Abstract
Poor prenatal nutrition, acting through epigenetic processes, induces persistent changes in offspring phenotype. We investigated the effect of maternal fat intake on polyunsaturated fatty acid (PUFA) status and on the epigenetic regulation of Fads2, encoding Δ6 desaturase (rate limiting in PUFA synthesis), in the adult offspring. Rats (n=6 per dietary group) were fed either 3.5% (w/w), 7% (w/w) or 21% (w/w) butter or fish oil (FO) from 14 days preconception until weaning. Offspring (n=6 males and females per dietary group) were fed 4% (w/w) soybean oil until postnatal day 77. 20:4n-6 and 22:6n-3 levels were lower in liver phosphatidylcholine (PC) and phosphatidylethanolamine and plasma PC (all P<.0001) in offspring of dams fed 21% than 3.5% or 7% fat regardless of type. Hepatic Fads2 expression related inversely to maternal dietary fat. Fads2 messenger RNA expression correlated negatively with methylation of CpGs at −623, −394, −84 and −76 bases relative to the transcription start site (all P<.005). Methylation of these CpGs was higher in offspring of dams fed 21% than 3.5% or 7% fat; FO higher than butter. Feeding adult female rats 7% fat reduced 20:4n-6 status in liver PC and Fads2 expression and increased methylation of CpGs −623, −394, −84 and −76 that reversed in animals switched from 7% to 4% fat diets. These findings suggest that fat exposure during development induces persistent changes, while adults exhibit a transient response, in hepatic PUFA status in offspring through epigenetic regulation of Fads2. Thus, epigenetic regulation of Fads2 may contribute to short- and long-term regulation of PUFA synthesis.
Keywords: Maternal dietary fat, Early life programming, Liver, Arachidonic acid, Docosahexaenoic acid, Δ6 desaturase
1. Introduction
During the past 50 years, Western populations have experienced changes in both type and amount of dietary fat [1]. For example, the introduction of margarine increased the dietary intake of the 18:2n-6 and so increased the dietary n-6:n-3 polyunsaturated fatty acid (PUFA) ratio and also increased consumption of trans fatty acids [1]. Furthermore, populations in developing countries migrating from rural to urban areas experience a persistent dietary change from a relatively low fat (LF) diet with a high monounsaturated fatty acid and PUFA content to a diet with a Western fat content and composition [2]. Such geographical and dietary transition is associated with increased prevalence of the metabolic syndrome and cardiovascular disease [3]. One explanation for these health effects is that environmental exposures during early life, including nutrition, can induce changes in the phenotype of the offspring that have implications for future risk of disease [4]. However, the mechanism by which maternal dietary fat intake induces long-term changes in the phenotype of the offspring has not been explored in detail.
In rodents, feeding a high-saturated fat diet during pregnancy and/or pregnancy and lactation induced in the offspring vascular dysfunction [5,6] and impaired control of lipid and glucose metabolism [7–14]. Furthermore, the offspring of mice fed a diet enriched in trans fatty acids during pregnancy and lactation showed growth retardation and impaired glucose homeostasis [15].
There is some evidence that changes in the regulation of membrane fatty acid composition in the offspring that persist beyond the period of exposure to the altered diet may be involved in phenotypes induced by fat exposures during development. Feeding rats diets containing 21% (w/w) total fat enriched in either saturated, n-6 PUFA, trans fatty acids or fish oil (FO) during pregnancy and lactation induced lower proportions of 20:4n-6 and 22:6n-3 in the aorta of adult offspring [16]. Since the diet fed after weaning contained only the precursors 18:2n-6 and 18:3n-3, these findings suggest that a maternal high-saturated fat diet reduced capacity for 20:4n-6 and 22:6n-3 synthesis [16]. Furthermore, the type of fat fed to pregnant and lactating mice influenced the effect of the fat fed after weaning on the fatty acid composition of liver and heart in the offspring [16]. Feeding adult rats a high fat (HF) diet reduced the messenger RNA (mRNA) expression of Fads2 that encodes Δ6 desaturase the rate-limiting enzyme in 20:4n-6 and 22:6n-3 synthesis in liver [17]. Thus, altered transcriptional regulation of Fads2 and/or Fads1, which encodes Δ5 desaturase, represents one potential mechanism by which maternal fat intake may induce persistent changes in the PUFA content of cell membranes in the offspring.
A number of studies have shown that long-term phenotypic changes induced by unbalanced prenatal nutrition involve altered epigenetic regulation of specific genes [18]. However, there is limited information about the effect of maternal dietary fat on epigenetic processes in the offspring. Fads2 transcription has been shown to be regulated by the methylation status of its promoter in rodents [16,19]. Feeding HF diets to pregnant and lactating rats induced increased methylation of specific CpG dinucleotides in the Fads2 promoter and lower mRNA expression in adult offspring aorta [16]. One CpG at −394 bases from the transcription start site was hypermethylated in the offspring of dams fed an HF diet and was shown to be involved directly in the regulation of Fads2 expression. Feeding an HF diet of undisclosed composition to pregnant and lactating mice induced global hypomethylation and lower average methylation of specific genes in the brain of the offspring [20]. Supplementation of U937 leukaemia cells with 20:5n-3 induced demethylation of specific CpG dinucleotides in the CCAAT/enhancer-binding protein-δ promoter [21]. Thus, we hypothesised that variations in the type and amount of fat consumed by pregnant and lactating rats induce changes in liver and plasma PUFA status in their offspring by a mechanism that involves altered epigenetic regulation of hepatic Fads genes.
