Abstract
Background and Aims
Species of Cactaceae are well adapted to arid habitats. Determinate growth of the primary root, which involves early and complete root apical meristem (RAM) exhaustion and differentiation of cells at the root tip, has been reported for some Cactoideae species as a root adaptation to aridity. In this study, the primary root growth patterns of Cactaceae taxa from diverse habitats are classified as being determinate or indeterminate, and the molecular mechanisms underlying RAM maintenance in Cactaceae are explored. Genes that were induced in the primary root of Stenocereus gummosus before RAM exhaustion are identified.
Methods
Primary root growth was analysed in Cactaceae seedlings cultivated in vertically oriented Petri dishes. Differentially expressed transcripts were identified after reverse northern blots of clones from a suppression subtractive hybridization cDNA library.
Key Results
All species analysed from six tribes of the Cactoideae subfamily that inhabit arid and semi-arid regions exhibited determinate primary root growth. However, species from the Hylocereeae tribe, which inhabit mesic regions, exhibited mostly indeterminate primary root growth. Preliminary results suggest that seedlings of members of the Opuntioideae subfamily have mostly determinate primary root growth, whereas those of the Maihuenioideae and Pereskioideae subfamilies have mostly indeterminate primary root growth. Seven selected transcripts encoding homologues of heat stress transcription factor B4, histone deacetylase, fibrillarin, phosphoethanolamine methyltransferase, cytochrome P450 and gibberellin-regulated protein were upregulated in S. gummosus root tips during the initial growth phase.
Conclusions
Primary root growth in Cactoideae species matches their environment. The data imply that determinate growth of the primary root became fixed after separation of the Cactiodeae/Opuntioideae and Maihuenioideae/Pereskioideae lineages, and that the genetic regulation of RAM maintenance and its loss in Cactaceae is orchestrated by genes involved in the regulation of gene expression, signalling, and redox and hormonal responses.
Keywords: Root development, determinate growth of primary root, Cactaceae, Cactoideae, Opuntioideae, Maihuenioideae, Pereskioideae, adaptation to aridity, differential gene expression
INTRODUCTION
The Cactaceae family constitutes a large group of highly specialized species well adapted to arid habitats. A number of adaptations of the shoot (Britton and Rose, 1919; Mauseth, 1999, 2006) and root (Dubrovsky and North, 2002; Dubrovsky and Shishkova, 2013) system enable many Cactaceae to survive and flourish in water-limited environments. As the plant shoot is easier to study than the root, our understanding of shoot adaptations of Cactaceae to aridity is more advanced than that of root adaptations. However, the shallow root systems of Cactaceae are known to enable the rapid uptake of scarce precipitation. Furthermore, lateral root primordia have been reported to initiate and develop on the roots of Ferocactus acanthodes and Opuntia ficus-indica during drought, but only to emerge following rainfall (North et al., 1993; Dubrovsky et al., 1998b). Another adaptation is determinate growth of the primary root (Dubrovsky, 1997a). Whereas the determinate growth of lateral and adventitious roots has been described for several taxa (Boke, 1979; Varney and McCully, 1991; Shishkova et al., 2008), that of the primary root, observed soon after germination, represents a unique phenomenon in angiosperms, as the primary root is capable of indeterminate growth for extended periods in most species. The differentiating epidermal cells form root hairs that cover the entire root tip, and these serve as a hallmark of determinate root growth. Previously, we described determinate primary root growth in some Sonoran Desert Cactaceae. The primary root of Stenocereus gummosus, S. thurberi, S. pruinosus, S. standlei and Fecocactus peninsulae grows for only 2–3 d (Dubrovsky, 1997a, b, 1999), whereas that of Pachycereus pringlei grows for 8–10 d (Dubrovsky and Gomez-Lomelí, 2003). During this time, only a few cell division cycles occur in the root apical meristem (RAM). Thereafter, the RAM is exhausted and all cells at the root tip differentiate (Dubrovsky et al., 1998a). No post-embryonic quiescent centre (QC) was established in S. gummosus, and limited QC activity was observed in P. pringlei (Rodríguez- Rodríguez et al., 2003). These observations link RAM organization and activity with determinate growth of the primary root in the studied Cactaceae. Furthermore, we have shown that programmed cell death is not involved in RAM exhaustion (Shishkova and Dubrovsky, 2005).
Meristem exhaustion induces the initiation of lateral roots, which in S. gummosus, S. thurberi and F. peninsulae start to emerge 3–5 after germination. Such rapid formation of a compact root system provides seedlings with a selective advantage under desert conditions (Dubrovsky 1997a, b). Roots regenerated from S. gummosus and F. peninsulae calli also exhibit determinate growth, suggesting that this developmental programme is maintained in different root types of Cactaceae (Shishkova et al., 2007).
The Cactaceae family includes approx. 1500–1800 species (Anderson, 2001; Hunt et al., 2006) of perennial succulent plants widely distributed in North and South America, with only one species, Rhipsalis baccifera, occurring in the Asian and African tropics (Barthlott, 1983). Since the 19th century, three subfamilies of Cactaceae, i.e. Cactoideae, Opuntioideae and Pereskioideae, were widely recognized (Anderson, 2001). However, the International Cactaceae Systematics Group currently recognizes four subfamilies, namely Cactoideae, Opuntioideae, Maihuenioideae and Pereskioideae (Hunt et al., 2006). All Cactaceae have specialized short shoots, or areoles, which produce spines; the areoles of Opuntioideae, in addition to, or instead of, spines, develop short hair-like prickles named glochids (Hunt and Taylor, 2002).
Cactoideae and Opuntioideae are stem succulents with a photosynthetic stem cortex. Cactoideae, the largest Cactaceae subfamily, are divided into seven tribes and around 110 genera with major species diversity (Hunt et al., 2006). Cactoideae can be found from Canada to Southern Patagonia in distinct habitats ranging from arid and semi-arid regions to tropical rainforests (Anderson, 2001). All Cactoideae are leafless and perform crassulacean acid metabolism (CAM) photosynthesis only in a succulent stem (Nobel and Hartsock, 1986).
The Opuntioideae subfamily consists of 15–18 genera belonging to five tribes (Majure et al., 2012) and inhabits arid, semi-arid and mesic environments from Canada to almost the southern tip of South America. Some Opuntioideae species have prominent leaves, which are responsible for the majority of CO2 uptake by daytime C3 photosynthesis (Nobel and Hartsock, 1986), while the leaves of most species are small and ephemeral. The stems and leaves of Opuntioideae can also perform CAM photosynthesis as the plant matures or in response to environmental changes (Nobel and Hartsock, 1986).
The Maihuenia genus, which has only two species and was originally classified as a member of the subfamily Pereskioideae, was placed in a separate subfamily, Maihuenioideae, based on its morphology and molecular phylogenetic data (Wallace, 1995a, b; Edwards et al., 2005; Hunt et al., 2006; Metzing and Kiesling, 2008). Maihuenia species, which are restricted to cool arid and semi-arid regions of Argentina and Chile, are caespitose shrubs with short succulent stems, small persistent terete leaves and fleshy taproots (Leuenberger, 1997; Anderson, 2001; Nyffeler et al., 2008). Their primary photosynthetic type is C3, but limited CAM cycling is thought to exist in both the leaves and stems (Nobel and Hartsock, 1986; Mauseth, 1999; Nyffeler et al., 2008).
In contrast to members of the Opuntioideae, Cactoideae and Maihuenioideae subfamilies, those of Pereskioideae grow in relatively mesic habitats, and are not able to store large amounts of water in the stem, leaf and/or root tissues (Mauseth, 1999). This subfamily contains only one paraphyletic genus Pereskia, which includes 17 species (Butterworth and Wallace, 2005). Although photosynthetic studies have mostly been carried out on greenhouse-grown plants, Pereskia species are thought to perform primarily C3 photosynthesis in the broad persistent leaves, but also to possess weak CAM-cycling abilities to reassimilate respired CO2 (Rayder and Ting, 1981; Nobel and Hartsock, 1986; Edwards and Díaz, 2006). Edwards and Donoghue (2006) have also reported evidence for inducible full CAM photosynthesis in species from both paraphyletic Pereskia clades from North and South America. It is widely accepted that Pereskia members possess primitive or ancestral features of the Cactaceae family (Leuenberger, 1986; Anderson, 2001).
Here, we surveyed the incidence of determinate growth of the primary root in species from the four Cactaceae subfamilies, with an emphasis on the Cactoideae subfamily. To obtain insight into the molecular mechanisms involved in RAM exhaustion in Cactaceae, we also identified some Cactaceae genes induced early in primary root development and repressed upon RAM exhaustion. We report that all members of six tribes of the largest Cactoideae subfamily (i.e. Cacteae, Cereeae, Trichocereeae, Notocacteae, Pachycereeae and Rhipsalideae) analysed in this study exhibit determinate growth of the primary root, while it was not an obligate characteristic for the two species of the seventh tribe, Hylocereeae. This finding suggests that, within this subfamily, the type of primary root growth depends on the degree of water deficit experienced by the seedlings. Our preliminary results for species from another three subfamilies, Opuntioideae, Pereskioideae and Maihuenioideae, show that, although the primary root growth pattern is more variable in species of these subfamilies, seedlings of Opuntioideae exhibit mostly determinate growth of the primary root, while those of Pereskioideae and Maihuenioideae exhibit mostly indeterminate growth of the primary root. Our data suggest that determinate growth of the primary root evolved after separation of the Cactoideae and Opuntioideae lineages from those of the Pereskioideae and Maihuenioideae.
