N-ras−/− CD8+ T cells have an intrinsic defect in Eomes expression resulting in impaired generation of protective memory cells that can be rescued by enforced Eomes expression.
Abstract
Signals from the TCR that specifically contribute to effector versus memory CD8+ T cell differentiation are poorly understood. Using mice and adoptively transferred T lymphocytes lacking the small GTPase N-ras, we found that N-ras–deficient CD8+ T cells differentiate efficiently into antiviral primary effectors but have a severe defect in generating protective memory cells. This defect was rescued, although only partly, by rapamycin-mediated inhibition of mammalian target of rapamycin (mTOR) in vivo. The memory defect correlated with a marked impairment in vitro and in vivo of the antigen-mediated early induction of T-box transcription factor Eomesodermin (Eomes), whereas T-bet was unaffected. Besides N-ras, early Eomes induction in vitro required phosphoinositide 3-kinase (PI3K)–AKT but not extracellular signal-regulated kinase (ERK) activation, and it was largely insensitive to rapamycin. Consistent with N-ras coupling Eomes to T cell memory, retrovirally enforced expression of Eomes in N-ras–deficient CD8+ T cells effectively rescued their memory differentiation. Thus, our study identifies a critical role for N-ras as a TCR-proximal regulator of Eomes for early determination of the CD8+ T cell memory fate.
After antigen exposure in the presence of appropriate signals, naive CD8+ T lymphocytes undergo clonal proliferation and gain the ability to traffic to peripheral sites while they can differentiate into effector CTL able to lyse target cells and actively secrete IFN-γ. CD8+ T lymphocytes can also differentiate into long-lived memory CTL. After the initial phase of expansion, most activated CD8+ T lymphocytes die, leaving a population of memory precursors (Kaech and Wherry, 2007; Williams and Bevan, 2007). Compared with effector CTL, memory precursors are not terminally differentiated (Joshi and Kaech, 2008) and may remain as resting memory cells or redifferentiate into cytotoxic effectors. These are critical for a rapid and potent response upon secondary antigen encounter and enhanced control of infection (Kaech and Wherry, 2007).
T cell memory differentiation involves multiple phenotypic and functional changes, and a growing body of evidence suggests that the early stages of the immune response are crucial in determining the fate of responding CD8+ T lymphocytes (Obar and Lefrançois, 2010; Rutishauser and Kaech, 2010). Signals received through the TCR must be integrated with others from costimulatory molecules and cytokine and chemokine receptors, and together direct the outcome of the response (Kaech and Wherry, 2007; Williams and Bevan, 2007). Which and how these diverse signals regulate the generation of the long-lived memory T lymphocytes is still being defined. Particularly, little is known about which signals proximal or even related to the TCR regulate these differentiation processes (Teixeiro et al., 2009). Recently, the balance of expression between transcription factors eomesodermin (Eomes) and T-bet has been proposed to be critical in determining whether CD8+ T cells adopt memory or effector fates, with Eomes being associated to memory commitment (Intlekofer et al., 2005; Banerjee et al., 2010). The mammalian target of rapamycin (mTOR) kinase plays a critical role in determining CD8+ T cell fate (Araki et al., 2009; Pearce et al., 2009) and affects Eomes and T-bet levels at least after IL-12 signaling (Rao et al., 2010; Li et al., 2011), but more mechanistic information remains to be elucidated. Specifically, there is little information on which early TCR signals regulate the expression of these transcription factors in antigen-responding CD8+ T lymphocytes and thereby determine T cell memory commitment.
A wide variety of extracellular stimuli activate guanine nucleotide binding proteins of the Ras family which, cycling as a binary signal switch, control multiple cellular responses (Olson and Marais, 2000). The different isoforms of classical Ras proteins (H-ras, N-ras, and K-ras 4A and 4B) have conserved effector binding domains but differ substantially in their carboxyl-terminal region, which is important for selective membrane association, compartmentalization (Mor and Philips, 2006), and activation (Ibiza et al., 2008). In nonlymphoid cell lines, N-ras has been implicated in controlling Stat1 and apoptosis (Castellano et al., 2007). All Ras isoforms are expressed in lymphocytes and have been collectively implicated in signaling downstream of the TCR for T-lymphocyte development and function by using T cell lines or transgenic mice expressing a dominant-negative Ras protein that inhibits all Ras isoforms (Scheele et al., 2007). More recently, the analysis of mice specifically lacking N-ras showed that this Ras isoform appears not to be essential for thymocyte development (Pérez de Castro et al., 2003; Iborra et al., 2011), although it is involved in CD4+ Th1 polarization and immune responses (Iborra et al., 2011). Here, we sought to determine whether the N-ras isoform is necessary for mature CD8+ T lymphocyte differentiation and function after a viral infection. Our results show that in CD8+ T lymphocytes, N-ras is a key mediator of early signals downstream of the TCR that control Eomes but not T-bet expression and thereby the generation of functional protective memory CD8+ T lymphocytes but not effector cells.
RESULTS
Impact of N-ras deficiency on OT-I TCR transgenic CD8+ T lymphocyte activation and effector functions in vitro
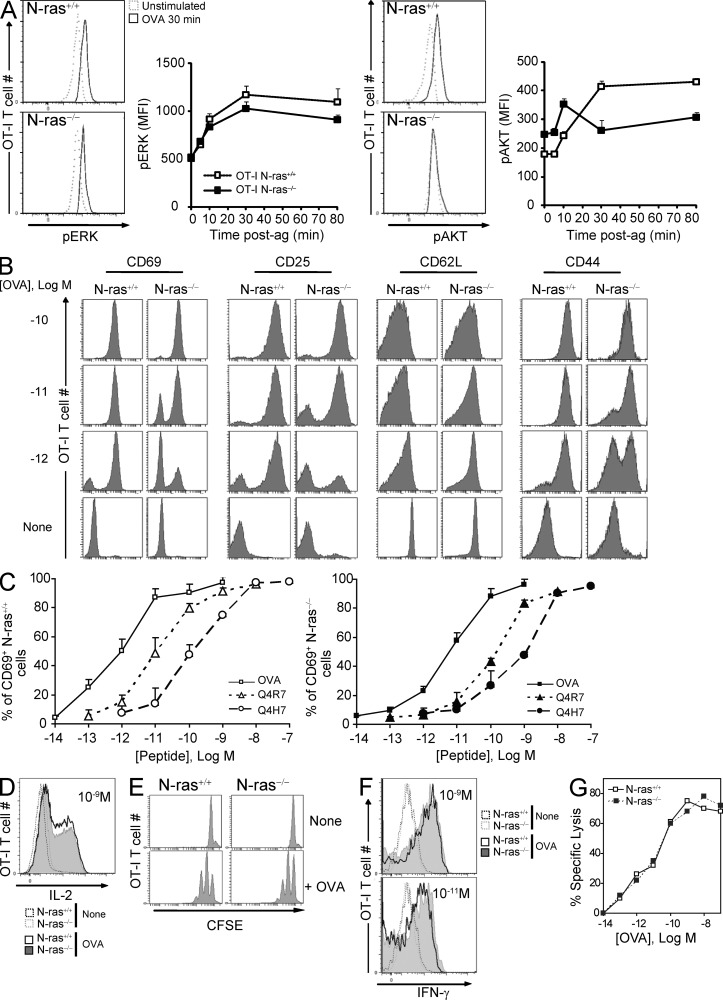
Several lines of evidence suggest that N-ras is a particularly important Ras isoform in T cell lines (Perez de Castro et al., 2004), thymocytes, and for polarization of mature CD4+ T lymphocytes. To test whether N-ras is necessary for mature CD8+ T lymphocyte activation after antigen stimulation, we generated N-ras−/− mice expressing the transgenic OT-I TCR specific for OVA peptide 257SIINFEKL264 presented by H-2Kb. We first assessed early TCR signaling events after antigen stimulation in vitro. Similar to what has been previously shown for N-ras−/− thymocytes (Pérez de Castro et al., 2003; Iborra et al., 2011), N-ras deficiency barely affected extracellular signal-regulated kinases (ERK) activation (Fig. 1 A). In contrast, phosphorylation of AKT at T306, a phosphoinositide 3-kinase (PI3K)–dependent event, was clearly deregulated in the absence of N-ras. Accordingly, nonstimulated N-ras−/− cells exhibited increased basal levels of phospho-AKT compared with wild-type counterparts (Pérez de Castro et al., 2003), and upon exposure to antigen they were unable to sustain it (Fig. 1 A).
Figure 1.
Effect of N-ras deficiency on activation and function of in vitro antigen-stimulated OT-I CD8+ T lymphocytes. (A) CD8+ T lymphocytes were isolated by negative selection from the spleen of CD45.1+ N-ras+/+ and N-ras−/− OT-I TCR transgenic mice. They were stimulated for the indicated times with CD45.1− WT splenocytes loaded with 10−9 M OVA peptide at an E/T ratio of 1/6, stained for CD45.1 and for intracellular pERK and pAKT, and analyzed by FACS (n = 2 experiments; results are expressed as mean ± SEM). (B) Alternatively, purified OT-I lymphocytes were stimulated at an E/T ratio of 1/5 with N-ras+/+ DC pulsed with titrated concentrations of OVA peptide, stained for CD69 and CD25 48 h later, or for CD44 and CD62L 72 h later, and gated on CD8+ cells for FACS. Results are expressed as mean ± SEM. (C) OT-I lymphocytes were stimulated for 16 h as in B with titrated concentrations of OVA peptide or of the two weaker partial agonist peptides Q4R7 and Q4H7, and CD69 expression was monitored. Results are expressed as mean ± SD. (D) To measure intracellular IL-2 production, brefeldin A was added for 16 h after 3-h stimulation with 10−9 M OVA peptide. (E) To measure proliferation, CFSE-labeled purified lymphocytes were co-cultured with N-ras+/+ mDC prepulsed with 10−7 M OVA peptide at an E/T ratio of 1/5, and analyzed by FACS 72 h later. (F) OT-I lymphocytes were stimulated as in D for 72 h, harvested, washed, and restimulated with OVA-pulsed DC. IFN-γ production was determined 16 h later by ICS. (G) To determine the cytotoxic activity, a 51Cr release assay was performed culturing OVA-specific CTL lines generated from WT or N-ras−/− OT-I mice with 51Cr-labeled target cells and with titrated concentrations of OVA peptide. Specific lysis is shown as the mean of triplicate wells. Data shown in B–G are representative of at least three independent experiments.
After stimulation with 10−10 M OVA peptide, N-ras−/− OT-I CD8+ T lymphocytes were comparable to WT cells in terms of up-regulation of activation markers (CD69, CD25, and CD44) and down-regulation of CD62L, but less sensitive to decreasing concentrations of either OVA (Fig. 1, B and C) or the weaker altered peptides Q4R7 and Q4H7 (Daniels et al., 2006), derived from the original OVA peptide (Fig. 1 C). Also, when stimulated with OVA peptide, N-ras−/− OT-I CD8+ T cells exhibited IL-2 production (Fig.1 D) and proliferation (Fig. 1 E) comparable to WT counterparts. Importantly, regardless of the abundance of antigen upon restimulation, both cell types displayed similar IFN-γ secretion (Fig. 1 F) and cytotoxic activity (Fig. 1 G). Thus, despite reduced sensitivity to antigen and impaired early AKT activation, N-ras−/− CD8+ T cells were capable of activation and acquisition of hallmark effector functions.
