth via the ROS pathway is unknown.
Compared with the wild type and single knockout mutants, the elongation of primary roots of PLDζ1 and PLDζ2 (PHOSPHOLIPASE D) double knockout mutants was slower (Li et al., 2006), indicating that either PLDζ1 or PLDζ2 can function to promote apical growth. However, only loss of PLDζ2 results in lower concentrations of phosphatidic acid (PA) under low-P conditions. This raises the possibility that PA generated by PLDζ genes may promote root elongation under P deficiency. Another study indicates that PA interacts with AtPDK1 (PHOSPHOINOSITIDE-DEPENDENT PROTEIN KINASE 1), stimulates a protein kinase cascade, and promotes root apical growth and initiation (Anthony et al., 2004). It has been speculated that AtPDK1 is the target for PA under low-P conditions.
The available data have indicated that both LPI1 and LPI2 regulate the growth of the primary root under P deprivation (Sánchez-Calderόn et al., 2006). Further lpi lines had neither a drastic reduction in cell length in the primary root nor a decreased cell number in the root elongation zone (Sánchez-Calderón et al., 2006), suggesting that LPI1 maintains an indeterminate root development programme under low P conditions. Notably, in addition to the insensitivity of growth inhibition of the primary root to P deprivation, the low-P-insensitive mutant lpi showed a reduction in the expression of genes involved in the P-starvation rescue system PHOSPHATE TRANSPORTER 1 (AtPT1), AtPT2, PURPLE ACID PHOSPHATASE 1 (PAP1), ACID PHOSPHATASE 5 (AtCP5) and INDUCED BY PHOSPHATE STARVATION 1 (AtIPS1) (Casamitjana-Martínez et al., 2003). It has been speculated that the transcriptional or post-transcriptional regulation of SHR, SCR, PLETHORA1 (PLT1), PLETHORA2 (PLT2) and/or CLV3/ESR-like gene CLE19 (AtCLE19) could be negatively regulated by LPI1 and LPI2, depending on P availability (Sánchez-Calderόn et al., 2006). These results suggest that high sensitivity to P starvation plays a regulatory role in primary root growth.
Recent molecular studies in arabidopsis and rice (O. sativa) have identified several genes and TFs involved in P absorption, transport and remobilization, and regulation of primary root elongation under low P availability. Arabidopsis has a family of high-affinity Pi transporters which includes nine members, named PHOSPHATE TRANSPORTER 1;1–9 (PHT1;1–9), accounting for 70 % of the total root P transport activity (Shin et al., 2004). Phosphate transport double mutants, plt1;1 plt1;4, exhibit stunted plant growth, including slow growth of primary roots (Shin et al., 2004). Recently, it was found that PHT1;5 played a negative role in primary root growth in response to low P supply (Nagarajan et al., 2011). PHO2 was shown to be involved in phloem transport of P between shoots and roots or in regulating leaf P concentration (Dong et al., 1998). In rice, LTN1 (the homologue of arabidopsis PHO2, LOC_Os05g48390) can alter P-deficiency-dependent root morphology including the promoted elongation of the primary and adventitious roots in ltn1 mutants (Hu et al., 2011). This implies that LTN1 has a negative role in primary root development in response to P deficiency. ZAT6, a Cys-2/His-2 zinc finger TF, also negatively regulates primary root growth, but the regulation is independent of the P status in arabidopsis (Devaiah et al., 2007b). PHOSPHATE STARVATION RESPONSE 1 (AtPHR1), a MYB TF, activates a sub-set of P starvation-induced genes, and phr1 showed a reduced response of AtIPS1::GUS to P starvation (Rubio et al., 2001). Zhou et al. (2008b) found that OsPHR2, the homologue of AtPHR1, was a key regulator for P starvation signalling in rice, and OsMYB2P-1-over-expressing arabidopsis displayed an enhanced induction rate of primary roots. OsMYB2P-1, an R2R3 MYB TF, was identified from microarray data by monitoring the expression profile of rice (O. sativa ssp. japonica) seedlings exposed to a P-deficient medium (Dai et al., 2012). Compared with OsMYB2P-1-over-expressing rice grown in P-deficient media, the reduced expression of OsMYB2P-1 in wild-type rice led to suppression of the growth rate of primary roots. Similarly, arabidopsis plants over-expressing OsMYB2P-1 exhibited longer primary roots and more lateral roots than wild-type arabidopsis plants (Dai et al., 2012). These results suggest that OsMYB2 plays a positive regulatory role in the modulation of primary root elongation in response to P deficiency.
Lateral root development in response to low P
Lateral roots play an important role in P acquisition by increasing soil exploration (Zhu et al., 2005a), the absorptive surface of the root system (Pérez-Torres et al., 2008) and P solubilization (Lynch, 1995, 2007). Many dicotyledonous plants, such as arabidopsis, cotton (Gossypium spp.) and soybean (Glycine max), generate several orders of lateral roots resulting from repeated branching of a primary root, whereas the root systems of cereal crops, such as rice (O. sativa) and maize (Z. mays), are principally composed of adventitious roots (Hochholdinger et al., 2004; Osmont et al., 2007). Phosphorus supply affects the growth and proliferation of lateral roots. Studies have shown that low P favours lateral root growth by reducing primary root elongation and increasing lateral root elongation and density in arabidopsis (Williamson et al., 2001; Linkohr et al., 2002; Reymond et al., 2006). In common bean, P availability regulates adventitious rooting (Borch et al., 1999; Miller et al., 2003), and two major QTLs accounted for 19–61 % of the total phenotypic variation for adventitious root traits under low-P conditions (Lynch et al., 2006).
Under normal conditions, lateral roots originate from a small number of pericycle cells that are adjacent to the xylem pole and initiate a series of asymmetric and transverse divisions (Torrey, 1950). The organization of cells in lateral roots is analogous to that in primary roots, but the numbers of cell files in each layer are more variable (Dolan et al., 1993). Early development of lateral roots, which may recapitulate embryonic and primary root formation, can be separated into five major stages: initiation, establishment and emergence of lateral root primordia, followed by meristem activation and maintenance (Malamy and Benfey, 1997). The pattern of lateral root formation close to the root tip under P deficiency is comparable with that of roots with damaged primary root meristemic activity, including destruction of meristemic activity by cell ablation or physical decapitation of the primary root meristem, eliciting an increase in lateral root number (Torrey, 1950; López-Bucio et al., 2002). It has been suggested that low-P stress not only reduces the rate of cell division but also inhibits cell growth in the root elongation zone (Sánchez-Calderón et al., 2006). Under low P concentrations, the mitotic activity is relocated to the sites of lateral root formation, which leads to increased lateral root density (Tyburski et al., 2012). Similar to the primary root tip, the manner of cell differentiation in older lateral roots occurs within the root apical meristem (RAM), which is followed by an inhibition of lateral root elongation (Nacry et al., 2005; Sánchez-Calderón et al., 2005). As mentioned above, the reduced growth of primary roots correlates with a decrease in the number of cells in the elongation zone and a corresponding decrease in the distance between the root tip and the first lateral root (Williamson et al., 2001). Therefore, as for the whole root system, lateral growth is favoured over elongation of the primary axis at the cellular level. Interestingly, each lateral root can produce more lateral roots, and a complex root system is constructed by the reiteration of a single developmental process.
The response of lateral roots to P deficiency, however, shows species and genotypic variations. In maize, some genotypes show an increase in the number and length of lateral roots while others show the opposite effect. In this crop, the mature root system is mainly composed of nodal roots, so the maintenance of nodal root formation could result in an increased proportion of root length in the adventitious root system when the overall growth is inhibited by P deficiency (Bayuelo-Jiménez et al., 2011). Maize plants have a fibrous root system that is generally more deeply distributed than the basal roots of the tap-root system, such as in common bean and soybean. In maize–soybean intercropping, soybean genotypes with shallower roots might be able to take up P from topsoil, and could avoid competition for sub-soil P with maize (Tang et al., 2005). Similar results have been observed in adventitious roots of common bean genotypes. Consequently, P-induced adventitious rooting could vary widely among common bean genotypes (Miller et al., 2003), and this trait is moderately heritable (Lynch et al., 2006). Architectural traits associated with promoted topsoil foraging in common bean are shallower basal roots, increased adventitious rooting and greater dispersion of lateral branching from the basal roots (Lynch, 2007; Ramaekers et al., 2010). These imply that plants grown with a low P supply change the angle of basal roots in favour of outward rather than downward growth, resulting in a shallower and broader root system. This point was supported by the observed correlation between the ability of bean cultivars to reduce root angle in low-P and yield in P-poor soils (Bonser et al., 1996). As mentioned above, a shallow root system exploits topsoil resources efficiently, which is advantageous in low-P soils. However, this may inadvertently lead to reduced water uptake. Indeed, drought tolerance in common bean has been associated with rooting depth (Sponchiado et al., 1989; Sanders and Markhart, 1992).
Genetic analyses have identified a suit of genes that control lateral root development under low P supply (Table 1). Three low-P-resistant root lines (lpr1-1, lpr1-2 and lpr1-3) of arabidopsis were reported to have reduced formation of lateral roots in low-P conditions (López-Bucio et al., 2005). Svistoonoff et al. (2007) verified that LPR1 and LPR2 in arabidopsis lines were strongly induced by P starvation, and that this induction was closely related to elongation of primary and lateral roots. The phosphate transport double mutant plt1; 1plt1; 4 showed faster lateral root growth but slower primary root growth than wild-type plants under low P availability (Shin et al., 2004). This supports the notion that the response to low P could be different in primary roots as compared with lateral roots. Also, PLDζ genes have been suggested to be involved in these different responses to low P between primary and lateral roots because these genes promote primary root elongation but inhibit lateral root elongation under low-P conditions (Li et al., 2006).
The typical increase in the number of lateral roots under low-P conditions was observed in the low-P-insensitive mutant lpi1 but not in lpi2 (Sánchez-Calderόn et al., 2006), implying that LPI2 regulates lateral root formation whereas LPI1 is not involved in lateral root induction by P deprivation. Genetic and molecular analyses have revealed that all lpr1 mutants are allelic to BIG, which is required for normal auxin transport in arabidopsis (López-Bucio et al., 2005). This provides genetic evidence that lateral root formation is not a direct consequence of the observed meristem exhaustion in the low-P response but could be mediated by an independent alteration of auxin sensitivity of pericycle cells during P deprivation. The PLEIOTROPIC DRUG RESISTANCE 2 (pdr2) mutant displays hypersensitive responses to P deficiency, showing higher density of lateral root meristems under the stress (Ticconi et al., 2004). It indicates that PDR2 is necessary for maintenance of root meristem function when external P supply is limited. The pdr2 phenotype in low P is strikingly similar to the auxin-conditional ABERRANT LATERAL ROOT FORMATION 3 (alf3) root phenotype (Celenza et al., 1995). The alf3 mutation causes primary root growth arrest and death, followed by increased formation of lateral root primordia that fail to mature and subsequently die. As auxin fails to rescue or mimic the pdr2 root phenotype, and auxin sensitivity of primary root growth is not altered in phr2 seedlings, it is unlikely that auxin is directly involved in the response of root meristems to low external P availability. Another study has shown that a class of transcriptional regulators mediating growth and developmental responses to auxins [AUXIN RESPONSE FACTOR 7 (ARF 7) and AUXIN RESPONSE FACTOR 19 (ARF 19)] are required for lateral root formation under P starvation and that this response involves an SCFTIR1 [a ubiquitin-protein ligase (E3)]-dependent signalling mechanism (Pérez-Torres et al., 2008). These reveal that an auxin response signal plays a key role in the adaptation of lateral root formation under P deficiency.