MATERIALS AND METHODS
Plant materials, growth conditions and microscopy
Twenty-five species of Cactoideae (Table 1), one species of Maihuenioideae, three species of Pereskioideae and six species of Opuntioideae were examined in this study (Table 2). For most species, seeds were bulked from plants in natural populations and, for a few species, from cultivated plants (Supplementary Data Table S1). Details of the material studied and voucher specimens are included in Tables 1 and 2, and Supplementary Data Table S1. Seeds of Cactoideae, Maihuenioideae and Pereskioideae were pre-sterilized for 10 min in 70 % ethanol, sterilized for 25 or 40 min in 60 % commercial bleach supplemented with ·08 % Triton X-100 and washed four times for 10 min each in sterile distilled water. For the Opuntioideae, seeds were subjected to an additional initial treatment with concentrated sulfuric acid for 90 min. Sterilized seeds were germinated in Petri dishes containing ·2× LS (Linsmaier and Skoog) medium [contains salts formulated by Murashige and Skoog and vitamins formulated by Linsmaier and Skoog (1965); Phyto Technology Laboratories, Lenexa, KS, USA] buffered to pH 5·7 and solidified with ·8 % bacto-agar (BD DIFCO, Franklin Lakes, NJ, USA). Plants were cultivated in vertically oriented Petri dishes maintained at 28 °C, with a 12 h light/dark photoperiod, and 80 µmol m−2 s−1 light intensity. Root growth along the medium surface was recorded. Roots that either were submerged in the medium or had a root tip that did not make contact with the medium surface were disregarded. Petri dishes with seedlings were scanned using an Epson Perfection 4490 scanner with 1200 ppi resolution or photodocumented using either a Leica V-Lux1 digital camera or a stereoscope Olympus S2X7 (Olympus, Tokyo, Japan) equipped with an Infinity 1 digital camera. Roots were cleared using the method of Malamy and Benfey (1997), with minor modifications (Dubrovsky et al., 2006), and observed and photographed under an Axiovert 200M microscope (Zeiss, Oberkochen, Germany) equipped with Nomarski optics.
Table 1.
Primary root growth pattern and habitat of the Cactoideae species studied
| Tribe | Species | Root growth pattern | Habitat |
|---|---|---|---|
| Cacteae | Aztekium ritteri (Boed.) Boed.1,4 | D | A/SA |
| Mammillaria carnea Zucc. ex Pfeiff.1,4 | D | SA | |
| Stenocactus multicostatus (Hildm.) A. Berger ex A.W. Hill1,4 | D | SA | |
| S. crispatus (DC.) A. Berger ex A.W. Hill1,3 | D | SA | |
| Ferocactus latispinus (Haw.) Britton and Rose1,3 | D | SA | |
| F. peninsulae (F.A.C. Weber) Britton and Rose5,7 | D | A/SA | |
| Cereeae | Cereus aethiops Haw.2,6 | D | SA |
| C. haenkeanus F.A.C. Weber ex K. Schum.1,2 | D | SA | |
| Melocactus matanzanus León1,4 | D | SA | |
| Trichocereeae | Acanthocalycium spiniflorum (K. Schum.) Backeb.4,6 | D | SA |
| Cleistocactus smaragdiflorus (F.A.C. Weber) Britton4,6 | D | SA | |
| Denmoza rhodacantha (Salm-Dyck) Britton and Rose1,4 | D | SA | |
| Gymnocalycium gibbosum (Haw.) Pfeiff. ex Mittler1,2 | D | SA | |
| Echinopsis leucantha (Gillies ex Salm-Dyck) Walp.4,6 | D | SA | |
| Lobivia formosa (Pfeiff.) Dodds1,3 | D | SA | |
| Trichocereus angelesiae R. Kiesling1,4 | D | SA | |
| T. candicans (K. Schum.) Britton & Rose1,4 | D | SA | |
| Notocacteae | Pyrrhocactus bulbocalyx (Werderm.) Backeb.1,3,6 | D | SA |
| P. catamarcensis (Speg.) F. Ritter4,6 | D | A | |
| Parodia ayopayana Cárdenas1,4 | D | SA | |
| P. submammulosa (Lem.) R. Kiesling4,6 | D | SA | |
| Notocactus haselbergii A. Berger1,3 | D | SA | |
| Pachyceeae | Carnegiea gigantea (Engelm.) Britton and Rose1,2 | D | A |
| Echinocereus cinerascens Lem.1,3 | D | SA | |
| E. grusonii Von Zeisold1,4 | D | SA | |
| Lemaireocereus hollianus (F.A.C. Weber) Britton and Rose1,3 | D | SA | |
| Pachycereus pringlei (S. Watson) Britton and Rose5,9 | D | A | |
| P. schottii (Engelm.) Hunt1,3 | D | A | |
| Stenocereus gummosus (Engelm.) A. Gibson and K.E. Horak.5,7 | D | A | |
| S. pruinosus (Otto ex Pfeiff.) Buxb.3,8 | D | A/SA | |
| S. thurberi, (Engelm.) Buxb.4,8 | D | A | |
| S. standleyi (J. G. Ortega) Buxb.3,8 | D | A/SA | |
| Hylocereae | Epiphyllum phyllanthus (L.) Haw.1,4 | I | M |
| Hylocereus sp.1,3 | I/D | M | |
| Rhipsalideae | Rhipsalis cruciformis (Vell.) A. Cast.1,4 | D | M* |
| R. baccifera (J.S. Muell.) Stearn1,3 | D | M* | |
| R. floccosa Salm-Dyck ex Pfeiff.1,4 | D | M* | |
| R. lumbricoides (Lem.) Lem. ex Salm-Dyck2,6 | D | M* | |
| Pfeiffera ianthothele (Monv.) F.A.C. Weber1,2 | D | M* |
I, indeterminate primary root growth; D, determinate primary root growth. A, arid; SA, semi-arid; M, mesic; M*, obligate epiphyte from mesic habitat.
1 Species analysed in this study. 2 Fewer than 10 seedlings examined. 3 From ten to 29 seedlings examined. 4 From 30 to 100 seedlings examined. 5 Hundreds to thousands of seedlings examined. 6 Species germinated in Petri dishes on filter paper (M. L. Las Peñas, unpubl. res.). 7 Dubrovsky (1997b). 8 Dubrovsky (1999). 9 Dubrovsky and Gomez-Lomeli (2003).
Table 2.
Primary root growth pattern and habitat of species from three Cactaceae subfamilies (preliminary data)
| Subfamily | Species | Root growth pattern | Habitat |
|---|---|---|---|
| Opuntioideae | Opuntia puberula Pfeiff. | D/I | SA |
| O. streptacantha Lem. | D/I | SA | |
| O. xoconostle syn. O. matudae Scheinvar | D/I | SA | |
| O. tehuacana S.Arias & U.Guzmán | D/I | SA | |
| Maihueniopsis darwinii (Hensl.) F. Ritter | D/I | A | |
| M. boliviana (Salm-Dyck) R. Kiesling | D/I | A | |
| Maihuenioideae | Maihuenia patagonica (Phil.) Britton & Rose | I/(D?) | A |
| Pereskioideae | Pereskia aculeata Mill. | I | M |
| P. bahiensis Gürke | I | M | |
| P. grandifolia Haw. | I | M |
I, indeterminate primary root growth; D, determinate primary root growth. A, arid; SA, semi-arid; M, mesic.
Phylogenetic tree
A phylogenetic tree was constructed using the sequence data available in GenBank for the trnL-trnF, trnK-matK and rpl16 regions (Hernández-Hernández et al., 2011). CLC Genomics Workbench 5·5 was used for alignments, joining alignments, and tree construction and manipulation. The sequences were aligned individually for each locus, the free ends were removed and the alignments were joined into a single matrix. A maximum likelihood (ML) phylogenetic tree (Felsenstein, 1981) was constructed using the Maximum Likelihood Phylogeny tool, Jukes–Cantor substitution model and the Neighbor–Joining algorithm (Saitou and Nei, 1987). The root was set above the Pereskia aculeata node.
Preparation and analysis of a subtracted cDNA library
Tips (1 mm) of the S. gummosus primary root were cut with a razor blade, collected in liquid nitrogen and stored at –75 °C. The first sample contained approx. 2000 primary root tips in the initial phase of development, when the RAM was still present. The second sample contained approx. 3000 primary root tips in the terminal growth phase, when the RAM was already exhausted. Total RNA and poly(A)+ mRNA were extracted from both samples using TRIzol reagent (Invitrogen) and the ‘Dynabeads® mRNA Purification Kit’ (Dynal-Invitrogen), respectively. Both extractions were performed according to the manufacturer's instructions. All steps of the suppression subtractive hybridization (SSH) were performed according to the instructions in the PCR-Select cDNA Subtraction Kit manual (Clontech). Forward subtraction was performed using initial phase cDNA as a tester and terminal phase cDNA as a driver; reverse subtraction was performed using terminal phase cDNA as a tester and initial phase cDNA as a driver. Secondary PCR products were cloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA). Differentially expressed cDNA clones induced during the initial stage of primary root development were selected using the Differential Screening Kit (Clontech) according to the manufacturer's instructions, with a minor modification: digoxigenin (DIG)-labelled probes were used instead of [α-32P]dCTP-labelled probes. DIG labelling and detection were performed using a PCR DIG Probe Synthesis Kit and DIG Nucleic Acid Detection Kit (Roche), respectively, according to the manufacturer's instructions. Pixel density of clones hybridized with forward and reverse subtraction libraries were measured with ImageJ (National Institutes of Health; http://rsbweb.nih.gov/ij/). Selected clones that showed the highest differential expression were sequenced, annotated by BLASTX and BLASTP (http://www.ncbi.nlm.nih.gov/) using the Ref-Seq protein database and deposited in GenBank under accession numbers KC415065–KC415071.
Quantitative RT–PCR analysis
Stenocereus gummosus root tips in the initial or terminal growth phases were collected and total RNA was isolated as described above. Quantitative (real-time) reverse transcription–PCR (RT–PCR) was performed in an iQ5 Multicolor Real-time PCR Detection System (Bio-Rad; Hercules, CA, USA). Reactions were set up using a one-step RT-PCR Kit with SYBR® Green (Bio-Rad) according to the manufacturer's instructions; 100 ng of DNase-treated RNA was used for each reaction, except for the negative control. Stenocereus gummosus homologues of the arabidopsis genes At4G24550 (clathrin adaptor complex subunit) and At5G65050 (MADS-box protein AGL31), described as stably expressed genes in Czechowski et al. (2005), were used for transcript normalization. The relative expression values normalized with two reference genes were calculated according to Vandesompele et al. (2002). Primers used and some methodological details are described in Supplementary Data Table S2.
