Abstract
We investigated the effects of individual (IC) and group (GC) diet and exercise counseling in men with metabolic syndrome. Participants received exercise instruction and exercise load was monitored. IC participants received individual diet counseling sessions and general consultations at baseline and monthly. GC participants received a group diet counseling session at baseline and general consultations at baseline and monthly. In the IC group, body mass index (BMI) percent body fat, waist circumference, diastolic blood pressure, low-density lipoprotein cholesterol, glycosylated hemoglobin A1c, and liver function levels were reduced significantly after 3 months, whereas in the GC group, waist circumference and levels of liver function were reduced. Exercise load was negatively correlated with change in BMI and waist circumference in the IC group, and positively correlated with changes in high-density lipoprotein cholesterol levels in all subjects and in the GC group. Diet and exercise counseling, especially IC, may benefit patients with metabolic syndrome.
Keywords: counsel, diet, exercise, metabolic syndrome, waist circumference
Introduction
Obesity has recently emerged as a global health concern. Indeed, patients with metabolic syndrome are now recognized as a high-risk group for cardiovascular diseases (CVD), next to patients with hypercholesterolemia.1,2 Metabolic syndrome involves having a clinical disorder based on abdominal obesity, including atherogenic dyslipidemia, elevated blood pressure, and high blood glucose and/or insulin resistance. In Japan, a report from the Ministry of Health, Labor and Welfare showed an increase in the number of individuals with metabolic syndrome, associated with the westernization of lifestyle.3 It has been demonstrated that individuals with a combination of 3 or more risk factors out of obesity, hyperglycemia, hyperlipidemia and hypertension had a 10.56-times increased relative risk for acute myocardial infarction.4 In addition to the above-mentioned classical risk factors, obesity is associated with several co-morbidities such as sleep apnea/sleep-disordered breathing and nonalcoholic fatty liver disease, which are themselves recognized as causes of CVD.4–6 For exsample, recent epidemiological data suggest that gamma-glutamyl transferase (GGT) is not only a biomaker of hepatobiliary diseases, but also a surrogate marker of metabolic and cardiovascular risk7–10.
Based on these evidences, It is necessary to take measures to prevent CVD in patients with metabolic syndrome.
A change in lifestyle that includes alterations in diet and exercise can not only improve glucose tolerance11–13 but also reduce the magnitude of several other cardiovascular risk factors such as obesity, dyslipidemia, high blood pressure,14–16 and metabolic syndrome,17,18 thereby decreasing the risk of CVD. Treatment recommendations in patients with nonalcoholic fatty liver disease, for example, include weight reduction through both diet and physical activity, in addition to weight-loss surgery for extreme obesity.19 Nutritional therapy may also be beneficial and cost-effective, although there have been no convincing randomized controlled trials investigating the effects of nutritional education in patients with metabolic syndrome or obesity.20,21
It is often difficult for patients to change their behaviors and environment. Henkin and colleagues22 suggested that lack of long-term compliance to the reduction of dietary saturated fat remains a problem, even in individuals who receive intensive initial training and show early favorable responses. Effective and useful methods for nutrition and exercise education included as part of a total health promotion plan are therefore necessary in order to improve metabolic disorders. A public health program that includes group-based or individual counseling could provide this. Individual counseling is reported to be effective in the prevention of type 2 diabetes mellitus,11,14 and group-based diabetes education programs have been shown to result in clinical improvements.15
In this study, we assessed two types of diet and exercise counseling—individual counseling (IC) and group counseling (GC)—and evaluated the effects of each type of counseling on various metabolic parameters, including anthropometric profiles [body mass index (BMI), percent body fat, waist circumference, and blood pressure], lipid and glucose metabolism, and hepatic function. We hypothesized that IC is more effective than GC. The goal of this study was to determine the extent to which each type of counseling can improve physical profiles and metabolic disorders in patients with metabolic syndrome, in order to plan an effective total health promotion plan for company employees.
Method
Participants
The study group consisted of patients with metabolic syndrome who underwent an employee health checkup at their workplace between April 2005 and March 2006. A total of 2,295 employees were screened. In accordance with the definition by the Japanese Committee for the Diagnostic Criteria of Metabolic Syndrome,23 metabolic syndrome was defined as the presence of visceral obesity (waist circumference = 85 cm or more in men) and 2 or more of the following abnormalities: (i) triglycerides ≥ 150 mg/dL and/or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, (ii) systolic blood pressure (SBP) ≥ 130 mmHg and/or diastolic blood pressure (DBP) ≥ 85 mmHg, and (iii) fasting glucose ≥ 110 mg/dL. Individuals were excluded from the study if they suffered from malignant diseases, inflammatory diseases, a history of atherosclerotic or cardiac diseases, virus or auto-immune hepatitis and cirrhosis, renal diseases, thyroid diseases, arthropathy or other physical problems. Patients with medication-treated diabetes mellitus, dyslipidemia, and primary or secondary hypertension were also excluded.
The study protocol was explained to 161 men who were diagnosed with metabolic syndrome and randomized into two groups. Prior to the start of the study, some participants dropped out for personal reasons that included loss of interest in the study, work transfer, change in schedule and other unknown causes. Ultimately, 37 and 54 men were enrolled in the IC and GC groups respectively. Informed consent was obtained from all study participants. The study protocol was approved by the Human Ethics Committee of Shinko Kakogawa Hospital (presently Kakogawa East City Hospital) and carried out according to the Declaration of Helsinki.
