Abstract
Background
There is a large body of literature on competitive interactions among plants, but many studies have only focused on above-ground interactions and little is known about root–root dynamics between interacting plants. The perspective on possible mechanisms that explain the outcome of root–root interactions has recently been extended to include non-resource-driven mechanisms (as well as resource-driven mechanisms) of root competition and positive interactions such as facilitation. These approaches have often suffered from being static, partly due to the lack of appropriate methodologies for in-situ non-destructive root characterization.
Scope
Recent studies show that interactive effects of plant neighbourhood interactions follow non-linear and non-additive paths that are hard to explain. Common outcomes such as accumulation of roots mainly in the topsoil cannot be explained solely by competition theory but require a more inclusive theoretical, as well as an improved methodological framework. This will include the question of whether we can apply the same conceptual framework to crop versus natural species.
Conclusions
The development of non-invasive methods to dynamically study root–root interactions in vivo will provide the necessary tools to study a more inclusive conceptual framework for root–root interactions. By following the dynamics of root–root interactions through time in a whole range of scenarios and systems, using a wide variety of non-invasive methods, (such as fluorescent protein which now allows us to separately identify the roots of several individuals within soil), we will be much better equipped to answer some of the key questions in root physiology, ecology and agronomy.
Keywords: Root, interaction, competition, facilitation, resource, intraspecific, interspecific, green fluorescent protein, red fluorescent protein, nuclear magnetic resonance, positron emission tomography, rhizotrons
INTRODUCTION
Interactions among plants
Coexisting plants rely on a restricted number of essential resources such as nutrients, water and light. Interactions among plants therefore involve a large potential for competition for limited resources (Grace and Tilman, 1990; Keddy, 2006), because these resources are usually found at an optimum during specific time periods and at specific locations. According to ecological theory, strong competition could lead to so-called ‘competitive exclusion under limiting similarity’ from a community of plants, i.e. species are more likely to go extinct from the community as a consequence of competition with species having similar traits (Hutchinson, 1957; Newman, 1973). Plant species have developed a wide variety of different traits, however, that allow species to occupy different niches in time and space, and this is considered one of the key reasons why such a high diversity of plant species can coexist in a given habitat (this is known as niche complementarity theory).
Plants can interact with each other in both negative and positive ways, and these interactions can be either direct or indirect. Competition forms a classical example of a negative interaction (e.g. competition between plants for limiting resources) whereas facilitation forms a classical example of a positive one. In the latter case, the main focus has often been on relatively extreme abiotic environments such as arid habitats, where nurse plant interactions between benefactors and beneficiary species (Brooker et al., 2008) include the provision of beneficial microclimates for some species to establish themselves in the community (e.g. Saguaro cacti can often only survive the seedling stage when growing under a desert shrub). Another form of facilitation is that of nitrogen (N) or N facilitation, whereby the neighbours of N2-fixing species benefit from the extra N input into the system that the N2-fixer brings (Spehn et al., 2002; Temperton et al., 2007). Such N facilitation interactions seem to include both issues of direct interactions in relation to sharing of resources (possible direct uptake by a neighbour of N exuded by an N2-fixer via mycorrhizal hyphae) as well as indirect interactions in relation to more optimal use of total resources when the roots of non-fixers forage in the vicinity of N2-fixers (Temperton et al., 2007).
Interactions can also be indirect when the negative effect of one species A on another species B, may benefit species C. This effect is a combination of both competition and facilitation (Brooker et al., 2008). Such interactions are no doubt also occurring below ground and affecting the outcome of root–root interactions.
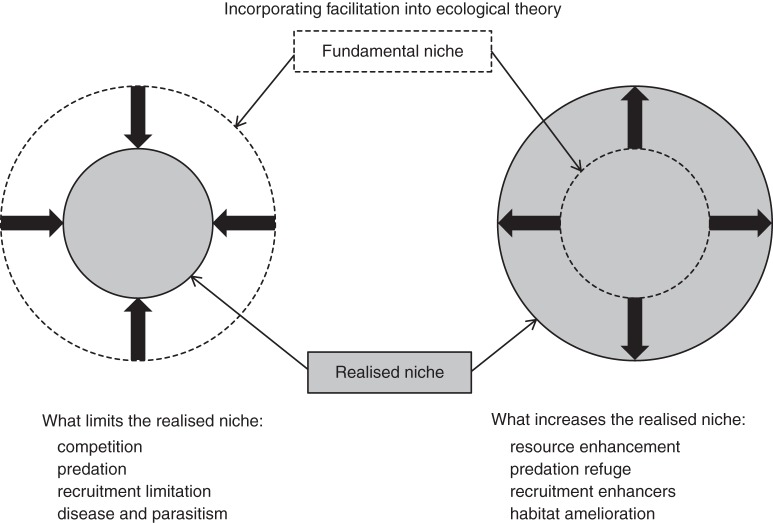
Competitive (negative) interactions between plants have been considered one of the key driving forces shaping plant communities (Grace and Tilman, 1990) over much of the history of ecology (defined as the study of how organisms interact with each other and their environment). This is especially true in population ecology (Harper, 1977) even though, in community ecology, theories of succession have long considered both negative and positive interactions between species (Connell and Slatyer, 1977). Recently some researchers (Bruno et al., 2003; Brooker et al., 2008) have presented strong cases for also considering that we often only measure the outcome of interactions between plants in our experiments, and yet assume that the net effect is only related to competitive interactions (whereas positive facilitative interactions may also play a role). In addition, positive interactions such as nurse-plant effects in more extreme arid environments may actually extend the fundamental niche of the benefiting species, rather than reduce it as in the traditional fundamental and realized niche model of Hutchinson (1957); see Fig. 1. Valiente Banuet et al. (2006) present evidence that during climate change from the Tertiary to the Quaternary where conditions changed from humid to arid, newly evolved species provided a nurse-plant function to older species (adapted to the wetter environment) and thus allowed a much larger number of species to make the transition to the new climate. Such positive interactions between species can therefore also promote the coexistence of species, and constitutes a different mechanism for the evolution of high diversity than that of niche complementarity driven by competitive interactions. Many researchers who study both facilitation and competition in interactions, find that the two phenomena can occur at the same time, or alternate sequentially over a time period (Brooker and Callaghan, 1998), such that it is imperative that we consider both issues when studying interactions.
Fig. 1.
Conceptual figure on root–root interactions and fundamental and realized niches. Adding effects of facilitation on realized niches to our overall perspective on root–root interactions. Hutchinson's fundamental and realized niche concept (1957) has been seen as where competition, predation, disease and parasitism and recruitment limitation limit the realized niche compared with the possible fundamental niche for a species. Recently, Bruno et al. (2003) have broadened the concept to include the possibility that positive interactions happening during facilitation could actually increase the size of the realized niche compared with the fundamental niche. Adapted from Bruno et al. (2003), based on Hutchinson (1957).
Considering interactions among plants in general, one can also detect a historical development in ecology of focusing more on certain conceptual aspects of interactions and much less on other aspects of interaction. Plant–plant interactions have been traditionally viewed as competition processes, with few authors studying facilitation as potential mechanisms between plant community performances.
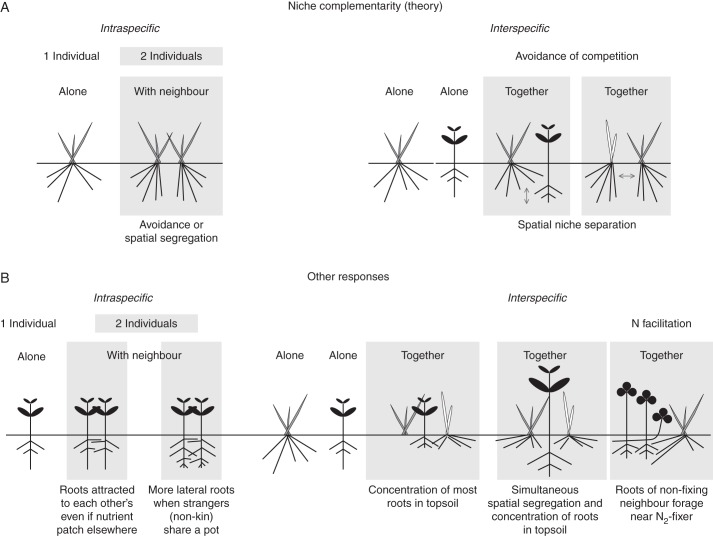
Interestingly, focusing more on one aspect has occurred in the field of mechanisms behind plant–plant interactions, i.e. whether interactions are resource driven or non-resource driven. In line with the strong focus on competitive interactions, plant interactions have been viewed mainly from the perspective of the availability of resources (e.g. nutrients and light) primarily affecting interaction outcomes (resource-driven or resource-depletion mechanisms). In contrast, non-resource-driven mechanisms, such as direct negative chemical and physicochemical effects that occur during allelopathic interactions between plants, were studied extensively up until the 1970s to 1980s, but then went out of fashion due to methodological difficulties related to separating chemical from physical effects as well as indirect effects of herbivores (Callaway, 2002) which had not always been taken into account. Recent research on plant communication and behaviour, however, has opened up our perspective towards non-resource-driven interactions once again, and provides impetus to consider both resource-driven and non-resource-driven mechanisms when studying plant interactions (see Fig. 2).
