Abstract
Rationale
Male rats escalate methamphetamine (meth) intake during long access meth self-administration, show enhanced reinstatement of meth seeking, and exhibit meth-induced memory impairments. However, the impact of long access daily meth self-administration on reinstatement and cognitive dysfunction has not been assessed in females, even though clinical studies on meth addiction have shown differences between men and women.
Objectives
This study determined whether male and freely-cycling female rats: 1) escalate meth-intake in a 6-h daily access period relative to 1-h access; 2) show different sensitivity to meth primed reinstatement after short and long access conditions; and 3) show deficits in novel object and object in place recognition memory.
Methods
Male and female Long-Evans rats self-administered meth in limited (1-h/day) or extended (6-h/day) daily access sessions. After 21 days, meth access was discontinued and rats entered an abstinence period. On the 7th and 14th days of abstinence, rats were assessed for recognition memory using tests for: a) novel object recognition memory, and b) object-in-place memory. Rats were tested for reinstatement of meth-seeking following extinction of responding.
Results
Female rats self-administered more meth and escalated intake faster than males during extended, but not limited, daily access. Both males and females in the extended, but not limited, access groups showed memory deficits on both tasks. Female rats showed greater reinstatement to meth-seeking with lower doses of meth priming injections than males.
Conclusions
Relative to males, females were equally susceptible to meth-induced memory deficits, but exhibited higher meth intake and greater relapse to meth-seeking.
Keywords: methamphetamine, object recognition, reinstatement, relapse, self-administration, sex differences
Introduction
Methamphetamine (meth) addiction in men and women is highly debilitating; however, differences exist between men and women in the epidemiology of meth use and treatment response outcomes (reviewed in (Dluzen and Liu 2008). For example, women typically report initial meth use at a younger age (Dluzen and Liu 2008) and transition faster from recreational use to dependence, with greater dependency (Rawson et al. 2005). Women also exhibit higher comorbid neuropsychiatric disorders and suicidal tendencies than their male counterparts (Hser et al. 2005) and prefer meth to other drugs, while men will use an alternative drug if meth is unavailable (Brecht et al. 2004). Although not tested empirically, this preference or “loyalty” to meth in women might be attributed to the drugs ability to suppress appetite, elevate mood, and/or enhance energy (Brecht et al. 2004; Dluzen and Liu 2008).
In animal models, female rodents have increased sensitivity to meth’s motor stimulating effects relative to males (Milesi-Hallé et al. 2007; Schindler et al. 2002), but not to the conditioned rewarding effects of the drug (Schindler et al. 2002). Female rats generally exhibit enhanced sensitivity during all phases of the self-administration/extinction/reinstatement model to various drugs of abuse (Lynch and Carroll 2000). The majority of reports using psychostimulant drugs to study sex differences have focused on cocaine. To date, very few studies of sex differences in meth self-administration and reinstatement have been published (Holtz et al. 2011; Kucerova et al. 2009; Roth and Carroll 2004). In general, more female rats acquire self-administration for cocaine and meth and this acquisition is faster than males (Hu et al. 2004; Lynch and Carroll 1999; Roth and Carroll 2004). However, not all drug self-administration studies have shown uniform results in this regard (Caine et al. 2004; Haney et al. 1995; Kosten et al. 2004). Females also reach higher break points on a progressive ratio schedule for cocaine and meth reinforcement, indicating a willingness to exert greater behavioral output for the drugs (Lynch and Carroll 2000; Roberts et al. 1989).
Self-administration models of drug taking rely on response-contingent drug delivery and as a consequence possess high face validity (Markou et al. 1993). Extended daily drug access conditions add several hallmark characteristics of addiction, including escalation of drug intake over time (Ahmed et al. 2000; Kitamura et al. 2006), compulsive drug-seeking (Vanderschuren and Everitt 2004), and increased motivation for the drug (Paterson and Markou 2003). Further, this translationally relevant administration procedure impacts several cognitive domains in male rats that are reminiscent of characteristics found in human meth addicts including memory, attention, impulsivity, and sensory motor gating (Dalley et al. 2005; Hadamitzky et al. 2011; Parsegian et al. 2011; Reichel et al. 2012; Reichel et al. 2011; Rogers et al. 2008).
Reports from our laboratory have shown that male rats given extended 6-hr access to self-administered meth escalated drug intake and increased meth seeking relative to rats with only 1- or 2-hr/day access periods (Rogers et al. 2008; Schwendt et al. 2009). Further, males given 6-hr daily access showed deficits in object recognition memory and object-in-place recognition memory (Reichel et al. 2012; Reichel et al. 2011; Rogers et al. 2008). The novel object recognition task determines whether a rat can discern between a novel and a previously experienced object; whereas, the object-in-place task requires rats to remember previously encountered items in novel locations (Warburton and Brown 2009). These tasks rely on the exploratory nature of rats (Berlyne 1950), rather than learning response contingent behavior or a Pavlovian association. Single trial object recognition tasks test some components of episodic memory (i.e., what, when, or where) and do not involve heuristic learning or changes in motivational state (Dickerson and Eichenbaum 2010; Ennaceur 2010). Whether females exhibit similar memory impairments on these tasks following meth self-administration has not been established. Further, the degree to which females show escalation of meth intake or a heightened reinstatement to meth seeking has not been characterized. To this end, we determined whether freely cycling female rats relative to male rats: 1) escalate meth-intake during extended (6-hr) and limited (1-hr) access periods, 2) exhibit meth-induced memory deficits, and 3) show enhanced sensitivity to meth-primed reinstatement.
