Abstract
Background:
1,3-dimethylamylamine (a constituent of geranium), alone and in combination with caffeine, is widely used within dietary supplements. We have recently determined the hemodynamic effects of 1,3-dimethylamylamine and caffeine alone and in combination, using a single ingestion study. However, no study has determined the hemodynamic effects of these ingredients following chronic use. Moreover, no study has determined the effects of these ingredients on bloodborne variables related to health and safety. Therefore, the purpose of this investigation was to assess the hemodynamic and hematologic profile of two different dietary supplements containing 1,3-dimethylamylamine and caffeine (in addition to other ingredients), before and after two weeks of daily intake.
Methods:
7 men (24.9 ± 4.2 yrs) ingested the dietary supplement Jack3d™, while 4 men and 2 women (22.5 ± 1.8 yrs) ingested the dietary supplement OxyELITE Pro™ once per day for two weeks. On days 1 and 15, resting heart rate (HR), systolic (SBP), and diastolic (DBP) blood pressure were measured and rate pressure product (RPP) was calculated. Fasting blood samples were analyzed for complete blood counts, comprehensive metabolic panel, and lipid panel. These tests were done prior to ingestion of supplement. On days 1 and 15 following blood collection, subjects ingested the assigned supplement (2 servings) and HR, SBP, DBP, and RPP were recorded at 30, 60, 90, and 120 minutes post-ingestion.
Results:
After 14 days of treatment, resting HR, SBP, DBP, and RPP were not increased (P > 0.05). No significant changes were noted in any measured bloodborne variable, with the exception of an increase in fasting blood glucose with ingestion of Jack3d™ (P = 0.02). In response to acute intake of the supplements, HR, DBP, and RPP were not increased statistically (P > 0.05). SBP was increased with OxyELITE Pro™ (P = 0.03), but not with Jack3d™ (P = 0.09). Compared to pre-ingestion and in general, both supplements resulted in an increase in SBP, DBP, and RPP from 5%–15%, with a peak occurring at the 60 or 90 minute post-ingestion time.
Conclusion:
Acute ingestion of OxyELITE Pro™, but not Jack3d™, results in an increase in SBP. Chronic intake of two servings per day of OxyELITE Pro™ or Jack3d™ over a 14 day period does not result in an elevation in resting HR, SBP, DBP, or RPP. No significant changes are noted in any measured bloodborne variable following 14 days of ingestion, with the exception of blood glucose with Jack3d™. Longer term intervention studies inclusive of larger sample sizes are needed to extend these findings.
Keywords: 1,3-dimethylamylamine; caffeine; blood pressure; heart rate; blood
Background
The use of nutritional supplements by the general population continues to rise, despite little direct scientific evidence in support of the use of many products.1 Perhaps more importantly, few safety data are available pertaining to several of the ingredients commonly contained within finished products.2 One such ingredient of interest is 1,3-dimethylamylamine, often combined with caffeine and other agents in an attempt to provide a stimulant “cocktail.” The popularity of 1,3-dimethylamylamine continues to increase, as evidenced by its widespread use within many performance and lipolytic dietary supplements.
Suggested to be found within geranium,3 1,3-dimethylamylamine is widely used within the dietary supplement industry, in particular as a component of weight/fat loss products, as well as those targeting enhanced exercise performance. Unfortunately, very little is known about this ingredient, in particular as it pertains to oral ingestion by human subjects; something that we describe in detail in our recent report.4
In our prior work, we determined the effect of 1,3-dimethylamylamine alone (at two different dosages) and in combination with caffeine on the hemodynamic response to acute intake (2 hours post-ingestion).4 In particular, we measured heart rate (HR), as well as systolic (SBP) and diastolic (DBP) blood pressure, and calculated rate pressure product (RPP) as an indicator of myocardial work. Our results indicated an increase in SBP in particular, with acute ingestion of 1,3-dimethylamylamine alone and in combination with caffeine. However, replication of this work is necessary. Moreover, to our knowledge, no study has determined the hemodynamic effects of 1,3-dimethylamylamine combined with caffeine following chronic use, which may differ from that observed with only single ingestion. Nor has any study determined the combined effects of these ingredients on bloodborne variables related to health and safety. Considering that many finished products now contain a combination of 1,3-dimethylamylamine and caffeine, and individuals using such products do so daily or semi-daily, we sought to determine the hemodynamic and hematologic profile of two different dietary supplements containing these ingredients before and after two weeks of daily intake. This was performed using an open label study design, with measurement of resting HR and blood pressure, in addition to bloodborne variables, on days 1 and 15. We also included a measurement of hemodynamic variables following acute intake of the supplements on days 1 and 15. In terms of acute intake, we hypothesized that the supplements would increase blood pressure, in particular SBP, as noted in our previous work. In terms of chronic intake, we hypothesized that no significant changes would be noted in any measured variable, due to the acute nature of the stimulant effect.
Methods
Subjects
Healthy men and women participated in this study. All subjects completed a medical history and physical activity questionnaire to determine eligibility. No subject smoked cigarettes or had self reported diagnosed disease of cardiovascular or metabolic origin. Subjects were active and considered to be exercise-trained, as they performed aerobic (eg, jogging, running, cycling, swimming) and anaerobic (eg, sprinting, resistance training) exercise for the past several years (based on self report). Subject descriptive characteristics are presented in Table 1. All experimental procedures were performed in accordance with the Helsinki Declaration. The University of Memphis Human Subjects Committee approved all experimental procedures (H10-77 and H10-78). Subjects provided verbal and written consent prior to participating in this study.
Table 1.
