Abstract
Accumulation evidence shows that β-amyloid (Aβ) is a neurotoxic and accumulation of Aβ is responsible for the pathology of Alzheimer's disease (AD). However, it is currently not fully understood what makes Aβ toxic and accumulated. Previous studies demonstrate that Aβ is a suitable substrate for glycation, producing one form of the advanced glycation endproducts (AGEs). We speculated that Aβ-AGE formation may exacerbate the neurotoxicity. To explore whether the Aβ-AGE is more toxic than the authentic Aβ and to understand the molecular mechanisms, we synthesized glycated Aβ by incubating Aβ with methylglyoxal (MG) in vitro and identified the formation of glycated Aβ by fluorescence spectrophotometer. Then, we treated the primary hippocampal neurons cultured 8 days in vitro with Aβ-AGE or Aβ for 24 h. We observed that glycation exacerbated neurotoxicity of Aβ with upregulation of receptor for AGE (RAGE) and activation of glycogen synthase kinase-3 (GSK-3), whereas simultaneous application of RAGE antibody or GSK-3 inhibitor reversed the neuronal damages aggravated by glycated Aβ. Thereafter, we found that Aβ is also glycated with an age-dependent elevation of AGEs in Tg2576 mice, whereas inhibition of Aβ-AGE formation by subcutaneously infusion of aminoguanidine for 3 months significantly rescued the early cognitive deficit in mice. Our data reveal for the first time that the glycated Aβ is more toxic. We propose that the glycated Aβ with the altered secondary structure may be a more suitable ligand than Aβ for RAGE and subsequent activation of GSK-3 that can lead to cascade pathologies of AD, therefore glycated Aβ may be a new therapeutic target for AD.
Keywords: Glycated Aβ, receptor for advanced glycation endproducts, aminoguanidine, glycogen synthase kinase-3
Senile plaques (SP) and neurofibrillary tangles are hallmark pathologies in the brains of Alzheimer's disease (AD).1, 2 The major component in the plaques is β-amyloid (Aβ), a peptide of 39–43 amino acids, produced from amyloid precursor protein (APP) by β-secretase pathway.3 Numerous studies show that the Aβ-induced neurotoxicity is responsible for the pathology of AD.4 However, what makes Aβ more toxic and which forms of Aβ are more toxic are elusive.
The plaques in the AD brains are colocalized with the advanced glycation endproducts (AGEs), and the plaque-enriched fractions contain approximately threefold higher AGE adducts than that of the age-matched controls,5 suggesting that Aβ may be glycated. The long-live proteins are preferentially modified to form AGEs and the stability of Aβ makes it an ideal substrate for non-enzymatic glycation and formation of AGEs. Although in vitro studies show that Aβ can be glycated and the glycated Aβ contribute to the Aβ accumulation,5, 6 it is currently not characterized whether Aβ is also glycated in vivo to form Aβ-AGEs and the role of Aβ-AGE in the pathogenesis of AD.
Accumulation of AGEs in the brains may impair neural cells through direct covalent crosslinking to the substrates7, 8 or binding to the surface AGE receptors, namely RAGEs.9 The ligand–receptor interaction may perturb cell functions by activating receptor-mediated signal transduction pathways.10 Interestingly, Aβ has been identified as a ligand of RAGE.11 RAGE is overexpressed in the AD brains and acts as a binding site for Aβ at the plasma membrane of neurons, microglial cells, and endothelial cells of the vessel wall.11 Upregulation of RAGE mediates Aβ-induced oxidative stress,12 activation of nuclear factor-κB,11 neuronal expression of macrophage colony-stimulating factor,13 and cell death.14 Recent studies show that RAGE-dependent signaling contributes to an impaired learning/memory in AD-like transgenic models.15 It is currently unknown whether RAGE mediates neurotoxicity induced by glycated Aβ.
Formation of AGEs involves nonenzymatic reactions of reducing sugars or dicarbonyl compounds, such as methylglyoxal (MG) and glyoxal, and abnormal glucose metabolism or oxidative stress can lead to the formation of the reactive dicarbonyl compounds.16 Aminoguanidine (AG) is a prototype scavenging agent that prevents the formation of AGEs from the dicarbonyl precursors both in vitro and in vivo.17 AG reduces tissue AGEs accumulation and inhibits the vascular and renal manifestations induced by experimental diabetes18 or AGEs administration.5 Recent studies show that AG prevents hippocampal alterations in streptozotocin-induced dementia in rat19 and protected neuroblastoma cells against the neurotoxic effects of MG.20
Based on the previous findings, we hypothesized that glycated Aβ in vitro could exacerbate the neurotoxicity of Aβ, and in vivo inhibition of AGEs partially constituted by Aβ-AGE could restore early cognitive decline of AD.
Results
Glycation exacerbates neurotoxicity of Aβ in hippocampal neurons
To synthesize Aβ-AGE in vitro, Aβ1-42 was incubated with MG for 1 month. The production of Aβ-AGE was identified with fluorescence spectrophotometer measuring AGE-specific fluorescence at emission of 440 nm and excitation of 370 nm. We observed that the fluorescence of Aβ-AGE was about seven times as much as Aβ1-42 (data not shown), suggesting Aβ-AGE had been successfully produced in vitro.
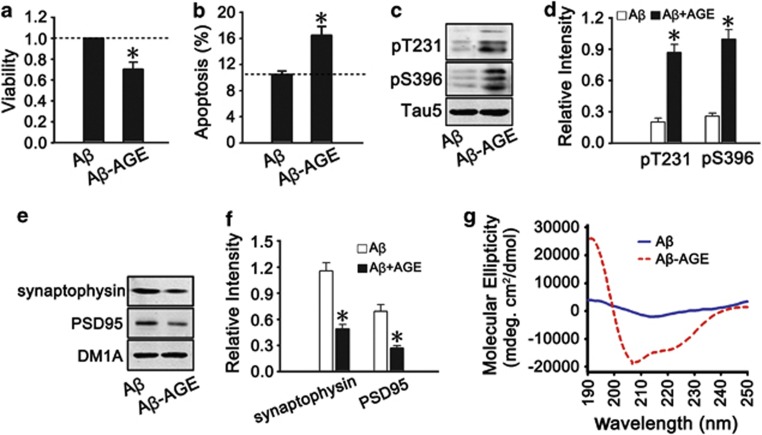
To explore whether Aβ-AGE is more toxic than authentic Aβ, 8-DIV embryonic hippocampal neurons were treated with Aβ or Aβ-AGE for 24 h. We found that Aβ-AGE was more toxic than Aβ in decreasing cell viability, increasing cell apoptosis, inducing tau hyperphosphorylation, and reducing synaptic proteins (Figures 1a–f). By circular dichroism (CD) spectra analysis, we found that Aβ-AGE displayed a significantly different profile (secondary structure) from Aβ (Figure 1g), which may underlie exacerbating toxicity of Aβ-AGE.