RESULTS
Determinate growth of the primary root occurred in all studied members of six Cactoideae tribes
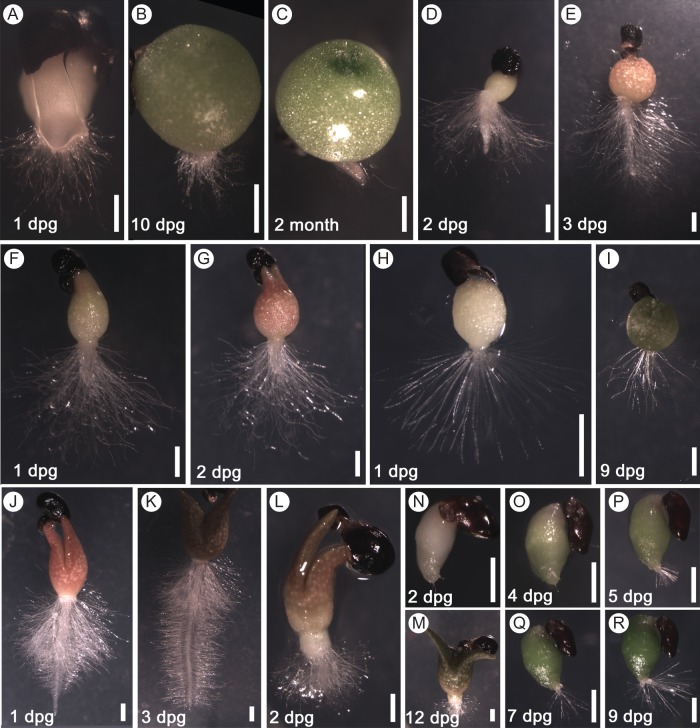
The type of primary root growth was analysed in 25 species belonging to the seven tribes of Cactoideae. Five species from the Cacteae tribe, Aztekium ritteri, Ferocactus latispinus, Mammillaria carnea, Stenocactus crispatus and S. multicostatus, all exhibited determinate primary root growth (Table 1). In different Cacteae species, the primary root growth lasted from 1 to 2 d or from 3 to 5 d. For example, the very short primary root of most S. crispatus seedlings stopped growing within the first day post-germination (dpg) and in some seedlings by 2 dpg. By this time, all primary root cells had differentiated and the root tip was covered in long root hairs (Fig. 1A). A seedling of S. crispatus at 1 dpg is shown in Fig. 1A. This specimen was viewed from the bottom of the Petri dish, as its very short primary root was hidden by abundant long root hairs when photographed from above. For approx. 2 weeks, no new roots developed and only the shoot grew (Fig. 1B). Later, lateral roots started to develop, but they also remained extremely short in most S. crispatus plants during the first few months of growth (Fig. 1C). On the other hand, a few S. crispatus seedlings had longer primary and lateral roots (Supplementary Data Fig. S1A).
Fig. 1.
Determinate primary root growth in seedlings of the Cactoideae subfamily. (A–C) Stenocactus crispatus, Cacteae tribe; (D, E) Melocactus matanzanus, Cereeae tribe, (F, G) Trichocereus candicans, Trichocereeae tribe; (H, I) Parodia ayopayana, Notocacteae tribe; (J–M) two types of seedlings of Pachycereus schottii, Pachycereeae tribe: a seedling of the first type (J, K) exhibited a longer primary root and of the second type (L, M) had a very short primary root; (N–R) Rhipsalis floccosa, Rhipsalideae tribe. (A, F, H, L) Primary root growth of S. crispatus, T. candicans, P. ayopayana and the second type of P. schottii seedlings, respectively, was terminated by 1 dpg. (B, G, I, M) The shoot of S. crispatus, T. candicans, P. ayopayana and the second type of P. schottii seedling, respectively, continued to develop after primary root growth ceased. (C) The same S. crispatus plant as in (A) and (B) developed a couple of short lateral roots with determinate growth by the age of 2 months. (D, J) The growing primary root of M. matanzanus at 2 dpg and of the first type of P. schottii seedling at 1 dpg. (E, K) Primary root growth of M. matanzanus and of the first type of P. schottii seedlings stopped by 3 dpg. (N, O) The primary root of the Rhipsalis floccosa seedling essentially did not develop; the same seedling is shown. (P–R) The first and second very short lateral roots developed by 5 and 9 dpg, respectively, and both exhibited a determinate growth pattern. Scale bars = 1 mm.
In the Cereus haenkeanus and Melocactus matanzanus species of the Cereeae tribe, primary root growth continued for 2–4 d. The primary roots of M. matanzanus seedlings grew for 1–2 d and the root tip was not covered in root hairs (Fig. 1D). Primary root growth stopped by 3 dpg or sometimes 4 dpg (Fig. 1E). After RAM exhaustion, the primary root was covered in long root hairs and its final length varied from 2 to 5 mm in different seedlings (Fig. 1E; Supplementary Data Fig. S1B). In M. matanzanus seedlings, the first lateral root emerged within about a week after RAM exhaustion, on either a distal, median or proximal part of the primary root (Supplementary Data Fig. S1C).
In all five species analysed from the Trichocereeae tribe (i.e. Denmoza rhodacantha, Gymnocalycium gibbosum, Lobivia formosa, Trichocereus angelesiae and T. candicans), the time to RAM exhaustion was very short and the primary root stopped growing by 1 dpg or sometimes 2 dpg, reaching a length of 1–4 mm. A seedling of T. candicans with long hairs covering the whole primary root is shown at 1 and 2 dpg (Fig. 1F and G, respectively). Similarly to M. matanzanus, the first lateral root emerged during the first week after germination (Supplementary Data Fig. S1D).
The primary root of seedlings from three species of the Notocacteae tribe (Notocactus haselbergii, Parodia ayopayana and P. submmamulosa) also only grew for a very short period of time and reached <1 to 2 mm in length. The primary root of P. ayopayana seedlings stopped growing and was totally covered in long root hairs by 1 dpg (Fig. 1H). Furthermore, it remained the only root of the seedling for a couple of weeks (Fig. 1I). By 2–4 weeks after germination, short lateral roots had emerged (Supplementary Data Fig. S1E).
The highest variation in both the duration of primary root growth time and primary root length was found in species of the Pachycereeae tribe, including Carnegiea gigantea, Echinocereus cinerascens, E. grusonii and Pachycereus schottii (syn. Lophocereus schottii), which were analysed in this study, as well as Pachycereus pringlei and Stenocereus species that were analysed previously. The duration of primary root growth varied from 2–3 d in some species to 8–10 d in others. Moreover, we noticed that for two species, C. gigantea and P. schottii, seedlings from the same seed lots possessed different primary root phenotypes. The primary root of the first group of seedlings grew for 3–6 d and reached a length of 10–20 mm (Fig. 1J, K), while that of the second group reached only a few millimetres in length and grew for no longer than 1 d (Fig. 1L, M). Despite having very short roots for 10–14 dpg, the shoots in the second group of seedlings developed similarly to those of seedlings with longer primary roots (Fig. 1M). After 10–14 dpg, a single lateral root developed in the seedlings of the second group, which grew for a similar period of time and reached a similar final length to that of the primary root of the seedlings from the first group (Supplementary Data Fig. S1F, G). Unfortunately, the seed germination percentage was very low for both species, and we were not able to perform a quantitative analysis of this phenomenon.
In the species examined from the Rhipsalideae tribe (i.e. Pfeiffera ianthothele, Rhipsalis cruciformis, R. baccifera and R. floccosa), the primary root grew for 1 d only and was very short. Figure 1N and O show the same R. floccosa seedling at 2 and 4 dpg, respectively, with a progressively developing shoot and poorly developed primary root. The first very short lateral root of this seedling that emerged and terminated growth within 1 d was developed by 5 dpg (Fig. 1P). At 7 dpg, a second lateral root emerged (Fig. 1Q), which also terminated growth rapidly, attaining a very short length, and developed root hairs almost ten times longer than the root itself (Fig. 1R).
In conclusion, all species analysed from the six tribes mentioned above had determinate primary root growth that could last between 1 and 10 d, depending on the species, producing a final root length ranging from <1 mm to ∼60 mm. Lateral roots also exhibited determinate growth.
Most Hylocereeae seedlings exhibit indeterminate primary root growth
Only two species from the Hylocereeae tribe were analysed: Epiphyllum phyllanthus and cultivated Hylocereus sp. The primary root of E. phyllanthus seedlings clearly had indeterminate growth: in all seedlings that developed well (25 out of 33 analysed), the root continued to grow slowly for 4 weeks after germination, reaching the base of the 12 cm Petri dish (Fig. 2A–C). Remarkably, some lateral roots of the E. phylanthus seedlings exhibited determinate root growth (Supplementary Data Fig. S1H). Eight germinated seeds stopped growth soon after germination and failed to develop into normal seedlings (Supplementary Data Fig. S1I). The primary root growth of another member of Hylocereeae, a cultivated Hylocereus species with red pulp, which could be a hybrid, exhibited the same tendency, but was more variable. Among the 14 seedlings analysed, the primary roots of nine seedlings grew slowly for >3 weeks, those of three seedlings stopped growth by 2 weeks after germination, and those of another two did not develop. Few lateral and adventitious roots exhibited determinate root growth. These observations indicate that at least in these two species of this tribe the primary root growth pattern is mostly indeterminate.
Fig. 2.