Characteristics of subjects using OxyELITE Pro™ and Jack3d™.
| Variable | OxyELITE Pro™ (N = 6: 4 men and 2 women) | Jack3d™ (N = 7 men) |
|---|---|---|
| Age (yrs) | 22.5 ± 1.8 | 24.9 ± 4.2 |
| Height (cm) | 173.8 ± 12.5 | 178.2 ± 8.6 |
| Weight (kg) | 65.9 ± 8.6 | 83.8 ± 9.4 |
| BMI (kg · m−2) | 21.9 ± 2.1 | 26.5 ± 3.3 |
| Body fat (%) | 13.8 ± 9.7 | 14.3 ± 5.2 |
| Waist (cm) | 72.8 ± 3.8 | 86.4 ± 7.9 |
| Hip (cm) | 95.4 ± 5.2 | 103.7 ± 5.5 |
| Waist:hip | 0.76 ± 0.04 | 0.83 ± 0.05 |
| Resting heart rate (bpm)* | 67.6 ± 9.7 | 60.1 ± 5.2 |
| Resting systolic blood pressure (mmHg)* | 104.0 ± 5.5 | 112.6 ± 13.8 |
| Resting diastolic blood pressure (mmHg)* | 63.8 ± 9.7 | 64.3 ± 8.0 |
| Years anaerobic exercise training | 1.6 ± 1.9 | 7.1 ± 6.5 |
| Hours per week anaerobic exercise | 1.8 ± 2.1 | 6.8 ± 2.4 |
| Years aerobic exercise training | 5.8 ± 5.6 | 4.1 ± 5.2 |
| Hours per week aerobic exercise | 3.5 ± 1.8 | 1.5 ± 1.3 |
Notes: Data are mean ± SD.
Measured during screening visit; prior to pre (day 1) testing.
Conditions and testing
Following all screening procedures, subjects reported to the lab in the morning hours (0600–0900) on two different occasions separated by 14 days. The time of day for testing was matched for each subject. Procedures described below were identical for both test sessions (and for both supplements tested). The dietary supplements used in this investigation were provided by USPlabs (Dallas, TX) and contained a combination of 1,3-dimethylamylamine and caffeine, in addition to other ingredients which may have contributed to the measured effect. Specifically the two supplements used were: (1) OxyELITE Pro™ (containing a proprietary blend of bauhinia purpurea, bacopa monniera, 1,3-dimethylamylamine, cirsium oligophyllum, rau-wolscine extract, and caffeine [100 mg per capsule]) and (2) Jack3d™ (containing a proprietary blend of arginine alpha-ketoglutarate, creatine monohydrate, beta alanine, 2 caffeine, 1,3-dimethylamylamine, and schizandrol A). Supplements were produced in accordance with Good Manufacturing Practices. For OxyELITE Pro™, subjects ingested two servings/capsules of the dietary supplement (both during the acute intake assessment [on days 1 and 15] and each day during the chronic intake period). For Jack3d™, subjects ingested two servings/ scoops of the dietary supplement mixed in water (both during the acute intake assessment [on days 1 and 15] and each day during the chronic intake period). This was an open label study. No placebo conditions were included in this initial investigation, as we were mainly interested in potential changes in our measured variables across time (both with the acute intake study and with the chronic intake study). This lack of a placebo condition is indeed a limitation of the present design. Subjects’ self-report of daily intake, coupled with our collection of remaining supplements at the conclusion of the 14-day supplementation period, determined compliance to supplementation.
On days 1 and 15 (pre and post intervention), subjects reported to the laboratory in a fasted state (≥10 hours), without caffeine consumption during the past 10 hours. Subjects were asked not to exercise or to perform any strenuous physical activity for the 24 hours prior to each testing day. Following a 10 minute rest period, HR (via 60 second palpation of the radial artery), as well as SBP and DBP (via auscultation using a dual-earpiece stethoscope by two trained technicians) were measured. RPP was calculated as: HR × SBP. A blood sample was then obtained. These tests (HR, blood pressure, and blood) were done prior to ingestion of the supplements. Subjects then ingested the assigned supplement in the presence of an investigator. At 30, 60, 90, and 120 minutes post-ingestion, HR, SBP, and DBP were measured, and RPP was calculated. Subjects remained in the lab and rested during this time (read, watched television, worked on the computer, listened to music) and no food was allowed during the two hour post intake period. However, water was allowed ad libitum, and was measured and matched for each subject on both days of testing. Following testing on day 1, subjects were provided with their assigned supplement and given instructions on how/when to ingest (ie, 2 capsules or 2 scoops daily—preferably ≥6 hours prior to bedtime).
Blood collection and biochemistry
A total of two venous blood samples were taken from subjects during the course of the study: day 1 and day 15. Blood was processed and sent to Laboratory Corporation of America for analysis of complete blood count, comprehensive metabolic panel, and lipid panel. These measures were included for a comprehensive assessment of bloodborne variables, as is routinely performed as part of a physical examination. They were also included within the design, as this is common practice within many dietary supplement studies focused on blood related “safety” variables. We had no directional hypotheses related to the impact of the supplements on these measures. The complete blood count was determined using an automated cell counter (Coulter LH750). The comprehensive metabolic panel was determined using automated procedures (Roche/Hitachi Modular). The lipid panel was determined using enzymatic procedures (Roche/ Hitachi Modular).
Dietary intake and physical activity
Subjects were asked to maintain their usual diet and physical activity patterns over the course of the entire two week study period. However, they were asked to refrain from strenuous activity during the 24 hours prior to each test day. As the tested dietary supplements have been noted anecdotally to result in appetite suppression, subjects were asked to rate their overall appetite before and after the intervention using a visual analog scale (0 = no hunger at all; 10 = extreme hunger).
Statistical analysis
Resting, pre/post intervention data (hemodynamic, bloodborne, and appetite) for both supplements were compared using a one way analysis of variance (ANOVA); a statistical technique used to evaluate whether there are differences between the mean values across different groups. In regards to the acute intake study, a 2 (pre/post intervention) × 5 (time) ANOVA was used. For the pre/post intervention effect to be significant (P ≤ 0.05), a measureable difference was observed between day 1 and day 15 data. Likewise, for the time effect to be significant (P ≤ 0.05), a measureable difference was observed between data collected at the various times before and following acute intake of the supplements (Pre, 30, 60, 90, and 120 minutes post-ingestion). Percent change values for all hemodynamic data were also calculated and compared using a 2 (pre/post intervention) × 5 (time) ANOVA. Tukey post hoc testing was used when needed, to determine where specific differences may have existed. No attempt was made to compare data between the two supplements, as we were attempting to characterize the effects of using supplements containing 1,3-dimethylamylamine and caffeine, and not make direct comparisons between such supplements. All analyses were performed using JMP statistical software (version 4.0.3, SAS Institute, Cary, NC). Statistical significance was set at P ≤ 0.05. The data are presented as mean ± SEM, except for subject descriptive characteristics (mean ± SD).
