Abstract
Purpose
To inhibit DNA double-strand break repair in tumor cells by delivery of a single chain antibody variable region fragment (ScFv 18-2) to the cell nucleus. ScFv 18-2 binds to a regulatory region of the DNA-dependent protein kinase (DNA-PK), an essential enzyme in the nonhomologous end-joining pathway, and inhibits DNA end-joining in a cell-free system and when microinjected into single cells. Development as a radiosensitizer has been limited by the lack of a method for intranuclear delivery to target cells. Here we investigate a delivery method based on folate receptor-mediated endocytosis.
Methods and Materials
A recombinant ScFv 18-2 derivative was conjugated to folate via a scissile disulfide linker. Folate-ScFv 18-2 was characterized for its ability to be internalized by tumor cells and to influence behavior of ionizing radiation-induced repair foci. Radiosensitization was measured in a clonogenic survival assay. Survival curves were fitted to a linear-quadratic model and between-group differences were evaluated by an F test. Sensitization ratios were determined based on mean inhibitory dose.
Results
Human KB and NCI-H292 lung cancer cells treated with the folate-conjugated scFv show significant radiosensitization (P<0.001). Sensitization enhancement ratios were 1.92 ± 0.42 for KB cells and 1.63 ± 0.13 for NCI-H292 cells. Studies suggest that treatment inhibits repair of radiation-induced DSBs, as evidenced by the persistence of γ-H2AX-stained foci and by inhibition of staining with anti-DNA-PKcs phosphoserine 2056.
Conclusions
Folate-mediated endocytosis is an effective method for intranuclear delivery of an antibody-derived DNA repair inhibitor.
Keywords: Radiosensitization, scFv, DNA-dependent protein kinase, folate-mediated delivery, nonhomologous end joining, DNA repair
Introduction
The DNA-dependent protein kinase (DNA-PK) regulates non-homologous end joining, the main pathway for repair of DNA double-strand breaks induced by clinically relevant doses of ionizing radiation. Loss of DNA-PKcs function results in radiosensitization (1). Conversely, increased levels of DNA-PK activity correlate with tumor cell radioresistance (2). DNA-PK inhibitors are thus potential radiosensitizers (3).
We previously described a single chain antibody variable fragment (ScFv 18-2) that binds to the DNA-PK catalytic subunit (DNA-PKcs) and inhibits DSB repair activity in a cell-free system (4). Microinjection of ScFv 18-2 into single human cells sensitizes them to an otherwise sublethal (1.5 Gy) dose of radiation (4).
The properties of ScFv 18-2 suggest that it might be useful as a radiosensitizer. Therapeutic agents based on antibodies and antibody fragments are widely used, but are generally directed against molecules present on the exterior surface of tumor cells. By contrast, DNA-PKcs is separated from the extracellular milieu by the plasma membrane and the nuclear envelope. Prior work has shown that transferrin and folate receptors can be used to deliver proteins and nanoparticles into tumor cells (5, 6). Receptor-mediated delivery is attractive because of its demonstrated potential for clinical translation (6). We hypothesized that receptor-mediated endocytosis might serve as an effective means for delivery of ScFv 18-2 as a radiosensitizer. We describe here synthesis and characterization of a folate-conjugated ScFv 18-2 derivative. Folate-ScFv is internalized by human cancer cells, enters the nucleus, and sensitizes cells to ionizing radiation.
Methods and Materials
ScFv expression, purification, and conjugation
ScFv 18-2 was derived from the anti-DNA-PKcs monoclonal antibody, mAb 18-2 (7). The MBP-ScFv 18-2 NLS LC2 derivative (8) or ovalbumin control protein were incubated with 10- to 20-fold molar excess of Traut’s reagent (2-iminothilane HCl, Pierce Biotechnology, Rockford, IL (9)). Products were isolated by PD-10 desalting chromatography (GE Healthcare, Piscataway, NJ), reacted with a 10-fold excess of folate-hydrazido-(2-pyridyldithiopropionate) (folate-SS-Pyr) for 1 h at room temperature (Fig. 1a), then desalted again.