Fig. 2.
Outcomes of root–root interactions with intraspecific and interspecific scenarios. (A) Outcome scenarios according to competition theory whereby growing with neighbours leads to avoidance of competition and spatial segregation, including spatial (or other) niche separation leading to niche complementarity in interspecific situations. (B) Evidence from direct studies of root–root interactions that cannot solely be explained by competition theory. In intraspecific scenarios, roots of different individuals have been found to be attracted to one another despite a nutrient patch being positioned elsewhere (Cahill et al., 2010), or more lateral roots developed if individuals were not related to one another suggesting kin recognition (Dudley and File, 2008). In interspecific scenarios, an accumulation of roots in the topsoil has often been found, or a simultaneous accumulation in topsoil and spatial segregation (Lehmann et al., 1998). We hypothesize that this is partly due to the roots of non-N2-fixing neighbours foraging closer to the N2-fixing species and that this form of facilitation may explain some but not all of the interaction outcomes found in direct studies. More inclusive use of a range of theories as we as new non-destructive techniques will provide much-needed help explaining surprising outcomes often found.
Going one step further even, based on evidence from the fields of facilitation/competition, biodiversity and ecosystem functioning as well as ecological assembly, it is becoming increasingly clear that seemingly opposing theories on mechanisms of interaction can often operate in one system at the same time. A classic example is in grassland biodiversity and ecosystem functioning experiments: both positive complementarity effects as well as sampling effects of dominant species (two opposing mechanisms that are usually suggested to explain higher productivity in diverse mixtures) often drive productivity at the same time (Hooper et al., 2005; Marquard et al., 2009). The key aspect to consider is that as nutrients are taken away from the grassland via mowing, the positive complementarity interactions increase and sampling effects decrease. This understanding is the kind of integrative and usefully applied knowledge needed for the application of scientific knowledge in practice.
A key aspect of plant interactions takes place below ground and involves roots interacting either directly or indirectly. Nevertheless, because of methodological limitations of studying phenomena below ground our knowledge of root–root interactions is more limited than for above-ground interactions. Many interaction studies can follow the outcome of interactions dynamically above ground during an experiment, whereas the outcome of interactions below ground can often only be measured at the end of an experiment using destructive methods.
Following underground dynamics of root architecture and growth requires new techniques to allow us to follow interactions over time and space. Only once we have access to more information, on how roots interact dynamically over time and space, will we be able to address a whole range of ecological theories in relation to interaction outcomes. For root–root interactions such integrated knowledge can only be generated via repeated investigations of root systems throughout their development, utilizing non-destructive methodologies. Thus we generally advocate a more inclusive approach of studying seemingly opposing mechanisms to explain root performance in both agronomy and ecology.
Objectives
The objective of this paper is to provide a synopsis of key findings on root–root interactions within both ecological and agricultural research, focusing mainly on studies that have directly investigated interactions amongst roots of different individuals to address key questions in ecology and agronomy. One key aspect of our overall objective is to consider the wider perspective, e.g. including both facilitation and competition and both non-resource-driven and resource-driven mechanisms when studying plant interactions. We make the case for taking this wider perspective into account when studying root–root interaction. We extend classical approaches for a more inclusive approach to using a wide variety of up-and-coming methods to study root–root interactions non-destructively, including fluorescent proteins, nuclear magnetic resonance, combined magnetic resonance imaging (MRI) and positron emission tomography (PET), and high-throughput phenotyping.
Root–root interactions between individuals and among species is a young, exciting and challenging field of investigation. A large number of important questions on the nature of root–root interactions for individual plant fitness as well as how plant communities are structured still remain to be answered. In addition, as methods for studying root–root interactions are currently developing rapidly, this should allow research in this field to make considerable progress in root physiology, ecology and agronomy, such as what mechanisms lie behind an accumulation of roots in the topsoil, or whether we can come up with a conceptual framework to explain the various outcomes of root–root interactions. In this article we do not address interactions between roots and nutrients (de Kroon et al., 2012) (unless this process was studied at the same time as root–root interactions), roots and mycorrhizae (Hodge, 2004) or roots and herbivores/pathogens (Bezemer et al., 2010; Scheu, 2001).
ROOT–ROOT INTERACTIONS: THE CHALLENGING HIDDEN HALF
Roots live in a complex and heterogeneous environment that is both biotic and abiotic, living and dead. Bidirectional biological and physico-chemical interactions occur within and in-between plants and soil but we do not address this issue explicitly here as it is beyond the scope of this article and is addressed in another article in this special issue (Carminati and Vetterlein, 2013; see also Gregory, 2007; Eshel and Beeckman, 2012). Roots are more difficult to study than the above-ground part of plants, but there is an urgent need to understand how a root system behaves in different environments (Gregory, 2007; Lynch, 2007; Eshel and Beeckman, 2012). Plants growing together share the same soil volume and, therefore, the same resources, which means that they have to rearrange their root systems in order to favour their access to resources (Robinson, 1994). Casper and Jackson (1997) show that below-ground competition occurs essentially for water, nutrients and space, which is more complex than competition above ground. Root competition, defined as the reduction in the availability of soil resources caused by another root (Ricklefs and Miller, 1999) was often the only interaction process taken in consideration in the past. Positive interactions also occur among plants, however, and above-ground studies have found that both competition and facilitation can affect plant performance and affect community structure.
As discussed above, the outcome of interactions is often complex and non-linear in its response to the biotic and abiotic environment (see Fig. 2). It is becoming increasingly important to understand both how dynamic plants adapt to dynamic spatial and temporal changes in the resources in their environment (Schurr et al., 2006; Walter et al., 2009; Poorter et al., 2012), as well as how plant interactions between roots of different species have non-additive effects on the outcome of these interactions (de Kroon, 2007). We advocate that as root–root interaction studies are on the brink of major breakthroughs (mainly due to improved methods of studying them in situ or under controlled conditions), we would benefit from more inclusion in our approach: including both positive and negative interactions, and resource-driven as well as non-resource-driven interactions.
Roots are usually a black box and studies have often focused on growing plants in unnatural controlled conditions without soil in different media, so that the roots become visible. In addition, in natural communities it has traditionally been extremely difficult to separate the root systems of different individuals as well as individuals of different species. There is an urgent need to study root interaction and to be able to identify roots from different plants throughout space and time. A prerequisite for such an approach would be the ability to distinguish roots of different individuals and species.
Recent technological advances have made it possible to distinguish roots of different plant species and roots of different individual plants in situ. Here, we report on some of the most promising, non-destructive technologies that will be able to contribute to this task. They are beginning to allow us to study root interactions dynamically in plant mixtures from both an ecological perspective and an agronomic perspective. For a more extensive review on mostly destructive technologies identifying plant roots see Rewald et al. (2012).
WHAT WE KNOW SO FAR FROM BOTH INDIRECT AND DIRECT STUDIES OF ROOT–ROOT INTERACTIONS
What we know from indirect studies of root–root interactions
In biodiversity ecosystem-functioning experiments in grasslands, where above-ground overyielding in more diverse mixtures is commonly found, only a few studies have looked broadly at overall root biomass below ground (i.e. the net outcome of root interactions) in relation to the diversity of species interacting. Outcomes show a whole range of responses: ranging from 50 % overyielding (Tilman et al., 2001; Dimitrakopoulos and Schmid, 2004; Reich et al., 2004) to no overyielding (Spehn et al., 2000; Gastine et al., 2003) or even underyielding in mixtures versus monocultures (Bessler et al., 2009). Because soil nutrient levels were similar in the temperate mesic grassland systems where no overyielding or underyielding was found [e.g. Spehn et al. (2000) or Bessler et al. (2009)], the effect does not seem to be solely related to soil nutrient availability but it may be related to the exact interaction outcomes of the different mixtures or perhaps to the strength of facilitative interactions in relation to overall N acquisition and the interplay of positive and negative interactions over time (Marquard et al., 2009).
