THE CASE
A 57-year-old man with a history of prostate cancer treated with robot-assisted laparoscopic prostatectomy and subsequent salvage pelvic irradiation was referred to our institution with a 2-year history of intermittent pelvic pain and recurrent symptomatic urinary tract infections (UTIs). He had signifi-cant worsening perineal pain refractory to conservative treatment, intermittent gross hematuria, and total urinary incontinence.
His medical history was significant for diabetes, hypertension, hyperlipidemia, and morbid obesity with a body mass index of 44 kg/m2. He had been diagnosed with prostate cancer and treated operatively 3 years before the present evaluation. His preoperative prostate-specific antigen (PSA) level was 9.8 ng/mL. The postoperative pathologic finding was Gleason grade 3+4 with a 4-mm positive margin at the apex. Extended bilateral pelvic lymph node dissection revealed no metastatic disease. His initial postoperative PSA level was undetectable; however, he developed PSA recurrence 12 months after surgery. He subsequently received 35 fractions of high-dose (78 Gy) salvage radiotherapy to the prostate bed during an 8-week period. His PSA values have been undetectable since then.
The review of his systems was negative, except as above. The physical examination findings were significant only for suprapubic and pelvic girdle tenderness. Knee flexion produced perineal pain, but he was without sensory or motor deficits.
Because his PSA values had been undetectable, the differential diagnosis focused on nononcologic etiologies for his pelvic pain and recurrent UTIs. As an initial step in his evaluation, laboratory assessments were performed. A complete blood count revealed a mildly elevated white blood cell count of 12,400/μL. The other parameters on his complete blood count were normal. His urinalysis demonstrated 235 red blood cells/high power field and was leukocyte esterase and nitrite positive. A urine culture grew Pseudomonas aeruginosa and vancomycin-resistant Enterococcus faecium. His basic metabolic panel was within normal limits.
Given the patient's history of pelvic radiotherapy, his gross hematuria was first investigated with office cystoscopy because of a heightened suspicion for bladder cancer or bladder neck contracture. This examination revealed an apparent calcification attached to the bladder mucosa just proximal to the vesical neck. The patient was brought to the operating room for examination under anesthesia and bladder biopsy. The mucosal biopsy specimens were negative for malignancy and demonstrated mild chronic cystitis. An indwelling urethral catheter was placed, and oral ciprofloxacin and nitrofurantoin were started on the basis of his previous urine culture findings.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis for the noted cystoscopic findings included an erosive bladder stone vs a foreign body retained from the previous surgery, such as a surgical clip. Pseudomonas is a urease-splitting bacteria known to increase the risk of struvite and matrix stone formation. However, this apparent calcification did not have the appearance of a bladder stone and was fixed to the anterior bladder wall at the level of the urethrovesical anastomosis. This raised the suspicion for a process related to the patient's previous prostate cancer treatments. Urothelial carcinoma and obstructive uropathy were thought unlikely, given the normal cystoscopy and serum creatinine findings, but upper tract imaging was needed. Chronic cystitis, either infectious or from the radiation, is enough to cause hematuria. However, the calcification seen on cystoscopy warranted additional inquiry.
ADDITIONAL TREATMENT AND PATHOLOGIC EVALUATION
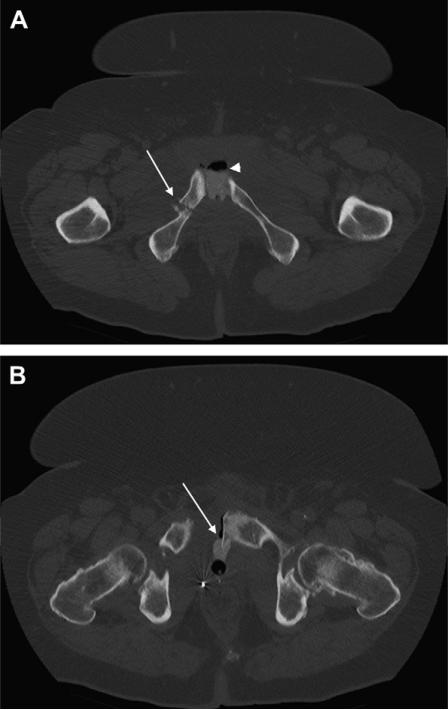
Given his previous findings, additional laboratory tests were performed. The erythrocyte sedimentation rate and C-reactive protein were determined and were elevated at 113 mm/h and 167.8 mg/L, respectively. These values were suggestive of a heightened systemic inflammatory response. Additionally, high levels of C-reactive protein—100 mg/L—can be a sensitive, but not a specific, indicator of osteomyelitis.1 This concern, coupled with the possibility of an upper tract source of his hematuria, prompted axial computed tomography (CT) of the abdomen and pelvis with intravenous contrast and delayed imaging. The CT scan revealed normal ureters and kidneys but demonstrated sclerosis of the sacrum, diastasis of the pubic symphysis, and fractures of the bilateral superior and inferior pubic rami. Also, extravasation of contrast from the bladder anteriorly into the space of Retzius and through the pubic symphysis was seen on the delayed images. The calcification visualized on cystoscopy was consistent with a bone fragment extending from the left pubic symphysis into the bladder. These findings were concerning for radiation-induced osteonecrosis of the pelvis (Fig. 1).