Polynucleotide phosphorylase (PNPase) is involved in P sensing and signalling, as suggested by the constitutive expression of P-responsive genes in their corresponding mutants (Ticconi et al., 2004; Marchive et al., 2009). The pnp mutant produces numerous, short and highly branched lateral roots under low-P conditions. SIZ1 (encodes an arabidopsis SUMO E3 ligase) has been found to inhibit lateral root formation at low P, as demonstrated by the increased lateral root response of the siz1 mutant (Miura et al., 2011). X.M. Wang et al. (2010) suggest that LPR1 functions independently of the PHR1/SIZ1 signalling pathway to regulate root growth under P starvation in arabidopsis.
Transcriptome analysis reveals several TFs involved in lateral root response to P deficiency. WRKY DNA-BINDING PROTEIN 75 (WRKY75), a member of the WRKY TFs, is up-regulated during P deficiency and negatively regulates lateral root growth which is independent of P status in plants (Devaiah et al., 2007a). In contrast, the OsPHR2-over-expressing plants showed a higher sensitivity to P deficiency with an enhanced induction rate of adventitious roots than wild-type plants (Zhou et al., 2008b). ZAT6, a Cys-2/His-2 zinc finger TF, positively regulates lateral root growth, and this regulation is independent of the P status in plants (Devaiah et al., 2007b). Arabidopsis plants over-expressing OsMYB2P-1 exhibited more lateral roots than wild-type arabidopsis plants (Dai et al., 2012). These results suggest that MYB2 plays a positive regulatory role in lateral root development during P deficiency.
Development of root hairs in response to low P
It has been known for some time that low P causes increased extension of root hairs in many plant species (Foehse and Jungk, 1983). Among the root traits, increased root hair proliferation is an early response of plants to low P (Ma et al., 2001, 2003; Jain et al., 2007). In addition, the root hair/P response is a localized cellular response and is specific for P (Bates and Lynch, 1996). At a P concentration of 1 µm, representative of many low-P soils, the root hair length of arabidopsis can exceed 1 mm. Yet at a P concentration of 1000 µm, root hair length is decreased to 0·3 mm, and it is suppressed completely at 3000 µm P (Bates and Lynch, 2000a). However, high P supply does not completely suppress root hair growth, suggesting that high-P plants maintain the potential for plasticity. Bates and Lynch (2000a) pointed out that low P increased root hair length by increasing both the rate and duration of hair elongation, and suggested that P deficiency accelerated and prolonged metabolic activity in elongating hairs. Overall, the increase in the root hair density and length in response to P deficiency is a general and well-researched phenomenon in plant biology (Péret et al., 2011).
The plasticity response of root hairs is relatively faster than root growth and branching, thus root hair number has been used as a measure of root response to low P in some specific plants. For example, genetic variation in root hair length and plasticity was suggested to be an appropriate target for marker-aided selection to improve the P efficiency in maize (Zhu et al., 2005b). The presence of root hairs in maize, particularly dense root hairs of the main axis and nodal first-order laterals, was associated with plant performance at low P (Bayuelo-Jiménez et al., 2011). In addition, root hairs can grow to terminal length, change the effective root radius and absorb available P prior to the increase in root surface area by lateral root branching. Therefore, root hair growth may represent the earliest morphological response to changing environmental conditions.
Interestingly, a maize mutant defective in root hair growth shows normal growth and development under field conditions (Wen and Schnable, 1994). This raises a question about the importance of root hairs in P acquisition under field conditions. Later experiments using root hair mutants showed that root hairs facilitate P uptake by increasing the absorptive surface area of the root (Bates and Lynch, 2000b). Genetic variation in root hair length and density in maize is controlled by several major QTLs (Zhu et al., 2005b), suggesting that this trait could be selected in breeding programmes through marker-assisted selection. This offers the possibility of quick screening of a large number of maize accessions based on variation in root hairs (Lynch and Brown, 2008) and further dissection of genetic control of root hair formation. Importantly, the effect of P deficiency on root hair development should be investigated in soil experiments where P cannot easily diffuse to the root surface.
Root hairs are long tubular outgrowths of specialized root epidermal cells that play a critical role in water and nutrient uptake from soils (Peterson and Farquhar, 1996). Each root hair is derived from a single epidermal cell, and only certain epidermal cells termed ‘trichoblasts’ actually develop into root hair-bearing cells. Trichoblasts are smaller and more densely cytoplasmic cells than the surrounding hairless epidermal cells (Leavitt, 1904). Root hair distribution can be grouped into three categories in angiosperms (Datta et al., 2011). In type I, all epidermal cells are morphologically identical before hair initiation, and any cell in the epidermis can possibly develop into a hair cell. Type I appears to be the most widespread pattern in plants, including tomato and lettuce (Pemberton et al., 2001), and can be significantly affected by environmental conditions (Tsai et al., 2004). In type II, a root hair develops from two smaller cells produced by asymmetric cell division of an epidermal cell in the meristematic zone, and the larger cell remains hairless. For type III, occurring in most members of the Brassicaceae family, including arabidopsis, the hair cells occur in files separated by 1–3 files of non-hair cells.
Root hair development in plants can be divided into three phases: cell specification, initiation and elongation (Schiefelbein, 2000). These processes are of crucial importance for building the structure of the plant body, and developing and maintaining its interface with the environment. It has been reported that the increase in root hair density in plants grown with low P can be explained by alterations in trichoblast file number, trichoblast length and/or percentage of trichoblast cells forming hairs (Ma et al., 2001; Zhang et al., 2003). Phosphorus-deficient plants of arabidopsis had smaller but more cortical cells, and hence a larger number of root-hair-bearing epidermal cell files than P-adequate plants (Zhang et al., 2003). Schmidt and Schikora (2001) found only a small number of ectopic hairs in arabidopsis plants after transfer from a very high-P (2·5 mm) to a no-P medium. These ectopic hairs might result from the stress of transfer, and are unlikely to contribute to the enhanced root hair density under low P.
Numerous experimental observations indicate that root hair development involves different cellular and genetic processes (Schiefelbein, 2000; Foreman and Dolan, 2001). During the past years, there has been a surge in research activity in root hair development, particularly in arabidopsis. Grierson et al. (2001) reported that >40 genes in arabidopsis affect root hair initiation and development, and most of these genes might be responsive to P deficiency. Two extensive reviews on the root hair development genes and cell patterning information have been provided by Griersona and Schiefelbein (2002) and Péret et al. (2011). Our review here provides further insight into the signalling pathways based on isolation of genes affected in low-P-insensitive mutants that regulate changes in root hair growth in response to low P availability (Table 1).
Several TFs have been shown to control root hair growth during P deficiency. For instance, low P strongly affected root hair length in the phr1 and phr1ph1 double mutants of arabidopsis, whereas it did not change root hair length in the phl1 mutant (Bustos et al., 2010). These authors concluded that this effect on root hair length reflected a lack of correct protection against the stress inherent in P deficiency, and that increased PHR1 activity increased reproductive success in these stress conditions. In contrast, a mutation in the PHR1 regulator SIZ1 enhanced the sensitivity of arabidopsis plants to P deficiency, resulting in a greater number of root hairs (Miura et al., 2011). These indicate that transcriptional repression responses are an integral part of adaptive responses to the stress. Except for the PHR-like gene, the two major arabidopsis transporters involved in P uptake are the PHOSPHATE TRANSPORTER1 (Pht1) family members, Pht1;1 and Pht1;4 (Shin et al., 2004). Both of these transporters are localized in the root epidermis (Karthikeyan et al., 2002; Mudge et al., 2002), an appropriate site for P perception. Irrespective of the P regime, the Pht1;5 over-expressors showed significant increases in both the number and length of root hairs compared with the wild type (Nagarajan et al., 2011). Moreover, low P controls root hair length by modulating steady-state levels of ROOT HAIR DEFECTIVE 6-LIKE4 (RSL4) transcript and protein, which requires the phosphate signalling activity of LPR1 and LPR2 (Yi et al., 2010), implying that LPR1 interacts with downstream components of the P-sensing pathway. Recently, two glycerophosphodiester phosphodiesterase genes (GPX-PDE1 and GPX-PDE2) were characterized from white lupin (Cheng et al., 2011). These two genes were highly expressed in root hairs, epidermal cells and vascular bundles, particularly in P-deficient plants. All of these results suggest that genes involved in P metabolism such as secondary metabolism, P scavenging and remobilization, plant hormone metabolism, and signal transduction play a role in modulating low-P-induced root hair development. However, the double knockouts of PLDζ1 and PLDζ2 in arabidopsis affect root elongation but do not affect root hair patterning under P deficiency (Li et al., 2006). Thereby, the relationship between genes involved in the pathway of P response and metabolism and the early phase of root epidermal cell specification should be considered in future research.
Cluster root formation in response to low P
Cluster roots, specialized tertiary lateral root structure, are densely clustered secondary roots with determinant growth and are a feature of the Proteaceae and several other plant species (Johnson et al., 1996; Watt and Evans, 1999; Skene, 2000; Shane and Lambers, 2005; Shu et al., 2005; Lambers et al., 2006). Recent research has revealed clearly that the morphological and physiological features of cluster roots (such as release of protons and carboxylates) play a key role in the high P efficiency (Shane et al., 2003; Shen et al., 2003; Cheng et al., 2011; Lambers et al., 2011). Several reviews have covered this aspect (e.g. Hinsinger et al., 2003; Lambers et al., 2006). It appears that cluster root formation may be an alternative strategy to arbuscular mycorrhiza (AM) formation, as in most Proteaceae and also white lupin. Also, some plants, such as Casuarina, develop both cluster roots and AM (Lambers et al., 2008). This review will not discuss the roles of AM in P acquisition because many excellent publications have covered various aspects of AM (e.g. Jasper et al., 1979; Bolan, 1991; Brundrett, 2002, 2004, 2009; Lynch, 2007; Smith and Smith, 2011; Smith et al., 2011).
White lupin is the tool of choice to improve our understanding on how P nutrition affects cluster root formation and development (Liu et al., 2005). Increased formation of cluster roots is the earliest response to P deficiency in seedlings of white lupin grown in a hydroponic culture system without P supply (Neumann et al., 2000). This rapid development of cluster roots within several days could be an advantage, particularly in extreme environments.
Formation of cluster roots appears to be mainly induced by P deficiency. However, it is argued whether external P (rooting media) or internal P concentration (in shoot or root) controls the formation of cluster roots. Some studies supported the idea that availability of external P in soil patches was important in enhancing cluster root formation (Shu et al., 2007). However, it was also suggested that shoot P concentrations regulated the initiation, growth and functioning of cluster roots. Suppression of cluster root formation by foliar P application (Marschner et al., 1987) and high internal P concentration (Keerthisinghe et al., 1998; Shane et al., 2003; Pearse et al., 2006) suggests that induction is determined by the internal P concentration rather than by P levels of the substrata. Later, Li et al. (2008) and Zhou et al. (2008a) demonstrated that the increased cluster root formation of white lupin was particularly regulated by the internal P concentration of the shoots, rather than by the P supply in the root medium or total P concentration in the roots. However, it was proposed that enhanced P uptake by cluster roots in turn had a negative effect on cluster root formation and root exudation (Keerthisinghe et al., 1998; Shen et al., 2003). These results indicate that shoot-derived signals transmit the message of P deficiency to stimulate cluster root formation and that adequate P supply to plants inhibits cluster root formation to avoid excessive loss of carbon from the root system.
Interestingly, a previous study using a split-root system of white lupin showed that local P supply had an additional effect on the formation of cluster roots, and that this cluster root formation depended on an interaction between local P supply and shoot P concentration (Shane et al., 2003). Importantly, if a high concentration of P in the shoot is a signal for prevention of cluster root formation, different species with inherent differences in maximum growth rate and P uptake may differ in the foliar P concentration that either enhances or suppresses cluster root formation (Abdolzadeh et al., 2010). In conclusion, on the one hand, shoot P concentration is responsible for formation of the cluster root in P-deficient plants. On the other hand, increased P concentration in shoots resulted from cluster root formation, which is partly due to stimulation by external P availability in soil.
