Abstract
There is emerging evidence for an under-recognized hepatitis E virus (HEV) as a human pathogen. Among different reasons for this neglect are the unsatisfactory performance and under-utilization of commercial HEV diagnostic kits; for instance, the number of anti-HEV IgM kits marketed in China is about one-fifth of that of hepatitis A kits. Over the last two decades, substantial progress has been achieved in furthering our knowledge on the HEV-specific immune responses, antigenic features of HEV virions, and development of serological assays and more recently prophylactic vaccines. This review will focus on presenting the evidence of the importance of HEV infection for certain cohorts such as pregnant women, the key antigenic determinants of the virus, and immunogenicity and clinical efficacy conferred by a newly developed prophylactic vaccine. Robust immunogenicity, greater than 195-fold and approximately 50-fold increase of anti-HEV IgG level in seronegative and seropositive vaccinees, respectively, as well as impressive clinical efficacy of this vaccine was demonstrated. The protection rate against the hepatitis E disease and the virus infection was shown to be 100 % (95 % CI 75–100) and 78 % (95 % CI 66–86), respectively.
Keywords: Hepatitis E, Hepatitis E virus, Antigenic determinants, Vaccine, Immunogenicity, Vaccine efficacy
Introduction
Hepatitis E virus (HEV) is a major cause of acute hepatitis, along with much better recognized hepatitis A virus [1]. It is estimated that one-third of the world’s population has been infected with the virus [2]. An estimated 14 million symptomatic cases of HEV infection, with 300,000 deaths and 5,200 stillborns, occur annually in the world, mainly in developing countries of the Indian subcontinent, Southeast and Central Asia, the Middle East, and northern and western parts of Africa [3]. Hepatitis E refers to liver disease caused by the HEV, a small non-enveloped virus with single-stranded RNA genome. The virus, originally identified in 1983, has four distinct genotypes but with only one serotype. Genotypes 1 and 2 infect humans only, whereas genotypes 3 and 4 also infect pigs and several other mammalian species.
There has been increased attention on HEV and the associated disease in recent years owing to the increased number of reports of autochthonous patients from many developed countries. Vaccine development and better disease management are expected to emerge with increased knowledge on pathogenesis and virology of HEV. This review focuses on the hepatitis E disease, the pathogen HEV, key antigenic determinants of HEV, and the development and performance of a recombinant protein-based prophylactic vaccine.
Hepatitis E disease and the virus
HEV as a human pathogen has been largely overlooked for many years, except in certain outbreaks. In addition to epidemics at different scales and during humanitarian crises among refugee populations without access to clean water, sporadic cases and small clusters of HEV infections have been recognized throughout the world in recent years. Nelson et al. [4] recently reviewed the emerging evidence of HEV infection as an under-recognized pathogen in patients with hepatitis in developed countries.
Acute and chronic HEV infections among certain populations, such as transplant recipients and other immunocompromised individuals including HIV/AIDS patients, were also increasingly reported in recent years [4–6]. Evolution into chronic hepatitis was evidenced by persistently elevated aminotransferase levels, which is quite common during the post-surgery phase for organ transplantation patients. Approximately 65.9 % of 85 patients with HEV infection were identified to have chronic hepatitis after solid-organ transplantation due to immunosuppression [7]. In an earlier report, 8 out 14 patients had developed chronic disease as a result of HEV infection plus organ transplantation, as confirmed by persistently elevated aminotransferase levels, serum HEV RNA, and histologic features of chronic hepatitis [6]. Reducing the level of immune suppression led to spontaneous viral clearance in one-third of the patients [7]. Because of the unreliability of antibody tests in this immunocompromised population, direct molecular assay is necessary for diagnosis of HEV infection.
Unique to HEV, high mortality among pregnant women particularly during the third trimester distinguishes HEV from other causes of acute viral hepatitis. During the large-scale phase III trial of an HEV vaccine, the treatment showed similar safety profiles and comparable immunogenicity among some women who had become pregnant during the clinical trials. The retrospective analysis of the safety and immunogenicity showed the real promise of the vaccine in protecting this population which is most vulnerable to this virus [8].
Recent surveillance data suggested that in rural Bangladesh acute hepatitis, most of it likely hepatitis E, is responsible for approximately 10 % of pregnancy-associated deaths. If the death rates due to acute hepatitis are representative of South Asia in general, as many as 10,500 maternal deaths each year in this region alone may be attributable to hepatitis E and, moreover, could be prevented with existing vaccines [9]. Two HEV vaccines have gone through clinical evaluation with one having been licensed recently in China. This underscores the importance of testing the vaccine among pregnant women and could be truly life-saving once widely adopted for women of child-bearing ages. The molecular basis for the grave outcome of HEV infection among pregnant women is not clear, although progress is being made by several laboratories across the globe working on the virology of HEV.
Though HEV does not grow well in cell culture, several aspects of its biology and pathogenesis have been elucidated using animal models and cell transfection studies, and by analogy with other related viruses. The HEV is a spherical, non-enveloped, single-stranded, positive-sense RNA virus that is approximately 32–34 nm in diameter and is the only member in the family Hepeviridae and genus Hepevirus. While genotype 1 is predominantly associated with large epidemics in developing countries, genotype 3 has recently emerged as a clinically significant pathogen in developed countries. The clinical manifestations and the laboratory abnormalities of hepatitis E are not distinguishable from those caused by other hepatitis viruses, except the grave outcome among pregnant women. This may have caused significant under-reporting of HEV as a pathogen for hepatitis cases. In recent years, the number of reported hepatitis E cases in China grew rapidly with more HEV diagnostic kits being used in the clinical testing laboratories. Interestingly, the growth rate of the reported hepatitis E cases nicely matches the growth rate of the diagnostic kit (anti-HEV IgM kit) sold on the Chinese market (Fig. 1). Thus, the increase in hepatitis E cases reflects better diagnosis with increased adoption of the anti-HEV IgM diagnostic kit, rather than a true increase of HEV infection or the disease.