Figure 1.
Aβ-AGE with a special CD profile is more toxic to hippocampal neurons than the authentic Aβ. (a–f) The hippocampal neurons cultured 8 DIV were treated with Aβ or Aβ-AGE for 24 h, then the viability of the neurons was analyzed by using CCK-8 kit (a); the apoptosis rate was examined by flow cytometry (b); the level of tau phosphorylation at Thr231 and Ser396 (c and d) and synaptic proteins (e and f) were analyzed by western blot. Levels of phosphorylated tau and synaptic proteins were normalized, respectively, to Tau-5 and DM1A. (g) Aβ or Aβ-AGE was prepared in vitro as described in the methods and the structural property was measured by CD spectra analysis. n=3, *P<0.01 versus Aβ group
Activation of RAGE and glycogen synthase kinase-3 (GSK-3) mediates Aβ-AGE-exacerbated neurotoxicity in hippocampal neurons and Tg2576 mice
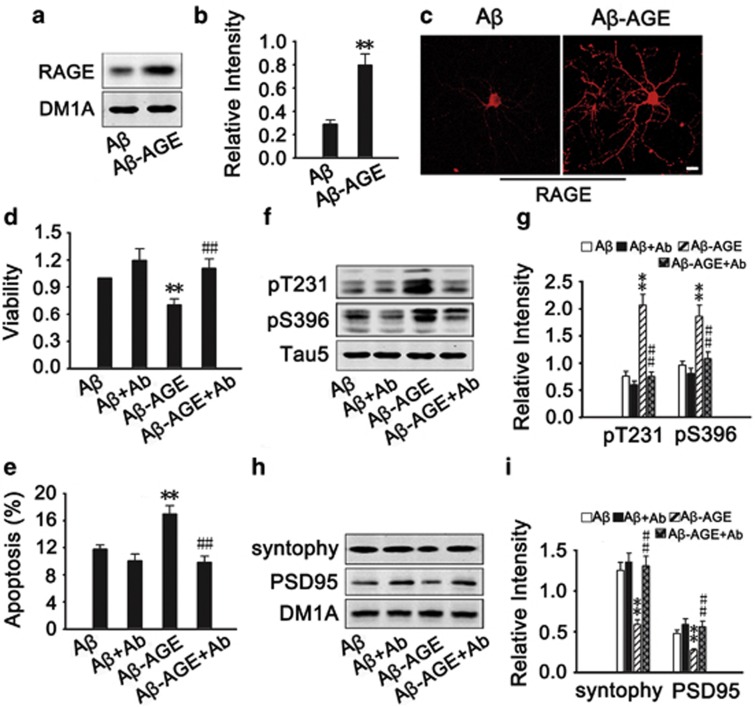
RAGE is the common receptor of Aβ and AGEs. To verify whether Aβ-AGE induces the neurotoxicity through RAGE, we first measured the level of RAGE after Aβ or Aβ-AGE treatment. We found that both Aβ-AGE and Aβ increased RAGE level, but the level of RAGE was even higher in Aβ-AGE group (Figures 2a–c). By using RAGE antibody to block the receptor, we found that blockage of RAGE almost abolished the Aβ-AGE-induced reduction of cell viability (Figure 2d), elevation of apoptotic rate (Figure 2e), tau hyperphosphorylation (Figures 2f and g) and deficits of synaptic proteins (Figures 2h and i). These data indicate that Aβ-AGE may be a more suitable ligand for RAGE than Aβ in exacerbating the Aβ-induced neurotoxicity.
Figure 2.
Upregulation of RAGE mediates the exacerbated Aβ-AGE toxicity in hippocampal neurons. (a–c) The hippocampal neurons cultured 8 DIV were treated with Aβ or Aβ-AGE for 24 h, then the level of RAGE was analyzed by western blot and immunofluorescence staining. (d–i) The hippocampal neurons cultured 8 DIV were pre-incubated with or without RAGE antibody (Ab) before treatment of Aβ or Aβ-AGE for 24 h, then the viability (d), the apoptotic rate (e), levels of the phosphorylated tau (f and g), and synaptic proteins (h, i) were measured. The level of RAGE and synaptic proteins was normalized to DM1A, and phosphorylated tau was normalized to Tau-5. n=3, *P<0.01 versus Aβ group; #P<0.01 versus Aβ-AGE group. Scale bar in c, 50 μm
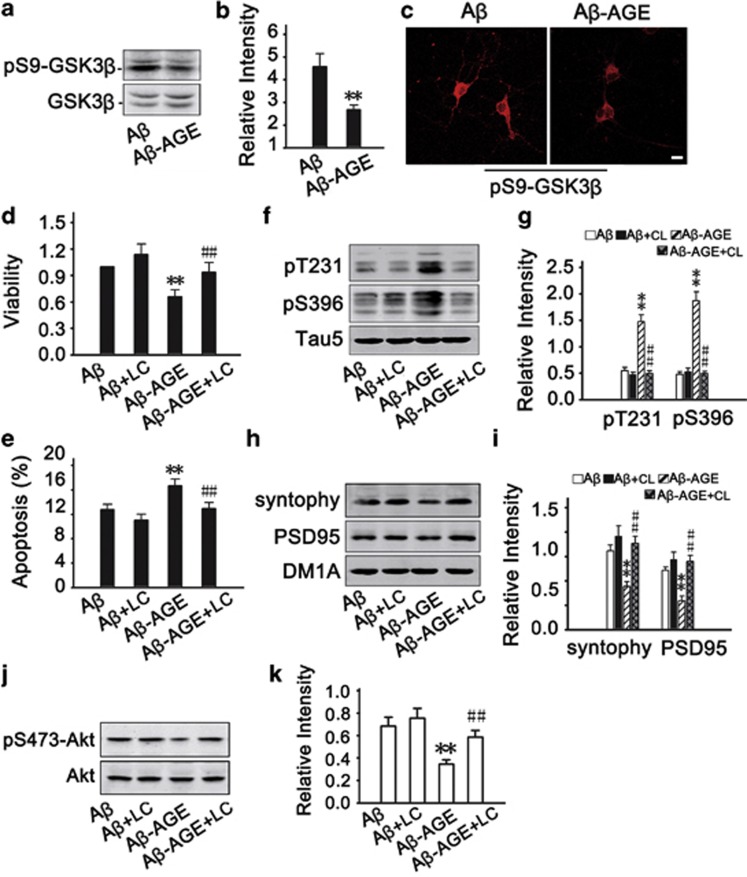
We have recently reported that AGEs induces cognitive impairment in SD rats through RAGE/GSK-3 pathway.21 To explore the involvement of GSK-3 in Aβ-AGE-induced neural impairments, we measured the activity-dependent phosphorylation of GSK-3β at Ser9 by western blot (Figures 3a and b) and immunofluorescence (Figure 3c). The phosphorylated GSK-3β at Ser9 decreased significantly in Aβ-AGE group than in Aβ group, suggesting that higher GSK-3 activity in Aβ-AGE group than the Aβ group. These data indicate that upregulation of GSK-3 may be involved in Aβ-AGE-induced toxicities. To further verify the role of GSK-3, we used inhibitor of GSK-3. We found that simultaneous inhibition of GSK-3 by LiCl attenuated the Aβ-AGE-induced reduction of cell viability (Figure 3d), elevation of apoptosis rate (Figure 3e), tau hyperphosphorylation (Figures 3f and g) and decline of synaptic proteins (Figures 3h and i). These data suggest that GSK-3 may mediate the Aβ-AGE-induced exacerbation of neural impairments.