Results
Appetite data
Appetite was lower from pre (6.3 ± 0.5) to post (4.3 ± 0.6) intervention for OxyELITE Pro™ (P = 0.04). However, appetite was not different from pre (6.1 ± 0.1) to post (6.1 ± 0.5) intervention for Jack3d™ (P = 1.00). It is possible that ingredients aside from 1,3-dimethylamylamine and caffeine, which are present within OxyELITE Pro™ but not within Jack3d™, may have contributed to these findings.
Resting data (pre-supplementation): hemodynamic and biochemical
When determined prior to the acute intake of the supplements, no change from pre (day 1) to post (day 15) intervention was noted for HR (Table 2), SBP (Table 3), DBP (Table 4), or RPP (Table 5) for subjects ingesting either supplement for the two week period (P > 0.05). In fact, a slight (non-statistical) reduction in HR, SBP, and RPP were observed following treatment with OxyELITE Pro™. No significant changes (P > 0.05) were observed in complete blood count (Table 6) or lipid panel (Table 7) data for either supplement. Likewise, no significant changes (P > 0.05) were noted in comprehensive metabolic panel data for either supplement (Table 8), with the exception of blood glucose with ingestion of Jack3d™ (P = 0.02).
Table 2.
Heart rate (bpm) before and following ingestion of OxyELITE Pro™ or Jack3d™ pre (day 1) and post (day 15) daily supplement use.
| Time |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1)* | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Pre | 64.8 ± 2.9 | 60.9 ± 2.6 | 58.3 ± 2.7 | 60.4 ± 2.5 |
| 30 min | 62.8 ± 2.7 | 56.1 ± 2.8 | 55.6 ± 2.2 | 58.4 ± 3.2 |
| 60 min | 68.8 ± 6.1 | 61.7 ± 2.4 | 61.4 ± 2.1 | 59.1 ± 2.2 |
| 90 min | 71.4 ± 6.9 | 60.9 ± 3.6 | 59.7 ± 2.0 | 59.0 ± 2.4 |
| 120 min | 68.8 ± 4.8 | 61.9 ± 3.1 | 62.1 ± 0.9 | 60.6 ± 2.3 |
Notes: Data are mean ± SEM. OxyELITE Pro™:
Pre/Post intervention (P = 0.008; Pre intervention higher than Post intervention), Time (P = 0.46); Pre/Post intervention × Time (P = 0.95). Jack3d™: Pre/Post intervention (P = 0.83), Time (P = 0.22); Pre/Post intervention × Time (P = 0.88).
Table 3.
Systolic blood pressure (mmHg) before and following ingestion of OxyELITE Pro™ or Jack3d™ pre (day 1) and post (day 15) daily supplement use.
| Time |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1) | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Pre | 103.0 ± 4.0 | 99.0 ± 5.4 | 109.0 ± 2.9 | 109.3 ± 3.3 |
| 30 min | 106.9 ± 7.5 | 104.1 ± 4.2 | 112.4 ± 6.3 | 122.3 ± 3.1 |
| 60 min* | 118.1 ± 5.1 | 113.0 ± 4.1 | 121.7 ± 5.1 | 121.4 ± 5.4 |
| 90 min* | 119.2 ± 3.6 | 110.2 ± 5.8 | 115.7 ± 4.8 | 122.7 ± 3.1 |
| 120 min* | 115.5 ± 5.2 | 110.0 ± 5.5 | 115.7 ± 5.4 | 122.1 ± 5.7 |
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.11),
Time (P = 0.03; 60, 90, and 120 min Post-ingestion higher than Pre [P < 0.05]); Pre/Post intervention × Time (P = 0.98). Jack3d™: Pre/Post intervention (P = 0.12), Time (P = 0.09); Pre/Post intervention × Time (P = 0.77).
Table 4.
Diastolic blood pressure (mmHg) before and following ingestion of OxyELITE Pro™ or Jack3d™ pre (day 1) and post (day 15) daily supplement use.
| Time |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1) | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Pre | 63.0 ± 3.8 | 62.0 ± 1.5 | 63.3 ± 3.3 | 64.6 ± 4.1 |
| 30 min | 66.3 ± 4.9 | 63.8 ± 2.0 | 68.9 ± 5.3 | 74.0 ± 2.2 |
| 60 min | 69.2 ± 5.1 | 68.3 ± 2.2 | 71.7 ± 5.0 | 73.7 ± 4.8 |
| 90 min | 67.1 ± 6.1 | 65.7 ± 2.7 | 68.4 ± 3.5 | 75.1 ± 1.8 |
| 120 min | 66.1 ± 4.2 | 65.2 ± 3.7 | 70.4 ± 5.2 | 72.7 ± 3.6 |
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.59), Time (P = 0.62); Pre/Post intervention × Time (P = 0.99). Jack3d™: Pre/Post intervention (P = 0.18), Time (P = 0.19); Pre/Post intervention × Time (P = 0.96).
Table 5.
Rate pressure product before and following ingestion of OxyELITE Pro™ or Jack3d™ pre (day 1) and post (day 15) daily supplement use.
| Time |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1)* | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Pre | 6634.3 ± 193.4 | 6033.7 ± 442.1 | 6381.3 ± 398.9 | 6606.6 ± 343.3 |
| 30 min | 6778.3 ± 733.9 | 5840.3 ± 393.8 | 6309.3 ± 545.9 | 6920.6 ± 481.0 |
| 60 min | 8228.7 ± 1068.8 | 6938.8 ± 245.0 | 7496.9 ± 463.0 | 7209.7 ± 470.2 |
| 90 min | 8573.1 ± 977.9 | 6637.8 ± 330.9 | 6935.1 ± 432.5 | 7268.6 ± 413.4 |
| 120 min | 8053.4 ± 907.4 | 6766.8 ± 363.5 | 7207.6 ± 411.2 | 7454.9 ± 486.9 |
Notes: Data are mean ± SEM. OxyELITE Pro™:
Pre/Post intervention (P = 0.004; Pre intervention higher than Post intervention), Time (P = 0.09); Pre/Post intervention × Time (P = 0.88). Jack3d™: Pre/Post intervention (P = 0.44), Time (P = 0.20); Pre/Post intervention × Time (P = 0.91).