To test this idea, rats were fed diets containing graded amounts of fat enriched in either saturated fatty acids (butter) or in 20:5n-3 and 22:6n-3 FO before and during pregnancy and during lactation. Male and female offspring were fed an LF diet in which the only PUFA were 18:2n-6 and 18:3n-3 until postnatal day 77. The effects of the maternal diets on PUFA status were determined by measuring the fatty acid composition of offspring liver and plasma phospholipids. The effects of maternal dietary fat on the epigenetic regulation of PUFA biosynthesis in the offspring were assessed by hepatic Fads1 and Fads2 mRNA expression and the methylation of specific CpG dinucleotides in the Fads2 promoter. We also compared the effect of differences in maternal fat intake on the epigenetic regulation of hepatic Fads2 in the offspring with the effect of feeding adult non-pregnant female rats different fats.
2. Materials and methods
2.1. Animal procedures
All animal procedures were in accordance with the British Home Office Animals (Scientific Procedures) Act 1986. Virgin female Wistar rats weighing 200–250 g were fed diets based on the AIN-93G formulation [22] containing either 3.5% (LF), 7% [adequate fat (AF)] or 21% (HF) (w/w) total fat derived from either butter, predominately saturated and monounsaturated fatty acids, or FO, predominately n-3 PUFA (Supplemental Table 1), from 14 days before conception and throughout pregnancy and lactation (n=6 per diet). The AF diets provided an adequate amount of fat to support pregnancy and lactation in rats [22] and so represent the reference group. All diets contained the same amount of vitamin E to provide sufficient antioxidant capacity at the highest level of FO intake. Litters were culled to 8 pups (equal numbers of males and females) within 24 h of spontaneous birth. Offspring (n=6 males or females, 1 male and 1 female in each litter per maternal diet) were weaned at 28 day onto AIN-93M diet containing 4% (w/w) soybean oil (Supplemental Table 1) and maintained on this diet until postnatal day 77 when they were fasted for 12 h and then killed by CO2 asphyxiation. Dams were fasted for 12 h and then killed on postpartum day 28.
In order to compare the effects of differences in fat exposure during development with the effect on adult animals, virgin female rats (200–250 g) were either maintained on AIN-93M for 13 weeks (n=6) and then killed, fed AF FO for 9 weeks (the period equivalent to the dams that were fed this diet) and then killed (n=6) or transferred from AF FO to AIN-93M for 4 weeks and then killed (n=6). All females were fasted for 12 h before killing by CO2 asphyxiation.
Blood was collected by cardiac puncture into tubes containing lithium heparin and plasma separated from cells by centrifugation. Plasma was stored at −80°C. Livers were removed immediately into liquid nitrogen and stored at −80°C.
2.2. Measurement of fatty acid composition
Plasma and liver fatty acid compositions were measured as described [23]. Briefly, liver (approximately 100 mg) was powdered under liquid nitrogen and total lipids extracted with chloroform and methanol [24]. Plasma (0.8 ml) was extracted with chloroform and methanol [24]. Triacylglycerol (TAG), phosphatidylcholine (PC) and phosphatidylethanolamine (PE) were isolated by solid-phase extraction using 100 mg BondElut aminopropyl silica cartridges (Agilent, Stockport, Cheshire, UK) [23,25] and converted to fatty acid methyl esters (FAMEs) by incubation with methanol containing 2% (v/v) sulphuric acid [23]. The proportions of individual fatty acids were measured by gas chromatography using BPX70 fused silica capillary column (30 m × 0.25 mm × 0.25 μm) (SGE Europe Ltd., Milton Keynes, Buckinghamshire, UK) on an Agilent 6890 gas chromatograph equipped with flame ionisation detection [23]. FAMEs were identified by their retention times relative to standards and quantified using Chemstation software (Agilent).
2.3. Measurement of Fads1 and Fads2 mRNA expression
Real-time reverse transcription (RT) polymerase chain reaction (PCR) was carried out essentially as described [26]. Total RNA was isolated from liver using Tri Reagent (Sigma, Poole, Dorset, UK). Complementary DNA was prepared and amplified using real-time RT PCR. The level of Fads2 mRNA was measured by QuantiTect assay QT00186739 (Qiagen, Crawley, West Sussex, UK) and Fads1 mRNA by QuantiTect assay QT00188664 (Qiagen). Samples were analysed in duplicate and expression of the individual transcripts was normalised to cyclophilin (QuantiTect assay QT00177394; Qiagen) [27], which did not differ in transcript level between groups of offspring [28]. Expression relative to cyclophilin was determined by the standard curve method [29].
2.4. Measurement of DNA methylation
The level of methylation of individual CpG dinucleotides in the Fads2 promoter was measured in a region between 0 and 766 bases upstream from the transcription start site (Supplemental Fig. 1) using sodium bisulphite pyrosequencing [16]. This region encompasses CpGs that have been shown previously to be involved in regulation of Fads2 transcription [16]. Genomic DNA was prepared and bisulphite conversion was carried out using the EZ DNA methylation kit (ZymoResearch, Irvine, CA, USA). The pyrosequencing reaction was carried out using primers listed in Supplemental Table 2. Modified DNA was amplified using KAPA2G Robust Hot Start Taq DNA polymerase (Labtech, Ringmer, East Sussex, UK). PCR products were immobilised on streptavidin–sepharose beads (GE Healthcare UK Ltd., Amersham, Buckinghamshire, UK), washed, denatured and released into annealing buffer containing the sequencing primers. Pyrosequencing was carried out using the SQA kit on a PSQ 96MA machine (Biotage, Uppsala, Sweden), and the percentage methylation was calculated using the Pyro Q CpG software (Biotage). Within assay, precision was between 0.8% and 1.8% and detection limits were 2–5% methylation.
