Abstract
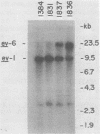
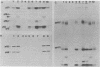
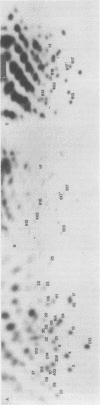
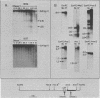
The endogenous avian provirus ev-1 is widespread in white leghorn chickens. Although it has no major structural defects, ev-1 has not been associated with any phenotype and is ordinarily expressed at a very low level. In this report, we describe a chicken embryo (Number 1836) cell culture containing both ev-1 and ev-6 which spontaneously expressed the ev-1 provirus. This culture released a high level of noninfectious virions containing a full complement of virion structural (gag) proteins but devoid of reverse transcriptase activity or antigen. These virions contained 70S RNA closely related to the genome of Rous-associated virus type 0, but identifiable as the ev-1 genome by oligonucleotide mapping. A fraction of the RNA molecules in the 70S complex were unusual in that they were polyadenylated 100 to 200 nucleotides downstream of the usual polyadenylation site. Eight sibling embryo cultures did not share this unusual phenotype with 1836, indicating that it was not inherited. However, an identical phenotype was inducible in the sibling cultures by treatment with 5-azacytidine, an inhibitor of DNA methylation, and the induced expression was stable for more than 10 generations. Analysis of chromatin structure and DNA methylation of the ev-1 provirus in 1836 cells revealed the presence (in a fraction of the proviruses) of both DNase I hypersensitive sites in the long terminal repeats and in gag and a pattern of cleavage sites for methyl-sensitive restriction endonuclease not found in a nonexpressing sibling. These results lend strong support to the role of DNA methylation in the control of gene expression. Additionally, they explain the lack of phenotype associated with ev-1 as due to a combination of its low expression and defectiveness in pol and env.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astrin S. M., Buss E. G., Haywards W. S. Endogenous viral genes are non-essential in the chicken. Nature. 1979 Nov 15;282(5736):339–341. doi: 10.1038/282339a0. [DOI] [PubMed] [Google Scholar]
- Astrin S. M., Crittenden L. B., Buss E. G. Ev 2, a genetic locus containing structural genes for endogenous virus, codes for Rous-associated virus type 0 produced by line 72 chickens. J Virol. 1980 Jan;33(1):250–255. doi: 10.1128/jvi.33.1.250-255.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrin S. M., Robinson H. L., Crittenden L. B., Buss E. G., Wyban J., Hayward W. S. Ten genetic loci in the chicken that contain structural genes for endogenous avian leukosis viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1105–1109. doi: 10.1101/sqb.1980.044.01.119. [DOI] [PubMed] [Google Scholar]
- Baker B., Robison H., Varmus H. E., Bishop J. M. Analysis of endogenous avian retrovirus DNA and RNA: viral and cellular determinants of retrovirus gene expression. Virology. 1981 Oct 15;114(1):8–22. doi: 10.1016/0042-6822(81)90248-8. [DOI] [PubMed] [Google Scholar]
- Bauer G., Temin H. M. Radioimmunological comparison of the DNA polymerases of avian retroviruses. J Virol. 1980 Mar;33(3):1046–1057. doi: 10.1128/jvi.33.3.1046-1057.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- Coffin J. M., Champion M., Chabot F. Nucleotide sequence relationships between the genomes of an endogenous and an exogenous avian tumor virus. J Virol. 1978 Dec;28(3):972–991. doi: 10.1128/jvi.28.3.972-991.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Czernilofsky A. P., DeLorbe W., Swanstrom R., Varmus H. E., Bishop J. M., Tischer E., Goodman H. M. The nucleotide sequence of an untranslated but conserved domain at the 3' end of the avian sarcoma virus genome. Nucleic Acids Res. 1980 Jul 11;8(13):2967–2984. doi: 10.1093/nar/8.13.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Mason W. S., Linial M. Synthesis and processing of polymerase proteins of wild-type and mutant avian retroviruses. J Virol. 1980 Oct;36(1):62–78. doi: 10.1128/jvi.36.1.62-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R., Shaikh R., Mason W. S. Identification of an avian oncovirus polyprotein in uninfected chick cells. Cell. 1978 May;14(1):89–104. doi: 10.1016/0092-8674(78)90304-5. [DOI] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Halpern M. S., Bolognesi D. P., Friis R. R., Mason W. S. Expression of the Major Viral Glycoprotein of Avian Tumor Virus in Cells of chf(+) Chicken Embryos. J Virol. 1975 May;15(5):1131–1140. doi: 10.1128/jvi.15.5.1131-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward W. S., Braverman S. B., Astrin S. M. Transcriptional products and DNA structure of endogenous avian proviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1111–1121. doi: 10.1101/sqb.1980.044.01.120. [DOI] [PubMed] [Google Scholar]
- Hishinuma F., DeBona P. J., Astrin S., Skalka A. M. Nucleotide sequence of acceptor site and termini of integrated avian endogenous provirus ev1: integration creates a 6 bp repeat of host DNA. Cell. 1981 Jan;23(1):155–164. doi: 10.1016/0092-8674(81)90280-4. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Vogt P. K., Bishop J. M., Varmus H. E. Endogenous proviruses of random-bred chickens and ring-necked pheasants: analysis with restriction endonucleases. Virology. 1981 Jan 15;108(1):222–229. doi: 10.1016/0042-6822(81)90540-7. [DOI] [PubMed] [Google Scholar]