In many of the biodiversity experiments, the role of N2-fixing legume species and their positive fertilizing (N facilitation) effect on their neighbours forms one of the key drivers of overyielding, but see van Ruijven and Berendse (2009) for positive diversity effects in experiments without legumes. Temperton et al. (2007) found that four different phytometer species planted into every plot of a grassland biodiversity experiment belonging to four different plant functional groups responded differently to the presence of legume neighbours as well as overall surrounding species richness. A more detailed study of the different capacities of legume species to fix atmospheric N (N derived from atmosphere, % Ndfa) and how surrounding diversity affects %Nfda showed large differences in fixing capacities between species, but also that surrounding diversity had a strong if temporary effect on %Ndfa as well as the above-ground productivity of the legumes (Roscher et al., 2011). So far, due to difficulties in separating roots of different species in the field, such studies have been mainly limited to measuring plant performance above ground. According to facilitation and competition theory (see Fig. 1) we would expect that roots of non-fixing neighbours around legumes may tend to grow towards the legume roots, where N brought into the system from the atmosphere would be expected to accumulate (either via decomposition of legume tissues or direct transfer of fixed N to neighbours). We suggest that this aspect of facilitation known as ‘N facilitation’ (as opposed to above-ground nurse-plant interactions) will probably play a key role in affecting root distribution and performance and the net outcome may depend as much on N facilitation as on competitive interactions (see Fig. 1). As seen in Fig. 2B, we hypothesize an attraction between roots of N2-fixers and their neighbours, although we do not have any clear evidence for this attraction yet. An initial study using MRI techniques to visualize root–root interactions dynamically and quantitatively between soya, maize and sainfoin (see Fig. 5, later) found that root systems growing next to a neighbour (regardless of the species identity of the neighbour) tended to grow towards the edge of the pot and not in the area between the individuals [see Rascher et al. (2011) for method and Fig. 5 for an example]. This finding is different to the results of Cahill et al. (2010) who observed that roots of two individuals grew towards each other even though a nutrient patch was not located between the root systems. Postma and Lynch (2012) modelled productive three-way interactions between bean, squash and maize plants in relation to mobile and immobile nutrients; they found that enhanced N capture in polycultures was not directly related to the amount of N fixed by the bean plant, implying ‘N sparing’. N sparing would involve the non-N2-fixing neighbours profiting from extra soil N available due to the bean fixing atmospheric N2. These initial results point to the need to use new techniques [both destructive and non-destructive as outlined here and by Rewald et al. (2012)] and to dynamically scan a wide range of species interactions during species establishment to assess the relative roles of different types of interaction on interaction outcomes.
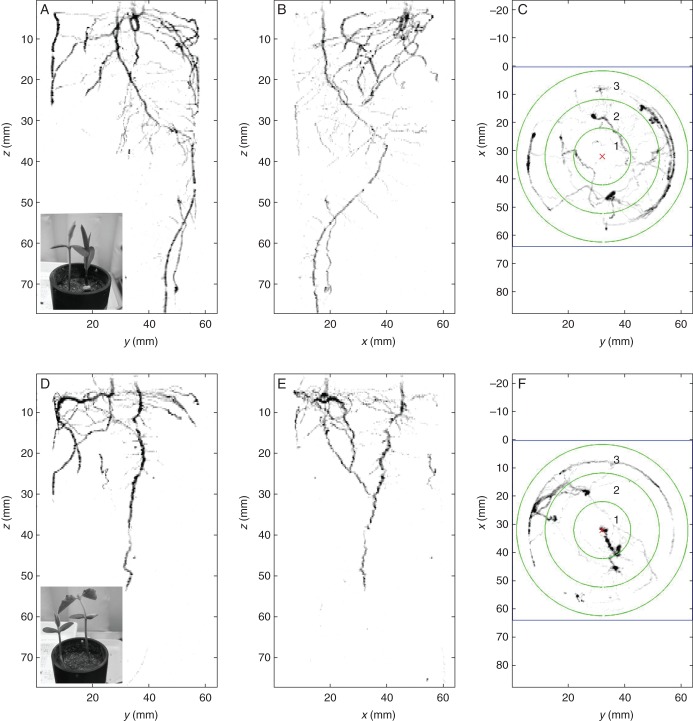
Fig. 5.
Quantifying root–root interactions between plant individuals using MRI. MRI images of root distribution of soya grown with maize (A–C, interspecific) or with another soya individual as a neighbour (D–F, intraspecific) at 14 d after transplanting seedlings into pots. (A, B) and (D, E) show two different 3-D side views; (C) and (F) show 2-D top views (projection in the axial plane) with specification of three concentric rooting zones (see Rascher et al., 2011 and fig. 7 therein for more details of the quantification method and MRI specifications). In this example, for the soya–maize interaction 9.3, 34.1 and 56.6 % or the total roots were found in concentric circles 1, 2 and 3, respectively (1 being the inner circle). For the soya–soya interaction we found 20.3, 30.2 and 49.5 % of the roots in concentric circles 1, 2 and 3, respectively.
What we know from direct studies of root–root interactions
Recent developments in molecular and cellular biology, plant physiology and ecology suggest that plants are not only able to sense their abiotic environment, but can also sense each other and communicate using a range of mechanisms. Plants can sense self/non-self (Karban and Shiojiri, 2009) as well as kin (Dudley and File, 2008), and can show swarming behaviour akin to animals (Ciszak et al., 2012). Recent work exposing Arabidopsis thaliana plants to root exudates of siblings, strangers and their own roots found that kin recognition required soluble chemical signalling, and that self-recognition (at least in this species) is a separate identity recognition system to kin recognition (Biedrzycki et al., 2010). Interaction studies using different species indicate that the sensing of plant neighbours is integrated with information about availability of resources in a complex, non-additive way (de Kroon, 2007). Cahill et al. (2010), for example, provided Abutilon theophrasti with either uniform nutrients, a patch in the centre or a patch at the edge of a pot and grew plants alone or with an intraspecific neighbour; intriguingly even with a patch at the edge of the pot, two neighbouring plant root systems grew more towards each other, even if less so than when the patch of nutrients was between the two individuals. Similarly, Gersani et al. (2001) found that Glycine max produced substantially more lateral roots when growing with non-related neighbour versus alone.
We suggest that this whole range of root reactions to not only nutrient availability but also plant neighbourhood now needs evaluating within a larger framework than competition and niche complementarity theory (see conceptual Figs 1 and 2). A whole variety of root responses to neighbours has now been found, many including an increase (e.g. Gersani et al. 2001) or a concentration of roots in the topsoil (e.g. Bessler et al., 2009; Mommer et al. 2010; see section on this topic below and Fig. 2). We generally advocate a more inclusive approach, involving consideration of seemingly opposing mechanisms and theories for both agronomy and ecology; very often in ecology two seemingly opposing mechanisms are put forward to explain a pattern occurring at the same time. One example of this is both complementarity and sampling effects enhancing productivity in biodiversity ecosystem-functioning experiments (Marquard et al., 2009) where both complementarity and sampling effects often occur at the same time. Similarly, in ecological assembly both abiotic and biotic characteristics of a system can act as filters only allowing certain plant species to establish themselves; the crux is both abiotic and biotic factors react interactively and at the same time on the filter system. Thus, only studying one aspect of the system provides us with a biased view (see Fig. 2). Based on niche complementarity theory and avoidance of competitive exclusion, for example, many authors have postulated and a number have found spatial segregation and avoidance of neighbours when plants are grown together. Alternatively reactions can involve several quite different responses; de Kroon (2007) found that the roots of wild strawberry (that grows clonally and spreads widely within natural communities) were attracted towards ground ivy roots, whereas the latter avoided the strawberry neighbourhood. It may be that the growth strategy of a species within a plant community (de Kroon, 2007) drives whether plants avoid or are attracted to neighbouring root systems of different species. Mommer et al. (2010) found below ground overyielding in mixtures compared with monocultures of grassland species, but this effect was not due to vertical niche differentiation of the roots as hypothesized; they suggest that competitive strength within a dominance hierarchy may be driving the actual response (see Fig. 2. Dominance hierarchies will be in some way related to the ecological strategy of a plant (e.g. the CSR triangle of Grime, 1974) but no one has yet tried to disentangle the perhaps different roles of the two aspects in root responses to interactions.
Indeed some studies have found so-called niche-pre-emption (Mwangi et al., 2007) whereby interaction with a dominant competitor leads a species to adjust its foraging strategy and to forage in less optimal areas or for less-available nutrient forms. The study of Ashton et al. (2010), however, studying labelled-N uptake of four alpine species (also grown alone or with other species), did not confirm this theory; they found more evidence for plasticity of resource use rather than niche complementarity in terms of avoiding taking up the same form of N as the neighbour. The dominant species increased their uptake of the main N source when interacting with other species, but the inferior species did not adjust its N uptake strategy.
In a minirhizotron study of Faget et al. (2012), where maize was grown alone and with Italian ryegrass as a neighbour and roots of different species were separated using a green fluorescent protein technique (see section on quantifying root–root interactions between plant individuals using fluorescent markers), the mean root density of maize was significantly reduced by the presence of a neighbour, but the relative root distribution in space was not affected by the ryegrass, it was only delayed in time. Mommer et al. (2010) used an innovative DNA-based technique to separate the roots of different grassland species and postulated that heterogeneous nutrient availability and presence of different neighbours may lead to additive effects on root distribution, but the authors found that the interaction of the two effects led to surprising reactions in the roots systems. Roots accumulated in the topsoil when interactions between species occurred.
The phenomenon of the accumulation of roots in the topsoil in mixed assemblages
A considerable number of studies of root dynamics both under controlled and field conditions have found that, in many cases, most of the root biomass accumulates in the topsoil. In many cases, 70–95 % of all roots found in the community were found in the first 10 or 15 cm of the soil (Bessler et al., 2009; Mommer et al., 2010). Lehmann et al. (1998) found that intercropped annual Sorghum crop and perennial Acacia trees had a higher root length density in the topsoil than in the tree monocultures, despite a simultaneous separation of root systems during intercropping. Schroth and Zech (1995) found most roots of nine legume trees in the upper 10 cm of soil, compared with the five out of 12 tree species, in an arid part of India, which were found in 15–30 cm soil depth (rather than 0–15 cm; Toky and Bisht, 1992). According to niche complementarity theory, species growing together need to have different traits, such as different rooting depth capacities, to be able to coexist. Intriguingly some studies that set out to demonstrate this spatial segregation of roots have not been able to find the effect, instead they often found that roots accumulate in the topsoil or, as Lehmann et al. (1998) found, both topsoil accumulation and spatial segregation can happen at the same time (see Fig. 2 for conceptual visualization of this).