Methods and Procedures
Subjects
Sixty-six male and female Long-Evans rats (Charles-River) were housed on a reversed 12:12 light-dark cycle in a temperature- and humidity-controlled vivarium. Adult male rats weighed 250-300 g and adult females were 180-200 g at the time of delivery. Rats were individually housed and received ad libitum water throughout the study and 20-30 g of standard rat chow (Harlan, Indianapolis, IN, USA) daily until self-administration stabilized, at which time animals were maintained ad libitum. Procedures were conducted in accordance with the “Guide for the Care and Use of Laboratory Rats” (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 1996) and approved by the IACUC of the Medical University of South Carolina.
Apparatus
Self-administration
Self-administration chambers (30×20×20 cm, Med Associates) were housed inside sound-attenuating cubicles containing two retractable levers, two stimulus lights, a speaker, and a house light. Tubing extended through a spring leash attached to a swivel (Instech) and a balanced metal arm. A 10 ml syringe mounted on a pump outside the sound-attenuating cubicle supplied the drug infusion.
Object recognition
Object recognition testing occurred on a round open field (98-cm diameter, 3.5-thickness, 65 cm above the floor) painted gray. A video camera recorded the sessions. The objects consisted of combinations of a PVC pipe (6.4 × 3.8 cm), a light bulb (8.9 cm), a tennis ball, a plastic bottle 12 × 5 cm), plastic blocks, and a glass jar (4.5 × 2 cm) (adapted from Reichel et al., 2011 & 2012).
Drugs
Methamphetamine hydrochloride was purchased from Sigma (St. Louis, MO, USA). Meth (dissolved in sterile saline) was administered IV at a volume of 17.5-20 μg/50 μl infusion or given IP at doses ranging from 0.01-3.0 mg/kg. Drugs used for anesthesia were ketamine (Vedco Inc, St Joseph, MO, USA), xylazine (Lloyd Laboratories, Shenandoah, IA, USA), equithesin (sodium pentobarbital 4 mg/kg, chloral hydrate 17 mg/kg, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol, 10 % ethanol solution), and ketorolac (Sigma, St. Louis, MO, USA). During self-administration, catheters were flushed with heparin (Elkins-Sinn, Cherry Hill, NJ, USA) and cephazolin (Schein Pharmaceuticals, Florham Park, NJ, USA). Catheter patency was verified with methohexital sodium (Eli Lilly, Indianapolis, IN, USA).
Surgery
Anesthesia consisted of IP injections of ketamine (66 mg/kg), xylazine (1.3 mg/kg), and equithesin (0.5 ml/kg). Ketorolac (2.0 mg/kg, IP) was given before surgery as an analgesic. One end of a silastic catheter was inserted into the external right jugular; the other ran subcutaneously, exited from a small incision on the back, and attached to an infusion harness (Instech Solomon, Plymouth Meeting, PA, USA). Cephazolin (10 mg/0.1 ml) was given post surgery and during recovery along with 0.1 ml 70 U/ml heparinized saline. During self-administration, rats received an IV infusion (0.1 ml) of 10 U/ml heparinized saline before each session. After each session, catheters were flushed with cefazolin and 0.1 ml 70 U/ml heparinized saline.
Methamphetamine self-administration
Following at least 5 days of recovery, rats were given daily 1 hr sessions to self-administer meth on a fixed ratio 1 schedule of reinforcement (The experimental time line is depicted in Fig. 1). Following 7 days of 1-hr/day meth access, half of the rats were given 6-hr/day sessions for 14 days. The other half remained in the 1-hr/day access condition. During the sessions, a response on the active lever resulted in a 2-s infusion and presentation of a light + tone stimulus complex, followed by a 20 s time out. Males received 20 μg and females 17.5 μg meth per bolus to account for body size differences. Responses occurring during the time out and on the inactive lever were recorded without scheduled consequences.
Fig. 1.
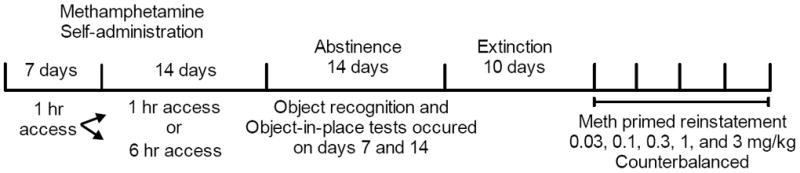
Experimental protocol. All rats experienced 21 days of meth self-administration, memory tests, extinction, and meth primed reinstatement testing.
Abstinence, extinction, and reinstatement
Following meth self-administration, rats were placed into abstinence and subsequent extinction before reinstatement testing. During 14 days of abstinence, rats were transported, handled, weighed, and fed but not placed in the self-administration chamber. Extinction consisted of daily 2-hr sessions for at least 10 days and responding on either lever had no scheduled consequences. Extinction criterion was ≤25 presses for two consecutive days. When criterion was met, rats experienced five counterbalanced meth-primed reinstatement tests (0.03, 0.1, 0.3, 1.0, and 3.0 mg/kg). During the meth-prime tests, responding on either lever had no scheduled consequences. Daily extinction sessions occurred between reinstatement tests.
