Loss of antiproliferative gene TOB1 results in more severe EAE driven by augmented pathogenic T cell responses.
Abstract
Reliable biomarkers corresponding to disease progression or therapeutic responsiveness in multiple sclerosis (MS) have not been yet identified. We previously reported that low expression of the antiproliferative gene TOB1 in CD4+ T cells of individuals presenting with an initial central nervous system (CNS) demyelinating event (a clinically isolated syndrome), correlated with high risk for progression to MS. We report that experimental autoimmune encephalomyelitis (EAE) in Tob1−/− mice was associated with augmented CNS inflammation, increased infiltrating CD4+ and CD8+ T cell counts, and increased myelin-reactive Th1 and Th17 cells, with reduced numbers of regulatory T cells. Reconstitution of Rag1−/− mice with Tob1−/− CD4+ T cells recapitulated the aggressive EAE phenotype observed in Tob1−/− mice. Furthermore, severe spontaneous EAE was observed when Tob1−/− mice were crossed to myelin oligodendrocyte glycoprotein–specific T cell receptor transgenic (2D2) mice. Collectively, our results reveal a critical role for Tob1 in adaptive T cell immune responses that drive development of EAE, thus providing support for the development of Tob1 as a biomarker for demyelinating disease activity.
The initial event in multiple sclerosis (MS) is commonly an acute neurological attack caused by inflammation in one or more sites in the central nervous system (CNS), a presentation referred to as a clinically isolated syndrome (CIS). Approximately 80% of CIS patients develop clinically definite MS (CDMS) within 3 yr (half within 2 yr), and only 10% do not advance to MS after 15 yr (Brex et al., 2002; Hauser and Goodin, 2012). We previously identified a gene expression signature in peripheral blood CD4+ T cells of individuals at CIS diagnosis that highly correlates with a rapid evolution to CDMS (Corvol et al., 2008). This signature includes the up-regulation of genes that promote T cell activation, proliferation, and survival, as well as down-regulation of genes that promote apoptosis and cell quiescence. One of the most differentially expressed genes in that signature was TOB1 (transducer of ERBB2-1), showing a sevenfold down-regulation compared with expression in CIS subjects who progressed at a slower pace. Remarkably, 92% of patients with this signature converted into CDMS within 9 mo of CIS diagnosis, whereas only 20% of patients without this gene expression profile converted in the same period of time. TOB1 is a member of the Tob/Btg1 family of anti-proliferative (APRO) proteins that regulate cell growth. Tob1 has been shown to modulate the activity of several transcription factors and other molecules involved in cellular differentiation and quiescence (Yoshida et al., 1997), including SMADs, ERKs, and CTNNB, underscoring its potential functional diversity within cell differentiation and proliferation pathways (Yoshida et al., 2003a; Xiong et al., 2006; Tzachanis et al., 2007; Kennedy et al., 2009; Winkler, 2010). Tob1 was found to be highly expressed in anergic or quiescent CD4+ lymphocytes and its inhibition augmented CD3-mediated responses, whereas Tob1 overexpression in primary T cells led to cell cycle arrest (Tzachanis et al., 2001). Thus, TOB1 deficiency (or down-regulation), as observed in CIS patients at risk of conversion to CDMS, may contribute to differentiation and proliferation of proinflammatory T cells that, in turn, promote CNS autoimmunity.
RESULTS AND DISCUSION
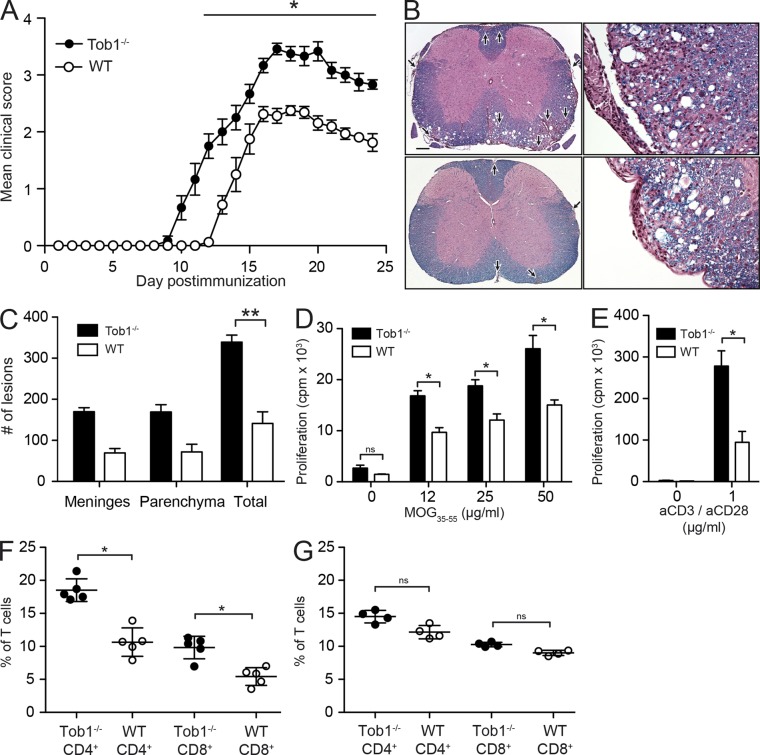
We investigated the role of Tob1 in EAE, an animal model which reproduces many of the clinical, immunological, and histopathological aspects of MS (Zamvil and Steinman, 2003), including multifocal infiltration of autoreactive T lymphocytes across the blood–brain barrier, leading to CNS inflammation, demyelination (Raine et al., 1999; Lucchinetti et al., 2000; Onuki et al., 2001; Pedotti et al., 2003; Sobel and Moore, 2008), damage to axons and neurons (Trapp et al., 1998; Peterson et al., 2001; Zipp et al., 2006), and signs of neurological disease (Hauser and Goodin, 2012). Immunization of Tob1−/− mice (on a C57BL/6 background) with myelin oligodendrocyte glycoprotein (MOG) peptide 35–55 (MOG35-55) resulted in an earlier disease onset, an increase in the maximum clinical score, and greater sustained disease severity compared with WT (Fig. 1 A and Table 1). Histological examination revealed larger and more numerous inflammatory/demyelinating foci in the brain and spinal cord of Tob1−/− mice compared with WT controls (Fig. 1, B and C). The observed EAE phenotype in Tob1−/− mice correlates well with our previous observations in CIS subjects, in which individuals with low expression of TOB1 in CD4+ T cells progressed more rapidly (Corvol et al., 2008).