Fig. 1.
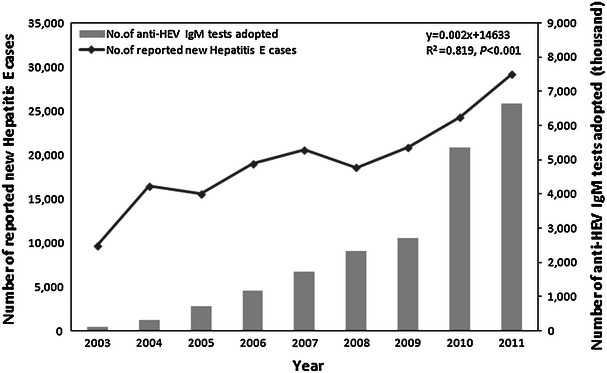
Reported new cases of hepatitis E and sales of anti-HEV IgM test kits in China (2003–2011). More reported hepatitis E cases due to expanded utilization of the diagnostic kits as indicated by similar trending of anti-HEV IgM kit consumption and number of reported cases in China. The estimated number (1 unit = 1,000 tests) of HEV diagnostic kit (anti-HEV IgM kit) sold in China on an annual basis (data were courteously provided by Qiu ZX, Wantai Biologics, Beijing). The jump of anti-HEV IgM testing numbers since 2009 can be explained by the national policy that recent HEV infection had to be excluded before issuing food health certificates since September 2009. The increased number of reported hepatitis E cases (data source, Chinese Ministry of Health, http://www.moh.gov.cn/publicfiles//business/htmlfiles/mohjbyfkzj/s2907/index.htm) on an annual basis for the past decade showed a similar trend with the increasing number of anti-HEV IgM kits sold in the Chinese market
Better understanding of the HEV virology and human serological response would certainly help to improve the disease management and likely facilitate the vaccine design and development. The viral genome is a polyadenylated, single-stranded, positive-sense RNA of about 7.2 kb with a short non-coding region at both the 5′ and the 3′ ends. It consists of three discontinuous and partially overlapping open reading frames (ORFs). The largest ORF is ORF 1, encoding non-structural proteins including methyltransferase, protease, helicase, and RNA-dependent RNA polymerase [10]. ORF2 encodes a structural protein—the only protein of the viral capsid. The smallest ORF, ORF3, encoding a phosphoprotein, was shown to be involved extensively in viral evasion of the immune system and regulation of viral replication and capsid assembly [11–18]. HEV is a non-cytopathic or weakly cytopathic virus, with immune-pathology playing an important role in the pathogenic mechanism of HEV infection. Not surprisingly, the immune system directs the immune response mainly against the capsid protein pORF2. Therefore, most of the diagnostic kits are designed to detect the antibodies in sera against HEV pORF2 epitopes. Better disease management relies on robust-performing diagnostic kits to confirm the viral infection.
Clinical biomarkers for diagnosis and lessons learned from animal models
The lack of a standardized assay for clinical diagnosis is still an issue, leading to likely under-reporting of HEV being the pathogen for acute hepatitis cases. Even today, there is no US Food and Drug Administration (FDA)-approved diagnostic kit for hepatitis E in the USA. Although there is a World Health Organization (WHO) reference reagent for antibody against HEV available through the National Institute for Biological Standards and Control (NIBSC) since 1997, it is not being commonly included in routine testing or used as a standard for comparing different tests. This reference reagent is human serum containing antibody against HEV with assigned unitage at 100 U/ml; in this paper, we refer to it as 100 Wu/ml. Even with the same set of serum samples, the results could be quite different depending on the anti-HEV kits used in the assay. As Zhang et al. [19] indicated, the prevalence rate of antibodies detected by another assay (IgG kit by GenLabs) using antigen not including the E2s domain was much lower at the plateau. In addition, although results obtained by the Wantai E2-based anti-HEV IgG assay showed full conversion with a robust plateau at 100 %, the seroconversion was delayed, reaching an incomplete prevalence rate and with the prevalence rate declining after having peaked at a suboptimal level for those infected animals [20]. Similarly with clinical samples, comparative studies of the Wantai E2 assay and GL peptide-based assay showed lower limits of detection for these two anti-HEV IgG kits: 0.25 and 2.5 Wu/ml, respectively. With a tenfold higher assay sensitivity, much better results were obtained with the Wantai E2 kit: (a) better detection for positivity in human sera from confirmed cases (98 vs. 56 %); (b) IgG levels remained positive for longer periods of time post infection; and (c) resulted in a substantially higher estimate of seroprevalence in blood donors (16.2 vs. 3.6 %) with the same set of samples [21]. The main difference between the two assays is the antigen used. The GL assay uses a mixture of peptides from both pORF2 and pORF3 specified by the C-terminal of pORF2 (aa 613–660), C-terminal of pORF3 (aa 91–123), and the full-length pORF3 [22]. As revealed by the cryo-electron microscopy (EM) and X-ray crystallographic structures of the HEV virus-like particle (VLP), the protruding E2s domain of viral capsid was clearly identified as the region with immune-dominant epitopes as shown in Fig. 2 [23–27]. The lack of E2 domain in the GL assay resulted in the inability of the GL assay to detect the immuno-dominant epitopes in the dimeric E2 domain. In contrast, the E2 assay uses the recombinant protein pE2, with ORF2 (aa 394–606), correctly presenting the dimerized form of the E2s domain with the conformational epitopes preserved just like those in the virions.
Fig. 2.