CD8+ T lymphocyte primary responses to viral infection are not impaired by N-ras deficiency in vivo
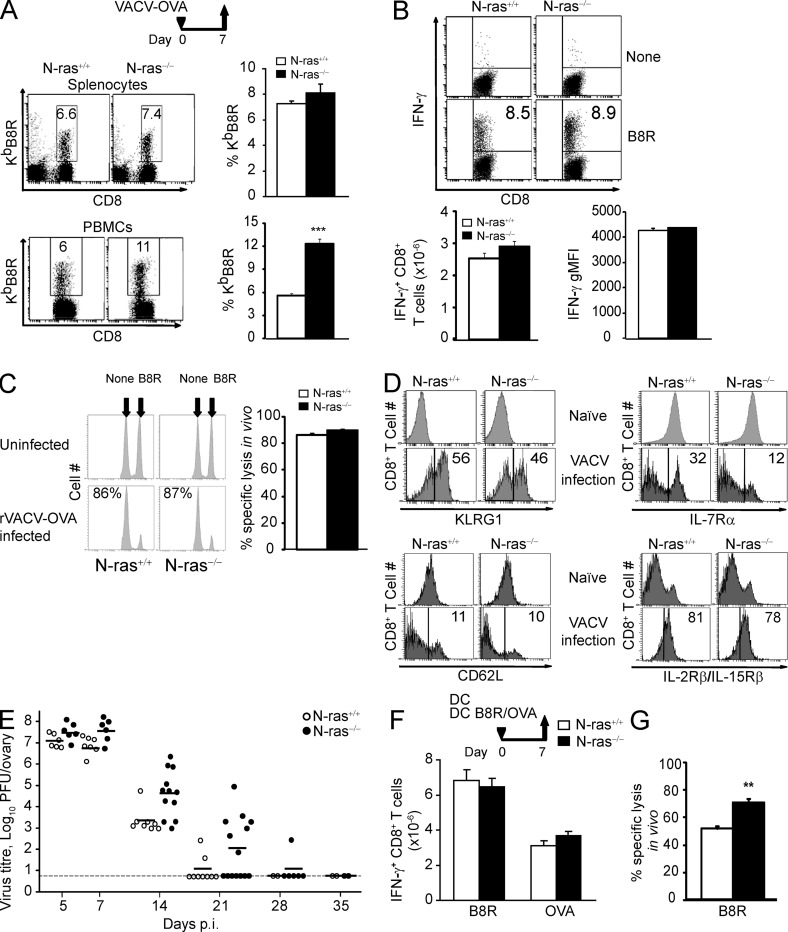
Next, we sought to determine the role of N-ras in CD8+ T lymphocyte primary responses in vivo. We infected WT and N-ras−/− mice with a recombinant vaccinia virus (VACV) expressing full length OVA protein (rVACV-OVA) and analyzed the CD8+ T lymphocyte responses to OVA and VACV immunodominant 20TSYKFESV27 (B8R) peptides. At the peak of the primary response, day 7, the frequency of antigen-specific CD8+ T cells in the spleen and blood was as high in the spleen and higher in blood in N-ras−/− compared with WT mice when assessed by ex vivo KbB8R pentamer staining (Fig. 2 A) or by intracellular IFN-γ expression (Fig. 2 B), with N-ras−/− cells producing amounts of IFN-γ on a per cell basis equivalent to those of WT counterparts (Fig. 2 B, far right). The cytotoxic activity of CD8+ T lymphocytes in vivo was also unaffected by the absence of N-ras, as both type of mice cleared peptide-bearing cells with the same efficiency (Fig. 2 C). Similar results were obtained for the CD8+ T cell response to OVA peptide (not depicted). Furthermore, up-regulation of KLGR1 and down-regulation of CD62L, events associated with acquisition of a terminal effector phenotype, were moderately reduced or similar, respectively, in N-ras−/− cells compared with WT counterparts (Fig. 2 D, left). Sustained expression of receptors for IL-15 and IL-7, a distinctive feature of putative memory precursors among antigen-responding cells (Williams and Bevan, 2007), was differentially affected by the N-ras deficiency. IL-2Rβ/IL-15Rβ was equally expressed on both cell types. On the contrary, the number of CD127+ cells was lower in N-ras−/− mice at the peak of the primary response (Fig. 2 D, right), but they gradually reached the levels of wild-type cells in the weeks following primary infection (not depicted).
Figure 2.
N-ras–deficient mice mount efficient primary CD8+ T lymphocyte effector responses after VACV infection and after DC immunization. WT and N-ras−/− mice were i.p. infected with rVACV-OVA (A–E) or immunized with a mixture of mDC loaded with B8R and OVA peptides (F and G). On day 7 p.i., splenocytes and PBMCs were harvested and the frequency of B8R-specific T cells within the CD8+ population (A) and the number of B8R-specific splenic CD8+ T cells producing IFN-γ, as well as their geometric MFI of IFN-γ expression, were determined ex vivo (B; n = 4, 2 experiments; results are expressed as mean ± SEM). ***, P < 0.0005. (C) In vivo function of day 7 p.i. primary CD8+ T lymphocytes was assessed in in vivo killing assays (n = 5, two experiments). The numbers in the histograms indicate the percentage of specific lysis. Results are expressed as mean ± SEM. (D) On day 7 p.i., surface expression of KLRG1, IL-7Rα (CD127), CD62L, and IL-2Rβ/IL-15Rβ (CD122) in CD8+ splenocytes from naive mice or in CD8+, H-2KbB8R+ splenocytes from infected mice was measured and a representative histogram per molecule is shown. The numbers within the histograms indicate the percentage of cells. (E) Infectious virus titer in the ovary was determined on the indicated days p.i. Dots represent titers for each individual ovary and the horizontal dashes represent the mean for each time point. Detection limit is indicated by the long horizontal line. (F and G) On day 7 after DC immunization, the number of B8R-and OVA-specific splenic CD8+ T cells producing IFN-γ were determined ex vivo as in B (n = 5, two experiments; F) and in vivo killing assays were performed as in C (n = 5, two experiments; G). **, P < 0.005. Results (F and G) are expressed as mean ± SEM.
Both N-ras+/+ and N-ras−/− mice were able to similarly control VACV infection and were thus exposed to a similar antigen load (Fig. 2 E). Furthermore, after immunization with B8R and OVA peptide-loaded DC, which selectively induce the CD8+ compartment while providing a less inflammatory priming environment (Badovinac et al., 2005), both types of mice also generated a fully functional primary response (Fig. 2, F and G). Thus, N-ras seemed to be dispensable also in vivo for acquisition of the two main functional hallmarks of effector CD8+ T lymphocytes, namely IFN-γ production and cytotoxic activity.
N-ras deficiency impairs CD8+ T lymphocyte memory and secondary responses
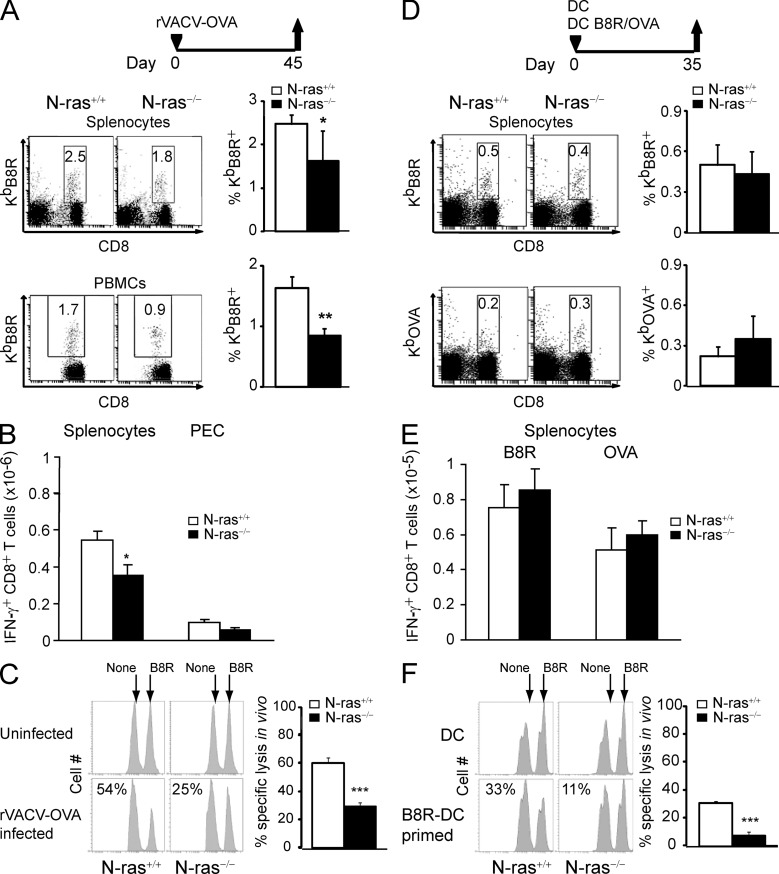
Furthermore, we studied the memory response to VACV infection or DC immunization and found that the frequencies and numbers of B8R-specific IFN-γ–producing CD8+ T cells at the memory stage were mildly but consistently lower in infected (Fig. 3, A and B) or similar in DC-vaccinated N-ras−/− mice (Fig. 3, D and E). In both settings, however, N-ras−/− mice were clearly less efficient than WT animals in eliminating B8R-bearing splenocytes in vivo (Fig. 3, C and F).
Figure 3.
Defective memory responses of N-ras–deficient mice upon VACV infection or DC immunization. WT and N-ras−/− mice were either infected with rVACV-OVA (A–C) or immunized with a mixture of mDC loaded with B8R and OVA peptides (D–F). (A and B) On day 45 after infection, splenocytes and PBMCs were harvested and the frequency of B8R-specific T cells within the CD8+ population (A) and the number of B8R-specific splenic CD8+ T cells producing IFN-γ (B) were determined ex vivo (n = 4, two experiments). **, P < 0.005. (C) In vivo function of day 45 p.i. memory T cells was assessed in an in vivo killing assay (n = 6, two experiments). (D and E) On day 35 after DC immunization, the frequency of B8R- and OVA-specific T lymphocytes within the CD8+ population (D) and the number of B8R- and OVA-specific splenic CD8+ T cells producing IFN-γ (E) were determined ex vivo (n = 6, two experiments). (F) In vivo function of day 35 p.i. memory T cells was assessed in an in vivo killing assay (n = 6, two experiments). Results are expressed as mean ± SEM. ***, P < 0.0005.