Füllner et al. (2012) exposed barley plants to a simulation of more natural soil temperature regimes using pots under controlled conditions and found that a gradient in root temperature enhanced overall productivity, as well as causing most of the root mass to accumulate in the top of the pots. They suggest that micro-organisms will function more optimally under a warmer temperature (20 °C rather than 10 °C) and hence affect soil mineralization rates at different depths. We suggest that given new insights into plant as well as plant–microbe communication there may be other mechanisms occurring that promote an accumulation of root biomass in the topsoil. This is clearly an area of promise for further research, linking both effects under controlled and field conditions, using a variety of different species and including measurements of microbe activity.
Different foci depending on the target: crops or wild species
It is worth considering how the related but separate fields of agricultural and ecological research affect the types of questions asked in root research as well as the types of methods developed and used.
In high-input intensive agriculture, in which single crops are usually grown, and productivity and yield are optimized with a range of herbicides, fertilizers and pesticides, key root-research questions relate to determining the size and architecture of the root system of crops and reducing weed competition, tillage intensity and its effects on soil erosion and compaction (Qin et al., 2005), nitrate leaching (Herrera et al., 2010) and chemical residues (Ploey, 1989) in the ecosystem, as well as increasing the absorption of nutrients (Noulas et al., 2010) and water (Palta et al., 2011) and, as a result, nutrient and water-use efficiency and crop tolerance to abiotic stress (Okubara et al., 2009).
For example, rooting depth and root uptake capacity of plants affect the potential for nitrate to leach out of the system (Kristensen and Thorup-Kristensen, 2004). The information coming out of such studies is used to design crop rotations where a succeeding crop with a deep and fibrous root system (e.g. winter wheat) takes up, before it leaches, the residual nitrate left by a preceding crop with a shallow root system (e.g. potato). This indicates one of the key differences between rooting scenarios in intensive agricultural versus more natural perennial systems; in the former, roots of a new crop tend to be occupying a space with many niche opportunities, whereas in natural systems (unless dealing with primary succession after major disturbances) a newcomer species is often faced with a relatively tightly packed system with a limited number of niche spaces available.
Soil erosion is reduced by crops that cover the ground extensively or/and provide rich residues that maintain or increase soil organic carbon content. Breeding crop plants with deep and prolific root systems is not only a strategy to minimize soil erosion but also to sequester carbon and mitigate the effects of global climate change (Kell, 2011). Another aspect of importance is the density with which crops are sown and how this affects their performance (Weiner et al., 2001) or how root systems respond to different crop-management alternatives (Herrera et al., 2010). Studies of such phenomena will per se focus on root uptake capacity, root productivity and distribution, and the relationship of these factors to nutrient and water-use efficiency.
In ecology, root dynamics under heterogeneous nutrient availability and mycorrhizal symbioses (Hodge, 2003, 2004), with and without herbivores and pests and pathogens (Bezemer et al., 2010; Scheu, 2001), as well as competitive and facilitative interactions between individuals (Brooker et al., 2008), usually form the main focus. In addition, changing interactions between plants (sometimes including roots) across environmental gradients and how this relates to diversity of plants in communities are common (Choler et al., 2001; Huber et al., 2007). Thus questions of speed and efficiency of root foraging, as well as root distribution, and how these affect nutrient-use efficiency, overall plant and population performance and finally diversity of the community are central to below-ground ecology in plants. One key difference to the agricultural perspective therefore is the focus on interactions between populations and species and the attempt to scale up from the outcome of the interactions to the importance of these interactions for the whole community of plants or the ecosystem. Such scaling up from root–root interactions to the whole community is currently in its infancy, but we foresee that new avenues of investigation we highlight here (but see also Rewald et al., 2012) promise interesting discoveries over the next 10 years or so. New avenues are opening up in part due to new methods of studying interspecific interactions (see section on quantifying root–root interactions between plant individuals using fluorescent markers), but also if we are more inclusive of a variety of different perspectives. Taking facilitation into account as often as we consider competitive interactions, or considering non-resource-driven as well as resource-driven competition (see Figs 1 and 2).
Glover et al. (2010) provide an innovative comparison and assessment of the ecosystem services and sustainable production provided by perennial plant vegetation compared with intensive annual crops. In particular, one key difference between more natural and agronomic systems is that the level of available soil nutrients is often an order of magnitude higher for crop plants versus perennial plants, due to fertilization. Will this affect the overall response of interacting root systems, and how does an evolutionary history of growing with many different neighbours play into the root response (in a grasslands versus a crop system)? The availability of a limiting resource will no doubt affect root responses to interactions between plants as much as the plasticity of responses of the species within the system (including dominance hierarchies and potential for facilitative interactions) but we still know very little about the effect sizes of these different factors. This kind of comparison is set to produce successful results when also comparing various responses of roots to nutrients and neighbourhoods and attempting to disentangle which combinations of species may be most sustainably productive (as in the traditional maize–bean–squash combination in Mexico that has a 1000-year history (Postma and Lynch, 2012) whilst, at the same time, maintaining high levels of diversity (in the case of perennial grasslands; see Bullock et al. 2001).
There is a large body of literature on two-species intercropping using annual and perennial species, particularly in agriculture (Høgh-Jensen et al., 2006; Neumann et al., 2007; Naumann et al., 2010). Although widespread in the tropics and sub-tropics (Lehmann et al., 1998), the agricultural and environmental potential of intercropping more diverse systems than two-species polycultures has barely been investigated in temperate regions. Agroforestry, particularly in the semi-tropics and the tropics, thus provides a link between the intensive agricultural perspective and the ecological perspective. Here, a few species are usually intercropped to provide more beneficial microclimates for subordinate species (shading and increased litter input to soils) often stimulating overall community production. Both overyielding (Lehmann et al., 1998) and underyielding of the main focus crop (Martin et al., 1999) have been found but, more importantly, intercropping should usually lead to stronger root–root interactions. Increasing root density by increasing the number of plant species and/or plant density via intercropping systems also leads to improved soil productivity (Bullock, 1992) and soil structure via biopore formation (Nakamoto, 2000). This aspect of intercropping and the relative role of both facilitation and competition below ground deserve more attention. Both agronomy and ecology can now profit enormously from recent advances in methodology; that will allow us to have a better understanding of root–root interactions.
RECENT ADVANCES IN METHODOLOGY OF DIRECT RELEVANCE TO ROOT–ROOT INTERACTIONS
Quantifying root–root interactions between plant individuals and species using fluorescent markers
To be able to distinguish between root systems of different plants sharing the same volume within the soil or within a container, fluorescence markers can be useful. For example, Faget et al. (2009, 2012) used genetically transformed maize (Zea mays) expressing green fluorescent protein (GFP) to study root–root interaction of maize plants grown with its wild-type or with Italian ryegrass (Lolium multiflorum) or soybean (Glycine max) as neighbours. The plants were grown in containers installed with horizontal minirhizotrons and the images were obtained using a new minirhizotron imaging system allowing the observation of roots expressing the GFP (Faget et al., 2010). It was then possible to study the horizontal and vertical distributions and temporal patterns of each root species in detail.
Even though the potential is enormous, the application of this approach in agricultural studies is still incipient. This potential includes the possibility of increasing our understanding about plant interactions between crops grown in association such as in intercropping, living mulches and agroforestry but also in the most common crop systems composed of single species. In single cropping systems there is a need to understand better root–root interactions between single plants of the same crop species but also between crops and weeds. Huge efforts have been made to identify root traits that may increase drought tolerance (Gregory et al., 2009) or nutrient-use efficiency (Palta et al., 2007). Because root traits show great plasticity (Cholick et al., 1977) and because the presence of neighbours affects processes such as leaf orientation (Maddonni et al., 2002), modifications in root architecture due to the presence of intraspecific neighbours can also be expected. Therefore experiments evaluating root responses both with intraspecific and interspecific neighbours are needed in agronomy.