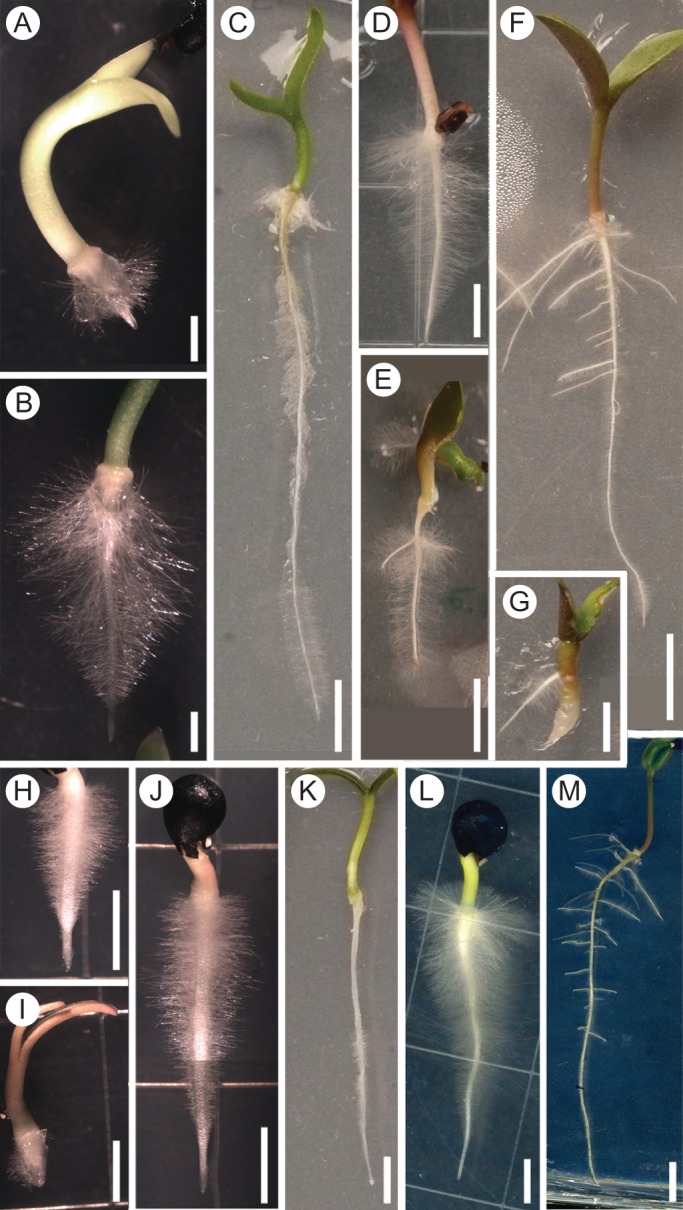
Primary root growth in seedlings of the Hylocereeae tribe (Cactoideae), and Opuntioideae, Maihuenioideae and Pereskioideae subfamilies. (A–C) The primary roots of all developed seedlings of Epiphyllum phyllantus, which belongs to the Hylocereeae tribe from the Cactoideae family, exhibited indeterminate root growth. (D–G) The primary roots of Opuntia puberula, Opuntioideae, seedlings exhibited determinate growth (E), indeterminate growth (D, F) or failed to develop at all (G). The primary root of most Maihuenia patagonica, Maihuenioideae, seedlings (H–K) and of all Pereskia aculeata, Pereskioideae (L, M) seedlings exhibited indeterminate growth. (I) Some Maihuenia patagonica seedlings exhibited determinate primary root growth. (A) Two dpg; (B) same seedling as in (A), 4 dpg; (C) 20 dpg; (D) 3 dpg; (E) 10 dpg; (F) 10 dpg; (G) 10 dpg; (H) 1 dpg; (I) 2 dpg; (J) same seedling as in (H), 2 dpg; (K) 20 dpg; (L) 3 dpg; (M) 14 dpg. Scale bars: (A, B) = 2 mm, (D, G–J, L) = 5 mm, and (C, E, F, K, M) = 10 mm.
Most seedlings of Opuntioideae exhibit determinate primary root growth
During our analysis of the primary root growth pattern in the Opuntioideae subfamily, two problems were encountered: a very low germination percentage and a high contamination of seedlings by fungi and bacteria. Treatment with concentrated sulfuric acid prior to seed sterilization alleviated these problems only for Opuntia puberula and O. streptacantha. Primary root growth was examined for a few seedlings from each of four other species: Opuntia tehuacana, O. xoconostle, Maihueniopsis boliviana and M. darwinii. The growth pattern and particularly the primary root length in these species were variable. Most seedlings of O. puberula and O. streptacantha exhibited determinate primary root growth (Table 3), although the processes of RAM exhaustion and root tip cell differentiation took variable amounts of time, from 2 to 12 d, and the primary roots varied greatly in final length (Fig. 2E–G). Moreover, the primary roots of a few plants continued to grow for 2–3 weeks, reaching the base of vertically oriented 12 cm square Petri dishes. The primary roots of some seedlings either did not develop or only reached a limited length of 2–7 mm (Fig. 2G, Table 3). Seedlings of both species readily developed lateral and adventitious roots of variable length, which also exhibited determinate growth (Supplementary Data Fig. S1J).
Table 3.
Primary root growth pattern of two Opuntioideae species
| Determinate PR growth |
PR did not grow or grew only briefly |
Indeterminate PR growth |
PR inside the testa | ||||
|---|---|---|---|---|---|---|---|
| Species | No. of plants | PR length | No. of plants | PR length | No. of plants | PR length | No. of plants |
| O. puberula | 50 | 15–35 mm | 14 | 1–7 mm | 1 | >70 mm | 27 |
| O. streptacantha | 12 | 11–45 mm | 8 | Approx. 2 mm | 3 | >70 mm | – |
PR, primary root.
We examined two germinated seeds of O. tehuacana. One seed gave rise to a seedling that exhibited determinate growth of the primary root, which reached 17 mm in length. The other seed, which was polyembryonic, produced two viable seedlings, neither of which formed primary roots. The primary roots of two O. xoconostle seedlings grew poorly, both reaching just 2 mm in length. For another species, M. darwinii, we were only able to monitor the development of one primary root, and the RAM of this root was exhausted by 9 dpg. The 14 remaining M. darwinii seedlings examined either became contaminated or their primary root tip failed to make contact with the medium. Of the two M. boliviana seedlings studied, one showed signs of contamination by 6 dpg, and the primary root of the other lost contact with the medium by 4 dpg. During the period in which these seedlings were studied, their primary roots grew and did not show signs of cell differentiation in the root tip.
Thus, in most seedlings examined in species from the Opuntioideae, the primary root growth was determinate; however, this trait may be variable and more studies are needed to confirm this conclusion.
Most seedlings of Maihuenioideae and all seedlings of Pereskioideae exhibit indeterminate primary root growth
For the Maihuenioideae subfamily, the primary root growth of several seedlings from two populations of Maihuenia patagonica (one population with a diploid cytotype and the other with a tetraploid cytotype) was analysed. Of 15 seedlings belonging to the tetraploid population, the primary roots of 12 continued to grow until they reached the base of the 12 cm Petri dish, usually by 2–3 weeks after germination (Fig. 2H, J, K). Thus, most seedlings from this population exhibit indeterminate growth of the primary root, at least during the period of study. Some of these plants formed lateral roots while others did not during the period of observation. The primary roots of another three seedlings from the same population stopped growth very soon after germination, reaching only a few millimetres in length, and became completely covered in root hairs (Fig. 2I). In the first 2 weeks after germination, the shoots of these seedlings developed similarly to those of plants with indeterminate growth of the primary root (Supplementary Data Fig. S1L). One of these plants developed only one short lateral root by 10 dpg, which stopped growing after reaching a length of 4 mm, while several larger lateral roots of different length were developed by another plant (Supplementary Data Fig. S1K, M). Of the three seedlings examined, only the seedling with long lateral roots survived by 3 weeks after germination.
Finally, three species of Pereskioideae from South America were analysed in this study: Pereskia aculeata, P. bahiensis and P. grandifolia. The primary root of most plants of these species was analysed only for the first 10 dpg, at which time the primary root of all seedlings was still growing and the RAM was still present (Fig. 2L, M).
We have reported previously that the presence of root hairs on the root tip indicates differentiation of all tissues, and the absence of root hairs on the root tip indicates the presence of meristematic and/or elongation zones (Dubrovsky, 1997b). To illustrate this, images of the cleared primary roots of one species of each Cactaceae subfamily are shown in Fig. 3. The tip of a growing primary root is shown in Fig. 3A, C, E and F for T. angelesiae (Cactoideae), O. streptacantha (Opuntioideae), M. patagonica (Maihuenioideae) and P. aculeata (Pereskioideae), respectively. Figure 3B and D shows the primary root tips of T. angelesiae and O. streptacantha after RAM exhaustion.
Fig. 3.
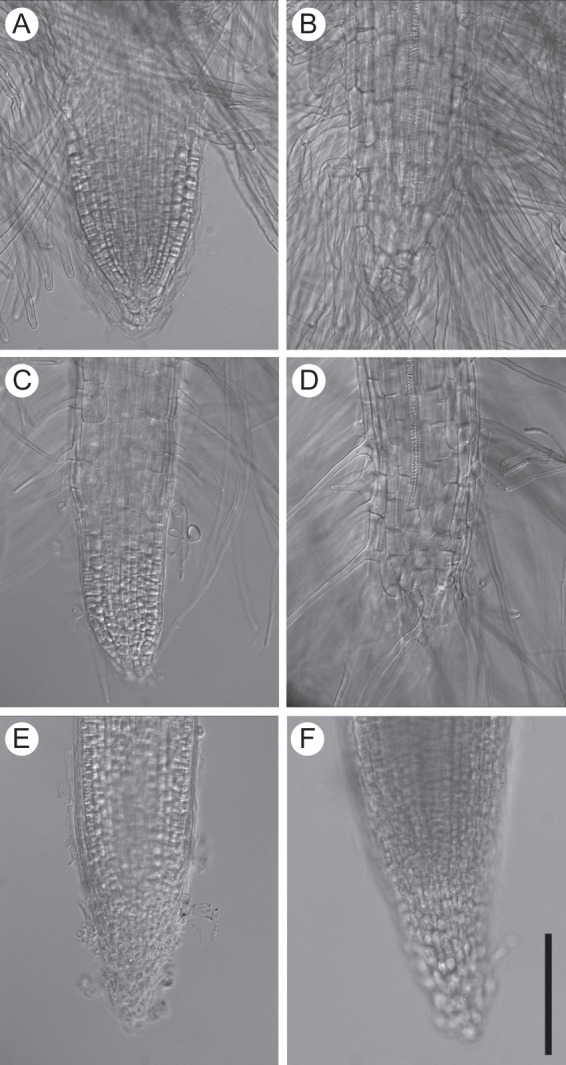
Cleared primary root tips of Cactaceae species from the four subfamilies. (A, B) Trichocereus angelesiae, Cactoideae; (C, D) Opuntia streptacantha, Opuntioideae; (E) Maihuenia patagonica, Maihuenioideae; and (F) Pereskia aculeata, Pereskioideae. The approximate age of the seedlings at the time of the root excision was (A) <1 dpg; (B, C) 2 dpg; and (D–F) 10 dpg. (A, C, E, F) Growing roots; (B, D) roots that terminated growth. Scale bar = 100 µm.
