Abstract
The past decade has witnessed a surge in the development of immunomodulatory approaches to combat a broad range of human diseases, including cancer, viral infections, autoimmunity and inflammation as well as in the prevention of transplant rejection. Immunomodulatory approaches mostly involve the use of monoclonal antibodies or recombinant fusion proteins that target cell surface signalling molecules on immune cells to drive immune responses towards the desired direction. Advances in our understanding of the human immune system, along with valuable lessons learned from the first generation of therapeutic biologics, are aiding the design of the next generation of immunomodulatory biologics with better therapeutic efficacy, minimized adverse effects and long-lasting clinical benefit. The recent encouraging results from antibodies targeting programmed cell death protein 1 (PD1) and B7 homolog 1 (B7H1; also known as PDL1) for the treatment of various advanced human cancers show that immunomodulatory therapy has come of age.
Immunomodulatory biologics can be utilized to treat immune-related diseases in broad therapeutic areas. The immune response can be dampened in hyperactive immune conditions such as transplant rejection as well as autoimmune or inflammatory diseases, or stimulated to reverse hypoactive immune responses in cancer or chronic bacterial or viral infections. Unlike the traditional and mainstream monoclonal antibody (mAb)- and recombinant fusion protein (RFP)-based therapeutics, which neutralize or deplete targets or target positive cells1,2, immunomodulatory biologics engage and manipulate cell surface signalling molecules on host immune cells to modulate antigen-specific T cell receptor (TCR) and B cell receptor (BCR) signals to control the direction and magnitude of lymphocyte responses. Such cell surface signalling molecules include those previously known as co-signalling (both co-stimulatory and co-inhibitory) molecules3 as well as membrane receptors that are involved in adhesion and migration, and can be divided into two major gene families: the immunoglobulin (Ig) superfamily and the tumour necrosis factor (TNF)–TNF receptor (TNFR) superfamily. Among Ig molecules, the B7–CD28 family members have crucial roles in modulating TCR and BCR signals and they influence the outcome of lymphocyte-mediated immune responses4,5.
Immunomodulatory biologics can generally be classified into two groups based on their mechanisms of action: antagonists (blocking or neutralizing the interaction between receptors and ligands) or agonists (inducing signalling via the receptor by mimicking the ligand). Neutralizing mAbs against disease-facilitating cytokines have become important non-steroidal therapeutic options to treat autoimmune and inflammatory diseases (reviewed in ref. 1).
As a general approach to promote tumour- or pathogen-specific immune responses, one can enhance either lymphocyte priming and maturation in the lymphoid organs or effector functions in the periphery by engaging distinctive co-stimulatory pathways — for example, the CD28, CD137 (also known as 4-1BB or TNFRSF9), CD27 and CD40 pathways — using agonistic reagents (Fig. 1). Alternatively, immune activation can be promoted through the blockade of co-inhibitory pathways by antagonists: for example, the programmed cell death protein 1 (PD1)–B7 homolog 1 (B7H1; also known as PDL1) pathway and the cytotoxic T lymphocyte antigen 4 (CTLA4) pathway (Fig. 1). Conversely, lymphocyte activation can be inhibited to suppress unwanted immunity by either blocking co-stimulatory receptors or triggering a negative regulatory pathway. Immune activation enhanced by co-stimulatory receptors is generally initiated through membrane proximal kinase activation and followed by phosphorylation cascades, whereas co-inhibitory receptors such as CTLA4, PD1 and B- and T lymphocyte attenuator (BTLA) recruit phosphatases to reverse activation-induced phosphorylation events. However, owing to the temporal and spatial differential expression of co-signalling molecules during immune activation and their differential involvement in cancer or autoimmune diseases, it is crucial to understand the mechanism of individual pathways to design the most efficacious therapeutic biologics with minimized immune-related side effects.
Figure 1. Cell surface signalling molecules as important therapeutic targets.
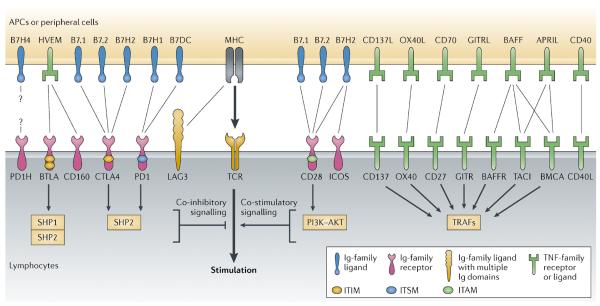
Co-signalling is a complex event that is coordinated through a network of ligand–receptor interactions on the cell surface, with both co-stimulatory and co-inhibitory capacities. The direction and outcome of immune responses are ultimately decided by the interplay of these complicated and often counterbalancing network interactions. Co-signalling pathways are thus important targets for therapeutic intervention. The immunoglobulin (Ig) superfamily and the tumour necrosis factor (TNF)–TNF receptor (TNFR) superfamily are two major gene families of cell surface signalling molecules. Important co-inhibitory receptors that are expressed on lymphocytes include cytotoxic T lymphocyte antigen 4 (CTLA4), programmed cell death protein 1 (PD1), B- and T lymphocyte attenuator (BTLA), lymphocyte activation gene 3 (LAG3), CD160 and the PD1 homolog (PD1H), whereas CD28, inducible co-stimulator (ICOS), CD137 (also known as 4-1BB), CD27, OX40, glucocorticoid-induced TNFR-related protein (GITR), CD40 ligand (CD40L), B cell activation factor receptor (BAFFR), transmembrane activator and CAML interactor (TACI) and B cell maturation antigen (BCMA) are the main co-stimulatory receptors. Co-signalling ligands, including B7 ligand members and TNF ligands, are mainly expressed by antigen-presenting cells (APCs). APRIL, a proliferation-inducing ligand; B7H1, B7 homolog 1; GITRL, GITR ligand; HVEM, herpesvirus entry mediator; ITAM, immunoreceptor tyrosine-based activation motif; ITIM, immunoreceptor tyrosine-based inhibitory motif; ITSM, immunoreceptor tyrosine-based switch motif; MHC, major histocompatibility complex; OX40L, OX40 ligand; PI3K, phosphoinositide 3-kinase; TCR, T cell receptor; TRAF, TNFR-associated factor.
The first immunomodulatory biologic to reach the market was the mAb muromonab (Orthoclone OKT3; Janssen-Cilag), directed against human CD3. Muromonab stimulates an initial T cell expansion followed by T cell depletion resulting from activation-induced cell death; it received approval from the US Food and Drug Administration (FDA) in 1986 for the prevention of transplant rejection. Since then, three additional immunomodulatory biologics have been approved for clinical use in the United States (Table 1); all of these target or are derived from the immune checkpoint receptor CTLA4. Furthermore, several immunomodulatory biologics are in different phases of clinical trials for the treatment of cancer as well as autoimmune and inflammatory conditions (Table 2).
Table 1.
FDA-approved biologics that target immunomodulatory pathways
| Target | Generic name (trade name) | Company | Type of biologic | Pathways | Roles | Indication (for which initially approved) | Year of approval |
|---|---|---|---|---|---|---|---|
| Monoclonal antibodies | |||||||
| CD3E | Muromonab-CD3 (Orthoclone OKT3) | Janssen-Cilag | Murine IgG2a | TCR | T cell priming and activation | Kidney transplantation rejection | 1986 |
| CTLA4 | Ipilimumab(Yervoy) | Bristol-Myers Squibb | Human IgG1 | CTLA4 | T cell activation | Metastatic melanoma | 2011 |
| Recombinant fusion proteins | |||||||
| CD80 and CD86 | Abatacept (Orencia) | Bristol-Myers Squibb | CTLA4-human IgG1 | CD28-B7.1, CD28-B7.2, B7H2 | T cell activation, T cell tolerance | Rheumatoid arthritis | 2005 |
| CD80 and CD86 | Belatacept (Nulojix) | Bristol-Myers Squibb | CTLA4-human IgG1 | CD28-B7.1, CD28-B7.2, B7H2 | T cell activation, T cell tolerance | Kidney transplantation rejection | 2011 |
B7H2, B7 homolog 2; CD3E, T cell surface glycoprotein CD3 ε-chain; CTLA4, cytotoxic T lymphocyte antigen 4; FDA, US Food and Drug Administration; IgG1, immunoglobulin G1; TCR, T cell receptor.
Table 2.
Biologics targeting immunomodulatory pathways in clinical trials
| Name | Companies | Type of biologic | Pathways | Roles | Indications | Trial phase |
|---|---|---|---|---|---|---|
| Immunoglobulin family | ||||||
| Tremelimumab | Medlmmune/AstraZeneca | CTLA4-specific human IgG2 | CTLA4-B7.1, CTLA4-B7.2, B7H2 | T cell priming and activation | Solid tumours | II |
| Galiximab | Cancer and Leukemia Group B (CALGB)/Biogen Idec |
B7.1-specific chimeric IgG1 | B7.1 | B cell proliferation | Lymphoma | II |
| BMS-936558 | Bristol-Myers Squibb/Medarex | PD1-specific human IgG4 | PD1-B7H1, PD1-B7DC | T cell activation and tolerance | Multiple cancers; HCV | III |
| CT-011 | CureTech | PD1-specific humanized IgG1 | PD1-B7H1, PD1-B7DC | T cell activation and tolerance | Advanced solid tumours; HCV | II |
| MK-3475 | Merck/Schering-Plough | PD1-specific IgG4 | PD1-B7H1, PD1-B7DC | T cell activation and tolerance | Advanced or metastatic solid tumours | I |
| AMP224 | Amplimmune/GlaxoSmithKline | B7DC and human IgG1 fusion protein | PD1-B7H1, PD1-B7DC | T cell activation and tolerance | Multiple cancers | I |
| BMS-936559 | Bristol-Myers Squibb | B7H1-specific human IgG4 | PD1-B7H1 | T cell activation and tolerance | Advanced or recurrent solid tumours | I |
| MPDL3280A | Genentech/Roche | B7H1-specific engineered human IgG1 | PD1-B7H1 | T cell activation and tolerance | Solid tumours | I |
| MEDI4736 | Medlmmune/AstraZeneca | B7H1-specific engineered human IgG1 | PD1-B7H1 | T cell activation and tolerance | Solid tumours | I |
| MEDI-570 | Medlmmune/AstraZeneca | ICOS-specific human IgG | ICOS-B7H2 | T cell-dependent B cell response | SLE | I |
| AMG 557 | Amgen | B7H2-specific human IgG | ICOS, CD28, CTLA4 | T cell-dependent B cell response | SLE, psoriasis | I |
| MGA271 | Macrogenics | B7H3-specific, ADCC-enhanced humanized IgG1 | B7H3 | T cell activation and tolerance | Solid tumours | I |
| IMP321 | Immutep | LAG3 and human IgG1 fusion protein | LAG3-MHCII | DC maturation and T cell activation | Multiple cancers | I/II |
| TNF family | ||||||
| BMS-663513 | Bristol-Myers Squibb | CD137-specific human IgG4 | CD137 | T cell activation | Solid tumours | I/II |
| PF-05082566 | Pfizer | CD137-specific human IgG | CD137 | T cell activation | Lymphoma | I |
| CDX-1127 | Celldex | CD27-specific human IgG1 | CD27 | T cell activation | Multiple cancers | I |
| Anti-OX40 | Providence Health & Services | OX40-specific mouse IgG | OX40 | CD4 T cell activation | Prostate cancer | II |
| huMAb OX40L | Genentech/Roche | OX40L-specific human IgG1 | OX40-OX40L | CD4 T cell activation | Asthma | II |
| TRX518 | GITR Inc. | GITR-specific humanized IgG1 | GITR-GITRL | T cell activation | Solid tumours | I |
| Atacicept | ZymoGenetics/EMD Serono | TACI and human IgG1 fusion protein | TACI, BCMA and BAFFR | B cell activation and antibody production | SLE, rheumatoid arthritis, multiple sclerosis and optic neuritis | II/III |
| CP-870,893 | Pfizer | CD40-specific human IgG1 | CD40 | APC activation and B cell maturation | Multiple cancers | I |
| Lucatumumab | Novartis | CD40-specific human IgG1 | CD40 | APC activation and B cell maturation | Lymphoma and leukaemia | I/II |
| Dacetuzumab | Seattle Genetics | CD40-specific humanized IgG1 | CD40 | APC activation and B cell maturation | Lymphoma and multiple myeloma | II |
ADCC, antibody-dependent cell-mediated cytotoxicity; APC, antigen-presenting cell; B7H1, B7 homolog 1; BAFFR, B cell activation factor receptor; BCMA, B cell maturation antigen; CTLA4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; GITR, glucocorticoid-induced TNFR-related protein; GITRL, GITR ligand; HCV, hepatitis C virus; ICOS, inducible co-stimulator; IgG1, immunoglobulin G1; LAG3, lymphocyte activation gene 3; MHCII, major histocompatibility complex class II; OX40L, OX40 ligand; PD1, programmed cell death protein 1; SLE, systemic lupus erythematosus; TACI, transmembrane activator and CAML interactor; TNF, tumour necrosis factor.