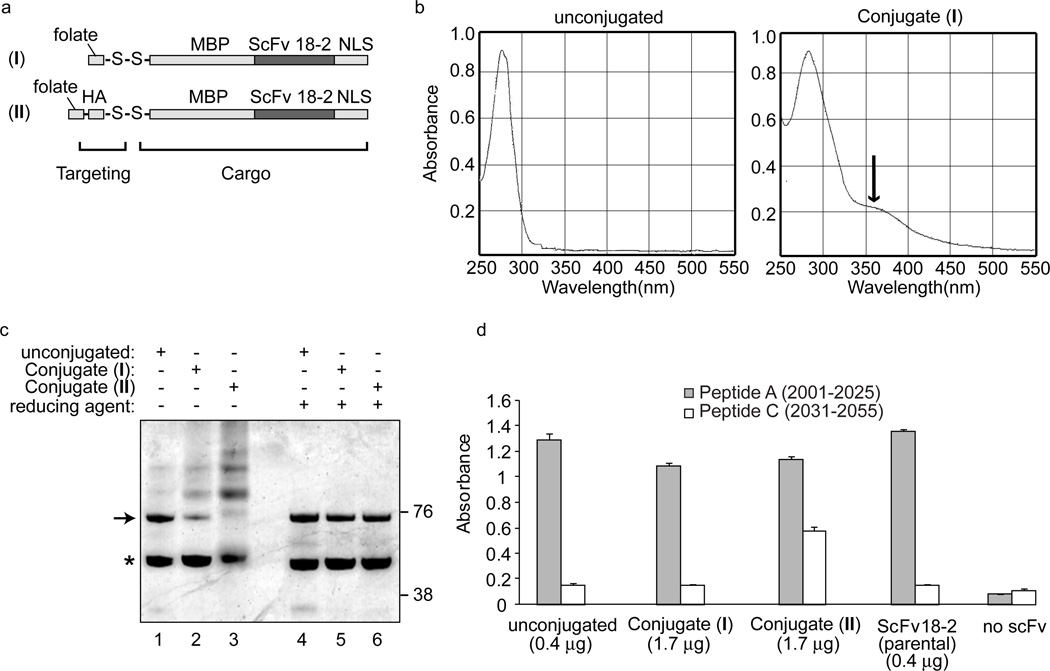
Figure 1. Folate conjugation.
a. Strategy. ScFv 18-2 NLS or ovalbumin were conjugated via a disulfide bond to folate or a folate-hemagglutinin peptide conjugate. b. Spectra of ScFv 18-2 before and after conjugation. Arrow denotes folate absorbance. c. SDS-PAGE analysis of folate-scFv conjugates. Arrow denotes unmodified MBP-ScFv 18-2 NLS; asterisk denotes free MBP contaminant. Molecular weight markers are indicated in kDa. Dithiothreitol (50 mM) was present in sample buffer where indicated (reducing agent). d. Peptide ELISA performed as described in Methods and Materials using indicated proteins.
Alternatively, folate-N-hydroxy succinimide (folate-NHS) (10) was coupled to the N-terminus of an endosome-disruptive hemagglutinin (HA) peptide (GLFGAIAGFIENGWEGMIDGC). The product was separated by Sephadex G-15 chromatography (GE Healthcare). ScFv or ovalbumin was activated with succinimidyl-3-(2-pyridyldithiopropionate) (SPDP, Thermo Scientific, Rockford, IL), incubated with 4-fold excess of folate-HA, and the conjugates were separated by Sephadex G-50 chromatography (GE Healthcare). Peptide enzyme-linked immunosorbent assay was performed as described (4) using Peptide A (DNA-PKcs residues 2001–2025, biotin-KKKYIEIRKEAREAANGDSDGPSYM) or Peptide C (DNA-PKcs residues 2031–2055, biotin-LADSTLSEEMSQFDFSTGVQSYSYS).
Cell culture, flow cytometry, and imaging
KB cells (ATCC # CCL-17) were grown in folate-free RPMI 1640 medium with 10% FBS. The KB cell line was originally thought to be derived from an oral carcinoma, but was subsequently found to have been established via HeLa cell contamination. NCI-H292 (ATCC # CRL-1848) mucoid epidermoid non small cell lung cancer cells were grown in RPMI 1640 medium with 10% FBS. For flow cytometry, cells were fixed with 2% paraformaldehyde, blocked with 2% BSA, and incubated with anti-folate receptor mAb (Mov18/ZEL, 1:200, Alexis Biochemicals, San Diego, CA) and FITC-goat anti-mouse immunoglobulin F(ab)2 (DAKO Cytomation Glostrup, Demark). FITC-ScFv 18-2 NLS was prepared by incubating ScFv 18-2 with 5-fold excess FITC (Invitrogen) at pH 8.0 for 30 min at 37 °C followed by PD-10 chromatography. For immunofluorescence, cells were seeded in 8-well slides (Lab-Tek Chamber Slide, Nunc, Rochester, NY). Staining was performed using rabbit anti-E tag (1:200, Abcam, Cambridge, MA) or mouse anti-DNA-PKcs mAb-2 (Clone 25-4, 1:100, Thermo Scientific, Fremont, CA) with Alexa Fluor 594-anti-rabbit IgG, or Alexa Fluor 488-anti-mouse IgG secondary antibodies (Molecular Probes, Inc., Eugene, OR). Slides were mounted using VECTASHIELD medium with DAPI (Vector Laboratories, Burlingame, CA. Staining of repair foci was performed using rabbit anti-DNA-PKcs pS2056, anti-DNA-PKcs pT2638 (1:500, Abcam, Cambridge, MA) or anti-γ-H2AX (1:500, clone JBW301, Millipore, Billerica, MA).
