Abstract
Organometallic ruthenium complex of quinolone antibacterial agent ofloxacin [(η6-p-cymene)RuCl(O,O-oflo)]·2.8H2O (1·2.8H2O) was isolated and its crystal structure was determined. In this »piano-stool« complex, quinolone is bidentately coordinated to the metal through the ring carbonyl and one of the carboxylic oxygen atoms. Interactions of the title complex with DNA were studied by spectroscopic methods (electronic, fluorescence, CD) and atomic force microscopy (AFM). It was established that the electrostatic attraction between the ruthenium complex and DNA in a solution is important for the binding since interactions were observed only in a solution with low ionic strengths. An induced CD (ICD) signal was observed in the solution of DNA and title complex which proves the interaction between ruthenium and macromolecule. Competitive binding between cisplatin and 1 to DNA revealed that cisplatin prevents binding of 1. Our experiments revealed that binding of the title complex to DNA occurs also if guanine N7 is protonated. AFM has shown that title complex provokes DNA shrinkage. Preliminary biological tests have also been performed.
Ruthenium anticancer complexes have been extensively studied and two of them, NAMI-A and KP1019, respectively have successfully entered clinical trials1. Organometallic ruthenium complexes are also potential anticancer agents that show promising activity2.
Ofloxacin (ofloH, Scheme 1) belongs to the group of quinolone antibacterial agents and is successfully used in clinical practice3. The target of the quinolones is the enzyme DNA gyrase and it is also well established that quinolones interact with calf thymus DNA4. The mechanism of action of these drugs is not fully understood, but several authors have stressed the importance of magnesium ions in these interactions5. It is well-known that metal ions coordinate to quinolones and some complexes exert biological activity6. The synthesis and study of metal complexes with drugs used in clinical practice, which may exhibit synergistic activity, has attracted much attention as an approach to new drug development7. Crystal structures of ofloxacin complexes with Cu, Zn, Co and Mg were reported before6b, 8. We have prepared and characterized the first ruthenium organometallic complex of ofloxacin [(η6-p-cymene)RuCl(O,O-oflo)]·2.8H2O (1·2.8H2O). Since it is known that both, ruthenium and ofloxacin interact with DNA, it was also appealing to test how the title complex interacts with DNA.
Scheme 1.
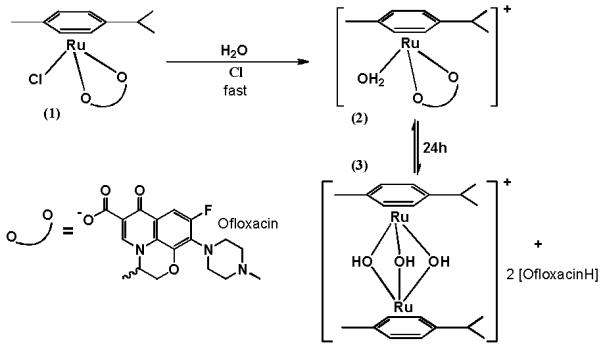
Main products of 1 hydrolysis are 2 and dimer 3.
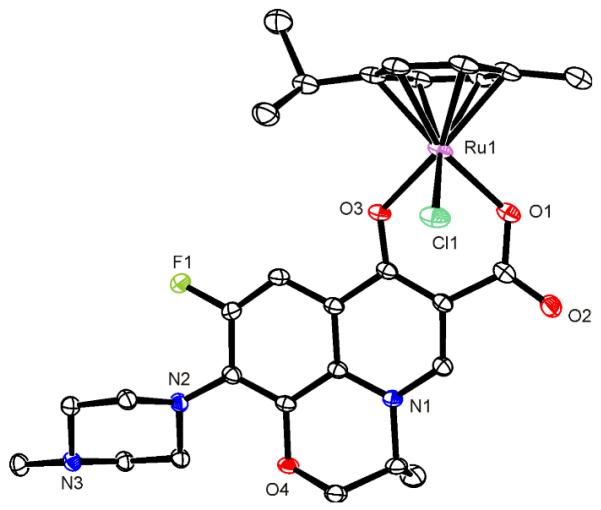
Title complex 1 can be prepared by treating the ligand with NaOH and ruthenium precursor ([RuCl(μ-Cl)(η6-p-cymene)]2, P1) in methanol. The microcrystallinic product was dissolved in CH2Cl2 and orange-brown crystals of 1·2.8H2O suitable for X-ray analysis were obtained by slow evaporation of a solvent. The complex adopts the pseudo-octahedral »piano-stool« geometry, with ruthenium(II) π-bonded to the p-cymene ring and σ-bonded to a chlorideand two oxygen atoms of the chelated quinolone ligand (Figure 1, Table S1).