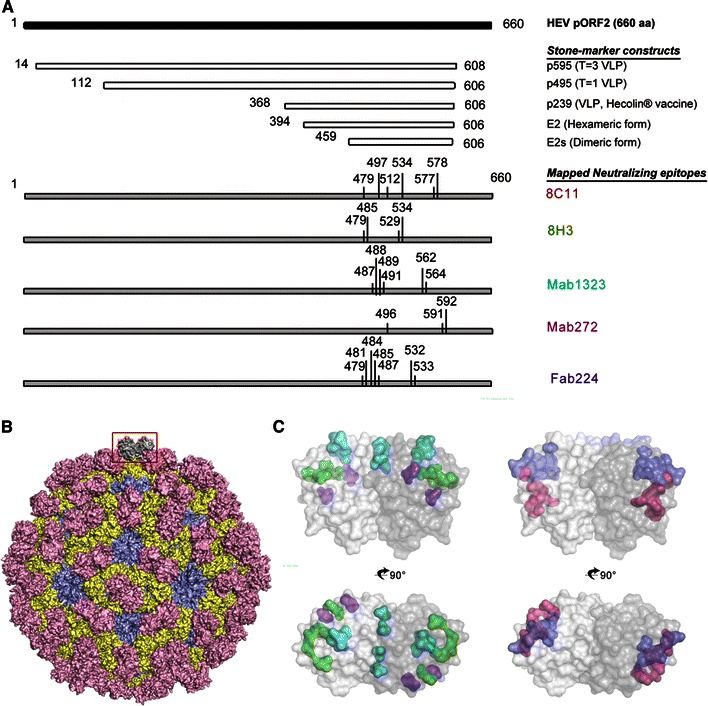
Key antigenic determinants on HEV pORF2. a Antigenic and assembly properties of truncated pORF2. b Crystal structure of HEV capsid protein. Crystal structure of T = 1 HEV-VLP (PDB:2ZTN). Mesh representations of dimeric E2s domains highlighted the neutralizing epitopes against several neutralizing monoclonal antibodies. c Key neutralizing epitopes on pORF2. All the identified neutralizing sites were mapped in E2s domain [23, 36, 48, 57, 61]
Different kits were used to report the epidemiology data in the field, causing a certain degree of confusion. When different antigens were utilized, different assay specificity and sensitivity were obtained, leading to different results or even conclusions on previous exposure to HEV by detection of IgG antibodies [28–30]. Therefore, precautions are needed when comparing the epidemiology data or animal serological data obtained with different assays.
Infected monkeys were resistant to secondary attack of symptomatic infection; however, only high levels of IgG antibody could provide complete protection from secondary asymptomatic infection [31]. In a monkey model, the IgG antibody response to the re-challenge varied from unaffected to up to tenfold enhancement, whereas the IgM response may or may not be detectable. These results clearly demonstrated the different dynamics of IgG and IgM during the primary and secondary HEV infections.
Therefore, four biomarkers—viral RNA, anti-HEV IgM, anti-HEV IgG (at least fourfold rise), and low avidity of anti-HEV IgG—are important in the diagnosis of HEV infection, particularly for patients presenting with acute hepatitis symptoms. This toolbox of genomic and immunological assays is valuable in furthering our understanding of the time course of HEV infection and the subsequent hepatitis. The knowledge gained from animal serology or clinical samples facilitated the design and development of a prophylactic vaccine during preclinical and clinical development of an efficacious vaccine [19]. In cases caused by primary infection, there was a clear separation between low avidity antibody produced soon after infection and high avidity antibody produced later with a certain maturation period [32–34]. Reinfection in humans are also indicated by much high IgG avidity in the acute phase of illness, with varying levels of IgM as reported in several studies [33–36]. The difficulty encountered with the individual markers in the diagnosis of hepatitis E could be partially overcome by using a combination of all four markers, i.e., viral RNA, anti-HEV IgM, rising IgG, and IgG avidity [35].
Besides the aforementioned serological biomarkers, anti-HEV IgA had been tested for its value in the diagnosis of hepatitis E. In a study with 68 patients, complete seropositivity was reported for all patients using the anti-HEV IgM or IgA assays [37]. In a more recent study, all three antibody assays were performed for serum samples from 81 acute hepatitis patients. The seropositivity rate was 9.9, 7.4, and 3.7 % for IgG, IgM, and IgA, respectively [38]. In both studies, good correlation between the anti-HEV IgA and anti-HEV IgM assays was observed, with both markers being more stringent markers for viremic HEV infection during the acute phase of HEV-associated hepatitis. Similar kinetics for IgM and IgA were reported for HEV infection [39], along with the longer detection period for IgM and IgA as compared to serum HEV RNA for the same set of samples [37]. Although anti-HEV IgA did not show additional value to anti-HEV IgM in the diagnosis of acute HEV infection, further data are needed to understand the diagnostic importance of anti-HEV IgA antibodies.
Key antigenic determinants of HEV
High immunoreactivity of serum samples was demonstrated to recombinant pORF2, particularly to the dimer form, but not to the monomer form. These samples include sera from laboratory-infected animals including non-human primates or from naturally infected individuals in the acute or convalescent phase of HEV-induced hepatitis [40, 41]. These results indicated the dominant immune response to the HEV capsid protein during viral infection. ORF2 encodes a single capsid protein of 660 aa, with the N-terminal 112 residues involved in packaging of the viral genome. The overall architecture of HEV capsid was elucidated first from the cryo-EM structure of the VLP and then the crystallographic structure of the VLPs of an N-terminal and C-terminal truncated fragment (aa 14–608 or aa 112–608) of pORF2 [23–27]. The general architecture of the viral capsid is as follows: (1) the capsid subunit consists of partial homodimer of the structural protein; (2) the S domain (aa 118–313) forms the virus shell, the P2 or P domain (aa 454–606) forms the protrusion projecting from the shell, and the P1 or M domain (aa 314–453) contributes two-, three-, and fivefold icosahedral symmetry of the virus capsid [26, 27]. The presence of the S domain is essential to form the T = 1 or T = 3 icosahedral VLP. In the absence of the S domain, the P1 and P2 domains were predicted to form smaller particles [42, 43].