2.5. Statistical analysis
After testing for normality, dietary groups were compared by analysis of variance (ANOVA) with sex and amount and type of maternal dietary fat as between-subject factors with Bonferroni's post hoc test (SPSS, Chicago, IL, USA). Data are presented as mean±S.D. Differences between means with a probability <.05 were assumed to be statistically significant. The strength of linear associations between outcome measures was assessed by Pearson's correlation analysis. Comparison between groups of adult rats fed different amounts of fat was by 1-way ANOVA with Bonferroni's post hoc test. Six rats per group provided statistical power of 80% for detecting a 5% difference between groups for all outcome measures with a probability of 5%.
3. Results
3.1. Maternal dietary fat intake altered liver PC and PE and plasma PC fatty acid composition in adult offspring
Maternal fasting plasma TAG showed the expected differences in fatty acid composition between groups indicating the effectiveness of the dietary regimen (Supplemental Table 3). Specifically, the proportions of 20:5n-3 and 22:6n-3 were higher in dams fed the FO diet compared to those fed butter and the level of these fatty acids was significantly greater in dams fed the HF diets compared to those fed LF and AF diets. 18:2n-6 did not differ between dams fed different amounts of butter but decreased in a dose-related manner in dams fed different amounts of FO.
The principal effects of differences in maternal dietary fat intake were on the proportions of 20:4n-6 and 22:6n-3 in offspring liver PC and PE and plasma PC. The proportions of 20:4n-6 and 22:6n-3 were significantly lower in liver PC from the offspring of dams fed HF diets compared to those fed the LF and AF diets. There was a minor effect of the fatty acid composition of the maternal diet on the proportion of 20:4n-6, but not 22:6n-3 (Table 1). The relationship of 20:4n-6 and 22:6n-3 in plasma PC from male and female offspring to the amount of fat in the maternal diet was similar to that in liver PC (Table 1). The proportions of 20:4n-6 and 22:6n-3 in liver PE showed an inverse relationship with the amount of fat in the maternal diet. This was accompanied by interactive effects of offspring sex and the type of fat in the maternal diet with the amount of dietary fat (Table 1).
Table 1.
Proportions of 20:4n-6 and 22:6n-3 in liver and plasma in adult offspring of rats fed diets with differing in fat contents during pregnancy and lactation
| Diet fat | Liver |
Plasma |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC |
PE |
PC |
||||||||||
| 20:4n-6 |
22:6n-3 |
20:4n-6 |
22:6n-3 |
20:4n-6 |
22:6n-3 |
|||||||
| Butter | FO | Butter | FO | Butter | FO | Butter | FO | Butter | FO | Butter | FO | |
| % total fatty acids | ||||||||||||
| Male | ||||||||||||
| LF | 29.6±0.8b | 27.9±2.3b | 6.9±0.7b | 6.8±0.8b | 29.0±1.0c | 27.4±3.sc | 12.4±1.6c | 13.3±2.1c | 27.2±0.3b | 26.1±0.3b | 7.7±0.4b | 6.8±0.3b |
| AF | 28.1±2.3b | 26.8±2.7b | 8.3±1.9b | 7.0±1.3b | 17.0±1.2b | 20.6±2.0b | 11.9±2.0b | 9.3±2.5b | 25.6±1.1b | 23.2±1.1b | 4.3±0.4a | 3.9±1.3a |
| HF | 22.2±2.6a | 20.0±2.1a | 4.9±1.1a | 5.2±0.7a | 9.2±1.9a | 11.5±2.5a | 10.3±0.4a | 6.7±0.7a | 21.4±1.1a | 18.5±3.1a | 3.8±0.9a | 2.7±08a |
| Female | ||||||||||||
| LF | 29.9±1.2b | 26.3±1.0b | 11.0±0.8b | 11.1±0.4b | 26.0±2.4c | 23.0±0.6c | 17.1±1.2c | 20.6±0.9c | 27.1±0.3c | 25.8±0.9c | 8.8±0.4b | 7.2±0.2b |
| AF | 27.2±2.1b | 26.1±1.4b | 10.1±3.2b | 12.0±1.2b | 20.0±1.3b | 18.7±3.2b | 12.2±1.3b | 14.9±1.7b | 24.7±1.2b | 23.2±2.1b | 6.0±0.5b | 5.5±0.6b |
| HF | 18.8±9.2a | 20.2±2.9a | 6.8±3.7a | 8.3±0.6a | 5.6±2.1a | 9.0±1.7a | 9.8±1.8a | 8.4±0.6a | 19.4±1.1a | 18.1±1.6a | 4.6±0.5a | 3.8±0.7a |
| ANOVA [P (F)] | ||||||||||||
| A | P<.0001 (69.0) | .002 (6.9) | <.0001 (319.4) | <.0001 (259.2) | <.0001 (68.4) | <.001 (126.0) | ||||||
| T | .02 (5.4) | – | – | – | <.0001 (17.2) | – | ||||||
| S | – | <.0001 (58.2) | <.0001 (16.0) | – | <.0001 (78.2) | <.0001 (241.1) | ||||||
| A × S | – | – | <.0001 (8.7) | <.0001 (13.4) | – | .001 (8.2) | ||||||
| S × T | – | .02 (6.1) | – | <.0001 (14.6) | – | – | ||||||
| A × T | – | – | .002 (7.1) | <.0001 (9.4) | .14 | – | ||||||
| A × S × T | – | – | .03 (3.7) | – | – | – | ||||||
Values are mean±S.D., n=6. Means with superscripts without a common letter for each fatty acid within a sex differ, P<.05; a<b<c. A, amount of maternal dietary fat; S, sex; T, type of maternal dietary fat. Degrees of freedom: A, 2; T, 1; S, 1; A × S, 2; S × T, 1; A × T, 2; A × S × T, 2.