Object recognition tests during abstinence from meth
During abstinence, rats were tested for object recognition and object-in-place recognition memory on days 7 and 14. In these tasks, yoked saline control rats (1/2 yoked to limited access and 1/2 to extended access) were used as a control group to provide baseline cognitive function measures in drug-naive rats. Half the rats were tested on day 7 for object recognition and half for object-in-place. The rats received the opposite test on day 14 of abstinence. The tasks occurred in 2 phases: familiarization and test. On the object recognition task, familiarization consisted of a 3 min session in which rats explored 2 identical objects. The memory test was conducted 90 min later by allowing rats to explore an object from the familiarization phase and a novel object for 3 min. Both familiar vs. novel objects and object placement were counterbalanced. On the object-in-place task, familiarization occurred during a 5 min session with 4 distinct objects positioned in adjacent corners. A memory test was conducted 90 min later by placing the rat in the apparatus for 3 min with the same objects, except that the position of two objects was changed (denoted throughout as “same” and “changed”, respectively). Behavior was recorded and stored with Noldus tracking software (EthoVision XT 7.0). An observer naive to the experimental conditions scored behavior with the event recorder of the software program. Object exploration was defined as sniffing or touching the object with the nose but not sitting, leaning, or standing on the objects. All objects and the apparatus were wiped down with 70% isopropyl alcohol between tests.
Estrous cycle monitoring
Females underwent habituation for vaginal cytology procedures during self-administration by taking vaginal samples before placement into the operant conditioning chamber. Samples were collected with a sterile saline-dipped pipette tip and smeared onto glass slides, stained with Quik-Dip Hematology Stain (Mercedes Medical, FL), examined using a light microscope set at 10X magnification, and classified according to previously published criteria (Marcondes et al. 2002).
Data analysis
Meth intake (mg/kg), recognition index, and lever presses served as the main dependent measures. For the object recognition test, the recognition index = (amount of time spent with novel objects)/(time spent with both objects). For the object in place test, the recognition index = (the time spent with changed objects)/(time with all objects). Meth intake during self-administration and lever responses during self-administration and extinction were analyzed with a 2 (intake) or 3 (lever presses) factor repeated measures analysis of variance (ANOVA) using General Linear Model in SPSS (Version 17.0). The between groups independent variables were sex (males vs. females), access condition (limited vs. extended access), and/or lever (active vs. inactive). The repeated measure was day (14 daily meth self-administration sessions or 10 extinction sessions). Recognition indices were analyzed with sex × access condition between subjects ANOVA. Reinstatement tests were analyzed with sex × access condition × meth dose ANOVA for the active and inactive levers separately. Tukey tests or Bonferroni adjustments were used for post hoc comparisons, when appropriate. Significance was set at p<0.05 for all tests and all data are presented as the mean ± SEM.
Results
Extended access females self-administer more meth than males
Extended access males (n=12) and females (n=13) showed increased active lever presses over the two weeks of meth self-administration, with females responding more on the meth paired lever than males (Fig. 2, sex main effect F(1,23)=4.76, p<0.05). No differences were found between limited access males (n=9) or females (n=12) active or inactive lever responding. Fig. 3 depicts total meth intake between groups over the self-administration period. Meth intake was similar in limited access rats. Extended access females had higher drug intake (mg/kg) relative to 6 hr males [Fig. 3A, sex main effect, F(1,23)=6.9, p<0.05]. Additionally, meth intake during the 14 days of long access increased for both sexes in the extended access condition [day main effect, F(13,299)=6.9, p<0.001], but sex did not interact with day [F<1]. To determine whether escalation occurred for each sex, separate analyses were conducted for males and females. Females self-administered more meth on days 9-11, 13, and 14 relative to the first day of long access [F(12,156)=5.17, p<0.001 and Bonferroni post hoc]. Males only increased intake on day 14 relative to the first day [F(11,143)=2.46, p<0.005 and Bonferroni post hoc].
Fig. 2.
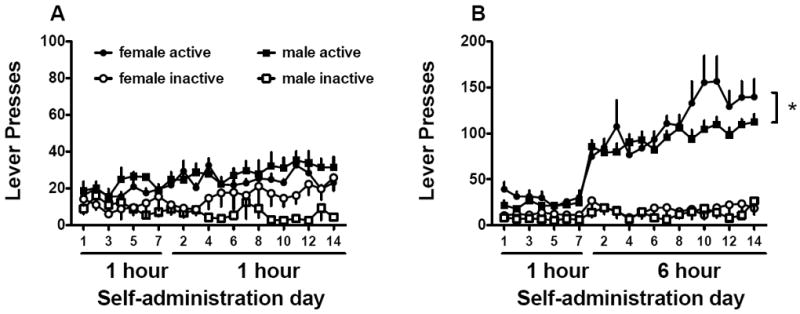
Sex differences in lever pressing during limited or extended meth access conditions. A) Lever presses of males and females in the limited access condition over the first 7 days of 1 hr/day access, followed by an additional 14 days 1 hr access. B) Lever presses of males and females in the extended access condition over the first 7 days of 1 hr/day access, followed by an additional 14 days 6 hr access.
