TO THE EDITOR: The co-expression of CD3 in B-cell lineage lymphomas or the aberrant co-expression of CD20 in T-cell lineage lymphomas has been rarely reported. We describe a 72-year-old female, who presented with systemic lymphadenopathy and a 2 cm-sized gastric mass. Biopsies of the lymph node and the gastric mass showed diffuse sheets of large, atypical lymphoid cells. The tumor cells were positive for B-cell antigens such as CD20 and CD79a as well as for the T-cell antigen CD3 and cytotoxic molecule TIA-1. In situ hybridization for Epstein-Barr virus-encoded RNA (EBER) showed positive signals in the nuclei of the majority of tumor cells. Molecular studies revealed rearrangements of the immunoglobulin heavy chain (IgH) region and the T-cell receptor (TCR)-γ genes, but not the TCR-β genes. The patient was treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP), which resulted in near-total remission. This case illustrates the difficulty of lineage determination of non-Hodgkin lymphomas with only pan-B- and pan-T-cell markers. Unlike the previously reported cases, the present case showed molecular markers of both the B- and T-cell lineages, which further complicates the interpretation. The association of CD3-positive diffuse large B-cell lymphoma (DLBCL) with the Epstein-Barr virus (EBV) warrants further study.
Lymphoma diagnosis is based on histomorphology, immunophenotyping, flow cytometry, and molecular studies. Lymphomas are broadly classified as being of the B-cell and T-cell lineages, using immunohistochemistry and/or flow cytometry. Among the markers to determine B- and T-cell lineages, CD20 and CD3 are the most commonly used. T-cell lymphomas with aberrant expression of the B-cell marker CD20 or B-cell lymphomas with aberrant expression of T-cell associated antigens such as CD5, CD43, CD7, CD2, CD4, and CD8 have been reported [1-5]. However, cases of B-cell lymphoma with CD3 co-expression are extremely rare [6, 7]. Herein, we report a case of diffuse large B-cell lymphoma (DLBCL) in an elderly patient, with an aberrant co-expression of the T-cell associated antigen CD3 and cytotoxic molecule TIA-1, an infection with EBV, and a dual rearrangement of the immunoglobulin heavy chain (IgH) and T-cell receptor (TCR)-γ genes, but not the TCR-β gene.
CASE
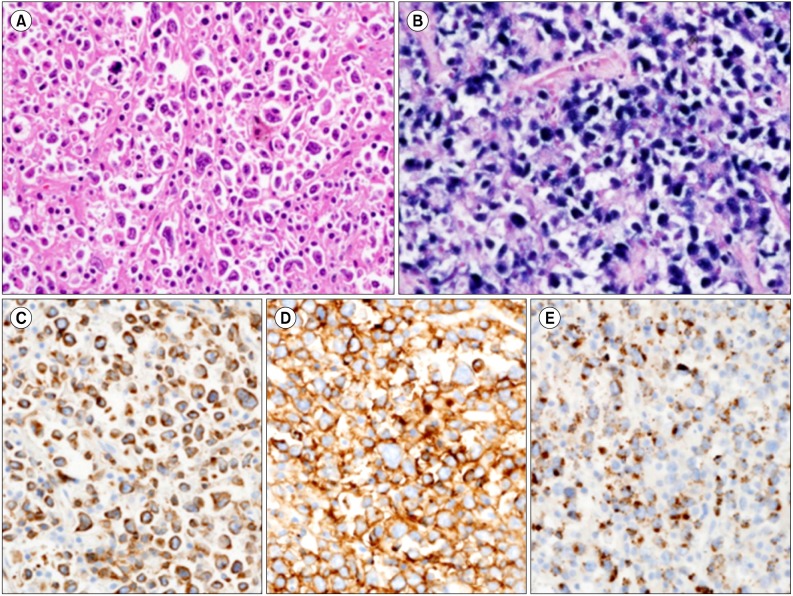
A 72-year-old, HIV-negative Korean female presented with easy fatigue and epigastric discomfort, which had been gradually worsening over a period of 4 months, and weight loss of 3 kg over a period of 1 month. She had a history of stroke, hypertension, and osteoporosis with spinal fractures. Laboratory tests on admission revealed an elevated serum lactate dehydrogenase level (489 IU/L; reference range, 120-250 IU/L) with a slightly decreased serum hemoglobin level (10.2 g/dL; reference range, 12-16 g/dL). Computed tomography (CT) scans of the neck, chest, and abdomen showed multiple lymph node enlargements in the left supraclavicular area and the retroperitoneum, with a 7.5 cm cystic