Abstract
BACKGROUND
The costs associated with a contemporary active surveillance strategy compared with immediate treatment for prostate cancer are not well characterized. The purpose of this study is to elucidate the health care costs of an active surveillance paradigm for prostate cancer.
METHODS
A theoretical cohort of 120,000 men selecting active surveillance for prostate cancer was created. The number of men remaining on active surveillance and those exiting to each of 5 treatments over 5 years were simulated in a Markov model. Estimated total costs after 5 years of active surveillance with subsequent delayed treatment were compared with immediate treatment. Sensitivity analyses were performed to test the effect of various surveillance strategies and attrition rates. Additional analyses to include 10 years of follow-up were performed.
RESULTS
The average simulated cost of treatment for 120,000 men initiating active surveillance with 5 years of follow-up and subsequent delayed treatment resulted in per patient cost savings of $16,042 (95% confidence interval [CI], $16,039-$16,046) relative to initial curative treatment. This represents a $1.9 billion dollar savings to the cohort. The strict costs of active surveillance exceeded those of brachytherapy in the ninth year of follow-up. A yearly biopsy within the active surveillance cohort increased costs by 22%, compared with every other year biopsy. At 10 years of follow-up, active surveillance still resulted in a cost benefit; however, the savings were reduced by 38% to $9944 (95% CI, $9941-$9948) per patient relative to initial treatment.
CONCLUSIONS
These data demonstrate that active surveillance represents a considerable cost savings over immediate treatment for prostate cancer in a theoretical cohort after 5 and 10 years of follow-up.
Keywords: prostate cancer, active surveillance, economics, Markov model, cost
INTRODUCTION
Prostate cancer is the most common malignancy diagnosed in American men and continues to represent a major source of medical expenditure.1 With >240,000 cases diagnosed each year and an often prolonged natural history, considerable costs can be accrued at all stages of prostate cancer treatment. As health care delivery costs continue to rise, increased attention must be paid to the costs of competing treatment modalities and how these relate to clinical outcomes.
Several options exist for management of localized prostate cancer. Recently, active surveillance has emerged as a viable treatment option for men with tumors of low- and potentially intermediate-risk potential.2-4 Active surveillance allows for temporary and often indefinite deferment of aggressive therapy for prostate cancer in men traditionally considered at low risk for prostate cancer-specific morbidity and mortality. Data from several series, notably Albertsen et al5 and Stattin et al,6 suggest that many men with low-risk prostate cancer will ultimately die of comorbid disease, and not of their cancer.
Within this context of potentially indolent disease, clinicians have historically advocated a watchful waiting paradigm for those men with significant medical comorbidities. However, in contrast to watchful waiting, an active surveillance protocol advocates a potential intention to treat and therefore imposes an often rigorous follow-up strategy, with frequent prostate-specific antigen (PSA) measurements, office visits, and prostate biopsies. A proportion of these men, approximately 30% over 5 years, will progress to more aggressive cancer and subsequently undergo treatment, most commonly with surgery or radiation.7 Aggressive diagnosis and treatment of prostate cancer remains the standard in the United States, with relatively few men selecting expectant management. However, surveillance of some low-risk tumors appears to be on the rise.8
Although the utilization of active surveillance remains relatively low, there are clearly demonstrated benefits of this paradigm. Active surveillance may reduce the risks of overtreatment, such as erectile dysfunction or urinary incontinence, while preserving the option for definitive treatment. This may effectively tailor treatment to the biologic behavior of an individual's specific prostate cancer.9 Furthermore, Manoharan and colleagues10 noted in an interesting analysis that the reimbursement for urologists using active surveillance may exceed the remuneration for other forms of initial prostate cancer treatment after 3 to 4 years of follow-up. In addition, Hayes et al11 recently evaluated the quality-adjusted life expectancy of prostate cancer treatments via an important theoretical decision analysis. These researchers noted that active surveillance represented the prostate cancer treatment modality with the highest quality-adjusted life years. Although their analysis did not include a strict cost-effectiveness calculation, the estimated costs for curative prostate cancer treatments have been well described. Wilson and colleagues12 in 2007 noted the Medicare costs for treatment of low- to intermediate-risk prostate cancer among 5 treatment modalities, with 5.5 years of follow-up. The cost in this series for external beam radiation therapy was $48,840 to $56,725; radical prostatectomy (RP) was $32,795 to $35,037, brachytherapy was $28,366 to $41,419, and watchful waiting was $31,789 to $31,871. It should be noted that costs within this series reflect the use of 3-dimensional conformal radiotherapy for treatment of prostate cancer. Contemporary practice is typically performed with more costly image-modulated or image-guided radiotherapy (IGRT).13
The costs associated with a contemporary active surveillance strategy incorporating delayed treatment compared with immediate treatment for prostate cancer are unknown. The purpose of this study is to better understand the health care costs associated with active surveillance using a theoretical cohort of 120,000 men with prostate cancer. Active surveillance, including possible exit to treatment each year, was compared with the cost of up-front treatment for a group of men via a simulation study.