Figure 1.
Tob1 deficiency exacerbates clinical and histological signs of EAE and increases myelin specific T cell responses. (A) Tob1−/− (n = 6) and WT (C57BL/6, n = 8) mice were immunized with MOG35-55 (these results are representative of three experiments). Mean disease score ± SEM is displayed. (B) Histology (Luxol fast blue–hematoxylin and eosin) of representative spinal cord samples. Meningeal and parenchymal inflammatory/demyelinating foci are indicated by arrows in Tob1−/− (top) and WT (bottom) mice. Bar: (left) 400 µm; (right) 80 µm. (C) Lesion quantification. (D) Proliferative responses in splenocytes of Tob1−/− and WT mice against MOG35-55. (E) Similar to D, but stimulation was performed with anti-CD3/anti-CD28. (F) Numbers of CD4+ and CD8+ spleen cells of MOG35-55 immunized (n = 4) Tob1−/− and WT mice. (G) Similar to F but with naive mice (n = 5). Experiments (C and G) are expressed as mean ± SD. *, P < 0.05; **, P < 0.01, Mann-Whitney U test. Data shown are representative two independent experiments.
Table 1.
EAE disease course
| EAE | Incidence | Mortality | Maximum clinical score | Day of onset |
| Tob1−/− | 21/21 (100%) | 9/21 (43%) | 4.2 ± 0.9 | 10.8 ± 1.9 |
| WT | 20/21 (95%) | 1/21 (5%) | 2.5 ± 1.0 | 13.8 ± 1.8 |
Data from three independent experiments are presented as means ± SD.
EAE exacerbation in TOB1−/− mice was associated with increased T cell proliferation in response to stimulation in vitro with MOG35-55 (Fig. 1 D) or nonselectively after T cell receptor stimulation via anti-CD3/anti-CD28 engagement (Fig. 1 E). Higher frequencies of CD4+ and CD8+ T cells were observed in Tob1−/− mice with EAE (Fig. 1 F) but not in unimmunized TOB1−/− mice (Fig. 1 G). These differences were more marked in CD4+ compared with CD8+ T cells. In this regard, it is noteworthy that Tzachanis et al. (2001) previously observed less marked down-regulation of Tob1 in anti-CD3/anti-CD28–stimulated CD8+ T cells and speculated that this may be related to a less central role of CD28 costimulation in CD8+ compared with CD4+ T cells. No difference in the frequency of dendritic cells (CD11c+), monocytes (CD11b+), or B cells (B220+) in response to antigen challenge was detected in vivo (unpublished data).
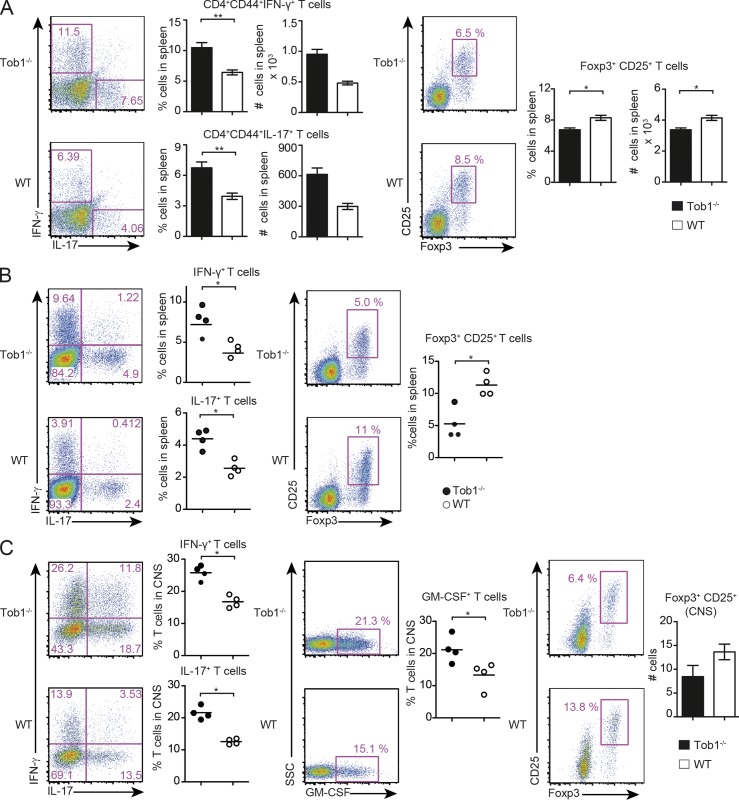
We also observed increases in both the number and proportion of activated splenic IFN-γ– and IL-17–producing CD4+CD44+ T cells in Tob1−/− mice shortly after immunization, whereas the number and proportion of CD4+CD25+Foxp3+ regulatory T cells (T reg cells) was decreased (Fig. 2 A). The same proinflammatory shift in T cell immune responses was observed after disease manifestation (Fig. 2 B). Notably, CD4+ T cells from naive Tob1−/− mice showed a small, but statistically significant, decrease in the proportion of CD25+Foxp3+ T reg cells (unpublished data). In association with an increased inflammatory response in the periphery, Tob1−/− mice also showed more severe CNS inflammation with a higher proportion of infiltrating Th1 and Th17 cells and less infiltrating T reg cells (Fig. 2 C). Recent studies highlighted the importance of GM-CSF regulating the encephalitogenicity of Th1 and Th17 cells in EAE (Codarri et al., 2011; El-Behi et al., 2011). Therefore, we evaluated GM-CSF–expressing cells and observed elevated numbers in the CNS of Tob1−/− mice compared with WT controls (Fig. 2 C). Collectively, these results indicate that Tob1-deficient animals are characterized by excessive proinflammatory T cell responses promoting severe CNS autoimmunity.