lesion in the left perirenal space. A 2 cm-sized gastric mass with central ulceration was noted during esophagogastroduodenoscopy (EGD). A biopsy of the left supraclavicular lymph node showed a partial effacement of the nodal architecture with diffuse infiltration by large atypical tumor cells separated by collagenous septae (Fig. 1). There were also multiple foci of geographic necrosis. These cells had pleomorphic nuclei, vesicular chromatin, prominent macronucleoli, and numerous mitotic figures, with many bizarre Reed-Sternberg (RS)-like multinucleated cells. Admixed with the tumor cells were small numbers of mature small lymphocytes and macrophages. A gastric biopsy also revealed similar findings. Immunohistochemistry showed that the tumor cells were positive for CD20, CD79a, CD3, MUM-1, BCL-2, TIA-1, CD43, OCT-2, and BOB-1, weakly positive for PAX-5, but negative for CD2, CD5, CD4, CD8, granzyme B, TCR-β F1, TCR-γ receptor, BCL-6, CD10, ALK-1, CD30, CD15, myeloperoxidase, and CD163. In the background, there were only a few scattered T cells, some of which were positive for TIA-1. In situ hybridization for Epstein-Barr virus-encoded RNA (EBER) showed positive nuclear signals in almost all tumor cells. A quantitative real-time PCR for Epstein-Barr virus (EBV) in the patient's serum showed that the EBV load was high (131,000 copies/mL). Clonality studies using multiplex PCR with BIOMED-2 primers (InVivoScribe Technologies, San Diego, CA, USA) revealed a dual rearrangement of IgH genes and TCR-γ genes (Fig. 2), whereas a TCR-β gene rearrangement study failed to identify a monoclonal T-cell population. Based on the expression of almost all B-cell-associated antigens and the monoclonal rearrangement of IgH genes, the patient was diagnosed with EBV-positive DLBCL of the elderly with an aberrant expression of CD3 and TIA-1. Tumor cells were also noted in bone marrow biopsy and clot sections, assigning her to the Ann Arbor stage 4B. She received 6 cycles of rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP), which resulted in almost complete remission. A follow-up positron emission tomography (PET) scan 8 months after the diagnosis revealed the disappearance of hypermetabolic lesions from the left supraclavicular area, retroperitoneum, and stomach. However, a cystic lesion with calcifications in the perirenal space, which seemed to be non-neoplastic (e.g., a postinflammatory calcification, a pseudocyst, or an old hematoma), was persistently observed on 3 follow-up CT scans after chemotherapy (R-CHOP) with salvage radiotherapy (3,060 cGy/20 fx, 5 weeks) for the perirenal lesion.
Fig. 1.
(A) Pleomorphic large tumor cells with prominent nucleoli, vesicular chromatin, and scanty cytoplasm are admixed with small numbers of mature lymphocytes. (B) A positive in situ hybridization for EBER in tumor cell nuclei is seen. (C-E) The neoplastic cells are diffusely and strongly positive for the T-cell marker CD3 (C), the B-cell marker CD20 (D), and the cytotoxic molecule TIA-1 (E) (A-E, ×400).