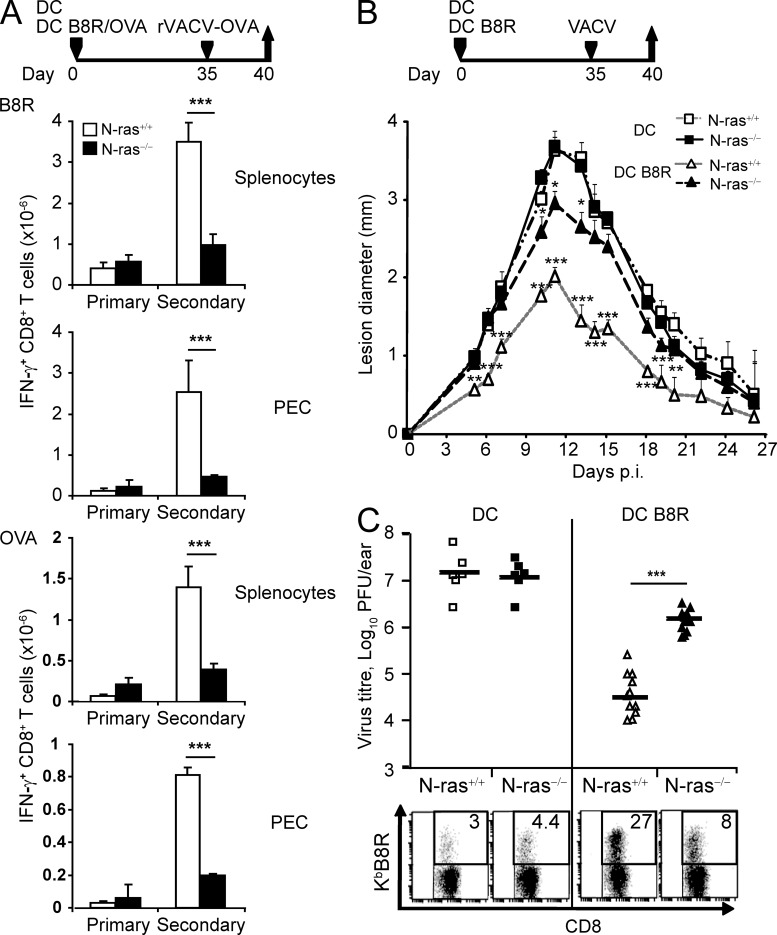
Next, we assessed the ability of N-ras−/− CD8+ T lymphocytes to mount rapid and potent recall responses against challenge infection, a hallmark of memory lymphocytes. A defective secondary response was found in N-ras−/− mice immunized with peptide-coated DCs. When vaccinated WT mice were challenged with virus, the number of splenic and peritoneal B8R- and OVA-specific CD8+ T cells was increased ∼12–23-fold with respect to nonpulsed DC as controls. Remarkably, in N-ras−/− mice, the number of CD8+ T cells specific for either antigen increased only two- to threefold as a consequence of priming (Fig. 4 A). Next, we tested the impact of N-ras deficiency on the protection against VACV challenge infection provided by memory CD8+ T lymphocytes. N-ras+/+ and N-ras−/− mice vaccinated with B8R-pulsed or nonpulsed DCs were infected in the ear pinnae with VACV (Tscharke and Smith, 1999). In unprimed mice, the development of dermal lesions and their resolution was not affected by N-ras deficiency (Fig. 4 B). In WT mice, vaccination with B8R-pulsed DCs significantly reduced dermal pathology, and virus titer in the infected ear was reduced ∼500-fold on day 6 (Fig. 4 C). In contrast, ear lesion development in B8R-DC–vaccinated N-ras−/− mice was closer to that of the unprimed controls throughout the infection, and virus titer was reduced only 10-fold (Fig. 4, B and C). The reduced frequency of B8R-specific CD8+ T lymphocytes in the respective retromaxillar draining lymph nodes after VACV challenge (Fig. 4 C, bottom) correlated with the impaired protection caused by N-ras deficiency. Collectively, these data indicate that N-ras signaling is required for the secondary response of memory CD8+ T lymphocytes that mediate protective immunity against a challenge infection.
Figure 4.
CD8+ T lymphocyte protective secondary responses are impaired in N-ras–deficient mice. (A) WT and N-ras−/− mice were injected with unpulsed mDC or mDC pulsed with B8R and OVA peptides. On day 35, mice were infected i.p. with rVACV-OVA and, 5 d later, IFN-γ production by B8R- and OVA-specific splenic and PEC CD8+ T lymphocytes was determined ex vivo (n = 4, two experiments). ***, P < 0.0005. (B) Mice primed with B8R-pulsed mDC were challenged with VACV intradermally in both ear pinnae on day 35. On indicated days after challenge infection, lesion diameter was determined with a digital caliper (n = 6 for unprimed mice, n = 8 for B8R-loaded mDC-primed mice; two experiments). Results in A and B are expressed as mean ± SEM. Statistics was performed comparing unprimed versus primed mice for either WT or N-ras−/− mice. (C) Infectious virus titers in the ears and frequency of B8R-specific T lymphocytes within the CD8+ population in the draining retromaxillar lymph nodes (bottom dot plots) were determined 6 d p.i. (n = 6 for mDC-injected mice, n = 12 for B8R-loaded mDC-primed mice, two experiments). Horizontal bars show the mean values. ***, P < 0.0005.
The defects of N-ras–deficient mice in generating memory and a secondary response are CD8+ T cell intrinsic
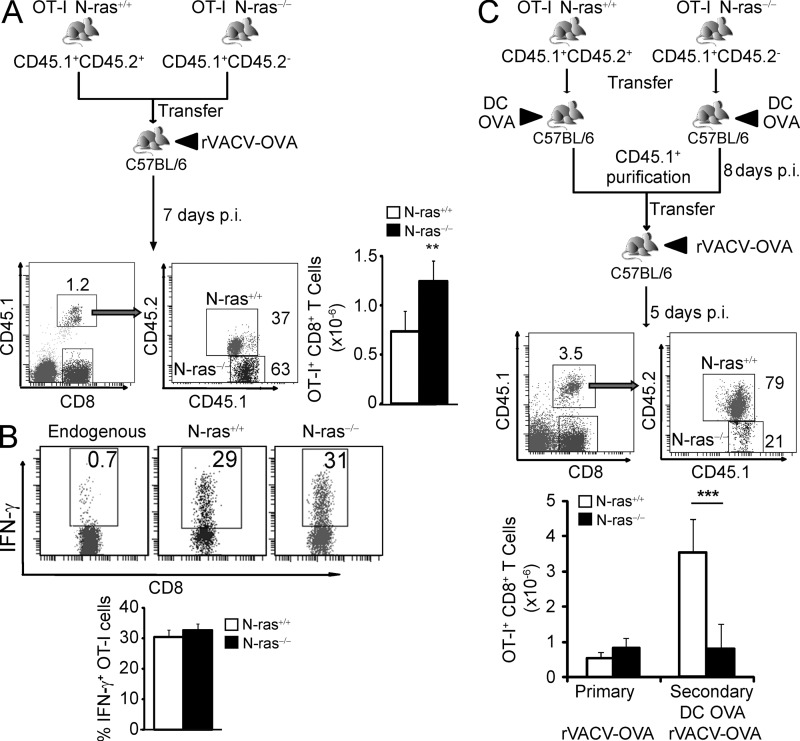
For the analysis of the primary response, small numbers of purified N-ras+/+ and N-ras−/− OT-I CD8+ T lymphocytes were cotransferred into the same congenic WT recipient. Upon primary infection, splenic N-ras−/− OT-I cells expanded better (Fig. 5 A) but were similar to WT OT-I cells in differentiating into IFN-γ–producing cells (Fig. 5 B). These results would also indicate that homing to the spleen is not affected by N-ras deficiency, as previously described for naive CD8+ T cells in N-ras–deficient mice and in OT-I TCR transgenic mice lacking N-ras (Pérez de Castro et al., 2003; Iborra et al., 2011).
Figure 5.
N-ras is intrinsically required in CD8+ T lymphocytes for an efficient secondary response but not for a primary response. (A and B) Around 200 purified CD8+ T cells from CD45.1+ CD45.2+ N-ras+/+ OT-I and CD45.1+ CD45.2− N-ras−/− OT-I TCR transgenic mice were cotransferred into the same CD45.1− CD45.2+ N-ras+/+ C57BL/6 recipients, which were then infected with rVACV-OVA. At 7 d p.i., the frequency and numbers of both N-ras+/+ and N-ras−/− OT-I cells (A) and ex vivo IFN-γ production by OVA-specific CD8+ T lymphocytes were determined (n = 4, two experiments) (B). Results are expressed as mean ± SEM. **, P < 0.005. (C) Around 200 purified CD8+ N-ras+/+ or N-ras−/− transgenic OT-I cells were separately transferred into N-ras+/+ recipients that were then immunized with mDCs pulsed with OVA peptide. From each recipient, CD45.1+ cells were purified 8 d after priming and 200 cells cotransferred into the same congenic recipients that were then infected with rVACV-OVA. The frequency and numbers of both splenic N-ras+/+ and N-ras−/− transgenic OT-I cells were determined 5 d after challenge (n = 3, 2 experiments, results are expressed as mean ± SEM). For comparison, naive OT-I cells were adoptively transferred into recipient mice that were subsequently infected as in A and euthanatized 5 d later (primary response; not depicted). ***, P < 0.0005.
Next, for the analysis of the secondary response, we transferred purified N-ras+/+ and N-ras−/− OT-I cells into WT congenic recipients and immunized them with DCs pulsed with the OVA peptide (Fig. 5 C). As expected from Fig. 2 F, both types of OT-I cells exhibited a comparable primary response. DC priming in a noninflammatory environment accelerates CD8+ T lymphocyte memory differentiation (Badovinac et al., 2005), quickly promoting their ability to undergo secondary expansion upon rechallenge. In addition, this approach was used because DC immunization did not result in any differences in the number of CD8+ T lymphocytes homing to the spleen (at 35 d post infection [p.i.] in Fig. 3 E, and not depicted for earlier times after DC immunization). Therefore, 8 d later, N-ras+/+ and N-ras−/− OT-I cells were isolated and cotransferred into new congenic recipients that were subsequently infected with rVACV-OVA. 5 d after challenge, we found higher frequencies as well as numbers of WT OT-I splenic cells than of N-ras−/− OT-I cells (Fig. 5 C). Therefore, the N-ras deficiency clearly caused a CD8+ T cell–intrinsic defect in the secondary response of memory-fated cells.
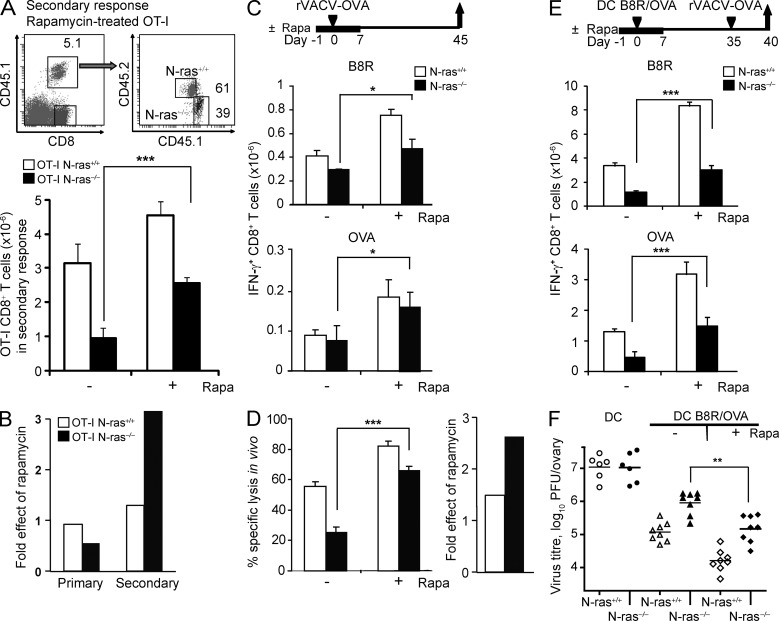
mTOR inhibition by rapamycin in vivo partially rescues the defect in memory differentiation and function of N-ras–deficient CD8+ T lymphocytes
A role for mTOR in regulating effector versus memory CD8+ T cell development has been recently unveiled with the use of mTORC1 inhibitor rapamycin (Araki et al., 2009; Pearce et al., 2009). We therefore explored whether the memory-promoting effect of rapamycin in vivo could rescue the defect in generation of functional memory caused by the N-ras deficiency (Fig. 6). To this end, rapamycin-treated or untreated N-ras+/+ and N-ras−/− OT-I cells were adoptively transferred into the same recipient mice, which were then immunized with antigen-loaded DC in the presence or absence of rapamycin and later infected with virus (as depicted in Fig. 5 C). Rapamycin treatment consistently ameliorated the secondary expansion of antigen-specific N-ras−/− cells, although only partly compared with WT counterparts treated with the inhibitor (Fig. 6 A). Nonetheless, while there was no differential effect from rapamycin on the antigen-driven primary expansion of OT-I CD8+ T cells of either genotype, rapamycin-treated N-ras−/− cells expanded ∼2.5-fold more than wild-type cells after secondary antigen challenge (Fig. 6 B).
Figure 6.