The FDA approval in 2011 of the CTLA4-specific mAb ipilimumab (Yervoy; Bristol-Myers Squibb) for the treatment of metastatic melanoma represented a major milestone for cancer immunotherapy. More recently, it was shown that an antibody (BMS-936558) targeting the co-inhibitory molecule PD1 induced significant and durable responses in several types of highly refractory tumours6. This antibody represents a newer generation of immunomodulatory biologics that stimulate highly effective and long-lasting host tumour immunity with controllable autoimmune toxicities.
Here, we examine the targets and mechanisms by which immunomodulation can be achieved, and discuss the development of immunomodulatory biologics for the treatment of cancer or autoimmune diseases.
Ig superfamily co-signalling molecules
The first Ig superfamily co-signalling molecules to be identified were the ligand and receptor pair B7–CD28, as reported in 1990 (Refs 7,8), and the family has rapidly expanded ever since. The CD28 receptor family members have a single Ig variable region-like (IgV) motif in their extracellular domains and include CD28, CTLA4, inducible co-stimulator (ICOS), PD1 and BTLA9. Their known ligands all belong to the family of B7 ligands, which have a typical structure of two Ig-like extracellular domains, an IgV motif and an Ig constant region-like (IgC) domain. Strictly speaking, these receptors and ligands should be termed `counter-receptors' because under certain circumstances some of these receptors can also serve as ligands and vice versa.
CD28–CTLA4–ICOS axis
Constitutively expressed on naive T cells, the receptor CD28 provides the primary co-stimulatory signal to promote naive T cell priming following the engagement of B7.1 (also known as CD80) or B7.2 (also known as CD86 and B70) — the CD28 ligands that are mainly expressed on antigen-presenting cells (APCs)7. CD28 activates phosphoinositide 3-kinase (PI3K)- and AKT-dependent signalling pathways as well as the mitogen-activated protein kinase (MAPK) cascade. As a result, it induces the expression of high amounts of interleukin-2 (IL-2) and an array of effector cytokines (both pro- and anti-inflammatory), it upregulates the survival factor B cell lymphoma XL (BCL-XL) and downregulates the cell cycle inhibitor cyclin-dependent kinase inhibitor 1B (CDKN1B; also known as p27Kip1) to promote T cell activation, differentiation and memory T cell formation10–12.
Conversely, CTLA4, a CD28 homologue induced on activated T cells, serves as a co-inhibitor to keep T cell responses in check following ligation of B7.1 and/or B7.2 (Refs 13,14). The cytoplasmic domain of CTLA4 contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) that is responsible for the recruitment of SHP family phosphatases, which reverse TCR activation-induced phosphorylation of signalling molecules. The essential negative function of CTLA4 in controlling T cell activation was demonstrated in CTLA4-deficient mice, which had fatal multi-organ destruction caused by uncontrolled lymphocyte proliferation and infiltration15,16.
ICOS, another homologue of CD28, is induced on activated T cells and constitutively expressed on follicular T helper (TFH) cells. It promotes T cell activation and T cell-dependent B cell responses by interacting with the ligand B7H2 (also known as ICOSL)17–20. It was recently found that B7H2 can also bind to CD28 and CTLA4 in humans (but not in mice), indicating a crosstalk and a possible synergistic relationship between CD28 and ICOS co-stimulatory pathways21 (Figs 2,3). In humans, the expression of B7.1 and B7.2 is largely restricted to professional APCs, whereas B7H2 is expressed not only on APCs but also induced on parenchymal cells, including endothelial and epithelial cells, under inflammatory conditions22. This differential expression pattern indicates that CD28 may regulate effector T cells following B7H2 engagement in the peripheral tissue, in addition to its role during the priming phase in lymphoid organs in response to B7.1 and/or B7.2 binding.
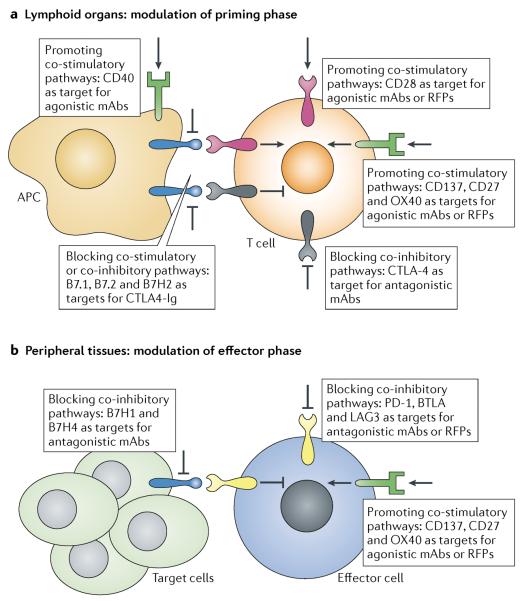
Figure 2. Immune modulation of the priming and the effector phase of lymphocyte activation.
a | To promote antigen-specific immune responses during priming in the lymphoid organs, distinctive co-stimulatory pathways can be engaged, such as CD28, CD137 (also known as 4-1BB), CD27, OX40 and CD40, by agonistic reagents or through blockade of the primary early checkpoint receptor cytotoxic T lymphocyte antigen 4 (CTLA4) by an antagonist. Vice versa, the CD28 co-stimulatory pathway can also be blocked by CTLA4–immunoglobulin (Ig) to inhibit T cell activation. b | To expand effector T cells or restore exhausted T cells in the peripheral organs, peripheral inhibitory pathways can be blocked, including the pathway mediated by B7 homolog 1 (B7H1) and programmed cell death protein 1 (PD1), B7H4, lymphocyte activation gene 3 (LAG3) as well as B- and T lymphocyte attenuator (BTLA); alternatively, co-stimulatory receptors on effector T cells — such as CD137 and OX40 — can be activated. mAb, monoclonal antibody.
Figure 3. B7–CD28 family and newly discovered interactions.
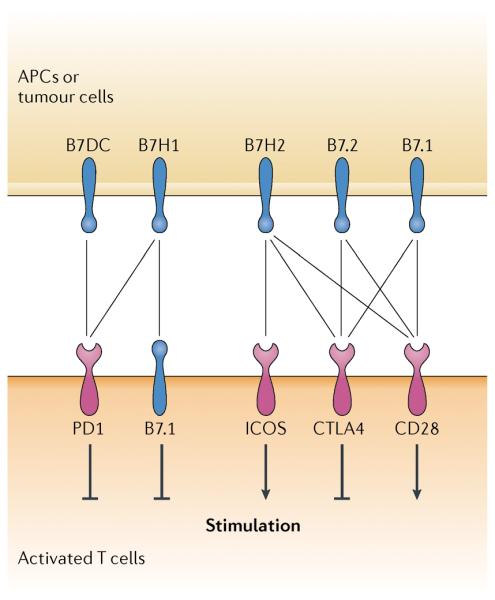
In addition to known interactions (see Fig. 1), B7 homolog 1 (B7H1) was recently found to interact with and deliver a negative signal through B7.1, which is expressed on activated T cells. Human B7H2 — also known as inducible co-stimulator ligand (ICOSL) — was identified to engage both CD28 and cytotoxic T lymphocyte antigen 4 (CTLA4) to modulate T cell activation in addition to ICOS. APC, antigen-presenting cell.
The central role of the CD28–CTLA4–ICOS axis in T cell activation has attracted much effort for therapeutic manipulation. Agonistic biologics that target CD28 or ICOS expand antigen-specific T cells and enhance the antitumour immune response23. In an original proof-of-principle experiment carried out two decades ago, ectopic expression of B7.1 in an immunogenic melanoma (K1735) cell line promoted local and systemic antitumour immunity in mice24. The B7-mediated antitumour effect has been attributed to the co-stimulation of both direct priming (antigens presented by the tumour)24 and cross-priming (antigens processed and presented by host APCs)25 of tumour-specific T cells.
B7.1– and B7.2–Ig RFPs targeting CD28 on naive T cells have also been shown to enhance T cell responses and facilitate tumour regression in several murine tumour models26–28. Similarly, a cancer vaccine using B7H2-positive tumour cells or the administration of a B7H2–Ig fusion protein, potentiating positive signals through ICOS and possibly through CD28, induces tumour-specific cytotoxic T lymphocyte (CTL) expansion and leads to tumour regression23,29,30. Instead of promoting co-stimulatory signals, the blockade of CTLA4 co-inhibitory pathways has also been shown to be effective in treating cancer. Infusion of a CTLA4-blocking mAb results in sustained immune activation and induces potent antitumour responses with limited autoimmunity in mouse models31–34. At least two underlying mechanisms have been proposed to explain the antitumour effect: first, the CTLA4-blocking mAb prevents the engagement of B7 ligands to CTLA4 expressed on activated T cells, allowing persistent activation through CD28; second, the CTLA4-blocking mAb binds to and inhibits immunosuppressive CD4+CD25+ regulatory T (TReg) cells, which constitutively express a high level of cell surface CTLA4 (Refs 35–37).
Ipilimumab, a human IgG1 CTLA4-specific mAb, was approved by the FDA in 2011 for the treatment of metastatic melanoma. Ipilimumab induced a sustained immune response and objective response rates of 10–15% in patients with advanced melanoma and significantly prolonged the survival of these patients38. In Phase III trials with ipilimumab, about 80% of patients had drug-related adverse events and 30% of patients had grade III severe immune-related toxicity with lymphocyte infiltration into multiple organs, causing dermatitis, colitis and hepatitis39,40. The adverse events associated with ipilimumab correlate with therapeutic responses, indicating that the drug blocks a central co-inhibitory pathway that affects both antitumour immunity and autoimmunity. Careful evaluation of treatment dosing, frequency and timing is thus critical to minimize immune-related adverse events while preserving antitumour efficacy. Ipilimumab and another CTLA4-specific mAb, tremelimumab, are currently being evaluated for the treatment of several other cancer indications as either a monotherapy or in combination with chemotherapy, an adjuvant, a dendritic cell (DC) vaccine or other biologics.
As an attractive approach to enhance co-stimulation, agonistic mAbs targeting CD28 or ICOS have been shown to strongly promote T cell proliferation and cytokine production in the presence of antigenic signals in vitro17,41. These mAbs could be especially potent when applied together with an antigen and/or a CTLA4 antagonist. Although co-stimulatory CD28-or ICOS-targeting mAbs have not yet reached clinical development, a `super-agonistic' CD28-targeting mAb, TGN1412, entered clinical trials based on its ability to stimulate naive human T cells without the need for a TCR signal. TGN1412 induced a severe systemic inflammatory response in healthy volunteers42, characterized by a massive pro-inflammatory cytokine storm 90 minutes after infusion, which was followed by multi-organ injury and lymphocyte depletion. Fortunately, all volunteers survived after receiving immunosuppressive treatments and cardiopulmonary support in an intensive care unit. The lesson learned from this trial thus cautioned against global non-discriminatory stimulation of naive T cell activation, especially with biologics that have antigen-independent mitogenic activity42.
