Abstract
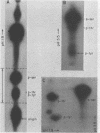
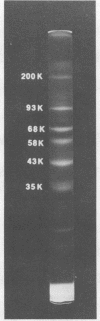
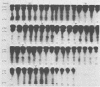
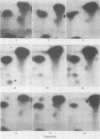
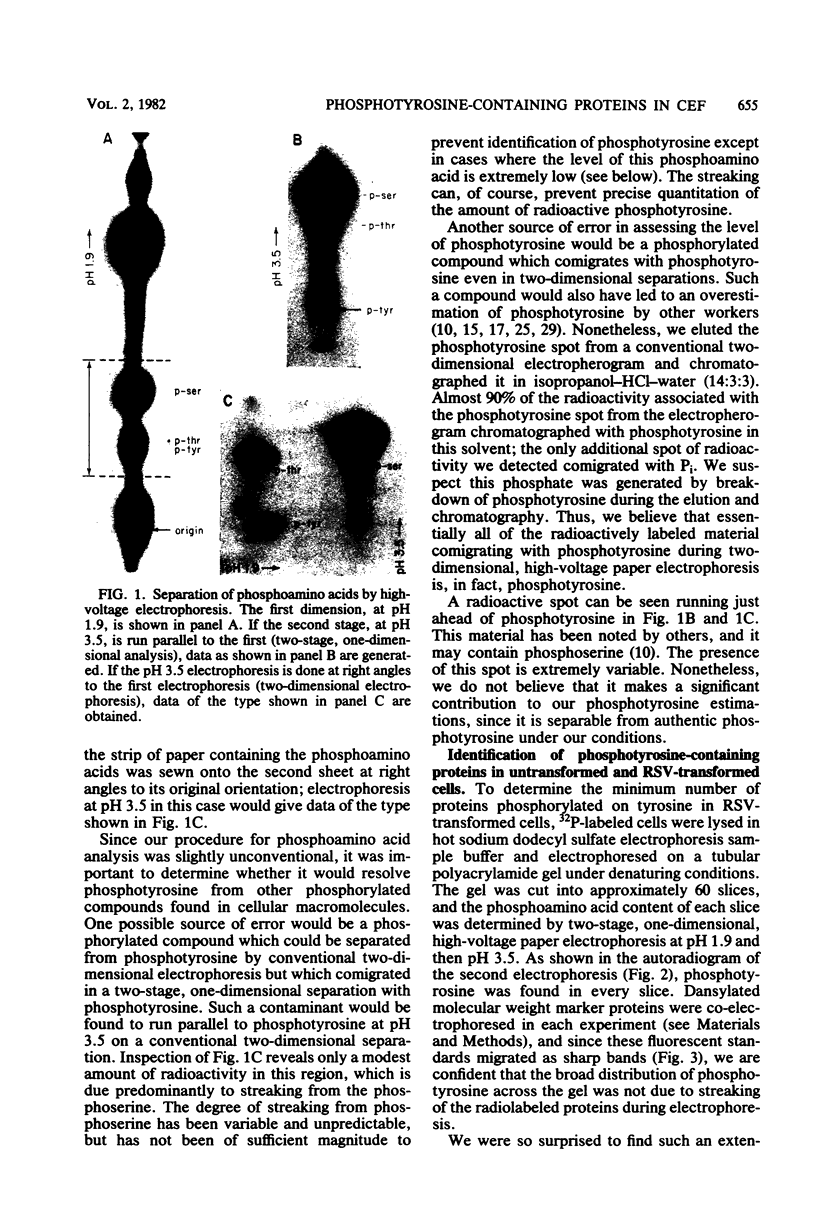
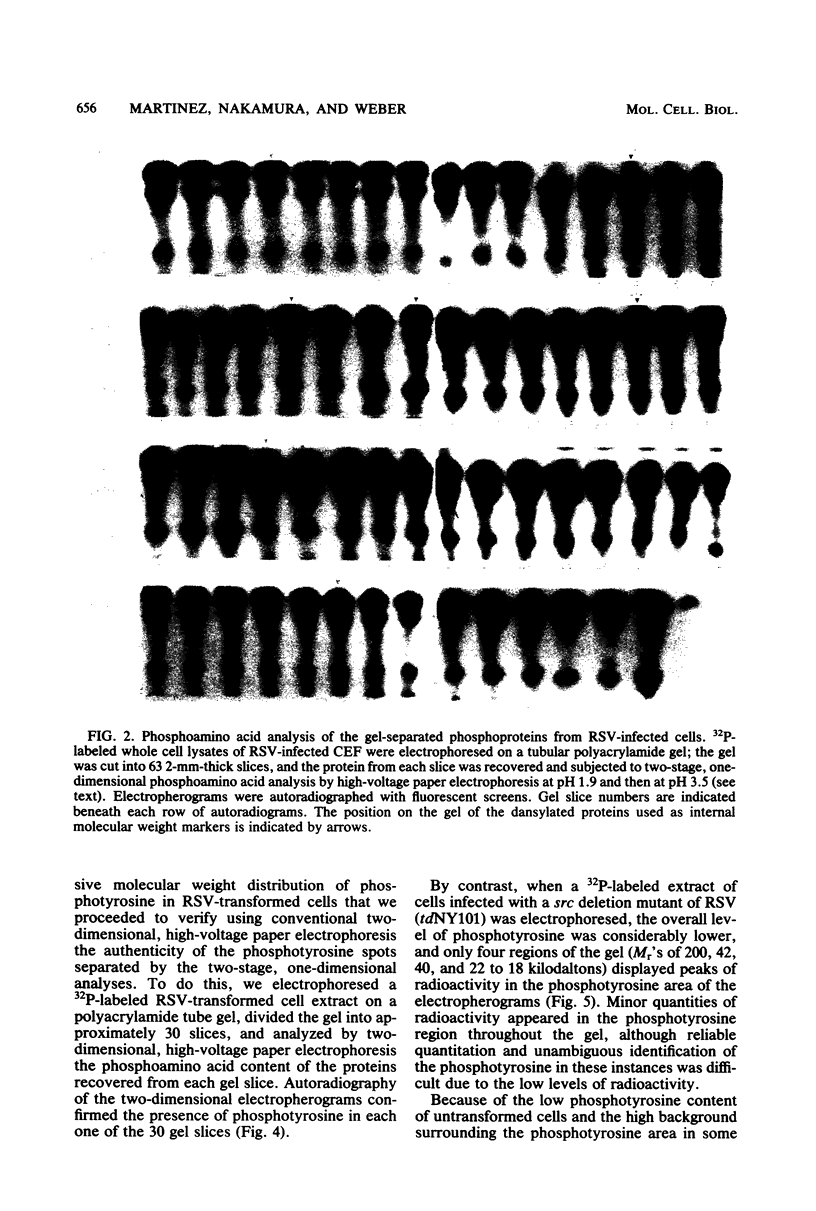
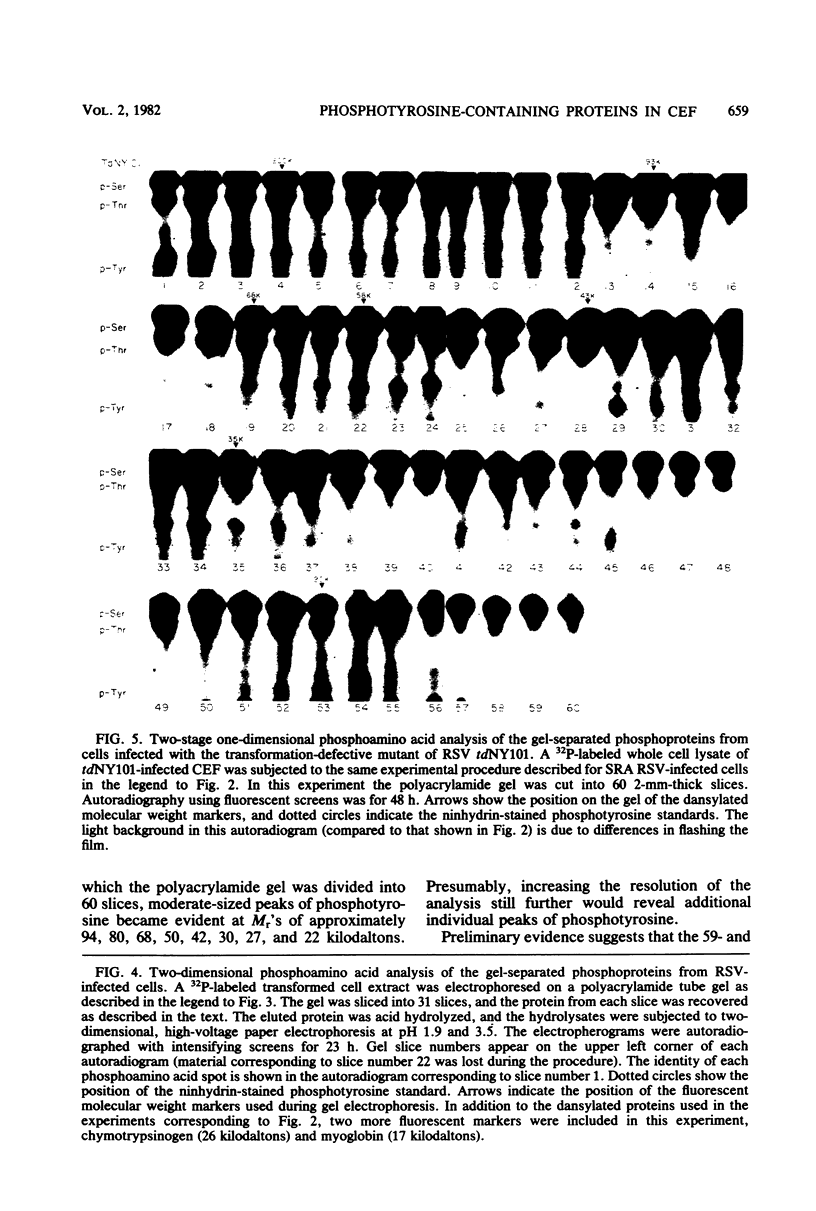
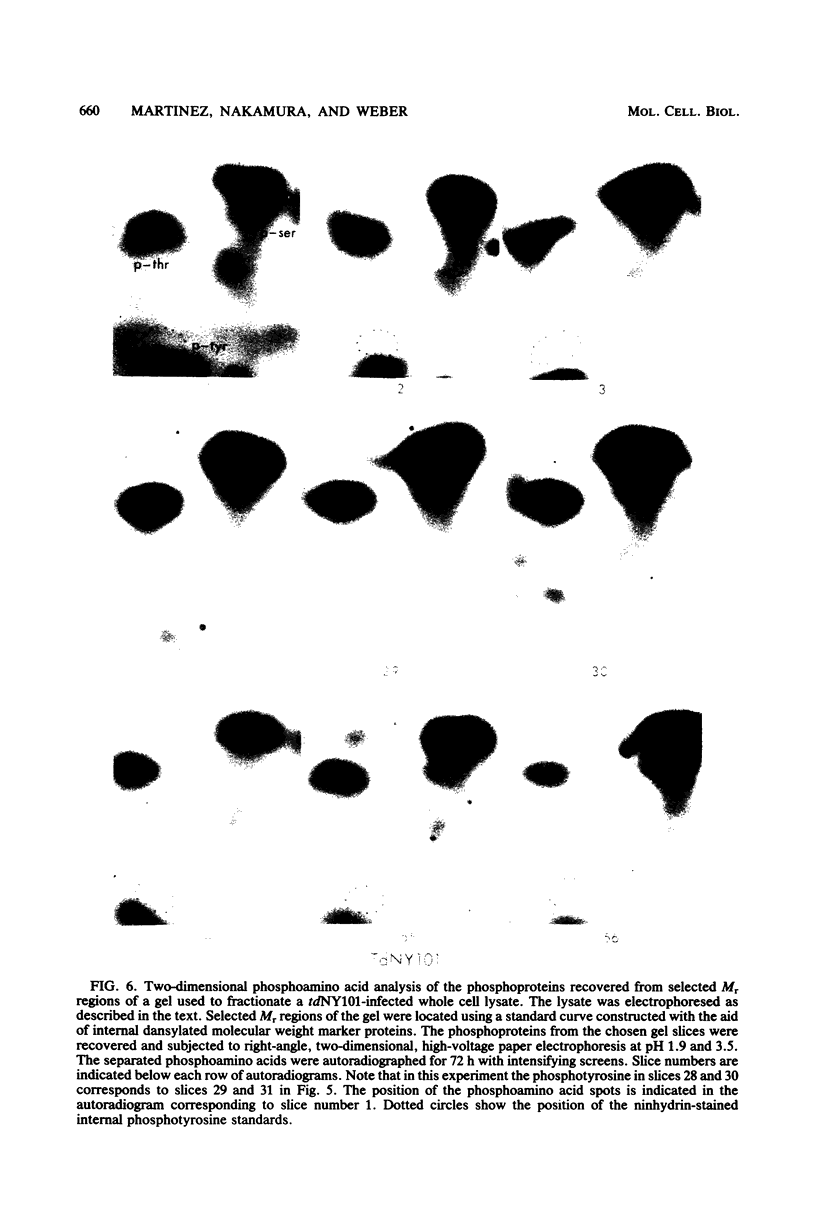
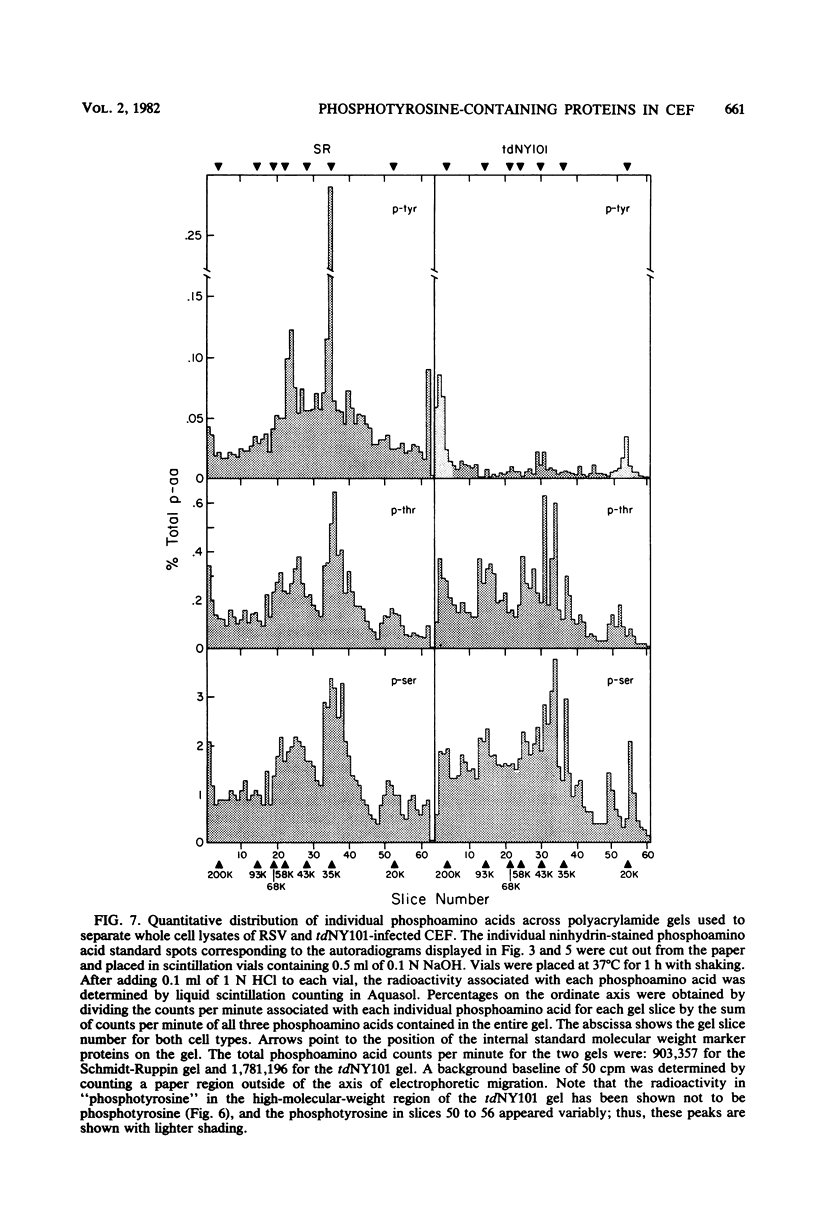
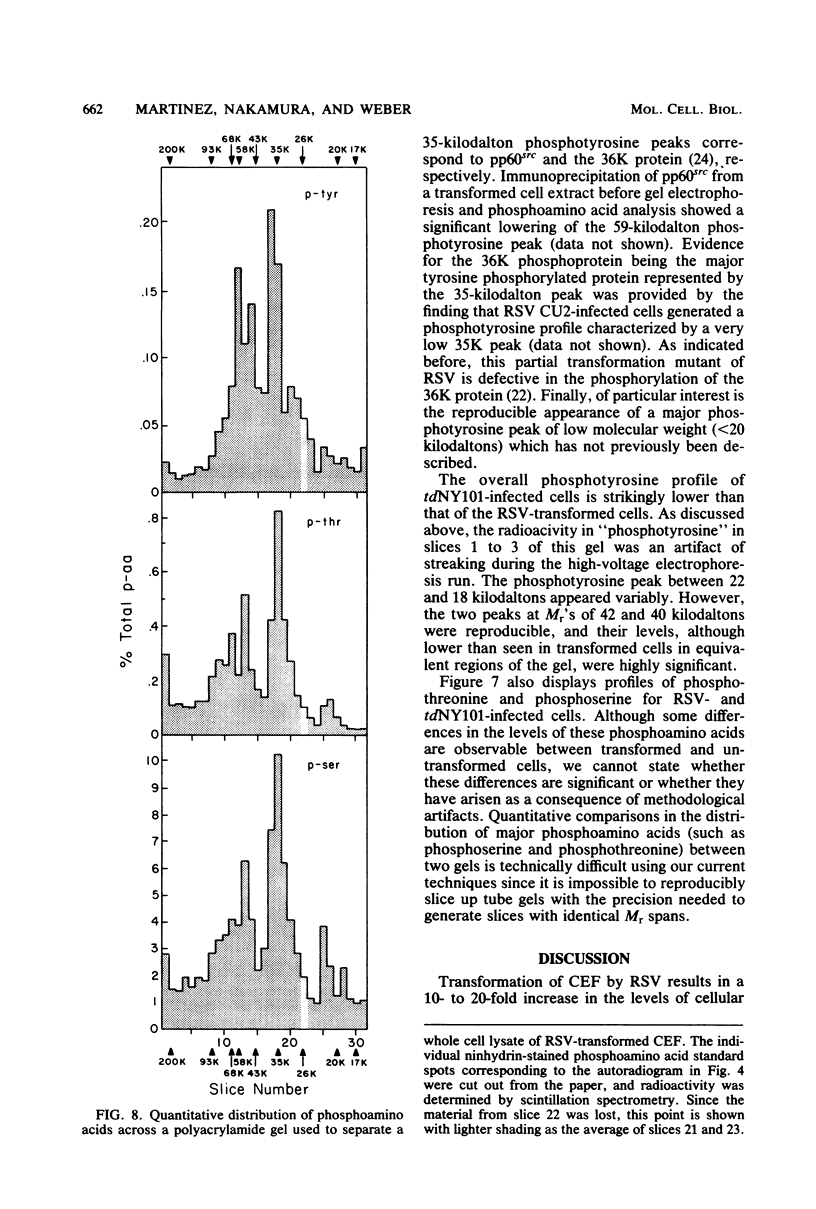
Phosphorylation on tyrosine residues mediated by pp60src appears to be a primary biochemical event leading to the establishment of the transformed phenotype in Rous sarcoma virus (RSV)-infected cells. To identify the cellular proteins that undergo tyrosine phosphorylation during transformation, a 32P-labeled RSV-transformed chicken embryo cell extract was analyzed by electrophoresis on a polyacrylamide gel. After slicing the gel into approximately 60 slices, phosphoamino acid analyses were carried out on the protein recovered from each gel slice. Phosphotyrosine was found in every gel slice, with two major peaks of this phosphoamino acid around Mr's