Apparatus
Blood pressure was measured while each participant was in a seated position using an automatic electronic sphygmomanometer (BP-203RV II, Nihon COLIN Corporation, Aichi, Japan). BMI was calculated by dividing body weight by the square of height (kg/m2). Percent body fat was determined by bioelectrical impedance analysis using TANITA BC-118E (TAN-ITA Corporation, Tokyo, Japan). Waist circumference at the umbilical level was measured in the late exhalation phase in a standing position.24,25 Alcohol consumption was calculated as grams per week from each participant’s interview, and estimated as average alcohol intake (in grams) per day.
Study protocol
The goal of this study was to evaluate the effect of diet and exercise counseling on various metabolic parameters, and to investigate the extent to which individual or group counseling can improve these metabolic parameters in patients with metabolic syndrome. Each participant was assessed for 3 months.
To encourage physical activity, at baseline and once monthly (4 times in total), all study participants received exercise instruction that included advice to maintain caloric expenditure above 300 kcal a day. To estimate exercise load by daily walking, study participants were monitored using a step-counter (KENZ lifecorder EX, SUZUKEN Co., Nagoya, Japan), which automatically calculated daily energy consumption (in kcal units). Data were collected at every session. Overtime work from 0 to 40 hours per month was recorded, yielding no difference between the IC group and the GC group. Since exercise load differed by approximately 100 to 350 kcal depending on the type of occupation, we did not separately evaluate exercise during working and non-working time, but rather measured the load in a whole day.
For diet therapy in the IC group, participants were asked to record their diet, alcohol consumption, and timing of meals for three consecutive days before the session at baseline and once monthly. These diet records were analyzed by dietitians. Based on dietitian reports, participants received individually tailored 1-hour counseling sessions, and were advised to maintain a well-balanced, low-fat diet with adequate calories. In addition, participants in the IC group received individual 1-hour general consultations regarding exercise and lifestyle by physicians and public health nurses at baseline and once monthly (4 times in total).
Participants in the GC group did not receive individual dietary analysis but participated in a 1-hour group session that included fundamental dietary advice by dietitians at baseline only. Additionally, individual general consultations by physicians and public health nurses were provided at baseline and once monthly (4 times total).
Body weight, percent body fat, and waist circumference were each measured by registered nurses at baseline and monthly. Blood sampling and analysis were performed at the beginning of the study and three months later.
Blood sampling and analysis
Blood samples were obtained between 9 AM and 11 AM following a 12-hour fast. Plasma triglycerides, HDL-C, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C, Kyowa Medex, Tokyo, Japan), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and GGT levels were determined by enzymatic methods, fasting plasma glucose and glycosylated hemoglobin A1c (HbA1c) by high-performance lipid chromatography (HPLC), high-sensitivity C-reactive protein (hsCRP) by a nephelometry method, and fasting plasma insulin levels by enzyme immunoassay. In accordance with homeostasis model assessment (HOMA),26 insulin resistance (HOMA-IR) was calculated as fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405.
Statistical analysis
Continuous variables were expressed as the mean ± standard deviation (SD) and analyzed using Student’s t-test or Pearson’s chi-square test as appropriate. Correlations were calculated using the formula for Pearson’s correlation coefficient. Probability values of less than 0.05 were considered statistically significant. All data were analyzed using Statview software version 4.0 (Abacus, Berkeley, CA, USA).
Results
Comparison of metabolic profiles between ic group and gc groups
There were no dropouts in either group throughout the course of the present study. Baseline characteristics of all participants are summarized in Table 1. Although there were no differences in anthropometric profiles, blood pressure, and lipid parameters between the IC and GC groups at baseline, the GC group showed significantly higher levels of AST, ALT, and cholinesterase. By contrast, the GC group showed significantly lower levels of HOMA-IR, HbA1c, and hsCRP. Since there were differences in some metabolic parameters at baseline between IC and GC groups, we compared the data before and after the study within each group.
Table 1.
Baseline characteristics of patients in IC and GC groups.
| IC (n = 37) | GC (n = 54) | |
|---|---|---|
| Age (years) | 51.1 ± 6.4 | 49.6 ± 5.0 |
| Alcohol intake (g/day) | 20.1 ± 18.8 | 21.7 ± 18.2 |
| BMI (kg/m2) | 27.3 ± 2.1 | 27.6 ± 2.3 |
| Percent body fat (%) | 31.0 ± 4.5 | 30.2 ± 4.4 |
| Waist circumference (cm) | 94.1 ± 5.2 | 95.3 ± 5.7 |
| SBP (mmHg) | 135.5 ± 15.9 | 136.1 ± 16.6 |
| DBP (mmHg) | 85.9 ± 9.9 | 87.8 ± 12.3 |
| TC (mg/dL) | 213.6 ± 30.1 | 210.8 ± 28.6 |
| LDL-C (mg/dL) | 130.4 ± 27.5 | 128.0 ± 26.7 |
| HDL-C (mg/dL) | 49.2 ± 10.3 | 49.9 ± 10.9 |
| Triglycerides (mg/dL) | 183.4 ± 84.5 | 191.6 ± 115.2 |
| Plasma glucose (mg/dL) | 103.8 ± 15.6 | 99.3 ± 11.6 |
| Plasma insulin (μU/ml) | 7.5 ± 5.5 | 5.9 ± 2.0 |
| HOMA-IR | 1.93 ± 1.60 | 1.45 ± 0.48a |
| HbA1c (%) | 5.43 ± 0.58 | 5.20 ± 0.48a |
| AST (IU/l) | 26.4 ± 8.3 | 34.7 ± 12.7b |
| ALT (IU/l) | 32.8 ± 18.2 | 47.4 ± 21.7b |
| ALP (U/l) | 251.5 ± 64.4 | 254.7 ± 85.8 |
| GGT (IU/l) | 60.0 ± 38.6 | 86.7 ± 79.5 |
| Cholinesterase (IU/l) | 364.1 ± 61.7 | 404.5 ± 73.9c |
| hsCRP (mg/l) | 0.25 ± 0.67 | 0.11 ± 0.17d |
Notes: Values are the mean ± SD.