Root interactions of only two species represent strictly the conditions often found in agricultural fields; however, for ecological studies there is an urgent need to develop approaches that allow one to investigate more complex communities that include a higher number of species in undisturbed or natural soil. The construction and adaptation of new imaging tools, and their application to different species, provides the potential to adapt this method to a broader ecological context, taking into account more than two species in one and the same situation. To achieve this, multiple plant species will have to be transformed with multiple variants of fluorescent proteins; to simplify, we could than discriminate a plant A in blue from a plant B in green or a plant C in yellow. For example, the maize genotype ETH-M72 was genetically transformed to include the gene that encodes GFP (ETH-M72GFP) and grown with the wheat genotype RFP BOBWHITE, genetically transformed to include the gene coding for the red fluorescent protein (RFP), and a third species, rapeseed (Brassica napus ‘Heros’), non-transformed, was used as a wild type (WT). Seeds of these three plants germinated and were grown on blotting paper before being photographed with a conventional camera (Fig. 3A). In Fig. 3A, from left to right, we can observe the seedlings of maize, wheat and rapeseed under conventional light (Fig. 3A). In Fig. 3B, at the same position, the camera was adapted with filters restricted for the GFP and RFP wavelengths, and the seedlings were excited with the appropriate wavelength following similar principles as those employed by Faget et al. (2010). Using this approach enables one to distinguish the species by the wavelength signature; maize is visible in green, wheat is visible in red and rapeseed (non-transformed) is only visible in the images taken with a conventional camera (Fig. 3A, B). The method can also be adapted to distinguish between root systems of different plant species grown along the transparent surface of minirhizotrons or rhizotrons (Fig. 3C, D). In another set-up, the same plant species, expressing the same fluorescent proteins, were sown together into rhizotrons filled with soil. In Fig. 3C, we can observe the latter three species at 12 d after sowing in an image taken at the soil/transparent-plate interface using a conventional camera. In this image (Fig. 3C), it was impossible to distinguish among the roots belonging to the different plant species. At the same time and position, a picture was taken (Fig. 3D) following the excitation of GFP and RFP. We can clearly identify the plant species of each root in green for maize, red for wheat and visible only in the conventional images for the WT rapeseed (see the white arrow in Fig. 3D).
Fig. 3.
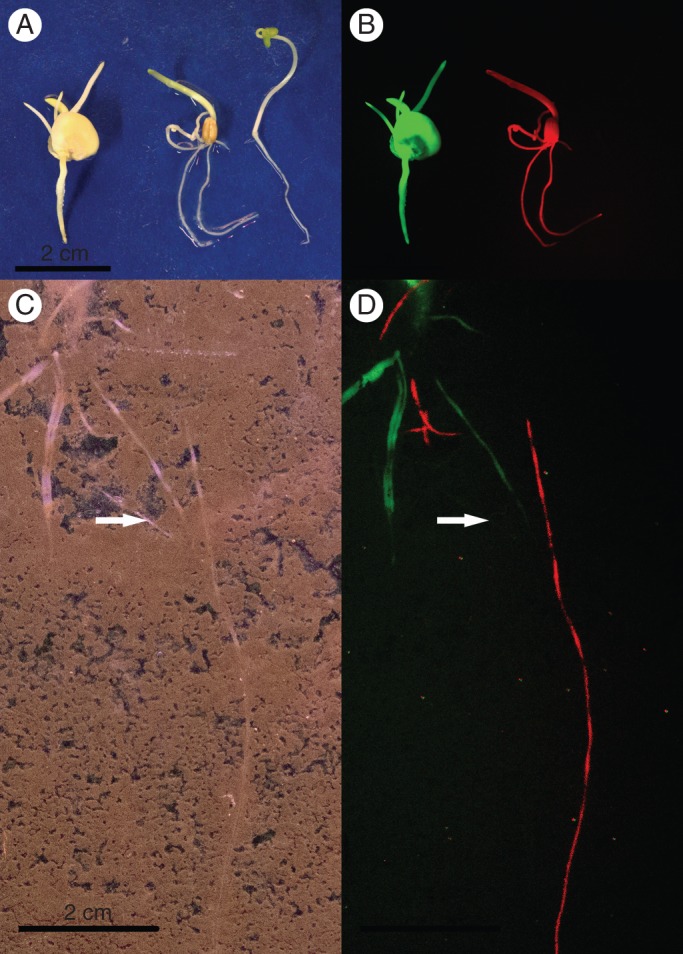
Quantifying root–root interactions between different species using fluorescent markers. (A, B) Images of seedlings (4 d after sowing). From left to right in each: transgenic maize expressing green fluorescent protein (GFP), transgenic wheat expressing red fluorescent protein (RFP) and rapeseed wild-type (WT) grown on blotting paper. (C, D) Images of a rhizotron filled with topsoil containing three plants (12 d after sowing). The arrow shows the wild-type non-transformed root of Colza rape which is then visible only in conventional images. From left to right in each: maize expressing GFP, wheat expressing RFP and rapeseed WT. Pictures were taken either with a conventional camera (Faget et al., 2009) (A, C) or with a modified camera that allowed the green and red fluorescence emission wave lengths to be captured. The images of GFP and RFP signals were then merged together (B, D). Plant material: the maize genotype ETH-M72 was genetically transformed to include the gene that encodes green fluorescent protein (gfp) (ETH-M72GFP). The wheat genotype RFP BOBWHITE 6h was genetically transformed to include the gene coding for the red fluorescent protein (rfp). The variety of Colza rape (Brassica napus ‘Heros’) was non-transformed and used as the wild type.
The evolution and adaptation of this technique facilitated the screening and identification of three different neighbouring species and their root interactions when growing alone, with one different neighbouring species or with more than one neighbouring species. Therefore, it is now possible to assess plant responses when they interact with several neighbours, as long as the species are available in the required transformed state. By targeting the appropriate plant species, it would now be possible to study within a rhizotron or minirhizotron system much more complex interactions, such as indirect ones, e.g. the impact of one species A on another species B, may benefit a third species C as described by Brooker et al. (2008). Many different colours of fluorescent proteins exist, and it would be possible to adapt this method to an entire plant community.
When considering potential limitations of the method as well as its strengths; the application of this method to a larger plant community will be mainly limited by the need to genetically transform the species of interest. This will be a question of time until a large range of species are available in transformed forms for fluorescent protein methods, but theoretically the method could be applied to all transformable biological organisms. In our view, the development of new camera systems specific to a targeted fluorescent protein will not be as limiting. The resolution of the camera is not a principal limiting factor as this technique was originally developed for studying roots using basic webcams with a resolution of 640*480 pixels. Another limitation in many countries will be that using transformed plants is forbidden in the field and will limit studies to more mechanistic experiments under controlled conditions, but insights from such experiments may nevertheless be very valuable for predicting field responses of species.
Minirhizotrons and rhizotrons are limited due to their artificial surfaces but they provide a simple way to study root dynamics in the field or close-to-field conditions and allow one to see the roots growing on the surface. Minirhizotrons provide good estimates of root density, whereas rhizotrons can supply information on root architecture and root length density. Applying the fluorescent protein technique will not introduce any new limitations to the already established rhizotron methods, in that the plants will still express the same genes and the background conditions in the contrast between roots and substrate will remain the same. If we would like to take only one measurement at the end of a growing season, for example, it would be more difficult to clearly identify separate root taxa because mainly the root tips and meristematic zones are stable and highly fluorescent at this time point. In this case it would be more useful to cut the shoots and inject dye into the roots for simple identification at the end of an experiment (Cahill et al., 2010).The main potential of the fluorescent protein technique is to be able to follow the dynamics of root interaction over time. As long as a new root enters the imaging area there is no limitation to distinguishing new roots from the roots already present because they are highly fluorescent in the meristematic zones.
Automation of the imaging process is another essential feature for the realization of more inclusive experiments. For increasing throughput when analysing root–root interactions of many plant species, automated phenotyping systems (de Dorlodot et al., 2005; Iyer-Pascuzzi et al., 2010; Nagel et al., 2012) will bring substantial benefit. Up to certain developmental stages of plants (depending on plant density), root systems of different plants can be distinguished visually. However, as soon as the roots of neighbouring plants start to overlap, a separation of root systems is impeded (Fig. 4). By equipping the camera systems of automated systems with appropriate filters and light-emitting devices to use certain excitation and emission wavelength, root systems of plants expressing different fluorescent proteins can be distinguished. This will enable us to analyse the plasticity of root traits influenced by neighbouring plants and quantify root–root interactions of plants grown in plant communities with the required throughput to address relevant ecological and agronomical questions. There is considerable potential for plant breeders to breed new crop varieties that perform particularly well under increasingly extreme climatic conditions in relation to a climate change. Since we know that more diverse systems are better at buffering themselves as an overall community against disturbances, intercropping may provide more resilient agricultural systems in a changing climate. As such, as plant fertilizers become more expensive with the scarcity of fossil fuels, breeders could focus on breeding varieties that perform particularly well in intercropping and/or changing climatic scenarios.
Fig. 4.

Analysing root–root interactions of plants grown in soil-filled rhizotrons. (A) Representative image of nine barley (Hordeum vulgare ‘Barke’) plants grown for 4 weeks after sowing in rhizotrons filled with black peat soil. The rhizotron was set to an inclination angle of 43° (with the transparent side facing downwards) to force roots to grow along the transparent plate of the rhizotron and make them visible and accessible for cameras (for more details, see Nagel et al., 2012). (B) A higher resolution image showing an area of interest (as indicated in A) with ×2.5 magnification.