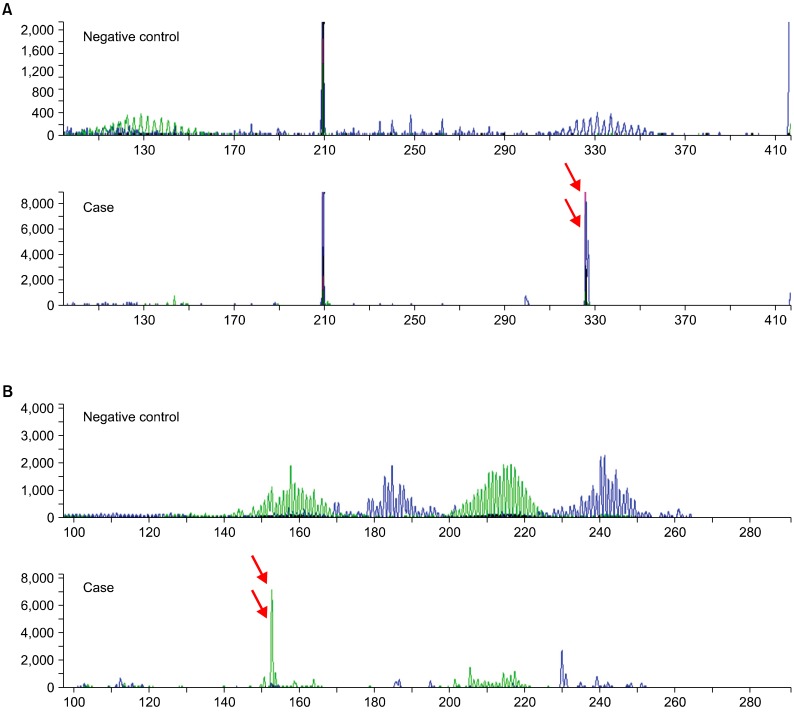
Fig. 2.
This tumor demonstrates dual rearrangement (red arrows) of IgH genes (A) and TCR-γ genes (B) by multiplex PCR.
DISCUSSION
Non-Hodgkin lymphomas (NHL) are assigned to B-cell and T-cell lineages based on immunoprofiling, flow cytometry, and molecular studies. We describe a case of a large cell lymphoma with the hybrid expression of both B- and T-cell markers, monoclonal gene arrangement of both lineages, and EBV infection. This case illustrates the difficulty of determining of the lineage of non-Hodgkin lymphoma using only pan-B-cell or pan-T-cell markers.
Based on the histopathologic features, the age of the patient, and the expression of EBER, CD20, MUM-1, BCL-2, OCT-2, and BOB-1, a diagnosis of EBV-positive DLBCL of the elderly was favored, despite the cytoplasmic expression of CD3 and TIA-1. Because classical Hodgkin lymphoma (cHL) in older patients is also often associated with EBV infection, it should be included as a major differential diagnosis. In EBV-positive DLBCL of the elderly, tumor cells are often positive for CD30; consequently, CD30 expression is not helpful for differential diagnosis. A strong, homogenous expression of CD20 in more than 50% of the RS-like cells in combination with the expression of B-cell specific transcription factors such as OCT-2 and BOB-1 support a diagnosis of EBV-positive DLBCL. In addition, the finding that over 30% of the cytotoxic T-cells in the background infiltrate expressed TIA-1 favors EBV-positive DLBCL over EBV-positive cHL.