Conversely, modulation of the B7–CD28 pathway can also induce strong immunosuppression and immune tolerance. CTLA4–Ig RFP, serving as a decoy receptor that disrupts B7–CD28 interactions, is a potent immunosuppressor. In addition to blocking the interactions between CD28 and B7.1, B7.2 and B7H2, CTLA4–Ig RFP engages B7.1 and B7.2 on DCs and negatively regulates DC function. Crosslinking of B7.1 and B7.2 by CTLA4–Ig on DCs induces the production of indoleamine 2,3-dioxygenase (IDO), an immunosuppressive enzyme involved in tryptophan metabolism, and suppresses T cell activation43. Through the inhibition of CD28 pathway activation, together with immune tolerance induction by IDO expression, CTLA4–Ig prolongs allograft survival and alleviates inflammatory conditions in several animal models of autoimmune disease, including diabetes, arthritis, multiple sclerosis, systemic lupus erythematosus (SLE) and colitis44.
Based on these studies, abatacept (Orencia; Bristol-Myers Squibb), a fusion protein composed of the extracellular domain of CTLA4 and the IgG1 Fc region, was the first biologic targeting the B7–CD28 family to be approved (in 2005, for patients with rheumatoid arthritis who have an inadequate response to TNF neutralization therapy)45. It is currently being evaluated in other autoimmune or inflammatory indications in which T cells are hyperactive or T cell activation is undesired, such as psoriasis, ulcerative colitis, type 1 diabetes, lupus nephritis and organ transplantation. Recently, a second-generation CTLA4–Ig product, belatacept (Nulojix; Bristol-Myers Squibb), harbouring two engineered point mutations with improved binding affinity to B7 ligands compared to abatacept, was approved for the prevention of human kidney transplantation rejection. In a Phase III kidney transplantation trial, belatacept had similar graft survival but was associated with better renal function and an improved cardiovascular and metabolic risk profile compared to the standard cyclosporine A treatment46,47. Belatacept is also in Phase I/II clinical trials for the treatment of rheumatoid arthritis and type 1 diabetes.
With three therapeutic biologics targeting CTLA4 or based on CTLA4 approved in the clinic, mAbs targeting the B7 ligand — including B7.1-specific and B7H2-specific mAbs — are also being evaluated in clinical trials. In addition to being the co-signalling ligand for CD28 and CTLA4 on professional APCs, B7.1 is expressed on B cells and certain types of lymphomas. Crosslinking of B7.1 by the mAb galiximab (IDEC-114) leads to growth arrest and apoptosis of normal B cells and B cell lymphomas48. In a completed Phase I/II trial treating relapsed or refractory follicular lymphoma, galiximab was well tolerated with no dose-limiting toxicities at the dose ranged tested49. Galiximab is currently being evaluated in Phase III trials to treat relapsed or refractory Hodgkin's lymphoma alone or in combination with rituximab (Rituxan; Biogen Idec/Genentech/Roche), a CD20-specific mAb.
ICOS was originally thought of as a redundant co-stimulatory receptor to CD28. However, owing to its inducible expression on activated T cells, ICOS has its own unique role in the late phase of T cell activation, memory T cell formation and the T cell-dependent B cell response. Constitutively expressed on TFH cells, the essential role of ICOS in B cell maturation and the antibody response is demonstrated by ICOS deficiency in both mouse models and humans20,50,51. Immunoglobulin class switching and memory B cell formation are severely impaired in ICOS-deficient mice as well as in ICOS-deficient patients, indicating that ICOS pathway blockade suppresses T- and B cell-mediated autoimmune conditions. In the NZB/NZW F1 mouse model of SLE and in a collagen-induced arthritis (CIA) model, B7H2 antibody treatment indeed led to a decrease in TFH cells as well as germinal centre B cells, and it inhibited disease progression52. The ICOS-targeting mAb MEDI-570 and the B7H2-targeting mAb AMG 557 are currently being evaluated in Phase I trials for the treatment of SLE.
B7H1, B7DC and PD1 pathways
B7H1 and its closest homologue B7DC (also known as PDL2) were identified as new B7 family members in 1999 (ref. 53) and 2001 (ref. 54), respectively, both sharing 20–23% protein sequence identity with B7.1 and B7.2 in their extracellular domain53,54. The B7H1 mRNA transcript has broad tissue distribution, including both lymphoid (thymus, bone marrow, spleen and lymph node) and non-lymphoid organs (heart, skeletal muscle, placenta, lung, kidney and liver)53. B7H1 is a cell surface protein that is constitutively expressed on APCs (macrophages and DCs). However, it is also inducibly expressed in a large variety of tissues and cell types, including epithelial and endothelial cells, in response to pro-inflammatory cytokines such as interferons (IFNs)55,56. By contrast, the expression of B7DC is mainly restricted to DCs and macrophages54,57.
PD1, a CD28 and CTLA4 homologue that was first identified in a T cell hybridoma undergoing apoptosis, is implicated in promoting programmed cell death58. PD1 is inducibly expressed on activated T cells, B cells, macrophages, DCs and monocytes but absent on their naive counterparts59–61. Upregulation of PD1 on these cells has been shown to inhibit both adaptive and innate immune responses59–61. In 2000, PD1 was found to be a receptor for B7H1 (ref. 62) and in 2001 it was found to be a receptor for B7DC63. PD1 contains an ITIM and an immunoreceptor tyrosine-based switch motif (ITSM) in its cytoplasmic region. Experimental data indicate that the ITSM but not the ITIM mediates SHP2 phosphatase recruitment and reverses activation-induced phosphorylation events58.
Genetic ablation has demonstrated the crucial function of PD1 in controlling lymphocyte activation and in maintaining peripheral tolerance. PD1-deficient mice have various types and degrees of autoimmune conditions, depending on strain backgrounds. PD1-deficient BALB/c mice have dilated cardiomyopathy, congestive heart failure and frequently suffer sudden death64, which is caused by the generation of high-titre autoantibodies against the heart-specific protein cardiac troponin I65. PD1-deficient C57BL/6 mice spontaneously develop arthritis and lupus-like disease at an advanced age (>14 months)66. PD1-deficient non-obese diabetic (NOD) mice develop early-onset type 1 diabetes67. The suppressive functions of PD1 in the periphery have been shown to be largely mediated through B7H1 rather than B7DC in vivo68,69, as only B7H1 is expressed in peripheral tissue.
The crucial function of the B7H1–PD1 axis in the control of human T cell activation and in maintaining peripheral tolerance appears to be exploited by tumour cells and by viruses during chronic viral infections70,71. B7H1 is overexpressed on many freshly isolated human tumours from different tissue origins71,72. The expression of B7H1 has been correlated with the progression and poor prognosis of certain types of human malignancies68,73. During chronic viral infections, B7H1 is persistently expressed on both lymphoid and peripheral tissues; meanwhile, PD1 is upregulated on virus-specific CTLs. The B7H1–PD1 pathway has been identified to contribute to T cell exhaustion74 — a T cell hyporeactive condition that occurs during chronic viral infections. Tumour- or virus-induced B7H1 appears to utilize multiple mechanisms to suppress and facilitate the evasion of host immune surveillance, including the promotion of T cell anergy, exhaustion, unresponsiveness and apoptosis, inducing the expansion of TReg cells as well as enhancing tumour-intrinsic resistance to killing and apoptosis70.
In a model of CTL-mediated killing of a human melanoma cell line (624mel), it was shown that B7H1-transfected 624mel cells increased the rate of apoptosis of 624mel-specific CTLs. The inclusion of a B7H1-specific mAb partially suppressed this apoptotic effect72. Consistent with this in vitro observation, in a mouse P815 tumour model, B7H1 transfectants also induced tumour-specific 2C transgenic T cell apoptosis72. Interestingly, when a different tumour-specific TCR transgenic CTL (P1A) was used in the same P815 system, the B7H1-transfected P815 model did not induce P1A apoptosis; rather, it promoted unresponsiveness — a phenomenon also known as `molecular shielding'. Under this condition, CTLs remain fully functional against B7H1-negative tumours but ignore B7H1-positive tumour cells75. Recently, another mechanism of B7H1-mediated immune evasion by tumour cells came to light. Upon PD1 ligation, tumour-associated B7H1 was shown to act as a receptor to deliver an anti-apoptotic signal to tumour cells through its intracellular domain, which renders these cells resistant to CTL lysis and FAS-induced apoptosis76. Most importantly, B7H1–PD1 blockade in vivo by a B7H1- or PD1-specific mAb promoted CTL expansion77 and accelerated tumour regression or viral clearance in many murine tumour models or murine models of chronic viral infection75,78. Therefore, B7H1 may facilitate multiple mechanisms to evade host antitumour or antiviral immunity in vivo.
The recent identification of B7.1 as a second receptor for B7H1 reveals the complexity of B7 family interactions and indicates a potential crosstalk between the CD28–CTLA4 and PD1 pathways79 (Fig. 3). Induced on activated T cells, B7.1 serves as an inhibitory receptor for B7H1 to suppress T cell responses in vitro79 and contributes to the formation of T cell tolerance in vivo80. Interestingly, the expression of B7.1 on TReg cells and its interaction with B7H1 expressed on APCs has a crucial role in TReg cell expansion during inflammatory responses in a mouse model of graft-versus-host disease (GVHD)81. The presence of two ligands (B7H1 and B7DC) for PD1 and two inhibitory receptors (PD1 and B7.1) for B7H1 suggests that biologics that target either PD1 or B7H1 have differential effects. One could speculate that an antagonistic PD1-specific Ab will block the PD1 pathway but leave B7.1–B7H1 interactions untouched, whereas a B7H1-blocking Ab could disrupt both B7H1–PD1 and B7.1–B7H1 pathways without interfering with the B7DC–PD1 interaction. Complete abrogation of B7H1- and PD1-mediated inhibitory pathways thus requires a combination strategy targeting both molecules.
At least seven therapeutic biologics targeting the human B7H1–B7DC–PD1 pathway are currently in clinical trials, and some of these agents have shown promising results. Three PD1-targeting mAbs (BMS-936558/MDX-1106, CT-011 and MK-3475), three B7H1-targeting mAbs (BMS-936559/MDX-1105, MPDL3280A and MEDI4736) and a B7DC–Ig RFP are being evaluated for the treatment of various advanced cancers or hepatitis C virus (HVC) infection. Two published studies of Phase I trials using BMS-936558/MDX-1106 (ref. 82) or CT-011 (ref. 83) as a monotherapy for the treatment of refractory solid tumours or haematological malignancies, respectively, have revealed that both PD1-targeting mAbs were generally well tolerated and had no dose-limiting toxicity up to 10 mg per kg body weight82,83. 14% of patients developed grade 3 or grade 4 drug-related adverse events and there were three deaths caused by pulmonary toxicity. Immune-related adverse events included colitis, hypothyroidism and polyarticular arthropathies, but in general these were milder than those observed in the trials with ipilimumab (the CTLA4-specific mAb).
A recent report summarizing the results of 296 patients treated with BMS-936558 showed significant objective responses (partial or complete) in 28% of patients with metastatic melanoma, 27% of patients with renal cell carcinoma and 18% of patients with non-small-cell lung cancer (NSCLC). The result in NSCLC is particularly encouraging, as patients with this type of cancer have responded poorly to immunotherapy in the past6. In this unusually large Phase I study, the results of all escalating doses were included in the analysis. However, for active doses (determined at 3 mg per kg or 10 mg per kg) the objective response rates were even higher. For example, treatment with 3 mg per kg of anti-PD1 therapy led to objective responses in 41% of patients with melanoma and 32% of patients with NSCLC. The clinical response was shown to be durable with a rare recurrence, indicating the formation of immune memory172. B7H1 overexpression in the tumour appeared to correlate with antitumour responses in a small patient cohort6,82, suggesting the potential use of B7H1 expression as a biomarker or inclusion criteria for this type of treatment. A recent study identified a strong association of mela noma-expressed B7H1 with the presence of tumourinfiltrating lymphocytes84. Tumours may thus upregulate B7H1 as an adaptive immune resistance mechanism in response to IFNγ released by tumour-infiltrating lymphocytes to suppress effector T cell function.