Clonogenic survival assays
Subconfluent cultures of KB or NCI-H292 cells were trypsinized and aliquots of 4–5 × 105 cells were distributed to 15 ml centrifuge tubes. Cells were washed with PBS, collected by centrifugation (1000 rpm for 5 min), resuspended in 500 µl of 100 µg/ml folate-protein, 2% BSA in folate-free RPMI 1640, and incubated at 37 °C for 3–4 h. Cells were washed with 10 ml of medium, collected, resuspended in 1 ml complete medium, and divided among triplicate T-25 flasks for 137Cs irradiation. Colonies were counted after 7 to 9 days. Survival curves were fitted to a linear quadratic model and differences between ScFv 18-2-treated and control groups were evaluated based on an F test (11). Mean inactivation dose was calculated by numeric integration (12).
There was some variability in recovery of viable cells following ScFv 18-2 treatment. Nominal plating efficiencies of non-irradiated cells were 15 – 37% for KB cells and 30 – 57% for H292 cells. This approximately 2.5-fold variation does not affect calculations of radiation survival, which are based on a ratio of colonies formed by irradiated and nonirradiated cells within an scFv treatment group.
Results
Synthesis of folate-conjugated ScFv 18-2
The receptor-mediated delivery strategy is based on the principle of joining two moieties via a scissile disulfide bond: a cargo moiety and a targeting moiety (Fig. 1a). After delivery, the disulfide linker undergoes cleavage in the intracellular reducing environment (13). The cargo moiety is MBP-ScFv 18-2-NLS, a derivative of ScFv 18-2 that contains a maltose binding protein (MBP) tag to promote expression as a soluble, periplasmic protein in E. coli and a nuclear localization signal to promote nuclear uptake (8). The targeting moiety is folic acid. To create conjugate (I), purified ScFv 18-2 was reacted with Traut’s reagent, then with folate-SS-Pyr as described in Methods and Materials. To create conjugate (II), SPDP-activated scFv was joined via a disulfide bond to a folate-coupled hemagglutinin (HA) cysteine peptide. The HA peptide is an endosome disruptor that may facilitate release of the cargo protein from the endosomal compartment following internalization (14).
Folate conjugation was evaluated by ultraviolet spectroscopy (Fig. 1b). Based on an absorbance peak at 360 nm, approximately 3–5 mol folate were present per mol of protein. The purity and oligomeric state of the ScFv 18-2, before and after conjugation, was evaluated by SDS-PAGE in the absence of reducing agent (Fig. 1c, lanes 1–3). Before conjugation, the major species were the 72.5 kDa MBP-ScFv 18-2 NLS (arrow) and a free MBP contaminant that arises during E. coli expression (asterisk) (8). With preparations of conjugates I and II, higher molecular weight products were observed, which likely reflect both disulfide-mediated oligomerization and folate coupling (lanes 2–3). The higher molecular weight species were reduced to their constituent polypeptides by heating in the presence of dithiothreitol, consistent with the presence of scissile disulfide linkages (lanes 4–6).
Binding to DNA-PKcs was evaluated by ELISA (Fig. 1d). Peptide A represents the ScFv 18-2 epitope and Peptide C represents a non-epitope DNA-PKcs sequence (4). Four proteins were evaluated: unconjugated MBP-ScFv 18-2 NLS, Conjugates I and II, and parental ScFv 18-2. Binding was observed in all cases; however approximately four times more of Conjugates I and II was needed to achieve the same level of binding as was seen with unconjugated ScFv 18-2, indicating four-fold loss of activity during conjugation. There was little binding to the control peptide except with Conjugate II where this nonspecific binding was significantly elevated (Fig. 1d).