Figure 3.
Activation of GSK-3β is involved in the exacerbated neurotoxicity of Aβ-AGE in hippocampal neurons. (a–c) Hippocampal neurons cultured 8 DIV were treated with Aβ or Aβ-AGE for 24 h, then the level of phosphorylated GSK-3β at Ser9 (inactive form) was measured by western blot (a and b) and immunofluorescence (c). (d–k) Hippocampal neurons cultured 8 DIV were pre-incubated with or without LiCl (inhibitor of GSK-3) before treatment of Aβ or Aβ-AGE for 24 h. Then the neuron viability (d), the apoptotic rate (e), levels of the phosphorylated tau (f and g), synaptic proteins (h and i), and Akt (j and k) were measured. The level of phosphorylated GSK-3β, phosphorylated tau, synaptic proteins, and phosphorylated Akt was normalized, respectively, to GSK-3β, Tau5, DM1A, and Akt. Li: LiCl. n=3, *P<0.01 versus Aβ group. #P<0.01 versus Aβ-AGE group. Scale bar in c, 50 μm
It is well known that Akt can phosphorylate GSK-3β at Ser9 and thus inhibit the kinase.22 Therefore, we measured the activity-dependent phosphorylation level of Akt. We found that phosphorylation of Akt at Thr473 was remarkably decreased after Aβ-AGE treatment (Figures 3j and k). These data suggest that inhibition of Akt may be upstream of the GSK-3 activation by Aβ-AGE.
Aβ is glycated to form Aβ-AGE with an age-dependent elevation of AGEs in the brains of Tg2576 mice
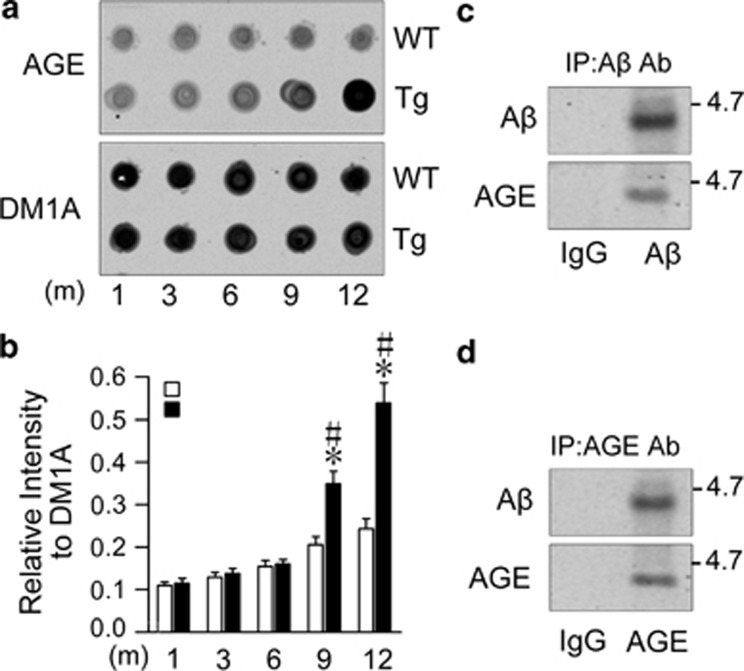
By western blot, we observed that the level of AGEs indeed increased significantly in hippocampus of a 9-month-old Tg2576 mice (Figures 4a and b). To verify whether Aβ is glycated, we analyzed the component of AGEs in a 9-month-old Tg2576 mice by coimmunoprecipitation and western blot. We found that Aβ was co-immunoprecipitated with an antibody against AGEs and vice versa (Figures 4c and d), suggesting that the glycated Aβ (Aβ-AGE) may be one of the major component of AGEs in Tg2576 mice.
Figure 4.
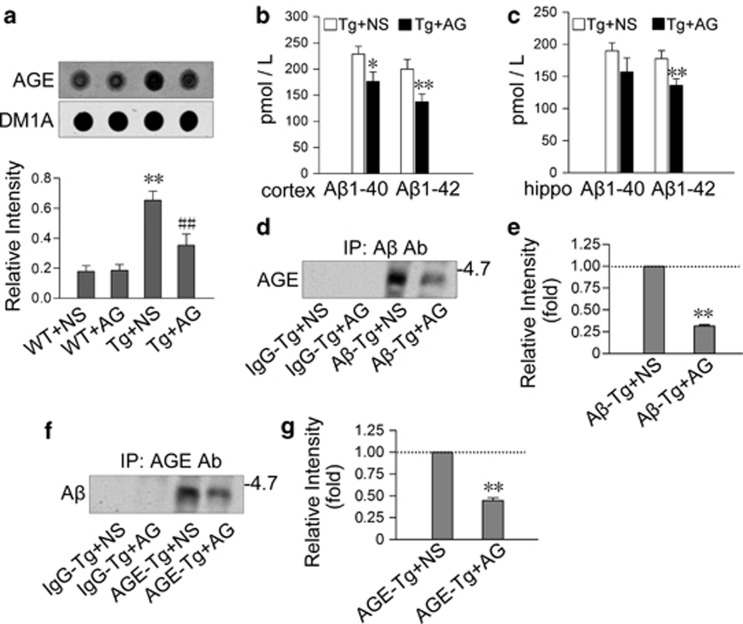
Aβ is glycated with an age-dependent increase of AGE in the brains of Tg2576 mice. (a and b) The hippocampal extracts from Tg2576 (Tg) or wild-type (WT) mice at 1, 3, 6, 9, and 12 months were analyzed by dot blot using anti-AGE antibody normalized against DM1A (b). (c and d) The hippocampal extracts from 9-month-old Tg mice were precipitated with AGE or Aβ or IgG antibody, and then the level of Aβ or AGE in the precipitate was measured by western blot using anti-Aβ (c) or anti-AGE (d) antibody. n=5, *P<0.01 versus WT group; #P<0.01 versus 6 months in Tg group
Early inhibiting the Aβ-AGE formation rescues cognitive impairment in Tg2576 mice
To verify the toxic roles of Aβ-AGE, we injected subcutaneously AG, an inhibitor of AGE formation, into Tg2576 mice for 3 months, started at 6-months old, and then measured the level of AGEs, Aβ, and Aβ-AGE by dot blot, ELISA and immunoprecipitation, respectively. We found that AG treatment decreased the levels of AGEs compared with normal saline (NS) group (Figure 5a) and Aβ in both of the cortex and the hippocampus (Figures 5b and c), simultaneously, the levels of AGE-associated Aβ and the Aβ-associated AGEs were reduced remarkably (Figures 5d–g). These data confirm that Aβ is glycated and AG inhibits the formation of Aβ-AGE in Tg2576 mice glycation.
