Abstract
Caffeine is consumed worldwide to enhance wakefulness, but the cellular mechanisms are poorly understood. Caffeine blocks adenosine receptors suggesting that adenosine decreases cortical arousal. Given the widespread innervation of the cerebral cortex by thalamic fibers, adenosine receptors on thalamocortical terminals could provide an efficient method of limiting thalamic activation of the cortex. Using a thalamocortical slice preparation and whole-cell patch clamp recordings, we examined whether thalamocortical terminals are modulated by adenosine receptors. Bath application of adenosine decreased excitatory postsynaptic currents (EPSCs) elicited by stimulation of the ventrobasal thalamus. Thalamocortical synapses onto inhibitory and excitatory neurons were equally affected by adenosine. Adenosine also increased the paired pulse ratio and the coefficient of variation of the EPSCs, suggesting that adenosine decreased glutamate release. The inhibition produced by adenosine was reversed by a selective antagonist of adenosine A1 receptors (CPT) and mimicked by a selective A1 receptor agonist (CPA). Our results indicate that thalamocortical excitation is regulated by presynaptic adenosine A1 receptors and provide a mechanism by which increased adenosine levels can directly reduce cortical excitability.
Keywords: somatosensory, glutamate, interneurons, spiny stellate cells
During quiescent states such as sleep or anesthesia, sensory stimuli have a reduced impact on the cerebral cortex (Livingstone and Hubel, 1981, Edeline et al., 2001). The mechanisms of sensory gating during sleep are still being elucidated; however, recent experiments suggest that some of the gating occurs at the level of the cerebral cortex during cortically generated slow (< 1 Hz) oscillations (Steriade et al., 1993a, Timofeev et al., 1996, Massimini et al., 2003, Rosanova and Timofeev, 2005). These oscillations involve a reduction in excitatory drive rather than an increase in cortical GABAergic inhibition (Timofeev et al., 2001) and are disrupted by thalamocortical stimulation (Steriade et al., 1993a) suggesting that a reduction in thalamocortical transmission is important for sensory gating.
Many previous studies have suggested that reduced thalamocortical transmission during sleep is caused by a reduction in depolarizing cholinergic inputs to the thalamus secondary to rising adenosine levels in the reticular nuclei (Rainnie et al., 1994, Porkka-Heiskanen et al., 2000, Basheer et al., 2004). In addition to its effects on the thalamus, adenosine also directly reduces cortical excitability by inhibiting the cortical release of acetylcholine (Materi et al., 2000), hyperpolarizing cortical neurons (McCormick and Williamson, 1989), and inhibiting the release of glutamate from cortical synapses (Vazquez and Sanchez-Prieto, 1997, Brand et al., 2001). However, it is not know whether adenosine directly affects the release of glutamate from thalamocortical terminals.
Our goal was to determine whether adenosine inhibits synaptic transmission at thalamocortical terminals. Previous studies have demonstrated that adenosine applied to the somatosensory cortex reduces the early components of the potentials evoked by peripheral sensory stimuli (Petrescu and Haulica, 1983, Addae and Stone, 1988) suggesting that thalamocortical excitation is modulated by adenosine receptors. In these studies it was not possible to discern whether the effects were due to decreased glutamate release from thalamocortical fibers or to hyperpolarization of the cortical neurons. Therefore, to determine whether adenosine receptors regulate release of glutamate from thalamocortical synapses, we examined the effects of adenosine on thalamocortical EPSCs in a thalamocortical slice preparation which is ideal for studies of the direct effects of agonists on thalamocortical synapses (Agmon and Connors, 1991, Gil et al., 1997, Porter and Nieves, 2004).
EXPERIMENTAL PROCEDURES
Thalamocortical Slices
The Institutional Animal Care and Use Committee of the Ponce School of Medicine in compliance with National Institute of Health (NIH) guidelines for the care and use of laboratory animals (Publication DHHS NIH 86-23) approved all procedures involving animals. Mice (13–21 days postnatal) were decapitated under deep anesthesia with halothane. The brain was removed and placed in ice cold artificial cerebral spinal fluid (ACSF) containing 126 mM NaCl, 3 mM KCl, 1.25 mM NaH2PO4, 1 mM MgSO4, 26 mM NaHCO3, 20 mM glucose and 2 mM CaCl2 and bubbled with 95% O2 and 5% CO2. As previously described (Agmon and Connors, 1991, Porter et al., 2001), 300 µm thick thalamocortical slices were cut with a Vibratome 1000 Plus (Vibratome, St. Louis, MO). Slices were incubated at room temperature in ACSF for at least an hour prior to experiments. 10 µM MK-801, a NMDA receptor blocker, was added during the incubation of slices from animals greater than 18 days old to attempt to increase neuronal survival (Schurr et al., 1995).