The supply of other nutrients such as nitrogen is involved in the development of cluster roots under low P supply. For example, low levels of N enhance P-deficiency-induced formation of cluster roots, whereas high N supply has inhibitory effects (Dinkelaker et al., 1995). The omission of P increased cluster root formation only under low N supply (Sas et al., 2002; Paungfoo-Lonhienne et al., 2009). The large overlap of genes differentially expressed in cluster roots under N and P deprivation indicates that a large number of genes involved in cluster root formation are not specific to a particular nutrient stress (Rath et al., 2010). In non-cluster-forming species, localized application of P combined with ammonium can significantly improve maize growth and nutrient use at the early stages by stimulating root proliferation and rhizosphere acidification (Jing et al., 2010). Similarly, localized supply of nitrate and P to acid sub-soil increases wheat root length and the number of root tips (Weligama et al., 2008). It is therefore of interest to evaluate the interaction between rhizosphere pH and N availability during low P availability in influencing cluster root formation.
In the past decade, a number of P-limitation-induced genes have been described in cluster roots of white lupin. These include a high-affinity phosphate transporter (LaPT1; Liu et al., 2001), a secreted acid phosphatase (LaSAP1; Wasaki et al., 1999; Miller et al., 2001), and a multidrug and toxin efflux gene (LaMATE; Uhde-Stone et al., 2005). The promoters of these genes contain one or more P1BS elements, which have been well characterized as upregulated in P-deficient cluster roots (Liu et al., 2001; Miller et al., 2001; Uhde-Stone et al., 2005). Apart from sugar signal genes, Uhde-Stone et al. (2003a) identified 35 expressed sequence tags (ESTs) or EST contigs showing enhanced expression of the corresponding genes in cluster roots of P-deficient white lupine. The role of the glycerophosphodiester phosphodiesterase gene (GPX-PDE1 and GPX-PDE2) products encoded by two ESTs in a P deficiency-induced phospholipid degradation pathway in cluster roots was proposed (Cheng et al., 2011). All these indicate that P metabolism, secondary metabolism and signal transduction govern interactions between P deficiency and cluster roots. However, the genetics of the low-P-induced cluster root modification have not been well developed, and many mutants specifically altered in this response need to be isolated.
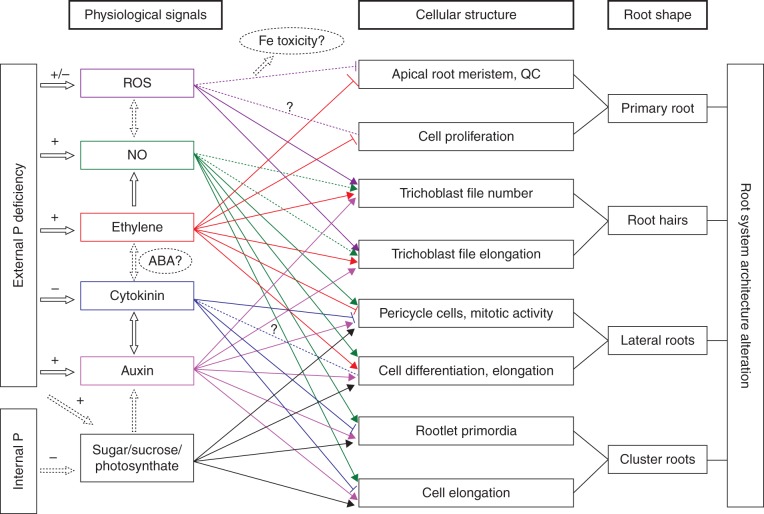
HOW DOES LOW P AVAILABILITY REGULATE ROOT ARCHITECTURE DEVELOPMENT?
Accumulating evidence suggests that a suite of classical signals involved in the process of root architecture development could be induced by low P. The role of these signals in the adaptive response of root growth to low P is complex. Some of these signals regulate only a specific change, whereas others modulate multiple effects. The mechanisms mediating the response of root architecture to P deficiency have not been fully understood, and here the current knowledge is summarized.
Sugars
Genetic studies show that low P availability induces sugar-dependent systematic expression of genes, which can modulate the root system architecture. Interactions between the sugar signal and systematic P starvation on plant responses have been explicitly demonstrated in several studies. For example, sugars modulate the expression of P deficiency-induced genes in white lupin (Liu et al., 2005; Tesfaye et al., 2007; Zhou et al., 2008a) and arabidopsis (Franco-Zorrilla et al., 2005; Jain et al., 2007; Karthikeyan et al., 2007; Müller et al., 2007; Hammond and White, 2008; Lei et al., 2011a). Li et al. (2008) reported that differences in citrate secretion, sugar metabolism and root cell proliferation were the main reasons for high P-deficiency tolerance of an L. albus ‘Kiev’ mutant. The plant displayed a specialized P-efficient root system with a high capacity for mobilizing external P and increased cell division in the root meristem under P starvation (Li et al., 2008). Meanwhile, Zhou et al. (2008a) showed that P starvation responses in white lupin roots were controlled by at least two sugar-signal-mediated regulation systems. One system regulates LaPT1 and LaPEPC3 gene expression and acts when P supply is low, and the other system regulates cluster root formation and LaSAP expression and acts even in P-sufficient medium if roots receive a sugar signal. This study suggests that the sugar signal interacts with other signals of signalling networks such as a local P-starvation signal or another systematic signal, or both. Therefore, it is probable that endogenously supplied sugar stimulated cluster root formation under low P as a signal, not as an energy source or a secondary effect.
In arabidopsis, exogenously supplied sugars increased lateral root density dependent on the P status in the rooting media, but did not affect tap-root length (Jain et al., 2007; Karthikeyan et al., 2007), implying that the sugar sensor participated in some aspects of the P-deficiency-response mechanism in lateral roots. The activity of the RAM has been proposed to determine the magnitude of P starvation responses. Increasing RAM activity by external sucrose application enhances the starvation responses, as reported by the expression of P starvation markers (Péret et al., 2011). This means that not only meristematic activity but also sugar signalling determine root developmental responses to P starvation in arabidopsis. It is worth noting that the expression of the microbial GPX-PDE genes induced by P stress is regulated in part by sugar availability (Santos-Beneit et al., 2009). Furthermore, a recent study has characterized these two genes (GPX-PDE1 and GPX-PDE2) from white lupin, which are highly expressed in root hairs, epidermal cells and vascular bundles, particularly in P-deficient plants (Cheng et al., 2011). However, whether sugars play a role in root hair development under low-P conditions is currently unclear.
It has been reported that sugars act as signalling molecules in plants to integrate environmental conditions and intrinsic developmental programmes modulated by multiple plant hormones. For example, sucrose is the main photosynthate translocated between shoot and root via the phloem, and a high root-to-shoot ratio of sucrose concentration and changes in concentrations of phytohormones such as cytokinins are required for P-starvation responses (Hammond and White, 2008). Furthermore, sucrose is proposed as a global regulator of a whole suite of P-starvation responses in arabidopsis (Lei et al., 2011a), implying that the sugar signal may act upstream in the low-P-induced root development pathway.
Auxins
Growing evidence suggests that auxins act as signalling intermediates in multiple pathways, including those involved in the responses of root architecture to low P supply. This was first supported by a study showing that exogenous application of auxins to P-adequate roots resulted in localized alterations in root architecture that mimicked those observed under low P (Gilbert et al., 2000; López-Bucio et al., 2002). It has been reported that changes in growth of lateral roots and cluster roots during low P are inter-related to auxin signalling (López-Bucio et al., 2002, 2005; Nacry et al., 2005; Jain et al., 2007). Lateral root development and the arrest of cell divisions in the apices in roots of P-starved plants result from changes in auxin transport and/or sensitivity (López-Bucio et al., 2005; Nacry et al., 2005). Moreover, both TRANSPORT INHIBITOR RESPONSE 1 (TIR1)- and AUXIN RESPONSE FACTOR 19 (ARF19)-dependent auxin signals are suggested to have roles in lateral root development under low P availability (Pérez-Torres et al., 2008). Furthermore, P-deprived pnp mutants develop aborted clusters of lateral roots, which are characterized by decreased auxin responsiveness and cell division, and cell death at the root tips (Marchive et al., 2009). Consequently, good evidence indicates that auxins are an important signal controlling the low-P-induced development of lateral roots. Many hormonally regulated developmental responses occurring in P-deficient arabidopsis also appear to be involved in cluster root formation (Cheng et al., 2011). In the process of P-induced cluster root formation, many genes involved in auxin synthesis and signalling are abundantly expressed (Vance et al., 2003; Yamagishi et al., 2011). Impaired auxin transport in white lupin roots decreased formation of cluster roots under low P supply (Gilbert et al., 2000). Therefore, the development of cluster roots under low P is also auxin related.
Similarly, the growth of root hairs is dependent on auxin signalling under P deficiency. This is supported by a study showing that the indole acetic acid (IAA) transport inhibitor CMPA produces a dramatic decrease in root hair elongation and root hair density of arabidopsis grown at low P availability (Bates and Lynch, 1996). This implies that an appropriate concentration of endogenous auxin and normal auxin transport in roots are critical for the regulation of low-P-induced root hair development. However, exogenous IAA increased both the density and elongation of root hairs by up to 3-fold under high P, but only slightly increased these parameters under low P, when root hair density and length were already high (Bates and Lynch, 1996). Future research should explore the role of auxin response and transport in regulating specific phases of root hair growth, such as root hair initiation and tip growth in response to low P.
Meanwhile, many other studies suggest that primary root and root hair growth in response to low-P stress are auxin independent (Williamson et al., 2001; Linkohr et al., 2002; Ticconi et al., 2004; Jain et al., 2007). Jain et al. (2007) found that auxin did not play a role in the determinate growth of the primary root exposed to localized P deficiency. Hence, it becomes clear that an auxin-dependent pathway involved in auxin transport or sensitivity and an auxin-independent pathway may coexist to modulate P-starvation-induced root architectural changes. These studies highlight the differential effects of auxin on P-deficiency-induced modulations of ontogenetically distinct root traits.
Other signalling pathways, including other phytohormones, may co-function with auxin in this process. For example, ethylene has been shown to act, at least in part, through changes in auxin biosynthesis and transport (Swarup et al., 2007; Grierson and Schiefelbein, 2009). Auxin and ethylene can interact in their biosynthesis and the response pathways, or, sometimes independently, regulate the same target genes (Stepanova et al., 2007). Additionally, auxin imposes a very rapid regulation of cytokinins and nitric oxide (NO) (see later). Strigolactone has recently been shown to act in concert with auxin to regulate lateral root development and shoot branching in arabidopsis differentially, depending on the P level in the growth medium (Ruyter-Spira et al., 2011). In other words, the interactions between phytohormones in regulating root architecture under nutrient stress are still equivocal, and deserve further investigation.
Ethylene
Ethylene has been shown to play a role in modifying root development in response to low P availability. Transcriptome analyses show that transcript levels for ethylene biosynthetic genes are increased in arabidopsis under low P (Thibaud et al., 2010). By altering ethylene biosynthesis or perception, several studies have suggested that ethylene plays a role in modulating root architecture under P deficiency (Borch et al., 1999; Ma et al., 2003; Zhang et al., 2003; Kim et al., 2008; Chacόn-Lόpez et al., 2011). However, the regulation of root architecture by low P depends on both the root type and the specific phase of root growth (e.g. lateral root and root hair formation or elongation). For example, addition of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) to arabidopsis seedlings grown in low-P conditions inhibited primary root growth and lateral root formation but had no influence on lateral root density (López-Bucio et al., 2002). Furthermore, the negative effect of ACC on lateral root formation and the reduced formation of lateral roots in response to low-P conditions in the ETHYLENE OVERPRODUCER 1 (eto1) and CONSTITUTIVE TRIPLE RESPONSE 1 (ctr1) mutants suggest that ethylene plays a negative rather than a positive role in lateral root induction.