Figure 1.
Computed tomography scans of (A) pelvis with contrast showing pelvic fracture of right inferior pubic rami fracture (arrow), pubic diastasis, and extravasation of contrast and air (arrowhead) between pubic symphysis and (B) small bone exostosis extending posteriorly from left pubic symphysis into the bladder (arrow).
The patient was referred to the orthopedic oncology clinic for additional evaluation. Plain films revealed patchy heterogeneous areas of sclerosis within the pubic rami and iliac wings, consistent with radiation-induced osteonecrosis. Although plain films provide an overview of the osseous anatomy and can show changes from chronic osteomyelitis, they are notoriously nonspecific. Because of the lack of a discrete site suspicious for osteomyelitis amenable to biopsy, a pelvic magnetic resonance imaging (MRI) scan was obtained. Although biopsy remains the reference standard, the MRI scan showed signal abnormalities in the right superior and inferior pubic rami that were highly suggestive of osteomyelitis.
At this point in the evaluation, the patient's pelvic pain had not responded to conservative management. A previous urine culture had identified specific organisms sensitive to the aforementioned antibiotics; thus, complete treatment followed by prophylaxis and lifestyle modification might have been a reasonable next step. Inflammation, perhaps compounded by the radiation cystitis, can persist for months after treatment and requires supportive care and symptomatic management. Nonetheless, given the multiple failed courses of antibiotics and imaging findings in the context of his clinical complaints, we believed that conservative management was not likely to be effective without surgical debridement. Resection of the involved bone and primary closure of the bladder defect would likely have improved his chronic pain and UTIs. However, consideration was given to the role of urinary diversion secondary to the patient's severe urinary incontinence and limited therapeutic options available for management of his incontinence. After careful consideration of his therapeutic options, the patient decided to proceed with cystectomy and ileal conduit urinary diversion at his pelvic debridement. Accordingly, combined cystectomy with ileal conduit and anterior pelvic resection with removal of the necrotic portions of the affected pubic rami was planned.
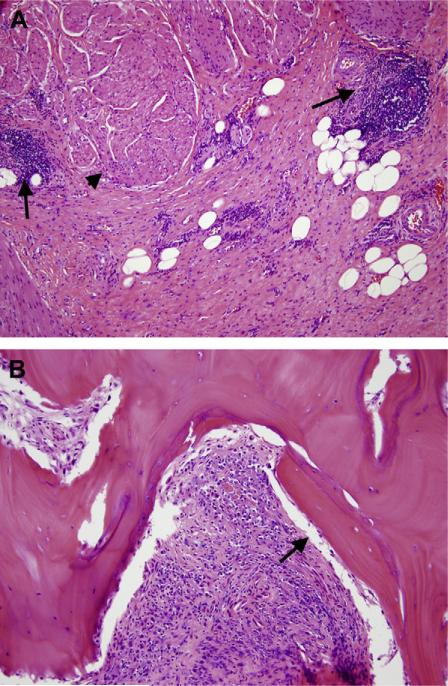
During the procedure, bone exostoses were visualized eroding into the bladder. Much of the anterior bladder was adherent to the pubic bone, which was removed en bloc. The anterior pelvis appeared moth-eaten and poorly vascularized and required extensive resection bilaterally. No frank abscess was encountered, and the surgical field was thoroughly irrigated. An omental flap was rotated into the pelvis to control the adhesions and fill the soft tissue defects. Histopathologic examination of the bladder and resected bone revealed transmural chronic cystitis (Fig. 2A), areas of chronic pelvic osteomyelitis, and foci of osteonecrosis (Fig. 2B). The patient's hospital course was uncomplicated, and the antibiotics were discontinued 48 hours postoperatively. At 8 months of follow-up, his urinary diversion remained functional and he was without any pelvic pain.