Electrophysiology
Slices were transferred to a submersion recording chamber mounted and perfused at 2–3 mL/min with room temperature ACSF. Neurons were visualized with infrared video microscopy using a 40× water immersion objective on an upright E600FN microscope (Nikon Instruments, Melville, NY). Since excitatory neurons generally had smaller, rounder somas than inhibitory neurons in layer IV, somatic size was used to select excitatory and inhibitory neurons to be recorded. Whole cell recordings were done with glass pipettes with a resistance of 3–5 MΩ when filled with an internal solution containing 12 mM KCl, 140 mM KGluconate, 0.2 mM EGTA, 10 mM HEPES, 0.3 mM GTP and 0.4 mM ATP (pH 7.3, 285 mOsm). As previously described (Connors and Gutnick, 1990, Porter et al., 2001), inhibitory and excitatory neurons were identified by their different patterns of action potential discharges in response to the injection of current pulses with a patch clamp amplifier (MultiClamp 700A, Axon Instruments, Union City, CA) in current clamp mode. Neurons were held in voltage clamp mode at −60 mV and EPSCs were evoked by stimulating the ventrobasal nucleus of the thalamus (A-M Systems, Carlsborg, WA) with paired stimuli (50 ms interstimulus interval) with a unipolar tungsten electrode (WPI, Sarasota, FL) every 10–15 seconds. Recordings were filtered at 4 kHz, digitized at 10 kHz, and saved to computer using pCLAMP9 (Axon Instruments, Union City, CA). Membrane potentials were not corrected for the junction potential. Recordings were not compensated for series resistance, but changes in series resistance were monitored throughout the recordings with a 500 ms hyperpolarizing pulse in every sweep. If the series resistance changed by more than 20% the experiment was eliminated from analysis. As previously described (Agmon et al., 1996, Beierlein and Connors, 2002), evoked EPSCs which exhibited latencies that varied by less than 1 ms were considered monosynaptic thalamocortical EPSCs and were included in the analysis. Since corticothalamic EPSCs exhibit paired pulse facilitation (Beierlein and Connors, 2002), we included in the analysis only inputs which displayed paired pulse depression (Fig. 5) like thalamocortical EPSCs examined in previous studies (Gil et al., 1997, Gibson et al., 1999, Porter et al., 2001).
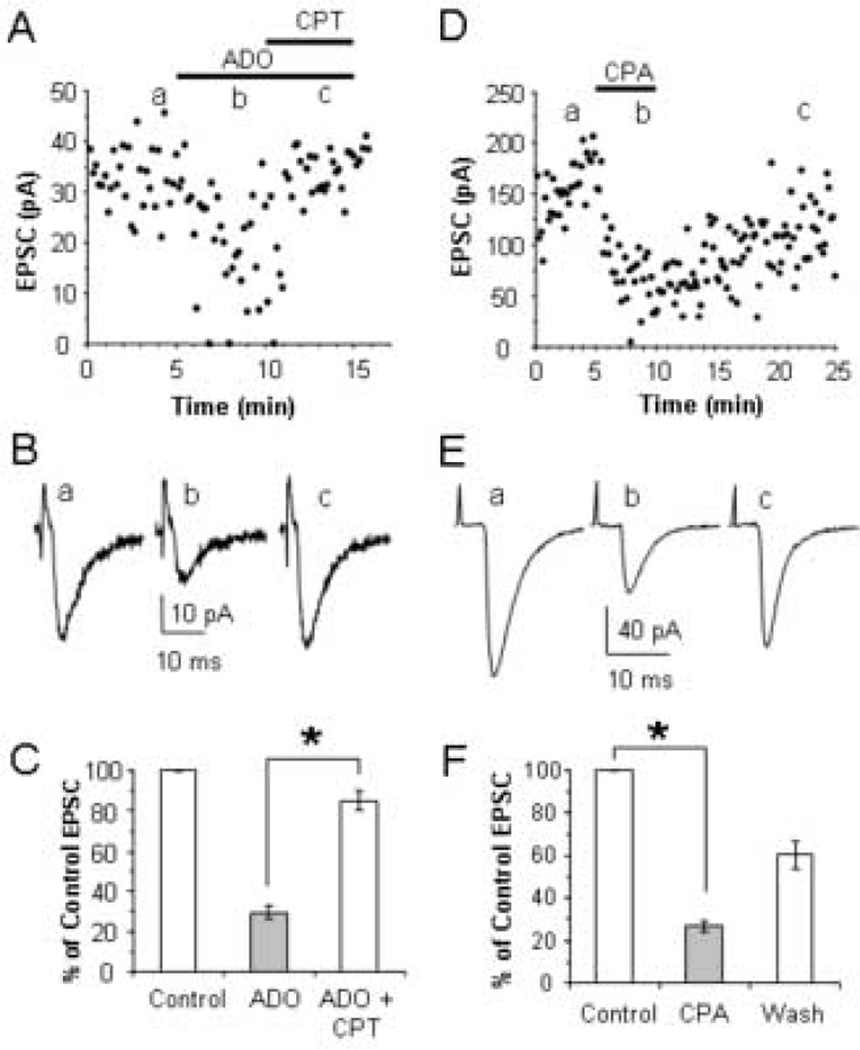
Fig. 5.
Adenosine A1 receptors mediate the EPSC reduction produced by adenosine. (A) Time course showing the reduction of evoked EPSCs by bath application of 100 µM adenosine and the reversal of adenosine’s effect by co-application of 1 µM CPT in a P13 neuron. (B) Average EPSCs corresponding to the average of 10 traces taken at the times indicated in A. (C) The average effect of adenosine and CPT expressed as a percent of the control EPSC in 10 cells. (D) Time course showing the reduction of evoked EPSCs by bath application of 1 µM CPA. (E) Average EPSCs corresponding to the average of 19 10 traces taken at the times indicated in D. (F) The average effect of CPA expressed as a percent of the control EPSC in 4 cells. * p < 0.001.