The detailed structure of the P2 and P1 domains and their functions were elucidated by the bacterially expressed peptides, E2s (aa 455–603), pE2 (aa 394–606), and p239 (aa 368–606). High resolution (<2 Å) crystal structures of the E2s domain (equivalent to the P2 domain) from genotypes 1 and 4 revealed an intimate interaction at the dimeric interface in the homodimer, happening in twofold direction of VLP [42]. A segment of the 66-aa extension to the N terminus of E2s (pE2) enabled the spontaneously assembly into hexamers in solution with neutral pH. Thus, the domain aa 394–458 is involved in the interaction between the dimeric domain to form the protrusions projecting from the surface of the virus shell [24]. For fivefold axial interaction, the region centered at Tyr-288 plays a key role in the capsid assembly [36]. Peptide pE2 contains 66 additional residues, and p239 contains another additional 26 residues, in the P1 domain (Fig. 2). The antigenicity or antibody binding activity of these peptides is quite similar [23]. The 66-aa extension in the P1 domain appears to stabilize the dimeric structure of pE2, rendering it a useful diagnostic agent, whereas the additional 26-aa extension in p239 results in the formation of multimeric VLP, rendering it more suitable as a vaccine candidate with much enhanced immunogenicity [24].
Overall structural features of the E2s domain were observed for four different genotypes, albeit there were some minor structural variations in the side chains of different residues. These observations are consistent with the serotyping based on immune reactivity to conclude with a single serotype for four different HEV genotypes [44]. The detailed functional profiles of the two kinds of genotypes represented by genotypes 1 and 2 for human infection only and by genotypes 3 and 4 for zoonosis remain unclear. The crystal structure of E2s, in complex with the Fab fragment of a neutralizing and protective monoclonal antibody 8C11, indicates that aa 497 is involved in determining the host specificity of the virus [44].
Experimental results indicated that human serum antibodies against HEV protected people from serious illness during outbreaks [45]. Furthermore, experimental HEV infections in animal models elicited an antibody response which results in protective immunity [46]. As with most other viruses [47–53], the outer protrusions on the virion surface of HEV, harboring major neutralizing and protective epitopes, are essential for host recognition [54]. Several recombinant proteins containing the E2s domain have been shown to induce protective immunity against liver injury in animals and some in human studies by homologous and heterologous HEV infection [41, 45, 55–60]. Efforts were made to characterize the neutralization sites located on the native virions or recombinant VLPs as probed with a large panel of monoclonal antibodies [61–67]. To date, all identified neutralizing sites were conformational and were mapped in the E2s domain of pORF2 and are composed with discontinuous peptide stretches (Fig. 2).
On the basis of the knowledge on HEV virology, virus-induced immune response, and epidemiology, various protein-based vaccines with truncated versions of capsid protein pORF2 (Fig. 2) were designed and tested in preclinical models. Two of the vaccine candidates have undergone clinical assessment with p239 being recently licensed in China.
Clinical evaluation of a prophylactic vaccine
To develop a prophylactic vaccine, one needs to understand as much as possible the disease course and the immune response from the natural infection. Clinically, HEV infection manifests in different ways from asymptomatic to fulminant hepatitis. About 20 % of infections are symptomatic in high disease endemic areas where genotype 1 is the predominant type. Among those with symptomatic hepatitis E disease, the probability of death is estimated at approximately 20 % for cases involving pregnant women and approximately 2 % for other cases [45, 68–71].
Symptomatic primary infection was shown to elicit a strong antibody response with anti-HEV IgG reaching a mean of 80.9 Wu/ml. Other types of infection, i.e., asymptomatic primary infection, symptomatic reinfection, and asymptomatic reinfection, only induced a modest antibody response of approximately 4 Wu/ml [19]. The fact that anti-HEV IgG antibody response to symptomatic reinfection and their peak ALT levels are both significantly lower than those in symptomatic primary infection suggested that pre-existing immunity limited the extent of the infection in these patients (Zhu et al., personal communication).
Recombinant VLPs, consisting of various lengths of the HEV capsid protein pORF2, were prepared to mimic the protective and neutralizing epitopes on the virion surface as probed and defined by different monoclonal antibodies (Fig. 2). These immunogens were designed to elicit protective immunity when injected into non-human primates or human volunteers during preclinical tests and clinical trials. The protective immunity conferred by an insect cell-expressed vaccine candidate, rHEV (Fig. 2), was first demonstrated in a phase II clinical trial conducted in Nepal, where the endemic stain is genotype 1, with vaccine against homologous genotype 1 strain [58]. Three vaccine doses elicited complete seroconversion in all participants. Although antibodies were undetectable in about half of them 2 years later, the vaccine was highly efficacious against symptomatic HEV infection for at least 2 years, i.e., 96 % (95 % CI 86–99) [58].
The doubt whether hepatitis E vaccine is protective against heterogenous virus infection in humans had been eliminated by a phase III trial conducted in China. This large-scale, randomized, controlled blinded clinical trial involved 112,604 subjects belonging to the same Chinese community described above, where the majority of HEV isolated from patients are genotype 4 [59]. The recombinant protein-based vaccine candidate, later branded as Hecolin®, contains 30 μg of HEV239 antigen encompassing 368–606 aa of ORF2 (Fig. 2) of HEV genotype 1 expressed in Escherichia coli. Three shots of vaccination elicited a robust antibody response with the anti-HEV IgG lasting for at least 2 years in most subjects. The vaccine-induced peak IgG antibody level was in-between that of symptomatic primary infection and that of asymptomatic infection [19].