Uptake of ScFv 18-2 conjugates
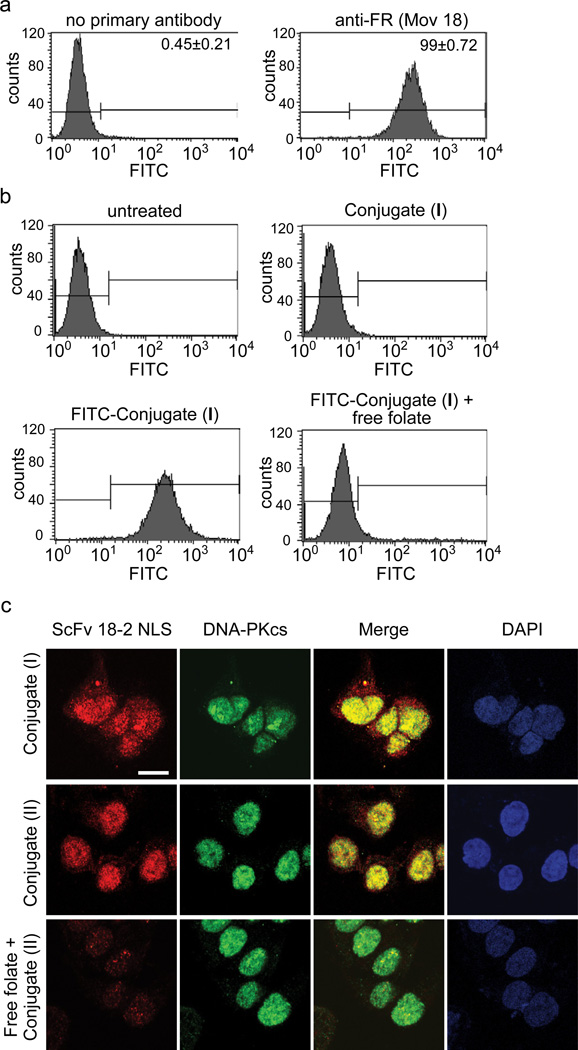
KB cells, which are well-studied folate receptor (FR)-α-positive human cancer cells, were used for uptake studies (6). FR-α status was confirmed by fluorescence-activated cell sorting using anti-folate receptor antibody (Fig. 2a). Binding of folate-ScFv 18-2 was investigated using a fluorescein isothiocyanate (FITC)-labeled derivative of Conjugate (I). This bound to the surface of KB cells and was competed by free folate, indicating folate-dependent interaction (Fig. 2b). Internalization and nuclear uptake were investigated by indirect immunofluorescence. Visual inspection indicates that both Conjugate (I) and Conjugate (II) entered the cells and were localized primarily in the nucleus (Fig. 2c). Somewhat more Conjugate (I) than Conjugate (II) was retained in the cytoplasm, consistent with the presence of an endosomal disruptor peptide in the latter, but the difference was slight. Staining of nuclear ScFv 18-2 and DNA-PKcs was coincident, consistent with binding of the intranuclear ScFv 18-2 to their targets. ScFv 18-2 uptake was competed by free folate, consistent with the results of the FACS analysis in Fig. 2b and indicating a FR-mediated mechanism of internalization. Because Conjugates I and II were nearly equivalent, and Conjugate (I) was easier to prepare, it was used for subsequent functional experiments.
Figure 2. Folate receptor mediated binding, uptake, and internalization.
a. FACS analysis of FR-α status for KB cells using anti-folate mAb. b. FACS analysis using FITC-Conjugate (I) or indicated control protein. Free folate competitor (1 mM) was present where indicated. c. Immunofluorescence analysis of KB cells incubated with indicated proteins in presence or absence of folate competitor. Representative confocal image is shown. Experiment was repeated three times with similar results. Scale bar is 10 µm. Staining with anti-E tag, anti-DNA-PKcs, and counterstaining with DAPI.
Folate-ScFv 18-2 treatment sensitizes cells to radiation
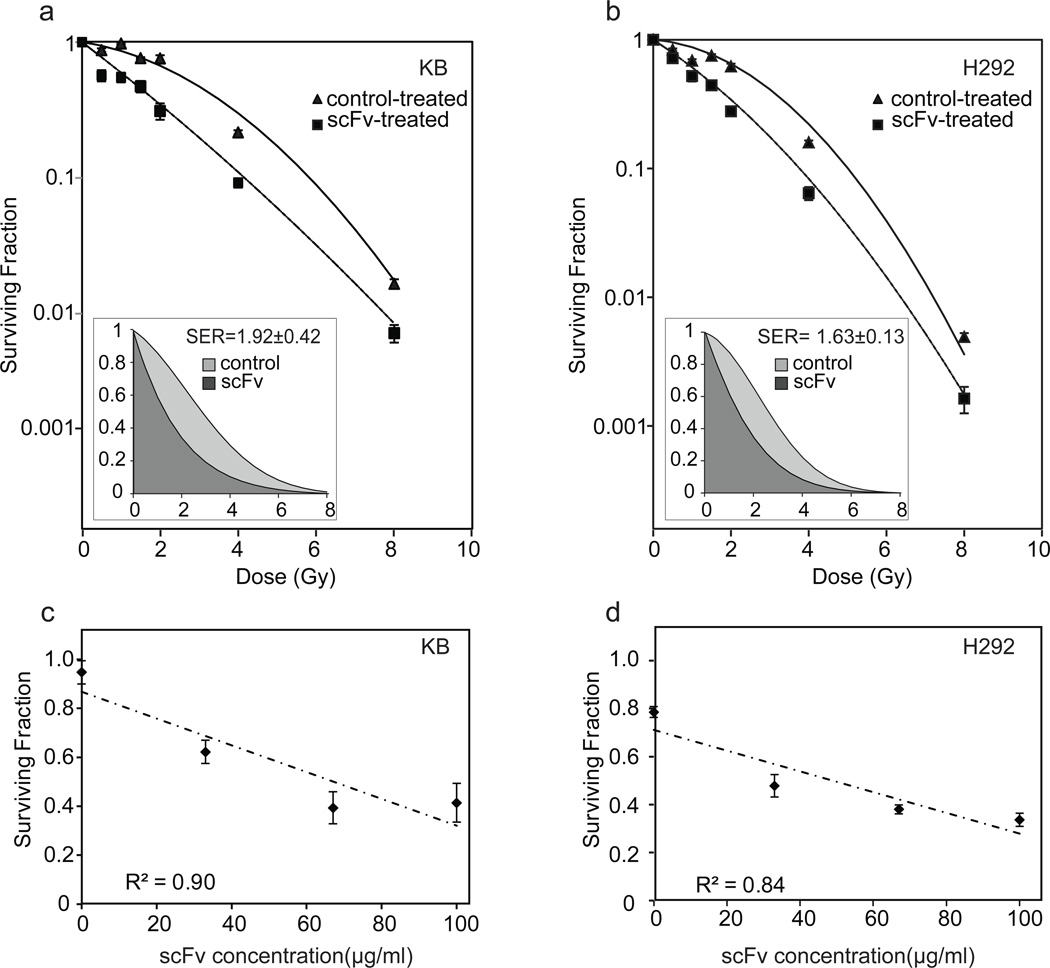
Clonogenic survival assays were performed to measure the effect of MBP-ScFv 18-2 NLS treatment on radiation sensitivity. Studies were performed in KB cells and NCI-H292 cells, a FR-α positive human nonsmall lung cancer (NSCLC) line. Cells were treated in suspension with control protein or Conjugate I, plated, irradiated, and incubated to allow colony formation. Plating efficiency at each radiation dose was compared with plating efficiency in the absence of radiation to determining surviving fraction. Folate-ScFv 18-2 treatment reduced survival, relative to control treatment, in both cell lines and at every radiation dose tested (Fig. 3 a,b). Data were fitted to a