To selectively examine the effects of adenosine on alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA) receptor-mediated currents, GABAA and N-methyl-D-aspartate (NMDA) receptors were respectively blocked with 10 µM bicuculline and 100 µM DL-2-amino-5-phosphonopentanoic acid (AP5) in all experiments. 6-cyano-7- nitroquinoxaline-2,3-dione (CNQX; n = 3) blocked the EPSCs confirming that the recorded EPSCs are AMPA receptor-mediated. Since kainate receptor-mediated thalamocortical EPSCs have much slower kinetics and largely disappear after postnatal day 8 (Kidd and Isaac, 1999), kainate receptors probably did not contribute to the evoked EPSCs.
Adenosine, 8-cyclopentyltheophylline (CPT), N6-cyclopentyladenosine (CPA), bicuculline, AP5, CNQX, and MK-801 were purchased from Sigma (St. Louis, MO).
Data analysis and statistics
Experiments were accepted for analysis if the effect of adenosine on the EPSCs reversed upon removal of the drug and there was no confounding di- or polysynaptic activity in the EPSCs. Data were analyzed using Clampfit (Axon Instruments, Union City, CA). Average EPSCs were taken from 10–50 consecutive traces including failures. The paired pulse ratio (PPR) was calculated from EPSCs evoked by paired stimuli as the mean amplitude of the EPSC evoked by the second stimulus (EPSC2) divided by the mean amplitude of the EPSC evoked by the first stimulus (EPSC1). The coefficient of variation (CV) was calculated as the standard deviation of the EPSC amplitude divided by the mean EPSC amplitude from 10 consecutive traces including failures. Concentration response curves were fit with the equation, y = min + (max−min)/(1+(x/EC50)Hillslope) using SigmaPlot (Aspire Software International, Leesburg, VA). Data were analyzed using t-tests or one-way ANOVA (Statistica, Statsoft, Tulsa, OK). After a significant main effect, post-hoc tests were done with the Tukey’s method. Statistical significance was set at p < 0.05. Values are reported as the mean ± the standard error of the mean (S.E.M.).
Morphology
In 10 experiments 5 mM biocytin was included in the recording solution to label the neurons for post hoc morphological identification of inhibitory and excitatory cells. At the end of the electrophysiological recordings, the slices were fixed overnight in 4% paraformaldehyde. Six of the labeled neurons were subsequently revealed with a standard advidin-biotin peroxidase procedure (Vectastain ABC kit, Vector Laboratories, Burlingame, CA) as previously described (Porter et al., 2001) and visualized with brightfield microscopy. Four of the biocytin labeled neurons were revealed by incubation with Streptavidin conjugated to Alexa Fluor 546 (5 µg/mL; Molecular Probes, Eugene, OR) for 2 hours after being permeablized with PBS containing 0.25% Triton X-100 for 2.5 hours. Images of the fluorescently labeled neurons were taken with a C1 confocal microscope (Nikon Instruments, Melville, NY) using a 40× objective on an upright microscope.
RESULTS
Adenosine reduces thalamocortical stimulation of inhibitory and excitatory neurons
To determine whether adenosine receptors modulate thalamocortical synaptic transmission, we examined the responses of thalamocortical inputs onto inhibitory and excitatory neurons in layer IV of the mouse barrel cortex to the application of adenosine receptor agonists in a thalamocortical slice preparation (Agmon and Connors, 1991). Neurons were classified as inhibitory or excitatory neurons based on their patterns of action potential discharges in response to injected current as previously described (Connors and Gutnick, 1990, Porter et al., 2001, Porter and Nieves, 2004). In response to injected current pulses, inhibitory neurons exhibited little or no spike frequency adaptation and large rapidly repolarizing afterhyperpolarizations (AHPs, Fig. 1A), while excitatory neurons displayed strong spike frequency adaptation and small, slowly repolarizing AHPs (Fig. 2A). Based on these criteria, a total of 28 inhibitory and 14 excitatory neurons were recorded. To confirm their classification, 6 inhibitory and 4 excitatory neurons were labeled with biocytin and their post hoc morphology was examined. The 6 inhibitory neurons exhibited non-pyramidal morphologies with aspiny dendrites (Fig. 1B) typical of cortical inhibitory neurons (Kawaguchi and Kubota, 1997, Wang et al., 1999, Porter et al., 2001), while the 4 excitatory neurons displayed small cell bodies with thin radiating spiny dendrites typical of excitatory spiny stellate neurons in layer IV (Fig. 2B; (Simons and Woolsey, 1984, Feldmeyer et al., 1999, Porter and Nieves, 2004).