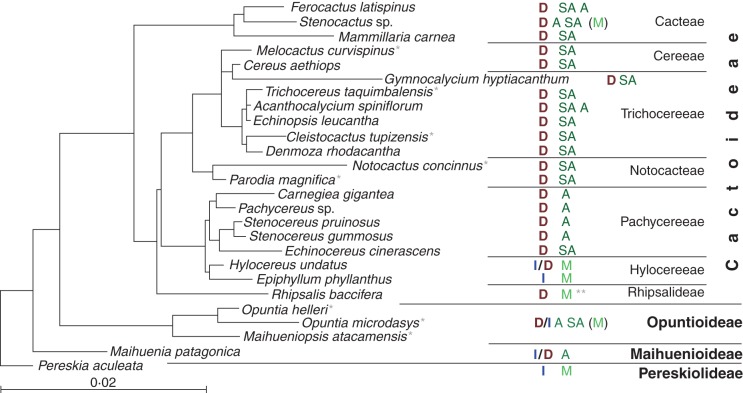
Our data of the primary root growth of seedlings from the Opuntioideae, Maihuenioideae and Pereskioideae subfamilies are preliminary; more detailed data will be presented elsewhere. However, these preliminary data allow us to suggest that the determinate growth of the primary root is a genetic trait developed as an adaptation to aridity (Fig. 4). To illustrate this notion, a phylogenetic tree was constructed based on the nucleotide sequences of three molecular markers, chloroplastic trnL-trnF and rpl6, and mitochondrial trnK-matK, available in GenBank. If the sequence of one or more of the markers was not available for the species examined, we used the three sequences of another species from the same genus, and such species are marked with an asterisk in Fig. 4. Within the Cactoideae and Opuntioideae lineages, the primary root growth pattern generally corresponded to growth in a particular habitat, i.e. determinate growth of the primary root was strongly correlated with arid and semi-arid environments. However, this situation is apparently different for Maihuenioideae and Pereskioideae, which seem to have separated from the first two subfamilies early in evolution (Fig. 4; Edwards et al., 2005; Bárcenas et al., 2011; Hernández-Hernández et al., 2011). Although members of the Maihuenioideae inhabit arid environments and those of the Pereskioideae more mesic environments, our data suggest that most seedlings of species from both of these subfamilies exhibit an indeterminate growth of the primary root.
Fig. 4.
Phylogenetic tree of some of the species analysed in this study, showing the relationship between the primary root growth pattern and plant habitat in different taxa. Cactaceae species with publicly available sequences for the three molecular markers, trnL-trnF, trnK-matK and rpl16, were used for the tree construction. When the three sequences were available only for another species of the same genus, the species was marked with one asterisk. For the Cactiodeae species used in this study, root growth pattern (D, determinate primary root growth; I, indeterminate primary root growth) corresponds to a particular habitat (A, arid; SA, semi-arid; M, mesic). The mesic habitat of obligate epiphytes from the Rhipsalis genus that experience water deficit is marked with two asterisks. For Opuntiodeae, preliminary data on the root growth pattern and habitat are summarized for all shown species of the subfamily.
Isolation of cDNA fragments of genes differentially expressed during primary root development
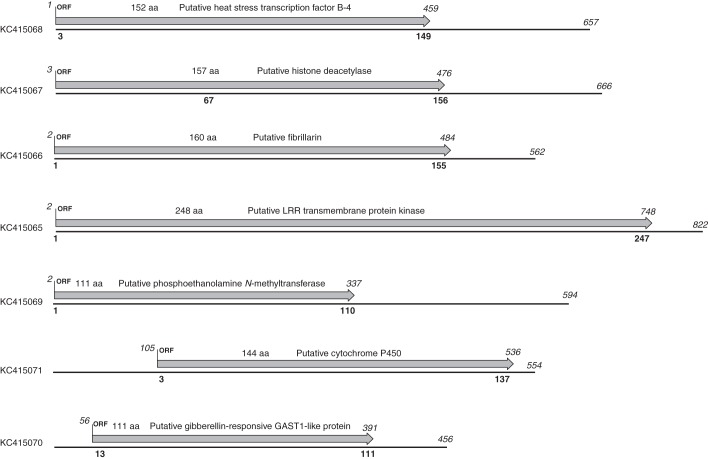
To identify and characterize genes involved in root meristem maintenance and determinate root growth, we constructed a SSH library of cDNA expressed in the primary root tips of S. gummosus during the initial root growth phase, when the RAM was still present. cDNA from the primary root tips in the terminal stage of development, when the RAM was already exhausted, was used for subtraction and analysis of differential gene expression. Differential screening of 384 clones from the library revealed the efficiency of subtraction to be approx. 20 %. Insertions from ten clones that showed the largest difference in hybridization signal were selected and sequenced. Putative function was assigned to seven of the corresponding genes (Fig. 5; Supplementary Data Table S3), whereas conserved domains were not found in the translated sequences of the remaining three. The length of the open reading frame and corresponding deduced protein sequence, as well as the extent of BLASTP homology are shown for each of seven cDNA fragments in Fig. 5. The parameters of the first and second informative BLASTP hits for each of the cDNAs are presented in Supplementary Data Table S3.
Fig. 5.
Open reading frames and putative functions assigned to the seven cDNA fragments upregulated in the primary root tip of S. gummosus during the initial growth phase. Numbers in italics above the arrow and the solid line refer to the nucleotide sequence, indicating the length of the fragment and the position of the open reading frame (ORF). Numbers in bold below the solid line refer to the deduced amino acid sequence, and indicate the start and end of the best BLASTP hit.
One of these genes, which is up-regulated in the primary root tip during the initial growth phase, encodes a homologue of the heat stress transcription factor B4 (HsfB4). The mutant phenotype of the At1g46264/NP_175142 arabidopsis homologue of this gene was described as schizoriza (scz; Mylona et al., 2002; Pernas et al., 2010; ten Hove et al., 2010). Another gene upregulated during the initial growth phase is a homologue of the plant-specific class 2 histone deacetylase (HD). It was reported that the AtHD2A, AtHD2B and AtHD2C homologues are involved in the repression of gene transcription (Wu et al., 2003). One more gene is an rRNA 2'-O-methyltransferase, fibrillarin 2 homologue. In animals and yeasts, multisubunit complexes containing fibrillarin are involved in numerous aspects of RNA biogenesis (Nicol et al., 2000). A putative leucine-rich repeat receptor-like protein kinase (LRR-RLK) is also upregulated during the initial growth phase. One of the arabidopsis LRR-RLKs, ERECTA, is involved in the regulation of plant development and responses to environmental stimuli (reviewed in van Zanten et al., 2009); erecta mutants of arabidopsis exhibit a compact shoot phenotype (Shpak et al., 2003). Another upregulated gene is a homologue of the phosphoethanolamine N-methyltransferase (PEAMT), or S-adenosyl-l-methionine:phosphoethanolamine N-methyltransferase. This enzyme is involved in the synthesis of phosphocholine, a precursor of phosphatidylcholine (Munnik et al., 1998; Bolognese and McGraw, 2000; Nuccio et al., 2000). Phosphatidylcholine is important either as a membrane structural component or in cellular signalling.
A conserved domain of the cytochrome P450 CYPX superfamily was found in the translated sequence of another cDNA induced in the primary root during the initial growth phase. Although the degree of identity and similarity between the deduced amino acid sequences of S. gummosus and proteins of other plant species is not very high (Supplementary Data Table S3), the closest protein homologues belong to the CYP718 family and to B and A subfamilies of the CYP716 family. The last transcript induced during the initial growth stage is a homologue of the plant-specific gibberellin-regulated GAST1-like genes, which in arabidopsis belong to the GASA (gibberellic acid-stimulated arabidopsis) family. These genes encode small secreted proteins with a conserved cysteine-rich domain and are involved in the hormone response, plant development and defence (Roxrud et al., 2007). The transcript upregulated in S. gummosus showed the highest degree of homology with GASA6/GASA4 members.
Upregulation of all but the CYP450 genes in the primary root tip of S. gummosus during the initial growth phase was confirmed by quantitative (real-time) RT–PCR (Fig. 6). The high and variable CT values (>30) obtained for CYP450 suggest that this gene was expressed at very low levels; therefore, the expression values calculated for this gene were not reliable.
Fig. 6.
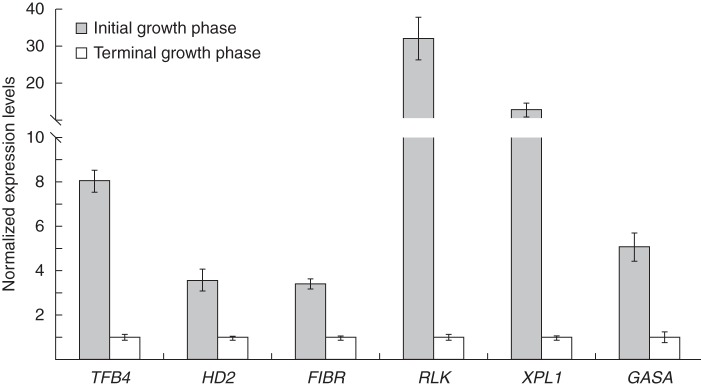
Quantitative (real-time) RT–PCR analysis of the expression of the Stenocereus gummosus genes induced in the initial growth phase. Relative expression was evaluated for RNA isolated from S. gummosus primary root tips during the initial or terminal growth phases. Transcript accumulation was normalized to the expression of two reference genes, SgCla and SgAGL31. A value of 1 was assigned to the expression during the terminal phase. Bars represent means ± s.e.m. from two independent biological replicates with three technical replicates.