Inhibition of mTOR in vivo with rapamycin partially rescues the N-ras–deficient CD8+ T cell–intrinsic defects in secondary responses, functional memory, and antiviral protective immunity. (A and B) Following a similar protocol to that depicted in Fig. 5 A for the study of the primary response, N-ras+/+ or N-ras−/− OT-I cells were treated or not with rapamycin and transferred into recipient mice. 1 d before rVACV-OVA infection, daily treatment or no treatment of recipient mice with rapamycin was started and maintained for 6 d. For the study of the secondary response after infection, a protocol similar to that depicted in Fig. 5 B was used, but cells were treated or not with rapamycin. 1 d before DC immunization, daily treatment or no treatment of recipient mice with rapamycin was started and maintained for 8 d. The frequency and numbers of splenic N-ras+/+ and N-ras−/− transgenic OT-I cells were determined 8 d after primary antigen exposure (not depicted) and 5 d after challenge, for which representative dot plots and the mean ± SEM data are shown (A). Fold effect of rapamycin treatment during the primary and secondary responses was calculated as the ratio of mean OT-I cell numbers in treated versus untreated animals (n = 3, two experiments; B). (C and D) WT and N-ras−/− mice were infected as in Fig. 3 A and treated or not with rapamycin as above. On day 45 p.i., IFN-γ production by B8R- and OVA-specific splenic CD8+ T lymphocytes (n = 3, two experiments, results are expressed as mean ± SEM) was determined ex vivo (C), and the cytotoxic activity specific for B8R peptide was assessed in vivo. Fold effect of rapamycin was calculated as in B (n = 3, two experiments, results are expressed as mean ± SEM; D). (E and F) WT and N-ras−/− mice were vaccinated with peptide-loaded mDC as in Fig. 4 A and treated or not with rapamycin as above. On day 35, mice were infected with rVACV-OVA, and 5 d later ex vivo IFN-γ production by B8R- and OVA-specific splenic CD8+ T lymphocytes was analyzed as a measure of functional secondary responses (n = 4, two experiments; E), whereas determination of infectious virus titers in the ovaries detected protective immunity (F). Horizontal bars show the mean values. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
Next, we determined the effects of rapamycin treatment in vivo on the memory response to rVACV-OVA infection. The numbers of B8R-specific and OVA-specific IFN-γ–producing CD8+ T cells were significantly increased in rapamycin-treated compared with untreated N-ras−/− mice, but to a lesser extent than in rapamycin-treated WT counterparts (Fig. 6 C). Similar results were obtained when the in vivo cytotoxic activity of memory cells was analyzed. Again, rapamycin ameliorated the capacity of N-ras−/− mice to eliminate antigen-bearing splenocytes in vivo (Fig. 6 D, left). Of note, the proportional improvement by rapamycin of the memory cytotoxic response was superior in N-ras−/− than in WT mice (Fig. 6 D, right).
We also tested the impact of rapamycin treatment on the recall response in DC-immunized mice. Also in this respect, rapamycin enhanced the expansion of responding memory CD8+ T cells (Fig. 6 E) and protective memory (Fig. 6 F) in N-ras−/− mice, but only partly in comparison with WT mice.
In all settings where CD8+ memory was assessed, the enhancing effects of rapamycin were more prominent in N-ras−/− than in WT mice or cells, but rapamycin-treated WT cells showed higher responses than their treated N-ras−/− counterparts. This supports the notion that the N-ras deficiency could affect other key events involved in memory differentiation that are not negatively regulated by mTOR.
N-ras deficiency caused an impairment of the antigen-mediated induction of T-box transcription factor Eomes, but not of T-bet
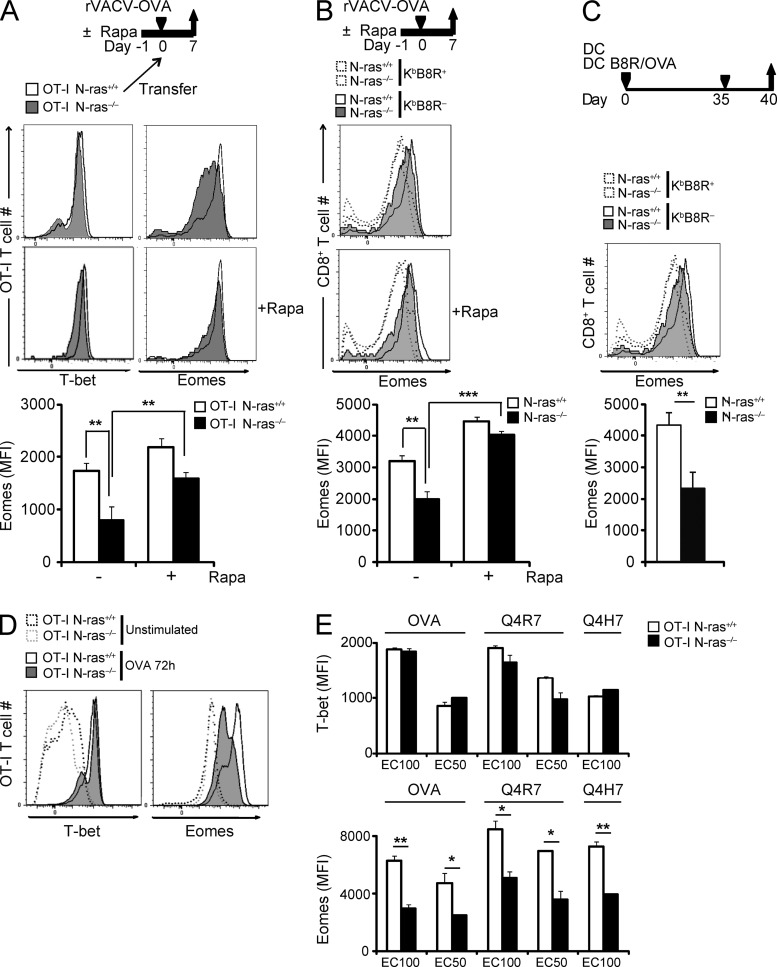
It has been proposed that the balance in the expression of transcription factors T-bet and Eomes impacts the effector versus memory T cell commitment (Intlekofer et al., 2005; Banerjee et al., 2010). To test the role of N-ras in the control of Eomes expression in differentiating CD8+ T lymphocytes in vivo, and the effect of inhibiting mTOR by rapamycin, naive N-ras+/+ and N-ras−/− OT-I cells, untreated or treated with rapamycin, were adoptively cotransferred into WT mice. Recipient mice were untreated or treated with the inhibitor and infected with rVACV-OVA. OT-I T cells were analyzed 7 d later ex vivo for expression of T-bet and Eomes (Fig. 7 A). T-bet expression was comparable in cells of both genotypes and mostly unaffected by rapamycin. In contrast, Eomes expression was significantly lower in N-ras−/− OT-I cells, and partly rescued by rapamycin treatment, as the rapamycin effect was superior in WT OT-I cells (Fig. 7 A). Similarly, KbB8R+ CD8+ T lymphocytes induced in N-ras−/− mice by VACV infection showed a consistently lower level of Eomes expression than their WT counterparts after primary infection, which was also rescued by rapamycin (Fig. 7 B). Furthermore, after viral challenge of DC-immunized mice, Eomes levels remained significantly lower in N-ras−/− compared with WT responding memory cells (Fig. 7 C). Together, these results suggest that N-ras−/− CD8+ T lymphocytes harbor a cell-intrinsic defect affecting Eomes but not T-bet expression during distinct stages of the in vivo CD8+ response.
Figure 7.
N-ras–deficient CD8+ T lymphocytes have an intrinsic deficiency in intracellular expression of Eomes transcription factor during the primary and secondary antiviral responses in vivo and in vitro. (A) Around 200 N-ras+/+ or N-ras−/− OT-I cells, either treated or not with rapamycin, were cotransferred as in Figs. 5 (A and B) and 6 (A and B) into the same N-ras+/+ recipients, which were then infected with rVACV-OVA and treated or not with rapamycin as in Fig. 6 (A and B) for 8 d. CD45.1+ OT-I cells were stained for intracellular T-bet and Eomes expression at 7 d p.i. and discriminated as WT or N-ras−/− according to CD45.2 expression (n = 3, two experiments). The graphs below represent mean ± SEM. (B) To study the primary response, WT and N-ras−/− mice were infected as in Fig. 2 A and treated or not with rapamycin as before. On day 7 p.i., Eomes expression was determined by FACS in CD8+ T lymphocytes and analyzed with respect to their staining with MHC H-2KbB8R pentamer (n = 3, two experiments). (C) For measuring the secondary response, WT and N-ras−/− mice were vaccinated with peptide-pulsed mDC and then infected as in Figs. 4 A and 6 (E and F). On day 5 p.i., Eomes expression and staining with H-2KbB8R pentamer was determined by FACS in CD8+ T lymphocytes (n = 3). The graphs below in B and C represent mean ± SEM Eomes expression in H-2KbB8R pentamer-positive CD8+ T lymphocytes. (D and E) Purified CD45.1+ N-ras+/+ and N-ras−/− OT-I CD8+ T lymphocytes were stimulated in vitro for 72 h at an E/T ratio of 1/6 with CD45.1− N-ras+/+ splenocytes loaded or not with nonlimiting OVA peptide concentration (10−9 M; D), or with OVA and the weak agonists Q4R7 and Q4H7 peptides at concentrations that induced either the maximal (EC100) or half-maximal (EC50) CD69 expression (as determined in Fig. 1 C; E). Cultures were then stained for CD45.1 and CD25 for gating and for transcription factors T-bet and Eomes and analyzed by FACS (three experiments). Results in E are expressed as mean ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
To further test whether N-ras is involved in regulating T-bet and Eomes in antigen-stimulated CD8+ T lymphocytes at earlier times, we analyzed induction of these transcription factors after exposure to antigen in vitro. As shown in Fig. 7 (D and E), the magnitude of T-bet induction was comparable in N-ras–sufficient and –deficient OT-I cells. In overt contrast, Eomes expression was consistently impaired in N-ras−/− OT-I cells after stimulation with antigen, which correlated with the memory defect of N-ras−/− mice.
Abundance of cognate antigen and the strength of TCR signals have been shown to impact effector versus memory fate decision (Zehn et al., 2009; Corse et al., 2011; Leignadier et al., 2011). However, the influence of these factors in the expression of fate-determining transcription factors, in particular that of T-bet and Eomes, had not been evaluated in detail. To test this, naive N-ras+/+ and N-ras−/− OT-I cells were stimulated with the strong OVA peptide agonist or with the weaker altered peptide ligands, Q4R7 and Q4H7. We tested concentrations promoting maximal (EC100) or half-maximal (EC50) CD69 induction response for each genotype and peptide (as determined in Fig. 1 C). By 72 h (Fig. 7 E), T-bet induction was comparable in N-ras–sufficient and –deficient OT-I cells irrespective of peptide affinity or concentration. In contrast, Eomes expression was always induced to a lesser extent in N-ras−/− compared with N-ras–sufficient cells. Collectively, these results confirm the strong dependence of Eomes expression on N-ras–mediated signaling and independently of the nature or abundance of the antigen.
Early induction of Eomes after TCR activation involves the PI3K–AKT but not the ERK pathway
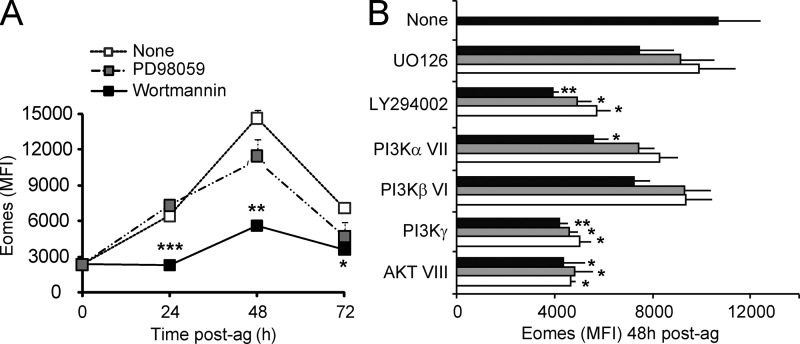
In T lymphocytes, early TCR-triggered activation of Ras results in downstream coupling to the ERK and PI3K pathways. To test the involvement of these pathways in the regulation of Eomes expression by the N-ras isoform, naive N-ras+/+ OT-I cells were stimulated with antigen in the presence or absence of PD98059, an inhibitor of MEK-1, an upstream activator of ERK kinases, or of wortmannin, a general PI3K inhibitor (Fig. 8 A). Inhibition of ERK activation had no major effect on Eomes induction, consistent with the lack of effect of N-ras deficiency on ERK activation (Fig. 1 A).