Figure 1.
ORTEP diagram of 1•2.8H2O with thermal ellipsoids drawn at the 30% probability level. Hydrogen atoms are omitted for clarity. Selected bond lengths (Å) and angles (deg): Ru1–Cl1 2.4183(7), Ru1–O1 2.069(2), Ru1–O3 2.0713(18), O1–C6 1.293(3), O3–C9 1.275(3), O1–Ru1–O3 85.30(7), O1–Ru1–Cl1 83.73(6), O3–Ru1–Cl1 86.92(6).
In contrast to most metal-quinolone complexes reported so far which are only sparingly soluble6a, title complex is easily soluble in water (solubility 0.016 M at room temperature). We have realized that after dissolution hydrolysis occurs which was already reported for other ruthenium organometallic complexes with various ligands9. We have studied these processes by NMR spectroscopy and ESI-MS and the results are similar to those obtained by the others9. It can be concluded that first quick hydrolysis of the chloride ion is taking place (product 2) which is followed by the formation of a dimer9 (Scheme 1, Figures S1-S3, Table S2). However, we can clearly see from NMR experiments (Figure S1) that even after a day a substantial amount of the first hydrolysis product (58 %) is still present in solution.
It was established that the interaction of the title complex 1 with DNA is observed only in a solution with low ionic strengths. This is a clear evidence that the electrostatic attraction between the ruthenium complex and DNA in a solution is important for the binding. We assume that negatively charged DNA interacts with the positively charged hydrolytic products of 1. Fluorescence data of solutions containing DNA and 1 (Figure S4) indicate two types of binding. The first type of binding coincides with ofloxacin binding mode with the binding constant K = 1.2·106 and a maximal number of bound ruthenium atoms per 1 DNA base pair n = 0.02 (K and n were determined from the curve equation). The second type of binding is well recognized at r > 0,02 (r is the ratio of total concentrations of fluorophores (kept constant in the experiment) and DNA base pairs in a solution) (Figure S4) and corresponds to the ruthenium complex binding with DNA. The latter type of binding was analyzed also with UV-VIS spectroscopy and CD titration experiments (see below; Figure 2). As we can conclude from the hydrolysis study (mentioned above), the main species in aqueous (as well as in 5 mM NaCl) solution of 1 are ruthenium containing hydrolytic products and free ofloxacin. Each of these can interact with DNA differently. We have established that CD spectrum obtained by mixing solutions of DNA and 1 (Figure 2) is not just a simple mathematical sum of DNA-ofloxacin and DNA-P1 spectra. Title complex does not have signals in CD spectrum. However, when DNA was added to the title complex solution an induced CD (ICD) spectrum was obtained (signal is out of DNA and ofloH absorption bands). ICD at λ > 380 nm corresponds to the interaction of ruthenium with DNA because from all reactants used only the precursor P1 has an absorption band around 400 nm. However, the shape of CD spectrum for DNA-P1 solution is substantially different in this region. From these facts we can propose that in the DNA complex with the hydrolytic products of 1, ruthenium is bound to the quinolone.
Figure 2.
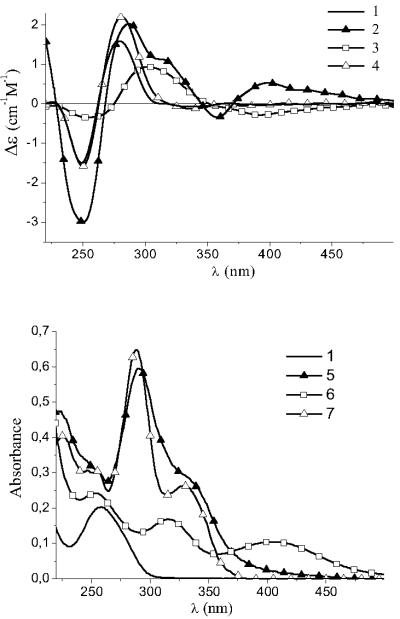
CD (above) and UV-VIS (below) spectra of free DNA (1), 1 (5), precursor P1 (6), ofloH (7) and DNA complexes with 1 (2), precursor P1 (3) and ofloH (4) in 5mM NaCl. C(1) = 2,1·10−5 M, C(P1) = 8·10−5 M, C(ofloH) = 2,2·10−5 M, C(DNA) = 7.5 10−5 (CD), 1.5 10−5 (UV-VIS) M (bp).
The analysis of ICD spectrum at constant 1 and different DNA concentrations (Figure S5) indicates the number of binding sites n=0.3 for 1. This result was obtained from the saturation of 1 binding with DNA from ICD dependence on r. It is essential to note that this analysis takes into account only DNA interaction with intact 2 which is accompanied with ICD and does not reflect other binding modes.