P < 0.0002;
P < 0.0005;
P < 0.01;
P < 0.05 vs. IC participants (unpaired Student’s t-test).
Abbreviations: IC, individual counseling; GC, group counseling; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405; HbA1c, glycosylated hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; hsCRP, high-sensitivity C-reactive protein.
Table 2 shows the effect of individual and group counseling on alcohol intake, anthropometric profiles, and blood pressure after 3 months. Alcohol intake did not change in either group. We could not assess exercise load before the study. After 3 months exercise load in the IC group appeared to be slightly greater than that in the GC group, but was not significantly different. In the IC group, BMI, percent body fat, waist circumference, and DBP levels were significantly reduced over time. A significant reduction in waist circumference was also observed in the GC group.
Table 2.
Age, exercise load, alcohol intake, body weight, body mass index, waist circumference, and blood pressure in IC and GC groups.
|
IC (n = 37)
|
GC (n = 54)
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age (years) | 51.1 ± 6.4 | – | 49.6 ± 5.0 | – |
| Alcohol intake (g/day) | 20.1 ± 18.8 | 17.9 ± 17.6 | 21.7 ± 18.2 | 21.7 ± 18.5 |
| Exercise load (kcal/day) | – | 373.4 ± 111.3 | – | 329.0 ± 113.3 |
| BMI (kg/m2) | 27.3 ± 2.1 | 26.7 ± 2.0a | 27.6 ± 2.3 | 27.5 ± 2.5 |
| Percent body fat (%) | 31.0 ± 4.5 | 28.5 ± 4.3a | 30.2 ± 4.4 | 30.6 ± 4.5d |
| Waist circumference (cm) | 94.1 ± 5.2 | 91.9 ± 4.9a | 95.3 ± 5.7 | 93.8 ± 5.6c |
| SBP (mmHg) | 135.5 ± 15.9 | 132.2 ± 16.8 | 136.1 ± 16.6 | 139.5 ± 14.9d |
| DBP (mmHg) | 85.9 ± 9.9 | 82.3 ± 9.8b | 87.8 ± 12.3 | 88.4 ± 14.3d |
Notes: Values are the mean ± SD.
vs. pre-IC participants at P < 0.0002;
vs. pre-IC participants at P < 0.02;
vs. pre-GC participants at P < 0.0001 (paired Student’s t-test);
vs. post-IC participants at P < 0.03 (unpaired Student’s t-test).
Abbreviations: IC, individual counseling; GC, group counseling; Pre, at baseline; Post, after study; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure.
As shown in Table 3, plasma levels of LDL-C and HbA1c were significantly reduced in the IC group. In contrast, plasma levels of insulin and HOMA-IR increased significantly in the GC group. In both groups, AST, ALT, GGT, and cholinesterase levels were significantly reduced.
Table 3.
Plasma analyses in IC and GC groups.
|
IC (n = 37)
|
GC (n = 54)
|
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| TC (mg/dL) | 213.6 ± 30.1 | 208.6 ± 32.0 | 210.8 ± 28.6 | 214.0 ± 36.8 |
| LDL-C (mg/dL) | 130.4 ± 27.5 | 122.5 ± 29.2* | 128.0 ± 26.7 | 132.2 ± 31.4 |
| HDL-C (mg/dL) | 49.2 ± 10.3 | 49.7 ± 11.2 | 49.9 ± 10.9 | 49.5 ± 11.0 |
| Triglycerides (mg/dL) | 183.4 ± 84.5 | 193.7 ± 141.2 | 191.6 ± 115.2 | 176.7 ± 112.7 |
| Plasma glucose (mg/dL) | 103.8 ± 15.6 | 104.5 ± 21.4 | 99.3 ± 11.6 | 105.1 ± 16.0 |
| Plasma insulin (μU/mL) | 7.5 ± 5.5 | 7.5 ± 6.3 | 5.9 ± 2.0 | 7.9 ± 4.9†,b |
| HOMA-IR | 1.93 ± 1.60 | 1.88 ± 1.52 | 1.45 ± 0.48a | 2.02 ± 1.16 |
| HbA1c (%) | 5.43 ± 0.58 | 5.36 ± 0.55* | 5.20 ± 0.48a | 5.27 ± 0.55 |
| AST (IU/l) | 26.4 ± 8.3 | 23.3 ± 5.9* | 34.7 ± 12.7a | 32.0 ± 11.2†,b |
| ALT (IU/l) | 32.8 ± 18.2 | 25.6 ± 11.1* | 47.4 ± 21.7a | 42.9 ± 21.8†,b |
| ALP (U/l) | 251.5 ± 64.4 | 251.9 ± 75.9 | 254.7 ± 85.8 | 247.2 ± 71.8 |
| GGT (IU/l) | 60.0 ± 38.6 | 50.2 ± 32.3* | 86.7 ± 79.5 | 77.5 ± 62.5†,b |
| Cholinesterase (IU/l) | 364.1 ± 61.7 | 343.6 ± 63.9* | 404.5 ± 73.9a | 387.2 ± 62.3†,b |
| hsCRP (mg/l) | 0.25 ± 0.67 | 0.28 ± 0.74 | 0.11 ± 0.17a | 0.12 ± 0.15 |
Notes: Values are the mean ± SD.