The first Phase I clinical trial report of 207 patients treated with a B7H1-targeting mAb (BMS-936559) showed a similar safety profile, with 9% of patients experiencing grade 3 or grade 4 toxicity — slightly lower than the 14% observed in the trials of PD1-targeting mAbs. Importantly, B7H1 blockade also induced durable tumour regression with objective response rates of 6–17% and stable disease rates of 12–41% among patients with cancer85. Because B7H1 is shown to be a major inhibitory ligand for PD1 in vivo, the lower efficacy of a B7H1-targeting mAb in comparison with a PD1-targeting mAb is somewhat unexpected. However, a direct comparison of the two different mAbs is difficult because the effect of a blocking antibody is also determined by its affinity or avidity, pharmacokinetics, stability, and so on. In addition to mAbs, a B7DC–IgG1 RFP (AMP-224) that targets the PD1 pathway is now in a Phase I trial for the treatment of advanced solid tumours.
The therapeutic principle of B7H1–PD1 blockade appears to be distinct to that of B7.1– or B7.2–CTLA4 blockade. As described above, antibodies targeting PD1 and B7H1 may work mainly by improving effector T cell functions in the tumour microenvironment, where this interaction largely occurs84. By contrast, CTLA4-specific mAbs may function by inhibiting naive T cell priming largely in lymphoid organs, where the B7.1– or B7.2–CTLA4 interactions take place. With high rates of durable clinical responses and a reasonable safety profile, B7H1–PD1 blockade could change the paradigm of cancer treatment and may become the building block for future combinations with other therapies. When CTLA4-specific therapy and B7H1–PD1 blockade are carefully combined with direct cancer-killing mechanisms such as traditional chemotherapy or radiation therapy, de novo priming and expansion of existing tumour-specific T cells could be greatly enhanced, as shown in animal tumour models86. PD1-specific mAbs in combination with tumour antigen-based vaccines, chemotherapy or other biologics (such as ipilimumab or rituximab) targeting multiple pathways are currently in clinical trials for the treatment of late-stage cancers (Table 3).
Table 3.
Combination therapies involving immunomodulatory biologics in clinical trials
| Biologic | Combination drugs | Targets in combination | Indications | Trial phase |
|---|---|---|---|---|
| Ipilimumab | Paclitaxel-carboplatin | CTLA4, chemotherapy | Non-small-cell lung cancer | III |
| Ipilimumab | Melphalan-dactinomycin | CTLA4, chemotherapy | Melanoma | II |
| Ipilimumab | Leuprolide-goserelin-degarelix | CTLA4, androgen deprivation | Prostate cancer | II |
| Ipilimumab | Interferon alfa-2b | CTLA4, IFNα | Melanoma | III |
| Ipilimumab | GM-CSF | CTLA4, GM-CSF | Melanoma | II |
| Ipilimumab | Bevacizumab | CTLA4, VEGFR | Melanoma | I |
| Ipilimumab | Dacarbazine | CTLA4, chemotherapy | Melanoma | II |
| Tremelimumab | CP-870893 | CTLA4, CD40 | Late-stage melanoma | I |
| Tremelimumab | DC vaccine | CTLA4, vaccine | Late-stage melanoma | II/III |
| Tremelimumab | BCG | CTLA4, adjuvant | Bladder cancer | I |
| Tremelimumab | Bicalutamide | CTLA4, androgen receptor | Prostate cancer | I |
| Galiximab | Rituximab | B7.1, CD20 | Non-Hodgkin's lymphoma | II |
| BMS-936558 | Ipilimumab | PD1, CTLA4 | Late-stage melanoma | I |
| BMS-936558 | IPH21 | PD1, KIR2DL1, KIR2DL2, KIR2DL3 | Advanced solid tumours | I |
| BMS-936558 | Peptide vaccine | PD1, peptide, adjuvant | Late-stage melanoma | I |
| BMS-936558 | Sunitinib and pazopanib | PD1, chemotherapy | Metastatic renal cell carcinoma | I |
| BMS-936558 | Gemcitabine, cisplatin, pemetrexed, paclitaxel, carboplatin, erlotinib and bevacizumab | PD1, chemotherapy, VEGFR | Late-stage non-small-cell lung cancer | I |
| BMS-936558 | Docetaxel | PD1, chemotherapy | Squamous cell non-small-cell lung cancer | III |
| MK-3475 | Carboplatin, paclitaxel, dacarbazine and temozolomide | PD1, chemotherapy | Advanced melanoma | II |
| CT-011 | Rituximab | PD1, CD20 | Lymphoma | II |
| CT-011 | FOLFOX | PD1, chemotherapy | Colorectal cancer | II |
| CT-011 | Gemcitabine | PD1, chemotherapy | Pancreatic cancer | II |
| CT-011 | DC vaccine | PD1, vaccine | Multiple myeloma, AML | II |
| CT-011 | Sipuleucel-T and cyclophosphamide | PD1, vaccine, chemotherapy | Prostate cancer | II |
| CT-011 | p53 peptide | PD1, p53 genetic vaccine | Advanced solid tumours | I |
| IMP321 | Peptide vaccine | LAG3, peptide | Melanoma | I/II |
| IMP321 | Gemcitabine | LAG3, chemotherapy | Pancreatic cancer | I |
| PF-05082566 | Rituximab | CD137, CD20 | Non-Hodgkin's lymphoma | I |
| Anti-OX40 | Radiation therapy and cyclophosphamide | OX40, chemotherapy, radiation therapy | Prostate cancer | I/II |
| Atacicept | Adalimumab (Humira) | BAFF-APRIL, TNF | Rheumatoid arthritis | II |
| CP-870,893 | Paclitaxel and carboplatin | CD40, chemotherapy | Advanced solid tumours | I |
| CP-870,893 | Peptide vaccine | CD40, vaccine | Melanoma | I |
| CP-870,893 | Gemcitabine | CD40, chemotherapy | Pancreatic cancer | I |
| Dacetuzumab | Rituximab, carboplatin and gemcitabine | CD40, CD20, chemotherapy | Lymphoma | II |
| Dacetuzumab | Lenalidomide and dexamethasone | CD40, chemotherapy | Multiple myeloma | I |
AML, acute myeloid leukaemia; APRIL, a proliferation-inducing ligand; BAFF, B cell activation factor; BCG, bacille Calmette–Guerin; CTLA4, cytotoxic T lymphocyte antigen 4; DC, dendritic cell; FOLFOX, folinic acid, 5-fluorouracil, oxaliplatin; GM-CSF granulocyte-macrophage colony-stimulating factor; IFNα, interferon-α; KIR2DL1, killer cell immunoglobulin-like receptor 2DL1; LAG3, lymphocyte activation gene 3; p53, tumour suppressor p53; PD1, programmed cell death protein 1; TNF, tumour necrosis factor; VEGFR, vascular endothelial growth factor receptor.
Ig receptors that are emerging as targets
In addition to CTLA4 and PD1, the discovery of several co-inhibitory pathways involving Ig family molecules (Fig. 2) — including BTLA, B7H4 (also known as VCTN1), lymphocyte activation gene 3 (LAG3), T cell immunoglobulin mucin 3 (TIM3) and the PD1 homolog (PD1H) (also known as V-domain Ig suppressor of T cell activation; VISTA) — has opened up new avenues for treating cancer and autoimmunity.
BTLA
BTLA is a CD28 receptor family member that was identified in 2003 (ref. 9). It has a single IgV extra-cellular domain but limited sequence identity with other CD28 family molecules, such as CD28 (11% sequence identity), CTLA4 (14% sequence identity) and PD1 (13% sequence identity)9. BTLA is expressed on T and B lymphocytes as well as subsets of DCs. Herpesvirus entry mediator (HVEM), a TNFR that is widely expressed in the haematopoietic system, was identified as a counter-receptor for BTLA87. BTLA contains two ITIMs in its cytoplasmic region, which recruit SHP1 and SHP2 phosphatases upon receptor activation by mAb crosslinking or ligand engagement. BTLA-deficient mice show enhanced T cell activation and exacerbated disease in models of autoimmunity and inflammation, including experimental autoimmune encephalomyelitis (EAE), SLE, airway inflammation in asthma and concanavalin A (ConA)-induced hepatitis9, indicating a suppressive function of BTLA in controlling T cell activation in vivo. Studies of peripheral blood mononuclear cells from patients with melanoma have revealed that BTLA is expressed at high levels on tumour-specific CTLs and inhibits T cell function upon its engagement by tumour-expressed HVEM88, suggesting that BTLA blockade might potentially improve T cell function and antitumour immunity. By contrast, in murine models of GVHD, BTLA-targeting agonistic mAbs suppress anti-host donor T cell responses89,90, indicating their potential therapeutic value in transplantation and autoimmune diseases.
B7H4
B7H4 (Refs 91–93) shares 22% and 23% sequence identity with human B7.1 and B7.2, respectively, and was first discovered in 2003. The B7H4 mRNA has a broad tissue distribution, whereas the B7H4 protein is mainly detectable in peripheral tissues, including the pancreas, ovary and prostate92. Although B7H4 is absent from naive haematopoietic cells, high levels of the protein were found in immunosuppressive tumour-associated macrophages and were associated with poor prognosis in patients with ovarian cancer94,95. A putative counter-receptor for B7H4 was detected on activated T cells but its identity has yet to be determined91,92. The inhibitory function of B7H4 on T cell-mediated immunity was initially reported by three groups91–93. An agonistic B7H4–Ig fusion protein inhibits T cell proliferation and cytokine production by inducing cell cycle arrest92. B7H4-deficient mice exhibit aggravated disease progression in models of EAE and rheumatoid arthritis91, further supporting the inhibitory role of B7H4 in vivo.
Interestingly, B7H4 was found on a high percentage of freshly isolated melanoma cells as well as ovarian, prostate and lung carcinoma cells96. B7H4 expression is associated with tumour progression and poor prognosis in multiple cancer types97–99, indicating that cancer cells might utilize this inhibitory mechanism to evade the host immune system. Blockade of the B7H4 pathway or tumour targeting using B7H4-specific mAbs is currently being evaluated in vivo in cancer models. Conversely, administration of an agonistic B7H4–Ig fusion protein alleviates disease phenotypes in the CIA model of rheumatoid arthritis and reduces the incidence of autoimmune diabetes in the NOD mouse model100,101, indicating its therapeutic potential in autoimmune diseases.
LAG3
First identified in 1990 (ref. 102), LAG3 is an Ig family receptor with a similar domain structure and 20% sequence identity to CD4 (ref. 103). Like CD4, LAG3 interacts with major histocompatibility complex (MHC) class II molecules. LAG3 has a broad expression pattern in the haematopoietic system, including activated T cells and TReg cells, plasmacytoid DCs, B cells, natural killer (NK) cells, natural killer T (NKT) cells and γδT cells103. The inhibitory function of LAG3 on activated T cells has been well documented. Crosslinking LAG3 by mAbs on activated T cells inhibits T cell proliferation and cytokine production induced by CD3 crosslinking in vitro104. Together with a group of T cell inhibitory receptors, including PD1, CTLA4, CD160 and the NK cell receptor 2B4 (also known as CD244), persistent expression of LAG3 on virus-specific CD8+ T cells has been associated with T cell exhaustion during chronic viral infection105. Importantly, a combination of LAG3-blocking mAbs with PD1-specific mAbs reverses the phenotype of exhausted T cells and improves virus clearance more effectively than treatment with a PD1-specific mAb alone in a murine model of chronic lymphocytic choriomeningitis infection105. Similarly, co-expression of LAG3 and PD1 on tumour-infiltrating lymphocytes is associated with impaired T cell function in patients with ovarian cancer. Dual blockade of the LAG3 and PD1 pathways improves tumour-specific T cell proliferation and cytokine production in vitro106,107. Targeting several T cell co-inhibitory pathways that contribute to the suppressive state of tumour-specific T cells might thus yield better clinical outcomes than the blockade of a single pathway.