However, in the present case, the expression of CD3, the most specific surface antigen for mature T-lymphocytes in pleomorphic large cells, led to a diagnostic dilemma. Although several reports describe aberrant expression of the B-cell marker CD20 in T-cell NHL or aberrant expression of T-cell-associated antigens such as CD2, CD4, CD7, and/or CD8 in B-cell NHL (B-NHL) [1-5], there are only 3 reports (9 cases in all) of CD3-positive B-NHL [6, 7]. Several mechanisms have been proposed to explain the aberrant expression of T-cell antigens by neoplastic B cells: 1) neoplastic transformation leading to the consequent derepression of genetic material, 2) neoplastic transformation of stem/progenitor cells, and 3) neoplastic expansion of a subpopulation of B cells which normally express T-cell antigens [6]. Another hypothesis is associated with the transcription factor encoded by the paired box 5 (PAX-5) gene [8]. PAX-5 activates B-cell-specific genes leading to B-cell lineage commitment and represses B-cell lineage-inappropriate genes, such as the T-cell specification gene, NOTCH1. It also encodes the transcription factor BSAP, which is critical for early B-cell lymphopoiesis and for maintaining B-lymphocyte differentiation. Immunoperoxidase staining for PAX-5 showed BSAP expression, which is highly indicative of B-cell differentiation and a B-cell lymphoma. Cobaleda et al. demonstrated that, in the absence of PAX-5, mature B lymphocytes can be transformed into functional T-lymphocytes [9]. In other words, B cells showing damaged and/or deregulated PAX-5 activity may occur in biphenotypic lymphomas that express PAX-5. This supports the hybrid features of this case, which showed weak immunopositivity for PAX-5. In addition, co-expression of CD3 most likely requires that repressed B-cell lineage-inappropriate genes such as NOTCH1 are activated [10].
Another dilemma encountered in the diagnosis of this case resulted from the observation that there were dual rearrangements in both cell lineages. Dual or aberrant rearrangements, in which B- or T-cell phenotype lymphomas show monoclonality of both cell lineages (so-called "lineage infidelity") have been reported [11-15]. The percentage of aberrant rearrangement is between 5% and 29% for both B- and T-cell lymphomas. Garcia et al. reported that 21% of B-NHL cases showed a monoclonal pattern in one of the TCR genes (TCR-β, 16%; TCR-γ, 7%), and 16% of T-NHL cases showed an IgH gene monoclonal pattern. Their study demonstrated that a TCR-γ gene analysis has higher sensitivity than a TCR-β gene analysis. Födinger et al. reported that 13% of B-cell lymphomas exhibited a clonal TCR-γ rearrangement and 29% of T-cell lymphomas showed a monoclonality of the IgH gene rearrangement.
Though the tumor in the present study showed a hybrid phenotype, the patient received R-CHOP therapy, with almost complete remission seen on a follow-up PET scan. The patient's response to R-CHOP treatment also supports the initial diagnosis of EBV-positive DLBCL with an aberrant expression of CD3 and TIA-1.
To the best of our knowledge, this is the first case of EBV-positive DLBCL with an aberrant expression of CD3, TIA, and dual monoclonal rearrangement of the IgH and TCR-γ genes. EBV infection may contribute to the transformation of B cells, with ensuing PAX-5 deregulation. This would result in a hybrid immunoreactivity pattern, with positivity for CD20, CD3, and TIA-1 and lineage infidelity. This case serves as a cautionary note of the diagnostic pitfalls involved in the lineage determination of NHL using only pan-B- and pan-T-cell markers. More extensive immunoprofiles and molecular studies are needed for an integrated interpretation and accurate diagnosis. Additionally, the association between the co-expression of CD20, CD3, and TIA-1 and EBV infection warrant further study.
Footnotes
No potential conflicts of interest relevant to this article were reported.