- Humphries E. H., Glover C., Weiss R. A., Arrand J. R. Differences between the endogenous and exogenous DNA sequences of Rous-associated virus-O. Cell. 1979 Nov;18(3):803–815. doi: 10.1016/0092-8674(79)90133-8. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Taylor S. M. Cellular differentiation, cytidine analogs and DNA methylation. Cell. 1980 May;20(1):85–93. doi: 10.1016/0092-8674(80)90237-8. [DOI] [PubMed] [Google Scholar]
- Ju G., Skalka A. M. Nucleotide sequence analysis of the long terminal repeat (LTR) of avian retroviruses: structural similarities with transposable elements. Cell. 1980 Nov;22(2 Pt 2):379–386. doi: 10.1016/0092-8674(80)90348-7. [DOI] [PubMed] [Google Scholar]
- Linial M., Fenno J., Burnette W. N., Rohrschneider L. Synthesis and processing of viral glycoproteins in two nonconditional mutants of Rous sarcoma virus. J Virol. 1980 Oct;36(1):280–290. doi: 10.1128/jvi.36.1.280-290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Roberts R. J. Directory of restriction endonucleases. Methods Enzymol. 1980;65(1):1–15. doi: 10.1016/s0076-6879(80)65003-4. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Eisenman R., Senior A., Ripley S. Low freqeuncy production of recombinant subgroup E avian leukosis viruses by uninfected V-15B chicken cells. Virology. 1979 Nov;99(1):21–30. doi: 10.1016/0042-6822(79)90033-3. [DOI] [PubMed] [Google Scholar]
- Robinson H. L. Inheritance and expression of chicken genes that are related to avian leukosis sarcoma virus genes. Curr Top Microbiol Immunol. 1978;83:1–36. doi: 10.1007/978-3-642-67087-9_1. [DOI] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele C. M., Hanafusa H. Proteins of helper-dependent RSV. Virology. 1971 Aug;45(2):401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Nunberg J. H., Schimke R. T. Size heterogeneity in the 3' end of dihydrofolate reductase messenger RNAs in mouse cells. Cell. 1980 Nov;22(2 Pt 2):361–370. doi: 10.1016/0092-8674(80)90346-3. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Brown S., Sen A., Eisenman R. Recombinant avian oncoviruses. II. Alterations in the gag proteins and evidence for intragenic recombination. Virology. 1979 Jan 30;92(2):463–481. doi: 10.1016/0042-6822(79)90150-8. [DOI] [PubMed] [Google Scholar]
- Shaikh R., Linial M., Coffin J., Eisenman R. Recombinant avian oncoviruses. I. Alterations in the precursor to the internal structural proteins. Virology. 1978 Jun 15;87(2):326–338. doi: 10.1016/0042-6822(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., Baluda M. A. Homology between avian oncornavirus RNAs and DNA from several avian species. J Virol. 1975 Dec;16(6):1492–1502. doi: 10.1128/jvi.16.6.1492-1502.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A., DeBona P., Hishinuma F., McClements W. Avian endogenous proviral DNA: analysis of integrated ev 1 and a related gs- chf- provirus purified by molecular cloning. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1097–1104. doi: 10.1101/sqb.1980.044.01.118. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Steimer K. S., Boettiger D. Envelope assembly mutant of rous sarcoma virus. J Virol. 1980 Dec;36(3):883–888. doi: 10.1128/jvi.36.3.883-888.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tereba A., Astrin S. M. Chromosomal localization of ev-1, a frequently occurring endogenous retrovirus locus in white Leghorn chickens, by in situ hybridization. J Virol. 1980 Sep;35(3):888–894. doi: 10.1128/jvi.35.3.888-894.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Recombinants between endogenous and exogenous avian tumor viruses: role of the C region and other portions of the genome in the control of replication and transformation. J Virol. 1980 Jan;33(1):238–249. doi: 10.1128/jvi.33.1.238-249.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsichlis P. N., Conklin K. F., Coffin J. M. Mutant and recombinant avian retroviruses with extended host range. Proc Natl Acad Sci U S A. 1980 Jan;77(1):536–540. doi: 10.1073/pnas.77.1.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R. Identification of a large polypeptide precursor of avian oncornavirus proteins. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1734–1738. doi: 10.1073/pnas.70.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Hackett P. B., Oppermann H., Ullrich A., Levintow L., Bishop J. M. Cell-free translation of avian sarcoma virus RNA: suppression of the gag termination codon does not augment synthesis of the joint gag/pol product. Cell. 1978 Oct;15(2):607–614. doi: 10.1016/0092-8674(78)90029-6. [DOI] [PubMed] [Google Scholar]
- Wigler M. H. The inheritance of methylation patterns in vertebrates. Cell. 1981 May;24(2):285–286. doi: 10.1016/0092-8674(81)90317-2. [DOI] [PubMed] [Google Scholar]
- Wu C., Bingham P. M., Livak K. J., Holmgren R., Elgin S. C. The chromatin structure of specific genes: I. Evidence for higher order domains of defined DNA sequence. Cell. 1979 Apr;16(4):797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., de Crombrugghe B., Pastan I. Identification of a functional promoter in the long terminal repeat of Rous sarcoma virus. Cell. 1980 Dec;22(3):787–797. doi: 10.1016/0092-8674(80)90555-3. [DOI] [PubMed] [Google Scholar]