There were relatively small differences between maternal dietary groups in the proportion of 20:5n-3 in offspring liver PC and in the proportion of 20:3n-6 in liver PE (Supplemental Tables 4 and 5). 18:2n-6 was significantly higher in liver PC and PE of the offspring of dams fed the HF diet compared to those fed the LF or AF diet (Supplemental Tables 4 and 5). There were no significant differences in the proportions of 18:2n-6 and low abundance n-3 and n-6 PUFA in plasma PC between maternal dietary groups (Supplemental Table 6).
3.2. Maternal dietary fat intake altered the mRNA expression of Fads2 in adult offspring liver
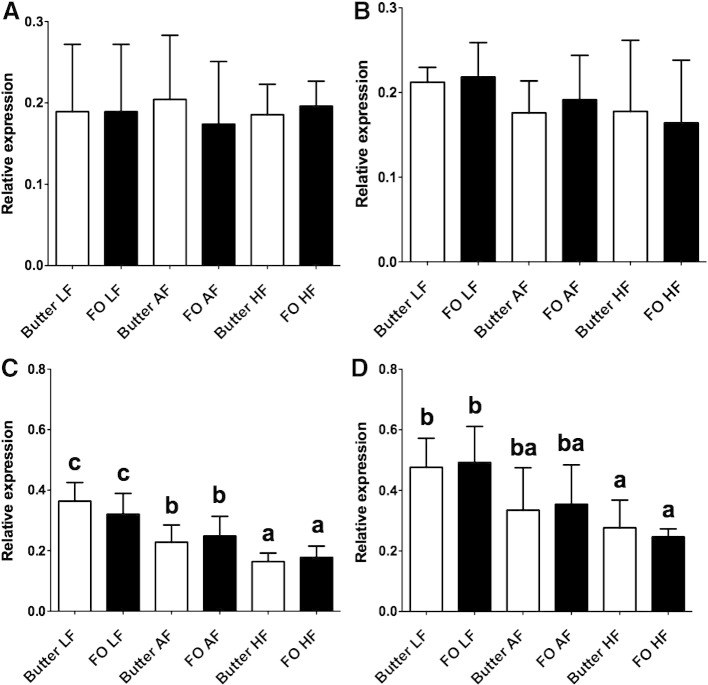
There were no statistically significant differences between groups in Fads1 mRNA expression in male or female offspring (Fig. 1A and B). There were significant effects of offspring sex [P=.002 (F 10.6)] and the amount of maternal dietary fat [P<.0001 (F 49.1)] on Fads2 mRNA expression in the liver of the offspring (Fig. 1C and D). Fads2 expression was significantly lower in male than female offspring. Increasing the amount of fat in the maternal diet decreased Fads2 mRNA expression in male and female offspring (Fig. 1C and D).
Fig. 1.
mRNA expression of Fads1 and Fads2 offspring liver. Values are mean±S.D. for n=6 offspring per maternal dietary group. Means without a common letter differ significantly (P<.05), a<b<c. Degrees of freedom: A, amount of maternal dietary fat, 2; T, type of maternal dietary fat, 1; S, sex, 1; A × S, 2; S × T, 1; A × T, 2; A × S × T, 2.
3.3. Maternal dietary fat intake altered the methylation of specific CpG dinucleotides in the Fads2 promoter in adult offspring liver
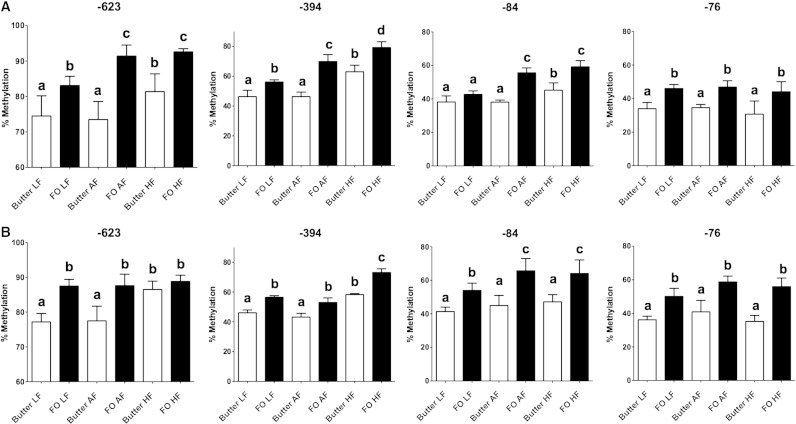
The methylation status of the 10 CpG dinucleotides in the proximal promoter of Fads2, which has been shown to contain the regulatory elements important for expression [16] (Supplemental Fig. 1), was measured in the liver of the offspring. CpGs at −722, −711, −678, −608, −585 and −65 bases from the transcription start site showed no significant difference in methylation between maternal dietary groups in male or female offspring (Supplemental Table 7). However, there were significant effects on the methylation of CpGs at −623, −394, −84 and −76 bases from the transcription start site of the amount [all P<.0001 (F 13–97)] and type [all P<.0001 (F 60–150)] of maternal dietary fat and of sex [CpG −623, P=.44 (F 4.2); CpG −394, P=.012 (F 6.8); CpGs −84 (F 20.1) and −76 (F 21.2), both P<.0001] (Fig. 2). There were significant interaction effects of the amount and type of maternal dietary fat and of sex on the methylation of CpG −623 [P=.03 (F 6.7)], CpG −394 [P=.007 (F 5.4)], CpG −84 [P=.01 (F 7.7)] and CpG −76 [P=.08 (F 5.9)]. Methylation of these four CpGs increased with the amount of fat in the maternal diet, although the level of methylation tended to be greater in offspring of dams fed FO than butter (Fig. 2).