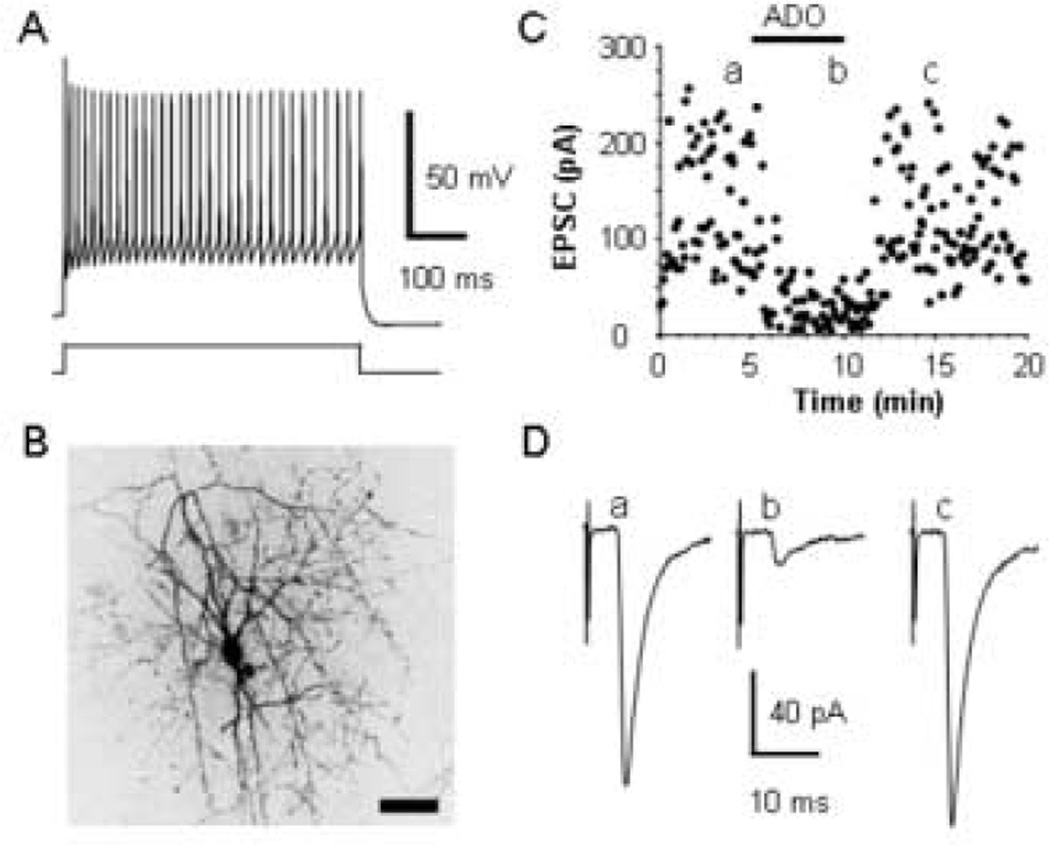
Fig. 1.
Adenosine inhibits thalamocortical EPSCs in an inhibitory neuron. (A) The pattern of action potential discharge in response to a 394 pA current step in a P15 inhibitory interneuron layer IV cell distinguished by the rapid, deep AHPs and lack of adaptation of the action potentials. (B) An image taken with a confocal microscope of the biocytin labeled inhibitory neuron that exhibited the pattern of action potential discharge in A. Images were taken at several focal plans and compiled into one image. Scale bar, 50 µm. (C) Time course showing the effect of 100 µM adenosine (ADO) on the evoked thalamocortical EPSCs in this interneuron. (D) Averages of 20 consecutive EPSCs taken at the times indicated in C.
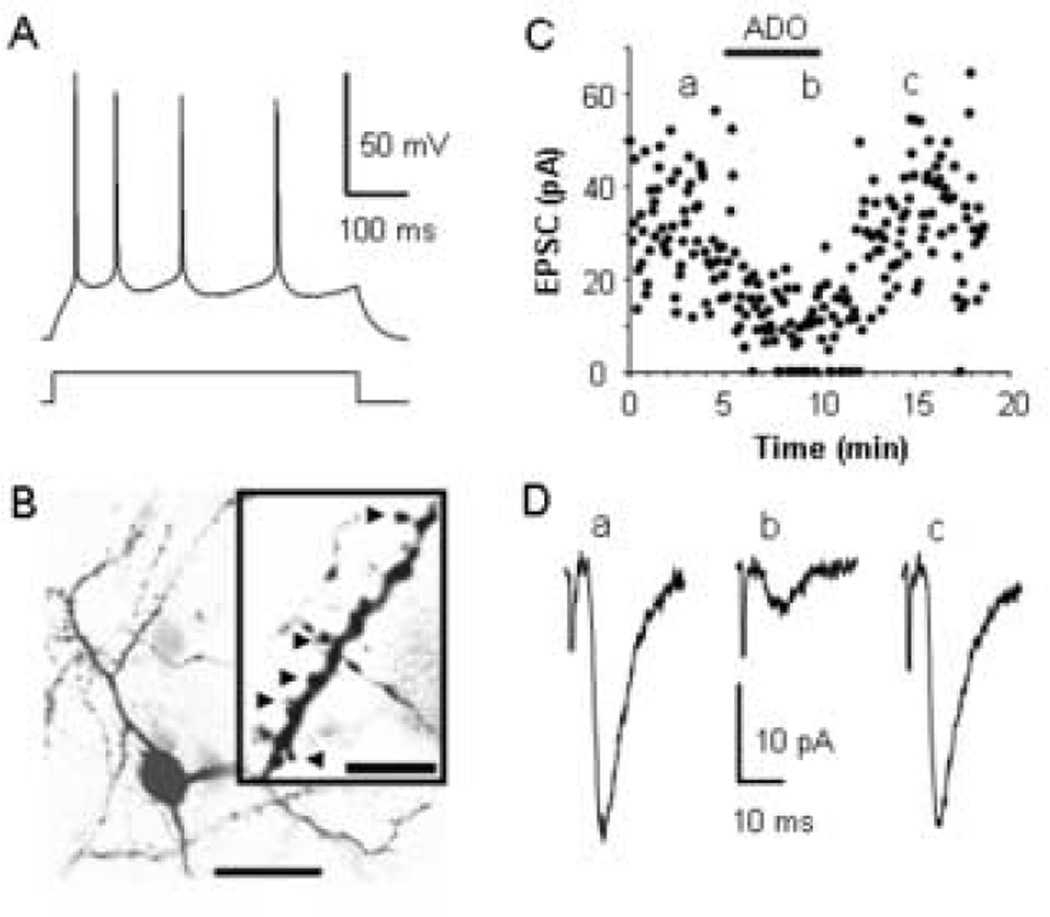
Fig. 2.