vs. pre-IC participants at P < 0.05;
vs. pre-GC participants at P < 0.05 (paired Student’s t-test).
vs. pre-IC participants at P < 0.05;
vs. post-IC participants at P < 0.05 (unpaired Student’s t-test).
Abbreviations: IC, individual counseling; GC, group counseling; Pre, at baseline; Post, after study; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-sensitivityß lipoprotein cholesterol; HOMA-IR, fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405; HbA1c, glycosylated hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; hsCRP, high-sensitivity C-reactive protein.
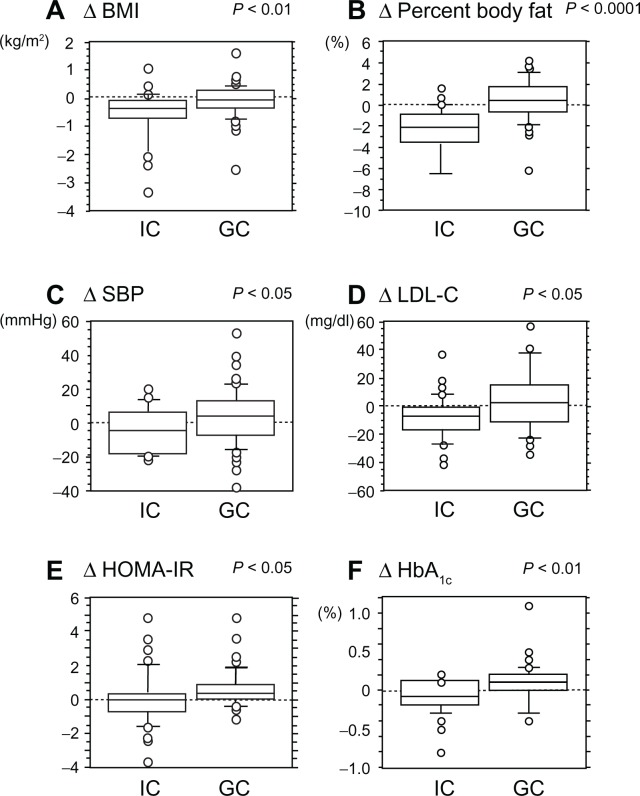
To confirm whether there was an increased effectiveness of IC versus GC, we computed a change score (Δ) for each parameter in Tables 2 and 3 by subtracting the value at baseline from the value after 3 months. Among these parameters, ΔBMI, Δ percent body fat, ΔSBP, ΔLDL-C, ΔHOMA-IR, and ΔHbA1c were each significantly greater in IC than in GC (Fig. 1). While both types of counseling were somewhat effective, these results suggest that IC led to the greatest improvement in various metabolic parameters.
Figure 1.
Comparison of differences in various parameters between IC and GC using an unpaired Student’s t-test.
Note: The Δ symbol represents change in parameter value from baseline to month 3 of counseling.
Abbreviations: IC, individual counseling; GC, group counseling; BMI, body mass index; SBP, systolic blood pressure; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405; HbA1c, glycosylated hemoglobin A1c.
To further evaluate the efficacy of IC and GC, we determined the number of participants who had initially been diagnosed with metabolic syndrome at baseline and still met the defined criteria for the disease at the end of the study. Table 4 shows the decrease in the number of participants who met the criteria of metabolic syndrome, greater waist circumference, dyslipidemia, high blood pressure, or high plasma glucose. Results suggest that both IC and GC improved various clinical factors in metabolic syndrome. Among these factors, the decrease in the number of participants with high blood pressure in the IC group was significantly different than that in the GC group.
Table 4.
Number of participants with metabolic syndrome, greater waist circumference, dyslipidemia, high blood pressure, or high plasma glucose in IC and GC groups.
|
IC
|
GC
|
P | |||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Metabolic syndrome | 37 | 20 | 54 | 31 | NS |
| Greater waist circumference | 37 | 36 | 54 | 52 | NS |
| Dyslipidemia | 24 | 19 | 33 | 23 | NS |
| High blood pressure | 27 | 18 | 41 | 36 | 0.035 |
| High plasma glucose | 17 | 11 | 12 | 9 | NS |
Notes: The data represent numbers of participants with metabolic syndrome, greater waist circumference, dyslipidemia, high blood pressure, or high plasma glucose. Metabolic syndrome was defined as the presence of 2 or more abnormalities in addition to visceral obesity (greater waist circumference: 85 cm or more in men). These three abnormalities are as follows: (i) dyslipidemia; triglycerides ≥ 150 mg/dL and/or high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, (ii) high blood pressure; systolic blood pressure (SBP) ≥ 130 mmHg and/or diastolic blood pressure (DBP) ≥ 85 mmHg, (iii) high plasma glucose; fasting glucose ≥ 110 mg/dL. Pearson’s chi-square test.