Although the precise role of ethylene in regulating root adaptations associated with P availability has not been fully understood, it appears that ethylene regulates root elongation via changes in both biosynthesis and responsiveness. Phosphorus-deficient roots produced twice as much ethylene per gram of dry matter as P-sufficient roots in common bean (Phaseolus vulgaris) (Borch et al., 1999). Enhanced ethylene production and altered ethylene sensitivity in P-deficient common bean were responsible for root responses to P deficiency (Borch et al., 1999). The angle of the basal roots in common bean can be modulated by sensitivity of the basal roots to ethylene stimulated by low P status (Basu et al., 2007). The ETHYLENE INSENSITIVE 1 (etr1) mutant of arabidopsis has normal responses in terms of lateral root formation and primary root inhibition when exposed to low-P conditions, whereas eto1 and ctr1 mutants are less responsive (López-Bucio et al., 2002). Likewise, ethylene-insensitive ‘Never-ripe’ tomato plants are unable to respond to low P with increased adventitious root development (Kim et al., 2008). These findings demonstrate that both the biosynthesis and responsiveness of ethylene regulate root elongation and some aspects of lateral root growth.
Direct sensing of low P and increased ethylene production are thought to induce the initiation and elongation of root hairs (Bates and Lynch, 1996; Zhang et al., 2003). Ethylene enhances root hair density by increasing the number of hair cells and shortening trichoblast cells to increase the number of hair cells per unit length (Zhang et al., 2003). The proportion of hair cells and root hair length were reduced in ethylene-insensitive mutants of arabidopsis, especially in the presence of low P (Zhang et al., 2003). Furthermore, a low-P signal may directly activate primary ethylene response genes involved in epidermal cell differentiation (Schmidt and Schikora, 2001). Hence, ethylene signalling may be utilized during the manifestation of P starvation responses as a way to fine-tune the adaptations.
Several lines of evidence indicate that ethylene stimulates auxin biosynthesis, and the synergistic interaction between them modulates root growth and root hair development (Osmont et al., 2007; Stepanova and Alonso, 2009). Evidence has been presented that root hair formation is modulated by the developmental pathways, and, for environmental stress, ethylene/auxin signalling is a predominant feature. Some of these pathways regulate only a specific change, whereas others may modulate interactive effects. However, the precise role of ethylene in regulating cluster root adaptations associated with P availability is still unclear.
Cytokinins
Under normal conditions, cytokinins are traditionally associated with stimulation of shoot growth and inhibition of root growth (Aloni et al., 2006). Cytokinins can reduce RAM activity, which results in a reduction of low-P responses (Lai et al., 2007). AtIPS1 and other P-starvation-inducible genes are repressed by cytokinins in roots of arabidopsis (Martín et al., 2000). In turn, low P represses the action of cytokinins by reducing the cytokinin concentration (Horgan and Wareing, 1980; Kuiper et al., 1988) and decreasing the expression of CYTOKININ RESPONSE 1 (CRE1), a cytokinin receptor (Franco-Zorrilla et al., 2002). Theoretically, a decrease in cytokinin concentration in plant roots during P deficiency could alleviate the inhibition of root growth. As expected, some studies have shown that cytokinins suppress lateral root initiation of arabidopsis plants under low-P conditions (López-Bucio et al., 2002) and acts negatively for increased root growth and other P-starvation responses (Martín et al., 2000; Franco-Zorrilla et al., 2002). Similarly, the expression of the gene for cytokinin oxidase/dehydrogenase (CKX) increases significantly in cluster roots of P-deficient white lupin (Vance et al., 2003). Also, many ESTs that annotate to cytokinin oxidase are found in mature segments of P-deficient cluster roots (Uhde-Stone et al., 2003b), suggesting that cytokinins are involved in cluster root development and maturation. Moreover, addition of cytokinin to white lupin significantly reduces the number of emerged cluster roots and inhibits cluster rootlet elongation (Neumann et al., 2000). Therefore, future research is warranted to clarify whether the stimulation of cluster root formation and development in P-deficient plants is partly due to the increased production of cytokinins induced by low P.
It is important to note that the changes in cytokinin signalling during P starvation are the secondary response, as a consequence of cross-talk between auxins and P signalling cascades. Auxins can impose a very rapid regulation of cytokinin biosynthesis (Nordström et al., 2004; Aloni et al., 2006). Conversely, by influencing auxin transport and homeostasis, cytokinins inhibit lateral root formation (Laplaze et al., 2007). These findings imply that auxins and cytokinins could interact at the metabolic level in controlling plant root development. Consequently, the repressor effect of cytokinins does not appear to be a consequence of changes in root development caused by this hormone, as it occurs in all root cell types and in root tissue formed both before and after cytokinin treatment (Franco-Zorrilla et al., 2005). This indicates a factor of potential importance for auxin–cytokinin-regulated root development. Apart from interacting with auxins, a complex interaction among cytokinins, sugars, auxins and P starvation signalling has been proposed (Franco-Zorrilla et al., 2005). There is no apparent difference in growth and development when seeds of arabidopsis were germinated under P deficiency, or in response to the growth regulators ACC, IAA or cytokinin (Hong et al., 2008). Therefore, future investigations should be focused on the multilevel interactions among sugars, auxins, ethylene and cytokinins in P-starvation-induced root architecture development, especially in the primary root and root hair growth.
Reactive oxygen species
Reactive oxygen species have been shown to be related to signal transduction responses, and play an important role in signalling, and hence affect root growth and development. Recently, it was reported that the redox status, probably mediated by jasmonic acid and ethylene, played a crucial role in the primary root meristem exhaustion process triggered by P starvation (Chacón-López et al., 2011). In response to P deficiency, changes in ROS concentration and distribution in specific root cells occurred, although different patterns were reported (Potters et al., 2002; Shin et al., 2005; Tyburski et al., 2009). These results suggest that ROS act positively in the root growth response to P deprivation.
The apex of the primary root has two areas of ROS production: the QC and the elongation zone (Jiang and Feldman, 2003; Liszkay et al., 2004). The oxidative environment in the QC and the root elongation zone are important for maintaining a low rate of cell division and increasing cell wall extensibility, respectively (Jiang and Feldman, 2003; Liszkay et al., 2004). Rapidly growing roots of plants in P-sufficient medium synthesize ROS in the root elongation zone and QC. Coincidentally, P deficiency is accompanied by a diminution of ROS accumulation in the elongation zone (Tyburski et al., 2009) and the root tip (Chacón-López et al., 2011). However, the research results of Tyburski et al. (2009) suggest that the mechanism responsible for growth inhibition of the primary root of arabidopsis under low P does not involve ROS accumulation, as would be expected from Fe toxicity, but rather a retranslocation of produced ROS. This is probably because of a typical pattern of ROS distribution in the root apex with the local maxima in the QC and root elongation zone.
It has been hypothesized that auxin, by regulating the ROS status of the discrete zones within the RAM, may act as a positional signal and mediate meristem patterning (Jiang and Feldman, 2003). ROS are also involved in the auxin-dependent regulation of cell elongation. The growth inhibition of maize roots by auxins is associated with a decrease in ROS production (Liszkay et al., 2004). Tyburski et al. (2012) demonstrated that the differences in the length of primary roots observed at low and high P are accompanied by changes in ascorbate content and redox status. Cells of the QC that accumulate high auxin levels are characterized by the oxidized status of ascorbate and glutathione and the overproduction of ROS (Tyburski et al., 2012). Interestingly, the localization of ROS after P deprivation differs from that observed under nitrogen and potassium deprivation. Phosphorus deficiency increases ROS in the root cortex, whereas N and K deficiencies enhanced ROS in the epidermis (Shin et al., 2005). Therefore, ROS production could be used as a measure of root response to nutrient deficiency.
Nitric oxide
Nitric oxide has been reported to be involved in diverse physiological and developmental processes in plants. It is also a second messenger in auxin signal transduction leading to root developmental processes. It has been reported that NO plays a key role in auxin-induced adventitious root development in cucumber (Cucumis sativus) (Pagnussat et al., 2003, 2004) and that NO increases root hair length and density in lettuce (Lactuca sativa) (Lombardo et al., 2006). Therefore, it is reasonable to hypothesize that NO is concerned with part of P-deficiency-induced root development. Evidence shows that NO plays a key role in the initiation of lateral roots in tomato and maize (Correa-Aragunde et al., 2004; Creus et al., 2005; Zandonadi et al., 2010), formation of crown roots in rice (Xiong et al., 2009a, b) and cluster root development in white lupin under low-P conditions (B.L. Wang et al., 2010; Meng et al., 2012).
It is worth mentioning that the patterns of NO production in the cluster roots of white lupin differ, depending on the root zone, developmental stage and P nutritional status. In addition, NO is involved in the root physiological response to Fe deficiency in tomato (Graziano and Lamattina, 2007) and enhances the citrate exudation in cluster roots of P-deficient white lupin (B.L. Wang et al., 2010). These findings imply that NO is a node in the possible shared signalling pathway in the formation of cluster roots under P or Fe deficiency. Yet the physiological basis of these processes and the regulatory mechanisms involved in them are unknown and warrant further investigation. Moreover, since both cytokinins and NO may act downstream of auxins to influence root growth, the interaction between cytokinins and NO is important in controlling root development in response to low P, and further work is also needed to understand its mechanisms.
Abscisic acid
Abscisic acid (ABA) plays a role in root development (Beaudoin et al., 2000; De Smet et al., 2003). Liang et al. (2007) showed that ABA could function as a growth inhibitor in certain cells, tissues or organs (e.g. shoot) and as a growth promoter in other organs (e.g. root). Later, it was suggested that ABA might possess dual functions: a growth inhibitor in the presence of severe drought stress and a promoter of root growth in the absence of stress or under moderate stress conditions (associated with relatively low endogenous ABA levels) (Cheng et al., 2002). The role of ABA in the P-stress response was addressed by Radin (1984) and Trull et al. (1997) who observed no differences in ABA concentration in P-deficient and P-sufficient cotton plants except when water stress was also applied. Trull et al. (1997) speculated on roles of ABA in influencing root development of the plant in response to P stress. There is indirect evidence showing that the ABA signal is involved in root growth induced by P deficiency, but no direct relationship between ABA signalling and P-response genes has been established. Interestingly, in castor bean (Ricinus communis), low P availability stimulated the xylem transport of ABA although it did not affect the concentration of ABA (Jeschke et al., 1997). Moreover, recent genetic and phenotypic analyses have revealed complex interactions among ABA, sucrose and ethylene signalling (Beaudoin et al., 2000; Ghassemian et al., 2000; Sharp, 2002; Sharp and LeNoble, 2002). For example, Spollen et al. (2000) showed that the stunted growth of ABA-deficient maize plants was caused by the overproduction of ethylene and that ABA may function to prevent the overproduction of ethylene. Earlier studies showed that N or P deficiency increases stomatal responsiveness to ABA, perhaps through changes in the cytokinin concentration (Radin, 1984; Radin and Hendrix, 1988). However, the action of ABA in lateral root development is thought to be independent of auxins (De Smet et al., 2003), but is modulated by nutrient availability (Signora et al., 2001). Considering the above discussion about cytokinins, it is speculated that ABA and cytokinins act oppositely in regulating root architecture development under low-P conditions.
Other nutrients
Recent studies have revealed that morphological changes of roots under P starvation are actually an outcome of complex interactions between P and other nutrients, such as N, Fe and Ca. Dinkelaker et al. (1995) reported that low levels of N enhanced formation of proteoid root under P deficiency, while high N levels exhibited an inhibitory effect. However, Sas et al. (2002) found that the addition of ammonium stimulated cluster root formation and proton excretion in white lupin under low-P conditions. Similarly, Jing et al. (2010) reported that localized application of P combined with ammonium can significantly improve maize root growth and nutrient use at early stages by stimulating root proliferation and rhizosphere acidification. Our research group recently observed that there was a distinct difference between root architecture of nitrate-fed and ammonium-fed arabidopsis under low P availability (Niu et al., 2012).