MATERIALS AND METHODS
Costs of Active Surveillance
A theoretical cohort of 120,000 men selecting active surveillance for prostate cancer was created. The numbers of men remaining on active surveillance and the proportion exiting to each of 5 treatments over the course of 5 years were simulated in a Markov model.14 The cohort size was chosen as a realistic approximation of a national at-risk cohort, given an approximate 50% rate of low-risk prostate cancer and a national prostate cancer incidence of approximately 240,000 men per year.1,8,15 The cycle length used for the model was selected as 1 year. At the end of each cycle, the cohort was redistributed with a proportion of men exiting active surveillance and entering active treatment. The probability of exiting active surveillance during each year was assumed to be 7% in follow-up years 1 through 5. Exit rates were decreased to 4.5% in years 6 through 10. This represents an exit percentage of 30% and 45% at 5 and 10 years of follow-up, respectively. These egress data were extracted from published active surveillance series.7,16-20
Upon exit from the active surveillance cohort, the patients then received delayed active treatment. The patients were distributed between different forms of common treatment for localized prostate cancer including: RP, IGRT with or without androgen deprivation therapy (ADT), prostate brachytherapy, and ADT monotherapy. The probability of receiving a given treatment once exiting active surveillance was assumed to be 0.4 for RP, 0.25 for IGRT, 0.1 for IGRT/ADT, 0.15 for brachytherapy, and 0.1 for ADT. These treatment distributions were extracted from published active surveillance series.7,16-20
Once a patient exited from active surveillance to treatment, he remained on that treatment and could not switch treatments or return to active surveillance. All men continued on active surveillance or treatment for 5 years, with no treatment discontinuations because of death or other factors. Costs were calculated as though a patient who switched treatment had done so at the beginning of a year. Each simulation was repeated 1000×, and the cost for a given approach from each simulation was stored in memory. The simulated costs were then used to calculate mean costs and z confidence intervals for each method. A standard discount rate of 5% per year was applied to all costs, to reflect the time value of money. Similar analyses were performed for 10 years of follow-up.
In addition, sensitivity analyses were conducted to determine the effect of variations on specific assumed variables and the observed outcomes. Specifically, the rates of conversion to active treatment after surveillance, frequency of prostate biopsy on active surveillance, and probability of selecting each treatment option were adjusted. The costs of active surveillance were then calculated from the simulation model as above. All simulation and statistical analysis was conducted using R, version 2.13.0 (R Development Core Team, 2011).
Costs of Immediate Curative Treatment
Treatment assignments for a theoretical sample of 120,000 men were simulated from a multinomial distribution, with the probability of each treatment as noted previously. The total cost was calculated as the cost of each treatment in each year and the proportion receiving each treatment in the simulated sample. Each of the above simulations was repeated 1000×, and the cost for a given approach from each simulation was stored in memory. The simulated costs were then used to calculate mean costs and z confidence intervals for each method.
Estimates of Treatment Cost
For this analysis, we focused on direct health care expenses for prostate cancer treatment based on hospital costs from a single academic medical center. Total weighted relative value units (RVUs) were used to calculate the standard cost per RVU multiplied by the RVU value to arrive at the standard cost per treatment. This is an institutionally derived formula used by the cost accountants at the University of California, Davis to derive true health care costs. Again, discounting was performed to account for the effect of passage of time on the value of costs and income. This was felt to be important, as the majority of the costs with immediate therapy are accrued early, whereas the costs of active surveillance accumulate over time. Inflation and rate increases were applied annually over the time course of the cost calculations, during the years 2007 through 2010.