Hecolin® vaccine, with a three-dose regime, elicited a robust immune response as indicated by the anti-HEV IgG level, much higher that of a natural infection (15.0 Wu in naïve group post immunization vs. 1.9 Wu from asymptomatic natural infection) as shown in Table 1. Hecolin® vaccine is highly protective for symptomatic HEV infection with efficacy of 100 % (95 % CI 72–100). More importantly, over 90 % of the symptomatic HEV infection cases in the placebo group were caused by heterogenous genotype 4 [58]. The determination of protection antibody level for symptomatic infection is prevented by the lack of cases in the vaccine group. Similar to the observation made in the Nepal trial of the GlaxoSmithKline vaccine, two shots of Hecolin® in 1 month induced immediate protection against hepatitis E for at least 5 months till the third shot. Hence the use of this prophylactic vaccine in the rapid control of an epidemic is justified.
Table 1.
Safety, immunogenicity, and efficacy conferred by vaccination with Hecolin® during a large-scale clinical trial
| Property | Performance | Ref. |
|---|---|---|
| Safety | In healthy adults: • Most adverse events were mild; vaccine-related serious AEs had not been observed • Rates of solicited local AEs were higher in the vaccine group than in the placebo group (13 vs. 7 %) and the rates of solicited systemic AEs were not significantly different between the two groups (20 vs. 20 %) • Reported AE of the entire immunized population was <2 % of both vaccine and placebo groups In pregnant women: • Appeared to be safe for mothers and fetus |
[5, 53] |
| Immunogenicity | Robust humoral response—Hecolin® induced, almost completely seroconversion, and reached sevenfold higher level of anti-HEV IgG (15 Wu/ml) than immunity induced by asymptomatic infection (1.9 Wu/ml) | [28] |
| Efficacy | 100 % (72–100) against hepatitis E disease; 78 % (66–86) against HEV infection; 100 % (9–100) against hepatitis E disease after receiving two shots in 1 month |
[28, 53] |
AE adverse event
Additional properties of Hecolin® and its performance during this trial are summarized in Table 1. Adverse events attributable to the vaccine were few and mild; within the closely monitored reactogenicity subset, solicited systematic adverse events within 72 after each vaccination were less than 20 % for both vaccinated group and placebo controls. Yet the reported systematic adverse events of the entire vaccinated cohort were only 2 % from both the vaccinated and the control groups [59]. Interestingly, in the duration of the study, we had the opportunity to observe some pregnancy in patients in the vaccinated group, and their newborn babies. All pregnant women went to full-term childbirth and no spontaneous abortion was observed; the babies were born without congenital abnormality [8].
Taken together, the findings showed that hepatitis E vaccine is highly immunogenic and protective for symptomatic infection caused by homologous or heterogenous HEV. It can effectively lower, but not entirely eliminate, the risk of asymptomatic infection, whereas it does confer complete protection from symptomatic hepatitis E disease. Both the virus breakthrough in vaccinated individuals and the reinfection in individuals with naturally acquired immunity are associated with a lower initial anti-HEV IgG antibody level. The protection level of vaccine-induced antibody has not been determined to date.
Conclusion and future perspective
This review summarizes the current understanding of the HEV virology, the key neutralization sites on the surface of the viral capsid, and the immunogenicity and clinical efficacy of a newly developed human vaccine. Protective immunity against hepatitis E can be obtained by natural exposure to the virus as well as by vaccination. Whereas the natural exposure to the virus left most individuals unprotected against reinfection, vaccination with Hecolin® showed superior results with a robust immune response and a virtually complete protective immunity against the disease in healthy adults. The value of vaccination in public health could be further realized by lowering the number of asymptomatic virus carriers who might be the reservoirs of food-borne or water-borne outbreaks. The latter is especially meaningful in areas endemic for genotype 1 or 2 viruses because of their singular human host. It is also expected that the vaccination should benefit high-risk populations such as pregnant women, patients with chronic liver diseases, and patients undergoing immunosuppressing therapy. Hepatitis E infection, which could be prevented with a vaccine, and the subsequent disease could be life-threatening among those cohorts. Much efforts and well-planned studies are needed to better understand the vaccine safety and efficacy in these corresponding populations.
Further studies are also needed to determine the duration of protection from vaccination, the need for boosters, and its efficacy in preventing disease when administered after exposure to HEV has occurred [72].
Although the vaccination can be an effective way to dramatically lower the HEV infection rate and to reduce the disease, the key prevention strategy to reduce disease burden of hepatitis E is improving the overall sanitation to root out the HEV in the water supply and food chains. Unfortunately, this may not be realized in the hyper-endemic areas. Two recombinant protein-based vaccine candidates showed a good safety profile, high immunogenicity, and high efficacy against symptomatic and/or asymptomatic HEV infection. One of the candidates was recently licensed in China [57–59]. A good post-launch vaccination adoption strategy for an effective prophylactic vaccine is expected to afford an effective means to reduce the morbidity and mortality caused by HEV infection. The first hepatitis E vaccine, Hecolin®, was launched in China in October 2012. The much-needed use of the vaccine in countries outside China is hindered by the fact that the hepatitis E vaccine is not on the priority vaccine list of WHO prequalification. Evaluation of Hecolin® in high-risk cohorts, such as pregnant women, children, and immunocompromised individuals, will be the focus of future clinical studies while the vaccine is being adopted for healthy adults on the basis of the current label claims.
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- aa
Amino acid
- EM
Electron microscopy
- GMC
Geometric mean concentration
- HEV
Hepatitis E virus
- ORF
Opening reading frame
- VLP
Virus-like particle
- Wu
WHO units of anti-HEV IgG
Contributor Information
Ning-Shao Xia, Email: nsxia@xmu.edu.cn.
James Wai-Kuo Shih, Phone: +86-592-2181661, FAX: +86-592-2181258, Email: jwshih@xmu.edu.cn.