It has been shown that P deficiency enhances Fe accumulation in arabidopsis, and Fe supply influences the response of root architecture and primary root elongation to P deficiency. When the Fe concentration in the P-deficient medium was reduced, recovery of primary root elongation was observed in arabidopsis without an increase in P availability (Ward et al., 2008). On the other hand, primary root inhibition is a well-documented response to the toxicity due to excess supply of some nutrients, including Fe (Marschner, 1995). This implies that manipulating Fe availability to a plant could be a valuable strategy for improving the tolerance of the plant to P deficiency.
Recently, it has been shown that increased Ca uptake enhances P availability by solubilization of Ca phosphates in the rhizosphere (Devau et al., 2010). Two mutants of arabidopsis, CATION EXCHANGER 1, 3 (cax1cax3) and INOSITOL-PENTAKISPHOSPHATE 2-KINASE 1 (ipk1), which are defective in two vacuolar Ca2+/H+ exchangers and impaired in inositol polyphosphate kinase, respectively, have a greater P concentration in shoots (Stevenson-Paulik et al., 2005). Our unpublished data showed that Mg availability is likely to play a role in affecting some aspects of root growth of arabidopsis through changes in cytosolic Ca2+ concentration. Thus, the combined application of other nutrients such as ammonium and Fe with P has the potential for modification of the root architecture. Consequently, nutrient interactions seem to be important for the manifestation of deficiency and toxicity symptoms in many plants, and root architecture under low-P conditions should be considered in the context of how it is influenced by the specific and non-specific interactions that P has with other nutrients.
CONCLUSIONS
Based on previous studies and the above discussion, a model can be generalized for how P deficiency regulates the development of root architecture (Fig. 1). This model is mainly based on models proposed by Cheng et al. (2002), Niu et al. (2011a, b) and Péret et al. (2011). Low P availability alters the local concentration, transport or sensitivity of intracellular compounds such as carbohydrates, especially sugars, auxins, ethylene, cytokinins, NO and ROS, and subsequently modulates the downstream genetic elements that control the initiation and maintenance of root development. Notably, this distinct pathway controlling the initiation and maintenance of root meristems in different root types may be involved in context-specific use of homologous factors or interactions that occur between these hormonal signalling pathways. Although there is much evidence to support this model, the current model of how the changes in root phenotype in response to low P availability are modified by the integrated output of these hormonal cross-talks is far from being complete due to the complexity of the synergistic, additive or antagonistic effects of phytohormones on signalling pathways. Nevertheless, the changes in root architecture to adapt to low-P conditions could greatly improve P-use efficiency. Understanding the relevant regulatory mechanisms would allow plant breeders to define the selection criteria for the development of P-efficient crops, thus reducing the use of fertilizers.
Fig. 1.
Conceptual model showing the potential low-P target points in the signalling events leading to the alteration of root architecture. Solid arrows indicate links established in the induction of root development, and dashed lines denote indirect or as yet undescribed pathways. A bar at the end of a line indicates inhibition. Different colours indicate different regulatory signals. This model is developed based principally on information from arabidopsis and white lupin.
ACKNOWLEDGEMENTS
We thank the anonymous reviewers for their constructive comments on the manuscript. This work was financially supported by the Chinese Ministry of Agriculture (201103004), the State Key Development Program for Basic Research of China (973 Program, no. 2009CB119003) and the National Key Project on Science and Technology of China (2012BAC17B02).
LITERATURE CITED
- Abdolzadeh A, Wang X, Veneklaas EJ, Lambers H. Effects of phosphorus supply on growth, phosphate concentration and cluster-root formation in three Lupinus species. Annals of Botany. 2010;105:365–374. doi: 10.1093/aob/mcp297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel S, Ticconi CA, Delatorre CA. Phosphate sensing in higher plants. Physiologia Plantarum. 2002;115:1–8. doi: 10.1034/j.1399-3054.2002.1150101.x. [DOI] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R, et al. The PLETHORA genes mediate patterning of Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Aloni R, Aloni E, Langhans M, Ullrich CI. Role of cytokinin and auxin in shaping root architecture: regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Annals of Botany. 2006;97:883–893. doi: 10.1093/aob/mcl027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andraski TW, Bundy LG. Relationships between phosphorus levels in soil and in runoff from corn production systems. Journal of Environmental Quality. 2003;32:310–316. doi: 10.2134/jeq2003.3100. [DOI] [PubMed] [Google Scholar]
- Anthony RG, Henriques R, Helfer A, et al. A protein kinase target of a PDK1 signaling pathway is involved in root hair growth in Arabidopsis. EMBO Journal. 2004;23:572–581. doi: 10.1038/sj.emboj.7600068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu P, Pal A, Lynch JP, Brown KM. A novel image-analysis technique for kinematic study of growth and curvature. Plant Physiology. 2007;145:305–316. doi: 10.1104/pp.107.103226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates TR, Lynch JP. Stimulation of root hair elongation in Arabidopsis thaliana by low phosphorus availability. Plant, Cell and Environment. 1996;19:529–538. [Google Scholar]
- Bates TR, Lynch JP. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. American Journal of Botany. 2000a;87:964–970. [PubMed] [Google Scholar]
- Bates TR, Lynch JP. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae) American Journal of Botany. 2000b;87:958–963. [PubMed] [Google Scholar]
- Batjes NH. A world data set for derived soil properties by FAO-UNESCO soil unit for global modelling. Soil Use and Management. 1997;13:9–16. [Google Scholar]
- Bayuelo-Jiménez JS, Gallardo-Valdéz M, Pérez-Decelis VA, Magdaleno-Armas L, Ochoa I, Lynch JP. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Research. 2011;121:350–362. [Google Scholar]
- Beaudoin N, Serizet C, Gosti F, Giraudat J. Interactions between abscisic acid and ethylene signaling cascades. The Plant Cell. 2000;12:1103–1106. doi: 10.1105/tpc.12.7.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beebe SE, Rao IM, Cajiao C, Grajales M. Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments. Crop Science. 2008;48:582–592. [Google Scholar]
- Bolan NS. A critical review on the role of mycorrhizal fungi in the uptake of phosphorus by plants. Plant and Soil. 1991;134:189–207. [Google Scholar]
- Bolland MDA, Brennan RF. Phosphorus, copper and zinc requirements of no-till wheat crops and methods of collecting soil samples for soil testing. Australian Journal of Experimental Agriculture. 2006;46:1051–1059. [Google Scholar]
- Borch K, Bouma TJ, Lynch JP, Brown KM. Ethylene: a regulator of root architectural responses to soil phosphorus availability. Plant, Cell and Environment. 1999;22:425–431. [Google Scholar]
- Bonser AM, Lynch J, Snapp S. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist. 1996;132:281–288. doi: 10.1111/j.1469-8137.1996.tb01847.x. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Coevolution of roots and mycorrhizas of land plants. New Phytologist. 2002;154:275–304. doi: 10.1046/j.1469-8137.2002.00397.x. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Diversity and classification of mycorrhizal associations. Biological Reviews. 2004;79:473–495. doi: 10.1017/s1464793103006316. [DOI] [PubMed] [Google Scholar]
- Brundrett MC. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. Soil Biology. 2009;9:281–298. [Google Scholar]
- Bustos R, Castrillo G, Linhares F, et al. A central regulatory system largely controls transcriptional activation and repression responses to phosphate starvation in Arabidopsis. PLoS Genetics. 2010;6:e1001102. doi: 10.1371/journal.pgen.1001102. http://dx.doi.org/10.1371/journal.pgen.1001102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell C, Grant BR, Theodorou ME, Harris L, Niere JO, Plaxton WC. The fungicide phosphonate disrupts the phosphate-starvation response in Brassica nigra seedlings. Plant Physiology. 1996;110:105–110. doi: 10.1104/pp.110.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Sheres B. Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem maintenance. Current Biology. 2003;13:1435–1441. doi: 10.1016/s0960-9822(03)00533-5. [DOI] [PubMed] [Google Scholar]
- Celenza JL, Grisafi PL, Fink GR. A pathway for lateral root formation in Arabidopsis thaliana. Genes and Development. 1995;9:2131–2142. doi: 10.1101/gad.9.17.2131. [DOI] [PubMed] [Google Scholar]
- Chacón-López A, Ibarra-Laclette E, Sánchez-Calderón L, Gutiérrez-Alanis D, Herrera-Estrella L. Global expression pattern comparison between low phosphorus insensitive 4 and WT Arabidopsis reveals an important role of reactive oxygen species and jasmonic acid in the root tip response to phosphate starvation. Plant Signaling and Behavior. 2011;6:382–392. doi: 10.4161/psb.6.3.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Xiong LM. The bifunctional abiotic stress signalling regulator and endogenous RNA silencing suppressor FIERY1 is required for lateral root formation. Plant, Cell and Environment. 2010;33:2180–2190. doi: 10.1111/j.1365-3040.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- Chen ZH, Nimmo GA, Jenkins GI, Nimmo HG. BHLH32 modulates several biochemical and morphological processes that respond to Pi starvation in Arabidopsis. Biochemical Journal. 2007;405:191–198. doi: 10.1042/BJ20070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Jenkins GI, Nimmo HG. Identification of an F-Box protein that negatively regulates Pi starvation responses. Plant and Cell Physiology. 2008;49:1902–1906. doi: 10.1093/pcp/pcn157. [DOI] [PubMed] [Google Scholar]
- Cheng LY, Bucciarelli B, Liu JQ, et al. White lupin cluster root acclimation to phosphorus deficiency and root hair development involve unique glycerophosphodiester phosphodiesterases. Plant Physiology. 2011;156:1131–1148. doi: 10.1104/pp.111.173724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, et al. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. The Plant Cell. 2002;14:2723–2743. doi: 10.1105/tpc.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell and Environment. 2003;26:1839–1850. [Google Scholar]
- Chiou TJ, Lin SI. Signaling network in sensing phosphate availability in plants. Annual Review of Plant Biology. 2011;62:185–206. doi: 10.1146/annurev-arplant-042110-103849. [DOI] [PubMed] [Google Scholar]
- Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–905. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- Creus CM, Graziano M, Casanovas EM, et al. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta. 2005;221:297–303. doi: 10.1007/s00425-005-1523-7. [DOI] [PubMed] [Google Scholar]
- Dai XY, Wang YY, Yang A, Zhang WH. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiology. 2012;159:169–183. doi: 10.1104/pp.112.194217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Kim CM, Pernas M, et al. Root hairs: development, growth and evolution at the plant–soil interface. Plant and Soil. 2011;346:1–14. [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inze D, Foyer CH, Zhang HM. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. The Plant Journal. 2003;33:543–555. doi: 10.1046/j.1365-313x.2003.01652.x. [DOI] [PubMed] [Google Scholar]