Moreover, we examined the number of participants who showed improvements in metabolic parameters (as indicated by a decreased value, except for HDL-C) in both groups. The proportion of participants exhibiting a lower BMI was higher in the IC group than in the GC group (IC: n = 32/37; GC: n = 28/54; χ2 = 11.73, P = 0.0006; data not shown). Similarly, IC resulted in an increased proportion of individuals with loss of percent body fat (IC: n = 32/37; GC: n = 23/54; χ2 = 17.69; P < 0.0001), decreased LDL-C (IC: n = 28/37; GC: n = 23/54; χ2 = 9.76, P = 0.002), decreased plasma glucose (IC: n = 20/37; GC: n = 13/54; χ2 = 8.54, P = 0.004), decreased HbA1c (IC: n = 19/37; GC: n = 12/54; χ2 = 8.29, P = 0.004), decreased plasma insulin (IC: n = 19/37; GC: n = 14/54; χ2 = 6.14, P = 0.013), and decreased HOMA-IR (IC: n = 21/37; GC: n = 14/54; χ2 = 8.82, P = 0.003).
Relationship between exercise load and various parameters
Correlation analyses demonstrated that exercise load was negatively correlated with ΔBMI and Δ percent body fat, and positively correlated with ΔHDL-C in all participants (total; Table 5). In the IC group, exercise load was negatively correlated with ΔBMI and Δ waist circumference. Exercise load was positively correlated with ΔHDL-C in the GC group. In addition, alcohol intake was weakly but significantly correlated with GGT (r = 0.302, P = 0.004) and LDL-C (r = 0.214, P = 0.044).
Table 5.
The correlation between exercise load and differences in various parameters in total, IC, and GC groups.
|
Total
|
IC
|
GC
|
||||
|---|---|---|---|---|---|---|
| r | P | r | P | r | P | |
| ΔBMI (kg/m2) | −0.323 | 0.002 | −0.480 | 0.003 | −0.104 | NS |
| ΔPercent body fat (%) | −0.227 | 0.030 | −0.185 | NS | −0.118 | NS |
| ΔWaist circumference (cm) | −0.179 | NS | −0.328 | 0.048 | −0.039 | NS |
| ΔSBP (mmHg) | −0.019 | NS | 0.080 | NS | −0.006 | NS |
| ΔDBP (mmHg) | 0.142 | NS | 0.255 | NS | 0.152 | NS |
| ΔTC (mg/dL) | −0.052 | NS | −0.020 | NS | −0.020 | NS |
| ΔLDL-C (mg/dL) | −0.066 | NS | −0.183 | NS | 0.076 | NS |
| ΔHDL-C (mg/dL) | 0.292 | 0.005 | 0.271 | NS | 0.304 | 0.026 |
| ΔTriglycerides (mg/dL) | −0.152 | NS | −0.085 | NS | −0.266 | NS |
| ΔPlasma glucose (mg/dL) | −0.039 | NS | 0.013 | NS | −0.006 | NS |
| ΔPlasma insulin (μU/mL) | −0.161 | NS | −0.142 | NS | −0.124 | NS |
| ΔHOMA-IR | −0.155 | NS | −0.114 | NS | −0.123 | NS |
| ΔHbA1c (%) | −0.107 | NS | −0.016 | NS | −0.076 | NS |
| ΔAST (IU/l) | −0.062 | NS | −0.136 | NS | −0.030 | NS |
| ΔALT (IU/l) | −0.136 | NS | −0.191 | NS | −0.085 | NS |
| ΔALP (U/l) | −0.059 | NS | −0.065 | NS | −0.096 | NS |
| ΔGGT (IU/l) | 0.110 | NS | −0.150 | NS | 0.230 | NS |
| ΔCholinesterase (IU/l) | −0.101 | NS | −0.074 | NS | −0.104 | NS |
| ΔhsCRP (mg/l) | −0.088 | NS | −0.216 | NS | 0.069 | NS |
Note: Δ in each parameter refers to the difference determined by subtracting the baseline value from the value after diet and exercise.
Abbreviations: IC, individual counseling; GC, group counseling; Pre, at baseline; Post, after study; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-sensitivity lipoprotein cholesterol; HOMA-IR, fasting plasma glucose (mg/dL) × fasting insulin (μU/mL)/405; HbA1c, glycosylated hemoglobin A1c; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase; hsCRP, high-sensitivity C-reactive protein.
Discussion
In this study, we demonstrated that with the exception of TG and HDL-C levels, IC and GC improved various clinical factors in metabolic syndrome. These results suggest that counseling for lifestyle change is an effective treatment for metabolic syndrome. Results implicated IC in particular in being a more effective treatment than GC. The lack of significant change found in TG and HDL-C levels may be due to continual intake of alcohol throughout the study, or to a non-significant difference in exercise loads between the two groups.