Figure 8.
Involvement of PI3Kγ and AKT but not of ERK in the induction of Eomes after antigen stimulation. Purified N-ras+/+ OT-I CD8+ T cells were pretreated or not with the indicated inhibitors for ERK kinase (PD98059) and PI3K (wortmannin), stimulated with 10−9 M OVA peptide as in Fig. 7 D, and incubated in the presence of the inhibitors for the indicated times (A), or similarly stimulated and treated with the inhibitors for ERK kinase (UO126), PI3K (LY294002), PI3Kα (inhibitor VII) PI3Kβ (VI), PI3Kγ, or AKT (VIII) at graded concentrations, and stained for Eomes after 48 h (B). Concentrations in B are indicated in Materials and methods and the highest to lowest depicted in black to gray to white bars, respectively. Statistical significance is indicated for untreated versus wortmannin treated cells in A (three experiments) and for untreated versus the different treatments in B (two experiments). Results are expressed as mean ± SEM. *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
In contrast, PI3K inhibition by wortmannin strongly suppressed antigen-mediated Eomes induction, mimicking the effect of the N-ras deficiency (Fig. 8 A). Together with data showing a deregulated antigen-induced AKT activity in N-ras−/− cells (Fig. 1 A), these results suggest that N-ras acts as a critical inducer of early Eomes expression via the PI3K–AKT pathway. To gain further insight, we analyzed the effect of specific PI3K and AKT pharmacological inhibitors on Eomes induction after antigen exposure (Fig. 8 B). Inhibition of PI3Kγ or AKT resulted in a marked decrease in Eomes induction, which was comparable to the effect of LY294002, a general PI3K inhibitor. Furthermore, interfering PI3Kα activity affected Eomes induction but only at the highest dose of inhibitor tested, and to a lesser extent than did PI3Kγ and AKT inhibitors, whereas PI3Kβ inhibition and ERK inhibition by UO126 had no significant effect. Together, these results suggest that PI3Kγ, most likely via AKT, but not the ERK pathway, is involved in regulating early Eomes expression in antigen-activated CD8+ T cells.
Impairment of the antigen-mediated induction of Eomes by N-ras deficiency was independent of mTOR at early priming times but partly rescued by mTOR inhibition at late times
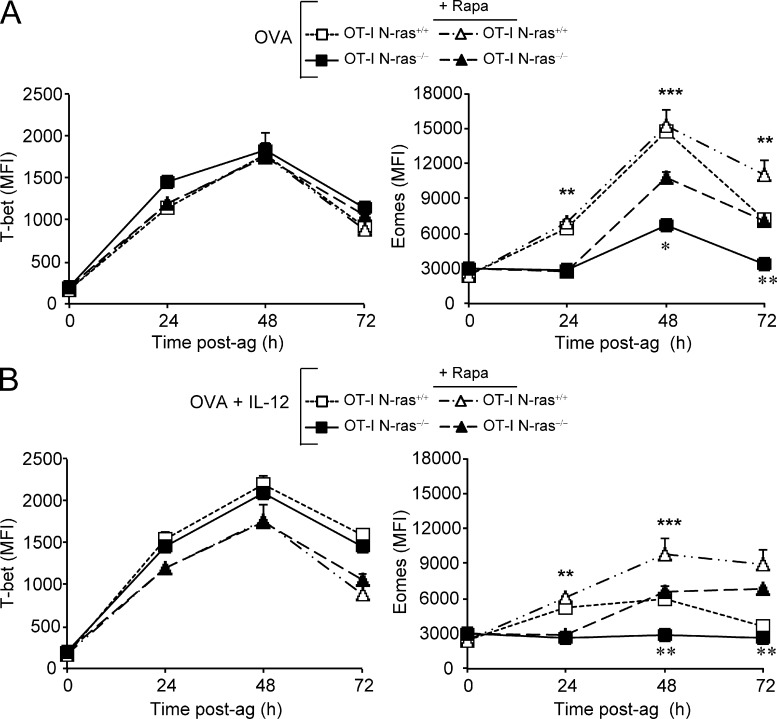
It has been suggested that mTOR activity regulates the balance between T-bet and Eomes expression in CD8+ T cells in response to antigen plus IL-12 (Rao et al., 2010). Moreover, the Ras pathway has recently been involved in TCR-induced mTOR activation (Gorentla et al., 2011), although the relevant Ras isoform was not identified. These findings prompted us to carefully analyze the kinetics and magnitude of T-bet and Eomes induction, and the effect of rapamycin, in N-ras–sufficient and –deficient CD8+ T cells at early and late times after antigen exposure in vitro (Fig. 9).
Figure 9.
Late involvement of mTOR in the induction of Eomes after antigen stimulation. Purified N-ras+/+ and N-ras−/− OT-I CD8+ T lymphocytes were stimulated in vitro as in Figs. 7 D and 8 and treated or not with rapamycin in the absence (A) or in the presence (B) of IL-12. At the indicated times, cultures were stained for CD45.1 and CD25 for gating and for transcription factors T-bet and Eomes and analyzed by FACS (three experiments for both A and B). Results are expressed as mean ± SEM. Statistical significance is indicated for untreated WT versus N-ras−/− cells (top asterisks) and for rapamycin treated versus untreated N-ras−/− cells (bottom asterisks). **, P < 0.005; ***, P < 0.0005.
Rapamycin had no effect on T-bet expression in WT or N-ras−/− OT-I cells stimulated only with antigen (Fig. 9 A) and, as previously shown for WT cells (Rao et al., 2010), reverted the IL-12–induced enhancement of T-bet expression in both cases (Fig. 9 B). In addition, in WT OT-I cells, rapamycin treatment had no effect on Eomes expression except at 72 h after antigen exposure (Fig. 9 A), suggesting that early Eomes induction occurs in an mTOR-independent manner. Only when Eomes expression was poorly or not induced in N-ras−/− OT-I cells (Fig. 9 A) or in either cell genotype stimulated in the presence of IL-12 (Fig. 9 B) did inhibition of mTOR consistently ameliorate Eomes expression at late time points (48–72 h). Thus, early control of Eomes induction in antigen-responding CD8+ T lymphocytes required N-ras and seemed to involve signaling pathways other than mTOR. These results correlate with the partial rescue of low Eomes expressing N-ras−/− CD8+ T cells by rapamycin in vivo. However, later during CD8+ T cell priming, N-ras and mTOR seem to converge at the level of Eomes to regulate its expression in an opposite manner.
Forced expression of Eomes rescues the CD8+ T cell–intrinsic defect in memory caused by the N-ras deficiency
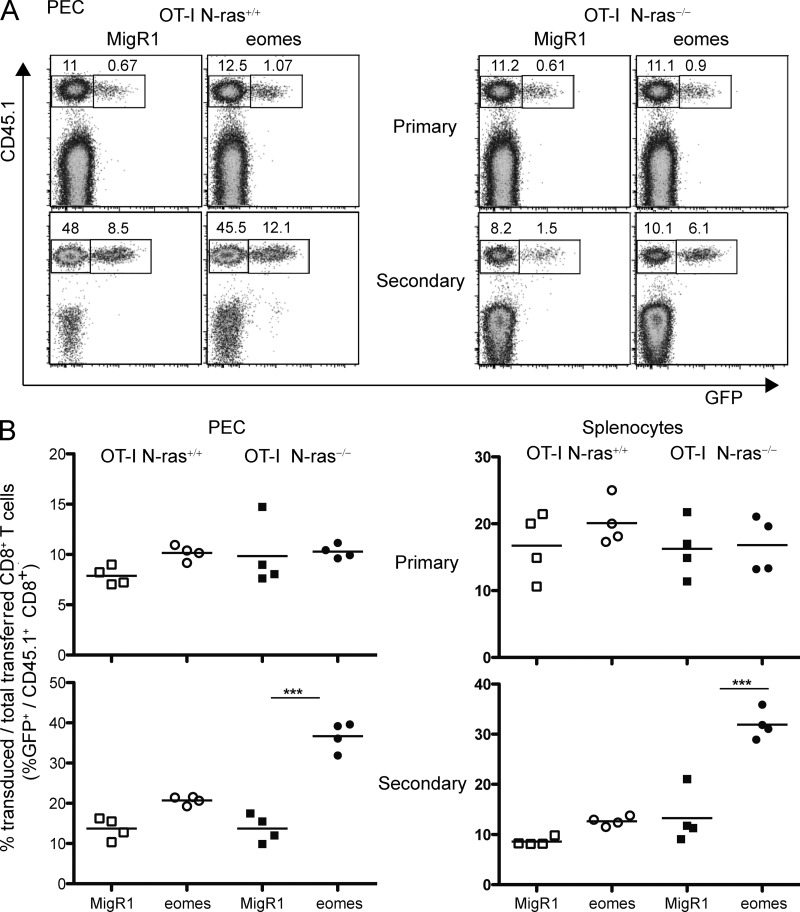
To test whether the defect in Eomes induction of N-ras−/− CD8+ T cells was associated with their intrinsic defect in generating functional memory cells, expression of Eomes was forced in N-ras−/− T lymphocytes. To this end, wild-type or N-ras−/− OT-I cells were activated with OVA peptide through the TCR, transduced with a retrovirus expressing Eomes and GFP, and adoptively transferred into recipient mice that were then infected with rVACV-OVA. As shown in Fig. 10, during the primary response transduced GFP+ and transferred CD45.1+ CD8+ T cells could be detected among PEC, and there was no difference in percentage between wild-type and N-ras−/− cells or between those transduced with control and with Eomes-expressing retroviruses (Fig. 10 A, top). After a secondary challenge with rVACV-OVA (Fig. 10 A, bottom), both transduced and nontransduced transferred wild-type OT-I cells expanded, as anticipated, and Eomes overexpression only conferred a minor benefit (Fig. 10 B, left). As also expected, N-ras−/− OT-I CD8+ cells were not able to expand, whether untransduced or transduced with MigR1 control retrovirus. In sharp contrast, forced expression of Eomes in these antigen-specific N-ras−/− cells allowed a highly significant secondary expansion in response to virus infection (Fig. 10, A and B, bottom). Similar results were obtained when responding cells in the spleen were analyzed. Again, forced expression of Eomes in N-ras−/− CD8+ T cells allowed their otherwise defective secondary expansion in response to viral infection, with the proportional improvement being superior in N-ras−/− cells to that in WT counterparts during the secondary but not the primary response (Fig. 10 B, right). Collectively, this data strongly supports a role for N-ras in coupling antigen receptor signaling to regulation of Eomes expression and to memory programming in CD8+ T lymphocytes.
Figure 10.
Forced retroviral expression of Eomes in vivo restores CD8+ memory generation in the absence of N-ras. N-ras+/+ or N-ras−/− OT-I CD45.1+/+ splenocytes were stimulated with OVA peptide in vitro for 24 h, CD8+ T cells purified, transduced with control GFP-expressing (MigR1), or with GFP-Eomes-expressing retroviruses and separately injected into naive CD45.1−/− CD45.2+/+ N-ras+/+ recipient mice that were infected concurrently or 14 d later with rVACV-OVA. On day 8 after primary exposure to antigen, and on day 5 after secondary infection, CD8+ PEC and splenocytes were analyzed by flow cytometry, as indicated. Representative dot plots of PEC (A) as well as graphs representing four individual mice in each group (B) of retrovirus-transduced (GFP+) and transferred (CD45.1+) CD8+ lymphocytes from PEC and spleen during the primary and secondary response, as indicated, are depicted. In A, numbers refer to percentage cells with respect to all CD8+ T lymphocytes, including GFP− CD45.1− endogenous CD8+ T cells. In B, numbers represent the percentage of GFP+ transduced cells with respect to total CD45.1+ transferred CD8+ T cells, excluding endogenous CD8+ T cells; horizontal bars show the mean values. A representative experiment out of two is shown. ***, P < 0.0005.