Active surveillance cost estimates were based on the current active surveillance protocol at the University of California, Davis. This included an initial office consultation, 2 prostate biopsies within the first 3 months (diagnostic and confirmatory), pathology costs, professional and technical fees, PSA values, and office visits every 3 months for 2 years, then every 6 months thereafter. Repeat prostate biopsy was performed after the second year of follow-up, then every other year. A sensitivity analysis was performed to assess the influence of yearly biopsy on active surveillance treatment costs. Individual costs for active surveillance are included in Table 1.
Table 1.
Discrete Costs of Active Surveillance
| Cost | Calculated Amount |
|---|---|
| Prostate biopsy | $1102 |
| Pathology costs | $660 |
| Professional/technical fees | $635 |
| Office consultation | $428 |
| Office visit | $118 |
| PSA measurement | $52 |
| Urologist reimbursement for biopsy | $433 |
Abbreviation: PSA, prostate-specific antigen.
Estimated surgical treatment costs included an initial office consultation, prostate biopsy, radical prostatectomy, pathology, anesthesia, inpatient costs, professional and technical fees, and office visits with PSA measurements every 3 months for 2 years, then every 6 months thereafter.21 Treatment costs were reflected as a median of open RP and robot-assisted laparoscopic RP costs. Early in the study period used for these cost calculations (2007-2010), a sizable proportion of prostatectomies (30%) were performed in an open fashion at our institution. In this series, the direct costs of robotic prostatectomy were higher than open prostatectomy, although the total costs were nearly offset by shorter hospital stay, reduced nursing costs, and decreased lab costs.
IGRT costs were estimated based on initial urology and radiation oncology consultations and a prostate biopsy with pathology costs. Treatment included computed tomography planning, IGRT plan, 42 radiation sessions, professional fees, and office visits every 6 months for 5 years with PSA measurements. For patients receiving IGRT with intermediate- or high-risk disease, we calculated costs associated with 6 months of androgen deprivation, including medication, pharmacy, and injection costs.
Brachytherapy costs included prostate biopsy with pathology costs, urology and radiation oncology consultations, professional and technical fees, dose planning, ultrasound guidance, anesthesia and medication costs, interstitial seed implantation, and office visits with PSA measurements every 6 months thereafter.
Primary ADT included urology consultation, prostate biopsy with pathology costs, luteinizing hormone-releasing hormone agonist, pharmacy and injection costs, professional and technical fees, office visits every 3 months, and PSA measurements every 6 months.
RESULTS
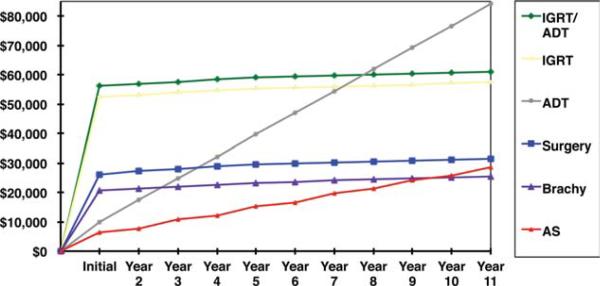
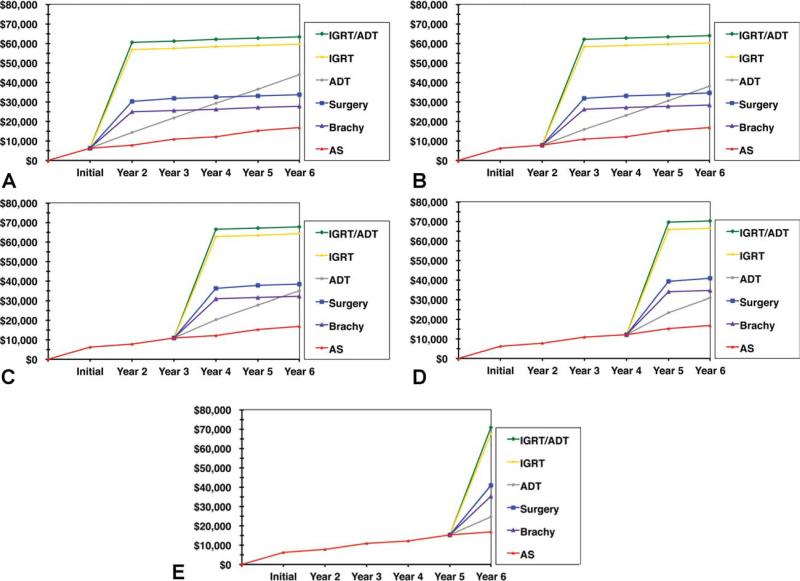
Total treatment costs with 5 and 10 years of follow-up and costs of initial monotherapy are listed in Figure 1 and Table 2, respectively. Total costs of active surveillance with delayed treatment over 5 years of follow-up are represented in Figure 2.