References
- 1.Teshale EH, Hu DJ, Holmberg SD. The two faces of hepatitis E virus. Clin Infect Dis. 2010;51(3):328–334. doi: 10.1086/653943. [DOI] [PubMed] [Google Scholar]
- 2.Purcell RH, Emerson SU. Prevention. In: Thomas HC, Lemon S, Zuckerman AJ, editors. Viral hepatitis. 3. Malden: Blackwell; 2005. pp. 635–645. [Google Scholar]
- 3.Aggarwal R. The global prevalence of hepatitis E virus infection and susceptibility: a systematic review. WHO/IVB/10.14. Viral hepatitis in the WHO South-East Asia region, 2011, SEA-CD-232; 2010.
- 4.Nelson KE, Kmush B, Labrique AB. The epidemiology of hepatitis E virus infections in developed countries and among immunocompromised patients. Expert Rev Anti Infect Ther. 2011;9(12):1133–1148. doi: 10.1586/eri.11.138. [DOI] [PubMed] [Google Scholar]
- 5.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med. 2009;361(10):1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 6.Kamar N, Selves J, Mansuy JM, Ouezzani L, Peron JM, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med. 2008;358(8):811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 7.Kamar N, Garrouste C, Haagsma EB, Garrigue V, Pischke S, Chauvet C, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology. 2011;140(5):1481–1489. doi: 10.1053/j.gastro.2011.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Wu T, Zhu FC, Huang SJ, Zhang XF, Wang ZZ, Zhang J, et al. Safety of the hepatitis E vaccine for pregnant women: a preliminary analysis. Hepatology. 2012;55(6):2038. doi: 10.1002/hep.25522. [DOI] [PubMed] [Google Scholar]
- 9.Labrique AB, Zaman K, Hossain Z, Saha P, Yunus M, Hossain A, et al. Epidemiology and risk factors of incident hepatitis E virus infections in rural Bangladesh. Am J Epidemiol. 2010;172(8):952–961. doi: 10.1093/aje/kwq225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koonin EV, Gorbalenya AE, Purdy MA, Rozanov MN, Reyes GR, Bradley DW. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: delineation of an additional group of positive-strand RNA plant and animal viruses. Proc Natl Acad Sci U S A. 1992;89(17):8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zafrullah M, Ozdener MH, Panda SK, Jameel S. The ORF3 protein of hepatitis E virus is a phosphoprotein that associates with the cytoskeleton. J Virol. 1997;71(12):9045–9053. doi: 10.1128/jvi.71.12.9045-9053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panda SK, Thakral D, Rehman S. Hepatitis E virus. Rev Med Virol. 2007;17(3):151–180. doi: 10.1002/rmv.522. [DOI] [PubMed] [Google Scholar]
- 13.Tyagi S, Korkaya H, Zafrullah M, Jameel S, Lal SK. The phosphorylated form of the ORF3 protein of hepatitis E virus interacts with its non-glycosylated form of the major capsid protein, ORF2. J Biol Chem. 2002;277(25):22759–22767. doi: 10.1074/jbc.M200185200. [DOI] [PubMed] [Google Scholar]
- 14.Tyagi S, Surjit M, Lal SK. The 41-amino-acid C-terminal region of the hepatitis E virus ORF3 protein interacts with bikunin, a kunitz-type serine protease inhibitor. J Virol. 2005;79(18):12081–12087. doi: 10.1128/JVI.79.18.12081-12087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyagi S, Surjit M, Roy AK, Jameel S, Lal SK. The ORF3 protein of hepatitis E virus interacts with liver-specific alpha1-microglobulin and its precursor alpha1-microglobulin/bikunin precursor (AMBP) and expedites their export from the hepatocyte. J Biol Chem. 2004;279(28):29308–29319. doi: 10.1074/jbc.M402017200. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Takahashi M, Hoshino Y, Takahashi H, Ichiyama K, Nagashima S, et al. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. J General Virol. 2009;90(Pt 8):1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]
- 17.Chandra V, Kalia M, Hajela K, Jameel S. The ORF3 protein of hepatitis E virus delays degradation of activated growth factor receptors by interacting with CIN85 and blocking formation of the Cbl-CIN85 complex. J Virol. 2010;84(8):3857–3867. doi: 10.1128/JVI.01994-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi M, Yamada K, Hoshino Y, Takahashi H, Ichiyama K, Tanaka T, et al. Monoclonal antibodies raised against the ORF3 protein of hepatitis E virus (HEV) can capture HEV particles in culture supernatant and serum but not those in feces. Arch Virol. 2008;153(9):1703–1713. doi: 10.1007/s00705-008-0179-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Li SW, Wu T, Zhao Q, Ng MH, Xia NS. Hepatitis E virus: neutralizing sites, diagnosis, and protective immunity. Rev Med Virol. 2012;22(5):339–349. doi: 10.1002/rmv.1719. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Ge SX, Huang GY, Li SW, He ZQ, Wang YB, et al. Evaluation of antibody-based and nucleic acid-based assays for diagnosis of hepatitis E virus infection in a rhesus monkey model. J Med Virol. 2003;71(4):518–526. doi: 10.1002/jmv.10523. [DOI] [PubMed] [Google Scholar]
- 21.Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82(5):799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- 22.Lee SD, Wang YJ, Lu RH, Chan CY, Lo KJ, Moeckli R. Seroprevalence of antibody to hepatitis E virus among Chinese subjects in Taiwan. Hepatology. 1994;19(4):866–870. doi: 10.1002/hep.1840190410. [DOI] [PubMed] [Google Scholar]
- 23.Xing L, Kato K, Li T, Takeda N, Miyamura T, Hammar L, et al. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T = 1 particle presenting native virus epitopes. Virology. 1999;265(1):35–45. doi: 10.1006/viro.1999.0005. [DOI] [PubMed] [Google Scholar]