DISCUSSION
During viral infections, antigen-specific naive CD8+ T lymphocytes give rise to both short-lived effectors and long-lived memory lymphocytes that contribute to the protective adaptive response. Many questions remain, however, as to the underlying molecular mechanisms and the temporal window when the effector versus memory fate decision is made. In particular, the nature and timing of TCR signals specifically required for programming the memory fate of CD8+ T lymphocytes have remained elusive.
Evidence exists that signaling differences may determine the fate of naive CD8+ T lymphocytes upon activation (Lauvau et al., 2001; Badovinac et al., 2005; Daniels et al., 2006; Tewari et al., 2006; Chang et al., 2007; Teixeiro et al., 2009). Several such fate-determining molecules regulating memory but dispensable for effector CD8+ T lymphocyte differentiation have been identified, and most of them are distal or unrelated to TCR-triggered pathways. These include surface receptor BTLA (Krieg et al., 2007), intracellular signaling molecules such as TRAF-6 and the mTOR kinase (Araki et al., 2009; Pearce et al., 2009), and transcriptional regulators such as Bcl-6, Eomes, MBD2, Id2, and TCF-1 (Ichii et al., 2002; Intlekofer et al., 2005; Cannarile et al., 2006; Kersh, 2006; Zhou et al., 2010).
In the present work, we report that naive N-ras−/− antigen-responding CD8+ T lymphocytes were able to efficiently expand and differentiate into cytolytic effectors but not into memory cells with protective function. As previously shown for CD4+ T cells (Iborra et al., 2011), CD8+ T cells from N-ras–deficient mice exhibited reduced sensitivity to antigen. However, this was seemingly without effect on antigen-mediated primary T cell expansion and acquisition of effector functions, either in vitro or in vivo. This is in agreement with previous reports (Zehn et al., 2009; Leignadier and Labrecque, 2010) and supports the existence of compensatory mechanisms enhancing antigen sensitivity in antigen-experienced T cells (Kumar et al., 2011). N-ras deficiency impinged instead on both the quantity and functional quality of memory cells, but more prominently on the latter, and severely compromised their protective antiviral function. This is consistent with previous work emphasizing the role of quality of memory T lymphocytes for efficient protective immunity (Lauvau et al., 2001). N-ras would thus partake in a CD8+ T lymphocyte memory differentiation program as a TCR-proximal and specific transducer of early signals.
The balance of expression of transcription factors Eomes and T-bet has been reported to influence whether CD8+ T lymphocytes commit to memory or effector cells (Intlekofer et al., 2005). High T-bet expression seems to favor effectors (Matsuda et al., 2007; Yeo and Fearon, 2011), whereas persistent Eomes expression is proposed to enhance memory lymphocyte differentiation (Joshi et al., 2007; Rao et al., 2010). This has been determined in response to IL-2 (Pipkin et al., 2010) or in contexts with designed antigen-presenting cells and with cytokines providing inflammatory (IL-12) or survival (IL-7 and IL-15) signals that may predominate over and potentially obscure early TCR signals (Intlekofer et al., 2005; Rao et al., 2010). When we focused on early antigen-mediated activation of naive CD8+ T cells in our in vitro system without added cytokines or costimulatory signals, we found that TCR-mediated induction of T-bet was independent of N-ras. Of interest, unlike antigen-exposed CD8+ T cells, activated CD4+ T lymphocytes required N-ras for T-bet induction and IFN-γ production (Iborra et al., 2011), suggesting that N-ras could be differentially required for TCR-elicited functions in CD4+ and CD8+ T cell lineages or depend on other concurrent signals.
With regard to Eomes, TCR-mediated signals inducing its expression and their relevance to effector versus memory fate choice had remained unclear. TCR engagement results in activation of the PI3K–AKT and ERK pathways downstream of active Ras (Smith-Garvin et al., 2009), although which Ras isoforms are specifically involved was unknown (Mor and Philips, 2006). We now report that antigen-responding CD8+ T cells lacking N-ras have a major defect in Eomes induction in vitro and in vivo. A similar defect is revealed when PI3K, particularly PI3Kγ, and AKT are pharmacologically inhibited in WT cells in vitro. Because antigen-stimulated N-ras−/− CD8+ T cells also showed an intrinsic impairment in sustaining PI3K–AKT activation, our data suggest that N-ras, through PI3Kγ–AKT, mediates TCR signals required for up-regulation of Eomes, and thus for concomitant programming of the memory phenotype. Nonetheless, the involvement of other PI3K family members (Delgado et al., 2009) in Eomes regulation cannot be presently excluded. In contrast, in antigen-responding CD8+ T cells, we observed that N-ras deficiency had no major effect on ERK activation, suggesting that the ERK pathway is dispensable for Eomes induction. Thus, N-ras would clearly stand out among other Ras isoforms, having much less impact on ERK than on the PI3K–AKT pathway, and thereby dominating the induction of Eomes and of memory in TCR-activated CD8+ T cells.
A critical role for mTOR in determining whether CD8+ T lymphocytes adopt an effector versus memory cell fate was identified using the mTORC1 inhibitor rapamycin (Araki et al., 2009; Pearce et al., 2009). Furthermore, rapamycin has been reported to inversely affect Eomes and T-bet levels in CD8+ T cells following antigen stimulation in the presence of IL-12, with the inhibitor favoring the persistence of Eomes over T-bet (Rao et al., 2010; Li et al., 2011). We found that rapamycin promoted Eomes expression in vitro while leaving T-bet unaffected, in both WT and N-ras−/− CD8+ T cells, but importantly, this happened only at late time points after exposure to antigen. In vivo, rapamycin treatment also enhanced Eomes expression in WT and N-ras−/− CD8+ T cells. However, Eomes expression in treated N-ras−/− cells rarely reached the levels of treated WT cells. This resulted in the partial rescue by rapamycin of each of the CD8+ T cell defects associated with N-ras deficiency in vivo. These concordant results may reflect a late downstream convergence of signaling pathways involving N-ras and mTOR at the level of Eomes, with N-ras and mTOR acting in opposite manners on Eomes expression. In this scenario, rapamycin treatment during priming would allow N-ras−/− CD8+ T cells to reach a minimal threshold of Eomes expression for memory commitment. However, because of the earlier, mTOR-independent defect on Eomes induction impinged by the N-ras deficiency, the mTOR inhibitor would only partially rescue the memory defect of N-ras−/− mice. Alternatively, N-ras could critically regulate, apart from Eomes, other memory fate-determining molecules in CD8+ T cells.
To date, only a handful of positive regulators of Eomes have been identified in CD8+ T cells, including Runx3 (Cruz-Guilloty et al., 2009), Notch (Cho et al., 2009), and TCF-1 (Zhou et al., 2010). Our study identified N-ras as a novel inducer of Eomes expression, but in contrast to the aforementioned ones, N-ras was distinctively required for programming memory but not effector CD8+ T cell fates. Furthermore, among the positive regulators of Eomes, N-ras would be the most proximally linked to the TCR. Suppression of Eomes has recently been shown to enhance IL-17 expression and Th17 differentiation (Ichiyama et al., 2011), which resembles the phenotype of TCR-activated CD4+ T lymphocytes in N-ras−/− mice (Iborra et al., 2011; unpublished data). This further supports the notion that N-ras, but not the other Ras isoforms, specifically couples TCR signaling and induction of Eomes.
A recent study in a fluorescent Eomes reporter mouse (Paley et al., 2013) showed that expression of Eomes was very stable along the effector-to-memory CD8+ T cell differentiation after acute viral infection. Notably, in agreement with previous reports (Intlekofer et al., 2005; Banerjee et al., 2010), Eomes was found to be equally expressed in memory precursors and terminal effectors. However, Eomes expression correlated with improved central memory formation but not with enhanced primary effector response. Consistent with this, we found that retrovirally forced expression of Eomes in N-ras−/− antigen-specific CD8+ T cells rescued their defective secondary expansion in response to viral infection while having no effect on their primary response. Collectively, these data support a direct, nonredundant and specific role of N-ras in coupling antigen receptor signaling to early Eomes induction and thus to memory development in CD8+ T lymphocytes. Our data also further support Eomes as a marker to reliably identify the memory potential of CD8+ T cells early after their response to antigen.
Because N-ras was dispensable for the generation and function of primary effector cells, our findings collectively support the concept that the TCR recruits distinct signaling pathways for programming effector and memory fates. Furthermore, they suggest that isoform-specific Ras inhibitors could be of value for specific control of pathogenic memory T lymphocytes, or alternatively as anti-cancer therapeutics without detrimental side effects on patients’ immunity.
MATERIALS AND METHODS
Mice and peptides.
N-ras+/+ mice (C57BL/6 strain) were purchased from Charles River. N-ras−/− mice (H-2b haplotype; Umanoff et al., 1995) were bred with C57BL/6 for six generations and subsequently crossed with OT-I TCR transgenic mice to generate OT-I TCR transgenic N-ras−/− mice. All animal studies were approved by CSIC and Instituto de Salud Carlos III’s Review Boards and were performed in accordance with national regulations.
Peptides 257SIINFEKL264 from OVA and 20TSYKFESV27 (B8R) from VACV soluble IFN-γR homologue (Moutaftsi et al., 2006) were synthesized in an Applera peptide synthesizer model 433A, purified, and determined to be homogeneous by HPLC analysis. OVA variant peptides Q4R7 (SIIQFERL) and Q4H7 (SIIQFEHL) were a gift of H. Van Santen (Centro de Biología Molecular Severo Ochoa, Madrid, Spain).
MHC peptide pentamers and antibodies.
The Pro5 MHC class I H-2K OVA and H-2KbB8R pentamers and FITC–anti-CD8α and anti-CD19 were obtained from ProImmune. PE–anti-CD8α, PE–anti-CD69, APC–anti-CD25, PE–anti-CD44, APC–anti-CD62L, PE–anti-IL-2, PE–anti–IFN-γ, biotinylated anti-CD45.1, APC–anti-CD45.1, PerCP-Cy5.5–anti-CD45.2, PE–anti-AKT phosphorylated at Thr 308, and PE–anti-ERK1/2 (pT202/pY204) were all anti–murine proteins antibodies (BD). PE–anti–mouse Eomes and PerCP-Cy5.5–anti–mouse/human T-bet were obtained from eBioscience. Antibody to mTOR phosphorylated at Ser 2481 was from Cell Signaling Technology.
Flow cytometry and intracellular staining of cytokines, phosphorylated proteins, and transcription factors.
Intracellular staining for IL-2 after primary activation of OT-I CD8+ T lymphocytes was performed after a 3-h stimulation with peptide-pulsed DCs. The co-cultures were then incubated overnight in the presence of brefeldin A before staining for FACS according to conventional procedures. In the ex vivo assays, cells were stimulated with an excess of peptide (1 µM) for up to 2 h and stimulated for a further 4 h in the presence of brefeldin A. Cells were then stained with FITC–anti-CD8α, fixed, and incubated with PE–anti–IFN-γ during permeabilization (Dako; Johnstone et al., 2004).
The intracellular staining for phosphorylated ERK, AKT, and mTOR, was performed as previously described (Krutzik and Nolan, 2003). In brief, after surface staining, the cells were fixed with formaldehyde, permeabilized with methanol, and stained with phosphospecific antibodies. The intracellular staining of T-bet and Eomes was performed using the Foxp3 fixation/permeabilization buffer (eBioscience), according to the protocol recommended for detecting nuclear antigens.
Events were acquired using a FACSCanto flow cytometer and the data were analyzed using FACSDiva software (BD). Representative dot plots or histograms of an individual mouse per group are shown in the figures. Percentage of stained cells was calculated and is indicated within dot plots. Percentage and mean fluorescence intensity (MFI) data from sets of experiments are graphed as the mean ± SEM. An average of 10,000 CD8+ cells was analyzed in each sample. Background activation obtained with nonpulsed cells (0–0.3%) was subtracted.