Figure 1.
Costs of primary treatment and active surveillance (AS) are shown, with follow-up costs at every other year biopsy. ADT, androgen deprivation therapy; Brachy, brachy-therapy; IGRT, image-guided radiotherapy.
Table 2.
Costs of Primary Treatment and Active Surveillance, With Follow-up Costs and Every Other Year Biopsy
| Treatment | Initial Cost | Cost at 5 Years of Follow-up | Cost at 10 years of Follow-up |
|---|---|---|---|
| Active surveillance | $6309 | $16,699 | $28,784 |
| Radical prostatectomy | $26,012 | $29,862 | $31,612 |
| IGRT | $52,531 | $55,681 | $57,431 |
| IGRT/androgen deprivation | $56,231 | $59,381 | $61,131 |
| Brachytherapy | $20,567 | $23,717 | $25,467 |
| Primary androgen deprivation | $10,055 | $47,055 | $84,055 |
Abbreviation: IGRT, image-guided radiotherapy.
Figure 2.
Total costs of active surveillance (AS) with delayed treatment after 5 years of follow-up are shown: (A) AS costs with exit within follow-up year 1; (B) AS costs with exit follow-up year 2; (C) AS costs with exit follow-up year 3; (D) AS costs with exit follow-up year 4; (E) AS costs with exit follow-up year 5. ADT, androgen deprivation therapy; Brachy, brachytherapy; IGRT, image-guided radiotherapy.
The simulated cost of 5 years of active surveillance with delayed active treatment for 120,000 men was compared with the simulated costs of initial monotherapy for 120,000 men to yield the cost savings within the theoretical active surveillance cohort. Calculated savings to the cohort were 1.9 billion dollars or $16,042 per patient (95% confidence interval [CI], $16,039-$16,046; P < .001) over 5 years. The cost savings within this active surveillance paradigm persisted at 10 years of follow-up, although the savings were reduced by 38% to $9944 (95% CI, $9941-$9948; P < .001) per patient relative to primary treatment. On an individual basis, men undergoing a period of active surveillance with subsequent delayed curative treatment incur substantially higher individual treatment costs. These men accrue not only the costs of their active surveillance treatment, but also sustain the added costs of delayed active treatment. On average, this represents an additional $17,919 per man over 5 years, above the costs accumulated under active surveillance, for a total treatment cost of $41,039 per man. The strict costs of active surveillance with every other year biopsy exceeded the costs of brachytherapy in the ninth year of follow-up and approached the cost of prostatectomy in the 10th year of follow-up (Fig. 1).
Sensitivity analyses were performed to test the assumptions of our surveillance treatment strategies and attrition rates. If the biopsy frequency was increased from every other year to yearly, a net cost savings persisted, although it decreased by 22% to $12,464 (95% CI, $12,461-$12,467) per individual. At 10 years of active surveillance follow-up, a yearly biopsy continued to result in a net savings to the cohort of $4996 (95% CI, $4993-$4999; P < .001) per man. However, the strict costs of active surveillance at 10 years of follow-up with a yearly biopsy exceed the costs of both brachytherapy and prostatectomy, at 6 and 8 years of follow-up, respectively. Not surprisingly, increasing the percentage of men who received IGRT and reducing the number of men treated with RP increased the costs to both the individual and the cohort. However, the net savings of active surveillance increased in this scenario, as active surveillance represents a less costly treatment paradigm relative to IGRT. A cost neutrality threshold was reached when at least 12% of men remained on active surveillance through the fifth year of follow-up. At 10 years of follow-up, cost neutrality was achieved if at least 15% of men persisted on active surveillance.
DISCUSSION
These data demonstrate that active surveillance results in considerable cost savings over immediate treatment for a theoretical cohort of 120,000 men with prostate cancer at both 5 and 10 years of follow-up. Although these findings may be somewhat intuitive, the magnitude of $1.9 billion dollar savings in this cohort is impressive. In addition, there are several important clinical and policy implications.