Figure 5.
Subcutaneous injection of AG prevents formation of Aβ-AGE in Tg2576 mice. Tg2576 (Tg) mice and the wild-type (WT) littermates at 6-month old were subcutaneously injected with AG (blocker of AGE formation) or NS for 3 months. (a) Level of AGE in the cortex extracts was measured by dot blot and quantitated by normalization against DM1A. **P<0.01 versus WT+NS group; ##P<0.01 versus Tg+NS group. (b and c) The levels of Aβ1-40 and Aβ1-42 in cortex and hippocampus were estimated by ELISA. hippo: hippocampus. *P<0.05, **P<0.01 versus Tg+NS group. (d–g) The cortex extracts were immunoprecipitated with IgG or AGE or Aβ antibody, and then the levels of Aβ-AGE or AGEs in precipitate were measured by western blot using anti-Aβ or anti-AGE antibody as indicated. **P<0.01 versus Aβ-Tg+NS (e) or AGE-Tg+NS group (g)
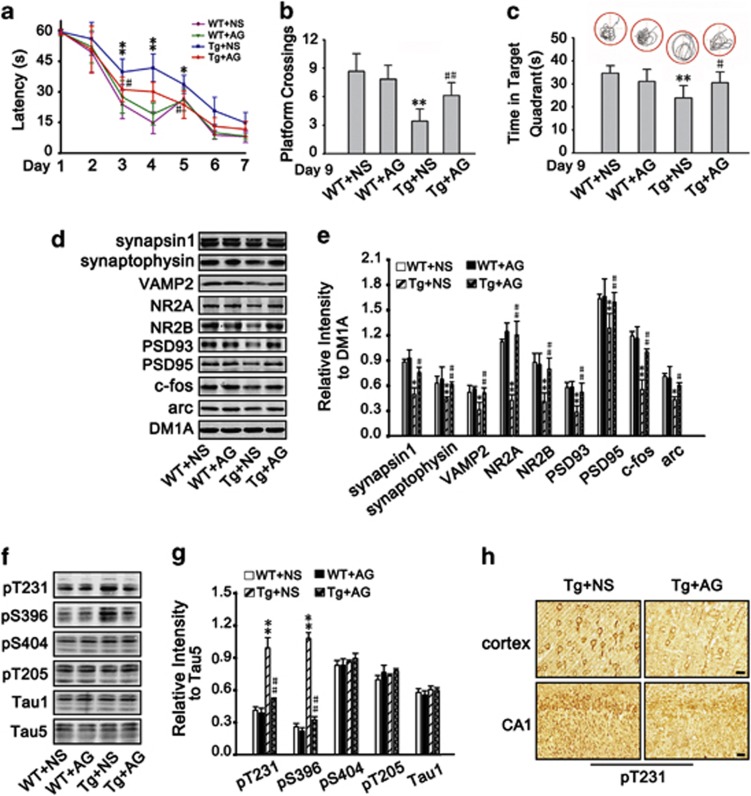
To test whether inhibition of Aβ-AGE by AG could rescue the cognitive impairments, we trained 9-month-old Tg2576 mice administrated with AG or NS for 3 months in water maze for 6 days, and tested the memory retention. We found that AG treatment improved the learning and memory of the mice, demonstrated by decreased latency (Figure 6a), increased platform crossings (Figure 6b) and increased time spent in target quadrant (Figure 6c). These data suggest that inhibition of Aβ-AGE can rescue learning and memory in Tg2576 mice.
Figure 6.
Subcutaneous injection of AG improves spatial memory and neuropathology in Tg2576 mice. (a and b) Tg2576 (Tg) mice and the wild-type (WT) littermates at 6-month old were subcutaneously injected with AG or NS for 3 months, then the spatial learning and memory were measured by Morris water maze. The mice were trained to remember the hidden platform in the maze for 6 days (learning process) and the latency (time to find platform) was recorded (a). The platform was removed at ninth day and the spatial memory was tested by measuring the time of platform quadrant crossing, the time stayed in the target quadrant and swimming paths (b and c). (d and e) The levels of presynaptic proteins (synapsin1, synaptophysin, and VAMP-2), postsynaptic proteins (NR2A, NR2B, PSD93, and PSD95) and the memory-related molecules (c-fos and arc) in hippocampus were measured by western blot and normalized against DM1A. (e and f) The phosphorylation levels of tau at Thr231, Ser396, Ser404, Thr205, and Tau-1 (Ser198/199/202) in hippocampus were measured by using phosphorylation site-specific antibodies as indicated in the blots normalized against total tau probed by Tau-5. (g) Slices of Tg mice treated with AG or NS were immunostained with antibody against phosphorylated tau at Thr231. b, c: n=8; d–g: n=3. *P<0.05, **P<0.01 versus WT+NS group; #P<0.05, ##P<0.01 versus Tg+NS group. s: second in a and c. Scale bar in h, 50 μm
We also found that both pre-synaptic (synapsin I, synaptophysin, and VAMP2) and post-synaptic proteins (NR2A, NR2B, PSD93, and PSD95), as well as memory-associated proteins (c-fos and arc) were downregulated in 9-month-old Tg2576 mice, and administration of AG restored the levels of the synaptic and memory-associated proteins (Figures 6d and e). In addition, tau was hyperphosphorylated at Thr231 and Ser396 but not at Ser404, Thr205, and Ser198/199/202 (Tau-1 epitope) in 9-month-old Tg2576 mice, and AG treatment attenuated tau hyperphosphorylation (Figures 6f–h). These data suggest that inhibiting formation of AGEs, which included Aβ-AGE, attenuates the neuropathology, which can contribute to the improved cognition of the mice. These in vivo data partially demonstrated the enhanced neurotoxicity of Aβ-AGE observed during in vitro experiments.