DISCUSSION
Determinate primary root growth is conserved in six Cactoideae tribes that inhabit mostly arid and semi-arid regions
Determinate growth of the primary root results in the rapid formation of the seedling root system and hence the rapid accumulation of shoot biomass. A greater shoot biomass provides an advantage for survival in drought conditions; for example, the survival rate of young seedlings of a Sonoran Desert species, S. thurberi, depends directly on plant biomass attained at the onset of drought (Dubrovsky, 1996). This developmental adaptation can be important for plant species grown in areas with limited rainfall. Therefore, we hypothesized that Cactaceae species inhabiting arid and semi-arid environments would exhibit determinate growth of the primary root, while species inhabiting mesic environments would exhibit indeterminate growth of the primary root. This hypothesis held for the species of the Cactiodeae and Opuntioideae subfamilies, but not for Maihuenioideae and Pereskioideae. Indeed, all Cactoideae from six tribes, Cacteae, Cereeae, Trichocereeae, Notocacteae, Pachycereeae and Rhipsalideae, examined either in this study or in our previous work (Table 1), exhibit determinate primary root growth (Fig. 1). Species from the first five tribes are distributed in arid and semi-arid regions, while many Rhipsalideae are epiphytic, with root systems that experience water deficit because of exposure to air. Root development in this interesting group has not been sufficiently studied, particularly in its native habitat, and awaits further investigation. In species from the five tribes of Cactoideae that inhabit arid and semi-arid zones, the timing of RAM exhaustion and root tip cell differentiation varies, but is usually completed by 10 dpg. The timing of RAM exhaustion appears to be a characteristic of a tribe and species. In contrast, E. phyllanthus from the Hylocereeae tribe, which tends to inhabit mesic environments, exhibited indeterminate root growth.
Opuntioideae species exhibit more variability in primary root growth
The results for the Opuntioideae subfamily, as well as for the Maihuenioideae and Pereskioideae subfamilies, can be considered as only preliminary, as we were only able to analyse a sufficient number of seedlings from two species, and gather some additional records for a few seedlings from four other species. Certainly, further analysis of primary root growth should be performed in more Opuntioideae species from different tribes to establish if the primary root growth pattern is conserved within this subfamily. Nevertheless, our data suggest that the determinacy of primary root growth is not as firmly genetically fixed in members of Opuntioideae as it is in most Cactoideae species. We have documented variation in primary root growth pattern within the same species of Opuntioideae; primary roots sometimes grew for extended periods, but, more frequently, the RAM was exhausted and primary root tip cells differentiated within a few days. In some seedlings, primary root growth was not induced at all, or the primary root grew for a very short period of time, reaching a final length of only a few millimetres, probably because the cells underwent expansion, but not division. In these seedlings, adventitious and/or lateral roots developed very soon after germination. Before sterilization and germination, seeds of Opuntioideae species were separated with a scalpel from the pulp to reduce fungal and bacterial contamination, and treated with sulfuric acid. Only a 90 min treatment with concentrated sulfuric acid, but not a 30 or 60 min treatment, resulted in seed germination of some of the species. The development of embryonic organs in the seedling could be affected by these severe treatments; either the mechanical seed peeling or the sulfuric acid treatment could account for the incomplete cotyledons found in some seedlings (Supplementary Data Fig. S1) or for the early arrest of primary root growth.
In Cactoideae species from six tribes, the timing of RAM exhaustion and root cell differentiation, as well as the final length of the primary root, varied slightly within the same species. In this case, most variation was found between species that belonged to different tribes. Nevertheless, the situation was different in Opuntioideae species. While primary roots of a few seedlings of O. puberula and O. streptacantha grew for long periods of time, indicating indeterminate growth, the RAM was exhausted much earlier in most seedlings, or the primary root did not develop at all. The same was true for the lateral and adventitious roots; a few grew for long periods and reached an appreciable length, while most stopped growing at 5–50 mm. Also, as described before (Boke et al., 1979), clusters of very short spur roots were formed. The species we analysed from the Opuntia genus inhabit semi-arid environments, where water accessibility tends to be variable. It would be interesting to determine if the primary root growth characteristics of Opuntioideae species distributed in arid zones varied to a similar extent as those in semi-arid zones. Although there is no apparent difference in the type of primary root growth found in Cactoideae members inhabiting arid and semi-arid regions, it is possible that variability in this feature could be lower in Opuntioideae species inhabiting more arid environments.
Indeterminate primary root growth does not depend on the habitat of Maihuenioideae and Pereskioideae species
According to our preliminary results, indeterminate primary root growth was found in three species from the Pereskioideae subfamily. A more complex pattern of primary root growth was found for M. patagonica, one of the two species that constitute the Maihuenioideae subfamily. For the first 2–3 weeks after germination, 12 of 15 seedlings exhibited indeterminate primary root growth, and another three seedlings had very short primary roots with determinate growth. Furthermore, indeterminate root growth seems to benefit M. patagonica seedling establishment, as the shoot of only one of the three seedlings that formed long lateral roots continued to develop for 4 weeks after germination. While Pereskia species mostly inhabit mesic environments, both species of the Maihuenia genus inhabit arid locations. Maihuenia patagonica grows in more arid environments than M. poepigii. Remarkably, a Hylocereus sp. plant that did not develop a primary root and had only a few roots of <1 mm long was able to develop a shoot during the 4 month observation period. These findings suggest that the determinate root growth of the Cactoideae and Opuntiodeae subfamilies arose after the separation of this lineage from the Maihuenioideae and Pereskioideae (Fig. 4). Subsequently, this feature might have been genetically fixed in the Cactoideae tribes that consistently experience water deficit.
Insight into the molecular mechanisms underlying RAM maintenance and exhaustion in Cactaceae
To decipher the molecular mechanisms underlying RAM maintenance and exhaustion and root development in Cactaceae with determinate root growth, we constructed a SSH subtracted library of cDNA fragments of genes induced in the primary root tip of S. gummosus soon after germination, when the RAM is active. The insertions from ten clones that displayed the highest changes in expression level between the initial and terminal phases of primary root growth were sequenced, and putative functions were assigned (Supplementary Data Table S3) for seven of them. Remarkably, some homologues of these S. gummosus genes are induced in the root tip of arabidopsis and/or soybean (Supplementary Data Table S4). The functions of these homologous genes and the corresponding mutant phenotypes are presented in Supplementary Data Table S5. One of these genes encodes the homologue of heat stress transcription factor B4 (also called SCZ in arabidopsis; Mylona et al., 2002). The arabidopsis homologue is expressed in the RAM and is required for QC specification, as well as for the formation of the ground tissue initial cells in the embryonic meristem and their activity in the primary root after germination (Pernas et al., 2010; ten Hove et al., 2010). Our finding suggests that the SCZ pathway, which interacts with the SHR/SCR pathway in arabidopsis, is also important during the initial phase of growth in the Cactaceae primary root.
Histone deacetylase (HD) 2, which is also induced in the primary root tip during the initial growth phase, represseses transcription by removing acetyl groups from the core histones. In arabidopsis, a reduction in the expression of some AtHD genes results in developmental defects (Wu et al., 2000; Tian and Chen, 2001; Xu et al., 2005). The upregulation of HD2 in the primary root tip of S. gummosus during the initial growth phase suggests that the repression of a particular set of genes soon after germination is important for root growth in Cactaceae.
Fibrillarin is one of the major proteins of the nucleolus and, together with RNA and proteins, forms multisubunit complexes. Recently, it was shown that arabidopsis recombinant fibrillarin 2 is able to interact in vitro with different rRNAs, including small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), small interfering RNAs (siRNAs) and viral RNAs (Rakitina et al., 2011). Furthermore, fibrillarin interacts with proteins (Nicol et al., 2000; Kim et al., 2007; Canetta et al., 2008). Therefore, upregulation of fibrillarin in the root tip soon after germination could have a wide spectrum of developmental effects that should be analysed in future studies.
The LRR-RLKs represent one of the largest protein families in plants. Although some of these proteins participate in signalling pathways that regulate plant development, as mentioned before for ERECTA LRR-RLK, the exact roles of many of them are still unclear. Phosphoethanolamine N-methyltransferase (PEAMT) in arabidopsis is encoded by three loci. The mutant phenotype for one of these genes is designated as xipotl1 (xpl1; Cruz-Ramírez et al., 2004). The similarity between the architecture of the xpl1 root and that of Cactaceae with determinate root growth, together with the finding that the homologous gene is upregulated in the S. gummosus primary root during the initial growth phase, suggest that the compounds produced in the phosphocholine/phosphatidylcholine biosynthetic pathways may be involved in the determinate root growth of Cactaceae.
Cytochromes P450 (CYPs) comprise a large superfamily of haemoproteins that catalyse the oxidation of multiple substrates. Several CYP716/718s, most similar to the S. gummosus CYP that is induced during the initial growth phase, are involved in diterpenoid and triterpernoid metabolism (Ro et al., 2005; Fukushima et al., 2011). Since gibberellins are diterpenoid, and brassinosteroids are triterpernoid compounds, the induction of CYP transcript during the initial growth phase may be related to the gibberellin- or brassinosteroid-mediated control of primary root development in Cactaceae. Upregulation during the initial growth phase of the transcript encoding a protein with homology to GAST1-like gibberellin-regulated proteins 4 and 6, antagonistically regulated by gibberellins and brassinosteroids (Bouquin et al., 2001), further reinforces this suggestion. These small GAST1-like peptides, which have highly conserved cysteine-rich domains, are believed to promote or repress gibberellin responses (Rubinovich and Weiss, 2010). The characterization of homologous GAST1-like proteins in different plant species suggests that they have diverse roles in plant development, including the regulation of cell division and cell elongation. It was suggested that these peptides are involved in redox regulation (Wigoda et al., 2006; Rubinovich and Weiss, 2010). The cellular redox status was shown to be important for the regulation of cell proliferation and for RAM organization and root development in several species (reviewed in Jiang and Feldman, 2005; De Tulio et al., 2010). The upregulation of the GAST1-like protein in the S. gummosus root tip indicates the importance of redox regulation in the determinate growth of the primary root of Cactaceae.