Adenosine inhibits thalamocortical EPSCs in an excitatory layer IV neuron. (A) The pattern of action potential discharge in response to a 34 pA current step in a P15 spiny stellate layer IV cell distinguished by the slow, shallow AHPs and significant adaptation. (B) A single focal plan image taken with a confocal microscope of the spiny stellate cell labeled with biocytin that exhibited the pattern of action potential discharges in A. Scale bar, 20 µm. Insert shows an enlargement of a dendrite exhibiting visible spines (Arrowheads). Scale bar, 5 µm. (C) Time course showing the inhibition and recovery of evoked EPSCs in the cell in B produced by bath application of 100 µM adenosine. (D) Averages of 20 consecutive EPSCs taken at the times indicated in C.
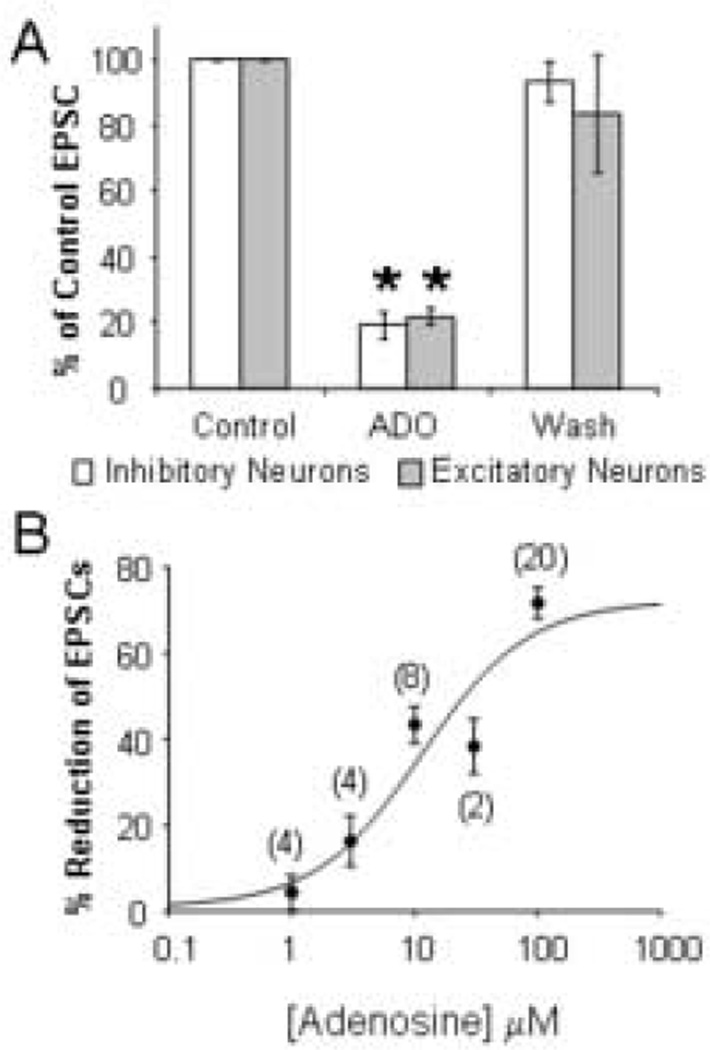
Monosynaptic thalamocortical excitatory postsynaptic currents (EPSCs) were evoked by stimulating the ventrobasal nucleus of the thalamus in the presence of bicuculline and AP5 to block GABAA and NMDA receptors, respectively. Bath application of 100 µM adenosine reversibly reduced the amplitude of the thalamocortical EPSCs recorded in both the inhibitory (Fig. 1C, D) and the excitatory neurons (Fig. 2C, D) indicating that adenosine modulates the thalamocortical activation of both inhibitory and excitatory circuits. As shown in Figure 3A, 100 µM adenosine reduced thalamocortical EPSCs in inhibitory neurons by 81 ± 4% (n = 7; t = 19.43, p < 0.0001) and by 78 ± 3% in excitatory neurons (n = 3; t = 28.99, p = 0.001). Since the reduction was equal in both cell types (t = 0.33, p = 0.75), the data from both excitatory and inhibitory neurons were grouped for the remaining analyses. The application of different concentrations of adenosine indicated that the reduction of thalamocortical EPSCs was dose-dependent with an EC50 of 11 µM (Fig. 3B), consistent with the EC50 of adenosine-mediated inhibition of EPSCs at hippocampal, calyx of Held, and hypothalamic synapses (Lupica et al., 1992, Oliet and Poulain, 1999, Kimura et al., 2003).
Fig. 3.