Imaging and quantifying root–root interactions between plant individuals using MRI and PET
To analyse root–root interactions not only in 2-D but in 3-D, magnetic resonance imaging (MRI) is a very powerful tool to visualize roots within soil substrates over time. We grew soya (Glycine max), maize (Zea mays) and sainfoin (Onobrychis viciifolia) in soil in tubular pots of 7 cm diameter and 20 cm depth, and combined either two individuals of the same species (intraspecific; Fig. 5A–C), different species (interspecific Fig. 5D–F) or a control individual growing alone (position in the centre or slightly to the side of the pot, equivalent to the positions of the two-individual treatments; see also Rascher et al., 2011). We mapped the development of both root systems through time over 2 weeks using magnetic resonance imaging (MRI) to visualize and quantify the outcome of root–root interactions. Three-dimensional space (3-D) data were translated into two dimensional (2-D) space images by using the axial projection of the tube from above and quantifying the percentage of total roots detectable within three concentric zones. The position of any root was determined as a particular distance from the central point in the tube (looking from above) and then categorized into three concentric zones (denoted 1, 2 and 3 in Fig. 5). This approach provided a quantifiable outcome from the root profiles over time; a quantification of total root mass found in the three concentric zones of the tube, allowing a treatment comparison of effects of intraspecific versus interspecific interactions on root distribution as well as effects of species identity. The full dataset is not included here, but an exemplary comparison of maize growing with maize or with soya is shown in Fig. 5 (for more technical details, see also Rascher et al., 2011). This non-invasive method allows one to follow the dynamic interaction of individual or different root systems with high precision and can also be linked to soil properties. A major breakthrough now would be to be able to separate the root systems of different plants and different species.
MRI is a volumetric (3-D) imaging techniques based on the detection of proton-containing compounds such as water. The interesting point here is that some substances, including some soil types, appear transparent using these techniques, and one can differentiate between water in roots and water in the soil due to different spatial positions of the water (Rascher et al., 2011).
As already discussed, a general problem to deal with in root–root interaction studies is to identify which root belongs to which plant. If roots of, for example, two plants growing in a pot could be continuously measured it would be possible to track individual roots from the beginning and to follow their growth over time. However, measuring replicates causes time gaps that make root tracking very difficult. In combination with MRI, positron emission tomography (PET) can be used to visualize roots of individual plants. Figure 6 shows two maize plants (1 and 2) that were grown in the same pot filled with soil and the roots were consecutively measured (and afterwards coregistered) with MRI (Fig. 6B–D; grey) and PET (coloured). When 11CO2 was administered to the shoots of both plants, roots of the two plants became radioactively labelled by import of recently radiolabelled photoassimilates (Fig. 6B). However, when 11CO2 was applied to the shoot of either plant 1 or 2 only the corresponding root system became radiolabelled (Fig. 6D, C) (Jahnke et al., 2009). This will be used to identify individual roots in mixed root systems and to attribute them to plant individuals in combined MRI–PET studies on below-ground processes. MRI–PET can thus be used, for example, to follow diurnal patterns in carbon allocation to roots or to dissect interactions between roots of individual plant (Jahnke et al., 2009).
Fig. 6.
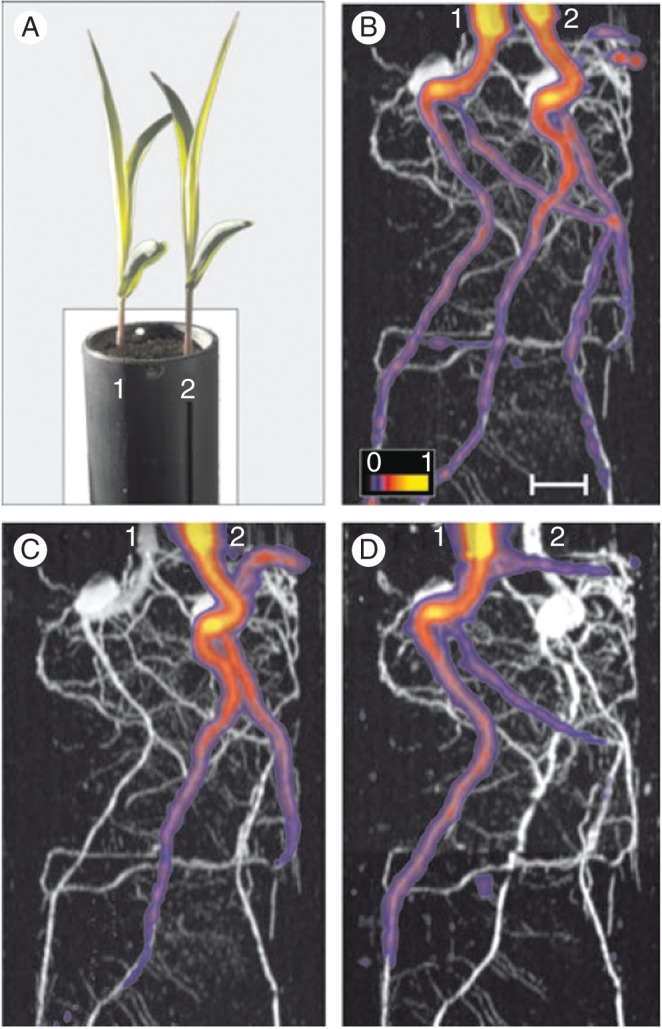
Quantifying root–root interactions between plant individuals using MRI–PET coregistration (after Jahnke et al., 2009). (A) Photograph of two maize plants (1 and 2) growing in a soil-filled pot. (B–D) Co-registered MRI (grey) and PET (colour) root images of the 18-d (B, C) and 19-d-old (D) plants. Radioactive 11CO2 was administered either to the shoots of both plants (B) or only to the shoot of plant 2 (C). The PET and MRI data in (B) and (C) were obtained on the 18-d-old plants and the MRI images are identical. On the following day, plant 1 shoot was radiolabelled and a new MRI image was obtained (D). Scale bar = 1 cm.
Other non-destructive techniques such as X-ray micro-computed tomography (micro-CT) (Gregory et al., 2003; Perret et al., 2007; Tracy et al., 2010; Flavel et al., 2012), which allow the imaging of root growth and root/soil interactions, could significantly contribute to our understanding of the processes involved in root–root interactions.
SOME BIG QUESTIONS THAT REMAIN, AND HOW CERTAIN TECHNIQUES AND MORE INCLUSIVE APPROACHES MAY HELP US TO ANSWER SOME OF THEM MORE EFFECTIVELY
Despite a vast literature on net effects of competitive interactions among plants, most studies have focused on two or maximum three species interactions in pots, whereas research at field scale has until very recently been restricted to being able to assess root length density or root productivity for the whole plant community below ground (see methods in the section about separating roots by species or functional groups using fluorescent proteins). In addition the net outcome of interactions has often been solely attributed to competitive interactions, when it is clear that facilitation, especially when N2-fixers are involved, can play a key role too. In particular, when scaling up from population studies to plant communities or the field, we need to be able to assess the importance of competitive or facilitative interactions for community structure and function, even if the intensity of the interaction between species may be strong.
The current fast development of methods to non-invasively and dynamically study root–root interactions as well as to screen a larger number of different species should allow us to now make some major steps forwards in ecology and agronomy. Using new methodological but also more inclusive theoretical and conceptual approaches as outlined in this paper, we suggest that we now have a better chance of answering the following key questions related to root–root interactions.
Key questions and issues to which research on root–root interactions can contribute
What overall picture of root–root interactions will start to emerge as we study more species, both natural and crop? Can we combine both types of research into one conceptual framework or do we need separate frameworks (related to an order of magnitude difference in availability of nutrients for crops versus natural species for example)?
Can we develop an interaction framework to explain why certain interactions lead to repulsion, some to neutral reactions and some to attraction between roots? What role would positive as well as negative interactions play in this framework?
Are there some conditions where the effects of neighbours are additive to the effects of availability of resources, or are interaction effects of the abiotic and biotic environment usually non-linear (de Kroon, 2007; Mommer et al., 2012)? For example, Mommer et al. (2012) recommend now developing a hierarchy of competitive strength between plants species which could help predict which ones would have more or less than additive effects.
What are the mechanisms contributing to the accumulation of roots in the topsoil commonly found in polycultures? When does this phenomenon occur and when not?
We suggest developing an overall hierarchy of interaction strength, based on both competitive and facilitative strength between species and how the outcome relates to plant performance. If N2-fixing plant species are involved in the interaction, for example, overall nutrient efficiency of the system will increase.
Following on from studies of the intensity of interactions, we need to be aware that intensity is not the same as importance at community level. Evaluating overall effect sizes of different factors such as land management, climate drivers as well as interactions on plant community structure and function will allow us to clarify the difference between intensity and importance of interaction (Brooker et al., 2005).
ACKNOWLEDGEMENTS
We thank Dagmar van Dussschoten, Jonas Bühler, Lea Märtin, Peter Blümler, Hanno Scharr and Uwe Rascher for productive discussions related to quantifying MRI images of roots interacting. We are indebted to Bernd Kastenholz and Ann-Katrin Kleinert for technical assistance during preparation of soil-filled rhizotrons and cultivation of barley plants. We also thank Prof. German Spangenberg for generously providing seeds of the wheat genotype RFP BOBWHITE.