Table 6.
Complete blood count data pre (day 1) and post (day 15) daily intake of OxyELITE Pro™ or Jack3d™.
| Variable |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1) | Post (day 15) | Pre (day 1) | Post (day 15) | |
| WBC (103 · μL−1) | 6.0 ± 0.4 | 6.1 ± 0.4 | 5.3 ± 0.3 | 5.1 ± 0.3 |
| RBC (106 · μL−1) | 4.3 ± 0.1 | 4.2 ± 0.2 | 4.6 ± 0.1 | 4.6 ± 0.1 |
| Hemoglobin (g · dL−1) | 13.4 ± 0.4 | 13.2 ± 0.4 | 14.6 ± 0.3 | 14.5 ± 0.2 |
| Hematocrit (%) | 39.2 ± 1.1 | 38.9 ± 1.6 | 41.7 ± 0.8 | 42.3 ± 0.7 |
| MCV (fL) | 92.0 ± 1.1 | 93.0 ± 1.7 | 90.0 ± 1.7 | 92.3 ± 1.3 |
| MCH (pg) | 31.4 ± 0.5 | 31.4 ± 0.6 | 31.5 ± 0.5 | 31.7 ± 0.5 |
| MCHC (g · dL−1) | 34.2 ± 0.4 | 33.9 ± 0.4 | 35.0 ± 0.2 | 34.3 ± 0.3 |
| RDW (%) | 13.3 ± 0.3 | 13.3 ± 0.3 | 12.9 ± 0.2 | 13.5 ± 0.3 |
| Platelets (103 μL−1) | 196.2 ± 14.8 | 198.3 ± 19.3 | 193.6 ± 12.9 | 198.4 ± 14.2 |
| Neutrophils (%) | 50.0 ± 2.4 | 51.5 ± 3.8 | 50.6 ± 2.6 | 47.0 ± 3.2 |
| Lymphocytes (%) | 40.3 ± 2.2 | 38.7 ± 4.0 | 36.4 ± 2.4 | 38.7 ± 3.1 |
| Monocytes (%) | 7.2 ± 0.6 | 6.7 ± 0.7 | 8.3 ± 0.5 | 8.9 ± 0.7 |
| Eosinophils (%) | 2.0 ± 0.4 | 2.5 ± 0.5 | 4.1 ± 0.7 | 4.9 ± 0.7 |
| Basophils (%) | 0.5 ± 0.2 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.6 ± 0.2 |
Notes: Values are mean ± SEM. OxyELITE Pro™: No significant difference noted from pre to post for any variable (P > 0.05). Jack3d™: No significant difference noted from pre to post for any variable (P > 0.05).
Table 7.
Lipid panel data pre (day 1) and post (day 15) daily intake of OxyELITE Pro™ or Jack3d™.
| Variable |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1) | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Cholesterol (mg · dL−1) | 164.5 ± 13.2 | 170.0 ± 14.5 | 145.1 ± 8.1 | 147.6 ± 8.8 |
| Triglycerides (mg · dL−1) | 87.8 ± 16.3 | 70.3 ± 9.1 | 74.1 ± 11.3 | 80.0 ± 11.3 |
| HDL-C (mg · dL−1) | 66.8 ± 5.9 | 69.3 ± 6.9 | 50.7 ± 4.7 | 52.3 ± 3.5 |
| VLDL-C (mg · dL−1) | 17.5 ± 3.2 | 14.2 ± 1.8 | 14.7 ± 2.2 | 16.0 ± 2.2 |
| LDL-C (mg · dL−1) | 80.2 ± 6.7 | 86.5 ± 9.0 | 79.7 ± 6.2 | 79.3 ± 7.7 |
| LDL-C/HDL-C | 1.2 ± 0.1 | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.6 ± 0.2 |
| Total:HDL-C | 2.5 ± 0.1 | 2.5 ± 0.2 | 2.9 ± 0.2 | 2.9 ± 0.2 |
Notes: Values are mean ± SEM. OxyELITE Pro™: No significant difference noted from pre to post for any variable (P > 0.05). Jack3d™: No significant difference noted from pre to post for any variable (P > 0.05).
Table 8.
Metabolic panel data pre (day 1) and post (day 15) daily intake of OxyELITE Pro™ or Jack3d™.
| Variable |
OxyELITE Pro™
|
Jack3d™
|
||
|---|---|---|---|---|
| Pre (day 1) | Post (day 15) | Pre (day 1) | Post (day 15) | |
| Glucose (mg · dL−1) | 78.2 ± 4.4 | 77.0 ± 3.1 | 86.4 ± 2.3* | 94.6 ± 2.0 |
| BUN (mg · dL −1) | 18.8 ± 3.9 | 17.0 ± 2.5 | 14.9 ± 1.1 | 16.9 ± 0.9 |
| Creatinine (mg · dL −1) | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.0 | 1.2 ± 0.0 |
| BUN:creatinine | 16.3 ± 2.8 | 17.2 ± 2.5 | 13.1 ± 1.8 | 13.9 ± 0.7 |
| Sodium (mmol · L − 1) | 138.2 ± 0.8 | 141.0 ± 1.6 | 141.1 ± 0.4 | 140.7 ± 0.4 |
| Potassium (mmol · L −1) | 4.7 ± 0.2 | 4.8 ± 0.4 | 4.7 ± 0.2 | 4.8 ± 0.2 |
| Chloride (mmol · L −1) | 101.3 ± 0.6 | 102.5 ± 1.1 | 102.1 ± 0.5 | 103.3 ± 0.5 |
| CO2 (mmol · L −1) | 28.5 ± 0.9 | 27.2 ± 0.9 | 28.1 ± 0.5 | 28.6 ± 0.6 |
| Calcium (mg · dL −1) | 9.1 ± 0.1 | 9.3 ± 0.2 | 9.3 ± 0.1 | 9.4 ± 0.1 |
| Protein (g · dL −1) | 6.7 ± 0.2 | 6.6 ± 0.2 | 6.6 ± 0.1 | 6.6 ± 0.1 |
| Albumin (g · dL −1) | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.5 ± 0.0 | 4.3 ± 0.1 |
| Globulin (g · dL −1) | 2.3 ± 0.1 | 2.2 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 |
| A:G | 2.0 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.1 |
| Bilirubin (mg · dL−1) | 0.4 ± 0.1 | 0.5 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.2 |
| Alk Phos (IU · L−1) | 64.2 ± 3.9 | 60.3 ± 4.4 | 64.1 ± 7.9 | 63.6 ± 8.4 |
| AST (SGOT) (IU · L−1) | 21.0 ± 2.2 | 25.7 ± 5.3 | 25.1 ± 2.6 | 30.7 ± 2.5 |
| ALT (SGPT) (IU · L −1) | 19.0 ± 1.9 | 16.8 ± 1.9 | 23.7 ± 2.8 | 27.6 ± 3.3 |
| GGT (IU · L · −1) | 22.1 ± 2.9 | 20.6 ± 3.2 | 16.1 ± 1.2 | 19.9 ± 2.3 |
Notes: Values are mean ± SEM. OxyELITE Pro™: No significant difference noted from pre to post for any variable (P > 0.05). Jack3d™:
Significant difference noted from pre to post for glucose (P = 0.02). No other significant difference noted from pre to post for any variable (P > 0.05).