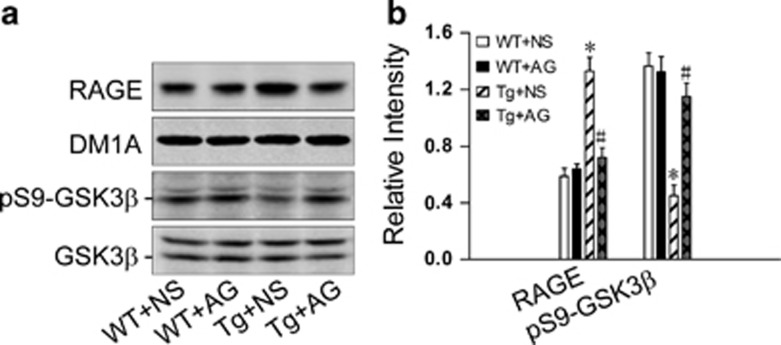
In view that the involvement of RAGE/GSK3 pathway in Aβ-AGE-induced exacerbation of neurotoxicity was found in hippocampal neurons, we also examined the level alteration of RAGE and activity of GSK-3 in Tg2576 mice after treatment with AG. We found that the level of RAGE increased and GSK-3 was activated in 9-month-old Tg2576 mice, whereas treatment of AG attenuated the upregulation of RAGE and GSK-3 in the mice (Figure 7). These in vivo data further support that RAGE and GSK-3 are participated in Aβ-AGE-induced neurotoxicity.
Figure 7.
Inhibition of Aβ-AGE formation suppresses RAGE upregulation and GSK-3β activation in Tg2576 mice. Tg2576 (Tg) or wild-type (WT) mice at 6-month old were injected subcutaneously with AG or NS for 3 months. At 9 month old, the mice were killed and levels of RAGE and the phosphorylated GSK-3β at Ser9 were estimated by western blot. RAGE and pS9-GSK-3β were normalized, respectively, to DM1A and total GSK-3β. n=3.*P<0.01 versus WT+NS group. #P<0.01 versus Tg+NS group
Discussion
In type 2 diabetes mellitus (T2D) patients, the consequence of the elevated blood glucose leads to the generation of AGEs. Previous study showed that the increased AGEs contribute to the failure of sensory nerve regeneration in diabetes,23 and administration of exogenous AGE-modified proteins modulates the maturation and functions of peripheral blood dendritic cells and neural stem cells.24 Epidemiological studies have shown that diabetes mellitus is an independent risky factor of AD.25, 26, 27, 28 However, the molecular mechanism is not fully understood. As the therapeutics advances for diabetes, the T2D patients will most likely live longer and thus the world may soon be facing the daunting challenge of dealing with a new population of AD sufferers with T2D.29 One of the hallmark lesion observed in AD brain is the formation of SPs, which are composed of the Aβ, derived from APP proteolysis. Studies suggest that accumulation of Aβ, is responsible for the age-related memory decline in AD model,30, 31, 32 however, it is not fully understood what may lead to Aβ accumulation and in which form Aβ may exert its toxic effects. Formation and accumulation of AGEs has been proposed to be involved in the evolution of AD.33 The level of AGEs is increased in the AD brains and the glycated Aβ accumulation accelerates Aβ deposition.5, 34 The proteins with prolonged turnover, such as tau in paired helical filament and Aβ in the AD brains,35 are favorable substrates for the formation of AGEs. Therefore, we speculate that the overproduced Aβ in Tg2576 mice may be glycated and the glycation may exacerbate the toxicity of Aβ.
To investigate the toxicity of glycated Aβ, we synthesized glycated Aβ (Aβ-AGE) in vitro and treated the hippocampal neurons cultured 8 days in vitro. We observed that the Aβ-AGE was more toxic than the authentic Aβ in decreasing the cell viability, increasing apoptosis, causing tau hyperphosphorylation and damaging the synapses. By CD spectra analysis, we also observed that the glycated Aβ showed an un-ordered secondary structure, which favors protein aggregation.36
RAGE, a cell surface-binding site for Aβ and AGEs, is upregulated in affected cerebral vessels, neurons, and microglia37, 38 when Aβ increases. RAGE mediates Aβ transport across the blood–brain barrier and accumulation in the brain.39 Transgenic mice overexpressing mutant human APP and RAGE in neurons displayed earlier stage deficits of spatial learning/memory and more serious neuropathologic changes.40 A growing body of evidence demonstrates that increased expression of RAGE allows for more profound RAGE-induced cellular perturbation.4142 To explore whether Aβ-AGE affects RAGE and whether RAGE is involved in the exacerbation of neural toxicity of Aβ-AGE, we measured the level of RAGE in hippocampal neurons in vitro. We found that the RAGE level was higher in Aβ-AGE-treated cells than in the Aβ-treated ones, whereas simultaneous application of RAGE antibody attenuated more significantly the neural damages induced by Aβ-AGE. These data imply that RAGE may have an important role in exacerbating the toxic effect of Aβ-AGE.
GSK-3 has been verified to participate in the pathogenesis of AD.43, 44 Upregulation of GSK-3 inhibits long-term potentiation and causes memory deficit.45 Based on our previous study that AGEs contribute to memory impairment through RAGE-mediated GSK-3 activation,21 we speculate that, with upregulation of RAGE, GSK-3 may be implicated in the exacerbated neural damages induced by Aβ-AGE. Indeed, our results showed that GSK-3 in hippocampal neurons in vitro was activated and simultaneous application of GSK-3 inhibitor attenuated the exacerbated pathological change of Aβ-AGE, suggesting the involvement of RAGE-dependent GSK-3 activation pathway. We also observed a more significant inhibition of Akt by Aβ-AGE than Aβ. The activity of GSK-3 is regulated by phosphatidyl inositol 3 kinase-Akt pathway,22 and upregulation of Akt attenuates the AGE-induced dysfunction of endothelial progenitor cells,46 which supports our data.
The in vitro results have shown that Aβ was the suitable substrate for the glycation and glycated Aβ appeared to be more neurotoxic than Aβ. Thus, we speculated that AGEs may be produced by glycating Aβ even in the early stage of Tg2576 mice, in which accumulation of Aβ contributes to the pathology. To test this, we measured the levels of AGEs and glycation of Aβ in hippocampus of Tg2576 mice. We found that the level of AGEs increased age dependently started from 9-month-old onward of the mice, and Aβ was co-immunoprecipitated with AGEs, suggesting Aβ is glycated to form Aβ-AGE. To verify the toxic role of AGEs, we used AG, an inhibitor of AGEs that can block glycation of proteins or peptides by glucose or its derivants, to suppress formation of AGEs. The AG was administrated subcutaneously for 3 months, started at 6 months old when the AGEs level was normal. We observed that infusion of AG decreased levels of AGEs and Aβ-AGE in hippocampus with significant improvement of spatial learning and memory of the mice. Simultaneously, levels of the hyperphosphorylated tau, synaptic, and memory-related proteins were remarkably restored in the hippocampus of mice. We also carried out the above experiments in the cortex of mice, the result of which was consistent with that in hippocampus. These data suggest that formation of Aβ-AGE may exaggerate AD pathologies.