of 59 and 36 kilodaltons. When the same analysis was performed with cells infected with a transformation-defective src deletion mutant of RSV (tdNY101), significant and reproducible peaks of phosphotyrosine were found in only 2 of 60 gel slices. These gel slices corresponded to Mr's of 42 and 40 kilodaltons. Identical results were obtained with normal uninfected chicken embryo fibroblasts. We conclude from these observations that pp60src or the combined action of pp60src and pp60src -activated cellular protein kinases cause the tyrosine-specific phosphorylation of a very large number of cellular polypeptides in RSV-transformed cells. In addition, untransformed cells appear to possess one or more active tyrosine-specific protein kinases which are responsible for the phosphorylation of a limited number of proteins. These proteins are different from the major phosphotyrosine-containing proteins of the transformed cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. P., Purich D., Stadtman E. R. Cascade control of Escherichia coli glutamine synthetase. Properties of the PII regulatory protein and the uridylyltransferase-uridylyl-removing enzyme. J Biol Chem. 1975 Aug 25;250(16):6264–6272. [PubMed] [Google Scholar]
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Anderson D. D., Beckmann R. P., Harms E. H., Nakamura K., Weber M. J. Biological properties of "partial" transformation mutants of Rous sarcoma virus and characterization of their pp60src kinase. J Virol. 1981 Jan;37(1):445–458. doi: 10.1128/jvi.37.1.445-458.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Beemon K., Devare S. G. Origin and functional properties of the major gene product of the Snyder-Theilen strain of feline sarcoma virus. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5158–5162. doi: 10.1073/pnas.77.9.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Changes in protein phosphorylation in Rous sarcoma virus-transformed chicken embryo cells. Mol Cell Biol. 1981 Feb;1(2):165–178. doi: 10.1128/mcb.1.