Acute intake of supplement: hemodynamics
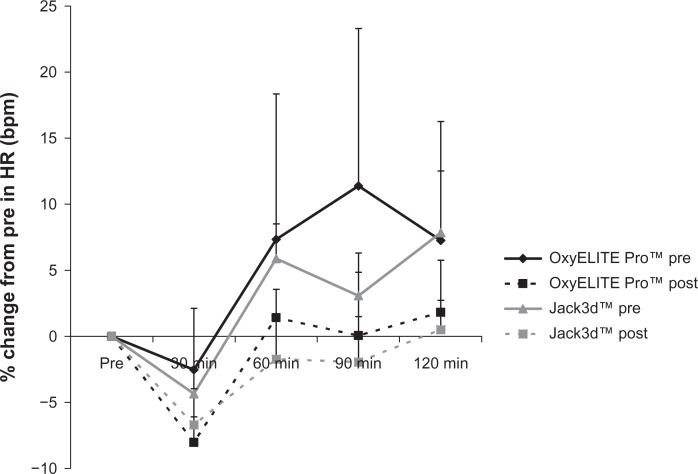
Following acute intake of the supplements, with regards to HR, the following effects were observed: For OxyELITE Pro™, a pre/post intervention effect was noted (P = 0.008), with values lower post intervention compared to pre intervention. No time (P = 0.46) or pre/post intervention × time effect was noted (P = 0.95). For Jack3d™, no pre/post intervention (P = 0.83), time (P = 0.22), or pre/post intervention × time effect was noted (P = 0.88). When expressing data as percent change from pre-ingestion, the following effects were noted: For OxyELITE Pro™, no pre/post intervention (P = 0.17), time (P = 0.41), or pre/post intervention × time effect was noted (P = 0.94). For Jack3d™, a pre/post intervention effect was noted (P = 0.01), with a lower response post intervention compared to pre intervention. A time effect was also noted (P = 0.01), with values at 120 minutes different than values at 30 minutes (P < 0.05). No pre/post intervention × time effect was noted (P = 0.59). Heart rate data are presented in Table 2 and Figure 1.
Figure 1.
Percent change from pre in heart rate for OxyELITE Pro™ and Jack3d™ pre (day 1) and post (day 15) daily supplement use.
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.17), Time (P = 0.41); Pre/Post intervention × Time (P = 0.94). Jack3d™: Pre/Post intervention (P = 0.01), Time (P = 0.01); Pre/Post intervention × Time (P = 0.59).
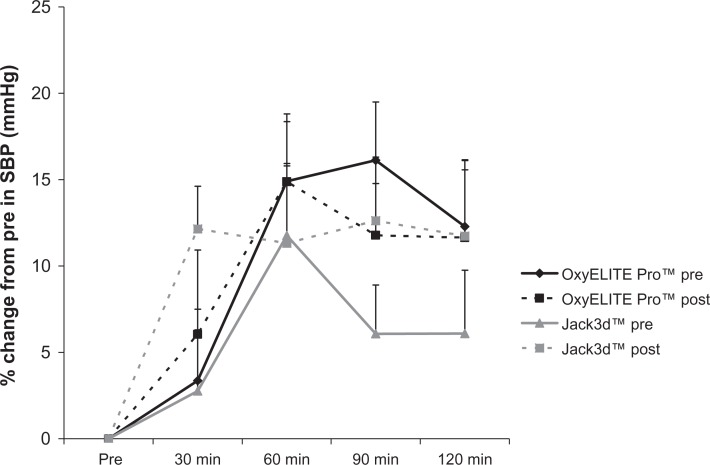
With regards to SBP, the following effects were observed: For OxyELITE Pro™, no pre/post intervention (P = 0.11) or pre/post intervention × time effect was noted (P = 0.98). However, a time effect was noted (P = 0.03), with values higher than pre-ingestion at 60, 90, and 120 minutes post-ingestion (P < 0.05). For Jack3d™, no pre/post intervention (P = 0.12), time (P = 0.09), or pre/post intervention × time effect was noted (P = 0.77). When expressing data as percent change from pre-ingestion, the following effects were noted: For OxyELITE Pro™, no pre/post intervention (P = 0.84) or pre/post intervention × time effect was noted (P = 0.92). However, a time effect was noted (P = 0.0004), with values higher than pre-ingestion at 60, 90, and 120 minutes post-ingestion (P < 0.05). For Jack3d™, a pre/post intervention effect was noted (P = 0.04), with a greater response post intervention compared to pre intervention. A time effect was also noted (P = 0.006), with values higher than pre-ingestion at 60, 90, and 120 minutes post-ingestion (P <0.05). No pre/post intervention × time effect was noted (P = 0.44). Systolic blood pressure data are presented in Table 3 and Figure 2.