Figure 2.
Tob1 deficiency increases proinflammatory T cell responses. Tob1−/− and WT mice were immunized with MOG35-55. (A) Spleen cells were analyzed before disease onset for proportion and numbers of IFN-γ–, IL-17–secreting cells (gated on CD4+CD44+ cells, day 8), and for Foxp3 expression (CD25+Foxp+, day 7) by CD4+ cells. Representative FACS staining profiles are shown including quantification (Th1/Th17 n = 6; T reg cells n = 4 mice per group). (B and C) 14 d after immunization, spleen cells (B) and CNS infiltrating cells (C) were evaluated for secretion of IFN-γ, IL-17, GM-CSF, and for expression of CD25 and Foxp3 by CD4+ cells. The proportion of Foxp3+CD25+ cells (gated on CD4+) in the CNS of Tob1−/− and WT mice are shown in the rightmost panel. Representative FACS staining profiles are shown in each case including quantification (n = 4–5 mice per group). For all experiments, *, P < 0.05; **, P < 0.01, Mann-Whitney U test. Data shown are representative of two independent experiments. Error bars indicate SEM. Horizontal bars indicate mean.
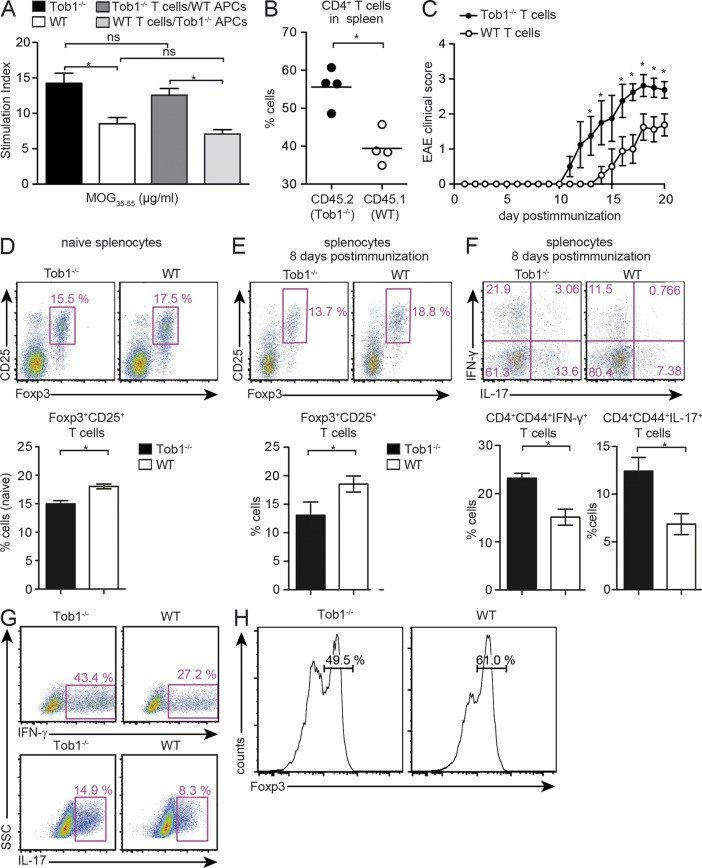
Increased proliferation of proinflammatory T cells in Tob1−/− mice could reflect a direct effect on T cells alone or, alternatively, Tob1 deficiency in APCs could influence T cell activation. To distinguish between these possibilities, splenocytes from Tob1−/− or WT mice immunized with MOG35-55 were used as APC for stimulation of CD4+ T cells isolated from immunized Tob1−/− or WT mice. An increased proliferative response toward MOG35-55 was observed when Tob1−/− T cells were stimulated with Tob1−/− APC compared with WT T cells stimulated with WT APC. More importantly, proliferative responses were augmented in all conditions in which Tob1−/− T cells were used, independent of whether they were stimulated with Tob1−/− or WT APC (Fig. 3 A). These results indicate that the proinflammatory phenotype of the Tob1−/− strain results from a direct effect on T cells and not on APC.
Figure 3.