Clinically, it is relevant to note that increasing the frequency of prostate biopsy to once a year, which is a common active surveillance paradigm at some institutions, results in significantly increased overall costs of active surveillance. A yearly biopsy protocol results in a net cost advantage at 5 years of follow-up relative to initial treatment; however, these savings are significantly minimized at 10 years of follow-up. Although it is not clear what biopsy or follow-up strategy should be implemented for those men on long-term active surveillance, a yearly biopsy regimen results in significantly elevated health care costs. This is largely due to the inherent costs of biopsy, as the expense of transrectal ultrasound, professional/technical fees, and pathology fees represent the principal drivers of active surveillance costs. Furthermore, men who undergo prolonged active surveillance and then subsequent delayed treatment incur very high individual treatment costs, in that they accumulate not only the nonnegligible costs of active surveillance, but the additional costs of delayed treatment. Although the data suggest that there may be no clinical consequence of this delayed treatment in terms of cancer-specific or overall survival,9,16 the accrued costs are impressive. Importantly, these costs to the cohort are offset by the majority of men remaining on an active surveillance protocol and realizing significant cost savings. Lastly, it is illuminating to note that the strict costs of active surveillance exceed brachytherapy and approach the costs of prostatectomy with prolonged active surveillance and follow-up. These observations highlight the challenge of rapidly identifying those men on an active surveillance paradigm who are destined to progress to curative treatment.
Several recent studies have estimated the costs and physician reimbursements of active surveillance compared with radical prostatectomy. Corcoran et al22 noted in an active surveillance paradigm, with 15 years of follow-up and annual conversion rates of 5% to 7% to RP, significant cost savings of 43% to 79% compared with upfront RP. Although this analysis calculated costs over a relatively long period of time, the study did not include costs of other common treatment modalities for prostate cancer, such as radiotherapy, that are substantially more expensive. As previously noted, Manoharan and colleagues demonstrated that urologist reimbursement for managing a man with active surveillance equals that of upfront RP after 3 to 4 years of surveillance.10 These data demonstrate that adoption of an active surveillance strategy can be financially rewarding, yet still result in net health care savings. However, their findings do not lend insight into the global economic burden of prostate cancer care, nor do they address how competing modalities influence the cost of treatment for prostate cancer.
Our cost analysis also has several limitations. These data represent results based upon a theoretical cohort using multiple assumptions obtained from published active surveillance series. Although these published series present consistent results and we have used sensitivity analyses to address a range of variation within our independent variables, these data are as reliable as our assumptions. Further limitations stem from the finding that treatment expenses within this model are based upon hospital costs at a single institution. Consequently, they may not be applicable in all health systems because of regional variations in costs and reimbursements. Moreover, our model did not incorporate other variables such as the opportunity costs of missed income or patient out of pocket expenses.
Another important limitation of our model is that we exclude costs associated with management of treatment-related complications as well as adjuvant or salvage therapies. However, as more complications are associated with radical therapy over surveillance, incorporating these costs into the model would likely only serve to widen the costs savings of an active surveillance paradigm. The need for adjuvant or salvage therapies after primary treatment of low-risk prostate cancer is also low and would be unlikely to significantly alter these findings. The rising incidence of postbiopsy sepsis23 is an issue that should be noted in the costs of an active surveillance paradigm. Although we did not incorporate these non-negligible costs in our paradigm, they would likely be offset at the cohort level by the significantly higher costs of complications associated with active treatment, such as incontinence, transfusion, erectile dysfunction, recurrence, adjuvant treatment, and secondary malignancy.
Our analysis did not include a strict cost-effectiveness calculation, as morbidity and mortality caused by prostate cancer over this short time period are exceedingly low for active surveillance patients. However, when evaluated in terms of the important quality of life benefits noted by Hayes et al,11 our data suggest that active surveillance represents a cost-effective option for treatment of low-risk prostate cancer. Long-term outcome data from ongoing prospective active surveillance trials are needed to further clarify the true cost-effectiveness of active surveillance for prostate cancer.