Figure 2.
Percent change from pre in systolic blood pressure for OxyELITE Pro™ and Jack3d™ pre (day 1) and post (day 15) daily supplement use.
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.84), Time (P = 0.0004); Pre/Post intervention × Time (P = 0.92). Jack3d™: Pre/Post intervention (P = 0.04), Time (P = 0.006); Pre/Post intervention × Time (P = 0.44).
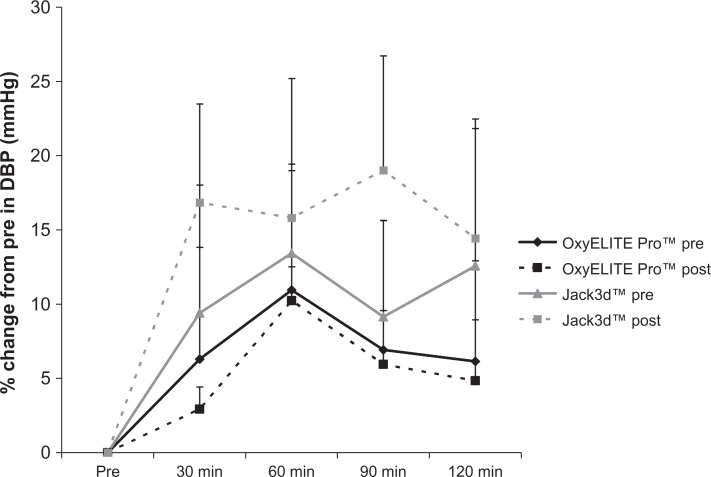
With regards to DBP, the following effects were observed: For OxyELITE Pro™, no pre/post intervention (P = 0.59), time (0.62), or pre/post intervention × time effect was noted (P = 0.90). For Jack3d™, no pre/post intervention (P = 0.18), time (P = 0.19), or pre/post intervention × time effect was noted (P = 0.96). When expressing data as percent change from pre-ingestion, the following effects were noted: For OxyELITE Pro™, no pre/post intervention (P = 0.71), time (P = 0.40), or pre/post intervention × time effect was noted (P = 0.99). For Jack3d™, no pre/post intervention (P = 0.34), time (P = 0.20), or pre/post intervention × time effect was noted (P = 0.95). Diastolic blood pressure data are presented in Table 4 and Figure 3.
Figure 3.
Percent change from pre in diastolic blood pressure for OxyELITE Pro™ and Jack3d™ pre (day 1) and post (day 15) daily supplement use.
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.71), Time (P = 0.40); Pre/Post intervention × Time (P = 0.99). Jack3d™: Pre/Post intervention (P = 0.34). Time (P = 0.20), Pre/Post intervention × Time (P = 0.95).
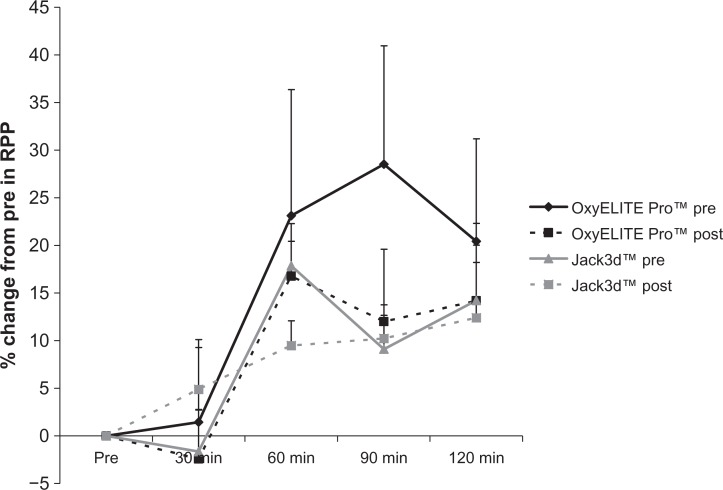
With regards to RPP, the following effects were observed: For OxyELITE Pro™, a pre/post intervention effect was noted (P = 0.004), with values lower post intervention compared to pre intervention. No time (P = 0.09) or pre/post intervention × time effect was noted (P = 0.88). For Jack3d™, no pre/post intervention (P = 0.44), time (P = 0.20), or pre/post intervention × time effect was noted (P = 0.91). When expressing data as percent change from pre-ingestion, the following effects were noted: For OxyELITE Pro™, no pre/post intervention (P = 0.22) or pre/post intervention × time effect was noted (P = 0.99). However, a time effect was noted (P =0.02), with values higher than pre-ingestion at 60, 90, and 120 minutes post-ingestion (P < 0.05). For Jack3d™, no pre/post intervention (P = 0.85) or pre/post intervention × time effect was noted (P = 0.48). However, a time effect was noted (P = 0.001), with values higher than pre-ingestion at 60 and 120 minutes post-ingestion (P < 0.05). Rate pressure product data are presented in Table 5 and Figure 4.
Figure 4.
Percent change from pre in rate pressure product for OxyELITE Pro™ and Jack3d™ pre (day 1) and post (day 15) daily supplement use.
Notes: Data are mean ± SEM. OxyELITE Pro™: Pre/Post intervention (P = 0.22), Time (P = 0.02); Pre/Post intervention × Time (P = 0.99). Jack3d™: Pre/Post intervention (P = 0.85), Time (P = 0.001); Pre/Post intervention × Time (P = 0.48).