Adenosine equally inhibits thalamocortical EPSCs in inhibitory and excitatory neurons. (A) Average % inhibition of thalamocortical EPSCs by 100 µM adenosine recorded in inhibitory (n = 7) and excitatory (n = 3) layer IV neurons. (B) Concentrationresponse curve for the reduction of thalamocortical EPSCs by adenosine. Numbers of experiments are given in parentheses. * p ≤ 0.001.
Adenosine acts presynaptically
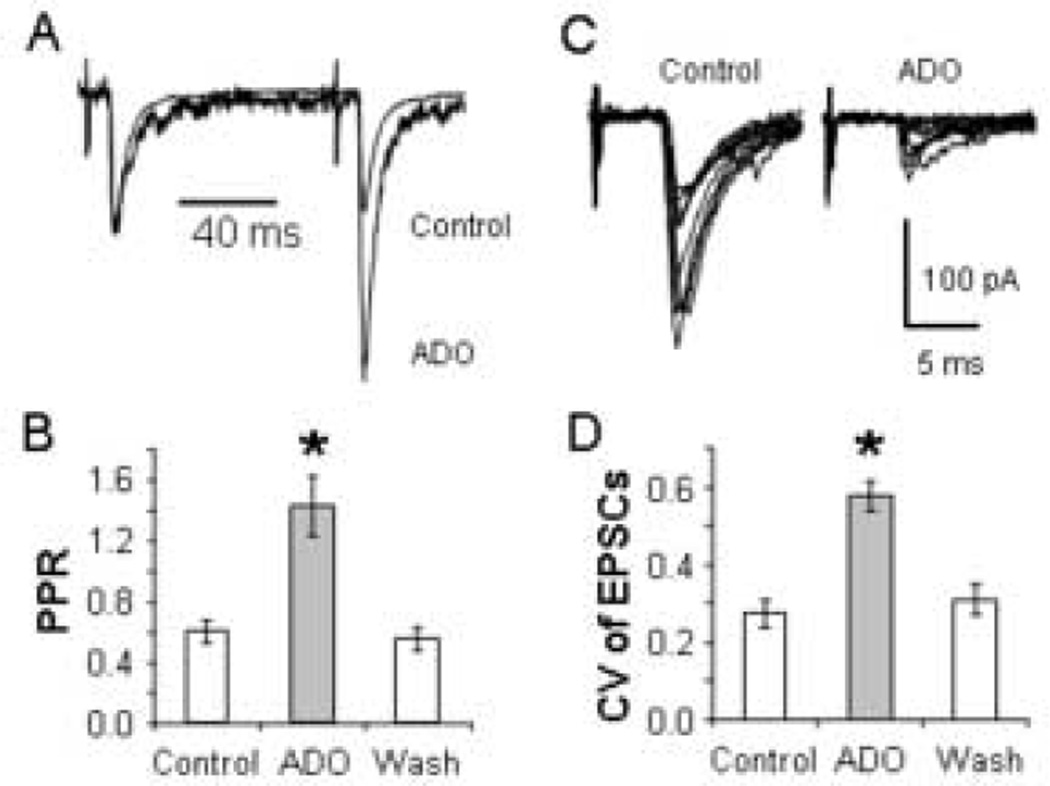
Adenosine has been shown to reduce neuronal excitation via two distinct mechanisms: 1) presynaptic adenosine receptors inhibit the release of the excitatory neurotransmitter glutamate (Lupica et al., 1992, Scanziani et al., 1992, Oliet and Poulain, 1999, Arrigoni et al., 2001, Shen and Johnson, 2003) and, 2) postsynaptic adenosine receptors activate inwardly activating K+ channels leading to neuronal hyperpolarization (Trussell and Jackson, 1985, Rainnie et al., 1994, Luscher et al., 1997). To determine whether the adenosine receptors are located on the thalamocortical terminals, we measured the effect of 100 µM adenosine on the paired pulse ratio (PPR) and the coefficient of variation (CV) of the thalamocortical EPSCs. Decreases in the probability of neurotransmitter release are correlated with increases in the PPR and CV of EPSCs (Zucker, 1989, Ohana and Sakmann, 1998). As shown in Fig. 4A (Control), paired stimulation of the thalamocortical fibers produced depressing EPSCs as reported previously (Gil and Amitai, 1996, Amitai, 2001). Scaling the first EPSCs to the same amplitude indicates that adenosine converted the synaptic depression into synaptic facilitation with a corresponding PPR increase from 0.8 to 2.0. Overlaying 10 consecutive traces before and during adenosine perfusion demonstrates that the CV was also increased from 0.48 to 0.70 in this neuron (Fig. 4C). Within the sample of neurons examined (n = 10), 100 µM adenosine increased the PPR from 0.61 ± 0.07 to 1.43 ± 0.19 and the CV from 0.27 ± 0.04 to 0.58 ± 0.04 (Fig. 4B, D). One-way ANOVA indicated a main effect of group for both the PPR (F (2,27) = 14.83; p < 0.001) and the CV (F (2,27) = 18.45; p < 0.001). Post hoc tests indicated that the PPR and CV were greater in the presence of adenosine than in either control or washout conditions (p < 0.001).
Fig. 4.
Adenosine increases the PPR and the CV of the thalamocortical EPSCs. (A) Average EPSCs in response to paired stimuli taken before (Control) and during adenosine application to the neuron in Figure 1 scaled to equalize the first EPSCs. (B) Average effect of 100 µM adenosine on PPR in 10 cells. (C) Overlays of 10 consecutive EPSCs under control conditions and during the application of adenosine showing that adenosine increased the CV. Data are from the same neuron as in A. (D) The average CV (n = 10) before, during, and after (Wash) 100 µM adenosine application. * p < 0.001.