linear-quadratic model using weighted, stratified linear regression (11). The difference between treatment groups was highly significant (P<0.001)) based on an F test. Mean inactivation dose (MID), determined as the integral of surviving fraction expressed in linear coordinates, provides a robust measure of radiosensitivity (12). Sensitivity enhancement ratios based on MID were 1.92±0.42 in KB cells and 1.63±0.13 in NCI-H292 cells, indicative of a strong radiosensitization effect (Fig. 3 a,b, inset). Survival at a clinically relevant 2 Gy dose was ScFv 18-2 concentration dependent, further demonstrating the robustness of the effect (Fig. 3 c, d).
Figure 3. Clonogenic survival assays.
a,b. Surviving fraction as a function of radiation dose for indicated cell lines treated with 100 µg/ml folate-ovalbumin or folate-ScFv 18-2 Conjugate (I) as indicated. Data were fitted to a linear-quadratic model by weighted, stratified linear regression. Inset shows surviving fraction plotted on a linear scale, with shading to indicate area under curves, which provides mean inactivation dose. Ratio of areas gives sensitization enhancement ratio. c,d. Surviving fraction as a function of folate-ScFv 18-2 concentration. Cells were treated with mixtures of folate-ovalbumin and Conjugate (I) such that total folate-protein concentration was held constant at 100 µg/ml for all groups. Each panel shows one representative experiment. Values are mean of three replicates, with standard errors shown. Experiment was performed twice with each cell line with similar results.
There was no evidence for single-agent toxicity of folate-scFv within the dose range used. Averaged across all experiments, nominal plating efficiency in the absence of radiation was 36 ± 8% for scFv-treated cells and 32 ± 16% for folate-ovalbumin treated cells. The difference is not significant based on a paired, 2-tailed t-test (P=0.68). As explained in Methods and Materials, comparisons between scFv dose groups were subject to more error than comparisons of irradiated and non-irradiated cells within an scFv dose group. Because of the rather broad error limits, the finding that the scFv had no effect as a single agent should be regarded as preliminary.
Effect of MBP-ScFv 18-2 NLS treatment on repair foci
We hypothesized that the radiosensitization observed with Conjugate I was attributable to interference with DNA-PKcs repair function. DSB repair occurs within cytologically detectable foci. Numerous phosphorylation and other post-translational modification events occur within these. If Conjugate I interferes with progression of DSB repair, this should be detectable by altered patterns of immunostaining with antibodies directed against specific phospho-epitopes in the repair foci.