Discussion
This study is the first to report the hemodynamic and hematologic effects of acute and chronic intake of dietary supplements containing a combination of 1,3-dimethylamylamine and caffeine in human subjects. Our data indicate that acute ingestion of the tested dietary supplements results in an increase in myocardial work (as measured specifically by SBP), which agree with our prior work using the isolated ingredients 1,3-dimethylamylamine and caffeine alone and in combination.4 It should be noted that the present findings are specific to an ingested dosage of two servings of the supplements. As we have noted in our prior work with 1,3-dimethylamylamine and caffeine, the increase in SBP is dose dependent.4 Therefore, use of only one serving of the tested supplements would likely result in less of a response as compared to what we report here. This is underscored by prior work indicating a dose-dependent effect for caffeine.6,7 Considering that 1,3-dimethylamylamine and caffeine are chief ingredients within both of the tested supplements, lowering the dosage would likely result in a lowering of the blood pressure response to acute ingestion. However, regardless of dose, if an increase in SBP was observed following chronic exposure to such dietary supplements, this may have clinical relevance in terms of increasing the risk for cardiovascular disease.5 We address this with our results for the 14-day intervention period, which indicate that no increase in resting HR or blood pressure is observed, nor are bloodborne variables negatively impacted with either supplement (with the possible exception of blood glucose with Jack3d™, as discussed below). These findings are underscored by our recent placebo controlled studies involving a 10-week intervention with Jack3d™ (with subjects using the product only on exercise training days) and an 8-week intervention involving daily intake of OxyELITEPro™, indicating similar findings as presented within for the 2-week intervention. That is, similar effects were noted across the intervention period for resting blood pressure and bloodborne safety variables for supplements and placebo (unpublished findings).
While no changes were noted in any bloodborne variable within the complete blood count and lipid panel, an increase was noted in fasting blood glucose from pre to post intervention with Jack3d™ treatment (Table 8). While caffeine intake is known to alter glucose homeostasis,8 this finding cannot be solely explained based on the caffeine (or the 1,3-dimethylamylamine), as similar findings would also be expected for subjects using OxyELITE Pro™ (unless other ingredients within OxyELITE Pro™ counteracted this effect). Therefore, it is possible that the creatine monohydrate within the Jack3d™ may be responsible for this effect. In support of this hypothesis, at least one published report documents the influence of creatine on glucose homeostasis in human subjects,9 by noting that supplemental creatine monohydrate at a daily dosage of 5 grams/day for 42 days resulted in a trend (P = 0.07) towards elevated fasting plasma glucose levels. If this were the case, this may be expected for all products containing creatine monohydrate. Aside from creatine, we are unaware of any data linking ingestion of the other ingredients contained within Jack3d™ (arginine alpha-ketoglutarate, beta alanine, schizandrol A) with impairments in glucose homeostasis. Moreover, our recent 10-week intervention study involving Jack3d™ did not result in an increase in fasting blood glucose, suggesting that the results obtained in the present study may have been influenced by confounding factors.
As noted in the Methods section, both of the supplements tested in this study contain additional ingredients that may be at least partly responsible for the noted findings. However, when comparing the findings for hemodynamic measures with those from our prior study using 1,3-dimethylamylamine alone and in combination with caffeine,4 the results are relatively similar. Coupled with the fact that many of the other ingredients included within these products are not known stimulants, it is likely that the simple combination of 1,3-dimethylamylamine and caffeine are responsible for the majority of the effect. It should also be noted that 1,3-dimethylamylamine is clearly responsible for some of the effect on the measured parameters, as caffeine alone does not result in the same magnitude of increase in SBP and RPP as compared to what we observed here.4,10
Although the effect of caffeine on cardiac rate and systemic blood pressure is well described,10 the same is not true for 1,3-dimethylamylamine, with only one published report documenting these effects.4 A suggested component of geranium,3 1,3-dimethylamylamine is becoming increasingly popular as a component of dietary supplements, in particular those targeting weight/fat loss and enhanced exercise performance. The action of 1,3-dimethylamylamine is cited as a simple aliphatic amine, functioning as a norepinephrine reuptake inhibitor and/or norepinephrine releasing agent. It is also suggested to stimulate smooth muscle and act as a vasoconstrictor.4 These effects certainly help to explain the elevation in blood pressure in response to acute ingestion. Anecdotal reports indicate a potential effect on appetite suppression, which is at least partially supported by our findings of decreased appetite with OxyELITE Pro™ treatment. However, no effect was noted for appetite in subjects using Jack3d™. Clearly, additional studies are needed to confirm our findings and to provide mechanistic data pertaining to the effect of 1,3-dimethylamylamine on hemodynamics, appetite, and associated parameters.
As expected based on the pharmacologic profiles of caffeine and of 1,3-dimethylamylamine, acute intake of dietary supplements containing these agents results in an increase in myocardial work. Specifically, SBP is increased significantly in response to treatment, while DBP, and RPP increase to a lesser extent. While such changes are common with weight loss supplements,11–16 in particular for those including caffeine and other stimulants, individuals using dietary supplements containing 1,3-dimethylamylamine alone or in combination with other stimulants should be advised to carefully follow label recommendations pertaining to dosing, and only use such products under the guidance of a qualified healthcare provider. Based on our data, which admittedly involved a very small number of subjects, it appears that such products should be avoided by individuals who are hypertensive (resting blood pressure ≥140/90 mmHg) or those who are pre-hypertensive (resting blood pressure ≥120/80 mmHg).
Finally, it is worth noting the various comments provided by subjects involved in this study. Related to the use of OxyELITE Pro™, comments included: heightened sense of focus and energy, improved workout intensity, sleeplessness, shakiness, feeling anxious, feeling of chills, sweating, nausea, tingling, and feeling of fatigue. Four subjects (3 women and 1 man) who began treatment with OxyELITE Pro™ decided not to complete the intervention period due to dislike for the supplement (sleeplessness, inability to focus, nausea, headaches, jittery). It should be noted that 2 of these 4 individuals had newly acquired employment obligations for which they were not willing to interfere with. Hence, by their own admission, any change in their usual daily routine which they may have attributed to intake of the OxyELITE Pro™ was deemed unacceptable. Four additional subjects were recruited in order to replace these subjects. Data are available only for those subjects who completed the study. Related to the use of Jack3d™, comments included: talkative, heightened sense of focus and energy, improved workout intensity, sleeplessness, feeling anxious, feeling of chills, tingling, and feeling of fatigue. Subjects tolerated Jack3d™ well and all who began treatment completed the entire two week intervention. While no severe adverse events were noted with either supplement, the above comments should be considered along with the objective data presented, as some individuals may find such effects unacceptable.