- Desnos T. Root branching responses to phosphate and nitrate. Current Opinion in Plant Biology. 2008;11:82–87. doi: 10.1016/j.pbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Devaiah BN, Karthikeyan AS, Raghothama KG. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiology. 2007a;143:1789–1801. doi: 10.1104/pp.106.093971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaiah BN, Nagarajan VK, Raghothama KG. Phosphate homeostasis and root development in Arabidopsis are synchronized by the zinc finger transcription factor ZAT6. Plant Physiology. 2007b;145:147–159. doi: 10.1104/pp.107.101691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devau N, Le Cadre E, Hinsinger P, Gérard F. A mechanistic model for understanding root-induced chemical changes controlling phosphorus availability. Annals of Botany. 2010;105:1183–1197. doi: 10.1093/aob/mcq098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinkelaker B, Hengeler C, Marschner H. Distribution and function of proteoid roots and other root clusters. Botanica Acta. 1995;108:183–200. [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, et al. Cellular organization of the Arabidopsis thaliana root. Development. 1993;119:71–84. doi: 10.1242/dev.119.1.71. [DOI] [PubMed] [Google Scholar]
- Dong B, Rengel Z, Delhaize E. Uptake and translocation of phosphate by pho2 mutant and wild-type seedlings of Arabidopsis thaliana. Planta. 1998;205:251–256. doi: 10.1007/s004250050318. [DOI] [PubMed] [Google Scholar]
- Foehse D, Jungk A. Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant and Soil. 1983;74:359–368. [Google Scholar]
- Foreman J, Dolan L. Root hairs as a model system for studying plant cell growth. Annals of Botany. 2001;88:1–7. [Google Scholar]
- Franco-Zorrilla JM, Martin AC, Solano R, Rubio V, Leyva A, Paz-Ares J. Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. The Plant Journal. 2002;32:353–360. doi: 10.1046/j.1365-313x.2002.01431.x. [DOI] [PubMed] [Google Scholar]
- Franco-Zorrilla JM, Martín AC, Leyva A, Paz-Ares JP. Interaction between phosphate-starvation, sugar, and cytokinin signaling in Arabidopsis and the roles of cytokinin receptors CRE1/AHK4 and AHK3. Plant Physiology. 2005;138:847–857. doi: 10.1104/pp.105.060517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahoonia TS, Nielsen NE, Joshi PA, Jahoor A. A root hairless barley mutant for elucidating genetic of root hairs and phosphorus uptake. Plant and Soil. 2001;235:211–219. [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P. Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. The Plant Cell. 2000;12:1117–1126. doi: 10.1105/tpc.12.7.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert GA, Knight JD, Vance CP, Allan DL. Proteoid root development of phosphorus deficient lupin is mimicked by auxin and phosphonate. Annals of Botany. 2000;85:921–928. [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Grierson CS, Parker JS, Kemp AC. Arabidopsis genes with roles in root hair development. Journal of Plant Nutrition and Soil Science. 2001;164:131–140. [Google Scholar]
- Grierson CS, Schiefelbein JW. Root hairs. The Arabidopsis Book. 2002;1:e0060. doi: 10.1199/tab.0060. http://dx.doi.org/10.1199/tab.0060 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grierson CS, Schiefelbein JW. Genetics of root hair formation. Plant Cell Monographs. 2009;12:1–25. [Google Scholar]
- Hammond JP, White PJ. Sucrose transport in the phloem: integrating root responses to phosphorus starvation. Journal of Experimental Botany. 2008;59:93–109. doi: 10.1093/jxb/erm221. [DOI] [PubMed] [Google Scholar]
- Hammond JP, White PJ. Sugar signaling in root responses to low phosphorus availability. Plant Physiology. 2011;156:1033–1040. doi: 10.1104/pp.111.175380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science. 2006;11:610–615. doi: 10.1016/j.tplants.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Hinsinger P, Plassard C, Tang CX, Jaillard B. Origins of root-mediated pH changes in the rhizosphere and their responses to environmental constraints: a review. Plant and Soil. 2003;248:43–59. [Google Scholar]
- Hirsch J, Misson J, Crisp PA, et al. A novel fry1 allele reveals the existence of a mutant phenotype unrelated to 5′ → 3′ exoribonuclease (XRN) activities in Arabidopsis thaliana roots. PLoS One. 2011;6:e16724. doi: 10.1371/journal.pone.0016724. http://dx.doi.org/10.1371/journal.pone.0016724 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, McCannon BC, Lynch JP. Optimization modeling of plant root architecture for water and phosphorus acquisition. Journal of Theoretical Biology. 2004;226:331–340. doi: 10.1016/j.jtbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany. 2004;93:359–368. doi: 10.1093/aob/mch056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. Plant root growth, architecture and function. Plant and Soil. 2009;321:153–187. [Google Scholar]
- Holanda FSR, Mengel DB, Paula MB, Carvaho JG, Bertoni JC. Influence of crop rotations and tillage systems on phosphorus and potassium stratification and root distribution in the soil profile. Communications in Soil Science and Plant Analysis. 1998;29:15–16. [Google Scholar]
- Hong YY, Pan XQ, Welti R, Wang XM. Phospholipase Dα3 is involved in the hyperosmotic response in Arabidopsis. The Plant Cell. 2008;20:803–816. doi: 10.1105/tpc.107.056390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan JM, Wareing PF. Cytokinins and the growth responses of seedlings of Betula pendula Roth. and Acer pseudoplatanus L. to nitrogen and phosphorus deficiency. Journal of Experimental Botany. 1980;31:525–532. [Google Scholar]
- Hu B, Zhu CG, Li F, et al. LEAF TIP NECROSIS1 plays a pivotal role in the regulation of multiple phosphate starvation responses in rice. Plant Physiology. 2011;156:1101–1115. doi: 10.1104/pp.110.170209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Poling MD, Karthikeyan AS, et al. Differential effects of sucrose and auxin on localized phosphate deficiency-induced modulation of different traits of root system architecture in Arabidopsis. Plant Physiology. 2007;144:232–247. doi: 10.1104/pp.106.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper DA, Robson AD, Abbott LK. Phosphorus and the formation of vesicular-arbuscular mycorrhizas. Soil Biology and Biochemistry. 1979;11:501–505. [Google Scholar]
- Jeschke WD, Holobradá M, Hartung W. Growth of Zea mays L. plants with their seminal roots only. Effects on plant development, xylem transport, mineral nutrition and the flow and distribution of abscisic acid (ABA) as a possible shoot to root signal. Journal of Experimental Botany. 1997;48:1229–1239. [Google Scholar]
- Jiang K, Feldman LJ. Root meristem establishment and maintenance: the role of auxin. Journal of Plant Growth Regulation. 2003;21:432–440. [Google Scholar]
- Jiang CF, Gao X, Liao L, Harberd NP, Fu XD. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin–DELLA signaling pathway in Arabidopsis. Plant Physiology. 2007;145:1460–1470. doi: 10.1104/pp.107.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Tang CX, Armstrong R, Sale P. Phosphorus supply enhances the response of legumes to elevated CO2 (FACE) in a phosphorus-deficient Vertisol. Plant and Soil. 2012;358:86–99. [Google Scholar]
- Jing J, Rui Y, Zhang F, Rengel Z, Shen J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Research. 2010;119:355–364. [Google Scholar]
- Johnson JF, Vance CP, Allan DL. Phosphorus deficiency in Lupinus albus (altered lateral root development and enhanced expression of phosphoenolpyruvate carboxylase) Plant Physiology. 1996;112:31–41. doi: 10.1104/pp.112.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Jain A, Held MA, Carpita NC, Raghothama KG. Phosphate starvation responses are mediated by sugar signaling in Arabidopsis. Planta. 2007;225:907–918. doi: 10.1007/s00425-006-0408-8. [DOI] [PubMed] [Google Scholar]
- Karthikeyan AS, Varadarajan DK, Mukatira UT, D'Urzo MP, Damsz B, Raghothama KG. Regulated expression of Arabidopsis phosphate transporters. Plant Physiology. 2002;130:221–233. doi: 10.1104/pp.020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keerthisinghe G, Hocking PJ, Ryan PR, Delhaize E. Effect of phosphorus supply on the formation and function of proteoid roots of white lupin (Lupinus albus L.) Plant, Cell and Environment. 1998;21:467–478. [Google Scholar]
- Kim HJ, Lynch JP, Brown KM. Ethylene insensitivity impedes a subset of responses to phosphorus deficiency in tomato and petunia. Plant, Cell and Environment. 2008;31:1744–1755. doi: 10.1111/j.1365-3040.2008.01886.x. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Bennett MJ. Auxin transport: a field in flux. Trends in Plant Science. 2006;11:382–386. doi: 10.1016/j.tplants.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kuiper D, Schuit J, Kuiper PJC. Effects of internal and external cytokinin concentrations on root growth and shoot to root ratio of Plantago major ssp Pleiosperma at different nutrient conditions. Plant and Soil. 1988;111:231–236. [Google Scholar]
- Lai F, Thacker J, Li YY, Doerner P. Cell division activity determines the magnitude of phosphate starvation responses in Arabidopsis. The Plant Journal. 2007;50:545–556. doi: 10.1111/j.1365-313X.2007.03070.x. [DOI] [PubMed] [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany. 2006;98:693–713. doi: 10.1093/aob/mcl114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Raven JA, Shaver GR, Smith SE. Plant nutrient-acquisition strategies change with soil age. Trends in Ecology and Evolution. 2008;23:95–103. doi: 10.1016/j.tree.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Lambers H, Finnegan PM, Laliberté E, et al. Phosphorus nutrition of proteaceae in severely phosphorus-impoverished soils: are there lessons to be learned for future crops? Plant Physiology. 2011;156:1058–1066. doi: 10.1104/pp.111.174318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, et al. Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell. 2007;19:3889–3900. doi: 10.1105/tpc.107.055863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt RG. Trichomes of the root in vascular cryptogams and angiosperms. Proceedings of the Boston Society of Natural History. 1904;31:273–313. [Google Scholar]
- Lei MG, Liu YD, Zhang BC, et al. Genetic and genomic evidence that sucrose is a global regulator of plant responses to phosphate starvation in Arabidopsis. Plant Physiology. 2011a;156:1116–1130. doi: 10.1104/pp.110.171736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei MG, Zhu CM, Liu YD, et al. Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis. New Phytologist. 2011b;189:1084–1095. doi: 10.1111/j.1469-8137.2010.03555.x. [DOI] [PubMed] [Google Scholar]
- Li HG, Shen JB, Zhang FS, Tang CX, Lambers H. Is there a critical level of shoot phosphorus concentration for cluster-root formation in Lupinus albus? Functional Plant Biology. 2008;35:328–336. doi: 10.1071/FP07222. [DOI] [PubMed] [Google Scholar]
- Li MY, Qin CB, Welti R, Wang XM. Double knockouts of phospholipases Dζ1 and Dζ2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiology. 2006;140:761–770. doi: 10.1104/pp.105.070995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WF, Perry PJ, Prafulla NN, Schmidt W. Ubiquitin-specific protease 14 (UBP14) is involved in root responses to phosphate deficiency in Arabidopsis. Molecular Plant. 2010;3:212–223. doi: 10.1093/mp/ssp086. [DOI] [PubMed] [Google Scholar]
- Liang Y, Mitchell DM, Harris JM. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Developmental Biology. 2007;304:297–307. doi: 10.1016/j.ydbio.2006.12.037. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HMO. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal. 2002;29:751–760. doi: 10.1046/j.1365-313x.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Liszkay A, Van Der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O2·−, H2O2, and ·OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiology. 2004;136:3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JQ, Uhde-Stone C, Li A, Vance C, Allan D. A phosphate transporter with enhanced expression in proteoid roots of white lupin (Lupinus albus L.) Plant and Soil. 2001;237:257–266. [Google Scholar]
- Liu JQ, Samac DA, Bucciarelli B, Allan DL, Vance CP. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. The Plant Journal. 2005;41:257–268. doi: 10.1111/j.1365-313X.2004.02289.x. [DOI] [PubMed] [Google Scholar]
- Liu L, Liao H, Wang XR, Yan XL. Adaptive changes of soybean genotypes with different root architecture to low phosphorus availability as related to phosphorus efficiency. Scientia Agricultura Sinica. 2008;41:1089–1099. [in Chinese] [Google Scholar]