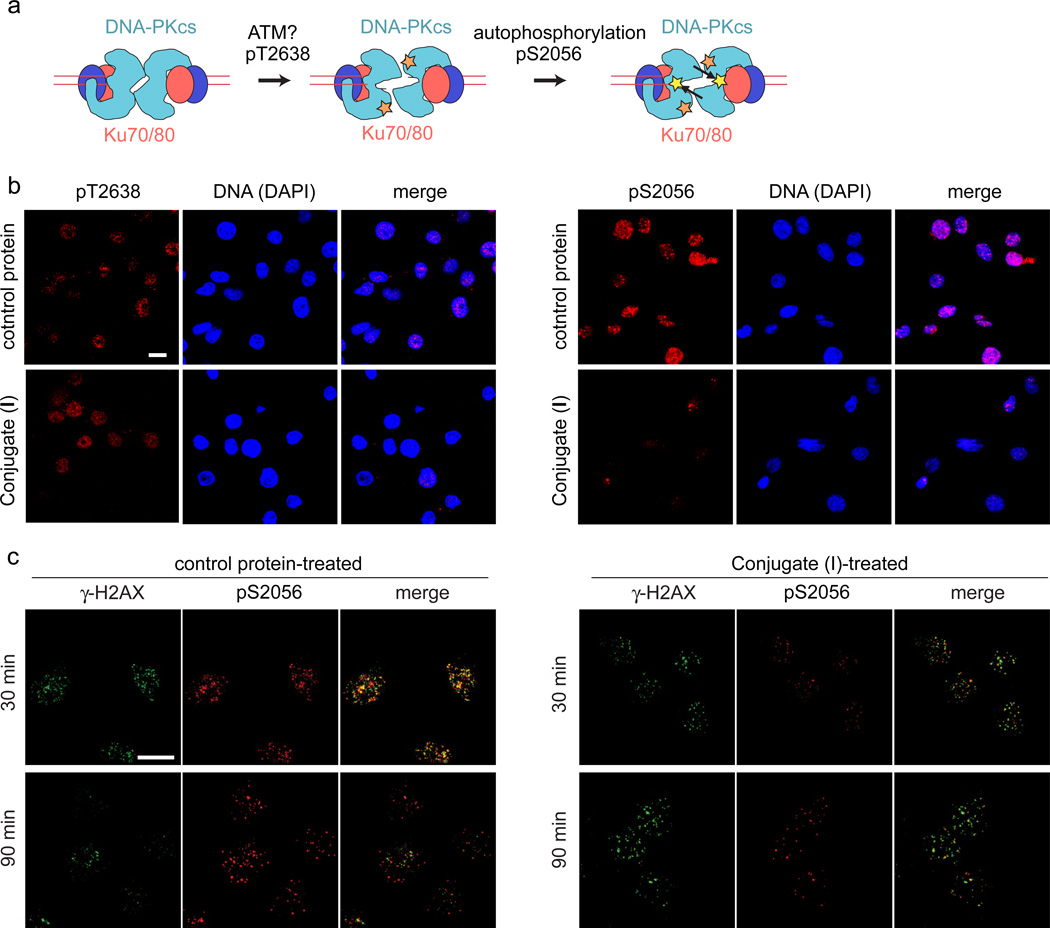
We performed immunostaining with antibodies directed against phosphorylation sites in DNA-PKcs. Although the pattern of DNA-PKcs phosphorylation is complex (1), phosphorylation of six sites spanning residues 2609–2647 is believed to be an early event, which is followed by phosphorylation of additional sites spanning residues 2023–2056 (Fig. 4a). Modification in the second cluster of sites occurs by DNA-PKcs autophosphorylation and may be associated with increased accessibility of DNA ends to processing enzymes (15). We irradiated ScFv 18-2- and control-treated KB cells at 0.5 Gy and were stained with antibodies to DNA-PKcs pT2638 (16) and pS2056 (17) after a 1 h recovery. Folate-ScFv 18-2 treatment did not affect immunostaining with anti-pT2638 (Fig. 4b, left), but markedly decreased immunostaining with anti-pS2056 (Fig. 4b, right). The latter site lies quite near the ScFv 18-2 epitope at residues 2000–2025. The ScFv 18-2 may interfere with phosphorylation at sites in the 2023–2056 cluster. Alternatively or in addition, the presence of bound ScFv 18-2 may hinder detection of phosphorylated residues in the immunostaining assay.