Compliance to behavior modifications in diet and physical activity is an important component of all treatments, and the role of therapeutic intervention in this process is not clearly established.27,28 It is known that some patients do not comply with dietary advice, while others might only maintain strict dietary control for a short time. Thus, adherence to dietary treatments is difficult to maintain and diet trials have been reported to have a high rate of dropout.29,30
It has been reported that the dropout rate of overweight patients consulted solely by dietitians (45%) was significantly greater than that of patients consulted by a doctor and dietitian (29%).29 Thompson and colleagues30 reported that patients with hypercholesterolemia receiving advice from dietitians experienced a greater reduction in total blood cholesterol than those receiving advice from doctors, suggesting that dietitians, as well-trained nutritional staff, can motivate and provide higher-quality diet intervention for patients requiring treatment. In the present study, the greatest advantage for study participants in the IC compared to the GC group was the opportunity for individual analysis of consecutive 3-day food records by a dietitian, as described in the study protocol. Participants in the IC group were given nutritional analysis sessions by dietitians followed by consultation with physicians and public health nurses, resulting in a greater improvement in metabolic disorders compared with those in the GC group. IC may enable health practitioners to give specific and appropriate advice, foster valuable discussion, and focus on concrete methods to improve metabolic parameters in each participant, resulting in a high motivation to maintain changes in diet and physical activity.
Some prospective studies have demonstrated that cardiorespiratory fitness is associated with the incidence of metabolic syndrome.31,32 As shown in Table 5, exercise load in the present study was negatively correlated with ΔBMI and Δ percent body fat, and positively correlated with ΔHDL-C in all participants. This may suggest that purposeful exercise in addition to regular work is effective in treating metabolic disorders in company employees. Walking is the most common form of physical activity. It is safe and easy, and improves a number of parameters of health. Therefore, brisk walking for at least 30 minutes daily can be recommended as the principal form of physical activity at the population level.33,34 The level of exercise load in our study was adequate to see improvement (373.4 kcal/day in the IC group and 329.0 kcal/day in the GC group, equivalent to around 10,000 steps a day), although an exercise load in excess of those observed in the present study may result in a greater amelioration of a number of metabolic factors. Proper and colleagues35 reported that individual face-to-face and repeated counseling sessions in the work-place positively influenced physical activity levels. In the current study, no differences in exercise levels at baseline were observed between the IC and GC groups, and there was no difference in exercise load per day between the two groups at the end of the study.
Although IC was more effective than GC for improvement of a number of parameters in the present study, IC is more costly and requires more dietitians than GC. The reason for the elevation in plasma insulin levels and HOMA-IR observed in the GC but not in IC group are unknown. However, as mentioned above, GC is an effective method for decreasing waist circumference. A combination therapy of GC and IC may produce longer-term benefits and be more cost-effective.
Henkin and colleagues22 demonstrated that serum lipid levels tend to return to basal levels after 6 months, even in individuals who receive intensive initial training and show an early favorable response. A systematic review of randomized controlled trials showed that although low-carbohydrate/high-protein diets have effects on reducing weight in obese persons, regain of weight is typically initiated after 6–12 months.36 Frequent assessment and repetition are needed to ensure patient understanding and to maintain patient motivation and the effects of health promotion. Thus, an example plan would include analyses of consecutive 3-day dietary records, anthropometric measures, blood analyses, and instruction and advice about exercise at baseline and at 3 months (twice total), combined with individual dietary consultation, and instruction and advice about exercise at 1 and 2 months.
It has been reported that moderate intensity aerobic exercise helps to normalize ALT levels in patients with nonalcoholic steatohepatitis.37 Reports from epidemiological studies also showed that an increase in serum GGT predicted onset of metabolic syndrome, incidence of CVD, and death, suggesting that GGT is a marker of metabolic and cardiovascular risk.7–10 Our results suggest that an exercise load of around 350 kcal/day may improve AST, ALT, GGT, and cholinesterase levels, if it is accompanied by dietary counseling.
Inflammation is a key mechanism in the development of cardiovascular and liver diseases and metabolic syndrome.1,6 In the present study, however, there was no significant improvement in hsCRP in either group. It has been reported that change in hsCRP concentration is related to changes in waist circumference and lipid metabolism, reflected by plasma triglycerides and free fatty acid levels.38 We observed no decrease in triglyceride levels in the present study, suggesting that an improvement in dyslipidemia may be necessary for the reduction of hsCRP or other inflammatory markers in metabolic syndrome.
There are clear limitations in this study. As described in the Participants section, we explained the study protocol to all participants, inquired as to whether they were interested and obtained their acceptance to participate in the study. A number of individuals, however, cancelled prior to receiving counseling because the study protocol was not what they wanted, or because they were no longer able to participate due to a work transfer, change in working hours (daytime, evening, or nighttime), change in schedule, or other unknown causes. Since all participants worked for the same company, we could not reduce bias arising from a lack of blinding. Ultimately 37 men were enrolled in the IC group and 54 men in the GC group. These problems with randomization and unbalanced numbers of participants may have led to differences in baseline levels of metabolic factors between the two groups. To account for this, we did not compare the levels of these factors between the two groups, but rather compared the baseline and post-study data within each group. Although there were no dropouts during the study, we cannot exclude the possibility of bias. Improved planning of the protocol itself, to consider employee working styles, may improve the results.