LITERATURE CITED
- Ashton IW, Miller AE, Bowman WD, Suding KN. Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology. 2010;91:3252–3260. doi: 10.1890/09-1849.1. [DOI] [PubMed] [Google Scholar]
- Bessler H, Temperton VM, Roscher C, et al. Aboveground overyielding in grassland mixtures is associated with reduced biomass partitioning to belowground organs. Ecology. 2009;90:1520–1530. doi: 10.1890/08-0867.1. [DOI] [PubMed] [Google Scholar]
- Bezemer TM, Fountain MT, Barea JM, et al. Divergent composition but similar function of soil food webs of individual plants: plant species and community effects. Ecology. 2010;91:3027–3036. doi: 10.1890/09-2198.1. [DOI] [PubMed] [Google Scholar]
- Biedrzycki ML, Jilany TA, Dudley SA, Bais HP. Root exudates mediate kin recognition in plants. Communicative & Integrative Biology. 2010;3:28–35. doi: 10.4161/cib.3.1.10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker R, Kikvidze Z, Pugnaire FI, et al. The importance of importance. Oikos. 2005;109:63–70. [Google Scholar]
- Brooker RW, Maestre FT, Callaway RM, et al. Facilitation in plant communities: the past, the present, and the future. Journal of Ecology. 2008;96:18–34. [Google Scholar]
- Bruno JF, Stachowicz JJ, Bertness MD. Inclusion of facilitation into ecological theory. Trends in Ecology and Evolution. 2003;18:119–125. [Google Scholar]
- Bullock DG. Crop rotation. Critical Reviews in Plant Sciences. 1992;11:309–326. [Google Scholar]
- Bullock JM, Pywell RF, Burke MJW, Walker KJ. Restoration of biodiversity enhances agricultural production. Ecology Letters. 2001;4:185–189. [Google Scholar]
- Cahill JF, Jr, McNickle GG, Haag JJ, Lamb EG, Nyanumba SM, Clair CCS. Plants integrate information about nutrients and neighbors. Science. 2010;328:1657. doi: 10.1126/science.1189736. [DOI] [PubMed] [Google Scholar]
- Callaway RM. The detection of neighbors by plants. Trends in Ecology and Evolution. 2002;17:104–105. [Google Scholar]
- Carminati A, Vetterlein D. Plasticity of rhizosphere hydraulic properties as a key for efficient utilization of scarce resources. Annals of Botany. 2013;112:277–290. doi: 10.1093/aob/mcs262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper BB, Jackson RB. Plant competition underground. Annual Review of Ecology and Systematics. 1997;28:545–570. [Google Scholar]
- Choler P, Michalet R, Callaway RM. Facilitation and competition on gradients in alpine plant communities. Ecology. 2001;82:3295–3308. [Google Scholar]
- Cholick FA, Welsh JR, Cole CV. Rooting patterns of semi dwarf and tall winter wheat cultivars under dryland field conditions. Crop Science. 1977;17:637–639. [Google Scholar]
- Ciszak M, Comparini D, Mazzolai B, et al. Swarming behavior in plant roots. PLoS ONE. 2012;7:e29759. doi: 10.1371/journal.pone.0029759. http://dx.doi.org/10.1371/journal.pone.0029759 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell JH, Slatyer RO. Mechanisms of succession in natural communities and their role in community stability and organization. American Naturalist. 1977;111:1119–1144. [Google Scholar]
- Dimitrakopoulos PG, Schmid B. Biodiversity effects increase linearly with biotope space. Ecology Letters. 2004;7:574–583. [Google Scholar]
- de Dorlodot S, Bertin P, Baret P, Draye X. Scaling up quantitative phenotyping of root system architecture using a combination of aeroponics and image analysis. Aspects of Applied Biology. 2005;73:41–54. [Google Scholar]
- Dudley SA, File AL. Yes, kin recognition in plants! Biology Letters. 2008;4:69–70. [Google Scholar]
- Eshel A, Beeckman T. Plant roots: the hidden half. New York, NY: CRC Press; 2012. [Google Scholar]
- Faget M, Herrera JM, Stamp P, Aulinger-Leipner I, Frossard E, Liedgens M. The use of green fluorescent protein as a tool to identify roots in mixed plant stands. Functional Plant Biology. 2009;36:930–937. doi: 10.1071/FP09125. [DOI] [PubMed] [Google Scholar]
- Faget M, Liedgens M, Stamp P, Fluetsch P, Herrera JM. A minirhizotron imaging system to identify roots expressing the green fluorescent protein. Computers and Electronics in Agriculture. 2010;74:163–167. [Google Scholar]
- Faget M, Liedgens M, Feil B, Stamp P, Herrera JM. Root growth of maize in an Italian ryegrass living mulch studied with a non-destructive method. European Journal of Agronomy. 2012;36:1–8. [Google Scholar]
- Flavel RJ, Guppy CN, Tighe M, Watt M, McNeill A, Young IM. Non-destructive quantification of cereal roots in soil using high-resolution X-ray tomography. Journal of Experimental Botany. 2012;63:2503–2511. doi: 10.1093/jxb/err421. [DOI] [PubMed] [Google Scholar]
- Fullner K, Temperton VM, Rascher U, et al. Vertical gradient in soil temperature stimulates development and increases biomass accumulation in barley. Plant, Cell & Environment. 2012;35:884–892. doi: 10.1111/j.1365-3040.2011.02460.x. [DOI] [PubMed] [Google Scholar]
- Gastine A, Scherer-Lorenzen M, Leadley PW. No consistent effects of plant diversity on root biomass, soil biota and soil abiotic conditions in temperate grassland communities. Applied Soil Ecology. 2003;24:101–111. [Google Scholar]
- Gersani M, Brown JS, O'Brien EE, Maina GM, Abramsky Z. Tragedy of the commons as a result of root competition. Journal of Ecology. 2001;89:660–669. [Google Scholar]
- Glover JD, Culman SW, DuPont ST, et al. Harvested perennial grasslands provide ecological benchmarks for agricultural sustainability. Agriculture, Ecosystems & Environment. 2010;137:3–12. [Google Scholar]
- Grace JB, Tilman D. Perspectives on plant competition. San Diego, CA: Academic Press; 1990. [DOI] [PubMed] [Google Scholar]
- Gregory PJ. Plant roots. Oxford: Blackwell Publishing; 2007. [Google Scholar]
- Gregory PJ, Hutchison DJ, Read DB, Jenneson PM, Gilboy WB, Morton EJ. Non-invasive imaging of roots with high resolution X-ray micro-tomography. Plant and Soil. 2003;255:351–359. [Google Scholar]
- Gregory PJ, Bengough AG, Grinev D, et al. Root phenomics of crops: opportunities and challenges. Functional Plant Biology. 2009;36:922–929. doi: 10.1071/FP09150. [DOI] [PubMed] [Google Scholar]
- Grime JP. Vegetation classification by reference to strategies. Nature. 1974;250:26–31. [Google Scholar]
- Harper JL. Population biology of plants. New York, NY: Academic Press; 1977. [Google Scholar]
- Herrera JM, Feil B, Stamp P, Liedgens M. Root growth and nitrate-nitrogen leaching of catch crops following spring wheat. Journal of Environmental Quality. 2010;39:845–854. doi: 10.2134/jeq2009.0306. [DOI] [PubMed] [Google Scholar]
- Hodge A. Plant nitrogen capture from organic matter as affected by spatial dispersion, interspecific competition and mycorrhizal colonization. New Phytologist. 2003;157:303–314. doi: 10.1046/j.1469-8137.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Høgh-Jensen H, Myaka F, Kamalongo D, Rasmussen J, Ngwira A. Effect of environment on multi-element grain composition of pigeonpea cultivars under farmers' conditions. Plant and Soil. 2006;285:81–96. [Google Scholar]
- Hooper DU, Chapin FS, Ewel JJ, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- Huber E, Wanek W, Gottfried M, et al. Shift in soil–plant nitrogen dynamics of an alpine–nival ecotone. Plant and Soil. 2007;301:65–76. [Google Scholar]
- Hutchinson GE. Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology. 1957;22:415–427. [Google Scholar]
- Iyer-Pascuzzi AS, Symonova O, Mileyko Y, et al. Imaging and analysis platform for automatic phenotyping and trait ranking of plant root systems. Plant Physiology. 2010;152:1148–1157. doi: 10.1104/pp.109.150748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnke S, Menzel MI, van Dusschoten D, et al. Combined MRI-PET dissects dynamic changes in plant structures and functions. The Plant Journal. 2009;59:634–644. doi: 10.1111/j.1365-313X.2009.03888.x. [DOI] [PubMed] [Google Scholar]
- Karban R, Shiojiri K. Self-recognition affects plant communication and defense. Ecology Letters. 2009;12:502–506. doi: 10.1111/j.1461-0248.2009.01313.x. [DOI] [PubMed] [Google Scholar]
- Keddy PA. eLS. Chichester: Wiley & Sons; 2006. Competition. http://dx.doi.org/10.1038/npg.els.0003162 . [Google Scholar]
- Kell DB. Breeding crop plants with deep roots: their role in sustainable carbon, nutrient and water sequestration. Annals of Botany. 2011;108:407–418. doi: 10.1093/aob/mcr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen HL, Thorup-Kristensen K. Root growth and nitrate uptake of three different catch crops in deep soil layers. Soil Science Society of America Journal. 2004;68:529–537. [Google Scholar]