- 24.Li TC, Takeda N, Miyamura T, Matsuura Y, Wang JC, Engvall H, et al. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J Virol. 2005;79(20):12999–13006. doi: 10.1128/JVI.79.20.12999-13006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xing L, Li TC, Mayazaki N, Simon MN, Wall JS, Moore M, et al. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J Biol Chem. 2010;285(43):33175–33183. doi: 10.1074/jbc.M110.106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guu TS, Liu Z, Ye Q, Mata DA, Li K, Yin C, et al. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc Natl Acad Sci U S A. 2009;106(31):12992–12997. doi: 10.1073/pnas.0904848106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita T, Mori Y, Miyazaki N, Cheng RH, Yoshimura M, Unno H, et al. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci U S A. 2009;106(31):12986–12991. doi: 10.1073/pnas.0903699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mast EE, Alter MJ, Holland PV, Purcell RH. Evaluation of assays for antibody to hepatitis E virus by a serum panel. Hepatitis E Virus Antibody Serum Panel Evaluation Group. Hepatology. 1998;27(3):857–861. doi: 10.1002/hep.510270331. [DOI] [PubMed] [Google Scholar]
- 29.Ghabrah TM, Tsarev S, Yarbough PO, Emerson SU, Strickland GT, Purcell RH. Comparison of tests for antibody to hepatitis E virus. J Med Virol. 1998;55(2):134–137. doi: 10.1002/(SICI)1096-9071(199806)55:2<134::AID-JMV9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Khudyakov Y, Kamili S. Serological diagnostics of hepatitis E virus infection. Virus Res. 2011;161(1):84–92. doi: 10.1016/j.virusres.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 31.Huang W, Zhang H, Harrison TJ, Lang S, Huang G, Wang Y. Cross-protection of hepatitis E virus genotypes 1 and 4 in Rhesus macaques. J Med Virol. 2008;80(5):824–832. doi: 10.1002/jmv.21140. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JZ, Im SW, Lau SH, Chau TN, Lai ST, Ng SP, et al. Occurrence of hepatitis E virus IgM, low avidity IgG serum antibodies, and viremia in sporadic cases of non-A, -B, and -C acute hepatitis. J Med Virol. 2002;66(1):40–48. doi: 10.1002/jmv.2109. [DOI] [PubMed] [Google Scholar]
- 33.Bendall R, Ellis V, Ijaz S, Thurairajah P, Dalton HR. Serological response to hepatitis E virus genotype 3 infection: IgG quantitation, avidity, and IgM response. J Med Virol. 2008;80(1):95–101. doi: 10.1002/jmv.21033. [DOI] [PubMed] [Google Scholar]
- 34.Bigaillon C, Tesse S, Lagathu G, Nicand E. Use of hepatitis E IgG avidity for diagnosis of hepatitis E infection. J Virol Methods. 2010;164(1–2):127–130. doi: 10.1016/j.jviromet.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 35.Huang S, Zhang X, Jiang H, Yan Q, Ai X, Wang Y, et al. Profile of acute infectious markers in sporadic hepatitis E. PLoS One. 2010;5(10):e13560. doi: 10.1371/journal.pone.0013560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seriwatana J, Shrestha MP, Scott RM, Tsarev SA, Vaughn DW, Myint KS, et al. Clinical and epidemiological relevance of quantitating hepatitis E virus-specific immunoglobulin M. Clin Diagn Lab Immunol. 2002;9(5):1072–1078. doi: 10.1128/CDLI.9.5.1072-1078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takahashi M, Kusakai S, Mizuo H, Suzuki K, Fujimura K, Masuko K, et al. Simultaneous detection of immunoglobulin A (IgA) and IgM antibodies against hepatitis E virus (HEV) is highly specific for diagnosis of acute HEV infection. J Clin Microbiol. 2005;43(1):49–56. doi: 10.1128/JCM.43.1.49-56.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elkady A, Tanaka Y, Kurbanov F, Hirashima N, Sugiyama M, Khan A, et al. Evaluation of anti-hepatitis E virus (HEV) immunoglobulin A in a serological screening for HEV infection. J Gastroenterol. 2007;42(11):911–917. doi: 10.1007/s00535-007-2109-x. [DOI] [PubMed] [Google Scholar]
- 39.Herremans M, Duizer E, Jusic E, Koopmans MP. Detection of hepatitis E virus-specific immunoglobulin A in patients infected with hepatitis E virus genotype 1 or 3. Clin Vaccine Immunol. 2007;14(3):276–280. doi: 10.1128/CVI.00312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang JZ, Ng MH, Xia NS, Lau SH, Che XY, Chau TN, et al. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J Med Virol. 2001;64(2):125–132. doi: 10.1002/jmv.1027. [DOI] [PubMed] [Google Scholar]
- 41.Im SW, Zhang JZ, Zhuang H, Che XY, Zhu WF, Xu GM, et al. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine. 2001;19(27):3726–3732. doi: 10.1016/S0264-410X(01)00100-1. [DOI] [PubMed] [Google Scholar]
- 42.Li SW, Zhang J, He ZQ, Gu Y, Liu RS, Lin J, et al. Mutational analysis of essential interactions involved in the assembly of hepatitis E virus capsid. J Biol Chem. 2005;280(5):3400–3406. doi: 10.1074/jbc.M410361200. [DOI] [PubMed] [Google Scholar]
- 43.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, et al. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine. 2005;23(22):2893–2901. doi: 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 44.Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, et al. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci U S A. 2011;108(25):10266–10271. doi: 10.1073/pnas.1101309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bryan JP, Tsarev SA, Iqbal M, Ticehurst J, Emerson S, Ahmed A, et al. Epidemic hepatitis E in Pakistan: patterns of serologic response and evidence that antibody to hepatitis E virus protects against disease. J Infect Dis. 1994;170(3):517–521. doi: 10.1093/infdis/170.3.517. [DOI] [PubMed] [Google Scholar]