To test whether adenosine also produced postsynaptic effects at the concentrations used in these experiments, we determined whether the membrane resistance or EPSC kinetics were affected by adenosine application. The application of 100 µM adenosine produced a small reduction in the membrane resistance (15 ± 4 %; n = 10; t = 3.94, p = 0.003) suggesting that adenosine also has some postsynaptic effects. However, 10 µM adenosine still produced a 43 ± 4 % reduction in the thalamocortical EPSCs (n = 8, t = 10.13, p < 0.001) without changing the membrane resistance (n = 8, t = 1.17, p = 0.28) indicating that adenosine-mediated reduction in EPSCs does not require a reduction in membrane resistance. To determine whether adenosine reduced the EPSCs by affecting the kinetics of the postsynaptic glutamate receptors, the decay of the thalamocortical EPSCs before and during adenosine application were fit with a mono-exponential function. Adenosine did not affect the decay time constants which were 3.4 ± 0.7 ms and 3.6 ± 0.7 ms before and during adenosine, respectively (n = 9; t = 0.56, p = 0.59). These results further suggest that adenosine was not acting postsynaptically to reduce the EPSCs. Taken together these results indicate that adenosine reduces the EPSCs by inhibiting the presynaptic release of glutamate from thalamocortical terminals.
Adenosine A1 receptors mediate the EPSC reduction
Of the four cloned adenosine receptors, A1, A2A, A2B, and A3, most of the inhibitory actions of adenosine on glutamate release have been ascribed to A1 adenosine receptors (Wu and Saggau, 1997, Dunwiddie and Masino, 2001). Therefore, we tested whether adenosine’s effect could be blocked by the selective A1 receptor antagonist, CPT, and mimicked by the selective A1 receptor agonist, CPA (Fredholm et al., 2001). The results shown in Fig 5 indicate that adenosine inhibited thalamocortical EPSCs through the stimulation of A1 adenosine receptors. Application of 100 µM adenosine reduced the thalamocortical EPSCs to 30 ± 4% of control values. Subsequent bath application of 1 µM CPT reversed the EPSC reduction produced by 100 µM adenosine to 85 ± 5% of control values (n = 10; t = 18.20, p < 0.001). Furthermore, the effect of adenosine was mimicked by the application of 1 µM CPA (Fig 5D–F). CPA reduced thalamocortical EPSCs by 73 ± 2% (n = 4; t = 32.56, p < 0.001). The reduction produced by CPA was not statistically different from the 75 ± 2% (n = 20) reduction produced by 100 µM adenosine (t = 0.31, p = 0.76). Together these results indicate that A1 adenosine receptors reduce thalamocortical transmission.
DISCUSSION
We examined the effects of adenosine on thalamocortical transmission. Our results demonstrate that adenosine inhibits thalamic excitation of cortical neurons. Adenosine produces this inhibition by activating adenosine A1 receptors on thalamocortical terminals which reduce glutamate release from thalamocortical synapses. Adenosine equally reduced thalamocortical inputs onto both inhibitory and excitatory neurons, suggesting that rising cortical adenosine levels would result in a global reduction in the impact of incoming sensory information. Therefore, these date provide a mechanism whereby cortical adenosine levels can directly modulate thalamocortical synapses and thereby reduce the influence of sensory inputs on cortical activity which is necessary for sleep.
Adenosine receptors modulate synaptic transmission by inhibiting neurotransmitter release (Lupica et al., 1992, Prince and Stevens, 1992) and by hyperpolarizing neuronal dendrites and cell bodies (Trussell and Jackson, 1985, Luscher et al., 1997). Our results indicate that adenosine modulated thalamocortical excitation largely by reducing neurotransmitter release, since the reduction in EPSCs was accompanied by an increase in the PPR and CV of the EPSCs. Furthermore, adenosine reduced the thalamocortical EPSCs even in the absence of a change in the membrane resistance or the kinetics of the postsynaptic receptors. The reversal of adenosine’s inhibition of EPSCs by CPT and the equivalent reduction produced by CPA indicate that A1 receptors mediate the effect of adenosine. These results are consistent with previous data indicating that presynaptic adenosine A1 receptors modulate the release of glutamate from intracortical (Brand et al., 2001, Marchi et al., 2002), hippocampal (Dunwiddie et al., 1984, Lupica et al., 1992), corticostriatal and thalamostriatal (Flagmeyer et al., 1997), supraoptic nucleus (Oliet and Poulain, 1999), laterodorsal tegmentum (Arrigoni et al., 2001), subthalamic nucleus (Shen and Johnson, 2003), and calyx of Held (Kimura et al., 2003) synapses.
Two earlier studies (Petrescu and Haulica, 1983, Addae and Stone, 1988) demonstrated that application of adenosine to the somatosensory cortex in vivo inhibits potentials evoked by stimulation of the periphery. As adenosine reduced the earliest components of the complex potentials, it was suggested that adenosine may inhibit thalamocortical inputs. Our results extend these earlier observations by confirming that adenosine does inhibit thalamocortical inputs and that A1 receptors mediate the inhibition. It remains to be determined if the modulation we observed is limited to specific thalamic projections to sensory cortex or also occurs in non-specific thalamocortical projections. Indirect evidence suggests that non-specific thalamocortical projections are also inhibited by adenosine. In slices from the prefrontal cortex, adenosine A1 receptors reduced serotonin-mediated enhancement of spontaneous EPSCs (Stutzmann et al., 2001). Since thalamic lesions eliminate the serotonin effect (Marek et al., 2001), these results suggest that thalamocortical synapses which arise from nonspecific thalamic nuclei are also modulated by adenosine A1 receptors.