References
- 1.Lee KY, Jeon SY, Hong JW, Kim YH, Song KH, Kim KH. CD20 positive T cell lymphoma involvement of skin. Ann Dermatol. 2011;23:529–535. doi: 10.5021/ad.2011.23.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohrmann RL, Arber DA. CD20-Positive peripheral T-cell lymphoma: report of a case after nodular sclerosis Hodgkin's disease and review of the literature. Mod Pathol. 2000;13:1244–1252. doi: 10.1038/modpathol.3880229. [DOI] [PubMed] [Google Scholar]
- 3.Yao X, Teruya-Feldstein J, Raffeld M, Sorbara L, Jaffe ES. Peripheral T-cell lymphoma with aberrant expression of CD79a and CD20: a diagnostic pitfall. Mod Pathol. 2001;14:105–110. doi: 10.1038/modpathol.3880265. [DOI] [PubMed] [Google Scholar]
- 4.Kaleem Z, White G, Zutter MM. Aberrant expression of T-cell-associated antigens on B-cell non-Hodgkin lymphomas. Am J Clin Pathol. 2001;115:396–403. doi: 10.1309/v8yg-8pp4-b4te-9x6j. [DOI] [PubMed] [Google Scholar]
- 5.Inaba T, Shimazaki C, Sumikuma T, Nakagawa M. T-cell associated antigen-positive B-cell lymphoma. Leuk Lymphoma. 2001;42:1161–1171. doi: 10.3109/10428190109097741. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Chen C, Lau S, et al. CD3-positive large B-cell lymphoma. Am J Surg Pathol. 2009;33:505–512. doi: 10.1097/PAS.0b013e318185d231. [DOI] [PubMed] [Google Scholar]
- 7.Aisenberg AC, Bloch KJ, Wilkes BM. Malignant lymphoma with dual B and T cell markers. Analysis of the neoplastic cells with monoclonal antibodies directed against T cell subsets. J Exp Med. 1981;154:1709–1714. doi: 10.1084/jem.154.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson M, Jerkeman M, Dictor M. Biphenotypic bigenotypic lymphoma with simultaneous expression of PAX5/BSAP and B- and T-cell markers. Eur J Haematol. 2007;79:159–165. doi: 10.1111/j.1600-0609.2007.00882.x. [DOI] [PubMed] [Google Scholar]
- 9.Cobaleda C, Jochum W, Busslinger M. Conversion of mature B cells into T cells by dedifferentiation to uncommitted progenitors. Nature. 2007;449:473–477. doi: 10.1038/nature06159. [DOI] [PubMed] [Google Scholar]
- 10.Wallentine JC, Perkins SL, Tripp SR, Bruggman RD, Bayerl MG. Diffuse large B-cell lymphoma with coexpression of CD3 in a pediatric patient: a case report, review of the literature, and tissue microarray study. J Pediatr Hematol Oncol. 2009;31:124–127. doi: 10.1097/MPH.0b013e31818b354a. [DOI] [PubMed] [Google Scholar]
- 11.García MJ, Martínez-Delgado B, Granizo JJ, Benítez J, Rivas C. IgH, TCR-gamma, and TCR-beta gene rearrangement in 80 B- and T-cell non-Hodgkin's lymphomas: study of the association between proliferation and the so-called "aberrant" patterns. Diagn Mol Pathol. 2001;10:69–77. doi: 10.1097/00019606-200106000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Krafft AE, Taubenberger JK, Sheng ZM, et al. Enhanced sensitivity with a novel TCRgamma PCR assay for clonality studies in 569 formalin-fixed, paraffin-embedded (FFPE) cases. Mol Diagn. 1999;4:119–133. doi: 10.1016/s1084-8592(99)80036-8. [DOI] [PubMed] [Google Scholar]
- 13.Chen YT, Godwin TA, Mouradian JA. Immunohistochemistry and gene rearrangement studies in the diagnosis of malignant lymphomas: a comparison of 152 cases. Hum Pathol. 1991;22:1249–1257. doi: 10.1016/0046-8177(91)90107-z. [DOI] [PubMed] [Google Scholar]
- 14.Pelicci PG, Knowles DM, 2nd, Dalla Favera R. Lymphoid tumors displaying rearrangements of both immunoglobulin and T cell receptor genes. J Exp Med. 1985;162:1015–1024. doi: 10.1084/jem.162.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Födinger M, Winkler K, Mannhalter C, Chott A. Combined polymerase chain reaction approach for clonality detection in lymphoid neoplasms. Diagn Mol Pathol. 1999;8:80–91. doi: 10.1097/00019606-199906000-00004. [DOI] [PubMed] [Google Scholar]