Fig. 2.
Methylation of specific CpG dinucleotides in the Fads2 promoter in liver of the offspring. Values are mean±S.D. for n=6 offspring per maternal dietary group. Means without a common letter differ significantly (P<.05), a<b<c. Degrees of freedom: A, amount of maternal dietary fat, 2; T, type of maternal dietary fat, 1; S, sex, 1; A × S, 2; S × T, 1; A × T, 2; A × S × T, 2.
Methylation of the Fads1 promoter was below 5%, the detection limit of the pyrosequencing assays in all samples measured (data not shown).
3.4. Methylation of specific CpGs in the Fads2 promoter was associated with the level of mRNA expression and 20:4n-6 and 22:6n-3 status
Fads2 mRNA expression correlated positively with the proportions of 20:4n-6 and 22:6n-3 in liver PC and PE and in plasma PC (Table 2). Methylation of CpG −394 showed a strong negative correlation with Fads2 mRNA expression in male and female offspring, while CpGs −623, −84 and −76 correlated weakly with Fads2 mRNA expression (Table 2). Methylation of CpG −394, but not CpGs −623, −84 and −76, correlated negatively with the proportions of 20:4n-6 and 22:6n-3 in liver PC and PE and in plasma PC in male and female offspring (Table 2).
Table 2.
Analysis of the relationship between the proportions of 20:4n-6 and 22:6n-3 in liver and plasma PC and Fads2 mRNA expression and methylation in liver in adult offspring of rats fed diets with differing in fat contents during pregnancy and lactation
| Liver |
Plasma |
Fads2 mRNA | |||||
|---|---|---|---|---|---|---|---|
| 20:4n-6 |
22:6n-3 |
20:4n-6 |
22:6n-3 |
||||
| PC | PE | PC | PE | PC | |||
| r (r2) | |||||||
| Male (n=24) | |||||||
| Fads2 mRNA | 0.57⁎⁎⁎ (0.32) | 0.61⁎⁎⁎ (0.37) | 0.31⁎ (0.1) | 0.54⁎⁎⁎ (0.29) | 0.51⁎⁎ (0.26) | 0.23⁎ (0.05) | |
| CpG −623 | −0.25 | −0.31 | −0.11 | −0.16 | −0.24 | −0.12 | −0.27⁎ (.07) |
| CpG −394 | −0.59⁎⁎ (0.35) | −0.57⁎⁎ (0.32) | −0.32⁎ (0.1) | −0.53⁎⁎ (0.28) | −0.57⁎⁎ (0.32) | −0.43⁎ (0.18) | −0.70⁎⁎⁎⁎ (0.49) |
| CpG −84 | −0.25 | −0.11 | −0.11 | −0.27 | −0.19 | −0.08 | −0.23⁎ (0.05) |
| CpG −76 | −0.1 | −0.22 | −0.08 | −0.19 | −0.23 | −0.29 | −0.15 |
| Female (n=24) | |||||||
| Fads2 mRNA | 0.42⁎ (0.18) | 0.60⁎⁎ (0.36) | 0.32⁎ (0.1) | 0.43⁎⁎ (0.18) | 0.49⁎⁎ (0.24) | 0.55⁎⁎⁎⁎ (0.3) | |
| CpG −623 | −0.17 | 0.12 | −0.15 | −0.17 | −0.23⁎ | −0.27 | −0.34⁎ (0.12) |
| CpG −394 | −0.70⁎⁎⁎⁎ (0.49) | −0.54⁎⁎ (0.29) | −0.27⁎ (0.07) | −0.78⁎⁎ (0.61) | −0.71⁎⁎⁎⁎ (0.5) | −0.63⁎⁎⁎⁎ (0.4) | −0.71⁎⁎⁎⁎ (0.5) |
| CpG −84 | −0.16 | 0.25 | −0.19 | −0.23 | −0.24 | −0.16 | −0.21⁎ (0.04) |
| CpG −76 | −0.20 | −0.19 | −0.412 | −0.19 | −0.14 | −0.26 | −0.01 |
P<.05.
P<.01.
P<.001.
P<.0001.