Figure 4. Effect of ScFv 18-2 treatment on DSB repair foci.
a. DNA-PK phosphorylation pathway. a. Schematic showing DNA-PK complexes at DNA ends, with Ku and DNA-PKcs indicated. Phosphorylation occurs first at a cluster of sites including residue 2638 (orange star) and is mediated in part by ATM kinase, then at a second cluster of sites including residue 2056 (yellow stars) by autophosphorylation (arrows). b. Immunofluorescence analysis of KB cells treated with 100 µg/ml of indicated proteins for 3 to 4 h, irradiated at 0.5 Gy, and allowed to recover for 1 h. Cells were stained with indicated phospho-specific antibodies and counterstained with DAPI. c. Immunofluorescence analysis of KB cells treated at 1 Gy and allowed to recover for 30 or 90 min. Scale bars are 10 µm.
To further confirm the effect of ScFv 18-2 treatment, we performed staining for the modified histone, γ-H2AX, a marker of unrepaired DSBs that peaks 10 to 30 min post-irradiation and diminishes over several hours, reflecting DSB repair and a return to cellular homeostasis (18). We irradiated cells at 1 Gy, allowed 30 or 90 min of recovery, then performed double immunostaining with anti-pS2056 (red) and anti-γ-H2AX (green) (Fig. 4c). In merged images of control protein-treated cells at 30 min post-irradiation, numerous dual-stained (yellow) foci are present, whereas at later times, as γ-H2AX diminishes, red (anti-S2056) foci predominate (Fig. 4c, left panels). In ScFv 18-2-treated cells, green (anti-γ-H2AX) foci predominate at 30 min and persist at 90 min (Fig. 4c, right panels). The differences in staining pattern confirm the deficit in anti-S2056 staining. Differences between treatment groups at the 90 minute timepoint are suggestive of a delay in DSB repair, although further studies of repair dynamics will be needed to confirm this. We saw a similar persistence of γ-H2AX staining scFv 18-2-microinjected cells (4).
Discussion
We describe an approach based on folate receptor-mediated endocytosis for intranuclear delivery of an antibody-derived radiosensitizer, ScFv 18-2. Our previous study of ScFv 18-2 was based on results in single, microinjected cells. The current study is based on results in a bulk cell population, which allowed for more accurate measurement of effects of clonogenic survival. The folate conjugated-ScFv 18-2 had a highly significant effect on survival and a robust sensitization enhancement ratio. Under the conditions used, we did not observe significant toxicity of folate-scFv as a single agent.
ScFv 18-2 binds with nanomolar affinity to an epitope unique to DNA-PKcs. The parent mAb and the derived scFv have no immunological cross-reactivity with other human proteins (4, 7). It is thus unlikely that ScFv 18-2 will have off-target effects on other kinases. This differs from small molecule inhibitors of DNA-PKcs, which target a catalytic domain that shares homology with other PI-3 kinase family members (19), reviewed in (3).
Mechanistic studies here and earlier (4) suggest that ScFv 18-2 sensitizes tumor cells by delaying DSB repair. An agent that delays or blocks DSB repair should potentiate the intrinsic sensitivity of tumor cells to radiation by increasing the probability that cells will undergo cell division before potentially lethal chromosome damage can be repaired. DSB repair inhibitors are predicted to have less effect on normal tissues, where cells divide less rapidly and intact DNA damage-dependent cell cycle checkpoint controls arrest cell division in the presence of unrepaired DNA damage. The use of the folate targeting mechanism, which takes advantage of the high levels of folate receptor on tumor cells (6) may further increase specificity for tumor over normal tissue. We note that receptor-mediated delivery of a single-chain antibody is a potentially general approach that could be extended to other repair proteins.
We anticipated that endosomal trapping of the cargo protein might present an obstacle to the use of folate receptor for intranuclear delivery, but this was not a major issue in practice. There is at least one natural example of efficient trafficking of an extracellular protein to the nucleus and indeed, to repair foci, which is epidermal growth factor receptor (20). We speculate that some element of the MCP-ScFv 18-2 NLS construct provides a similar natural capability for endosome escape.
The present studies were limited to cultured cells and have not been extended to a preclinical model. To do so will require development of methods for scale-up of expression and possibly re-engineering of the ScFv 18-2 to increase stability and purity. However, there should be no fundamental barrier to extension of the folate receptor-mediated delivery approach in vivo (6). Further development of scFv 18-2 as a radiosensitizer could impact treatment of cancers where radiotherapy is a major treatment modality, including inoperable NSCLC or head and neck squamous cell cancer.
Conclusions
Effective radiosensitization of tumor cells in vitro can be obtained through folate receptor-mediated intranuclear delivery of an antibody fragment that targets a DNA DSB repair enzyme.