Tob1 deficiency in T cells modulates T cell responses. (A) Whole spleen cells from immunized Tob1−/− and C57BL/6 mice were depleted of CD3+ cells and used as APC in co-culture with CD4+ T cells from Tob1−/− or WT T cells. 3H-thymidine incorporation in response to MOG35-55 is shown. (B) Rag1−/− mice were reconstituted with identical numbers of CD4+ cells from Tob1−/− (CD45.2) and transgenic WT (C57BL/6-CD45.1) mice. CD4+ T cells were isolated 12 d after immunization with MOG35-55 and quantified by FACS. Horizontal bars indicate mean. (C) Disease course after immunization with MOG35-55 of Rag1−/− mice reconstituted with CD4+ T cells from Tob1−/− or WT mice. Mean EAE disease score ± SEM is shown. (D and E) Proportion of splenic Foxp3-expressing cells (CD25+Foxp3+) by CD4+ cells in Rag1−/− mice either naive (D) or 8 d after immunization (E). (F) Proportion of splenic IFN-γ–, IL-17–secreting cells gated on CD4+CD44+ cells 8 d after immunization. (G) Polarization of T cells into a Th1 lineage was induced by IL-12 and polarization into a Th17 lineage was induced by IL-23, IL-6, and TGF-β. (H) Polarization of T cells into T reg cells was achieved with TGF-β. Intracellular cytokine staining for IFN-γ and IL-17 after 3 d in culture, and histograms for Foxp3 expression (gated on CD4+CD25+ cells) after 5 d in culture, are shown. Representative FACS staining profiles are shown including quantification (C, n = 4; D, n = 6; E, n = 4). For all experiments, data shown are representative of at least two independent experiments. *, P < 0.05, Mann-Whitney U test. Error bars indicate SEM.
To address whether Tob1 deficiency in T cells can directly or indirectly affect de novo T cell immune responses to myelin antigen in vivo, we studied proliferative responses after replenishing the T cell compartment of Rag1−/− mice with CD4+ cells from both Tob1−/− (CD45.2) and WT mice (C57BL/6-CD45.1) in a 1:1 ratio. In response to immunization with MOG35-55, the frequency of Tob1−/− T cells (CD4+CD45.2+) in the spleen was higher than the one of WT T cells (CD4+CD45.1+; Fig. 3 B). Together with our previous results, these data strongly support a role for Tob1 during adaptive T cell responses after MOG35-55 challenge in vivo. Furthermore, we reconstituted the T cell compartment of Rag1−/− mice with CD4+ cells from Tob1−/− mice and immunized animals with MOG35-55. As a control, CD4+ T cells from WT mice were used for reconstitution. After immunization, Rag1−/− reconstituted with Tob1−/− T cells showed an earlier disease onset and a more severe disease than Rag1−/− replenished with WT T cells, similar to that observed when EAE was compared in Tob1−/− mice and WT controls (Fig. 3 C). We also evaluated the proportion of Foxp3+ T cells in naive and MOG35-55-immunized Rag1−/− mice reconstituted with either Tob1−/− or WT CD4+ T cells, and detected significantly fewer T reg cells in the spleen of mice replenished with Tob1−/− T cells (Fig. 3, D and E). Interestingly, there was a more considerable reduction in the frequency of T reg cells in a lymphopenic environment (Fig. 3 D) in comparison with the slight decrease observed in naive Tob1−/− mice. Concomitantly, a higher proportion of activated (CD44+) IFN-γ– and IL-17–producing cells was observed in Rag1−/− mice receiving Tob1−/− T cells (Fig. 3 F). This suggests that the lower T reg cell population and increased Th1 and Th17 responses are intrinsic properties of T cells as a result of the loss of Tob1 expression. This is also supported by in vitro observations showing increased Th1 and Th17 differentiation (Fig. 3 G) and a reduced capability of naive Tob1−/− T cells to differentiate into T reg cells (Fig. 3 H). These results confirm that Tob1 deficiency restricted to T cells promotes encephalitogenic T cell responses and is sufficient to modulate adaptive T cell immune responses responsible for disease induction.
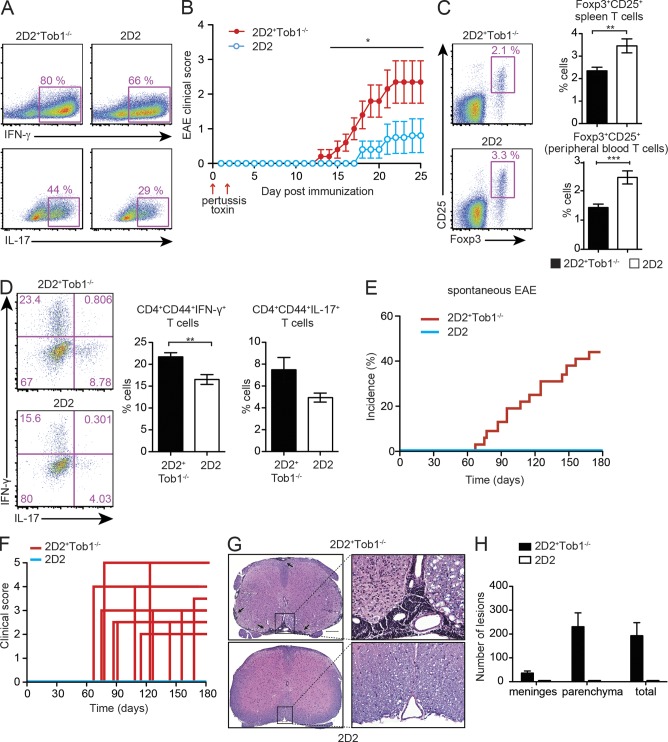
Activation and expansion of autoreactive myelin-specific Th1 and Th17 cells are known to take place during the initial phase of MS and EAE. To further evaluate whether Tob1 deficiency in myelin-specific T cells affects de novo development of Th1 and Th17 responses, we crossed MOG35-55 T cell receptor transgenic mice (2D2) with Tob1−/− mice. Naive CD4+ T cells isolated from both 2D2 and double transgenic 2D2+Tob1−/− mice were labeled with CFSE and stimulated with anti-CD3/CD28. CFSE dilution revealed greater proliferation of 2D2+Tob1−/− T cells compared with those from 2D2 mice (unpublished data). More importantly, the number of Th1 and Th17 cells observed after in vitro polarization of naive CD4+ T cells from 2D2+Tob1−/− mice was increased compared with 2D2 controls (Fig. 4 A). Considering the importance of Th1 and Th17 cells in the development of MS and EAE, and given that a previous study showed that EAE in 2D2 mice can be triggered by non–antigen-specific stimulation as pertussis toxin (ptx; Bettelli et al., 2003), we wondered whether 2D2+Tob1−/− mice were more prone to develop EAE under these conditions. Strikingly, after ptx administration alone, 2D2+Tob1−/− mice showed an earlier disease onset compared with 2D2 mice (Fig. 4 B). In addition, disease incidence and severity were increased in 2D2+Tob1−/− mice in comparison to 2D2 controls.