A second major limitation in this study was that we did not perform a detailed nutrient analysis of the food records provided by the participants. Recently, Otsuka and colleagues39 demonstrated that a lower intake of vitamin B6 and dietary fiber in men, or a lower intake of calcium, milk and dairy products and higher intake of cereal in women were related to the incidence of metabolic syndrome in Japanese people. A recent review indicates that the quantity and type of carbohydrate affects metabolic outcomes.40 Thus, in addition to analysis of total energy and nutrient balance, it may be important to analyze specific dietary factors in each individual. We were also unable to monitor exercise load before the study, and did not moderate alcohol consumption. The number of participants in this study was small and study duration was short. Further study of longer duration with more participants is needed in order to develop and provide a strong protocol, including a combination of individual and group-based counseling sessions with cooperation of medical staff such as physicians, nurses, and dietitians.
In conclusion, lifestyle diet and exercise counseling, especially individually, may improve physical profiles and metabolic disorders in patients with metabolic syndrome.
Acknowledgments
We wish to thank physicians Kenzo Yamashiro, Yoshio Ohnishi, and Isao Utaka, as well as Noriko Taitani, a public health nurse, and Rumiko Taniguchi, a registered nurse, dietitians Kiyomi Niboshi, Takao Kameda, and Hideo Ohira, and all staff engaged in laboratory examinations in Kakogawa East City Hospital for their valuable help with this study.
Footnotes
Competing Interests
Authors disclose no potential conflicts of interest.
Funding
Author(s) disclose no funding sources.
Author Contributions
Experiment conception and design: S.M-S. Data analysis: S.M-S., Y.F. First draft of manuscript: S.M-S., H.M. Contribution to writing of the manuscript: Y.F., H.M., M.T., M.H. All members agreed with results and conclusions, jointly developed the structure and arguments for the paper, made critical revisions and approved the final version of the manuscript.
Disclosures and Ethics
The study protocol was approved by the Human Ethics Committee of Shinko Kakogawa Hospital and carried out according to the Declaration of Helsinki. As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and protection of human research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 2.Teramoto T, Sasaki J, Ueshima H, et al. Metabolic syndrome. J Atheroscler Thromb. 2008;15(1):1–5. doi: 10.5551/jat.e580. [DOI] [PubMed] [Google Scholar]
- 3.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12(6):295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura T, Tsubono Y, Kameda-Takemura K, et al. Magnitude of sustained multiple risk factors for ischemic heart disease in Japanese employees: a case-control study. Jpn Circ J. 2001;65(1):11–7. doi: 10.1253/jcj.65.11. [DOI] [PubMed] [Google Scholar]
- 5.Poirier P, Giles TD, Bray GA, et al. Obesity and cardiovascular disease; pathophysiology, evaluation, and effect of weight loss. Arterioscler Thromb Vasc Biol. 2006;26(5):968–76. doi: 10.1161/01.ATV.0000216787.85457.f3. [DOI] [PubMed] [Google Scholar]
- 6.Loria P, Lonardo A, Bellentani S, Day CP, Marchesini G, Carulli N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: an open question. Nutr Metab Cardiovasc Dis. 2007;17(9):684–98. doi: 10.1016/j.numecd.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H. Gamma-glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation. 2005;112(14):2130–7. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(1):127–33. doi: 10.1161/01.ATV.0000251993.20372.40. [DOI] [PubMed] [Google Scholar]
- 9.Fraser A, Harris R, Sattar N, et al. Gamma-glutamyltransferase is associated with incident vascular events independently of alcohol intake: analysis of the British Women’s Heart and Health Study and Meta-Analysis. Arterioscler Thromb Vasc Biol. 2007;27(12):2729–35. doi: 10.1161/ATVBAHA.107.152298. [DOI] [PubMed] [Google Scholar]
- 10.Strasak AM, Kelleher CC, Klenk J, et al. Longitudinal change in serum gamma-glutamyltransferase and cardiovascular disease mortality: a prospective population-based study in 76,113 Austrian adults. Arterioscler Thromb Vasc Biol. 2008;28(10):1857–65. doi: 10.1161/ATVBAHA.108.170597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 12.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2005;28(11):2780–6. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 14.Blumenthal JA, Sherwood A, Gullette EC, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;160(13):1947–58. doi: 10.1001/archinte.160.13.1947. [DOI] [PubMed] [Google Scholar]
- 15.Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self-management strategies in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;18(2):CD003417. doi: 10.1002/14651858.CD003417.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Al-Mahmood AK, Ismail AA, Rashid FA, Azwany YN, Singh R, Gill G. Effect of therapeutic lifestyle changes on insulin sensitivity of non-obese hyperlipidemic subjects: preliminary report. J Atheroscler Thromb. 2007;14(3):122–7. doi: 10.5551/jat.14.122. [DOI] [PubMed] [Google Scholar]