3.5. Feeding adult females a higher fat diet enriched in FO induced transient changes in the methylation of the Fads2 promoter and its mRNA expression
There were significant effects of diet on the proportions of 20:4n-6 and 22:6n-3 [both P<.0001 (F 47.4 and F 31.8, respectively)] on Fads2, but not Fads1, mRNA expression and on the methylation of specific CpG dinucleotides [−62, P=.006 (F 19.7); −694, P=.0014 (F 21.8); −84, P<.0001 (F 33.1); −76, P=.0002 (F 24.4)] in offspring liver (Fig. 3). Feeding the FO AF diet to non-pregnant females for 9 weeks decreased the proportion of 20:4n-6 and increased the proportion of 22:6n-3 in liver PC compared to females maintained on the AIN-94M diet (Fig. 3A and B). This was accompanied by a significant decrease in Fads2 mRNA expression (Fig. 3D), but there was no difference in Fads1 mRNA expression between groups (Fig. 3C). Methylation of CpGs −623, −394, −84 and −76 in the Fads2 promoter was increased in females fed FO AF compared to those fed AIN-93M (Fig. 3E–H). However, transfer of females fed FO AF to AIN-93M for 4 weeks reversed the effects of the FO AF diet on the proportions of 20:4n-6 and 22:6n-3 in liver PC, Fads2 mRNA expression and methylation of individual CpG dinucleotides in the Fads2 promoter (Fig. 3).
Fig. 3.
Effect of feeding adult female rats a diet enriched in FO on the 20:4n-6 and 22:6n-3 in PC, Fads1 and Fads2 mRNA expression and methylation of specific CpG dinucleotides in the Fads2 promoter in liver. Values are mean±S.D. for n=6 rats dietary group. Means without a common letter differ significantly (P<.05), a<b<c. (A) and (B) are proportions of 20:4n-6 and 22:6n-3; (C) and (D) are Fads1 and Fads2 mRNA expression; (E–H) are methylation of specific CpG dinucleotides in the Fads2 promoter. Degrees of freedom: T, type of maternal dietary fat, 1; S, sex, 1; A × S, 2; S × T, 1; A × T, 2; A × S × T, 2.
4. Discussion
The findings of this study support the hypothesis that maternal fat intake during pregnancy and lactation alters the proportions of PUFA, specifically 20:4n-6 and 22:6n-3, in the liver of the adult offspring and that this is associated with altered epigenetic regulation of Fads2 transcription.
Increasing maternal fat intake during pregnancy and lactation has been shown to induce changes in the fatty acid composition of membrane phospholipids in the offspring that persist beyond the period when fatty acids are supplied from the mother may be expected to have been lost due to turnover [30,31]. The present findings show that the effects of the maternal diets on the fatty acid compositions of offspring liver and plasma phospholipids differed from those in maternal plasma TAG, a major substrate for supply of fatty acids to the offspring during pregnancy and lactation. This suggests that the maternal diets induced long-term changes in PUFA metabolism rather than persistence of fatty acids derived from the maternal diets in the offspring. Our findings are in general agreement with a previous report [31] but extend those observations by showing that the amount of fat consumed by dams exerted a substantially greater effect on the proportions of 20:4n-6 and 22:6n-3 in liver and plasma phospholipids than the fatty acid composition of the maternal diet. In the present study, all offspring were fed the same type of fat after weaning and so the findings are not directly comparable to those reported previously [30]. Since the only PUFA in the diet fed to the offspring after weaning were 18:3n-3 and 18:2n-6, one possible interpretation is that differences in 20:4n-6 and 22:6n-3 status reflect altered capacity for synthesis from these precursors. Thus, these findings suggest that differences in maternal dietary fat intake induce long-term changes in membrane PUFA content in the offspring by altering the metabolism of these fatty acids.
Our findings show for the first time that maternal fat intake was related inversely to Fads2, but not Fads1, mRNA expression in the offspring and, for example, that Fads2 mRNA expression predicted in males 32% and 10% and in females 18% and 36%, of the variation in the proportions of 20:4n-6 and 22:6n-3, respectively, in liver PC. This suggests that differences in the regulation of Fads2 transcription induced during development may be an important factor in future capacity to maintain liver and plasma 20:4n-6 and 22:6n-3 contents. However, while maternal dietary 7% fat appear to be a threshold for the effect of fat intake on PUFA status in the offspring, the effect on offspring Fads2 expression was graded between the different amounts of fat in the maternal diet. One possible explanation for the difference in the effect of the maternal diet on fatty acid composition and Fads2 expression is the influence of other factors regulating PUFA levels, such as rate of fatty acid turnover, which may mask the changes in gene expression at low to moderate maternal fat intakes.
We have shown previously that feeding rats an HF diet during pregnancy and lactation induced increased methylation of specific CpG dinucleotides in the Fads2 promoter and decreased mRNA expression of this gene in offspring aorta and that this was associated with dysregulation of vascular tone [16]. Furthermore, methylation of CpG −394 was shown using a Fads2 promoter-reporter gene construct to regulate Fads2 transcription directly [16]. Our present findings show, for the first time, that both the type and the amount of maternal dietary fat induce persistent changes in the methylation of four CpG dinucleotides in the liver Fads2 promoter in the liver of the adult offspring. Methylation levels of three CpGs, −394, −84 and −76, correlated negatively with the level of Fads2 mRNA. CpG −394 methylation was also negatively associated with the proportion of 20:4n-6 and 22:6n-3 in liver and plasma phospholipids and variation in the methylation at this locus predicted up to 59% of the variation in 20:4n-6 in males and 71% in females and 53% of the variation in 22:6n-3 in males and 78% in females. Together, these findings support the suggestion that Fads2 methylation is an important factor in regulating 20:4n-6 and 22:6n-3 levels. Polymorphisms in Fads1 and Fads2 have been shown to explain about 28% of the variation in 20:4n-6 in red blood cells in humans [32]. One implication of the present findings is that variations in the epigenetic regulation of Fads2 may also contribute significantly variation in 20:4n-6 and 22:6n-3 concentrations.