In view of observation of amplified RAGE-mediated GSK-3 activation induced by Aβ-AGE in hippocampal neurons, we also examined the expression of RAGE and GSK-3 in AG-treated Tg2576 mice. We found that inhibition of Aβ-AGE formation could attenuate upregulation of RAGE and activation of GSK-3, which provides the in vivo data supporting the participation of RAGE/GSK-3 pathway in the exacerbated neural dysfunctions induced by Aβ-AGE. We also found that the levels of Aβ1-40 and Aβ1-42 decreased with inhibition of AGEs. A recent study show that inhibition of RAGE with RAGE antibody or RAGE knockout could downregulate β-site APP cleaving enzyme 1 (BACE1),47 whereas activation of RAGE upregulate BACE1,48 which may partially explain the decreased Aβ after AG treatment observed in the current study.
Aβ is found in extracellular SP cores and is associated with neurodegeneration in later stages of AD. In contrast, recent studies suggest that accumulation of intraneuronal Aβ may be an early event in the pathogenesis of AD.49, 50 RAGE mediates intraneuronal transport of Aβ and neuronal dysfunction.12 The above studies demonstrate that Aβ also exits inside the neurons, especially in the early stage of AD. Oxidative stress and glucose metabolism disorder in AD lead to intracellular production of carbonyl compounds, which could modify intracellular Aβ to form Aβ-AGE. This may explain why we observed numerous AGEs inside the neurons.
In summary, we have found for the first time that the formation of Aβ-AGE exacerbates the toxicity of Aβ with the mechanisms involving activation of RAGE/GSK-3 pathway, and inhibition of AGEs including Aβ-AGE can restore the cognitive deficit in AD-like model mice, which reveal that the underlying mechanism of T2D to be related to AD may be through increasing formation of Aβ-AGE.
Materials and methods
Antibodies and chemicals
Mouse monoclonal antibody (mAb) AGE was from Tans Genic.Inc (Kumamoto, Japan). Rabbit polyclonal antibody (pAb) against tau phosphorylated at Ser396, Ser404, Thr231, and Thr205 were from Biosource (Camarillo, CA, USA). mAb Tau1 against tau unphosphorylated at Ser198/199/202, RAGE, and synapsin I were from Millipore (Billerica, MA, USA). mAb Tau5 against total tau was from Lab Vision Corp (Fremont, CA, USA). pAb GSK-3, pS9-GSK-3β, pS473-Akt, total Akt, and c-fos were from Cell Signaling Technology (Beverly, MA, USA); pAb Aβ, pT308-Akt, NR2A, NR2B, PSD93, PSD95, VAMP2, and Arc were from Abcam (Cambridge, UK). mAb DM1A against α-tubulin and mAb synaptophysin were from Sigma (St Louis, MO, USA). Peptide Aβ1-42 was from ChinaPeptides Co., Ltd (Shanghai, China). Anti-rabbit IRDye and anti-mouse IRDye were from Li-Cor Biosciences (Lincoln, NE, USA). BCA kit was from Pierce (Rockford, IL, USA). Peroxidase-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies were from Pierce (Rockford, IL, USA). Neurobasal and B27 were from Gibco (Grand Island, NY, USA).
Animals and treatment
APP transgenic mice (Tg2576 Mice) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). These mice overexpress human APP695 with a double mutation KM670/671NL. All mice were produced by the Experimental Animal Center of Tongji Medical College. The genotype was confirmed by PCR analysis of tail biopsies. All mice were kept under standard laboratory conditions: 12 h light and 12 h dark; lights on at 0600 hours; temperature: 22±2 °C; water and food ad libitum. All animal experiments were performed according to the ‘Policies on the Use of Animals and Humans in Neuroscience Research' revised and approved by the Society for Neuroscience in 1995.
Six-month Tg2576 or control C57BL mice were subcutaneously injected with 0.1 μl NS or AG (20 mg/ml) at 1000 hours for 3 months. Then, mice were trained for morris water maze test.
Preparation of Aβ-AGE
Aβ-AGE was prepared by incubating 100 mg/ml of Aβ1-42 with or without 0.5 mℳ MG in 0.1 M phosphate buffer, pH 7.2, at 37 °C for 1 month under sterile condition. After dialyzed against phosphate buffer for 48 h to remove MG, the prepared Aβ-AGE was sterilized by filtration and kept at −20 °C. The production of Aβ-AGE was identified with western blot and fluorescence spectrophotometer measuring AGE-specific fluorescence at emission of 440 nm and excitation of 370 nm (Perkin-Elmer, Waltham, MA, USA).
CD spectroscopy
CD spectra from Aβ or glycated Aβ (0.1 mg/ml) in water were taken using Jasco J-810 spectrapo-larimeter (Jasco international Co. Ltd, Tokyo, Japan). The CD spectrum was recorded in the range of 190–250 nℳ using a 0.1 cm path length quartz cuvette at 25 °C in continuous scanning mode. The acquisition parameters were 100 nℳ/min, with a 1.0-s response and a 1.0 nℳ band width. The data were accumulated over 10 runs, the presented data being the average. The results were expressed in term of molecular ellipticity [θ] in unit of deg.cm2/dmol.
Cell culture and treatment
Embryonic hippocampal neurons were cultured according to the procedure described previously.45 Primary hippocampal neurons at 8 DIV were treated with Aβ (100 nℳ) or Aβ-AGE (100 nℳ) for 24 h. Then, cells were harvested for western blot and immunofluorescent staining. For investigating underlying mechanism, 8-d hippocampal neurons were pre-incubated with or without RAGE antibody (10 μg/ml) or LiCl (4 mℳ) for 1 h before treatment with or without Aβ (100 nℳ) or Aβ-AGE (100 nℳ) for 24 h.
Morris water maze test
Morris water maze test was performed according to the procedure described previously.51 Briefly, the mice were trained to find a submerged platform by using a stationary array of cues outside the pool tub. The water was made opaque by using milk powder for chiaroscuro. Acquisition training consisted of a total of 28 trials, given as four spaced trials a day for 6 consecutive days. The probe tests were performed with the platform on the seventh day and by removing the platform on the ninth day. Swimming paths in probe test were monitored using an automatic tracking system. This system was used to record the swimming trace and calculate the latency to the platform and the time spent in each quadrant.
Western blot and dot blot
Hippocampus were homogenized and cells were lysed in a cooled buffer containing 10 mℳ Tris-HCl (pH 7.6), 50 mℳ NaF, 1 mℳ Na3VO4, 1 mℳ EDTA, 1 mℳ benzamidine, and 1 mℳ phenylmethylsulfonylfluoride and protease inhibitors mixture (1 mg/l each of a leupeptin, protininand pepstain A). The tissue homogenates and cell lysates were added with one-third volume of sample buffer containing Tris-HCl (pH 7.6) 200 mℳ, 8% SDS, 40% glycerol, and boiled in a water bath for 10 min. The lysates were centrifuged at 12 000 × g for 15 min at 4 °C. The protein concentration in the supernatant was measured by BCA kit according to manufacturer's instruction.