Determinate root growth, as an adaptation to aridity, was genetically fixed after the separation of Pereskioideae and Maihuenioideae from Cactoideae and Opuntioideae
Recently, several phylogenetic analyses of Cactaceae have been reported (Nyffeler et al., 2008; Bárcenas et al., 2011; Majure et al., 2012), one of which included representatives from 85 % of Cactaceae genera (Hernández-Hernández et al., 2011). In this report, the matK protein-coding gene, trnL-trnF and trnK-matK intergenic spacers, rpl16 intron plastid markers and the ppc nuclear marker were used for the ML phylogenetic analysis of the family. This study prompted the authors to propose the early divergence of Pereskioideae and Maihuenioideae from Cactoideae and Opuntioideae, and to confirm the paraphyletic origin of the Pereskia genus, as suggested earlier (Nyffeler, 2002; Arias et al., 2003, 2005; Edwards et al., 2005). Our findings suggest that determinate growth of the primary root as an adaptation for aridity originated at the time of or was genetically fixed after this separation. It seems that, in the case of Cactoideae species that exhibit determinate root growth and reside in mesic zones, this type of growth could be explained by specific microenvironmental conditions. For example, plants of the Mammilaria species that occur in seasonal tropical forests are usually found on well-drained, rocky mountain slopes (Anderson, 2001). The Rhipsalideae species from the evergreen tropical rainforests are epiphytes and also experience water deficit due to a lack of soil substrate (Dubrovsky and North, 2002). Therefore, most Cactoideae members occupy a niche in which they might experience water deficit and are never subjected to prolonged water surplus.
In conclusion, our analysis revealed that determinate root growth is more prevalent in Cactaceae than previously reported (Dubrovsky, 1997b; Dubrovsky and North, 2002). Our data suggest that determinate growth of primary roots became fixed after the separation of Cactoideae and Opuntioideae from Pereskioideae and Maihuenioideae. This trait is linked to arid and semi-arid environmental conditions, in which the first two subfamilies are distributed. Importantly, determinate primary root growth has hitherto only been reported in a single angiosperm family. To understand how this developmental programme is controlled, we identified a number of genes differentially expressed in the primary root during the initial growth phase, i.e. before RAM exhaustion, compared with during the terminal growth phase, i.e. after RAM exhaustion, in one Cactoideae species, S. gummosus. Our results suggest that the genetic regulation of RAM maintenance and its loss in Cactaceae seedlings might be orchestrated by RAM patterning genes, and by genes involved in the regulation of gene expression, redox and hormonal control. Further studies will shed light on how these and other factors control RAM activity in plants.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Salvador Arias, José Francisco Jiménez-Bremont, Francisco Molina-Freaner, José F. Martínez-Rodríguez, Clara Tinoco Ojanguren and Vladimir Basiuk for seed donation; Marcela Ramírez-Yarza, Shirley Ainsworth, Juan Manuel Hurtado, Roberto Rodríguez-Bahena, Paul Gaytan, Eugenio Lopez and Natalia Doktor for their excellent technical assistance; Elizabeth Cordoba and Verónica Lira-Ruan for critical reading of the manuscript; and Kathleen Farquharson and Philip White for valuable editorial suggestions. This work was supported by the Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica – Universidad Nacional Autónoma de México [IN204912 to S.S. and IN204312 to J.G.D.] and Consejo Nacional de Ciencia y Technología, Mexico [79736 to S.S. and 127957 to J.G.D.]. A travel research fellowship was awarded by Consejo Nacional de Investigación Científica y Tecnológica, Argentina, to M.L.L.P.
LITERATURE CITED
- Anderson EF. The cactus family. Portland, OR: Timber Press; 2001. [Google Scholar]
- Arias S, Terrazas T, Cameron KM. Phylogenetic analysis of Pachycereus (Cactaceae, Pachycereeae) based on chloroplast and nuclear DNA sequences. Systematic Botany. 2003;28:547–557. [Google Scholar]
- Arias S, Terrazas T, Arreola-Nava HJ, Vázquez-Sánchez M, Cameron KM. Phylogenetic relationships in Peniocereus (Cactaceae) inferred from plastid DNA sequence data. Journal of Plant Research. 2005;118:317–328. doi: 10.1007/s10265-005-0225-3. [DOI] [PubMed] [Google Scholar]
- Bárcenas RT, Yesson C, Hawkins JA. Molecular systematic of the Cactaceae. Cladistics. 2011;27:470–489. doi: 10.1111/j.1096-0031.2011.00350.x. [DOI] [PubMed] [Google Scholar]
- Barthlott W. Biogeography and evolution in Neo-and Paleotropical Rhipsalinae (Cactaceae) In: Kubitzki K, editor. Dispersal and distribution: an international symposium. Sonderband des Naturwissenschaftlichen Vereins in Hamburg. Vol. 7. Paul Parey Verlag: Hamburg/Berlin; 1983. pp. 241–248. [Google Scholar]
- Boke NH. Root glochids and root spurs of Opuntia arenaria (Cactaceae) American Journal of Botany. 1979;69:1085–1092. [Google Scholar]
- Bolognese CP, McGraw P. The isolation and characterization in yeast of a gene for Arabidopsis S-adenosylmethionine:phospho-ethanolamine N-methyltransferase. Plant Physiology. 2000;124:1800–1813. doi: 10.1104/pp.124.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquin T, Meier C, Foster R, Nielsen ME, Mundy J. Control of specific gene expression by gibberellin and brassinosteroid. Plant Physiology. 2001;127:450–458. [PMC free article] [PubMed] [Google Scholar]
- Britton NL, Rose JN. The Cactaceae: descriptions and illustrations of plants of the cactus family. Vol. 1. Washington, DC: Carnegie Institution of Washington, publication 248; 1919. Gibson Brothers Press. [Google Scholar]
- Butterworth CA, Wallace RS. Molecular phylogenetics of the leafy cactus genus Pereskia (Cactaceae) Systematic Botany. 2005;30:800–808. [Google Scholar]
- Canetta E, Kim SH, Kalinina NO, et al. A plant virus movement protein forms ringlike complexes with the major nucleolar protein, fibrillarin, in vitro. Journal of Molecular Biology. 2008;376:932–937. doi: 10.1016/j.jmb.2007.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Ramírez A, López -Bucio J, Ramírez-Pimentel G, et al. The xipotl mutant of Arabidopsis reveals a critical role for phospholipid metabolism in root system development and epidermal cell integrity. The Plant Cell. 2004;16:2020–2034. doi: 10.1105/tpc.103.018648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiology. 2005;139:5–17. doi: 10.1104/pp.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Tullio MC, Jiang K, Feldman LJ. Redox regulation of root apical meristem organization: connecting root development to its environment. Plant Physiology and Biochemistry. 2010;48:328–36. doi: 10.1016/j.plaphy.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG. Seed hydration memory in Sonoran Desert cacti and its ecological implication. American Journal of Botany. 1996;83:624–632. [Google Scholar]
- Dubrovsky JG. Determinate primary root growth in Stenocereus gummosus (Cactaceae), its organization and role in lateral root development. In: Altman A, Waisel Y, editors. Biology of root formation and development. New York: Plenum Publishing Corporation; 1997a. pp. 13–20. [Google Scholar]
- Dubrovsky JG. Determinate primary-root growth in seedlings of Sonoran Desert Cactaceae; its organization, cellular basis, and ecological significance. Planta. 1997b;203:85–92. [Google Scholar]
- Dubrovsky JG. Desarrollo de sistema radicular durante la ontogénesis de plantas del género Stenocereus (Cactaceae) In: Pimienta-Barrios E, editor. El Pitayo en Jalisco y especies afines en México. Guadalajara: Universidad de Guadalajara, Fundación Produce Jalisco AC; 1999. pp. 133–146. [Google Scholar]
- Dubrovsky JG, Gomez-Lomeli LF. Water deficit accelerates determinate developmental program of the primary root and does not affect lateral root initiation in a Sonoran Desert cactus (Pachycereus pringlei, Cactaceae) American Journal of Botany. 2003;90:23–831. doi: 10.3732/ajb.90.6.823. [DOI] [PubMed] [Google Scholar]
- Dubrovsky JG, North GB. Root structure and function. In: Nobel PS, editor. Cacti biology and uses. Berkeley, CA: University of California Press; 2002. pp. 1–56. [Google Scholar]
- Dubrovsky JG, Shishkova S. Developmental adaptations in roots of desert plants with special emphasis on cacti. In: Eshel A, Beeckman T, editors. Plant roots: the hidden half. CRC Press; 2013. [Google Scholar]
- Dubrovsky JG, Contreras-Burciaga L, Ivanov VB. Cell cycle duration in the root meristem of Sonoran Desert Cactaceae as estimated by cell-flow and rate-of-cell production methods. Annals of Botany. 1998a;81:619–624. [Google Scholar]
- Dubrovsky JG, North GB, Nobel PS. Root growth, developmental changes in the apex, and hydraulic conductivity for Opuntia ficus-indica during drought. New Phytologist. 1998b;138:75–82. [Google Scholar]
- Dubrovsky JG, Gambetta GA, Hernández-Barrera A, Shishkova S, González I. Lateral root initiation in Arabidopsis: developmental window, spatial patterning, density, and predictability. Annals of Botany. 2006;97:903–915. doi: 10.1093/aob/mcj604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards EJ, Donoghue MJ. Pereskia and the origin of the cactus life-form. American Naturalist. 2006;167:777–793. doi: 10.1086/504605. [DOI] [PubMed] [Google Scholar]
- Edwards EJ, Nyffeler R, Donoghue MJ. Basal cactus phylogeny: implication of Pereskia (Cactaceae) paraphyly for the transition to the cactus life form. American Journal of Botany. 2005;92:1177–1188. doi: 10.3732/ajb.92.7.1177. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Evolutionary trees from DNA sequences: a maximum likelihood approach. Journal of Molecular Biology. 1981;17:368–376. doi: 10.1007/BF01734359. [DOI] [PubMed] [Google Scholar]