Figure 2.
(A) Intermediate power of urinary bladder wall showing transmural fibrosis and chronic inflammation (arrows) mostly arranged in lymphoid aggregates and perivascular infiltrates in between smooth muscle bundles (arrowhead) of muscularis propria. (B) Intermediate power of osseous fragments showing intertrabecular granulation tissue with lymphoplasmacytic infiltrate and foci of bone necrosis (arrow indicates bone lacunae without osteocytes) and remodeling, consistent with chronic osteomyelitis. (Color version available online.)
DISCUSSION AND REVIEW OF THE LITERATURE PROVIDED BY MICHAEL COOKSON, M.D., M.M.H.C.
The present case featured a 57-year-old man with pelvic pain who presented >1 year after robot-assisted laparoscopic prostatectomy/bilateral pelvic lymph node dissection and salvage radiotherapy with intractable pubic pain, recurrent UTIs, hematuria, and total incontinence. Although postoperative pelvic pain is expected after radical prostatectomy, pain lasting >6 months is uncommon and should suggest alternative etiologies. Given these symptoms, the differential diagnosis is vast and includes chronic pelvic pain syndrome, urolithiasis, a retained foreign body, metastases to the bone, and pelvic joint dysfunction. Possible complications of radiotherapy include secondary bladder cancer, radiation-induced cystitis, proctitis, enteritis, and lymphedema. However, given the bone tenderness and imaging findings suggestive of an orthopedic etiology, the differential diagnosis can be narrowed down to 4 potential diagnoses: pelvic insufficiency fractures (PIFs), osteonecrosis (ON), osteomyelitis (OM), and osteitis pubis (OP).
PIFs are nontraumatic stress fractures of the pelvis with deficient elastic resistance. Postmenopausal osteoporosis is a common cause of PIFs; however, secondary causes such as glucocorticoid therapy, androgen deprivation therapy, and pelvic radiotherapy also play a role. As a potential treatment of PSA recurrence, radiotherapy directly affects mature bone by (1) increasing osteoblasts and osteoclasts, (2) impairing mineralization, and (3) causing microvascular injury, all leading to increased susceptibility to fracture and necrosis. Radiation-induced bone injury is more common with high dose (>60 Gy) than low-dose (50 Gy) radiotherapy.2
PIFs are a relatively rare complication of radiotherapy in patients with prostate cancer, with a cumulative 5-year incidence of 6.8%. The fractures typically occur >20 months after radiotherapy.3 In the urologic studies, reports of orthopedic complications of radiotherapy are lacking. For example, a recent study investigating patient-reported outcomes after high-dose radiotherapy for prostate cancer focused only on bowel, urinary, and sexual symptoms, with no mention of orthopedic complications.4 Most of the published data concerning bone complications of urologic cancer surrounds osseous metastases or the side effects of hormonal therapy.5
However, the association of pelvic irradiation and PIFs is well documented in gynecologic and colorectal studies, with a 5-year incidence of 8.2%-19.7%.6 In a notable series, elderly women who received radiotherapy for anal, cervical, and rectal cancer developed moderate rates of PIFs, with the greatest incidence among those treated for anal cancer.7 Other studies have noted a dose-dependent relationship of radiation and pelvic complications, with a predilection for solitary fractures at lower doses (>45 Gy) and an increased likelihood of ON at greater doses (60-77 Gy).6 Although these series often used adjunctive brachytherapy, the total pelvic radiation dosages were often similar to those used for treatment of prostate cancer and, although speculative, might suggest equivalent risk.