For western blot, equal amounts of protein were separated by 10% SDS-PAGE52 or tricine-SDS-PAGE (for Aβ)53 electrophoresis, and transferred to nitrocellulose membrane. The membranes were blocked with 5% nonfat milk dissolved in TBS-Tween-20 (50 mℳ Tris-HCl, pH 7.6, 150 mℳ NaCl, 0.2% Tween-20) for 1 h and probed with primary antibodies overnight at 4 °C. For dot blot,54 draw grid by pencil to indicate the region to be blotted. Using narrow-mouth pipet tip, adjust proteins to the same concentration, and spot 2 μl of samples onto the nitrocellulose membrane at the center of the grid. Let the membrane dry at 37 °C.
Then the blots were incubated with anti-rabbit or anti-mouse IgG conjugated to IRDye (800 CW) for 1 h at room temperature and visualized using the Odyssey Infrared Imaging System (Lincoln, NE, USA).
Co-immunoprecipitation
Cells were lysed in cooled lysis buffer (50 mℳ Tris-HCl, pH 7.4, 250 mℳ NaCl, 5 mℳ EDTA, 50 mℳ NaF, 0.1 mℳ Na3VO4, 0.1% Triton X-100) containing 1 mℳ phenylmethylsulfonylfluoride and protease inhibitors mixture for 30 min. The cell lysates were centrifuged at 12 000 × g for 10 min at 4 °C. The supernatants (0.5 mg) were incubated with indicated antibody at 4 °C overnight with gentle rotation, then mixed (20 μl) with the suspension of protein G-Sepharose beads (1:1) and incubated for 2 h at 4 °C with gentle rotation. The beads were collected by centrifugation and washed extensively with cell lysis buffer. The bound proteins were dissociated by boiling the beads in sample buffer and examined by dot blot.
Immunohistochemistry, immunofluorescence, and ELISA
The mice were killed by overdose of chloral hydrate and transcardially perfused with 50 ml NS, and then with 100 ml 4% cooled paraformaldehyde solution. The brains were post-fixed in the same fixation fluid for another 24 h at 4 °C. Coronal brain sections of hippocampal tissue were cut at 25 μm using a vibrate microtome (VT1000S, Leica, Solms, Germany). For each primary antibody, three to five consecutive sections from each brain were used. The immunoreaction was detected using horseradish peroxidase-labeled antibodies and visualized with diaminobenzidine (Sigma) tetrachloride system. The images were observed using a microscope (Olympus BX60, Olympus, Tokyo, Japan).
For cell studies, cells were cultured on coverslips and fixed with 4% paraformaldehyde. Rhodamine red-X- or Oregon Green 488-conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA) were used for immunofluorescence. The images were visualized using a laser two-photon confocal microscope (LSM510, Zeiss, Oberkochen, Germany).
The level of Aβ in cortical and hippocampal homogenates were measured by a sandwich ELISA kit using an anti-Aβ N-terminal antibody and an anti-Aβ1-40 or Aβ1-42 C-terminal antibody, according to the manufacturer's instructions (Biosource International, Camarillo, CA, USA).
Statistical analysis
Data were analyzed by using SPSS 11.0 statistical software (SPSS, Chicago, IL, USA). Statistical significance was determined by one-way ANOVA procedure followed by Duncan's post hoc test with 95% confidence and two-tailed Student's t-test. All data were expressed as mean±S.D.
Acknowledgments
This work was supported by the National Nature Scientific Fund of China (81171196), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (No 2012BAI10B03).
Glossary
- Aβ
β-amyloid
- AD
Alzheimer's disease
- AG
aminoguanidine
- AGEs
advanced glycation endproducts
- APP
amyloid precursor protein
- BACE1
b-site APP cleaving enzyme 1
- CD
circular dichroism
- MG
methylglyoxal
- GSK-3
glycogen synthase kinase-3
- NS
normal saline
- PHF
paired helical filament
- RAGE
receptor for AGEs
- SP
senile plaques
- Tg
Tg2576
- Tg2576 Mice
APP transgenic mice
- mAb
monoclonal antibody
- pAb
polyclonal antibody
The authors declare no conflict of interest.
Footnotes
Edited by A Verkhratsky
References
- Yan SD, Chen X, Schmidt AM, Brett J, Godman G, Zou YS, et al. Glycated tau protein in Alzheimer disease: a mechanism for induction of oxidant stress. Proc Natl Acad Sci USA. 1994;91:7787–7791. doi: 10.1073/pnas.91.16.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Uchida K, Harada T, Tsuboi M, Sato M, Kubo M, et al. Neurofibrillary tangles and the deposition of a Beta amyloid Peptide with a novel N-terminal epitope in the brains of wild tsushima leopard cats. PLoS One. 2012;7:e46452. doi: 10.1371/journal.pone.0046452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease and Down's syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, et al. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:4766–4770. doi: 10.1073/pnas.91.11.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawver JN, Schall HE, Petrofes Chapa RD, Zhu X, Murray IV. Amyloid-beta metabolite sensing: biochemical linking of glycation modification and misfolding. J Alzheimers Dis. 2012;30:63–73. doi: 10.3233/JAD-2012-112114. [DOI] [PubMed] [Google Scholar]
- Yan SD, Yan SF, Chen X, Fu J, Chen M, Kuppusamy P, et al. Non-enzymatically glycated tau in Alzheimer's disease induces neuronal oxidant stress resulting in cytokine gene expression and release of amyloid beta-peptide. Nat Med. 1995;1:693–699. doi: 10.1038/nm0795-693. [DOI] [PubMed] [Google Scholar]
- Byun K, Bayarsaikhan E, Kim D, Kim CY, Mook-Jung I, Paek SH, et al. Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer's disease. PLoS One. 2012;7:e37917. doi: 10.1371/journal.pone.0037917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- Shang L, Ananthakrishnan R, Li Q, Quadri N, Abdillahi M, Zhu Z, et al. RAGE modulates hypoxia/reoxygenation injury in adult murine cardiomyocytes via JNK and GSK-3beta signaling pathways. PLoS One. 2010;5:e10092. doi: 10.1371/journal.pone.0010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer's disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- Takuma K, Fang F, Zhang W, Yan S, Fukuzaki E, Du H, et al. RAGE-mediated signaling contributes to intraneuronal transport of amyloid-beta and neuronal dysfunction. Proc Natl Acad Sci USA. 2009;106:20021–20026. doi: 10.1073/pnas.0905686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Yan S, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, et al. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: a proinflammatory pathway in Alzheimer disease. Proc Natl Acad Sci USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadding A, Kaltschmidt B, Kaltschmidt C. Overexpression of receptor of advanced glycation end products hypersensitizes cells for amyloid beta peptide-induced cell death. Biochimica et Biophysica Acta. 2004;1691:67–72. doi: 10.1016/j.bbamcr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Fang F, Lue LF, Yan S, Xu H, Luddy JS, Chen D, et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer's disease. Faseb J. 24:1043–1055. doi: 10.1096/fj.09-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queisser MA, Yao D, Geisler S, Hammes HP, Lochnit G, Schleicher ED, et al. Hyperglycemia impairs proteasome function by methylglyoxal. Diabetes. 59:670–678. doi: 10.2337/db08-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Soulis-Liparota T, Cooper M, Papazoglou D, Clarke B, Jerums G. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40:1328–1334. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Biasibetti R, Swarowsky A, Leite MC, Quincozes-Santos A, Quilfeldt JA, et al. Hippocampal alterations in rats submitted to streptozotocin-induced dementia model are prevented by aminoguanidine. J Alzheimers Dis. 2009;17:193–202. doi: 10.3233/JAD-2009-1034. [DOI] [PubMed] [Google Scholar]