BM–derived DCs.
DCs were generated from BM progenitors. Freshly prepared BM cells were cultured in the presence of 200 U/ml GM-CSF (PeproTech) and fed with GM-CSF on days 3 and 6. After 7 d, nonadherent cells with a typical DC morphology and a myeloid DC phenotype (MHC class II+, CD11c+, and CD8−) were collected (Medina et al., 2009). Maturation of DCs was performed by the addition of 0.5 µg/ml LPS to cultures on day 7. Mature DCs (mDCs) showed a mature morphology and up-regulated CD40 and CD86 costimulatory molecules and were collected 1 h later for immunizations or 16 h later for other purposes.
In vitro stimulation of OT-I CD8+ T lymphocytes.
CD8+ T lymphocytes were isolated from the spleens of OT-I TCR transgenic N-ras+/+ and N-ras−/− mice by negative selection using Miltenyi magnetic beads. 1 × 106 CD8+ T cells were stimulated with either 5 × 106 WT DCs pulsed with titrated concentrations of OVA or agonist peptides or with 6 × 106 CD45.1− CD45.2+ WT splenocytes pulsed with 10−9 M OVA peptide. For some experiments, 2 ng/ml IL-12 (R&D Systems) was added to the cultures. When indicated, cells were pretreated 20 min before stimulation and kept thereafter with 20 nM PI3K inhibitor wortmannin (Sigma-Aldrich), 50 µM ERK kinase (MEK) inhibitor PD98059 (EMD Millipore), 10, 5, and 2.5 µM ERK kinase (MEK) inhibitor UO126 (Cell Signaling Technology), 10, 5, and 2.5 µM PI3K inhibitor LY294002 (Cell Signaling Technology), 250, 125, and 62.5 nM PI3Kα inhibitor VII (EMD Millipore), 1, 0.5, and 0.25 µM μM PI3Kβ inhibitor VI (EMD Millipore), 10, 5, and 2.5 µM PI3Kγ inhibitor (EMD Millipore), 1, 0.5, and 0.25 µM AKT inhibitor VIII (EMD Millipore), or 2.2 nM mTORC1 inhibitor rapamycin (Sigma-Aldrich).
Proliferation was assessed by labeling OT-I CD8+ T lymphocytes with 5 µM CFSE (Molecular Probes) and coculturing them with mDC prepulsed with 10−7 M OVA peptide. CFSE dilution was analyzed 72 h later.
CTL lines were generated by culturing purified OT-I CD8+ cells with 10−9 M SIINFEKL-pulsed DCs for 72 h at an E/T ratio of 5/1 before adding human rIL-2 (NCI Preclinical Repository). The CTL lines were then used 2 d later for in vitro 51Cr-release cytotoxicity assays at an E/T ratio of 5/1 (Samino et al., 2004).
Viral infection, cytotoxicity assay, DC immunization, and rapamycin treatment in vivo.
VACV strain WR and the recombinant virus encoding full-length OVA (rVACV-OVA) based on the WR strain were provided by J.W. Yewdell and J. Bennink (National Institutes of Health, Bethesda, MD). Stocks were grown in CV-1 monolayers and consisted of clarified sonicated cell extracts.
Mice were infected either i.p. with 106 PFU of rVACV-OVA or intradermally in the ears with 5 × 104 PFU of VACV WR (Tscharke and Smith, 1999). In this case, the evolution of the infection was monitored by measuring the diameter of the ear lesion with a digital caliper. For DC immunizations, mice were inoculated i.p. with a mixture of 5 × 105 mDCs pulsed with 1 µM B8R peptide and 5 × 105 mDCs pulsed with 1 µM OVA peptide, or with mDCs pulsed with only one of the peptides.
For virus titration ex vivo, the ventral and dorsal dermal sheets of infected mouse ears were separated using forceps and incubated with 50 µg/ml liberase CI (Sigma-Aldrich) for 1 h at 37°C to prepare ear homogenates. After five freeze-thaw cycles, ear and mechanically prepared ovary homogenates were serially diluted, inoculated onto CV-1 cells, and stained 24 h later with crystal violet. Each dot in the figures represents the virus titer in each ear or ovary from individual mice and the thick horizontal bars represent the mean values for each group.
Rapamycin (Sigma-Aldrich) was administered in 5% Tween 80 and 5% polyethylene glycol 400 (Sigma-Aldrich) in saline solution (Dehay et al., 2010). Rapamycin or vehicle was inoculated i.p. daily at a low dose (75 mg/kg per mouse; Araki et al., 2009) 1 d before immunization or infection and for an additional 7 d during the T lymphocyte expansion phase.
In vivo cytotoxicity assays with N-ras+/+ and N-ras−/− splenocytes were performed as previously described (Medina et al., 2009). N-ras+/+ and N-ras−/− splenocytes were each split in two populations, labeled with either a high or a low concentration of CFSE, and washed. N-ras+/+ and N-ras−/− CFSEhi cells were pulsed with OVA peptide, mixed with unpulsed CFSElo cells, their actual relative ratio in the mixture measured by cytometry, and injected i.p. into infected, immunized, or control syngeneic recipients. The peritoneal cavity was lavaged 16 h later. The cells were then analyzed by FACS to measure in vivo killing. Specific lysis was calculated using to the formula: [1 − (ratio unprimed/ratio primed) × 100], where the ratio unprimed is %CFSElo/%CFSEhi cells remaining in the control recipients and the ratio primed is %CFSElo/%CFSEhi cells remaining in the experimental recipients. Representative histograms of an individual mouse per group are shown in the figures. The numbers in the histograms indicate the percentage of specific lysis. Mean specific lysis data from sets of experiments are graphed as the mean ± SEM.
Adoptive transfer and evaluation of in vivo responses.
Around 200 CD8α+ purified OT-I T cells from N-ras+/+ mice (CD45.1+ CD45.2+) and/or from N-ras−/− mice (CD45.1+ CD45.2−) were mixed, their actual relative ratio in the mixture measured by cytometry, and adoptively transferred i.v. into the same intact congenic N-ras+/+ recipients (CD45.1− CD45.2+). In secondary response experiments, OT-I cells were first transferred into separate CD45.1− CD45.2+ recipients that were then immunized with mDCs pulsed with OVA peptide. Transgenic OT-I cells were purified ex vivo 8 d after priming in two steps. CD8+ T cells were first negatively selected and then enriched in CD45.1+ OT-I cells by positive selection using magnetic beads (Miltenyi Biotec). Equal numbers of primed N-ras+/+ and N-ras−/− OT-I cells (∼200) were then cotransferred into the same congenic N-ras+/+ recipients that were subsequently infected with rVACV-OVA. The number of splenic N-ras+/+ and N-ras−/− transgenic OT-I cells was determined 5 d after challenge. For some experiments, OT-I cells and recipients were treated with rapamycin. The number of CD45.1+ OT-I cells was determined after staining and FACS evaluation. The total number of adoptively transferred N-ras+/+ and N-ras−/− cells was calculated by multiplying the total cell count by the fraction of CD8+ CD45.1+ CD45.2+ or CD8+ CD45.1+ CD45.2− cell gates, respectively.
Retroviral transduction.
Retroviral vector MigR1 expressing GFP and bicistronic retrovirus expressing GFP and Eomes were obtained from S. Reiner (Columbia University Medical Center, New York, NY; Intlekofer et al., 2005). Retroviruses were packaged by transient transfection of 293T cells with the retroviral vector along with pCLeco and retroviral transduction of antigen-specific CD8+ T cells was performed following a previously described procedure (García-Peydró et al., 2003). In brief, splenocytes from CD45.1+ N-ras+/+ or N-ras−/− OT-I TCR transgenic mice were incubated with 10−9 M OVA peptide for 24 h, and CD8+ T cells were purified and then spin-infected with control or Eomes-expressing retrovirus-containing supernatants in the presence of 8 µg/ml polybrene. The retrovirally infected cells were transferred into N-ras+/+ CD45.2+ recipients (105 cells/mouse), which were infected with 106 PFU rVACV-OVA. To study the primary response, PECs were analyzed 8 d after first exposure to antigen. Some recipients were infected 14 d later and the secondary response was similarly analyzed 5 d p.i.
Statistical analysis.
Data are represented as means and SD or SEM. Statistical significance of differences between the means of experimental groups was determined using an unpaired two-tailed Student’s t test.
Acknowledgments
We thank S. Reiner and H.-H. Xue for the retrovirus constructs, L. López-Ferreras, M.L. Toribio, and M. García-Peydró for help with retroviral protocols, J.L. Rodríguez Fernández for the PI3K inhibitors, P. Boya for help with rapamycin, A. Anel and J. Pardo for helpful discussion, B. Alarcón for his initial insight, and Y. Laó, C. Mir, and S. Sánchez for technical assistance.
This work was supported by Spanish Ministerio de Ciencia e Innovación, Red Temática de Investigación Cooperativa en SIDA from Instituto de Salud Carlos III (ISCIII) and CSIC (M. Del Val); by Comunidad Autónoma de Madrid, Universidad Complutense de Madrid and Fondo de Investigaciones Sanitarias (E. Fernández-Malavé); by Fondo de Investigaciones Sanitarias and by Red Temática de Investigación Cooperativa en Cáncer from ISCIII (E. Santos); by ISCIII (S. Iborra); and by Ministerio de Ciencia e Innovación (S. Lázaro).
The authors declare that they have no conflicting financial interests.
S. Iborra, M. Ramos, S. Lázaro, F. Aguilar, D. López, E. Fernández-Malavé, and M. Del Val designed research and analyzed data; S. Iborra, M. Ramos, S. Lázaro, F. Aguilar, and E. Fernández-Malavé performed research; E. Santos and E. Fernández-Malavé provided mice; and S. Iborra, E. Fernández-Malavé, and M. Del Val wrote the paper.
Footnotes
Abbreviations used:
- Eomes
- eomesodermin
- ERK
- extracellular signal-regulated kinase
- mDC
- mature DC
- mTOR
- mammalian target of rapamycin
- p.i.