To get more details on the type of interaction between title complex and DNA competitive binding experiments with cisplatin, cis-[PtCl2(NH3)2], were performed. From these experiments it is clear that cisplatin and 1 compete for binding positions on DNA (Figure S6). Complex 1 (or cisplatin) was added into initial solution of DNA and after a day the second compound-cisplatin (or 1) was added. The concentration of cisplatin was 5·10−5 M since at this concentration almost all binding sites on DNA are occupied but its double stranded structure is still stable. For all systems under the study the spectra were recorded at the third day. The CD spectra have shown that 1 cannot bind to DNA after cisplatin has already coordinated (no typical ICD band appeared). On the other hand ICD bands (negative band at 345 nm and positive band at 400 nm) in solution of 1 and DNA do not disappear after the addition of cisplatin. This is a proof that also 1 strongly interacts with DNA and cisplatin cannot simply replace it. One possibility would be that there is a competition between cisplatin and 1 for the N7 guanine atom of DNA. However, our experiments at different pH values revealed that binding of the title complex occurs also when N7 is protonated (Figure S7). This is a proof that ruthenium binding to N7 does not occur (or at least is not crucial). An alternative interpretation is that the binding of cisplatin (or 1) causes the modification of DNA secondary structure and as a result the steric inconvenience prevents further binding of the second coordination compound to DNA. It is known that also cisplatin is prone to hydrolysis (one or both chlorides are displaced by aqua ligands). In the cell the resulting cationic species react with DNA to give numerous cisplatin-DNA adducts10. In the most important adduct cisplatin is chelated to two neighboring guanines at their N7 sites to form intrastrand cross-links, resulting in a kink of the DNA structure11. We can suppose, for example, that such Pt-N7 guanine binding and corresponding change of DNA geometry prevent ruthenium binding to DNA.
At the moment the details of the title complex-DNA binding are not known, but some assumptions can be done from our experimental results and the available literature data on similar systems (however, we should be aware that the systems are not ideally comparable). It is interesting to note a recently published structure of topoisomerase enzyme-DNA-moxifloxacin quinolone complex12. It was revealed that the role of metal (Mg2+) is very important. Magnesium is bidentately coordinated by the quinolone and four aqua ligands and mediates interactions with the DNA. There is no direct coordination between metal and DNA but coordinated water molecules are involved in hydrogen bonding with DNA nucleobases. Sadler et al. have studied the binding of various [(η6-arene)Ru(en)]2+ complexes to nucleotide phosphate groups. It was found that ruthenium interacts with phosphate though it was suggested that in DNA direct coordination of ruthenium to backbone phosphodiester groups is probably weak. However, electrostatic interactions and hydrogen bonding may be involved in the initial recognition of ruthenium complex prior to binding to guanine N7, which is similar to some platinum complexes13. We can therefore propose that also the positively charged hydrolytic products of 1 are first attracted to negatively charged DNA. After that binding of ruthenium to DNA occurs. Since our experiments revealed the binding to guanine N7 atoms is not crucial, other types of interaction (interaction with phosphate groups, hydrogen bonds, etc.) might be more important. It is also important to stress that our AFM experiments (Figure S8) have shown that title complex caused DNA shrinkage. At high concentration of 1 the formation of rather uniform compressed structures was observed, but not a condensation of DNA which was for example recently observed in ruthenium complexes that can intercalate into DNA14. Compound 1 was tested in in vitro tests against various microorganisms that are causing tropical diseases and in in vitro cytotoxicity experiments with rat skeletal myoblasts. The results have shown that compound 1 is moderately active against Trypanosoma b. rhodesiense, Trypanosoma cruzi and Plasmodium falciparum, while precursors P1 and ofloH are moderately active only against Plasmodium falciparum (Table S3). The enzyme inhibition tests (human topoisomerase IIα) have also been performed. Compound 1 shows no improved activity in comparison to free ofloH (Figure S9).
Supplementary Material
ACKNOWLEDGMENT
We thank for the financial support from the Slovenian Research Agency (J1-0200-0103-008; EN→FIST Centre of Excellence, Dunajska 156, SI-1000 Ljubljana) and the Ministry of Education and Science of Russian Federation and the Federal Agency of Education (grant AVTP “Development of science potential of High School” 4011). We thank Dr. B. Kralj and Dr. D. Žigon (Jožef Stefan Institute, Ljubljana, Slovenia) for mass spectral measurements. We thank Dr. J. Keiser and M. Kaiser (Swiss Tropical and Public Health Institute, Basel) for tropical disease tests.