Anterior pelvic fractures can be difficult to diagnosis because, in addition to pelvic pain, they can manifest as low back pain owing to increased motion posteriorly at the sacroiliac joints. Plain radiography, CT scan, and radionucleotide bone scans are all helpful in identifying PIFs. The latter might reveal the classic finding of symmetric, bilateral radionucleotide uptake in the sacrum, known as the H-sign.3 Treatment is symptomatic and involves nonsteroidal anti-inflammatory drugs and rest. A comprehensive history is critical, regardless of the etiology, because pathologic fractures can lead to a 20% increased risk of death among patients with cancer.8 The causal relationship between fracture risk and death, however, remains poorly defined.
In addition to PIFs, radiotherapy can also be complicated by ON. ON has several etiologies; however, the unifying pathophysiology is vascular compromise that results in necrosis at ≥1 bone sites. This most commonly occurs at the weight-bearing joints. Chronic glucocorticoid use, alcohol consumption, lupus, bisphosphonates, and radiotherapy are all known risk factors. ON is a recognized complication of radiotherapy for head and neck cancer but is a rarely reported complication of pelvic irradiation.9 One series noted a prevalence of 0.1%-1.3% for femoral head ON after pelvic irradiation.10 Specifically, the prevalence of pelvic ON among urologic patients is not well documented.
The symptoms of ON vary from mild discomfort to extreme pain and joint collapse that limits mobility. With pelvic ON, groin pain and discomfort on weight bearing are common. MRI is the most sensitive modality for diagnosing ON, showing a linear demarcation of healthy and devascularized bone on T1-weighted images. However, plain radiographs and bone density scans can also play a role. Treatment can be conservative for mild cases, and the use of bisphosphonates has been shown to slow the progression of ON.11 Moderate to severe cases can be treated surgically with core decompression, vascularized bone grafting, bone resection, or joint replacement.
Whenever PIFs and ON are considered as part of a differential diagnosis, clinicians must also be suspicious of OM—bacterial infection of the bones. Direct inoculation is more common than hematogenous spread and can be precipitated by chronic pressure ulcers, trauma, pelvic surgery, or, rarely, a chronic indwelling urethral catheter. OM is a known, albeit extremely rare, complication of many urologic procedures. Studies investigating the rate of OM after prostatectomy are uncommon. However, the estimated incidence of OM after bone-anchored bladder neck suspension is 1.3%.12
Patients with OM typically have severe pelvic pain with tenderness at the pubic symphysis, a wide-based gait, and pain with abduction of the hip. Fever can be absent, but inflammatory markers such as the erythrocyte sedimentation rate and C-reactive protein are typically elevated. MRI is the most sensitive modality, with CT and plain radiography less so. Because OM is infectious in etiology, biopsy and culture are the optimal methods to make a definitive diagnosis. In mild cases, a 6-8eweek course of directed antibiotic therapy can be sufficient.13 Surgical debridement and curettage is indicated for patients with severe complications that include pelvic diastasis, bladder perforation, ON, or antibiotic therapy failure.
The final consideration in the differential diagnosis for pelvic pain is OP, a self-limited, idiopathic inflammation of the pubic symphysis and its surrounding structures. OP is the most common inflammatory disease of the pubic symphysis and can occur after overuse in athletes, childbirth, or pelvic surgery. More importantly, OP is a reported complication of several urologic procedures, including prostatectomy, transurethral resection of the prostate, prostate biopsy, and prostate cryotherapy.14
Because they are both relatively rare conditions, the clinical distinction between OP and OM of the pubic symphysis is often obscured. Both conditions are characterized by chronic pelvic pain often radiating to the groin that is exacerbated by walking, pubic tenderness, a wide-based gait, and bony destruction, inflammatory changes or pubic diastasis on CT or MRI. Patients with either condition might have elevated acute phase reactants and erythrocyte sedimentation rate, mild leukocytosis, and fever. OP is thus, by necessity, a diagnosis of exclusion. Conservative treatment with nonsteroidal anti-inflammatory drugs, physical therapy, and, occasionally, steroid injections is usually sufficient. In a series of 70 nonathletes with OP, all but 1 improved with medical management.15 Although surgery is reserved for refractory cases, it should prompt a thorough investigation of alternative etiologies.
CONCLUSION
The present patient likely developed radiation cystitis and ON of the pubic bones, which subsequently led to chronic OM, bony pelvic fractures, and erosion of bone into bladder with resultant recurrent UTIs and hematuria. Orthopedic complications after pelvic radiation for urologic cancer are uncommon and are less well-documented than in the gynecologic and colorectal studies. Although these complications are rare, they can be progressive and debilitating if left untreated. An accurate diagnosis is key, because OM and ON are likely to require surgical therapy for symptomatic relief. Chronic pelvic pain after prostatectomy and pelvic radiotherapy not responsive to conservative treatment or antibiotics should prompt a thorough workup.