Significant sex differences are indicated (*p<0.05).
Fig. 3.
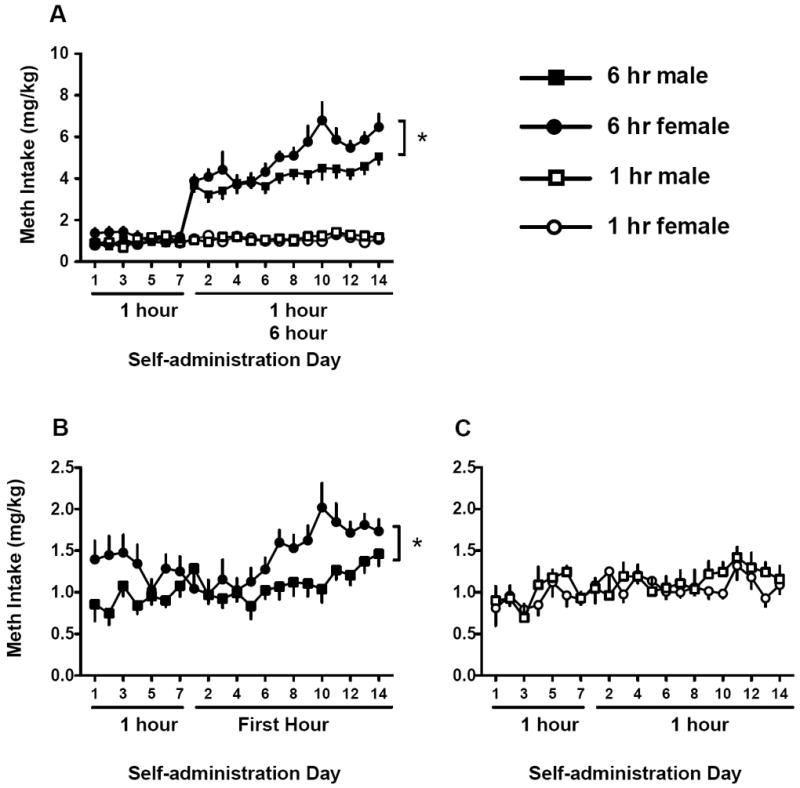
Sex differences in meth intake (mg/kg) during limited or extended meth access conditions. A) Meth intake of males and females over the first 7 days of 1 hr/day access, followed by 14 days of either 1 hr or 6 hr/day access. B) Meth intake during the first hour of the 6-hr meth access period for males and females. C) Meth intake of males and females in the 1-hr access condition. Significant sex differences are indicated (*p<0.05).
To directly compare the extended access to limited access groups, only the first hour of the 6-hr session was used in additional analyses (Fig. 3B and 3C). Over the 14 days, meth intake during the initial hour varied according to access condition and sex [day × access × sex interaction, F(13,546)=2.8, p<0.01]. Although both males and females in the 6 hr groups escalated intake during the first hour across the 14 days [day main effect, F(13,299)=6.2, p<0.001], females took more meth than males [sex main effect, F(1,23)=6.9, p<0.05]. No differences in meth intake were seen in 1-hr access rats (Fig. 3C). Throughout the 21 days of meth access, all groups gained weight at a steady pace and there were no differences in weight gain [mean body weight gain (g) ± SEM: limited access females = 27.6 ± 2.0, extended access females = 35.5 ± 3.0, limited access males = 39.0 ± 8.7, extended access males 30.4 ± 3.1].
Extended access males and females have impaired object recognition memory
During the familiarization phase, rats in control (male n=10; female n=10), 1 hr (male n=7, female n=9), and 6 hr (male n=8, female=13) groups had similar exploration values and approach indices. However, females had greater distance traveled scores than males, regardless of meth access condition (see Supplemental Table 1). Furthermore, values for exploration and approach were not significantly above 0.5 for any group, indicating equal exploration of both objects. Fig. 4A shows the comparisons between males and females in the various access conditions on the object recognition test. Extended access males and females had significantly lower recognition index scores, relative to controls [access condition main effect, F(2,51)=9.4, p<0.0001, and Bonferroni post hoc, p<0.05]. However, there were no sex differences or interaction. In general, males made more approaches to the novel object over the familiar objects than their female counterparts [sex main effect, F(1,52)=9.4, p<0.0001, Table 1], but there was no effect of access condition or an interaction. Females in the 6 hr access condition had higher distance traveled scores than controls [access condition main effect, F(2,57)=6.5, p<0.001] and males [sex main effect, F(1,57)=8.2, p<0.01] during the recognition memory test (Table 2).
Fig. 4.