A combination of a LAG3-specific mAb with a tumour vaccine also increases the number of tumour-infiltrating CTLs and promotes tumour regression in a murine tumour model108. A LAG3-targeting mAb thus holds great potential to improve the efficacy of tumour vaccines or PD1-targeting mAbs in patients with cancer. Meanwhile, an RFP of LAG3 and human IgG1 (IMP321) has shown adjuvant properties by activating DCs through MHCII crosslinking109. IMP321 enhanced the immunogenicity of influenza and hepatitis B virus (HBV) vaccines in Phase I trials110,111. As a monotherapy in a Phase I trial treating patients with advanced renal cell carcinoma, IMP321 showed no dose-limiting toxicity and no clinically significant adverse events in the dose range tested112. IMP321 is currently being evaluated in Phase I/II trials for the treatment of advanced melanoma or pancreatic cancer in combination with a peptide vaccine and/or chemotherapy.
PD1H
VISTA113, also called PD1H114, has been identified as an Ig family member with immune-inhibitory functions. It has the highest sequence similarity with PD1 and the extracellular domain structure of a single IgV. PD1H has a broad expression pattern in the haematopoietic system, including T cells, NK cells, macrophages, DCs and neutrophils, with the exception of B cells. Overexpression of PD1H on murine tumour cells increased their immunogenicity, and a PD1H-specific mAb exacerbated the development of EAE in mice113. Treatment with a single dose of PD1H-specific mAb treatment prevents the induction of GVHD and promotes survival in mouse models114, indicating the therapeutic value of PD1H-specific mAbs in manipulating immune responses.
The TNF and TNFR superfamilies
Members of the TNF and TNFR family provide divergent signals that are essential for haematopoiesis, the development of secondary lymphoid organs, innate and adaptive immune responses and bone absorption. For T and B lymphocytes, TNF and TNFR family molecules are essential for differentiation, effector functions and the formation of a memory T cell population115. Both TNF ligands and receptors have a trimeric structure as their basic units. According to their intracellular domain and cellular functions, TNFRs can be classified into three main groups: death domain-containing TNFRs, decoy TNFRs and TNFR-associated factor (TRAF)-recruiting TNFRs. Death domain-containing TNFR family members include CD95 (also known as FAS), TNFR1, death receptor 3 (DR3), DR4 (also known as TRAILR1), DR5 (also known as TRAILR2) and DR6. Crosslinking of death domain-containing TNFRs by TNF ligands triggers caspase activation and apoptosis, which have been extensively reviewed in the literature116,117. Several therapeutic agonistic mAbs targeting tumour-expressed death domain-containing TNFRs induce cancer cell destruction and are currently in clinical trials118–120. Decoy TNFRs, including decoy receptor 1 (DCR1; also known as TRAILR3), DCR2 (also known as TRAILR4), DCR3 and osteoprotegerin (OPG), are receptors without intracellular signalling domains. They bind and neutralize TNF ligands and block downstream signalling.
The rest — and the majority — of TNFRs belong to the third family: TRAF-recruiting TNFRs. Upon engagement with TNF ligands, these TNFRs recruit distinctive TRAF family adaptor proteins through intracellular domains to differentially activate MAPK signalling cascades, promote nuclear factor-κB (NF-κB) activation and enhance cellular proliferation and function. In addition to the successful neutralizing biologics against TNF for treating TNF-mediated inflammation, therapeutic biologics targeting other members of the TNF superfamily have been shown to be promising for the treatment of cancer, bacterial and viral infections as well as autoimmune diseases121.
Death domain-containing TNFR pathways
The engagement of death domain-containing TNFRs by TNF ligands or agonistic mAbs induces apoptosis, representing promising therapeutic strategies if tumour cells or inflammatory effector cells can be specifically targeted. Systematic administration of TNF, CD95 ligand (CD95L; also known as FASL) or agonistic mAbs directed at TNFRs, however, causes severe liver toxicity as a result of massive hepatocyte apoptosis116. By contrast, targeting TNF-related apoptosis-inducing ligand (TRAIL; also known as TNFSF10) receptor pathways has shown encouraging antitumour results in animal models117. TRAIL, a soluble TNF ligand, is expressed and secreted by activated lymphocytes and DCs. TRAIL engages two death domain-containing receptors, TRAIL receptor 1 (TRAILR1; also known as DR4) and TRAILR2 (also known as DR5), to induce apoptosis. TRAIL can also be neutralized by binding to three decoy receptors, TRAILR3 (also known as DCR1), TRAILR4 (also known as DCR2) and OPG.
TRAILR1 has a low expression on normal tissues compared with TNFR1 and FAS, and has been shown to negatively regulate the innate immune response. By contrast, TRAILR2 is induced on activated lymphocytes and overexpressed on some tumour cells. In line with the expression pattern of TRAILR1 and TRAILR2, agonistic mAbs directed against TRAILR-positive tumour cells cause limited toxicity to normal tissues. Alternatively, a single-chain variable fragment (scFv) targeting a tumour surface antigen can be fused with CD95L and TRAIL, which can provide tumour-specific targeting and delivers the trimeric CD95L and TRAIL fusion protein locally to the tumour site. Meanwhile, prodrugs consisting of apoptosis-inducing TNF ligands (such as TNF, CD95L and TRAIL) fused with a protease cleavage sequence are being developed. For these drugs, TNF ligands are only activated upon entering the tumour microenvironment, where they are proteolytically released by tumour-associated proteases117.
Several therapeutic biologics targeting the TRAIL–TRAILR pathway are currently in clinical trials for the treatment of cancer. Two recombinant human TRAIL proteins, dulanermin and AMG 951, in combination with rituximab, bevacizumab (Avastin; Roche/Genentech), cetuximab (Erbitux; Bristol-Myers Squibb/Lilly) or chemotherapy, are being evaluated for the treatment of metastatic colorectal cancers, non-Hodgkin's lymphoma and NSCLC. Mapatumumab (HGS-ETR1), an agonistic human TRAILR1-targeting mAb, is being used to treat solid tumours alone or in combination with chemotherapy. In completed Phase I/II trials treating non-Hodgkin's lymphoma, colorectal cancer and NSCLC, mapatumumab was well tolerated without hepato toxicity. In a clinical trial of non-Hodgkin's lymphoma, 2 out of 40 patients experienced complete responses and one further patient had a partial response173. Similarly, three TRAILR2-targeting mAbs — conatumumab, lexatumumab and CS-1008 — are in clinical trials for multiple cancers, alone or in combination with other mAbs or chemotherapy regimens. Recently, results were reported of Phase I trials using conatumumab and lexatumumab to treat advanced solid tumours118–120. Conatumumab and lexatumumab were well tolerated and had no dose-limiting toxicity in the dose range tested. In summary, biologics targeting TRAIL and TRAILRs appear to have promising antitumour efficacy with limited hepatic toxicity.
BAFF–APRIL and BCMA–TACI–BAFFR pathways
B cell activation factor (BAFF; also known as BLYS and TNFSF13B) was first identified as a monocyte-expressed TNF family ligand that promotes B cell activation and was later shown to be one of the most important co-stimulatory ligands for B cell development and function122. BAFF and its homologue APRIL (a proliferation-inducing ligand) bind to B cell maturation antigen (BCMA) as well as transmembrane activator and CAML interactor (TACI) to promote B cell development, class switching and survival123,124. BAFF, but not APRIL, also binds to BAFF receptor (BAFFR) to promote the development and survival of B2 cells and marginal zone B cells125.
In 2011, belimumab (Benlysta; Human Genome Sciences/GlaxoSmithKline), a human IgG1 mAb targeting BAFF, was the first drug to be approved by the FDA for the treatment of SLE in 50 years. Belimumab sequesters soluble and membrane-bound BAFF and suppresses B cell proliferation and antibody production, which significantly reduces levels of serum immunoglobulin and autoantibodies in patients with SLE, especially autoantibodies against nuclear and double-stranded DNA. A reduction in various circulating B cell populations was observed in the treatment group, which prompted the evaluation of belimumab in multiple clinical trials for the treatment of other B cell-mediated autoimmune diseases, including rheumatoid arthritis and Sjögren's syndrome, as well as B cell malignancies such as Waldenström's macro-globulinaemia125. Moreover, atacicept, a fusion protein of TACI and human Ig with the ability to neutralize BAFF and APRIL and thus block BCMA–TACI–BAFFR activation, is currently in different phases of clinical trials to treat SLE, rheumatoid arthritis, relapsing multiple sclerosis, optic neuritis and B cell malignancies, as a monotherapy or in combination with chemotherapy drugs or other biologics (TNF- and CD20-targeting biologics)125.
CD40L-CD40 pathway
CD40 is expressed on B cells, DCs and macrophages, and has an essential function in controlling T cell-dependent B cell responses, including B cell proliferation, survival, Ig production, class switching and memory B cell formation126. CD40 ligand (CD40L; also known as CD154) is expressed on activated T cells, B cells and other myeloid cells. CD40L mutations have been identified in humans and are associated with hyper IgM syndrome, which is characterized by defects in Ig class switching and germinal centre formation. Similarly, mice in which CD40l or CD40 is genetically ablated have profound defects in humoral immune responses126. Antagonistic mAbs targeting the CD40L–CD40 pathway have thus been in development for the treatment of autoimmune conditions since 2004.
Conversely, recombinant CD40L protein and CD40-targeting agonistic mAbs have the ability to promote antitumour immunity in animal models and are thus being evaluated in several cancer trials. In clinical trials, CD40L-targeting mAbs have been shown to cause severe thrombosis in patients by unexpectedly inducing massive platelet activation. By contrast, CD40-targeting mAbs have proven to be a safer approach for either blocking the CD40–CD40L pathway (to treat autoimmune conditions) or for inducing CD40 signalling (to promote antitumour immunity).
In completed Phase I trials of two agonistic CD40-targeting mAbs, CP-870,893 and dacetuzumab, treating solid tumours or B cell non-Hodgkin's lymphoma, respectively, no dose-limiting toxicity and dose-dependent adverse events were observed and the mAbs were associated with objective responses127,128. It was originally believed that CD40 crosslinking first activates APCs, which in turn promote the priming and expansion of tumour-specific T cells. However, a new underlying mechanism of agonistic CD40-targeting mAbs in cancer treatment has recently emerged. CP-870,893, in combination with the chemotherapy agent gemcitabine, has shown efficacy against pancreatic ductal adenocarcinoma by directly activating tumour-infiltrating macrophages to destroy the tumour stroma independently of T cell activities129.
CD137L–CD137 pathway
CD137 was first cloned from a T cell cDNA library in 1989 (ref. 130). Subsequent studies showed that CD137 has a broad inducible expression pattern in both the haematopoietic system (including T and B lymphocytes, NK and NKT cells, monocytes and DCs) and in non-haematopoietic cells (including epithelial, endothelial and smooth muscle cells)131. CD137 ligand (CD137L) is mainly expressed on APCs (DCs, B cells and macrophages) and is inducibly expressed on activated T cells and endothelial cells. In line with its expression pattern, CD137 signalling modulates both innate and adaptive immune responses. In the presence of a TCR signal, agonistic CD137-targeting mAbs co-stimulate the proliferation of both CD4+ and CD8+ T cells, induce cytokine production, enhance the cytotoxic activity of CTLs and protect T cells from activation-induced cell death132. A recent report shows that a CD137-targeting mAb stimulates memory T cell expansion in the absence of cognate antigens133 and improves CD8 memory T cell survival. CD137 crosslinking on NK cells stimulates NK cell proliferation and IFNγ production134, whereas the engagement of CD137 on DCs promotes DC activation by enhancing cytokine production and B7 upregulation135.
Consistent with their immune-activating properties in vitro, agonistic CD137-targeting mAbs have been shown to be potent antitumour reagents that utilize multiple mechanisms in many animal tumour models136,137. The antitumour effects of these CD137 agonists have also been validated by CD137L–Fc RFPs138, CD137 aptamers (agonistic synthetic nucleic acid species)139 and CD137L-transfected tumour cells121. Using targeted depletion, CD8+ T cells have been shown to be essential for the antitumour effects of agonistic CD137-targeting mAbs in vivo; CD4+ T cells, NK cells, DCs and non-haemato poietic cells also have roles — depending on the models used136,137,140. In addition to the direct effects on immune cells, CD137-targeting mAbs upregulate cell adhesion molecules including intercellular adhesion molecule 1 (ICAM1) and vascular cell adhesion molecule 1 (VCAM1) on endothelial cells to recruit and retain activated T cells at the tumour site141. Furthermore, in a peptide-induced T cell anergy model, administration of agonistic CD137-targeting mAbs prevented anergy formation and also reversed established anergy142, suggesting that agonistic CD137-targeting mAbs could potentially disrupt immune tolerance in the tumour microenvironment or during chronic viral infections.