Conclusion
In conclusion, we report that the finished products OxyELITE Pro™ and Jack3d™, both of which contain a combination of 1,3-dimethylamylamine and caffeine, do not elevate resting HR, SBP, DBP, or RPP when ingested daily for 14 days. Moreover, no significant changes were noted in any measured bloodborne variable following 14 days of ingestion, with the exception of blood glucose with ingestion of Jack3d™. Acute ingestion of these products results in an increase in SBP, while leading to a statistically insignificant increase in DBP and RPP. Considering that these data were generated using two servings of the supplements, it is likely that a single serving would have resulted in a lesser change. Additional acute studies are needed to replicate our findings. In addition, intervention studies involving a larger subject sample monitored over an extended period of time are needed to more fully elucidate the effects of 1,3-dimethylamylamine and caffeine on hemodynamic and hematologic variables.
Acknowledgments
Funding for this work was provided in part by USPlabs, LLC (owner of OxyELITE Pro™ and Jack3d™) and the University of Memphis.
Footnotes
Competing Interests
Financial support for this work was provided in part by USPlabs, LLC. None of the authors have a financial interest in this company. RJB has received research funding or acted as consultant to other nutraceutical and dietary supplement companies. All other authors declare no competing interests.
Authors’ Contributions
TMF, CGM, REC, and RJA were responsible for data collection, blood collection and processing, data entry, and assistance with manuscript preparation. RJB was responsible for the study design, statistical analyses, and manuscript preparation. All authors read and approved of the final manuscript.
Disclosures
Author(s) have provided signed confirmations to the publisher of their compliance with all applicable legal and ethical obligations in respect to declaration of conflicts of interest, funding, authorship and contributorship, and compliance with ethical requirements in respect to treatment of human and animal test subjects. If this article contains identifiable human subject(s) author(s) were required to supply signed patient consent prior to publication. Author(s) have confirmed that the published article is unique and not under consideration nor published by any other publication and that they have consent to reproduce any copyrighted material. The peer reviewers declared no conflicts of interest.
References
- 1.Jiang T. Re-thinking the dietary supplement laws and regulations 14 years after the Dietary Supplement Health and Education Act implementation. Int J Food Sci Nutr. 2009;60(4):293–301. doi: 10.1080/09637480701777977. [DOI] [PubMed] [Google Scholar]
- 2.Fu PP, Chiang HM, Xia Q, et al. Quality assurance and safety of herbal dietary supplements. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009;27(2):91–119. doi: 10.1080/10590500902885676. [DOI] [PubMed] [Google Scholar]
- 3.Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996 Feb;25(1):82–5. [Google Scholar]
- 4.Bloomer RJ, Harvey IC, Farney TM, Bell ZW, Canale RE. Effects of 1,3-dimethylamylamine and caffeine alone or in combination on heart rate and blood pressure in healthy men and women. Physician and Sportsmedicine. 2011;39(3):111–20. doi: 10.3810/psm.2011.09.1927. [DOI] [PubMed] [Google Scholar]
- 5.Sasai H, Sairenchi T, Irie F, et al. Long-term exposure to elevated blood pressure and mortality from cardiovascular disease in a Japanese population: the Ibaraki Prefectural Health Study. Hypertens Res. 2010 doi: 10.1038/hr.2010.173. [DOI] [PubMed] [Google Scholar]
- 6.Kaplan GB, Greenblatt DJ, Ehrenberg BL, et al. Dose-dependent pharmacokinetics and psychomotor effects of caffeine in humans. J Clin Pharmacol. 1997;37(8):693–703. doi: 10.1002/j.1552-4604.1997.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40(9):1243–55. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 8.Dekker MJ, Gusba JE, Robinson LE, Graham TE. Glucose homeostasis remains altered by acute caffeine ingestion following 2 weeks of daily caffeine consumption in previously non-caffeine-consuming males. Br J Nutr. 2007;98(3):556–62. doi: 10.1017/S0007114507730738. [DOI] [PubMed] [Google Scholar]
- 9.Rooney KB, Bryson JM, Digney AL, Rae CD, Thompson CH. Creatine supplementation affects glucose homeostasis but not insulin secretion in humans. Ann Nutr Metab. 2003;47(1):11–5. doi: 10.1159/000068908. [DOI] [PubMed] [Google Scholar]
- 10.Cornelis MC, El-Sohemy A. Coffee, caffeine, and coronary heart disease. Curr Opin Clin Nutr Metab Care. 2007;10(6):745–51. doi: 10.1097/MCO.0b013e3282f05d81. [DOI] [PubMed] [Google Scholar]
- 11.Bloomer RJ, Canale RE, Blankenship MM, Hammond KG, Fisher-Wellman KH, Schilling BK. Effect of the dietary supplement Meltdown on catecholamine secretion, markers of lipolysis, and metabolic rate in men and women: a randomized, placebo controlled, cross-over study. Lipids Health Dis. 2009;8:32. doi: 10.1186/1476-511X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bui LT, Nguyen DT, Ambrose PJ. Blood pressure and heart rate effects following a single dose of bitter orange. Ann Pharmacother. 2006;40(1):53–7. doi: 10.1345/aph.1G488. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman JR, Kang J, Ratamess NA, Rashti SL, Tranchina CP, Faigenbaum AD. Thermogenic effect of an acute ingestion of a weight loss supplement. J Int Soc Sports Nutr. 2009;6:1. doi: 10.1186/1550-2783-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haller CA, Benowitz NL, Jacob P., 3rd Hemodynamic effects of ephedra-free weight-loss supplements in humans. Am J Med. 2005;118(9):998–1003. doi: 10.1016/j.amjmed.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Haller CA, Jacob P, 3rd, Benowitz NL. Enhanced stimulant and metabolic effects of combined ephedrine and caffeine. Clin Pharmacol Ther. 2004;75(4):259–73. doi: 10.1016/j.clpt.2003.11.375. [DOI] [PubMed] [Google Scholar]
- 16.Vukovich MD, Schoorman R, Heilman C, Jacob P, 3rd, Benowitz NL. Caffeine-herbal ephedra combination increases resting energy expenditure, heart rate and blood pressure. Clin Exp Pharmacol Physiol. 2005;32(1–2):47–53. doi: 10.1111/j.1440-1681.2005.04152.x. [DOI] [PubMed] [Google Scholar]