- 46.Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, et al. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A. 1994;91(21):10198–10202. doi: 10.1073/pnas.91.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, Estes MK. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286(5438):287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 48.Dong J, Dong L, Mendez E, Tao Y. Crystal structure of the human astrovirus capsid spike. Proc Natl Acad Sci U S A. 2011;108(31):12681–12686. doi: 10.1073/pnas.1104834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pokidysheva E, Zhang Y, Battisti AJ, Bator-Kelly CM, Chipman PR, Xiao C, et al. Cryo-EM reconstruction of dengue virus in complex with the carbohydrate recognition domain of DC-SIGN. Cell. 2006;124(3):485–493. doi: 10.1016/j.cell.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 50.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393(6686):648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371(6492):37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, et al. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384(6605):179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 53.Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393(6686):705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 54.Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, et al. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog. 2009;5(8):e1000537. doi: 10.1371/journal.ppat.1000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li F, Torresi J, Locarnini SA, Zhuang H, Zhu W, Guo X, et al. Amino-terminal epitopes are exposed when full-length open reading frame 2 of hepatitis E virus is expressed in Escherichia coli, but carboxy-terminal epitopes are masked. J Med Virol. 1997;52(3):289–300. doi: 10.1002/(SICI)1096-9071(199707)52:3<289::AID-JMV10>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 56.Robinson RA, Burgess WH, Emerson SU, Leibowitz RS, Sosnovtseva SA, Tsarev S, et al. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr Purif. 1998;12(1):75–84. doi: 10.1006/prep.1997.0817. [DOI] [PubMed] [Google Scholar]
- 57.Zhang M, Emerson SU, Nguyen H, Engle RE, Govindarajan S, Gerin JL, et al. Immunogenicity and protective efficacy of a vaccine prepared from 53 kDa truncated hepatitis E virus capsid protein expressed in insect cells. Vaccine. 2001;20(5–6):853–857. doi: 10.1016/S0264-410X(01)00399-1. [DOI] [PubMed] [Google Scholar]
- 58.Shrestha MP, Scott RM, Joshi DM, Mammen MP, Jr, Thapa GB, Thapa N, et al. Safety and efficacy of a recombinant hepatitis E vaccine. N Engl J Med. 2007;356(9):895–903. doi: 10.1056/NEJMoa061847. [DOI] [PubMed] [Google Scholar]
- 59.Zhu FC, Zhang J, Zhang XF, Zhou C, Wang ZZ, Huang SJ, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376(9744):895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- 60.Purdy MA, McCaustland KA, Krawczynski K, Tam A, Beach MJ, Tassopoulos NC, et al. Expression of a hepatitis E virus (HEV)-trpE fusion protein containing epitopes recognized by antibodies in sera from human cases and experimentally infected primates. Arch Virol. 1992;123(3–4):335–349. doi: 10.1007/BF01317268. [DOI] [PubMed] [Google Scholar]
- 61.Schofield DJ, Glamann J, Emerson SU, Purcell RH. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol. 2000;74(12):5548–5555. doi: 10.1128/JVI.74.12.5548-5555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng J, Dai X, Chang JC, Lopareva E, Pillot J, Fields HA, et al. Identification and characterization of the neutralization epitope(s) of the hepatitis E virus. Virology. 2001;288(2):203–211. doi: 10.1006/viro.2001.1093. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Gu Y, Ge SX, Li SW, He ZQ, Huang GY, et al. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine. 2005;23(22):2881–2892. doi: 10.1016/j.vaccine.2004.11.065. [DOI] [PubMed] [Google Scholar]
- 64.He J, Kuschner RA, Dewar V, Voet P, Asher LV, Vaughn DW. Characterization of monoclonal antibodies to hepatitis E virus (HEV) capsid protein and identification of binding activity. J Biomed Sci. 2007;14(5):555–563. doi: 10.1007/s11373-007-9172-4. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Dai X, Shan X, Meng J. The Leu477 and Leu613 of ORF2-encoded protein are critical in forming neutralization antigenic epitope of hepatitis E virus genotype 4. Cell Mol Immunol. 2008;5(6):447–456. doi: 10.1038/cmi.2008.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takahashi M, Hoshino Y, Tanaka T, Takahashi H, Nishizawa T, Okamoto H. Production of monoclonal antibodies against hepatitis E virus capsid protein and evaluation of their neutralizing activity in a cell culture system. Arch Virol. 2008;153(4):657–666. doi: 10.1007/s00705-008-0045-6. [DOI] [PubMed] [Google Scholar]
- 67.Xing L, Wang JC, Li TC, Yasutomi Y, Lara J, Khudyakov Y, et al. Spatial configuration of hepatitis E virus antigenic domain. J Virol. 2011;85(2):1117–1124. doi: 10.1128/JVI.00657-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guthmann JP, Klovstad H, Boccia D, Hamid N, Pinoges L, Nizou JY, et al. A large outbreak of hepatitis E among a displaced population in Darfur, Sudan, 2004: the role of water treatment methods. Clin Infect Dis. 2006;42(12):1685–1691. doi: 10.1086/504321. [DOI] [PubMed] [Google Scholar]
- 69.Bryan JP, Iqbal M, Tsarev S, Malik IA, Duncan JF, Ahmed A, et al. Epidemic of hepatitis E in a military unit in Abbotrabad, Pakistan. Am J Trop Med Hyg. 2002;67(6):662–668. doi: 10.4269/ajtmh.2002.67.662. [DOI] [PubMed] [Google Scholar]
- 70.Clayson ET, Vaughn DW, Innis BL, Shrestha MP, Pandey R, Malla DB. Association of hepatitis E virus with an outbreak of hepatitis at a military training camp in Nepal. J Med Virol. 1998;54(3):178–182. doi: 10.1002/(SICI)1096-9071(199803)54:3<178::AID-JMV6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 71.Rein DB, Stevens GA, Theaker J, Wittenborn JS, Wiersma ST. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55(4):988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 72.Goel A, Aggarwal R. Prevention of hepatitis E: another step forward. Future Microbiol. 2011;6(1):23–27. doi: 10.2217/fmb.10.151. [DOI] [PubMed] [Google Scholar]