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson E., Erikson R. L. Identification of a cellular protein substrate phosphorylated by the avian sarcoma virus-transforming gene product. Cell. 1980 Oct;21(3):829–836. doi: 10.1016/0092-8674(80)90446-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Purchio A. F., Erikson E., Collett M. S., Brugge J. S. Molecular events in cells transformed by Rous Sarcoma virus. J Cell Biol. 1980 Nov;87(2 Pt 1):319–325. doi: 10.1083/jcb.87.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S., Duesberg P. H., Hanafusa H. Transformation-defective mutants of Rous sarcoma virus with src gene deletions of varying length. J Virol. 1977 Dec;24(3):910–914. doi: 10.1128/jvi.24.3.910-914.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Nakamura K. D., Weber M. J. Phosphorylation of a 36,000 Mr cellular protein in cells infected with partial transformation mutants of rous sarcoma virus. Mol Cell Biol. 1982 Feb;2(2):147–153. doi: 10.1128/mcb.2.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppermann H., Levinson A. D., Levintow L., Varmus H. E., Bishop J. M., Kawai S. Two cellular proteins that immunoprecipitate with the transforming protein of Rous sarcoma virus. Virology. 1981 Sep;113(2):736–751. doi: 10.1016/0042-6822(81)90202-6. [DOI] [PubMed] [Google Scholar]