In addition to their profound antitumour effects, agonistic CD137-targeting mAbs surprisingly display anti-inflammatory properties in several autoimmune disease models, including EAE143, chronic GVHD144, CIA145, colitis146, asthma147, type 1 diabetes in NOD mice148 and SLE-like disease in Fas (lpr)-deficient or NZB/NZW F1 mice149,150. The underlying mechanism of the anti-inflammatory effects of agonistic CD137-targeting mAbs includes apoptosis of autoreactive T cells and B cells as well as expansion of TReg cells. Conversely, in HBV-transgenic mice, agonistic CD137-targeting mAbs induced liver toxicity mediated by CD8 T cell expansion and the accumulation of CTLs as a result of enhanced resistance to activation-induced cell death151. Repetitive injection of CD137-targeting mAbs152 or systemic transgenic CD137L expression153 progressively depletes B cells in vivo, indicating the potential for inducing B cell toxicity when the CD137 pathway is persistently activated.
In a completed Phase I/II trial treating late-stage cancers including melanoma, renal cell carcinoma and ovarian cancer, BMS-663513 — a fully human agonistic CD137-targeting mAb — caused manageable autoimmune adverse effects when administrated at dose levels ranging from 0.3 mg per kg to 10 mg per kg. Following treatment with BMS-663513, 6% of patients with melanoma had partial responses, and 17% of patients with melanoma and 14% of patients with renal cell carcinoma had no tumour progression for more than 6 months154. In a separate Phase I trial treating advanced solid tumours, however, severe liver toxicity (grade 4 hepatitis) was observed with BMS-663513 monotherapy, especially at high doses, leading to the termination of the trial. Detailed biomarker studies from the completed Phase I/II trial indicated that the 0.3 mg per kg dose of BMS-663513 was sufficient to elicit antitumour activity without causing significant liver toxicity, indicating that a low dosing regimen could potentially strike the balance between antitumour efficacy and autoimmunity.
OX40L–OX40 pathway
OX40 shares many functional similarities with the CD137 receptor in the control of T cell activation, expansion, survival and memory T cell formation, but with a preferential effect on CD4+ T cells115. This is in line with the OX40 expression pattern, which is restricted to CD4+ cells and CD4+FOXP3+ (forkhead box P3-positive) TReg cells. Like biologics targeting CD137, OX40-targeting agonistic mAbs show therapeutic efficacy against tumours in several animal models. Depletion experiments indicate that CD8+ and CD4+ T cells, and sometimes NKT cells, are the cellular components that mediate the antitumour effects of OX40-specific mAbs in vivo. With high levels of OX40 expression on TReg cells, one agonistic OX40-specific mAb has recently been shown to prevent the generation of the inducible TReg (iTReg) cell population in the tumour microenvironment and thus improve the antitumour immune response155. Furthermore, in a murine B16 tumour model the combination of the chemotherapy drug cyclophosphamide with an OX40-specific agonistic mAb induced profound TReg cell deletion through activation-induced cell death in the tumour microenvironment and led to the regression of established tumours156. An OX40-specific mAb sponsored by Providence Health & Services is currently in Phase I trials for the treatment of metastatic prostate cancer as a monotherapy or in combination with radiation therapy and cyclophosphamide.
Conversely, owing to its prominent role in controlling CD4+ T cell activation and T cell-dependent B cell responses, blockade of the OX40–OX40L pathway alleviates the severity of inflammatory or autoimmune diseases in several murine models, including EAE, CIA and airway inflammation115. RO4989991, a human OX40L-targeting mAb, is being evaluated in Phase II trials for the treatment of asthma.
CD27–CD70 pathway
CD27 (also known as TNFRSF7), another CD137-related TNFR, is mainly expressed on CD4+ T cells, CD8+ T cells, B cells and NK cells, whereas its counter-receptor CD70 is expressed on DCs as well as T and B lymphocytes115. In contrast to the absence of CD137 on naive T cells, CD27 is highly expressed on these cells. It is downregulated on activated T cells after repetitive antigenic stimulation, and CD27 downregulation is associated with enhanced effector functions. Crosslinking of CD27 by mAbs or CD70 fusion proteins co-stimulates the proliferation of both CD4+ and CD8+ T cells as well as cytokine production in vitro and in vivo. It has been shown that CD27 mainly promotes T cell survival rather than stimulating cell cycle progression157. In a model of influenza infection, CD27 was crucial for the expansion of virus-specific T cells and the accumulation of CTLs in the lung. Similarly, agonistic CD27-targeting mAbs expanded tumour-specific CTLs and eradicated established tumours in multiple murine tumour models158.
Engineered human T cells expressing a chimeric antigen receptor (CAR) with an intracellular region consisting of the CD3 ζ-chain signalling domain and a CD27 intracellular co-stimulatory motif show substantially improved expansion, survival and effector functions in vitro compared with T cells expressing the CAR–CD3 ζ-chain alone. When infused into tumour-bearing mice in a xenograft model, CAR–CD3–CD27 T cells showed increased survival and enhanced effector functions, leading to tumour regression159. Similarly, CAR-expressing T cells with CD3 ζ-chains and a CD137 or CD28 intracellular motif showed increased expansion and eradicated xenograft tumours in vivo. Interestingly, among the three constructs, the CD27 and CD137 intracellular motifs conferred better survival in vivo than the CD28 construct159, indicating that targeting multiple co-stimulatory receptors might have added value by enhancing different aspects of T cell activation and effector functions. CDX-1127, a human IgG1 agonistic mAb targeting CD27, is being evaluated in a Phase I trial treating haematological and solid tumours.
GITRL–GITR pathway
Glucocorticoid-induced TNFR-related protein (GITR; also known as AITR and TNFRSF18) has a broad distribution on haematopoietic cells, including natural TReg cells and activated T, B, NK and myeloid cells115. Unlike most other TNF ligands, GITR ligand (GITRL) has limited expression on APCs but it is constitutively expressed on peripheral tissues and presumably engages GITR on tissue-infiltrating immune cells. GITR-targeting agonistic mAbs co-stimulate T cell proliferation and cytokine production in vitro, and induce tumour regression in vivo through the activation of CD4+ T cells, CD8+ T cells and NK cells in several tumour models160,161. With its prominent expression on natural TReg cells, GITR has been a target for manipulating TReg function. GITR-targeting agonistic mAbs expand CD4+CD25− T cells, abrogate CD4+CD25+ TReg cell-mediated suppression and induce autoimmune gastritis and colitis in animal models162,163. However, evidence indicates that the expansion of CD4+ effector cells, rather than TReg inhibition, is the primary mechanism underlying the antitumour effects mediated by GITR-targeting mAbs164. TRX518, a humanized GITR-targeting mAb, is currently in Phase I clinical trials treating patients with late-stage melanoma.
Combination therapy
Biologics targeting individual Ig or TNFR family members, as described above, have shown promising therapeutic effects as monotherapies. Meanwhile, several immunomodulatory biologics targeting complementary pathways or combinations of immunomodulatory biologics with traditional chemotherapy, radiation therapy, vaccination or targeted depletion have shown promising synergistic effects in animal models and are being extensively explored in ongoing clinical trials (Table 3).
Combinations with direct tumour-killing therapy
Traditional chemotherapy and radiation therapy, together with depleting mAbs or treatment with small-molecule inhibitors, all directly target and kill cancer cells, leading to the destruction of the tumour stroma and the release of tumour antigens. When coupled with these direct killing mechanisms, immunomodulatory biologics promote the priming and expansion of existing tumour-specific T cells and their de novo generation, with a potential to form long-lasting and self-sustained antitumour responses. In recent years, small-molecule inhibitors targeting tumours that harbour mutated BRAF (vemurafenib (Zelboraf; Plexxikon/Roche)) or translocated BCR–ABL (imatinib (Gleevec; Novartis)) have shown high initial response rates in clinical trials165. However, the duration of the antitumour response is limited owing to acquired drug resistance. A combination of these fast-acting small-molecule inhibitors with immune co-inhibitory blockade — for example, with CTLA4-specific or PD1-specific mAbs — could promote the priming and expansion of tumour-specific CTLs against multiple tumour antigens and/or epitopes, prevent the generation of escape variants or drug-resistant mutant cancer cells and induce sustained T cell responses.
Combinations with cancer vaccines
As validated in numerous animal models of cancer, tumour vaccine approaches — including whole-tumour lysate, tumour antigen peptide or DC vaccines — greatly enhance the efficacy of immunomodulatory biologics. One main drawback of the vaccine approach is that both antigen-specific effector T cells and suppressive TReg cells are expanded following antigenic stimulation. Although antigen-based vaccination was shown to frequently activate tumour-specific CTLs in the bloodstream, with strong antitumour functions in vitro, these cells are often inhibited in the tumour microenvironment owing to the presence of inhibitory molecules on tumour cells as well as the presence of suppressive cells in the tumour stroma, such as TReg cells, tumour-associated macrophages and myeloid suppressor cells. A combination of PD1 blockade with a tumour vaccine plus TReg cell depletion is being evaluated in several clinical trials (Table 3).
Combinations with other immunomodulatory biologics and/or targeting of multiple cellular components
Combining agonistic mAbs targeting co-stimulatory receptors with agents that induce the blockade of co-inhibitory receptors is another highly effective approach for stimulating efficient antitumour immunity and has been validated in various animal tumour models: for example, the combination of CD137-targeting agonistic mAbs with CTLA4 or PD1 blockade75. One such combination, a CD40-targeting mAb plus a CTLA4-targeting mAb, is being tested in a Phase I trial for the treatment of late-stage melanoma. However, careful trial design and close clinical monitoring is required to monitor potentially enhanced autoimmunity in such a combination therapy.
In xenograft tumour models, it was recently demonstrated that CD137-targeting agonistic mAbs activate NK cells and synergize with tumour-targeting biologics and/or biologics that induce antibody-dependent cell-mediated cytotoxicity, such as rituximab (a CD20-specific mAb), cetuximab (a mAb targeting epidermal growth factor receptor) and the HER2 (also known as ERBB2)-targeting mAb trastuzumab (Herceptin; Roche/Genentech), thus paving the way for a sequential antibody combination therapy in the clinic166. PF-05082566, an agonistic CD137-targeting mAb, is being tested for the treatment of CD20+ non-Hodgkin's lymphoma in combination with rituximab in a Phase I trial.
Combination targeting of several tumour-killing effector cell populations, such as T cells and NK cells, is also being evaluated in clinical trials. IPH21, a blocking antibody against NK inhibitory receptors (killer cell immunoglobulin-like receptor 2DL1 (KIR2DL1), KIR2DL2 and KIR2DL3), induces NK cell activation and was combined with PD1 blockade (using BMS-936558) to treat advanced solid tumours. IPH21–BMS-936558 combination treatment intends to utilize both innate and adaptive tumour-specific killing mechanisms to achieve a better clinical outcome than PD1 blockade alone.