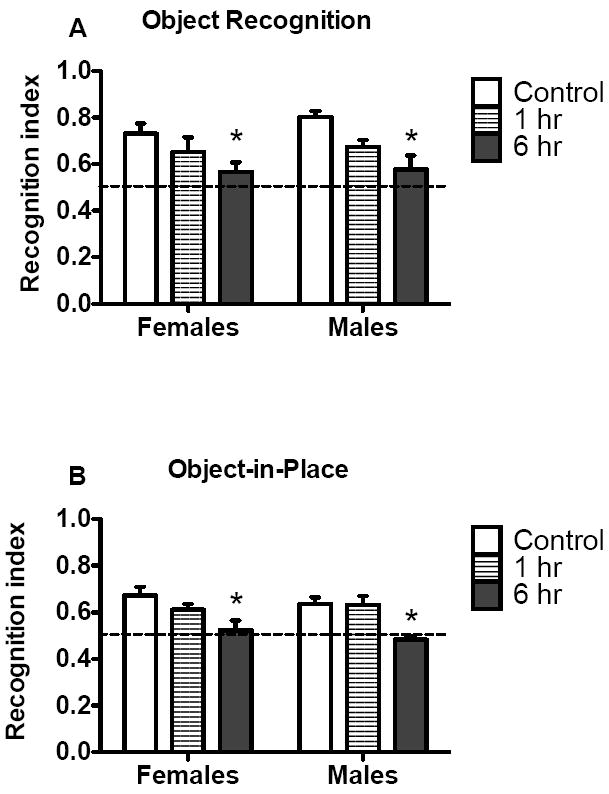
Object recognition and object in place data from rats that self-administered meth in daily 1 or 6 hr session and controls. A) Object recognition index for control, 1 hr, and 6 hr groups on test day. Data are represented as an index between the time spent exploring the novel object/time with novel + familiar objects. B) Object in place recognition index for control, 1 hr, and 6 hr groups on test day. Data are represented as an index between time spent exploring objects in the changed position/time with both sets of objects. Significant differences from control are indicated (*p<0.05).
Table 1.
Index of approach to objects and distance traveled during object recognition testing.
| Approach to Objects (novel / novel + familiar objects)
| ||
|---|---|---|
| Access Condition | Males | Females |
| Control | .58 ± .03 | .43 ± .04 |
| 1 hour | .57 ± .04 | .40 ± .05 |
| 6 hour | .52 ± .05 | .47 ± .03 |
|
| ||
| Distance Traveled (cm)
| ||
| Access Condition | Males | Females |
|
| ||
| Control | 3155 ± 123 | 3236 ± 263 |
| 1 hour | 3254 ± 226 | 3369 ± 319 |
| 6 hour | 3477 ± 377 | 4908 ± 405*† |
Significantly greater than males,
significantly greater than same sex control
Table 2.
Index of approach to objects and distance traveled during object-in-place testing.
| Approach to Objects (changed / changed + same objects)
| ||
|---|---|---|
| Access Condition | Males | Females |
| Control | .49 ± .04 | .54 ± .04 |
| 1 hour | .53 ± .04 | .55 ± .03 |
| 6 hour | .55 ± .03 | .48 ± .03 |
|
| ||
| Distance Traveled (cm)
| ||
| Access Condition | Males | Females |
|
| ||
| Control | 2951 ± 344 | 3525 ± 198 |
| 1 hour | 3551 ± 418 | 3856 ± 341 |
| 6 hour | 3846 ± 350 | 4944 ± 460*† |
Significantly greater than males;
significantly greater than same sex control
Extended access males and females have impaired object-in-place recognition memory
During familiarization, control (male n=10; female n=10), 1 hr (male n=7, female n=9), and 6 hr (male n=8, female=13) groups had similar exploration values and approach indices. However, females had greater distance traveled scores than males, regardless of meth access condition (see Supplemental Table 2). Further, values for exploration and approach were not significantly above 0.5 for any group, indicating equal exploration of all objects. As shown in Fig. 4B, extended access males and females had significantly lower recognition index scores relative to controls [access condition main effect, F(2,50)=10.2, p<0.001, and Bonferroni post hoc, p<0.05]. However, there were no sex differences or interaction, nor were there any differences between sex or access condition on approach to the objects (Table 2). Females in the 6 hr access condition had higher distance traveled scores than controls [access condition main effect, F(2,57)=6.5, p<0.01] and males [sex main effect, F(1,57)=8.2, p<0.01] during the object-in-place memory test (Table 2).
Meth seeking is greater in females relative to males during meth-primed reinstatement
Following 14 days of abstinence, rats were placed back into the self-administration context for at least 10 days of extinction. All groups demonstrated similar extinction responding over the course of 10 days [day effect, F(9,270)=32.38, p<0.0001, data not shown], and no group differences or interactions were evident. Inactive lever responding also decreased across the 10 days for all groups [day effect, F(9,270)=11.07, p<0.0001, data not shown].
During reinstatement testing (Fig. 5), active lever presses for males and females varied according to meth dose and access condition [sex × access condition × dose interaction, F(4,116)=2.56, p<0.05]. For rats in the extended access condition (Fig. 5A), females (n=10) reinstated to a greater degree after a 1 mg/kg meth injection, relative to males (n=8) at the same dose [p<0.05]. In the limited access condition (Fig. 5B), females (n=9) showed greater meth seeking than males (n=6) at the 0.3 mg/kg dose. Females, regardless of access condition, responded more on the inactive lever than males after a 1 mg/kg meth dose [sex × dose interaction, F(4,116)=7.33, p<0.001, and Tukey post hoc, p<0.05]. Females were freely cycling and tested for reinstatement based on extinction criterion, rather than specific estrous cycle phase.