- post infection
- PI3K
- phosphoinositide 3-kinase
- VACV
- vaccinia virus
References
- Araki K., Turner A.P., Shaffer V.O., Gangappa S., Keller S.A., Bachmann M.F., Larsen C.P., Ahmed R. 2009. mTOR regulates memory CD8 T-cell differentiation. Nature. 460:108–112 10.1038/nature08155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badovinac V.P., Messingham K.A., Jabbari A., Haring J.S., Harty J.T. 2005. Accelerated CD8+ T-cell memory and prime-boost response after dendritic-cell vaccination. Nat. Med. 11:748–756 10.1038/nm1257 [DOI] [PubMed] [Google Scholar]
- Banerjee A., Gordon S.M., Intlekofer A.M., Paley M.A., Mooney E.C., Lindsten T., Wherry E.J., Reiner S.L. 2010. Cutting edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 185:4988–4992 10.4049/jimmunol.1002042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarile M.A., Lind N.A., Rivera R., Sheridan A.D., Camfield K.A., Wu B.B., Cheung K.P., Ding Z., Goldrath A.W. 2006. Transcriptional regulator Id2 mediates CD8+ T cell immunity. Nat. Immunol. 7:1317–1325 10.1038/ni1403 [DOI] [PubMed] [Google Scholar]
- Castellano E., De Las Rivas J., Guerrero C., Santos E. 2007. Transcriptional networks of knockout cell lines identify functional specificities of H-Ras and N-Ras: significant involvement of N-Ras in biotic and defense responses. Oncogene. 26:917–933 10.1038/sj.onc.1209845 [DOI] [PubMed] [Google Scholar]
- Chang J.T., Palanivel V.R., Kinjyo I., Schambach F., Intlekofer A.M., Banerjee A., Longworth S.A., Vinup K.E., Mrass P., Oliaro J., et al. 2007. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 315:1687–1691 10.1126/science.1139393 [DOI] [PubMed] [Google Scholar]
- Cho O.H., Shin H.M., Miele L., Golde T.E., Fauq A., Minter L.M., Osborne B.A. 2009. Notch regulates cytolytic effector function in CD8+ T cells. J. Immunol. 182:3380–3389 10.4049/jimmunol.0802598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse E., Gottschalk R.A., Allison J.P. 2011. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. J. Immunol. 186:5039–5045 10.4049/jimmunol.1003650 [DOI] [PubMed] [Google Scholar]
- Cruz-Guilloty F., Pipkin M.E., Djuretic I.M., Levanon D., Lotem J., Lichtenheld M.G., Groner Y., Rao A. 2009. Runx3 and T-box proteins cooperate to establish the transcriptional program of effector CTLs. J. Exp. Med. 206:51–59 10.1084/jem.20081242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M.A., Teixeiro E., Gill J., Hausmann B., Roubaty D., Holmberg K., Werlen G., Holländer G.A., Gascoigne N.R., Palmer E. 2006. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 444:724–729 10.1038/nature05269 [DOI] [PubMed] [Google Scholar]
- Dehay B., Bové J., Rodríguez-Muela N., Perier C., Recasens A., Boya P., Vila M. 2010. Pathogenic lysosomal depletion in Parkinson’s disease. J. Neurosci. 30:12535–12544 10.1523/JNEUROSCI.1920-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado P., Cubelos B., Calleja E., Martínez-Martín N., Ciprés A., Mérida I., Bellas C., Bustelo X.R., Alarcón B. 2009. Essential function for the GTPase TC21 in homeostatic antigen receptor signaling. Nat. Immunol. 10:880–888 10.1038/ni.1749 [DOI] [PubMed] [Google Scholar]
- García-Peydró M., de Yébenes V.G., Toribio M.L. 2003. Sustained Notch1 signaling instructs the earliest human intrathymic precursors to adopt a gammadelta T-cell fate in fetal thymus organ culture. Blood. 102:2444–2451 10.1182/blood-2002-10-3261 [DOI] [PubMed] [Google Scholar]
- Gorentla B.K., Wan C.K., Zhong X.P. 2011. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 117:4022–4031 10.1182/blood-2010-08-300731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibiza S., Pérez-Rodríguez A., Ortega A., Martínez-Ruiz A., Barreiro O., García-Domínguez C.A., Víctor V.M., Esplugues J.V., Rojas J.M., Sánchez-Madrid F., Serrador J.M. 2008. Endothelial nitric oxide synthase regulates N-Ras activation on the Golgi complex of antigen-stimulated T cells. Proc. Natl. Acad. Sci. USA. 105:10507–10512 10.1073/pnas.0711062105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S., Soto M., Stark-Aroeira L., Castellano E., Alarcón B., Alonso C., Santos E., Fernández-Malavé E. 2011. H-ras and N-ras are dispensable for T-cell development and activation but critical for protective Th1 immunity. Blood. 117:5102–5111 10.1182/blood-2010-10-315770 [DOI] [PubMed] [Google Scholar]
- Ichii H., Sakamoto A., Hatano M., Okada S., Toyama H., Taki S., Arima M., Kuroda Y., Tokuhisa T. 2002. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat. Immunol. 3:558–563 10.1038/ni802 [DOI] [PubMed] [Google Scholar]
- Ichiyama K., Sekiya T., Inoue N., Tamiya T., Kashiwagi I., Kimura A., Morita R., Muto G., Shichita T., Takahashi R., Yoshimura A. 2011. Transcription factor Smad-independent T helper 17 cell induction by transforming-growth factor-β is mediated by suppression of eomesodermin. Immunity. 34:741–754 10.1016/j.immuni.2011.02.021 [DOI] [PubMed] [Google Scholar]
- Intlekofer A.M., Takemoto N., Wherry E.J., Longworth S.A., Northrup J.T., Palanivel V.R., Mullen A.C., Gasink C.R., Kaech S.M., Miller J.D., et al. 2005. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6:1236–1244 10.1038/ni1268 [DOI] [PubMed] [Google Scholar]
- Johnstone C., de León P., Medina F., Melero J.A., García-Barreno B., Del Val M. 2004. Shifting immunodominance pattern of two cytotoxic T-lymphocyte epitopes in the F glycoprotein of the Long strain of respiratory syncytial virus. J. Gen. Virol. 85:3229–3238 10.1099/vir.0.80219-0 [DOI] [PubMed] [Google Scholar]
- Joshi N.S., Kaech S.M. 2008. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J. Immunol. 180:1309–1315 [DOI] [PubMed] [Google Scholar]
- Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. 2007. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 27:281–295 10.1016/j.immuni.2007.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech S.M., Wherry E.J. 2007. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 27:393–405 10.1016/j.immuni.2007.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersh E.N. 2006. Impaired memory CD8 T cell development in the absence of methyl-CpG-binding domain protein 2. J. Immunol. 177:3821–3826 [DOI] [PubMed] [Google Scholar]
- Krieg C., Boyman O., Fu Y.X., Kaye J. 2007. B and T lymphocyte attenuator regulates CD8+ T cell-intrinsic homeostasis and memory cell generation. Nat. Immunol. 8:162–171 10.1038/ni1418 [DOI] [PubMed] [Google Scholar]
- Krutzik P.O., Nolan G.P. 2003. Intracellular phospho-protein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 55A:61–70 10.1002/cyto.a.10072 [DOI] [PubMed] [Google Scholar]
- Kumar R., Ferez M., Swamy M., Arechaga I., Rejas M.T., Valpuesta J.M., Schamel W.W., Alarcon B., van Santen H.M. 2011. Increased sensitivity of antigen-experienced T cells through the enrichment of oligomeric T cell receptor complexes. Immunity. 35:375–387 10.1016/j.immuni.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Lauvau G., Vijh S., Kong P., Horng T., Kerksiek K., Serbina N., Tuma R.A., Pamer E.G. 2001. Priming of memory but not effector CD8 T cells by a killed bacterial vaccine. Science. 294:1735–1739 10.1126/science.1064571 [DOI] [PubMed] [Google Scholar]
- Leignadier J., Labrecque N. 2010. Epitope density influences CD8+ memory T cell differentiation. PLoS ONE. 5:e13740 10.1371/journal.pone.0013740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leignadier J., Rooney J., Daudelin J.F., Labrecque N. 2011. Lowering TCR expression on naive CD8+ T cells does not affect memory T-cell differentiation. Immunol. Cell Biol. 89:322–325 10.1038/icb.2010.80 [DOI] [PubMed] [Google Scholar]
- Li Q., Rao R.R., Araki K., Pollizzi K., Odunsi K., Powell J.D., Shrikant P.A. 2011. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 34:541–553 10.1016/j.immuni.2011.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda J.L., George T.C., Hagman J., Gapin L. 2007. Temporal dissection of T-bet functions. J. Immunol. 178:3457–3465 [DOI] [PubMed] [Google Scholar]
- Medina F., Ramos M., Iborra S., de León P., Rodríguez-Castro M., Del Val M. 2009. Furin-processed antigens targeted to the secretory route elicit functional TAP1−/−CD8+ T lymphocytes in vivo. J. Immunol. 183:4639–4647 10.4049/jimmunol.0901356 [DOI] [PubMed] [Google Scholar]
- Mor A., Philips M.R. 2006. Compartmentalized Ras/MAPK signaling. Annu. Rev. Immunol. 24:771–800 10.1146/annurev.immunol.24.021605.090723 [DOI] [PubMed] [Google Scholar]
- Moutaftsi M., Peters B., Pasquetto V., Tscharke D.C., Sidney J., Bui H.H., Grey H., Sette A. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24:817–819 10.1038/nbt1215 [DOI] [PubMed] [Google Scholar]
- Obar J.J., Lefrançois L. 2010. Early events governing memory CD8+ T-cell differentiation. Int. Immunol. 22:619–625 10.1093/intimm/dxq053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M.F., Marais R. 2000. Ras protein signalling. Semin. Immunol. 12:63–73 10.1006/smim.2000.0208 [DOI] [PubMed] [Google Scholar]
- Paley M.A., Gordon S.M., Bikoff E.K., Robertson E.J., Wherry E.J., Reiner S.L. 2013. Technical advance: fluorescent reporter reveals insights into eomesodermin biology in cytotoxic lymphocytes. J. Leukoc. Biol. 93:307–315 10.1189/jlb.0812400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce E.L., Walsh M.C., Cejas P.J., Harms G.M., Shen H., Wang L.S., Jones R.G., Choi Y. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 460:103–107 10.1038/nature08097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez de Castro I., Diaz R., Malumbres M., Hernández M.I., Jagirdar J., Jiménez M., Ahn D., Pellicer A. 2003. Mice deficient for N-ras: impaired antiviral immune response and T-cell function. Cancer Res. 63:1615–1622 [PubMed] [Google Scholar]
- Perez de Castro I., Bivona T.G., Philips M.R., Pellicer A. 2004. Ras activation in Jurkat T cells following low-grade stimulation of the T-cell receptor is specific to N-Ras and occurs only on the Golgi apparatus. Mol. Cell. Biol. 24:3485–3496 10.1128/MCB.24.8.3485-3496.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipkin M.E., Sacks J.A., Cruz-Guilloty F., Lichtenheld M.G., Bevan M.J., Rao A. 2010. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 32:79–90 10.1016/j.immuni.2009.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao R.R., Li Q., Odunsi K., Shrikant P.A. 2010. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 32:67–78 10.1016/j.immuni.2009.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutishauser R.L., Kaech S.M. 2010. Generating diversity: transcriptional regulation of effector and memory CD8 T-cell differentiation. Immunol. Rev. 235:219–233 [DOI] [PubMed] [Google Scholar]
- Samino Y., López D., Guil S., de León P., Del Val M. 2004. An endogenous HIV envelope-derived peptide without the terminal NH3 group anchor is physiologically presented by major histocompatibility complex class I molecules. J. Biol. Chem. 279:1151–1160 10.1074/jbc.M305343200 [DOI] [PubMed] [Google Scholar]
- Scheele J.S., Marks R.E., Boss G.R. 2007. Signaling by small GTPases in the immune system. Immunol. Rev. 218:92–101 10.1111/j.1600-065X.2007.00530.x [DOI] [PubMed] [Google Scholar]
- Smith-Garvin J.E., Koretzky G.A., Jordan M.S. 2009. T cell activation. Annu. Rev. Immunol. 27:591–619 10.1146/annurev.immunol.021908.132706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeiro E., Daniels M.A., Hamilton S.E., Schrum A.G., Bragado R., Jameson S.C., Palmer E. 2009. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 323:502–505 10.1126/science.1163612 [DOI] [PubMed] [Google Scholar]
- Tewari K., Walent J., Svaren J., Zamoyska R., Suresh M. 2006. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc. Natl. Acad. Sci. USA. 103:16388–16393 10.1073/pnas.0602565103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke D.C., Smith G.L. 1999. A model for vaccinia virus pathogenesis and immunity based on intradermal injection of mouse ear pinnae. J. Gen. Virol. 80:2751–2755 [DOI] [PubMed] [Google Scholar]
- Umanoff H., Edelmann W., Pellicer A., Kucherlapati R. 1995. The murine N-ras gene is not essential for growth and development. Proc. Natl. Acad. Sci. USA. 92:1709–1713 10.1073/pnas.92.5.1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.A., Bevan M.J. 2007. Effector and memory CTL differentiation. Annu. Rev. Immunol. 25:171–192 10.1146/annurev.immunol.25.022106.141548 [DOI] [PubMed] [Google Scholar]
- Yeo C.J., Fearon D.T. 2011. T-bet-mediated differentiation of the activated CD8+ T cell. Eur. J. Immunol. 41:60–66 10.1002/eji.201040873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D., Lee S.Y., Bevan M.J. 2009. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 458:211–214 10.1038/nature07657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Yu S., Zhao D.M., Harty J.T., Badovinac V.P., Xue H.H. 2010. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 33:229–240 10.1016/j.immuni.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]