Acknowledgments
Funding Support: This work was supported in part by the National Institutes of Health, K-12 Paul Calabresi Career Development Award for Clinical Oncology, grant CA-90625, to K. A. Keegan; M. J. Resnick is supported by the Veterans Affairs National Quality Scholars Program and the T. J. Martell Foundation.
Footnotes
Financial Disclosure: M. J. Resnick is a financially affiliated consultant for Bayer Healthcare, and M. S. Cookson is a financially affiliated consultant for Endo, Myriad, and Spectrum. The authors declare that they have no relevant financial interests.
References
- 1.Harris JC, Caesar DH, Davison C, et al. How useful are laboratory investigations in the emergency department evaluation of possible osteomyelitis? Emerg Med Australas. 2011;23:317–330. doi: 10.1111/j.1742-6723.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 2.Holt GE, Griffin AM, Pintilie M, et al. Fractures following radiotherapy and limb-salvage surgery for lower extremity soft-tissue sarcomas: a comparison of high-dose and low-dose radiotherapy. J Bone Joint Surg Am. 2005;87:315–319. doi: 10.2106/JBJS.C.01714. [DOI] [PubMed] [Google Scholar]
- 3.Iğdem S, Alço G, Ercan T, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77:818–823. doi: 10.1016/j.ijrobp.2009.05.059. [DOI] [PubMed] [Google Scholar]
- 4.Talcott JA, Rossi C, Shipley WU, et al. Patient-reported long-term outcomes after conventional and high-dose combined proton and photon radiation for early prostate cancer. JAMA. 2010;303:1046–1053. doi: 10.1001/jama.2010.287. [DOI] [PubMed] [Google Scholar]
- 5.Lee RJ, Saylor PJ, Smith MR. Treatment and prevention of bone complications from prostate cancer. Bone. 2011;48:88–95. doi: 10.1016/j.bone.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys. 2008;70:1183–1188. doi: 10.1016/j.ijrobp.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 7.Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294:2587–2593. doi: 10.1001/jama.294.20.2587. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;15:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 9.Micha JP, Goldstein BH, Rettenmaier MA, et al. Pelvic radiation necrosis and osteomyelitis following chemoradiation for advanced stage vulvar and cervical carcinoma. Gynecol Oncol. 2006;101:349–352. doi: 10.1016/j.ygyno.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Dzik-Jurasz AS, Brooker S, Husband JE, et al. What is the prevalence of symptomatic or asymptomatic femoral head osteonecrosis in patients previously treated with chemoradiation? A magnetic resonance study of anal cancer patients. Clin Oncol (R Coll Radiol) 2001;13:130–134. doi: 10.1053/clon.2001.9236. [DOI] [PubMed] [Google Scholar]
- 11.Agarwala S, Shah S, Joshi VR. The use of alendronate in the treatment of avascular necrosis of the femoral head: follow-up to eight years. J Bone Joint Surg Br. 2009;91:1013–1018. doi: 10.1302/0301-620X.91B8.21518. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RP, Tchetgen MB, Sand PK, et al. Incidence of pubic osteomyelitis after bladder neck suspension using bone anchors. Urology. 2004;63:704–708. doi: 10.1016/j.urology.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Andonian S, Rabah DM, Aprikian AG. Pseudomonas aeruginosa sacroiliitis and osteomyelitis of pelvic bones after radical prostatectomy. Urology. 2002;60:698. doi: 10.1016/s0090-4295(02)01862-9. [DOI] [PubMed] [Google Scholar]
- 14.Seigne JD, Pisters LL, von Eschenbach AC. Osteitis pubis as a complication of prostate cryotherapy. J Urol. 1996;156:182. [PubMed] [Google Scholar]
- 15.Kavroudakis E, Karampinas PK, Evangelopoulos DS, et al. Treatment of osteitis pubis in non-athlete female patients. Open Orthop J. 2011;5:331–334. doi: 10.2174/1874325001105010331. [DOI] [PMC free article] [PubMed] [Google Scholar]