A modest increase in the fat intake of adult non-pregnant female rats induced decreased liver PC 20:4n-6 and Fads2 mRNA expression and increased the methylation of the same CpGs that were altered in the offspring of dams fed an HF diet. However, in contrast to the persistent effect of the maternal HF diet on the epigenetic regulation of Fads2, the changes induced in Fads2 methylation and expression and in 20:4n-6 status in adults did not persist after a ‘wash out’ period of feeding AIN-93M. These findings suggest that there is epigenetic plasticity in the Fads2 gene in adulthood and that fat intake can alter the DNA methylation of its promoter. However, unlike exposure to an HF diet during development that induced a persistent change on Fads2 methylation and expression, there appears in adulthood to be a homeostatic mechanism that returns the level of methylation and transcription of the original state after the period of HF feeding ended. This suggests that although DNA methylation is regarded as a mechanism that induces stable changes in gene expression, methylation of specific CpGs in the Fads2 promoter appears to be relatively dynamic. Szyf has proposed that an equilibrium between DNA methyltransferases and, as yet to be identified, demethylases is involved [33], although current experimental evidence is largely circumstantial. One implication of these findings is that short-term changes in the methylation of the Fads2 promoter may contribute to the regulation of its expression by dietary fat [17].
The proportions of PUFA in cell membranes modify cell function by modulating the biophysical properties of the lipid bilayer and by determining the composition of substrate pools for the synthesis of lipid second messengers [34]. Thus, induction of long-term changes in PUFA synthesis may have important consequences for tissue function and potentially for disease risk, although the effect in humans may differ from that in rats because of differences between species in dietary PUFA intake, particularly of preformed 20:4n-6 and 22:6n-3. Female rats and humans maintain higher levels of 22:6n-3 in liver and/or plasma phospholipids and appear to synthesise more 22:6n-3 than males [34]. Although the precise biological function of this sex difference is not known, it has been suggested to be associated with the ability to increase 22:6n-3 status in pregnancy to meet the demands of the developing fetus and neonate [25,35]. Thus, variations in maternal fat intake during pregnancy and lactation acting via altered epigenetic regulation of Fads2 transcription in the female offspring may, in turn, have an adverse effect on the capacity of the female offspring to synthesise long-chain PUFA during their pregnancy. If so, pregnant and lactating females who consume an HF diet, even a diet enriched in n-3 long-chain PUFA, may be less able to provide adequate amounts of these fatty acids to their offspring for optimal growth and development and so negatively influence their offspring's future health.
Footnotes
Sources of support: The research leading to these results was supported by a grant from the British Heart Foundation (PG/08/126/26417).
Conflict of interest: The authors declare no conflict of interest.
Author contributions to the manuscript: G.C.B., K.A.L., C.T., P.C.C. and M.A.H. designed the study; S.P.H., N.A.I., C.S., C.K., A.C. and A.F. conducted the research. G.C.B. analysed the data and wrote the paper. All authors read and approved the final manuscript.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.jnutbio.2012.09.005.
Contributor Information
Karen A. Lillycrop, Email: k.a.lillycrop@southampton.ac.uk.
Graham C. Burdge, Email: g.c.burdge@southampton.ac.uk.
Appendix A. Supplementary data
Hoile Supplemental material 13 Sept 2012.doc
References
- 1.British Nutrition Foundation . Unsaturated fatty acids: nutritional and physiological significance: the report of the British Nutrition Foundation's task force. Chapman & Hall; London: 1992. [Google Scholar]
- 2.Cordain L., Eaton S.B., Sebastian A., Mann N., Lindeberg S., Watkins B.A. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K.S. Cardiovascular diseases in the developing countries: dimensions, determinants, dynamics and directions for public health action. Public Health Nutr. 2002;5:231–237. doi: 10.1079/phn2001298. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman P.D., Hanson M.A., Cooper C., Thornburg K.L. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor P.D., Khan I.Y., Hanson M.A., Poston L. Impaired EDHF-mediated vasodilatation in adult offspring of rats exposed to a fat-rich diet in pregnancy. J Physiol. 2004;558:943–951. doi: 10.1113/jphysiol.2002.018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage J.A., Lakasing L., Taylor P.D., Balachandran A.A., Jensen R.I., Dekou V. Developmental programming of aortic and renal structure in offspring of rats fed fat-rich diets in pregnancy. J Physiol. 2005;565:171–184. doi: 10.1113/jphysiol.2005.084947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ainge H., Thompson C., Ozanne S.E., Rooney K.B. A systematic review on animal models of maternal high fat feeding and offspring glycaemic control. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.149. [DOI] [PubMed] [Google Scholar]
- 8.Cerf M.E. High fat programming of beta-cell failure. Adv Exp Med Biol. 2010;654:77–89. doi: 10.1007/978-90-481-3271-3_5. [DOI] [PubMed] [Google Scholar]
- 9.Cerf M.E., Louw J. High fat programming induces glucose intolerance in weanling Wistar rats. Horm Metab Res. 2010;42:307–310. doi: 10.1055/s-0030-1248303. [DOI] [PubMed] [Google Scholar]