Fig. 5.
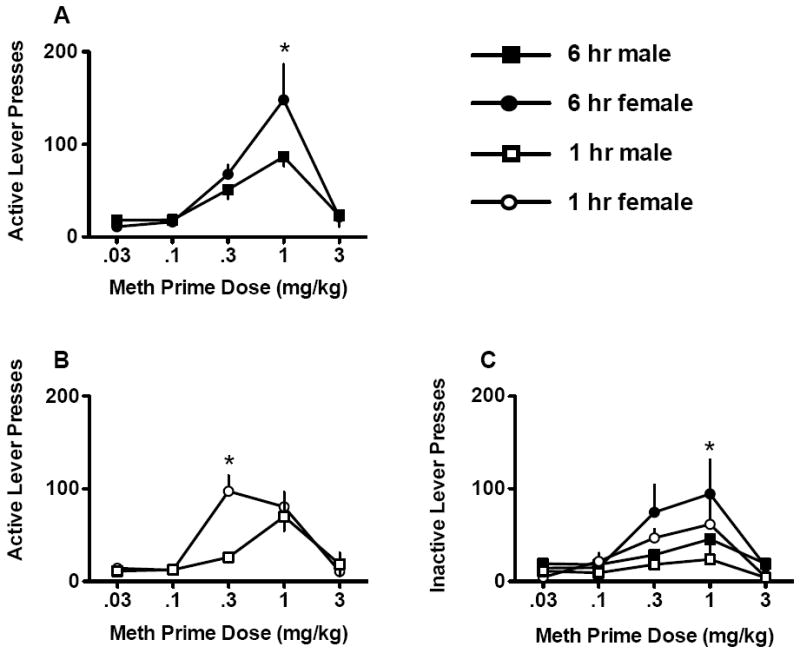
Meth primed reinstatement to meth seeking across a range of priming doses of meth. A) Active lever presses for males and females in the 6 hr access condition. B) Active lever presses for males and females in the 1 hr access condition. C) Inactive lever presses for males and females in both access conditions. Significant sex differences are indicated (*p<0.05).
Discussion
To date, studies have devoted minimal focus on sex differences in methamphetamine addiction using preclinical models of drug taking and seeking (Holtz et al. 2011; Kucerova et al. 2009; Roth and Carroll 2004). None of these previous studies have examined cognitive consequences following chronic meth in females. Here, we found greater sensitivity to the primary reinforcing effects of daily meth in females relative to males as evidenced by greater escalation of meth intake and meth seeking. Interestingly, both sexes were equally impaired on tests of recognition memory during abstinence from extended access meth.
Both sexes escalated intake during daily-extended access meth self-administration conditions with enhanced meth consumption in females relative to males. This sex difference is consistent with previous findings on cocaine and meth intake during extended access conditions (Lynch and Taylor 2004; 2005; Roth and Carroll 2004). Roth and Carroll (2004) reported that more females acquire self-administration and show greater meth intake. In the current study, lever responses and intake were the same for males and females during the initial 7 days of 1 hr access, indicating equal ability to learn the operant behavior and conditioned associations. This difference is likely due to the methodology used during acquisition, as the earlier study was specifically designed to assess acquisition and our study focused on the escalation of meth intake. Taken together, increased drug consumption combined with greater escalation demonstrates that females are more sensitive to the primary rewarding aspects of psychostimulant drugs (Roth et al. 2004). The progression into addiction suggests that alterations in endogenous set points produce deviations from regular use patterns to disordered drug taking (Ahmed and Koob 1999). The sex difference in meth intake only occurred in the long access condition, as males and females on limited daily access (1 hr) took similar amounts of meth and did not escalate their intake, consistent with a previous report in which males and females in a 2 hr daily access condition self-administered similar amounts of meth (Holtz et al. 2011). Interestingly, similar intake between males and females did not engender similar meth seeking during reinstatement testing. Females in both access conditions exhibited increased meth seeking, resulting in a leftward shift in the dose response curve. As we expected, extended access females reinstated to a greater extent than males. However, limited access females also reinstated to a much greater extent relative to males, in spite of similar meth intake during self-administration, an effect also reported after 2 hr limited access conditions (Holtz et al. 2011). Accordingly, these data suggest that females have increased propensity to relapse to meth seeking relative to males, regardless of previous meth use history.
Males and females displayed similar cognitive function on both object recognition tasks, as rats with limited or no meth history had normal recognition memory regardless of sex, whereas extended access males and females exhibited impaired object recognition memory. Importantly, memory performance remained consistent whether rats were tested at 7 or 14 days of abstinence. We have previously shown meth-induced cognitive impairments in males following extended access to self-administered meth on both object recognition (Reichel et al. 2011; Rogers et al. 2008) and object-in-place tasks (Reichel et al. 2012). Importantly, all groups explored and approached the objects equally during familiarization on both tasks, indicating similar interest in novelty reward (Supplemental Tables 1 and 2). Female rats are somewhat impaired on spatial memory tasks relative to males (Simpson and Kelly 2012). The object-in-place task, however, is less reliant on spatial memory or navigation, but instead requires that the rats must remember specific objects in specific locations. When given multiple familiarization sessions and long intertrial intervals (24 hr), females outperform males on object-in-place tasks (Saucier et al. 2008); however, using one familiarization session and a 90 min interval, we observed similar performance on both tasks. As such, determination of sex differences in recognition memory is dependent on task demand, as well as task parameters (Ennaceur et al. 2005; Simpson and Kelly 2012).