- Radke K., Gilmore T., Martin G. S. Transformation by Rous sarcoma virus: a cellular substrate for transformation-specific protein phosphorylation contains phosphotyrosine. Cell. 1980 Oct;21(3):821–828. doi: 10.1016/0092-8674(80)90445-6. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Todaro G. J., Fryling C., Stephenson J. R. Human transforming growth factors induce tyrosine phosphorylation of EGF receptors. Nature. 1981 Jul 16;292(5820):259–262. doi: 10.1038/292259a0. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Ball E. H., Singer S. J. Vinculin: a cytoskeletal target of the transforming protein of Rous sarcoma virus. Cell. 1981 Apr;24(1):165–174. doi: 10.1016/0092-8674(81)90512-2. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K. Temperature-sensitive transformation by Rous sarcoma virus and temperature-sensitive protein kinase activity. J Virol. 1980 Jan;33(1):220–229. doi: 10.1128/jvi.33.1.220-229.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Stadtman E. R. 5'-adenylyl-O-tyrosine. The novel phosphodiester residue of adenylylated glutamine synthetase from Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3769–3771. [PubMed] [Google Scholar]
- Smart J. E., Oppermann H., Czernilofsky A. P., Purchio A. F., Erikson R. L., Bishop J. M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc Natl Acad Sci U S A. 1981 Oct;78(10):6013–6017. doi: 10.1073/pnas.78.10.6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot D. N., Yphantis D. A. Fluorescent monitoring of SDS gel electrophoresis. Anal Biochem. 1971 Nov;44(1):246–253. doi: 10.1016/0003-2697(71)90367-8. [DOI] [PubMed] [Google Scholar]
- Tse Y. C., Kirkegaard K., Wang J. C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J Biol Chem. 1980 Jun 25;255(12):5560–5565. [PubMed] [Google Scholar]
- Weber M. J., Friis R. R. Dissociation of transformation parameters using temperature-conditional mutants of Rous sarcoma virus. Cell. 1979 Jan;16(1):25–32. doi: 10.1016/0092-8674(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]