In conclusion, men with prostate cancer represent a source of considerable health care expenditure in the United States. Short-term follow-up data suggest active surveillance is feasible and safe for the management of low-risk prostate cancer. Active surveillance with selective intervention for those men with evidence of prostate cancer progression can result in significant cost savings over immediate curative treatment. From a policy perspective, it is important to recognize that health care dollars represent a limited resource. Furthermore, they also signify an opportunity cost, in that what we spend for 1 treatment limits the ability to spend for others. Given the extent of the cost savings, the significant underutilization of active surveillance as a treatment paradigm, and impending health care reform, as clinicians, we are missing an important clinical and policy opportunity by not advocating active surveillance for our patients with low-risk prostate cancer. As the costs of health care rise, through the utilization of progressively more expensive technologies, the relative clinical and economic benefits of an active surveillance paradigm for the treatment of low-risk prostate cancer will likely become increasingly attractive.
Acknowledgments
FUNDING SOURCES
This work was supported in part by the National Institutes of Health, K-12 Paul Calabresi Career Development Award for Clinical Oncology, CA-90625 to KAK.
We thank Noel Sousa and Carrie Pattinson in the University of California at Davis Decision Support/Cost Accounting Office for assistance with financial data.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Dall'Era MA, Cooperberg MR, Chan JM, et al. Active surveillance for early-stage prostate cancer: review of the current literature. Cancer. 2008;112:1650–1659. doi: 10.1002/cncr.23373. [DOI] [PubMed] [Google Scholar]
- 3.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Cowan JE, Hilton JF, et al. Outcomes of active surveillance for men with intermediate-risk prostate cancer. J Clin Oncol. 2011;29:228–234. doi: 10.1200/JCO.2010.31.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albertsen PC, Moore DF, Shih W, et al. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29:1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102:950–958. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dall'Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112:2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 8.Krahn MD, Zagorski B, Laporte A, et al. Healthcare costs associated with prostate cancer: estimates from a population-based study. BJU Int. 2010;105:338–346. doi: 10.1111/j.1464-410X.2009.08758.x. [DOI] [PubMed] [Google Scholar]
- 9.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28:126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 10.Manoharan M, Eldefrawy A, Katkoori D, Antebi E, Soloway MS. Comparison of urologist reimbursement for managing patients with low-risk prostate cancer by active surveillance versus total prostatectomy. Prostate Cancer Prostatic Dis. 2010;13:307–310. doi: 10.1038/pcan.2010.34. [DOI] [PubMed] [Google Scholar]
- 11.Hayes JH, Ollendorf DA, Pearson SD, et al. Active surveillance compared with initial treatment for men with low-risk prostate cancer: a decision analysis. JAMA. 2010;304:2373–2380. doi: 10.1001/jama.2010.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson LS, Tesoro R, Elkin EP, et al. Cumulative cost pattern comparison of prostate cancer treatments. Cancer. 2007;109:518–527. doi: 10.1002/cncr.22433. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen PL, Gu X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29:1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inadomi JM. Decision analysis and economic modelling: a primer. Eur J Gastroenterol Hepatol. 2004;16:535–542. doi: 10.1097/00042737-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol. 2011;29:3669–3676. doi: 10.1200/JCO.2011.34.9738. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz L. Active surveillance for prostate cancer: patient selection and management. Curr Oncol. 2010;17(suppl 2):S11–S17. doi: 10.3747/co.v17i0.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti SL, Dall'era M, Fradet V. Pathological outcomes of candidates for active surveillance of prostate cancer. J Urol. 2009;181:1628–1633. doi: 10.1016/j.juro.2008.11.107. [DOI] [PubMed] [Google Scholar]
- 19.Tosoian JJ, Trock BJ, Landis P. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185–2190. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 20.Carter HB, Kettermann A, Warlick C, et al. Expectant management of prostate cancer with curative intent: an update of the Johns Hopkins experience. J Urol. 2007;178:2359–2364. doi: 10.1016/j.juro.2007.08.039. discussion 2364-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans CP. Follow-up surveillance strategies for genitourinary malignancies. Cancer. 2002;94:2892–2905. doi: 10.1002/cncr.10525. [DOI] [PubMed] [Google Scholar]
- 22.Corcoran AT, Peele PB, Benoit RM. Cost comparison between watchful waiting with active surveillance and active treatment of clinically localized prostate cancer. Urology. 2010;76:703–707. doi: 10.1016/j.urology.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 23.Zaytoun OM, Vargo EH, Rajan R, et al. Emergence of fluoroquinolone-resistant Escherichia coli as cause of post-prostate biopsy infection: implications for prophylaxis and treatment. Urology. 2011;77:1035–1041. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]