Sex played an important role on activity during the sessions, with females exploring the apparatus more than males during familiarization and test. Furthermore, extended access females had higher activity than control females on both tests. This increased activity may simply reflect high levels of nondirected locomotion that was also evident during self-administration and reinstatement testing, as seen by high inactive lever responding. Whether non-discriminated lever responding is specific to meth or extends to other rewards remains to be determined. We previously found that extended access to meth did not increase locomotor activity in males during object recognition testing (Reichel et al. 2012; Reichel et al. 2011; Rogers et al. 2008), consistent with the current findings. Increased motor activity in the extended access females renders the object recognition deficit potentially subject to an alternative interpretation rather than solely due to memory impairment. For example, locomotor activity in an open field can also reflect anxiety. However, increased anxiety in the extended access females is unlikely, as they did not differ from female controls during the familiarization session when the environment was more novel. In addition, no differences were found in object interaction or approach that would indicate anxiety. Alternatively, locomotor activity may have served as a competitive behavior to object exploration. This scenario is also unlikely because approach to the objects showed no difference between controls and the long access females during the test. Taken together, memory deficits are the most parsimonious explanation of object recognition memory performance in both males and females.
The current study used freely cycling female rats to ascertain sex differences in motivational and cognitive factors following meth self-administration without gonadectomy and artificial manipulation of ovarian hormones. Intact animals of both sexes allow for closer parallels with human meth addicts. Consequently, object recognition tests were conducted based on abstinence day and reinstatement tests were based on extinction criterion, rather than specific cycle phase. We did, however, collect vaginal lumen samples to ensure representation of each cycle phase on each test (Supplemental Table 3). Estrous cycle has been shown to influence object recognition and object location memory (Paris and Frye 2008), cocaine self-administration, and reinstatement (Feltenstein et al. 2009; Feltenstein and See 2007; Lynch et al. 2000; Roberts et al. 1989). In general, females responded more during cocaine-primed reinstatement while in vaginal estrus, when estrogen levels are low (Fuchs et al. 2005; Kippin et al. 2005). In contrast, no estrous cycle effects were seen for nicotine-primed reinstatement in females with a history of nicotine self-administration (Feltenstein et al. 2011). Although the number of subjects per estrous cycle phase was limited in the current study, we did not see any clear pattern of cycle effects on meth-primed reinstatement. A full determination of specific cycle effects would require substantially more female subjects and future studies may focus specifically on the influence of estrous cycle in meth seeking. Such research in warranted as ovariectomized females self-administered less meth than ovariectomized females with estrogen replacement (Kucerova et al. 2009), indicating a potential role for gonadal hormones in meth addiction.
In conclusion, relative to males, females escalate meth intake to a greater extent during extended access conditions, show greater reinstatement to meth seeking regardless of prior meth history, and show equivalent degrees of object memory impairment. Given the complex nature of sex differences in meth addiction and relapse, future studies are warranted to determine the biological basis of these sex differences, as well as the relationship of motivational and cognitive factors in meth addiction in both males and females. By determining the nature of these factors, sex specific therapies may be targeted at meth-induced deficits and for relapse prevention.
Supplementary Material
Acknowledgments
This research was supported by NIDA grants DA016511 (RES), DA022658 (RES), HD055885 (CMR), Specialized Center on Research-Pilot Research Grant (CMR), and NIH grant C06 RR015455. The authors thank Meghin Gilstrap, Christina Johnson, and Melza van Roijen for technical assistance.