- de Kroon H. Ecology: how do roots interact? Science. 2007;318:1562–1563. doi: 10.1126/science.1150726. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Hendriks M, van Ruijven J, et al. Root responses to nutrients and soil biota: drivers of species coexistence and ecosystem productivity. Journal of Ecology. 2012;100:6–15. [Google Scholar]
- Lehmann J, Peter I, Steglich C, Gebauer G, Huwe B, Zech W. Below-ground interactions in dryland agroforestry. Forest Ecology and Management. 1998;111:157–169. [Google Scholar]
- Lynch JP. Roots of the second green revolution. Turner Review. Australian Journal of Botany. 2007;55:493–512. [Google Scholar]
- Maddonni GA, Otegui MaE, Andrieu B, Chelle M, Casal JJ. Maize leaves turn away from neighbors. Plant Physiology. 2002;130:1181–1189. doi: 10.1104/pp.009738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquard E, Weigelt A, Temperton VM, et al. Plant species richness and functional composition drive overyielding in a six-year grassland experiment. Ecology. 2009;90:3290–3302. doi: 10.1890/09-0069.1. [DOI] [PubMed] [Google Scholar]
- Martin RC, Greyson PR, Gordon R. Competition between corn and a living mulch. Canadian Journal of Plant Science. 1999;79:579–586. [Google Scholar]
- Mommer L, van Ruijven J, De Caluwe H, et al. Unveiling below-ground species abundance in a biodiversity experiment: a test of vertical niche differentiation among grassland species. Journal of Ecology. 2010;98:1117–1127. [Google Scholar]
- Mommer L, van Ruijven J, Jansen C, van de Steeg HM, de Kroon H. Interactive effects of nutrient heterogeneity and competition: implications for root foraging theory? Functional Ecology. 2012;26:66–73. [Google Scholar]
- Mwangi PN, Schmitz M, Scherber C, et al. Niche pre-emption increases with species richness in experimental plant communities. Journal of Ecology. 2007;95:65–78. [Google Scholar]
- Nagel KA, Putz A, Gilmer F, et al. GROWSCREEN-Rhizo is a novel phenotyping robot enabling simultaneous measurements of root and shoot growth for plants grown in soil-filled rhizotrons. Functional Plant Biology. 2012;39:891–904. doi: 10.1071/FP12023. [DOI] [PubMed] [Google Scholar]
- Nakamoto T. The distribution of wheat and maize roots as influenced by biopores in a subsoil of the Kanto loam type. Plant Production Science. 2000;3:140–144. [Google Scholar]
- Naumann A, Heine G, Rauber R. Efficient discrimination of oat and pea roots by cluster analysis of Fourier transform infrared (FTIR) spectra. Field Crops Research. 2010;119:78–84. [Google Scholar]
- Neumann A, Schmidtke K, Rauber R. Effects of crop density and tillage system on grain yield and N uptake from soil and atmosphere of sole and intercropped pea and oat. Field Crops Research. 2007;100:285–293. [Google Scholar]
- Newman EI. Competition and diversity in herbaceous vegetation. Nature. 1973;244:310. [Google Scholar]
- Noulas C, Liedgens M, Stamp P, Alexiou I, Herrera JM. Subsoil root growth of field grown spring wheat genotypes (Triticum aestivum L.) differing in nitrogen use efficiency parameters. Journal of Plant Nutrition. 2010;33:1887–1903. [Google Scholar]
- Okubara PA, Steber CM, DeMacon VL, Walter NL, Paulitz TC, Kidwell KK. Scarlet-Rz1, an EMS-generated hexaploid wheat with tolerance to the soilborne necrotrophic pathogens Rhizoctonia solani AG-8 and R. oryzae. Theoretical and Applied Genetics. 2009;119:293–303. doi: 10.1007/s00122-009-1038-x. [DOI] [PubMed] [Google Scholar]
- Palta JA, Finery IRP, Rebetzke GJ. Restricted-tillering wheat does not lead to greater investment in roots and early nitrogen uptake. Field Crops Research. 2007;104:52–59. [Google Scholar]
- Palta JA, Chen X, Milroy SP, Rebetzke GJ, Dreccer MF, Watt M. Large root systems: are they useful in adapting wheat to dry environments? Functional Plant Biology. 2011;38:347–354. doi: 10.1071/FP11031. [DOI] [PubMed] [Google Scholar]
- Perret JS, Al-Belushi ME, Deadman M. Non-destructive visualization and quantification of roots using computed tomography. Soil Biology and Biochemistry. 2007;39:391–399. [Google Scholar]
- Ploey JD. Erosional systems and perspectives for erosion control in European loess areas. Soil Technology Series. 1989;1:93–102. [Google Scholar]
- Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist. 2012;193:30–50. doi: 10.1111/j.1469-8137.2011.03952.x. [DOI] [PubMed] [Google Scholar]
- Postma JA, Lynch JP. Complementarity in root architecture for nutrient uptake in ancient maize/bean and maize/bean/squash polycultures. Annals of Botany. 2012;110:521–534. doi: 10.1093/aob/mcs082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin R, Stamp P, Richner W. Impact of tillage and banded starter fertilizer on maize root growth in the top 25 centimeters of the soil. Agronomy Journal. 2005;97:674–683. [Google Scholar]
- Rascher U, Blossfeld S, Fiorani F, et al. Non-invasive approaches for phenotyping of enhanced performance traits in bean. Functional Plant Biology. 2011;38:968–983. doi: 10.1071/FP11164. [DOI] [PubMed] [Google Scholar]
- Reich PB, Tilman D, Naeem S, et al. Species and functional group diversity independently influence biomass accumulation and its response to CO2 and N. Proceedings of the National Academy of Sciences of the USA. 2004;101:10101–10106. doi: 10.1073/pnas.0306602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewald B, Meinen C, Trockenbrodt M, Ephrath JE, Rachmilevitch S. Root taxa identification in plant mixtures: current techniques and future challenges. Plant and Soil. 2012;359:165–182. [Google Scholar]
- Ricklefs RE, Miller GL. Ecology. New York: W.H. Freeman; 1999. [Google Scholar]
- Robinson D. The responses of plants to nonuniform supplies of nutrients. New Phytologist. 1994;127:635–674. doi: 10.1111/j.1469-8137.1994.tb02969.x. [DOI] [PubMed] [Google Scholar]
- Roscher C, Thein S, Weigelt A, Temperton V, Buchmann N, Schulze E-D. N2 fixation and performance of 12 legume species in a 6-year grassland biodiversity experiment. Plant and Soil. 2011;341:333–348. [Google Scholar]
- van Ruijven J, Berendse F. Long-term persistence of a positive plant diversity–productivity relationship in the absence of legumes. Oikos. 2009;118:101–106. [Google Scholar]
- Scheu S. Plants and generalist predators as links between the below-ground and above-ground system. Basic and Applied Ecology. 2001;2:3–13. [Google Scholar]
- Schroth G, Zech W. Root length dynamics in agroforestry with Gliricidia sepium as compared to sole cropping in the semi-deciduous rainforest zone of West Africa. Plant and Soil. 1995;170:297–306. [Google Scholar]
- Schurr U, Walter A, Rascher U. Functional dynamics of plant growth and photosynthesis – from steady-state to dynamics – from homogeneity to heterogeneity. Plant, Cell & Environment. 2006;29:340–352. doi: 10.1111/j.1365-3040.2005.01490.x. [DOI] [PubMed] [Google Scholar]
- Spehn EM, Joshi J, Schmid B, Alphei J, Körner C. Plant diversity effects on soil heterotrophic activity in experimental grassland ecosystems. Plant and Soil. 2000;224:217–230. [Google Scholar]
- Spehn EM, Scherer-Lorenzen M, Schmid B, et al. The role of legumes as a component of biodiversity in a cross-European study of grassland biomass nitrogen. Oikos. 2002;98:205–218. [Google Scholar]
- Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N. Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia. 2007;151:190–205. doi: 10.1007/s00442-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- Toky OP, Bisht RP. Observations on the rooting patterns of some agroforestry trees in an arid region of north-western India. Agroforestry Systems. 1992;18:245–263. [Google Scholar]
- Tracy SR, Roberts JA, Black CR, McNeill A, Davidson R, Mooney SJ. The X-factor: visualizing undisturbed root architecture in soils using X-ray computed tomography. Journal of Experimental Botany. 2010;61:311–313. doi: 10.1093/jxb/erp386. [DOI] [PubMed] [Google Scholar]
- Valiente-Banuet A, Rumebe AV, Verdu M, Callaway RM. Modern Quaternary plant lineages promote diversity through facilitation of ancient Tertiary lineages. Proceedings of the National Academy of Sciences of the USA. 2006;103:16812–16817. doi: 10.1073/pnas.0604933103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Silk WK, Schurr U. Environmental effects on spatial and temporal patterns of leaf and root growth. Annual Review of Plant Biology. 2009;60:279–304. doi: 10.1146/annurev.arplant.59.032607.092819. [DOI] [PubMed] [Google Scholar]
- Weiner J, Griepentrog H-W, Kristensen L. Suppression of weeds by spring wheat Triticum aestivum increases with crop density and spatial uniformity. Journal of Applied Ecology. 2001;38:784–790. [Google Scholar]