Figure 4.
Tob1 deficiency in myelin-specific T cells favors development of spontaneous EAE. (A) Naive (CD4+CD44−CD62L+) T cells were isolated from MOG35-55 TCR-transgenic mice (2D2) and 2D2+Tob1−/− mice and cultured in the presence of purified APC and antigen (MOG35-55). Polarization of T cells into a Th1 lineage was induced by IL-12, and polarization into a Th17 lineage was induced by IL-23, IL-6, and TGF-β. Intracellular cytokine staining for IFN-γ and IL-17 after 3 d in culture is shown. (B) Incidence of EAE after administration of ptx in 2D2 (n = 5) and 2D2+Tob1−/− (n = 5) mice. Mean EAE disease score ± SEM is shown. (C) Splenocytes (n = 7) and peripheral blood (n = 14) of age-matched (60–80 d old) symptom-free 2D2 and 2D2+Tob1−/− mice were analyzed for the proportion of Foxp3-expressing (CD25+Foxp3+) CD4+ cells. (D) Similar to C but the proportion of activated (CD44+CD4+) IFN-γ– and IL-17–secreting splenocytes is shown. Error bars indicate SEM. (E and F) The incidence (E) and disease severity (F) of spontaneous EAE in 2D2 (n = 25) and 2D2+Tob1−/− (n = 32) is also increased compared with the 2D2 line. (G) Histology of representative spinal cord samples (Luxol fast blue–hematoxylin and eosin). Meningeal and parenchymal lymphoid infiltrates in a 93-d-old 2D2+Tob1−/− mouse (top). A representative spinal cord section from a 165-d-old 2D2 mouse is shown for comparison (bottom). Bar: (left) 200 µm; (right) 50 µm. (H) Lesion quantification of meningeal and parenchymal inflammatory foci. For all experiments, data shown are representative of two independent experiments. Error bars indicate SD. For all experiments, *, P < 0.05; **, P < 0.01; ***, P < 0.001, Mann-Whitney U test.
In most EAE models, disease is induced by immunization with autoantigen supplemented with adjuvant and administration of B. ptx. Given the strong effect seen on T cell proliferation, increased Th1 and Th17 responses, and a decreased T reg cell population, we hypothesized that Tob1 deficiency would promote spontaneous EAE in 2D2+Tob1−/− mice. Compared with 2D2, age-matched 2D2+Tob1−/− mice were characterized by a proinflammatory phenotype with a reduced proportion of T reg cells in the spleen and blood (Fig. 4 C), and a higher proportion of activated (CD44+) IFN-γ– and IL-17–producing cells (Fig. 4 D). Although 2D2 mice can develop spontaneous EAE with a low incidence and a mild clinical disease score (Bettelli et al., 2003), these mice did not develop spontaneous disease in our facility. In contrast, a higher rate of spontaneous disease was observed in 2D2+Tob1−/− mice (44%) than 2D2 control mice (0%; Fig. 4, E and F; Table 2). Histological examination of spinal cords revealed inflammatory foci and demyelination in 2D2+Tob1−/− mice, but not in 2D2 controls (Fig. 4, G and H).
Table 2.
Spontaneous EAE
| EAE | Incidence | Mortality | Maximum clinical score | Day of onset |
| 2D2+Tob1−/− | 14/32 (44%) | 2/32 (6%) | 3.3 ± 0.9 | 114 ± 33 |
| 2D2 | 0/25 (0%) | na | na | na |
Data are presented as means ± SD.
Altogether, our results allow us to speculate that interplay between the activation and expansion of autoreactive T cells and a low expression of TOB1 could underlie MS risk. Potentially, either or both of these contributors could be environmentally or genetically determined, or result from a combined influence of the two. In this regard, the latest genome-wide association study in MS revealed common DNA variants in >50 loci associated with disease susceptibility, many of which are involved in T cell activation pathways (International Multiple Sclerosis Genetics Consortium and Wellcome Trust Case Control Consortium 2 et al., 2011).