References
- Ahmed SH, Koob GF. Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology. 1999;146:303–12. doi: 10.1007/s002130051121. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology. 2000;22:413–21. doi: 10.1016/S0893-133X(99)00133-5. [DOI] [PubMed] [Google Scholar]
- Berlyne DE. Novelty and curiosity as determinants of exploratory behaviour. British Journal of Psychology. 1950;41:68–80. [Google Scholar]
- Brecht M-L, O’Brien A, von Mayrhauser C, Anglin MD. Methamphetamine use behaviors and gender differences. Addict Behav. 2004;29:89–106. doi: 10.1016/s0306-4603(03)00082-0. [DOI] [PubMed] [Google Scholar]
- Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29:929–42. doi: 10.1038/sj.npp.1300387. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Berry D, Milstein JA, Lääne K, Everitt BJ, Robbins TW. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005;30:525–37. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE, Liu B. Gender differences in methamphetamine use and responses: a review. Gender Medicine. 2008;5:24–35. doi: 10.1016/s1550-8579(08)80005-8. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: Methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Michalikova S, Bradford A, Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav Brain Res. 2005;159:247–66. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Byrd EA, Henderson AR, See RE. Attenuation of cocaine-seeking by progesterone treatment in female rats. Psychoneuroendocrinology. 2009;34:343–52. doi: 10.1016/j.psyneuen.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, Ghee SM, See RE. Nicotine self-administration and reinstatement of nicotine-seeking in male and female rats. Drug Alcohol Depend. 2012;121:240–246. doi: 10.1016/j.drugalcdep.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–9. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179:662–72. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Markou A, Kuczenski R. Extended access to methamphetamine self-administration affects sensorimotor gating in rats. Behav Brain Res. 2011;217:386–90. doi: 10.1016/j.bbr.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698:46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Lozama A, Prisinzano TE, Carroll ME. Reinstatement of methamphetamine seeking in male and female rats treated with modafinil and allopregnanolone. Drug Alcohol Depend. 2011;120:233–237. doi: 10.1016/j.drugalcdep.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hser YI, Evans E, Huang YC. Treatment outcomes among women and men methamphetamine abusers in California. J Sub Abuse Treatment. 2005;28:77–85. doi: 10.1016/j.jsat.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology. 2005;182:245–52. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio S, Koob G, Pulvirenti L. Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology. 2006;186:48–53. doi: 10.1007/s00213-006-0353-z. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Sanchez H, Zhang XY, Kehoe P. Neonatal isolation enhances acquisition of cocaine self-administration and food responding in female rats. Behav Brain Res. 2004;151:137–49. doi: 10.1016/j.bbr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Kucerova J, Vrskova D, Sulcova A. Impact of repeated methamphetamine pretreatment on intravenous self-administration of the drug in males and estrogenized or non- estrogenized ovariectomized female rats. Neuro Endocrinol Lett. 2009;30:663–70. [PubMed] [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–9. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology. 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology. 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–51. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Taylor JR. Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology. 2005;30:927–35. doi: 10.1038/sj.npp.1300656. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol. 2002;62:609–14. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold L, Caine S, Schulteis G, Koob G. Animal models of drug craving. Psychopharmacology. 1993;112:163–182. doi: 10.1007/BF02244907. [DOI] [PubMed] [Google Scholar]
- Milesi-Hallé A, McMillan DE, Laurenzana EM, Byrnes-Blake KA, Owens SM. Sex differences in (+)-amphetamine- and (+)-methamphetamine-induced behavioral response in male and female Sprague-Dawley rats. Pharmacol Biochem Behav. 2007;86:140–9. doi: 10.1016/j.pbb.2006.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–15. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian A, Glen WB, Lavin A, See RE. Methamphetamine self-administration produces attentional set-shifting deficits and alters prefrontal cortical neurophysiology in rats. Biol Psychiatry. 2011;69:253–259. doi: 10.1016/j.biopsych.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport. 2003;14:2229–2232. doi: 10.1097/00001756-200312020-00019. [DOI] [PubMed] [Google Scholar]
- Rawson R, Gonzales R, Obert JL, McCann MJ, Brethen P. Methamphetamine use among treatment-seeking adolescents in Southern California: participant characteristics and treatment response. J Sub Abuse Treatment. 2005;29:67–74. doi: 10.1016/j.jsat.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Reichel CM, Ramsey LA, Schwendt M, Mcginty JF, See RE. Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology. 2012;62:1119–26. doi: 10.1016/j.neuropharm.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Schwendt M, McGinty JF, Olive MF, See RE. Loss of object recognition memory produced by extended access to methamphetamine self-administration is reversed by positive allosteric modulation of metabotropic glutamate receptor 5. Neuropsychopharmacology. 2011;36:782–92. doi: 10.1038/npp.2010.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Rogers J, Santis S, See R. Extended methamphetamine self-administration enhances reinstatement of drug seeking and impairs novel object recognition in rats. Psychopharmacology. 2008;199:615–624. doi: 10.1007/s00213-008-1187-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Carroll ME. Sex differences in the acquisition of IV methamphetamine self-administration and subsequent maintenance under a progressive ratio schedule in rats. Psychopharmacology. 2004;172:443–9. doi: 10.1007/s00213-003-1670-0. [DOI] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neurosci and Biobehav Rev. 2004;28:533–46. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G. Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn. 2008;11:129–37. doi: 10.1007/s10071-007-0096-1. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Bross JG, Thorndike EB. Gender differences in the behavioral effects of methamphetamine. Eur J Pharmacol. 2002;442:231–5. doi: 10.1016/s0014-2999(02)01550-9. [DOI] [PubMed] [Google Scholar]
- Schwendt M, Rocha A, See R, Pacchioni A, Mcginty J, Kalivas P. Extended methamphetamine self-administration in rats results in a selective reduction of dopamine transporter levels in the prefrontal cortex and dorsal striatum not accompanied by marked monoaminergic depletion. J Pharma Exp Ther. 2009;331:555. doi: 10.1124/jpet.109.155770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. An investigation of whether there are sex differences in certain behavioural and neurochemical parameters in the rat. Behav Brain Res. 2012;229:289–300. doi: 10.1016/j.bbr.2011.12.036. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Brown MW. Findings from animals concerning when interactions between perirhinal cortex, hippocampus and medial prefrontal cortex are necessary for recognition memory. Neuropsychologia. 2009;48:2262–2272. doi: 10.1016/j.neuropsychologia.2009.12.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