- Fukushima EO, Seki H, Ohyama K, et al. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant and Cell Physiology. 2011;52:2050–2061. doi: 10.1093/pcp/pcr146. [DOI] [PubMed] [Google Scholar]
- ten Hove CA, Willemsen V, de Vries WJ, van Dijken A, Scheres B, Heidstra R. SCHIZORIZA encodes a nuclear factor regulating asymmetry of stem cell divisions in the Arabidopsis root. Current Biology. 2010;20:452–457. doi: 10.1016/j.cub.2010.01.018. [DOI] [PubMed] [Google Scholar]
- Hernández-Hernández T, Hernández HM, De-Nova JA, Puente R, Eguiarte LE, Magallon S. Phylogenetic relationships and evolution of growth form in Cactaceae (Caryophyllales, Eudicotyledoneae) American Journal of Botany. 2011;98:44–61. doi: 10.3732/ajb.1000129. [DOI] [PubMed] [Google Scholar]
- Hunt D, Taylor N. Studies in the Opuntioideae (Cactaceae). Milborne Port, UK: DH Books; 2002. Succulent Plant Research 6. [Google Scholar]
- Hunt D, Taylor N, Charles G. The new cactus lexicon. Milborne Port, UK: DH Books; 2006. [Google Scholar]
- Jiang K, Feldman LJ. Regulation of root apical meristem development. Annual Review of Cell and Developmental Biology. 2005;21:485–509. doi: 10.1146/annurev.cellbio.21.122303.114753. [DOI] [PubMed] [Google Scholar]
- Kim SH, Macfarlane S, Kalinina NO, et al. Interaction of a plant virus-encoded protein with the major nucleolar protein fibrillarin is required for systemic virus infection. Proceedings of the National Academy of Sciences, USA. 2007;104:11115–11120. doi: 10.1073/pnas.0704632104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuenberger BE. Pereskia (Cactaceae) Memoirs of the New York Botanical Garden. 1986;41:1–141. [Google Scholar]
- Leuenberger BE. Maihuenia: monograph of a Patagonian genus of Cactaceae. Botanische Jahrbücher. 1997;119:1–92. [Google Scholar]
- Linsmaier EM, Skoog F. Organic growth factor requirements of tobacco tissue cultures. Physiologia Plantarum. 1965;15:473–497. [Google Scholar]
- Majure LC, Puente R, Griffith MP, Judd WS, Soltis PS, Soltis DE. Phylogeny of Opuntia s.s. (Cactaceae): clade delineation, geographic origins, and reticulate evolution. American Journal of Botany. 2012;99:847–864. doi: 10.3732/ajb.1100375. [DOI] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- Mauseth JD. Anatomical adaptations to xeric conditions in Maihuenia (Cactaceae), a relictual, leaf-bearing cactus. Journal of Plant Research. 1999;112:307–315. [Google Scholar]
- Mauseth JD. Structure–function relationships in highly modified shoots of cactaceae. Annals of Botany. 2006;98:901–926. doi: 10.1093/aob/mcl133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzing D, Kiesling R. The study of cactus evolution: the pre-DNA era. Haseltonia. 2008;14:6–25. [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signaling in plants. Biochimica et Biophysica Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Mylona P, Linstead P, Martienssen R, Dolan L. SCHIZORIZA controls an asymmetric cell division and restricts epidermal identity in the Arabidopsis root. Development. 2002;129:4327–4334. doi: 10.1242/dev.129.18.4327. [DOI] [PubMed] [Google Scholar]
- Nicol SM, Causevic M, Prescott AR, Fuller-Pace FV. The nuclear DEAD box RNA helicase p68 interacts with the nucleolar protein fibrillarin and colocalizes specifically in nascent nucleoli during telophase. Experimental Cell Research. 2000;257:272–278. doi: 10.1006/excr.2000.4886. [DOI] [PubMed] [Google Scholar]
- Nobel PS, Hartsock TL. Leaf and stem CO2 uptake in the three subfamilies of the Cactaceae. Plant Physiology. 1986;80:913–917. doi: 10.1104/pp.80.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North GB, Huang B, Nobel PS. Changes in structure and hydraulic conductivity for root junctions of desert succulents as soil water status varies. Botanica Acta. 1993;106:126–135. [Google Scholar]
- Nuccio ML, Ziemak MJ, Henry SA, Weretilnyk EA, Hanson AD. cDNA cloning of phosphoethanolamine N-methyltransferase from spinach by complementation in Schizosaccharomyces pombe and characterization of the recombinant enzyme. Journal of Biological Chemistry. 2000;275:14095–14101. doi: 10.1074/jbc.275.19.14095. [DOI] [PubMed] [Google Scholar]
- Nyffeler R. Phylogenetic relationships in the cactus family (Cactaceae) based on evidence from trnK/matK and trnL-trnF sequences. American Journal of Botany. 2002;89:312–326. doi: 10.3732/ajb.89.2.312. [DOI] [PubMed] [Google Scholar]
- Nyffeler R, Eggli U, Ogburn M, Edwards E. Variations on a theme: repeated evolution of succulent life forms in the Portulacineae (Caryophyllales) Haseltonia. 2008;14:26–36. [Google Scholar]
- Pernas M, Ryan E, Dolan L. SCHIZORIZA controls tissue system complexity in plants. Current Biology. 2010;20:818–823. doi: 10.1016/j.cub.2010.02.062. [DOI] [PubMed] [Google Scholar]
- Rakitina DV, Taliansky M, Brown JW, Kalinina NO. Two RNA-binding sites in plant fibrillarin provide interactions with various RNA substrates. Nucleic Acids Research. 2011;39:8869–8880. doi: 10.1093/nar/gkr594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayder L, Ting IP. Carbon metabolism in two species of Pereskia (Cactaceae) Plant Physiology. 1981;68:139–142. doi: 10.1104/pp.68.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro DK, Arimura G, Lau SYW, Piers E, Bohlmann J. Loblolly pine abietadienol/abietadienal oxidase PtAO (CYP720B1) is a multifunctional, multisubstrate cytochrome P450 monooxygenase. Proceedings of the National Academy of Sciences, USA. 2005;102:8060–8065. doi: 10.1073/pnas.0500825102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Rodríguez JF, Shishkova S, Napsucialy-Mendivil S, Dubrovsky JG. Apical meristem organization and lack of establishment of the quiescent center in Cactaceae roots with determinate growth. Planta. 2003;217:849–857. doi: 10.1007/s00425-003-1055-y. [DOI] [PubMed] [Google Scholar]
- Roxrud I, Lid SE, Fletcher JC, Schmidt ED, Opsahl- Sorteberg HG. GASA4, one of the 14-member Arabidopsis GASA family of small polypeptides, regulates flowering and seed development. Plant and Cell Physiology. 2007;48:471–483. doi: 10.1093/pcp/pcm016. [DOI] [PubMed] [Google Scholar]
- Rubinovich L, Weiss D. The Arabidopsis cysteine-rich protein GASA4 promotes GA responses and exhibits redox activity in bacteria and in planta. The Plant Journal. 2010;64:1018–1027. doi: 10.1111/j.1365-313X.2010.04390.x. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor–joining method: a new method for reconstructing phylogenetic trees. Molecular Biology and Evolution. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shishkova S, Dubrovsky JG. Developmental programmed cell death in primary roots of Sonoran Desert Cactaceae. American Journal of Botany. 2005;92:1590–1594. doi: 10.3732/ajb.92.9.1590. [DOI] [PubMed] [Google Scholar]
- Shishkova S, García-Mendoza E, Castillo-Díaz V, Moreno NE, Arellano J, Dubrovsky JG. Regeneration of roots from callus reveals stability of the developmental program for determinate root growth in Sonoran Desert Cactaceae. Plant Cell Reports. 2007;26:547–557. doi: 10.1007/s00299-006-0269-4. [DOI] [PubMed] [Google Scholar]
- Shishkova S, Rost TL, Dubrovsky JG. Determinate root growth and meristem maintenance in angiosperms. Annals of Botany. 2008;101:319–340. doi: 10.1093/aob/mcm251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. The Plant Cell. 2003;15:1095–1110. doi: 10.1105/tpc.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Chen ZJ. Blocking histone deacetylation in Arabidopsis induces pleiotropic effects on plant gene regulation and development. Proceedings of the National Academy of Sciences, USA. 2001;98:200–205. doi: 10.1073/pnas.011347998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal reference genes. Genome Biology. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. research0034.I-0034.II. http://dx.doi.org/10.1186/gb-2002-3-7-research0034 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zanten M, Snoek LB, Proveniers MCG, Peeters AJM. The many functions of ERECTA. Trends in Plant Sciences. 2009;14:214–218. doi: 10.1016/j.tplants.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Varney GT, McCully ME. The branch roots of Zea. II. Developmental loss of the apical meristem in field-grown roots. New Phytologist. 1991;118:535–546. [Google Scholar]
- Wallace RS. A family-wide phylogeny, subfamilial and tribal relationships, and suggestions for taxonomic realignments. IOS Bulletin. 1995a;6:13. [Google Scholar]
- Wallace RS. Molecular systematic study of the Cactaceae: using chloroplast DNA variation to elucidate cactus phylogeny. Bradleya. 1995b;13:1–12. [Google Scholar]
- Wigoda N, Ben-Nissan G, Granot D, Schwartz A, Weiss D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. The Plant Journal. 2006;48:796–805. doi: 10.1111/j.1365-313X.2006.02917.x. [DOI] [PubMed] [Google Scholar]
- Wu K, Tian L, Malik K, Brown D, Miki B. Functional analysis of HD2 histone deacetylase homologues in Arabidopsis thaliana. The Plant Journal. 2000;22:19–27. doi: 10.1046/j.1365-313x.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- Wu K, Tian L, Zhou C, Brown D, Miki B. Repression of gene expression by Arabidopsis HD2 histone deacetylases. The Plant Journal. 2003;34:241–247. doi: 10.1046/j.1365-313x.2003.01714.x. [DOI] [PubMed] [Google Scholar]
- Xu CR, Liu C, Wang YL, Li LC, Chen WQ, Xu ZH, Bai SN. Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. Proceedings of the National Academy of Sciences, USA. 2005;102:14469–14474. doi: 10.1073/pnas.0503143102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.