A recent study showed that dominant active β-catenin (DA-Cat) transgenic mice experience delayed myelination and remyelination upon injury, thus implicating the Wnt pathway in this process for the first time (Fancy et al., 2009). Tob1 has also been shown to interact and antagonize β-catenin in zebrafish embryos, thus controlling dorsal development (Xiong et al., 2006). We speculate that Tob1−/− mice might have de-repressed β-catenin expression, thus mimicking the DA-Cat phenotype. If true, Tob1 would then be affecting the two connected, albeit distinct processes (i.e., inflammation and demyelination) underlying EAE and MS. Experiments to evaluate this hypothesis are underway.
In summary, these results demonstrate that Tob1 deficiency in T cells is capable of driving aberrant T cell immune responses favoring the development of autoimmune demyelinating disease. These data provide a biological rationale for the clinical observation that Tob1 expression influences the development of MS, support the development of Tob1-based expression profiling as a biomarker of disease activity in MS, and imply that increasing Tob1 expression on CD4+ cells represents a potential therapeutic strategy for MS and perhaps other autoimmune conditions.
MATERIALS AND METHODS
Mice.
Tob1−/− mice on a C57BL/6 background were obtained from the RIKEN Bioresource Center. These mice are derived from a line produced and maintained by T. Yamamoto (University of Tokyo, Tokyo, Japan; Yoshida et al., 2003b). Genotyping was performed as previously described (Ho et al., 2010). C57BL/6 and Rag1−/− female mice, 5–8 wk of age, were purchased from The Jackson Laboratory. MOG35–55-specific TCR transgenic mice were provided by V.K. Kuchroo (Harvard, Boston, MA). Tob1−/− and WT (Tob1+/+, C57BL/6) were obtained from a heterozygous breeding of Tob1+/− mice and further kept as separate colonies. All animal procedures were performed in compliance with experimental guidelines approved by the University of California, San Francisco committee on animal research (CAR).
Peptides.
Mouse MOG35-55 (MEVGWYRSPFSRVVHLYRNGK) was synthesized by AnaSpec.
EAE Induction.
7–10-wk-old female C57BL/6 and Tob1−/− mice were injected subcutaneously with 75 µg MOG35-55, in complete Freund’s adjuvant (DIFCO Laboratories). After immunization and 2 d later, mice received 300 ng (C57BL/6) ptx i.p. Active EAE in C57BL/6 Rag1−/− mice: 8 × 106 MACS-purified splenic CD4+ cells isolated from C57BL/6 or Tob1−/− mice were injected i.v. into naive C57BL/6 Rag1−/− mice. After cell transfer C57BL/6 Rag1−/− mice were immunized as stated above. Suboptimal immunization: 2D2 and 2D2+Tob1−/− mice were treated with 200 ng ptx on day 0 and 2 d after first ptx injection. For all experiments animals were observed daily, and clinical signs were assessed as follows: 0, no signs; 1, decreased tail tone; 2, mild monoparesis or paraparesis; 3, severe paraparesis; 4, paraplegia and/or quadraparesis; and 5, moribund or death. All animal experiments were conducted according to protocols approved by the local animal welfare committees.
Generation of Th1, Th17, and T reg cells.
Naive T cells (CD4+CD62L+CD44+) were obtained by magnetic cell sorting from TCR-transgenic 2D2, 2D2+Tob1−/−, Tob1−/−, and WT mice (purity >96%; Miltenyi Biotec). T cells were stimulated with 20 µg/ml MOG35-55 in the presence of WT-APC (T cell/APC ratio of 1:5). Th differentiation was induced using 3 ng/ml TGF-β, 20 ng/ml IL-23, and 20 ng/ml IL-6 for Th17 lineage or 10 ng/ml IL-12 for Th1 lineage (R&D Systems). 3 d after culture, cytokine production was analyzed using a FACS Canto flow cytometer (BD). T reg cell differentiation was conducted as previously described (Fantini et al., 2007).
T cell proliferation.
Spleen cells from Tob1−/− and C57BL/6 mice 14 d after immunization with MOG35-55 were depleted for CD3+ cells (Miltenyi Biotec) and used as APCs for CD4+ T cells. T cells were purified from Tob1−/− and C57BL/6 mice immunized with MOG35-55. Cells were cultured in 96-well microtiter plates at a concentration of 0.25 × 106 cells/ml and 20 µg/ml MOG35-55. Culture medium consisted of RPMI 1640 supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 100 Uml−1 penicillin, 0.1 mg/ml streptomycin, 5 × 10−5 M 2-mercaptoethanol, and 10% (vol/vol) fetal bovine serum. Cells were incubated for 48 h and pulsed for 18 h with 1 µCi/well [3H]-thymidine before harvesting.
Isolation of CNS infiltrating mononuclear cells.
Isolation of CNS infiltrating cells was performed as previously described (Schulze-Topphoff et al., 2009). In brief, mice were perfused using PBS. CNS tissue was manually cut into small pieces and incubated for 20 min in Hank’s buffered saline solution containing collagenase. Homogenate was resuspended in 30% Percoll (Sigma-Aldrich) and underlain with 70% Percoll and centrifuged for 30 min. Cells were harvested from the resulting interface.
Histopathology.
Brains and spinal cords of mice were fixed in 10% neutral-buffered formalin, sectioned, and stained with Luxol fast blue (LFB) and hematoxylin and eosin (H&E). Meningeal and parenchymal inflammatory lesions and areas of demyelination were quantified as previously described (Kuchroo et al., 1995; Stüve et al., 2006).