- Lombardo MC, Graziano M, Polacco JC, Lamattina L. Nitric oxide functions as a positive regulator of root hair development. Plant Signaling and Behavior. 2006;1:28–33. doi: 10.4161/psb.1.1.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology. 2002;129:244–256. doi: 10.1104/pp.010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Cruz-Ramírez A, Herrera-Estrella L. The role of nutrient availability in regulating root architecture. Current Opinion in Plant Biology. 2003;6:280–287. doi: 10.1016/s1369-5266(03)00035-9. [DOI] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, et al. An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiology. 2005;137:681–691. doi: 10.1104/pp.104.049577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Root architecture and plant productivity. Plant Physiology. 1995;109:7–13. doi: 10.1104/pp.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. Roots of the second green revolution. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Lynch JP, Brown KM. Topsoil foraging – an architectural adaptation of plants to low phosphorus availability. Plant and Soil. 2001;237:225–237. [Google Scholar]
- Lynch JP, Brown KM. Root strategies for phosphorus acquisition. Plant Ecophysiology. 2008;7:83–116. [Google Scholar]
- Lynch JP, Ho MD. Rhizoeconomics: carbon costs of phosphorus acquisition. Plant and Soil. 2005;269:45–56. [Google Scholar]
- Lynch JP, Ochoa IE, Blair MW. QTL analysis of adventitious root formation in common bean under contrasting phosphorus availability. Crop Science. 2006;46:1609–1621. [Google Scholar]
- Ma Z, Bielenberg DG, Brown KM, Lynch JP. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant, Cell and Environment. 2001;24:459–467. [Google Scholar]
- Ma Z, Baskin TI, Brown KM, Lynch JP. Regulation of root elongation under phosphorus stress involves changes in ethylene responsiveness. Plant Physiology. 2003;131:1381–1390. doi: 10.1104/pp.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral root of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Marchive C, Yehudai-Resheff S, Germain A, et al. Abnormal physiological and molecular mutant phenotypes link chloroplast polynucleotide phosphorylase to the phosphorus deprivation response in Arabidopsis. Plant Physiology. 2009;151:905–924. doi: 10.1104/pp.109.145144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschner H, Römheld V, Cakmak I. Root induced changes of nutrient availability in the rhizosphere. Journal of Plant Nutrition. 1987;10:1175–1184. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Martín AC, Del Pozo JC, Iglesias J, et al. Influence of cytokinins on the expression of phosphate starvation responsive genes in Arabidopsis. The Plant Journal. 2000;24:559–567. doi: 10.1046/j.1365-313x.2000.00893.x. [DOI] [PubMed] [Google Scholar]
- Meng ZB, Chen LQ, Suo D, Li GX, Tang CX, Zheng SJ. Nitric oxide is the shared signalling molecule in phosphorus- and iron-deficiency-induced formation of cluster roots in white lupin (Lupinus albus) Annals of Botany. 2012;109:1055–1064. doi: 10.1093/aob/mcs024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Ochoa I, Nielsen KL, Beck D, Lynch JP. Genetic variation for adventitious rooting in response to low phosphorus availability: potential utility for phosphorus acquisition from stratified soils. Functional Plant Biology. 2003;30:973–985. doi: 10.1071/FP03078. [DOI] [PubMed] [Google Scholar]
- Miller SS, Liu JQ, Allan DL, Menzhuber CJ, Fedorova M, Vance CP. Molecular control of acid phosphatase secretion into the rhizosphere of proteoid roots from phosphorus-stressed white lupin. Plant Physiology. 2001;127:594–606. [PMC free article] [PubMed] [Google Scholar]
- Miura K, Rus A, Sharkhuu A, et al. The Arabidopsis SUMO E3 ligase SIZ1 controls phosphate deficiency responses. Proceedings of the National Academy of Sciences, USA. 2005;102:7760–7765. doi: 10.1073/pnas.0500778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura K, Lee J, Gong QQ, et al. SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation. Plant Physiology. 2011;155:1000–1012. doi: 10.1104/pp.110.165191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollier A, Pellerin S. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany. 1999;50:487–497. [Google Scholar]
- Müller R, Morant M, Jarmer H, Nilsson L, Nielsen TH. Genome-wide analysis of the Arabidopsis leaf transcriptome reveals interaction of phosphate and sugar metabolism. Plant Physiology. 2007;143:156–171. doi: 10.1104/pp.106.090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge SR, Rae AL, Diatloff E, Smith FW. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. The Plant Journal. 2002;31:341–353. doi: 10.1046/j.1365-313x.2002.01356.x. [DOI] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, et al. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology. 2005;138:2061–2074. doi: 10.1104/pp.105.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan A, Reddy BK. Effect of phosphorus deficiency on the form of plant root system. In: Scaife A, editor. Plant nutrition. Vol. 2. Slough, UK: Commonwealth Agricultural Bureau; 1982. pp. 412–417. [Google Scholar]
- Nagarajan VK, Jain A, Poling MD, Lewis AJ, Raghothama KG, Smith AP. Arabidopsis Pht1;5 mobilizes phosphate between source and sink organs and influences the interaction between phosphate homeostasis and ethylene signaling. Plant Physiology. 2011;156:1149–1163. doi: 10.1104/pp.111.174805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann G, Massonneau A, Langlade N, et al. Physiological aspects of cluster root function and development in phosphorus-deficient white lupin (Lupinus albus L.) Annals of Botany. 2000;85:909–919. [Google Scholar]
- Niu YF, Jin CW, Jin GL, et al. Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana (L.) Heynh. under elevated CO2. Plant, Cell and Environment. 2011a;34:1304–1317. doi: 10.1111/j.1365-3040.2011.02330.x. [DOI] [PubMed] [Google Scholar]
- Niu YF, Jin GL, Chai RS, Wang H, Zhang YS. Responses of root hair development to elevated CO2. Plant Signaling and Behavior. 2011b;6:1414–1417. doi: 10.4161/psb.6.9.17302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu YF, Chai RS, Dong HF, Wang H, Tang CX, Zhang YS. Effect of elevated CO2 on phosphorus nutrition of phosphate-deficient Arabidopsis thaliana (L.) Heynh under different nitrogen forms. Journal of Experimental Botany. 2012 doi: 10.1093/jxb/ers341. http://jxb.oxfordjournals.org/content/early/2012/11/23/jxb.ers341.short. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström A, Tarkowski P, Tarkowska D, et al. Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin–cytokinin-regulated development. Proceedings of the National Academy of Sciences, USA. 2004;101:8039–8044. doi: 10.1073/pnas.0402504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. Hidden branches: developments in root system architecture. Annual Review of Plant Biology. 2007;58:93–113. doi: 10.1146/annurev.arplant.58.032806.104006. [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L. Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiology. 2003;132:1241–1248. doi: 10.1104/pp.103.022228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L. Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiology. 2004;135:279–286. doi: 10.1104/pp.103.038554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paungfoo-Lonhienne C, Schenk PM, Lonhienne TGA, et al. Nitrogen affects cluster root formation and expression of putative peptide transporters. Journal of Experimental Botany. 2009;60:2665–2676. doi: 10.1093/jxb/erp111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse SJ, Veneklaas EJ, Cawthray GR, Bolland MDA, Lambers H. Carboxylate release of wheat, canola and 11 grain legume species as affected by phosphorus status. Plant and Soil. 2006;288:127–139. [Google Scholar]
- Pemberton LMS, Tsai SL, Lovell PH, Harris PJ. Epidermal patterning in seedlings roots of eudicotyledons. Annals of Botany. 2001;87:649–654. [Google Scholar]
- Peterson RL, Farquhar ML. Root hairs: specialized tubular cells extending root surfaces. Botanical Review. 1996;62:1–40. [Google Scholar]
- Péret B, Clément M, Nussaume L, Desnos T. Root developmental adaptation to phosphate starvation: better safe than sorry. Trends in Plant Science. 2011;16:442–450. doi: 10.1016/j.tplants.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, et al. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell. 2008;20:3258–3272. doi: 10.1105/tpc.108.058719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potters G, De Gara L, Asard H, Horemans N. Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiology and Biochemistry. 2002;40:537–548. [Google Scholar]
- Radin JW. Stomatal responses to water stress and to abscisic acid in phosphorus-deficient cotton plants. Plant Physiology. 1984;76:392–394. doi: 10.1104/pp.76.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radin JW, Hendrix DL. The apoplastic pool of abscisic acid in cotton leaves in relation to stomatal closure. Planta. 1988;174:180–186. doi: 10.1007/BF00394770. [DOI] [PubMed] [Google Scholar]
- Ramaekers L, Remans R, Rao IM, Blair MW, Vanderleyden J. Strategies for improving phosphorus acquisition efficiency of crop plants. Field Crops Research. 2010;117:169–176. [Google Scholar]
- Rath M, Salas J, Parhy B, et al. Identification of genes induced in proteoid roots of white lupin under nitrogen and phosphorus deprivation, with functional characterization of a formamidase. Plant and Soil. 2010;334:137–150. [Google Scholar]
- Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant, Cell and Environment. 2006;29:115–125. doi: 10.1111/j.1365-3040.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- Richardson AE, Hocking PJ, Simpson RJ, George TS. Plant mechanisms to optimise access to soil phosphorus. Crop and Pasture Science. 2009;60:124–143. [Google Scholar]
- Rouached H, Arpat AB, Poirier Y. Regulation of phosphate starvation responses in plants: signaling players and cross-talks. Molecular Plant. 2010;3:288–299. doi: 10.1093/mp/ssp120. [DOI] [PubMed] [Google Scholar]
- Rubio V, Linhares F, Solano R, et al. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes and Development. 2001;15:2122–2133. doi: 10.1101/gad.204401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V, Bustos R, Irigoyen ML, Cardona-López X, Rojas-Triana M, Paz-Ares J. Plant hormones and nutrient signaling. Plant Molecular Biology. 2009;69:361–373. doi: 10.1007/s11103-008-9380-y. [DOI] [PubMed] [Google Scholar]
- Ruyter-Spira C, Kohlen W, Charnikhova T, et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: another belowground role for strigolactones? Plant Physiology. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, Lόpez-Bucio J A, Chacόn-Lόpez A, et al. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant and Cell Physiology. 2005;46:174–184. doi: 10.1093/pcp/pci011. [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, Lόpez-Bucio J, Chacόn-Lόpez A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiology. 2006;140:879–889. doi: 10.1104/pp.105.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders PL, Markhart AH. Interspecific grafts demonstrate root system control of leaf water status in water-stressed Phaseolus. Journal of Experimental Botany. 1992;43:1563–1567. [Google Scholar]
- Santos-Beneit F, Rodríguez-García A, Apel AK, Martín JF. Phosphate and carbon source regulation of two PhoP-dependent glycerophosphodiester phosphodiesterase genes of Streptomyces coelicolor. Microbiology. 2009;155:1800–1811. doi: 10.1099/mic.0.026799-0. [DOI] [PubMed] [Google Scholar]
- Sas L, Rengel Z, Tang CX. The effect of nitrogen nutrition on cluster root formation and proton extrusion by Lupinus albus. Annals of Botany. 2002;89:435–442. doi: 10.1093/aob/mcf066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtman DP, Reid RJ, Ayling SM. Phosphorus uptake by plants: from soil to cell. Plant Physiology. 1998;116:447–453. doi: 10.1104/pp.116.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein JW. Constructing a plant cell. The genetic control of root hair development. Plant Physiology. 2000;124:1525–1531. doi: 10.1104/pp.124.4.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W, Schikora A. Different pathways are involved in phosphate and iron stress-induced alterations of root epidermal cell development. Plant Physiology. 2001;125:2078–2084. doi: 10.1104/pp.125.4.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shane M, Lambers H. Cluster roots: a curiosity in context. Plant and Soil. 2005;274:101–125. [Google Scholar]
- Shane MW, De Vos M, De Roock S, Lambers H. Shoot P status regulates cluster-root growth and citrate exudation in Lupinus albus grown with a divided root system. Plant, Cell and Environment. 2003;26:265–273. [Google Scholar]