- Webster J, Urban C, Berbaum K, Loske C, Alpar A, Gartner U, et al. The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox Res. 2005;7:95–101. doi: 10.1007/BF03033780. [DOI] [PubMed] [Google Scholar]
- Biosa G, Addis MF, Tanca A, Pisanu S, Roggio T, Uzzau S, et al. Comparison of blood serum peptide enrichment methods by Tricine SDS-PAGE and mass spectrometry. J Proteomics. 2011;75:93–99. doi: 10.1016/j.jprot.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, et al. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Jimenez B, Dobler D, Moffatt S, Rabbani N, Streuli CH, Thornalley PJ, et al. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes. 2009;58:2893–2903. doi: 10.2337/db09-0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Sun ZL, Guo YJ, Yuan Y, Li L. PPARgamma-mediated advanced glycation end products regulation of neural stem cells. Mol Cell Endocrinol. 2009;307:176–184. doi: 10.1016/j.mce.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci USA. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67:505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Xu WL, von Strauss E, Qiu CX, Winblad B, Fratiglioni L. Uncontrolled diabetes increases the risk of Alzheimer's disease: a population-based cohort study. Diabetologia. 2009;52:1031–1039. doi: 10.1007/s00125-009-1323-x. [DOI] [PubMed] [Google Scholar]
- Han W, Li C. Linking type 2 diabetes and Alzheimer's disease. Proc Natl Acad Sci USA. 2010;107:6557–6558. doi: 10.1073/pnas.1002555107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, et al. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, et al. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 32:763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Loske C, Gerdemann A, Schepl W, Wycislo M, Schinzel R, Palm D, et al. Transition metal-mediated glycoxidation accelerates cross-linking of beta-amyloid peptide. Eur J Biochem. 2000;267:4171–4178. doi: 10.1046/j.1432-1327.2000.01452.x. [DOI] [PubMed] [Google Scholar]
- Kosik KS. Alzheimer's disease: a cell biological perspective. Science. 1992;256:780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Liu YH, Yin J, Li HL, Wang Q, et al. Peroxynitrite induces Alzheimer-like tau modifications and accumulation in rat brain and its underlying mechanisms. Faseb J. 2006;20:1431–1442. doi: 10.1096/fj.05-5223com. [DOI] [PubMed] [Google Scholar]
- Lue LF, Walker DG, Brachova L, Beach TG, Rogers J, Schmidt AM, et al. Involvement of microglial receptor for advanced glycation endproducts (RAGE) in Alzheimer's disease: identification of a cellular activation mechanism. Exp Neurol. 2001;171:29–45. doi: 10.1006/exnr.2001.7732. [DOI] [PubMed] [Google Scholar]
- Miller MC, Tavares R, Johanson CE, Hovanesian V, Donahue JE, Gonzalez L, et al. Hippocampal RAGE immunoreactivity in early and advanced Alzheimer's disease. Brain Res. 2008;1230:273–280. doi: 10.1016/j.brainres.2008.06.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–913. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Arancio O, Zhang HP, Chen X, Lin C, Trinchese F, Puzzo D, et al. RAGE potentiates Abeta-induced perturbation of neuronal function in transgenic mice. EMBO J. 2004;23:4096–4105. doi: 10.1038/sj.emboj.7600415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Origlia N, Righi M, Capsoni S, Cattaneo A, Fang F, Stern DM, et al. Receptor for advanced glycation end product-dependent activation of p38 mitogen-activated protein kinase contributes to amyloid-beta-mediated cortical synaptic dysfunction. J Neurosci. 2008;28:3521–3530. doi: 10.1523/JNEUROSCI.0204-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Toki S, Chowei H, Saito T, Nakano N, Hayashi Y, et al. Immunohistochemical distribution of the receptor for advanced glycation end products in neurons and astrocytes in Alzheimer's disease. Brain Res. 2001;888:256–262. doi: 10.1016/s0006-8993(00)03075-4. [DOI] [PubMed] [Google Scholar]
- Onishi T, Iwashita H, Uno Y, Kunitomo J, Saitoh M, Kimura E, et al. A novel glycogen synthase kinase-3 inhibitor 2-methyl-5-(3-{4-[(S )-methylsulfinyl]phenyl}-1-benzofuran-5-yl)-1,3,4-oxadiazole decreases tau phosphorylation and ameliorates cognitive deficits in a transgenic model of Alzheimer's disease. J Neurochem. 2011;119:1330–1340. doi: 10.1111/j.1471-4159.2011.07532.x. [DOI] [PubMed] [Google Scholar]
- Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, et al. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- Zhu LQ, Wang SH, Liu D, Yin YY, Tian Q, Wang XC, et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. J Neurosci. 2007;27:12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C, Ren Y, Tan H, He Z, Jiang Q, Wu J, et al. Rosiglitazone via upregulation of Akt/eNOS pathways attenuates dysfunction of endothelial progenitor cells, induced by advanced glycation end products. Br J Pharmacol. 2009;158:1865–1873. doi: 10.1111/j.1476-5381.2009.00450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Son SM, Jin SM, Hong HS, Shin DH, Kim SJ, et al. RAGE regulates BACE1 and Abeta generation via NFAT1 activation in Alzheimer's disease animal model. Faseb J. 2009;23:2639–2649. doi: 10.1096/fj.08-126383. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, et al. AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging. 33:e113–e127. doi: 10.1016/j.neurobiolaging.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, et al. Intraneuronal Abeta42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci. 2007;8:499–509. doi: 10.1038/nrn2168. [DOI] [PubMed] [Google Scholar]
- Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- Li XH, Xie JZ, Jiang X, Lv BL, Cheng XS, Du LL, et al. Methylglyoxal induces tau hyperphosphorylation via promoting AGEs formation. Neuromol Med. 2012;14:338–348. doi: 10.1007/s12017-012-8191-0. [DOI] [PubMed] [Google Scholar]
- Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- Zhou XW, Li X, Bjorkdahl C, Sjogren MJ, Alafuzoff I, Soininen H, et al. Assessments of the accumulation severities of amyloid beta-protein and hyperphosphorylated tau in the medial temporal cortex of control and Alzheimer's brains. Neurobiol Dis. 2006;22:657–668. doi: 10.1016/j.nbd.2006.01.006. [DOI] [PubMed] [Google Scholar]