- 17.Orchard TJ, Temprosa M, Goldberg R, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142(8):611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderssen SA, Carroll S, Urdal P, Holme I. Combined diet and exercise intervention reverses the metabolic syndrome in middle-aged males: results from the Oslo Diet and Exercise Study. Scand J Med Sci Sports. 2007;17(6):687–95. doi: 10.1111/j.1600-0838.2006.00631.x. [DOI] [PubMed] [Google Scholar]
- 19.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49(1):306–17. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pavlovich WD, Waters H, Weller W, Bass EB. Systematic review of literature on the cost-effectiveness of nutrition services. J Am Diet Assoc. 2004;104(2):226–32. doi: 10.1016/j.jada.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 21.Runge CF. Economic consequences of the obese. Diabetes. 2007;56(11):2668–72. doi: 10.2337/db07-0633. [DOI] [PubMed] [Google Scholar]
- 22.Henkin Y, Garber DW, Osterlund LC, Darnell BE. Saturated fats, cholesterol, and dietary compliance. Arch Intern Med. 1992;152(6):1167–74. [PubMed] [Google Scholar]
- 23.Matsuzawa Y. Metabolic syndrome-definition and diagnostic criteria in Japan. J Atheroscler Thromb. 2005;12(6):301. doi: 10.5551/jat.12.301. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida D, Toyomura K, Fukumoto J, et al. Waist circumference and cardiovascular risk factors in Japanese men and women. J Atheroscler Thromb. 2009;16(4):431–41. doi: 10.5551/jat.no539. [DOI] [PubMed] [Google Scholar]
- 25.Oka R, Kobayashi J, Miura K, et al. Difference between fasting and non-fasting triglyceridemia; the influence of waist circumference. J Atheroscler Thromb. 2009;16(5):633–40. doi: 10.5551/jat.406. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Lichtenstein AH, Appel LJ, Brands M, et al. Diet and lifestyle recommendations revision 2006: a scientific statement from the American Heart Association Nutrition Committee. Circulation. 2006;114(1):82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 28.Gill JM. Physical activity, cardiorespiratory fitness and insulin resistance: a short update. Curr Opin Lipidol. 2007;18(1):47–52. doi: 10.1097/MOL.0b013e328012b8bd. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard DA, Hyndman J, Taba F. Nutritional counseling in general practice: a cost effective analysis. J Epidemiol Community Health. 1999;53(5):311–6. doi: 10.1136/jech.53.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RL, Summerbell CD, Hooper L, et al. Relative efficacy of differential methods of dietary advice: a systemic review. Am J Clin Nutr. 2003;77(Suppl 4):1052S–7. doi: 10.1093/ajcn/77.4.1052S. [DOI] [PubMed] [Google Scholar]
- 31.Carnethon MR, Gidding SS, Nehgme R, Sidney S, Jacobs DR, Jr, Liu K. Cardiorespiratory fitness in young adulthood and the development of cardiovascular disease risk factors. JAMA. 2003;290(23):3092–100. doi: 10.1001/jama.290.23.3092. [DOI] [PubMed] [Google Scholar]
- 32.LaMonte MJ, Barlow CE, Jurca R, Kampert JB, Church TS, Blair SN. Cardiorespiratory fitness is inversely associated with the incidence of metabolic syndrome: a prospective study of men and women. Circulation. 2005;112(4):505–12. doi: 10.1161/CIRCULATIONAHA.104.503805. [DOI] [PubMed] [Google Scholar]
- 33.Rennie KL, McCarthy N, Yazdgerdi S, Marmot M, Brunner E. Association of the metabolic syndrome with both vigorous and moderate physical activity. Int J Epidemiol. 2003;32(4):600–06. doi: 10.1093/ije/dyg179. [DOI] [PubMed] [Google Scholar]
- 34.Lakka TA, Laaksonen DE. Physical activity in prevention and treatment of the metabolic syndrome. Appl Physiol Nutr Metab. 2007;32(1):76–88. doi: 10.1139/h06-113. [DOI] [PubMed] [Google Scholar]
- 35.Proper KI, Hildebrandt VH, Van der Beek AJ, Twisk JW, Van Mechelen W. Effect of individual counseling on physical activity fitness and health: a randomized controlled trial in a workplace setting. Am J Prev Med. 2003;24(3):218–26. doi: 10.1016/s0749-3797(02)00645-1. [DOI] [PubMed] [Google Scholar]
- 36.Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systemic review of randomized controlled trials of low-carbohydrate vs. low-fat/low-calorie diets in the management of obesity and its comorbidities. Obes Rev. 2009;10(1):36–50. doi: 10.1111/j.1467-789X.2008.00518.x. [DOI] [PubMed] [Google Scholar]
- 37.Sreenivasa Baba C, Alexander G, Kalyani B, et al. Effect of exercise and dietary modification on serum aminotransferase levels in patients with non-alcoholic steatohepatitis. J Gastroenterol Hepatol. 2006;21(1 Pt 1):191–8. doi: 10.1111/j.1440-1746.2005.04233.x. [DOI] [PubMed] [Google Scholar]
- 38.Dvoráková-Lorenzová A, Suchánek P, Havel PJ, et al. The decrease in C-reactive protein concentration after diet and physical activity induced weight reduction is associated with changes in plasma lipids, but not interleukin-6 or adiponectin. Metabolism. 2006;55(3):359–65. doi: 10.1016/j.metabol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Otsuka R, Imai T, Kato Y, Ando F, Shimokata H. Relationship between number of metabolic syndrome components and dietary factors in middle-aged and elderly Japanese subjects. Hypertens Res. 2010;33(6):548–54. doi: 10.1038/hr.2010.29. [DOI] [PubMed] [Google Scholar]
- 40.Wood RJ, Fernandez ML. Carbohydrate-restricted versus low-glycemic index diets for the treatment of insulin resistance and metabolic syndrome. Nutr Rev. 2009;67(3):179–83. doi: 10.1111/j.1753-4887.2009.00186.x. [DOI] [PubMed] [Google Scholar]