Footnotes
SUPPORTING INFORMATION PARAGRAPH Experimental details, characterization data of compound, X-ray diffraction data, ESI-MS, NMR, fluorescence, CD, AFM, Biological tests, Figures S1-S9, Tables S1-S3. Additional literature data. This material is available free of charge via the internet at:
REFERENCES
- 1.(a) Rademaker-Lakhai JM, van den Bongard D, Pluim D, Beijnen JH, Schellens JHM. Clin. Cancer Res. 2004;10:3717–3727. doi: 10.1158/1078-0432.CCR-03-0746. [DOI] [PubMed] [Google Scholar]; (b) Hartinger CG, Zorbas-Seifried S, Jakupec MA, Kynast B, Zorbas H, Keppler BK. J. Inorg. Biochem. 2006;100:891–904. doi: 10.1016/j.jinorgbio.2006.02.013. [DOI] [PubMed] [Google Scholar]; (c) Sava G, Alessio E, Bergamo A, Mestroni G. In: Topics in biological inorganic chemistry. Clarke MJ, Sadler PJ, editors. Springer Verlag; Berlin: 1999. pp. 143–169. [Google Scholar]
- 2.Hartinger CG, Dyson PJ. Chem. Soc. Rev. 2009;38(2):391–401. doi: 10.1039/b707077m. [DOI] [PubMed] [Google Scholar]
- 3.Brighty KE, Gootz TD, Andriole VT. The Quinolones. Academic Press; San Diego: 2000. [Google Scholar]
- 4.Morrissey I, Hoshino K, Sato K, Yoshida A, Hayakawa I, Bures MG, Shen LL. Antimicrob. Agents Chemotherapy. 1996;40:1775–1784. doi: 10.1128/aac.40.8.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitscher LA. Chem. Rev. 2005;105:559–592. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]
- 6.(a) Turel I. Coord. Chem. Rev. 2002;232:27–47. [Google Scholar]; (b) Macias B, Villa MV, Rubio I, Castineiras A, Borras J. J. Inorg. Biochem. 2001;84:163–170. doi: 10.1016/s0162-0134(01)00182-9. [DOI] [PubMed] [Google Scholar]; (c) Katsarou ME, Efthimiadou EK, Psomas G, Karaliota A, Vourloumis D. J. Med. Chem. 2008;51:470–478. doi: 10.1021/jm7013259. [DOI] [PubMed] [Google Scholar]
- 7.Christofis P, Katsarou M, Papakyriakou A, Sanakis Y, Katsaros N, Psomas G. J. Inorg. Biochem. 2005;99:2197–2210. doi: 10.1016/j.jinorgbio.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 8.(a) Drevensek P, Kosmrlj J, Giester G, Skauge T, Sletten E, Sepcic K, Turel I. J. Inorg. Biochem. 2006;100:1755–1763. doi: 10.1016/j.jinorgbio.2006.06.011. [DOI] [PubMed] [Google Scholar]; (b) Yu LC, Lai L, Xia R, Liu SL. J. Coord. Chem. 2009;62:1313–1319. [Google Scholar]
- 9.(a) Peacock AFA, Melchart M, Deeth RJ, Habtemariam A, Parsons S, Sadler PJ. Chem.-Eur. J. 2007;13:2601–2613. doi: 10.1002/chem.200601152. [DOI] [PubMed] [Google Scholar]; (b) Grguric-Sipka S, Stepanenko IN, Lazic JM, Bartel C, Jakupec MA, Arion VB, Keppler BK. Dalton Trans. 2009;17:3334–3339. doi: 10.1039/b822725j. [DOI] [PubMed] [Google Scholar]
- 10.Reedijk J. Eur. J. Inorg. Chem. 2009;10:1303–1312. [Google Scholar]
- 11.Jung YW, Lippard SJ. Chem. Rev. 2007;107:1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 12.Wohlkonig A, Chan PF, Fosberry AP, Homes P, Huang J, Kranz M, Leydon VR, Miles TJ, Pearson ND, Perera RL, Shillings AJ, Gwynn MN, Bax BD. Nat. Struct. Mol. Biol. 2010;17:1152–1153. doi: 10.1038/nsmb.1892. [DOI] [PubMed] [Google Scholar]
- 13.Chen HM, Parkinson JA, Morris RE, Sadler PJ. J. Am. Chem. Soc. 2003;125:173–186. doi: 10.1021/ja027719m. [DOI] [PubMed] [Google Scholar]
- 14.Sun B, Guan JX, Xu L, Yu BL, Jiang L, Kou JF, Wang L, Ding XD, Chao H, Ji LN. Inorg. Chem. 2009;48:4637–4639. doi: 10.1021/ic900102r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.