Challenges and perspectives
Over the past decade, cell surface signalling molecules have emerged as crucial targets for the treatment of cancer and immune disorders. Immunomodulatory mAbs or RFPs have had a chequered history as therapeutics but have finally become one of the most promising approaches for the treatment of human diseases. With the promise to induce long-lasting tolerance in autoimmunity or memory responses (immunity) against tumours, immunomodulatory biologics represent a distinctive class of drugs that target and correct aberrant immune responses. They might even hold the key to fully realizing the potential of cancer vaccines, which have resulted in more disappointment than success in recent clinical trials.
mAbs and RFPs are the preferred therapeutics for targeting cell surface signalling molecules because of their specificity, high affinity (in the nanomolar range), stability and the accessibility of their targets. By contrast, small-molecule inhibitors penetrate the cell membrane at ease and thus hold great advantage for targeting intracellular signalling pathways to modulate immune responses; such small-molecule inhibitors include cyclosporin A (targeting calcineurin), sirolimus (targeting mammalian target of rapamycin (mTOR)) and tasocitinib (targeting the Janus kinase (JAK) and signal transducer and activator of transcription (STAT) pathway)167. With the aid of structural analysis and high-throughput screening methods, small-molecule inhibitors disrupting immunomodulatory cell surface interactions have also been explored as a means to treat immune disorders168, including IL-2–IL-2 receptor (IL-2R)169, B7.1–CD28 (ref. 170) and CD40–CD40L171 pathway inhibitors, but these have had limited success in moving into the clinic. The common drawbacks for this group of small-molecule inhibitors include lack of specificity, low affinity (in the micromolar range) and the disassociation of biological effect with blocking activity. However, with an expanding repertoire of small molecules and the advancement of crystal structure analyses of surface interactions, the perfect small-molecule inhibitor might be waiting to be identified.
When a given cell surface signalling molecule target is a receptor, it is desirable that both agonistic and antagonistic biologics could be obtained so that all aspects of therapeutic options can be explored. Currently, a major technical hurdle in targeting a receptor is the difficulty of obtaining both agonistic and antagonistic reagents. For example, although a co-stimulatory mAb for CD28 was generated more than 20 years ago, antagonistic CD28-specific antibodies are not yet available for therapeutic manipulation. In theory, such a CD28-specific antagonist will be ideal for the suppression of primary immune responses because this strategy will preserve the interactions of B7.1 and B7.2 with CTLA4 for co-inhibition and would be more efficacious than a CTLA4–Ig fusion protein, which blocks both co-stimulation and co-inhibition. Similarly, although antagonistic PD1-specific mAbs have been generated to enhance immune responses against cancer and viral infections, we have yet to obtain a PD1-specific agonist that can suppress T and B cell immunity to explore its use for dampening inflammation and autoimmunity. Although it is relatively easy to identify and select therapeutic antagonistic mAbs when the interacting receptor and ligand are known, the identification of agonistic mAbs is not as straightforward. This is largely due to the lack of reliable assays in the human system. The recent development of humanized mice or selective human gene knock-in mice may provide possible solutions for overcoming this obstacle.
Acknowledgements
The authors thank B. Cadugan for manuscript editing. This work has been supported by the US National Institutes of Health (NIH) grants CA142779, CA121974, CA97085, CA16359, CA86721, CA113341 and AI72592.
Footnotes
Competing interests statement The authors declare no competing financial interests.
References
- 1.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nature Rev. Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 2.Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nature Rev. Immunol. 2010;10:317–327. doi: 10.1038/nri2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Yao S, Chen L. Cell surface signaling molecules in the control of immune responses: a tide model. Immunity. 2011;34:466–478. doi: 10.1016/j.immuni.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carreno BM, Collins M. The B7 family of ligands and its receptors: new pathways for costimulation and inhibition of immune responses. Annu. Rev. Immunol. 2002;20:29–53. doi: 10.1146/annurev.immunol.20.091101.091806. [DOI] [PubMed] [Google Scholar]
- 5.Chen L. Co-inhibitory molecules of the B7–CD28 family in the control of T-cell immunity. Nature Rev. Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- 6.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linsley PS, Clark EA, Ledbetter JA. T-cell antigen CD28 mediates adhesion with B cells by interacting with activation antigen B7/BB-1. Proc. Natl Acad. Sci. USA. 1990;87:5031–5035. doi: 10.1073/pnas.87.13.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linsley PS, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nature Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 10.Appleman LJ, van Puijenbroek AA, Shu KM, Nadler LM, Boussiotis VA. CD28 costimulation mediates down-regulation of p27kip1 and cell cycle progression by activation of the PI3K/PKB signaling pathway in primary human T cells. J. Immunol. 2002;168:2729–2736. doi: 10.4049/jimmunol.168.6.2729. [DOI] [PubMed] [Google Scholar]
- 11.Boise LH, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. [PubMed] [Google Scholar]
- 12.Parry RV, Rumbley CA, Vandenberghe LH, June CH, Riley JL. CD28 and inducible costimulatory protein Src homology 2 binding domains show distinct regulation of phosphatidylinositol 3-kinase, Bcl-xL, and IL-2 expression in primary human CD4 T lymphocytes. J. Immunol. 2003;171:166–174. doi: 10.4049/jimmunol.171.1.166. [DOI] [PubMed] [Google Scholar]
- 13.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 15.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 16.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 17.Hutloff A, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 18.Yoshinaga SK, et al. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402:827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 19.Dong C, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 20.McAdam AJ, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 21.Yao S, et al. B7-H2 is a costimulatory ligand for CD28 in human. Immunity. 2011;34:729–740. doi: 10.1016/j.immuni.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J. Immunol. 2005;175:4886–4896. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 23.Zuberek K, et al. Comparable in vivo efficacy of CD28/B7, ICOS/GL50, and ICOS/GL50B costimulatory pathways in murine tumor models: IFNγ-dependent enhancement of CTL priming, effector functions, and tumor specific memory CTL. Cell. Immunol. 2003;225:53–63. doi: 10.1016/j.cellimm.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen L, et al. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992;71:1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang AY, Bruce AT, Pardoll DM, Levitsky HI. Does B7-1 expression confer antigen-presenting cell capacity to tumors in vivo? J. Exp. Med. 1996;183:769–776. doi: 10.1084/jem.183.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sturmhoefel K, et al. Potent activity of soluble B7-IgG fusion proteins in therapy of established tumors and as vaccine adjuvant. Cancer Res. 1999;59:4964–4972. [PubMed] [Google Scholar]
- 27.Runyon K, et al. The combination of chemotherapy and systemic immunotherapy with soluble B7-immunoglobulin G leads to cure of murine leukemia and lymphoma and demonstration of tumor-specific memory responses. Blood. 2001;97:2420–2426. doi: 10.1182/blood.v97.8.2420. [DOI] [PubMed] [Google Scholar]
- 28.Liu A, Hu P, Khawli LA, Epstein AL. Combination B7–Fc fusion protein treatment and Treg cell depletion therapy. Clin. Cancer Res. 2005;11:8492–8502. doi: 10.1158/1078-0432.CCR-05-1411. [DOI] [PubMed] [Google Scholar]
- 29.Wallin JJ, Liang L, Bakardjiev A, Sha WC. Enhancement of CD8+ T cell responses by ICOS/B7H costimulation. J. Immunol. 2001;167:132–139. doi: 10.4049/jimmunol.167.1.132. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, et al. B7H costimulates clonal expansion of, and cognate destruction of tumor cells by, CD8+ T lymphocytes in vivo. J. Exp. Med. 2001;194:1339–1348. doi: 10.1084/jem.194.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwon ED, et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc. Natl Acad. Sci. USA. 1999;96:15074–15079. doi: 10.1073/pnas.96.26.15074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 34.Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J. Immunol. 2001;166:3908–3914. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 35.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi T, et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaehler KC, et al. Update on immunologic therapy with anti-CTLA-4 antibodies in melanoma: identification of clinical and biological response patterns, immune-related adverse events, and their management. Semin. Oncol. 2010;37:485–498. doi: 10.1053/j.seminoncol.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Hodi FS, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl Acad. Sci. USA. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phan GQ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thompson CB, et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc. Natl Acad. Sci. USA. 1989;86:1333–1337. doi: 10.1073/pnas.86.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suntharalingam G, et al. Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N. Engl. J. Med. 2006;355:1018–1028. doi: 10.1056/NEJMoa063842. [DOI] [PubMed] [Google Scholar]
- 43.Grohmann U, et al. CTLA-4–Ig regulates tryptophan catabolism in vivo. Nature Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 44.Sharpe AH, Freeman GJ. The B7–CD28 superfamily. Nature Rev. Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 45.Bluestone JA, St Clair EW, Turka LA. CTLA4Ig: bridging the basic immunology with clinical application. Immunity. 2006;24:233–238. doi: 10.1016/j.immuni.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Thompson RH, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl Acad. Sci. USA. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Larsen CP, et al. Belatacept-based regimens versus a cyclosporine A-based regimen in kidney transplant recipients: 2-year results from the BENEFIT and BENEFIT-EXT studies. Transplantation. 2010;90:1528–1535. doi: 10.1097/TP.0b013e3181ff87cd. [DOI] [PubMed] [Google Scholar]
- 48.Vinjamaram S, Czuczman MS, Hernandez-Ilizaliturri FJ. The use of galiximab in non-Hodgkin lymphoma. Clin. Lymphoma Myeloma. 2008;8:277–282. doi: 10.3816/CLM.2008.n.038. [DOI] [PubMed] [Google Scholar]
- 49.Czuczman MS, et al. Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed or refractory follicular lymphoma. J. Clin. Oncol. 2005;23:4390–4398. doi: 10.1200/JCO.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 50.Tafuri A, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 51.Grimbacher B, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nature Immunol. 2003;4:261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 52.Hu YL, Metz DP, Chung J, Siu G, Zhang M. B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J. Immunol. 2009;182:1421–1428. doi: 10.4049/jimmunol.182.3.1421. [DOI] [PubMed] [Google Scholar]
- 53.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nature Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 54.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp. Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 56.Wiendl H, et al. Human muscle cells express a B7-related molecule, B7-H1, with strong negative immune regulatory potential: a novel mechanism of counterbalancing the immune attack in idiopathic inflammatory myopathies. FASEB J. 2003;17:1892–1894. doi: 10.1096/fj.03-0039fje. [DOI] [PubMed] [Google Scholar]
- 57.Yamazaki T, et al. Expression of programmed death 1 ligands by murine T cells and APC. J. Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 58.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;1:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Agata Y, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 60.Yao S, et al. PD-1 on dendritic cells impedes innate immunity against bacterial infection. Blood. 2009;113:5811–5818. doi: 10.1182/blood-2009-02-203141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Said EA, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nature Med. 2010;16:452–459. doi: 10.1038/nm.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nature Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 64.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 65.Okazaki T, et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nature Med. 2003;9:1477–1483. doi: 10.1038/nm955. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 67.Wang J, et al. Establishment of NOD-Pdcd1−/− mice as an efficient animal model of type I diabetes. Proc. Natl Acad. Sci. USA. 2005;102:11823–11828. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tsushima F, et al. Interaction between B7-H1 and PD-1 determines initiation and reversal of T-cell anergy. Blood. 2007;110:180–185. doi: 10.1182/blood-2006-11-060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao S, Chen L. Reviving exhausted T lymphocytes during chronic virus infection by B7-H1 blockade. Trends Mol. Med. 2006;12:244–246. doi: 10.1016/j.molmed.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 71.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nature Rev. Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 72.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nature Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 73.Thompson RH, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 74.Zajac AJ, et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirano F, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005;65:1089–1096. [PubMed] [Google Scholar]
- 76.Azuma T, et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood. 2008;111:3635–3643. doi: 10.1182/blood-2007-11-123141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strome SE, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63:6501–6505. [PubMed] [Google Scholar]