DNA repair enzymes are attractive targets for development of cancer therapeutics. Previous studies have identified a single chain antibody variable fragment (ScFv 18-2) that binds to DNA-PK, a key regulator of nonhomologous end joining repair. Although the properties of ScFv 18-2 suggest that it might be useful as a radiosensitizer, a method for delivery to intracellular sites of DNA repair has been lacking. This study describes a delivery method based on folate receptor-mediated endocytosis. Folate-conjugated ScFv 18-2 is taken up by tumor cells in vitro, localizes to the nucleus, and sensitizes cells to radiation in clonogenic survival assays. Further development of ScFv 18-2 as a radiosensitizer could impact treatment of cancers where radiotherapy is a major treatment modality, including inoperable NSCLC or head and neck squamous cell cancer. Folate-mediated delivery of single chain antibodies is a general approach that could potentially be extended to other repair enzymes.
Acknowledgements
This work was supported by PHS grants CA 98239 and EY 018244, by a Georgia Research Alliance Venture Lab award, and by Apeliotus Technologies, Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest notification
WSD served as a consultant to Apeliotus Technologies, Inc. A potential conflict does exist.
References
- 1.Mahaney BL, Meek K, Lees-Miller SP. Repair of ionizing radiation-induced DNA double-strand breaks by non-homologous end-joining. Biochem J. 2009;417:639–650. doi: 10.1042/BJ20080413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirzen F, Nilsson A, Zhivotovsky B, et al. DNA-dependent protein kinase content and activity in lung carcinoma cell lines: correlation with intrinsic radiosensitivity. Eur J Cancer. 1999;35:111–116. doi: 10.1016/s0959-8049(98)00289-5. [DOI] [PubMed] [Google Scholar]
- 3.Bolderson E, Richard DJ, Zhou BB, et al. Recent advances in cancer therapy targeting proteins involved in DNA double-strand break repair. Clin Cancer Res. 2009;15:6314–6320. doi: 10.1158/1078-0432.CCR-09-0096. [DOI] [PubMed] [Google Scholar]
- 4.Li S, Takeda Y, Wragg S, et al. Modification of the ionizing radiation response in living cells by an scFv against the DNA-dependent protein kinase. Nucleic Acids Res. 2003;31:5848–5857. doi: 10.1093/nar/gkg775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian ZM, Li H, Sun H, et al. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev. 2002;54:561–587. doi: 10.1124/pr.54.4.561. [DOI] [PubMed] [Google Scholar]
- 6.Low PS, Kularatne SA. Folate-targeted therapeutic and imaging agents for cancer. Curr Opin Chem Biol. 2009;13:256–262. doi: 10.1016/j.cbpa.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Carter T, Vancurova I, Sun I, et al. A DNA-activated protein kinase from HeLa cell nuclei. Mol Cell Biol. 1990;10:6460–6471. doi: 10.1128/mcb.10.12.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiong H, Li S, Yang Z, et al. E. coli expression of a soluble, active single-chain antibody variable fragment containing a nuclear localization signal. Protein Expr Purif. 2009 doi: 10.1016/j.pep.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Traut RR, Bollen A, Sun TT, et al. Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome. Biochemistry. 1973;12:3266–3273. doi: 10.1021/bi00741a019. [DOI] [PubMed] [Google Scholar]
- 10.Guo W, Lee RL. Receptor-targeted gene delivery via folate-conjugated polyethylenimine. AAPS PharmSci. 1999;1:E19. doi: 10.1208/ps010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franken NA, Rodermond HM, Stap J, et al. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 12.Fertil B, Dertinger H, Courdi A, et al. Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res. 1984;99:73–84. [PubMed] [Google Scholar]
- 13.Atkinson SF, Bettinger T, Seymour LW, et al. Conjugation of folate via gelonin carbohydrate residues retains ribosomal-inactivating properties of the toxin and permits targeting to folate receptor positive cells. J Biol Chem. 2001;276:27930–27935. doi: 10.1074/jbc.M102825200. [DOI] [PubMed] [Google Scholar]
- 14.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, Yu Y, Gupta S, et al. Autophosphorylation of DNA-dependent protein kinase regulates DNA end processing and may also alter double-strand break repair pathway choice. Mol Cell Biol. 2005;25:10842–10852. doi: 10.1128/MCB.25.24.10842-10852.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soubeyrand S, Pope L, Pakuts B, et al. Threonines 2638/2647 in DNA-PK are essential for cellular resistance to ionizing radiation. Cancer Res. 2003;63:1198–1201. [PubMed] [Google Scholar]
- 17.Chen BP, Chan DW, Kobayashi J, et al. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. J Biol Chem. 2005;280:14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- 18.Kinner A, Wu W, Staudt C, et al. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008;36:5678–5694. doi: 10.1093/nar/gkn550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izzard RA, Jackson SP, Smith GC. Competitive and noncompetitive inhibition of the DNA-dependent protein kinase. Cancer Res. 1999;59:2581–2586. [PubMed] [Google Scholar]
- 20.Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–808. doi: 10.1038/ncb0901-802. [DOI] [PubMed] [Google Scholar]