- Sharp RE. Interaction with ethylene: changing views on the role of abscisic acid in root and shoot growth responses to water stress. Plant, Cell and Environment. 2002;25:211–222. doi: 10.1046/j.1365-3040.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME. ABA, ethylene and the control of shoot and root growth under water stress. Journal of Experimental Botany. 2002;53:33–37. [PubMed] [Google Scholar]
- Shen J, Rengel Z, Tang C, Zhang F. Role of phosphorus nutrition in development of cluster roots and release of carboxylates in soil-grown Lupinus albus. Plant and Soil. 2003;248:199–206. [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. The Plant Journal. 2004;39:629–642. doi: 10.1111/j.1365-313X.2004.02161.x. [DOI] [PubMed] [Google Scholar]
- Shin R, Berg RH, Schachtman DP. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant and Cell Physiology. 2005;46:1350–1357. doi: 10.1093/pcp/pci145. [DOI] [PubMed] [Google Scholar]
- Shimizu A, Yanagihara S, Kawasaki S, Ikehashi H. Phosphorus deficiency-induced root elongation and its QTL in rice (Oryza sativa L.) Theoretical and Applied Genetics. 2004;109:1361–1368. doi: 10.1007/s00122-004-1751-4. [DOI] [PubMed] [Google Scholar]
- Shu LZ, Shen JB, Rengel Z, Tang CX, Zhang FS. Growth medium and phosphorus supply affect cluster root formation and citrate exudation by Lupinus albus grown in a sand/solution split-root system. Plant and Soil. 2005;276:85–94. [Google Scholar]
- Shu LZ, Shen JB, Rengel Z, Tang CX, Zhang FS. Cluster root formation by Lupinus albus is modified by stratified application of phosphorus in a split-root system. Journal of Plant Nutrition. 2007;30:271–288. [Google Scholar]
- Signora L, De Smet I, Foyer CH, Zhang HM. ABA plays a central role in mediating the regulatory effects of nitrate on root branching in Arabidopsis. The Plant Journal. 2001;28:655–662. doi: 10.1046/j.1365-313x.2001.01185.x. [DOI] [PubMed] [Google Scholar]
- Skene KR. Pattern formation in cluster roots. Some developmental and evolutionary considerations. Annals of Botany. 2000;85:901–908. [Google Scholar]
- Smith SE, Smith FA. Roles of arbuscular mycorrhizas in plant nutrition and growth: new paradigms from cellular to ecosystems scales. Annual Review of Plant Biology. 2011;63:227–250. doi: 10.1146/annurev-arplant-042110-103846. [DOI] [PubMed] [Google Scholar]
- Smith SE, Jakobsen I, Grønlund M, Smith FA. Roles of arbuscular mycorrhizas in plant phosphorus nutrition: interactions between pathways of phosphorus uptake in arbuscular mycorrhizal roots have important implications for understanding and manipulating plant phosphorus acquisition. Plant Physiology. 2011;156:1050–1057. doi: 10.1104/pp.111.174581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology. 2000;122:967–976. doi: 10.1104/pp.122.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponchiado BN, White JW, Castillo JA, Jones PG. Root growth of four common bean cultivars in relation to drought tolerance in environments with contrasting soil types. Experimental Agriculture. 1989;25:249–257. [Google Scholar]
- Stepanova AN, Alonso JM. Ethylene signaling and response: where different regulatory modules meet. Current Opinion in Plant Biology. 2009;12:548–555. doi: 10.1016/j.pbi.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD. Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proceedings of the National Academy of Sciences, USA. 2005;102:12612–12617. doi: 10.1073/pnas.0504172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, et al. Root tip contact with low-phosphate media reprograms plant root architecture. Nature Genetics. 2007;39:792–796. doi: 10.1038/ng2041. [DOI] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, et al. Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. The Plant Cell. 2007;19:2186–2196. doi: 10.1105/tpc.107.052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JC, Mboreha IA, She LN, et al. Nutritional effects of soybean root architecture in a maize/soybean intercropping system. Scientia Agricultura Sinica. 2005;38:1196–1203. [in Chinese] [Google Scholar]
- Tesfaye M, Liu JQ, Allan DL, Vance CP. Genomic and genetic control of phosphate stress in legumes. Plant Physiology. 2007;144:594–603. doi: 10.1104/pp.107.097386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud MC, Arrighi JF, Bayle V, et al. Dissection of local and systemic transcriptional responses to phosphate starvation in Arabidopsis. The Plant Journal. 2010;64:775–789. doi: 10.1111/j.1365-313X.2010.04375.x. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. The Plant Journal. 2004;37:801–814. doi: 10.1111/j.1365-313x.2004.02005.x. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Lucero RD, Sakhonwasee S, et al. ER-resident proteins PDR2 and LPR1 mediate the developmental response of root meristems to phosphate availability. Proceedings of the National Academy of Sciences, USA. 2009;106:14174–14179. doi: 10.1073/pnas.0901778106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey JG. The induction of lateral roots by indoleacetic acid and root decapitation. American Journal of Botany. 1950;37:257–264. [Google Scholar]
- Trull MC, Guiltinan MJ, Lynch JP, Deikman J. The responses of wild-type and ABA mutant Arabidopsis thaliana plants to phosphorus starvation. Plant, Cell and Environment. 1997;20:85–92. [Google Scholar]
- Tsai SL, Harris PJ., Lovell PH. Bands of root hairs are produced in tomato (Lycopersicon esculentum) in response to specific combinations of thermoperiods and photoperiods. New Zealand Journal of Crop and Horticultural Science. 2004;32:121–129. [Google Scholar]
- Tyburski J, Dunajska K, Tretyn A. Reactive oxygen species localization in roots of Arabidopsis thaliana seedlings grown under phosphate deficiency. Plant Growth Regulation. 2009;59:27–36. [Google Scholar]
- Tyburski J, Dunajska K, Tretyn A. A role for redox factors in shaping root architecture under phosphorus deficiency. Plant Signaling and Behavior. 2010;5:64–66. doi: 10.4161/psb.5.1.10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyburski J, Dunajska-Ordak K, Skorupa M, Tretyn A. Role of ascorbate in the regulation of the Arabidopsis thaliana root growth by phosphate availability. Journal of Botany. 2012;2012:580342. http://dx.doi.org/10.1155/2012/580342 . [Google Scholar]
- Uhde-Stone C, Gilbert G, Johnson JMF, et al. Acclimation of white lupin to phosphorus deficiency involves enhanced expression of genes related to organic acid metabolism. Plant and Soil. 2003a;248:99–116. [Google Scholar]
- Uhde-Stone C, Zinn KE, Yáňez-Ramirez M, Li AG, Vance CP, Allan DL. Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiology. 2003b;131:1064–1079. doi: 10.1104/pp.102.016881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhde-Stone C, Liu JQ, Zinn KE, Allan DL, Vance CP. Transgenic proteoid roots of white lupin: a vehicle for characterizing and silencing root genes involved in adaptation to P stress. The Plant Journal. 2005;44:840–853. doi: 10.1111/j.1365-313X.2005.02573.x. [DOI] [PubMed] [Google Scholar]
- Vance CP. Plants without arbuscular mycorrhizae. Plant Ecophysiology. 2008;7:117–142. [Google Scholar]
- Vance CP, Uhde-Stone C, Allan DL. Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytologist. 2003;157:423–447. doi: 10.1046/j.1469-8137.2003.00695.x. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist. 2012;195:306–320. doi: 10.1111/j.1469-8137.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- Vu DT, Tang C, Armstrong RD. Tillage system affects phosphorus form and depth distribution in three contrasting Victorian soils. Australian Journal of Soil Research. 2009;47:33–45. [Google Scholar]
- Wang BL, Tang XY, Cheng LY, et al. Nitric oxide is involved in phosphorus deficiency-induced cluster-root development and citrate exudation in white lupin. New Phytologist. 2010;187:1112–1123. doi: 10.1111/j.1469-8137.2010.03323.x. [DOI] [PubMed] [Google Scholar]
- Wang X, Tang C, Guppy CN, Sale PWG. The role of hydraulic lift and subsoil P placement in P uptake of cotton (Gossypium hirsutum L.) Plant and Soil. 2009;325:263–275. [Google Scholar]
- Wang XM, Du GK, Wang XM, et al. The function of LPR1 is controlled by an element in the promoter and is independent of SUMO E3 ligase SIZ1 in response to low Pi stress in Arabidopsis thaliana. Plant and Cell Physiology. 2010;51:380–394. doi: 10.1093/pcp/pcq004. [DOI] [PubMed] [Google Scholar]
- Ward JT, Lahner B, Yakubova E, Salt DE, Raghothama KG. The effect of iron on the primary root elongation of Arabidopsis during phosphate deficiency. Plant Physiology. 2008;147:1181–1191. doi: 10.1104/pp.108.118562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasaki J, Omura M, Osaki M, et al. Structure of a cDNA for an acid phosphatase from phosphate-deficient lupin (Lupinus albus L.) roots. Soil Science and Plant Nutrition. 1999;45:439–449. [Google Scholar]
- Watt M, Evans JR. Proteoid roots. Physiology and development. Plant Physiology. 1999;121:317–323. doi: 10.1104/pp.121.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen TJ, Schnable PS. Analyses of mutants of three genes that influence root hair development in Zea mays (Gramineae) suggest that root hairs are dispensable. American Journal of Botany. 1994;81:833–842. [Google Scholar]
- Weligama C, Tang C, Sale PWG, Conyers MK, Liu DL. Localised nitrate application together with phosphorus enhances root proliferation of wheat and maximises rhizosphere alkalization in acid subsoil. Plant and Soil. 2008;312:101–115. [Google Scholar]
- Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology. 2001;126:875–882. doi: 10.1104/pp.126.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wissuwa M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiology. 2003;133:1947–1958. doi: 10.1104/pp.103.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, An LY, Lu H, Zhu C. Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta. 2009a;230:755–765. doi: 10.1007/s00425-009-0984-5. [DOI] [PubMed] [Google Scholar]
- Xiong J, Lu H, Lu KX, Duan YX, An LY, Zhu C. Cadmium decreases crown root number by decreasing endogenous nitric oxide, which is indispensable for crown root primordia initiation in rice seedlings. Planta. 2009b;230:599–610. doi: 10.1007/s00425-009-0970-y. [DOI] [PubMed] [Google Scholar]
- Yamagishi M, Zhou K, Osaki M, Miller SS, Vance CP. Real-time RT-PCR profiling of transcription factors including 34 MYBs and signaling components in white lupin reveals their P status dependent and organ-specific expression. Plant and Soil. 2011;342:481–493. [Google Scholar]
- Yi KK, Wu ZC, Zhou J, et al. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiology. 2005;138:2087–2096. doi: 10.1104/pp.105.063115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi K, Menand B, Bell E, Dolan L. A basic helix–loop–helix transcription factor controls cell growth and size in root hairs. Nature Genetics. 2010;42:264–267. doi: 10.1038/ng.529. [DOI] [PubMed] [Google Scholar]
- Zandonadi DB, Santos MP, Dobbss LB, et al. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta. 2010;231:1025–1036. doi: 10.1007/s00425-010-1106-0. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Lynch JP, Brown KM. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. Journal of Experimental Botany. 2003;54:2351–2361. doi: 10.1093/jxb/erg250. [DOI] [PubMed] [Google Scholar]
- Zhu JM, Kaeppler SM, Lynch JP. Mapping of QTL controlling root hair length in maize (Zea mays L.) under phosphorus deficiency. Plant and Soil. 2005a;270:299–310. [Google Scholar]
- Zhu JM, Kaeppler SM, Lynch JP. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theoretical and Applied Genetics. 2005b;111:688–695. doi: 10.1007/s00122-005-2051-3. [DOI] [PubMed] [Google Scholar]
- Zibilske LM, Bradford JM. Tillage effects on phosphorus mineralization and microbial activity. Soil Science. 2003;168:677–685. [Google Scholar]
- Zhou KQ, Yamagishi M, Osaki M, Masuda K. Sugar signalling mediates cluster root formation and phosphorus starvation-induced gene expression in white lupin. Journal of Experimental Botany. 2008a;59:2749–2756. doi: 10.1093/jxb/ern130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Jiao FC, Wu ZC, et al. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiology. 2008b;146:1673–1686. doi: 10.1104/pp.107.111443. [DOI] [PMC free article] [PubMed] [Google Scholar]