- 78.Iwai Y, et al. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl Acad. Sci. USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park JJ, et al. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116:1291–1298. doi: 10.1182/blood-2010-01-265975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yi T, et al. Host APCs augment in vivo expansion of donor natural regulatory T cells via B7H1/B7.1 in allogeneic recipients. J. Immunol. 2011;186:2739–2749. doi: 10.4049/jimmunol.1002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brahmer JR, et al. Phase I study of single-agent antiprogrammed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 84.Taube JM, et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl. Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mkrtichyan M, et al. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 2011;41:2977–2986. doi: 10.1002/eji.201141639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nature Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 88.Derre L, et al. BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination. J. Clin. Invest. 2010;120:157–167. doi: 10.1172/JCI40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sakoda Y, et al. Dichotomous regulation of GVHD through bidirectional functions of the BTLA-HVEM pathway. Blood. 2011;117:2506–2514. doi: 10.1182/blood-2010-08-301325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Albring JC, et al. Targeting of B and T lymphocyte associated (BTLA) prevents graft-versus-host disease without global immunosuppression. J. Exp. Med. 2010;207:2551–2559. doi: 10.1084/jem.20102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 92.Sica GL, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 93.Zang X, et al. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc. Natl Acad. Sci. USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kryczek I, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J. Exp. Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kryczek I, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 96.Choi IH, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J. Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 97.Arigami T, et al. Expression of B7-H4 in blood of patients with gastric cancer predicts tumor progression and prognosis. J. Surg. Oncol. 2010;102:748–752. doi: 10.1002/jso.21722. [DOI] [PubMed] [Google Scholar]
- 98.Jiang J, et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol. Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-H4 expression in human melanoma: its association with patients' survival and antitumor immune response. Clin. Cancer Res. 2011;17:3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 100.Azuma T, et al. Potential role of decoy B7-H4 in the pathogenesis of rheumatoid arthritis: a mouse model informed by clinical data. PLoS Med. 2009;6:e1000166. doi: 10.1371/journal.pmed.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang X, et al. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60:3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Triebel F, et al. LAG-3, a novel lymphocyte activation gene closely related to CD4. J. Exp. Med. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin. Ther. Targets. 2011;15:91–101. doi: 10.1517/14712598.2011.540563. [DOI] [PubMed] [Google Scholar]
- 104.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J. Immunol. 1998;161:4058–4065. [PubMed] [Google Scholar]
- 105.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nature Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T cell function to promote tumoral immune escape. Cancer Res. 2011;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc. Natl Acad. Sci. USA. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grosso JF, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J. Clin. Invest. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fougeray S, Brignone C, Triebel F. A soluble LAG-3 protein as an immunopotentiator for therapeutic vaccines: preclinical evaluation of IMP321. Vaccine. 2006;24:5426–5433. doi: 10.1016/j.vaccine.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 110.Brignone C, Grygar C, Marcu M, Perrin G, Triebel F. IMP321 (sLAG-3) safety and T cell response potentiation using an influenza vaccine as a model antigen: a single-blind phase I study. Vaccine. 2007;25:4641–4650. doi: 10.1016/j.vaccine.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 111.Brignone C, Grygar C, Marcu M, Perrin G, Triebel F. IMP321 (sLAG-3), an immunopotentiator for T cell responses against a HBsAg antigen in healthy adults: a single blind randomised controlled phase I study. J. Immune Based Ther. Vaccines. 2007;5:5. doi: 10.1186/1476-8518-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin. Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 113.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Flies DB, Wang S, Xu H, Chen L. Cutting edge: a monoclonal antibody specific for the programmed death-1 homolog prevents graft-versus-host disease in mouse models. J. Immunol. 2011;187:1537–1541. doi: 10.4049/jimmunol.1100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 116.Grewal IS. Overview of TNF superfamily: a chest full of potential therapeutic targets. Adv. Exp. Med. Biol. 2009;647:1–7. doi: 10.1007/978-0-387-89520-8_1. [DOI] [PubMed] [Google Scholar]
- 117.Abdulghani J, El-Deiry WS. TRAIL receptor signaling and therapeutics. Expert Opin. Ther. Targets. 2010;14:1091–1108. doi: 10.1517/14728222.2010.519701. [DOI] [PubMed] [Google Scholar]
- 118.Herbst RS, et al. A first-in-human study of conatumumab in adult patients with advanced solid tumors. Clin. Cancer Res. 2010;16:5883–5891. doi: 10.1158/1078-0432.CCR-10-0631. [DOI] [PubMed] [Google Scholar]
- 119.Doi T, et al. Phase 1 study of conatumumab, a pro-apoptotic death receptor 5 agonist antibody, in Japanese patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2010;68:733–741. doi: 10.1007/s00280-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 120.Wakelee HA, et al. Phase I and pharmacokinetic study of lexatumumab (HGS-ETR2) given every 2 weeks in patients with advanced solid tumors. Ann. Oncol. 2010;21:376–381. doi: 10.1093/annonc/mdp292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pardee AD, Wesa AK, Storkus WJ. Integrating costimulatory agonists to optimize immune-based cancer therapies. Immunotherapy. 2009;1:249–264. doi: 10.2217/1750743X.1.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 123.O'Connor BP, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Benson MJ, et al. Cutting edge: the dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 125.Mackay F, Schneider P. Cracking the BAFF code. Nature Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 126.Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 1998;16:111–135. doi: 10.1146/annurev.immunol.16.1.111. [DOI] [PubMed] [Google Scholar]
- 127.Vonderheide RH, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J. Clin. Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 128.Advani R, et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. J. Clin. Oncol. 2009;27:4371–4377. doi: 10.1200/JCO.2008.21.3017. [DOI] [PubMed] [Google Scholar]
- 129.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc. Natl Acad. Sci. USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vinay DS, Kwon BS. 4-1BB signaling beyond T cells. Cell. Mol. Immunol. 2011;8:281–284. doi: 10.1038/cmi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cannons JL, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 133.Zhu Y, Zhu G, Luo L, Flies AS, Chen L. CD137 stimulation delivers an antigen-independent growth signal for T lymphocytes with memory phenotype. Blood. 2007;109:4882–4889. doi: 10.1182/blood-2006-10-043463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J. Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 135.Wilcox RA, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J. Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 136.Melero I, et al. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nature Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- 137.Narazaki H, Zhu Y, Luo L, Zhu G, Chen L. CD137 agonist antibody prevents cancer recurrence: contribution of CD137 on both hematopoietic and nonhematopoietic cells. Blood. 2010;115:1941–1948. doi: 10.1182/blood-2008-12-192591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang N, et al. Targeted and untargeted CD137L fusion proteins for the immunotherapy of experimental solid tumors. Clin. Cancer Res. 2007;13:2758–2767. doi: 10.1158/1078-0432.CCR-06-2343. [DOI] [PubMed] [Google Scholar]
- 139.McNamara JO, et al. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Murillo O, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. Eur. J. Immunol. 2009;39:2424–2436. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 141.Palazon A, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res. 2011;71:801–811. doi: 10.1158/0008-5472.CAN-10-1733. [DOI] [PubMed] [Google Scholar]
- 142.Wilcox RA, et al. Ligation of CD137 receptor prevents and reverses established anergy of CD8+ cytolytic T lymphocytes in vivo. Blood. 2004;103:177–184. doi: 10.1182/blood-2003-06-2184. [DOI] [PubMed] [Google Scholar]
- 143.Sun Y, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J. Immunol. 2002;168:1457–1465. doi: 10.4049/jimmunol.168.3.1457. [DOI] [PubMed] [Google Scholar]
- 144.Kim J, et al. Stimulation with 4-1BB (CD137) inhibits chronic graft-versus-host disease by inducing activation-induced cell death of donor CD4+ T cells. Blood. 2005;105:2206–2213. doi: 10.1182/blood-2004-06-2080. [DOI] [PubMed] [Google Scholar]
- 145.Foell JL, et al. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004;113:89–98. doi: 10.1111/j.1365-2567.2004.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee J, et al. Administration of agonistic anti-4-1BB monoclonal antibody leads to the amelioration of inflammatory bowel disease. Immunol. Lett. 2005;101:210–216. doi: 10.1016/j.imlet.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 147.Sun Y, et al. Inhibition of Th2-mediated allergic airway inflammatory disease by CD137 costimulation. J. Immunol. 2006;177:814–821. doi: 10.4049/jimmunol.177.2.814. [DOI] [PubMed] [Google Scholar]
- 148.Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes. 2007;56:186–196. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 149.Sun Y, et al. Costimulatory molecule-targeted antibody therapy of a spontaneous autoimmune disease. Nature Med. 2002;8:1405–1413. doi: 10.1038/nm1202-796. [DOI] [PubMed] [Google Scholar]
- 150.Foell J, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB × NZW F1 mice. J. Clin. Invest. 2003;111:1505–1518. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang J, et al. CD137-mediated pathogenesis from chronic hepatitis to hepatocellular carcinoma in hepatitis B virus-transgenic mice. J. Immunol. 2010;185:7654–7662. doi: 10.4049/jimmunol.1000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lee SW, Salek-Ardakani S, Mittler RS, Croft M. Hypercostimulation through 4-1BB distorts homeostasis of immune cells. J. Immunol. 2009;182:6753–6762. doi: 10.4049/jimmunol.0803241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhu G, et al. Progressive depletion of peripheral B lymphocytes in 4-1BB (CD137) ligand/I-Eα-transgenic mice. J. Immunol. 2001;167:2671–2676. doi: 10.4049/jimmunol.167.5.2671. [DOI] [PubMed] [Google Scholar]
- 154.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin. Oncol. 2010;37:508–516. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 155.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J. Exp. Med. 2008;205:825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hirschhorn-Cymerman D, et al. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J. Exp. Med. 2009;206:1103–1116. doi: 10.1084/jem.20082205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J. Exp. Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sakanishi T, Yagita H. Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochem. Biophys. Res. Commun. 2010;393:829–835. doi: 10.1016/j.bbrc.2010.02.092. [DOI] [PubMed] [Google Scholar]
- 159.Song DG, et al. CD27 costimulation augments the survival and anti-tumor activity of redirected human T cells in vivo. Blood. 2011;119:696–706. doi: 10.1182/blood-2011-03-344275. [DOI] [PubMed] [Google Scholar]
- 160.Ramirez-Montagut T, et al. Glucocorticoid-induced TNF receptor family related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J. Immunol. 2006;176:6434–6442. doi: 10.4049/jimmunol.176.11.6434. [DOI] [PubMed] [Google Scholar]
- 161.Zhou P, L'Italien L, Hodges D, Schebye XM. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb-induced-immune activation and tumor immunity in CT26 tumors. J. Immunol. 2007;179:7365–7375. doi: 10.4049/jimmunol.179.11.7365. [DOI] [PubMed] [Google Scholar]
- 162.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nature Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 163.Uraushihara K, et al. Regulation of murine inflammatory bowel disease by CD25+ and CD25−CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J. Immunol. 2003;171:708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- 164.Stephens GL, et al. Engagement of glucocorticoid-induced TNFR family-related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 2004;173:5008–5020. doi: 10.4049/jimmunol.173.8.5008. [DOI] [PubMed] [Google Scholar]
- 165.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kohrt HE, et al. Effect of stimulation of natural killer cells with an anti-CD137 mAb on the efficacy of trastuzumab, cetuximab, and rituximab. J. Clin. Oncol. Abstr. 2012;30:2514. [Google Scholar]
- 167.Azimzadeh AM, Lees JR, Ding Y. Bromberg, J. S. Immunobiology of transplantation: impact on targets for large and small molecules. Clin. Pharmacol. Ther. 2011;90:229–242. doi: 10.1038/clpt.2011.106. [DOI] [PubMed] [Google Scholar]
- 168.Arkin MR, Wells JA. Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nature Rev. Drug Discov. 2004;3:301–317. doi: 10.1038/nrd1343. [DOI] [PubMed] [Google Scholar]
- 169.Emerson SD, et al. NMR characterization of interleukin-2 in complexes with the IL-2Rα receptor component, and with low molecular weight compounds that inhibit the IL-2/IL-Rα interaction. Protein Sci. 2003;12:811–822. doi: 10.1110/ps.0232803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Erbe DV, Wang S, Xing Y, Tobin JF. Small molecule ligands define a binding site on the immune regulatory protein B7.1. J. Biol. Chem. 2002;277:7363–7368. doi: 10.1074/jbc.M110162200. [DOI] [PubMed] [Google Scholar]
- 171.Margolles-Clark E, Umland O, Kenyon NS, Ricordi C, Buchwald P. Small-molecule costimulatory blockade: organic dye inhibitors of the CD40–CD154 interaction. J. Mol. Med. 2009;87:1133–1143. doi: 10.1007/s00109-009-0519-3. [DOI] [PubMed] [Google Scholar]
- 172.Lipson EJ, et al. Durable cancer regression off-treatment and effective re-induction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2012 Nov 20; doi: 10.1158/1078-0432.CCR-12-2625. doi:10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Younes A, et al. A Phase 1b/2 trial of mapatumumab in patients with relapsed/refractory non-Hodgkin's lymphoma. Br. J. Cancer. 2010;103:1783–1787. doi: 10.1038/sj.bjc.6605987. [DOI] [PMC free article] [PubMed] [Google Scholar]