- 10.Giraudo S.Q., Della-Fera M.A., Proctor L., Wickwire K., Ambati S., Baile C.A. Maternal high fat feeding and gestational dietary restriction: effects on offspring body weight, food intake and hypothalamic gene expression over three generations in mice. Pharmacol Biochem Behav. 2010;97:121–129. doi: 10.1016/j.pbb.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan E.L., Smith M.S., Grove K.L. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology. 2011;93:1–8. doi: 10.1159/000322038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naef L., Moquin L., Dal B.G., Giros B., Gratton A., Walker C.D. Maternal high-fat intake alters presynaptic regulation of dopamine in the nucleus accumbens and increases motivation for fat rewards in the offspring. Neuroscience. 2011;176:225–236. doi: 10.1016/j.neuroscience.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 13.Bruce K.D., Cagampang F.R., Argenton M., Zhang J., Ethirajan P.L., Burdge G.C. Maternal high-fat feeding primes steatohepatitis in adult mice offspring, involving mitochondrial dysfunction and altered lipogenesis gene expression. Hepatology. 2009;50:1796–1808. doi: 10.1002/hep.23205. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi R., Nakagawa Y., Liu Y.J., Fujisawa Y., Sai S., Nagata E. Effects of maternal high-fat diet on serum lipid concentration and expression of peroxisomal proliferator-activated receptors in the early life of rat offspring. Horm Metab Res. 2010;42:821–825. doi: 10.1055/s-0030-1261954. [DOI] [PubMed] [Google Scholar]
- 15.Kavanagh K., Sajadian S., Jenkins K.A., Wilson M.D., Carr J.J., Wagner J.D. Neonatal and fetal exposure to trans-fatty acids retards early growth and adiposity while adversely affecting glucose in mice. Nutr Res. 2010;30:418–426. doi: 10.1016/j.nutres.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelsall C.J., Hoile S.P., Irvine N.A., Masoodi M., Torrens C., Lillycrop K.A. Vascular dysfunction induced in offspring by maternal dietary fat involves altered arterial polyunsaturated fatty acid biosynthesis. PLoS One. 2012;7:e34492. doi: 10.1371/journal.pone.0034492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H.P., Nakamura M.T., Clarke S.D. Cloning, expression, and nutritional regulation of the mammalian delta-6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- 18.Burdge G.C., Lillycrop K.A. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- 19.Devlin A.M., Singh R., Wade R.E., Innis S.M., Bottiglieri T., Lentz S.R. Hypermethylation of Fads2 and altered hepatic fatty acid and phospholipid metabolism in mice with hyperhomocysteinemia. J Biol Chem. 2007;282:37082–37090. doi: 10.1074/jbc.M704256200. [DOI] [PubMed] [Google Scholar]
- 20.Vucetic Z., Kimmel J., Totoki K., Hollenbeck E., Reyes T.M. Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology. 2010;151:4756–4764. doi: 10.1210/en.2010-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ceccarelli V., Racanicchi S., Martelli M.P., Nocentini G., Fettucciari K., Riccardi C. Eicosapentaenoic acid demethylates a single CpG that mediates expression of tumor suppressor CCAAT/enhancer-binding protein delta in U937 leukemia cells. J Biol Chem. 2011;286:27092–27102. doi: 10.1074/jbc.M111.253609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves P.G. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 23.Burdge G.C., Wright P., Jones A.E., Wootton S.A. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. Br J Nutr. 2000;84:781–787. [PubMed] [Google Scholar]
- 24.Folch J., Lees M., Sloane-Stanley G.H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Burdge G.C., Hunt A.N., Postle A.D. Mechanisms of hepatic phosphatidylcholine synthesis in adult rat: effects of pregnancy. Biochem J. 1994;303:941–947. doi: 10.1042/bj3030941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burdge G.C., Lillycrop K.A., Phillips E.S., Slater-Jefferies J.L., Jackson A.A., Hanson M.A. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr. 2009;139:1054–1060. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- 27.Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 28.Lillycrop K.A., Phillips E.S., Jackson A.A., Hanson M.A., Burdge G.C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–1386. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 29.Cikos S., Bukovska A., Koppel J. Relative quantification of mRNA: comparison of methods currently used for real-time PCR data analysis. BMC Mol Biol. 2007;8:113. doi: 10.1186/1471-2199-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chechi K., Herzberg G.R., Cheema S.K. Maternal dietary fat intake during gestation and lactation alters tissue fatty acid composition in the adult offspring of C57Bl/6 mice. Prostaglandins Leukot Essent Fatty Acids. 2010;83:97–104. doi: 10.1016/j.plefa.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh P., Bitsanis D., Ghebremeskel K., Crawford M.A., Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533:815–822. doi: 10.1111/j.1469-7793.2001.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lattka E., Illig T., Koletzko B., Heinrich J. Genetic variants of the FADS1 FADS2 gene cluster as related to essential fatty acid metabolism. Curr Opin Lipidol. 2010;21:64–69. doi: 10.1097/MOL.0b013e3283327ca8. [DOI] [PubMed] [Google Scholar]
- 33.Szyf M. DNA methylation properties: consequences for pharmacology. Trends Pharmacol Sci. 1994;15:233–238. doi: 10.1016/0165-6147(94)90317-4. [DOI] [PubMed] [Google Scholar]
- 34.Burdge G.C., Calder P.C. Dietary alpha-linolenic acid and health-related outcomes: a metabolic perspective. Nutr Res Rev. 2006;19:26–52. doi: 10.1079/NRR2005113. [DOI] [PubMed] [Google Scholar]
- 35.Postle A.D., Al M.D., Burdge G.C., Hornstra G. The composition of individual molecular species of plasma phosphatidylcholine in human pregnancy. Early Hum Dev. 1995;43:47–58. doi: 10.1016/0378-3782(95)01663-n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hoile Supplemental material 13 Sept 2012.doc