Flow cytometry.
Single-cell suspensions were incubated with anti-CD16/CD32 (1:100) to prevent nonspecific antibody binding and stained with anti-CD4, -CD11c, -CD11b, -B220, and -CD3. Intracellular cytokine staining by CD4+ cells was analyzed by monitoring the expression of IFN-γ, IL-17, and GM-CSF (all 1:100; eBioscience). Foxp3 staining was performed according to the manufacturer’s protocol (eBioscience). For intracellular cytokine staining, T cells were stimulated with 50 ng/ml PMA plus 500 ng/ml ionomycin in the presence of 1 µl/ml GolgiStop (BD).
Statistical analysis.
Data are presented as mean ± SEM or SD. We examined significance between groups using the Mann-Whitney U test. A value of P ≤ 0.05 was considered significant.
Acknowledgments
We thank Dr. S.J. Karp for providing material and protocols for the genotyping of Tob1−/− mice.
This work was supported by the National Institutes of Health (NIH) R01 grants NS26799 (J.R. Oksenberg), NS049477 (J.R. Oksenberg), AI073737 (S.S. Zamvil), AI059709 (S.S. Zamvil), and NS063008 (S.S. Zamvil), and the National Multiple Sclerosis Society (NMSS) grants RG3622 and RG3913 (S.S. Zamvil). We also thank the generous contributions of the Robert Tillman Family Fund (S.E. Baranzini), Guthy Jackson Charitable Foundation (S.S. Zamvil), and the Maisin Foundation (S.S. Zamvil). S.E. Baranzini is a Harry Weaver Neuroscience scholar from the NMSS. U. Schulze-Topphoff is a fellow of the NMSS and the Deutsche Forschungsgemeinschaft (DFG; SCHU 2587/1-1). M. Varrin-Doyer is a fellow of the NMSS.
Authors declare no competing financial interests.
Footnotes
Abbreviations used:
- CDMS
- clinically definite MS
- CIS
- clinically isolated syndrome
- CNS
- central nervous system
- EAE
- experimental autoimmune encephalomyelitis
- MOG
- myelin oligodendrocyte glycoprotein
- MS
- multiple sclerosis
- ptx
- pertussis toxin
References
- Bettelli E., Pagany M., Weiner H.L., Linington C., Sobel R.A., Kuchroo V.K. 2003. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197:1073–1081 10.1084/jem.20021603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brex P.A., Ciccarelli O., O’Riordan J.I., Sailer M., Thompson A.J., Miller D.H. 2002. A longitudinal study of abnormalities on MRI and disability from multiple sclerosis. N. Engl. J. Med. 346:158–164 10.1056/NEJMoa011341 [DOI] [PubMed] [Google Scholar]
- Codarri L., Gyülvészi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., Becher B. 2011. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat. Immunol. 12:560–567 10.1038/ni.2027 [DOI] [PubMed] [Google Scholar]
- Corvol J.C., Pelletier D., Henry R.G., Caillier S.J., Wang J., Pappas D., Casazza S., Okuda D.T., Hauser S.L., Oksenberg J.R., Baranzini S.E. 2008. Abrogation of T cell quiescence characterizes patients at high risk for multiple sclerosis after the initial neurological event. Proc. Natl. Acad. Sci. USA. 105:11839–11844 10.1073/pnas.0805065105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M., Ciric B., Dai H., Yan Y., Cullimore M., Safavi F., Zhang G.X., Dittel B.N., Rostami A. 2011. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 12:568–575 10.1038/ni.2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S.P., Baranzini S.E., Zhao C., Yuk D.I., Irvine K.A., Kaing S., Sanai N., Franklin R.J., Rowitch D.H. 2009. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes Dev. 23:1571–1585 10.1101/gad.1806309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantini M.C., Dominitzki S., Rizzo A., Neurath M.F., Becker C. 2007. In vitro generation of CD4+ CD25+ regulatory cells from murine naive T cells. Nat. Protoc. 2:1789–1794 10.1038/nprot.2007.258 [DOI] [PubMed] [Google Scholar]
- Hauser S.L., Goodin D.S. 2012. Multiple Sclerosis and other demyelinating diseases. Harrison’s principles of internal medicine. Longo D.I., editor McGraw-Hill, New York: 3395-3409 [Google Scholar]
- Ho K.J., Do N.L., Otu H.H., Dib M.J., Ren X., Enjyoji K., Robson S.C., Terwilliger E.F., Karp S.J. 2010. Tob1 is a constitutively expressed repressor of liver regeneration. J. Exp. Med. 207:1197–1208 10.1084/jem.20092434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Multiple Sclerosis Genetics Consortium; Wellcome Trust Case Control Consortium 2; Sawcer S., Hellenthal G., Pirinen M., Spencer C.C., Patsopoulos N.A., Moutsianas L., Dilthey A., Su Z., Freeman C., Hunt S.E., et al. 2011. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 476:214–219 10.1038/nature10251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy K.A., Porter T., Mehta V., Ryan S.D., Price F., Peshdary V., Karamboulas C., Savage J., Drysdale T.A., Li S.C., et al. 2009. Retinoic acid enhances skeletal muscle progenitor formation and bypasses inhibition by bone morphogenetic protein 4 but not dominant negative beta-catenin. BMC Biol. 7:67 10.1186/1741-7007-7-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchroo V.K., Das M.P., Brown J.A., Ranger A.M., Zamvil S.S., Sobel R.A., Weiner H.L., Nabavi N., Glimcher L.H. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell. 80:707–718 10.1016/0092-8674(95)90349-6 [DOI] [PubMed] [Google Scholar]
- Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. 2000. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann. Neurol. 47:707–717 [DOI] [PubMed] [Google Scholar]
- Onuki M., Ayers M.M., Bernard C.C., Orian J.M. 2001. Axonal degeneration is an early pathological feature in autoimmune-mediated demyelination in mice. Microsc. Res. Tech. 52:731–739 10.1002/jemt.1057 [DOI] [PubMed] [Google Scholar]
- Pedotti R., DeVoss J.J., Youssef S., Mitchell D., Wedemeyer J., Madanat R., Garren H., Fontoura P., Tsai M., Galli S.J., et al. 2003. Multiple elements of the allergic arm of the immune response modulate autoimmune demyelination. Proc. Natl. Acad. Sci. USA. 100:1867–1872 10.1073/pnas.252777399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson J.W., Bö L., Mörk S., Chang A., Trapp B.D. 2001. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann. Neurol. 50:389–400 10.1002/ana.1123 [DOI] [PubMed] [Google Scholar]
- Raine C.S., Cannella B., Hauser S.L., Genain C.P. 1999. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann. Neurol. 46:144–160 [DOI] [PubMed] [Google Scholar]
- Schulze-Topphoff U., Prat A., Prozorovski T., Siffrin V., Paterka M., Herz J., Bendix I., Ifergan I., Schadock I., Mori M.A., et al. 2009. Activation of kinin receptor B1 limits encephalitogenic T lymphocyte recruitment to the central nervous system. Nat. Med. 15:788–793 10.1038/nm.1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel R.A., Moore G.R.W. 2008. Demyelinating Diseases. Greenfield’s neuropathology. Greenfield J.G., Love S., Louis D.N., Ellison D., Hodder Arnold, London: 1513-1608 [Google Scholar]
- Stüve O., Youssef S., Weber M.S., Nessler S., von Büdingen H.C., Hemmer B., Prod’homme T., Sobel R.A., Steinman L., Zamvil S.S. 2006. Immunomodulatory synergy by combination of atorvastatin and glatiramer acetate in treatment of CNS autoimmunity. J. Clin. Invest. 116:1037–1044 10.1172/JCI25805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp B.D., Peterson J., Ransohoff R.M., Rudick R., Mörk S., Bö L. 1998. Axonal transection in the lesions of multiple sclerosis. N. Engl. J. Med. 338:278–285 10.1056/NEJM199801293380502 [DOI] [PubMed] [Google Scholar]
- Tzachanis D., Freeman G.J., Hirano N., van Puijenbroek A.A., Delfs M.W., Berezovskaya A., Nadler L.M., Boussiotis V.A. 2001. Tob is a negative regulator of activation that is expressed in anergic and quiescent T cells. Nat. Immunol. 2:1174–1182 10.1038/ni730 [DOI] [PubMed] [Google Scholar]
- Tzachanis D., Li L., Lafuente E.M., Berezovskaya A., Freeman G.J., Boussiotis V.A. 2007. Twisted gastrulation (Tsg) is regulated by Tob and enhances TGF-beta signaling in activated T lymphocytes. Blood. 109:2944–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler G.S. 2010. The mammalian anti-proliferative BTG/Tob protein family. J. Cell. Physiol. 222:66–72 10.1002/jcp.21919 [DOI] [PubMed] [Google Scholar]
- Xiong B., Rui Y., Zhang M., Shi K., Jia S., Tian T., Yin K., Huang H., Lin S., Zhao X., et al. 2006. Tob1 controls dorsal development of zebrafish embryos by antagonizing maternal beta-catenin transcriptional activity. Dev. Cell. 11:225–238 10.1016/j.devcel.2006.06.012 [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Matsuda S., Yamamoto T. 1997. Cloning and characterization of the mouse tob gene. Gene. 191:109–113 10.1016/S0378-1119(97)00049-8 [DOI] [PubMed] [Google Scholar]
- Yoshida Y., von Bubnoff A., Ikematsu N., Blitz I.L., Tsuzuku J.K., Yoshida E.H., Umemori H., Miyazono K., Yamamoto T., Cho K.W. 2003a. Tob proteins enhance inhibitory Smad-receptor interactions to repress BMP signaling. Mech. Dev. 120:629–637 10.1016/S0925-4773(03)00020-0 [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Nakamura T., Komoda M., Satoh H., Suzuki T., Tsuzuku J.K., Miyasaka T., Yoshida E.H., Umemori H., Kunisaki R.K., et al. 2003b. Mice lacking a transcriptional corepressor Tob are predisposed to cancer. Genes Dev. 17:1201–1206 10.1101/gad.1088003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S.S., Steinman L. 2003. Diverse targets for intervention during inflammatory and neurodegenerative phases of multiple sclerosis. Neuron. 38:685–688 10.1016/S0896-6273(03)00326-X [DOI] [PubMed] [Google Scholar]
- Zipp F., Hartung H.P., Hillert J., Schimrigk S., Trebst C., Stangel M., Infante-Duarte C., Jakobs P., Wolf C., Sandbrink R., et al. ; CCR1 Antagonist Study Group 2006. Blockade of chemokine signaling in patients with multiple sclerosis. Neurology. 67:1880–1883 10.1212/01.wnl.0000244420.68037.86 [DOI] [PubMed] [Google Scholar]