Adenosine levels increase in the cholinergic basal forebrain during prolonged wakefulness (Porkka-Heiskanen et al., 1997). Given that the basal forebrain is a critical site for cortical arousal, the inhibition of neuronal activity in this structure by adenosine is believed to promote somnolence (Basheer et al., 2004) in part by decreasing excitation of the thalamus (Rainnie et al., 1994). Our results suggest that in addition to its effects on the basal forebrain, adenosine acts in the cerebral cortex to reduce excitability during sleep. In support of this, prolonged wakefulness increases adenosine concentrations in the cerebral cortex (Porkka-Heiskanen et al., 2000). The increase in cortical adenosine levels may serve to directly dampen thalamocortical excitation via stimulation of presynaptic A1 receptors and thereby weaken the impact of peripheral sensory stimuli on the cerebral cortex. Consistent with this hypothesis, it has been suggested that increases in cortical adenosine levels may mediate the decrease in excitatory drive onto cortical neurons that occurs during the slow oscillations in membrane potential seen in slow-wave sleep (Contreras and Steriade, 1995, Timofeev et al., 2001). In addition, prethalamic stimulation of the brachium conjunctivum produces smaller EPSPs in cortical neurons during the hyperpolarized phases of the slow oscillations (Timofeev et al., 1996). Both the reduced excitatory drive and decreased cortical responses could be due to inhibition of thalamocortical synapses by increased adenosine levels. Furthermore, a reduction in thalamocortical excitation may permit the cortex to generate the slow oscillations during slow-wave sleep (Steriade et al., 1993b), given that the slow oscillations occur in the absence of thalamocortical input and can be disrupted by thalamic stimulation (Steriade et al., 1993a).
In conclusion, our results demonstrate that presynaptic adenosine receptors modulate thalamocortical excitation. This modulation is likely to reduce thalamocortical transmission of sensory stimuli and, thereby, play an important role during sleep.
Acknowledgments
We are very grateful to Gregory Quirk and Edwin Santini for their many useful comments on an earlier version of the manuscript. This work was supported by National Institutes of Health Grant S06 GM08239 to J.T.P. D.F. was supported by National Institutes of Health Grants 5-T34-GM07732 and P20 RR-016470.
LIST OF ABBREVIATIONS
- APV
DL-2-amino-5-phosphonopentanoic acid
- CPT
8-cyclopentyltheophylline
- CPA
N6-cyclopentyladenosine
- AMPA
alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- EPSC
excitatory postsynaptic current
- ACSF
artificial cerebral spinal fluid
- NMDA
N-methyl-D-aspartate
- CNQX
6-cyano-7- nitroquinoxaline-2,3-dione
- CV
coefficient of variation
- PPR
paired pulse ratio
- AHP
afterhyperpolarization
REFERENCES
- 1.Addae JI, Stone TW. Purine receptors and kynurenic acid modulate the somatosensory evoked potential in rat cerebral cortex. Electroencephalogr Clin Neurophysiol. 1988;69:186–189. doi: 10.1016/0013-4694(88)90214-3. [DOI] [PubMed] [Google Scholar]
- 2.Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. doi: 10.1016/0306-4522(91)90333-j. [DOI] [PubMed] [Google Scholar]
- 3.Agmon A, Hollrigel G, O'Dowd DK. Functional GABAergic synaptic connection in neonatal mouse barrel cortex. J Neurosci. 1996;16:4684–4695. doi: 10.1523/JNEUROSCI.16-15-04684.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amitai Y. Thalamocortical synaptic connections: efficacy, modulation, inhibition and plasticity. Rev Neurosci. 2001;12:159–173. doi: 10.1515/revneuro.2001.12.2.159. [DOI] [PubMed] [Google Scholar]
- 5.Arrigoni E, Rainnie DG, McCarley RW, Greene RW. Adenosine-Mediated Presynaptic Modulation of Glutamatergic Transmission in the Laterodorsal Tegmentum. J Neurosci. 2001;21:1076–1085. doi: 10.1523/JNEUROSCI.21-03-01076.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004;73:379–396. doi: 10.1016/j.pneurobio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Beierlein M, Connors BW. Short-term dynamics of thalamocortical and intracortical synapses onto layer 6 neurons in neocortex. J Neurophysiol. 2002;88:1924–1932. doi: 10.1152/jn.2002.88.4.1924. [DOI] [PubMed] [Google Scholar]
- 8.Brand A, Vissiennon Z, Eschke D, Nieber K. Adenosine A(1) and A(3) receptors mediate inhibition of synaptic transmission in rat cortical neurons. Neuropharmacology. 2001;40:85–95. doi: 10.1016/s0028-3908(00)00117-9. [DOI] [PubMed] [Google Scholar]
- 9.Connors BW, Gutnick MJ. Intrinsic firing patterns of diverse neocortical neurons [see comments] Trends Neurosci. 1990;13:99–104. doi: 10.1016/0166-2236(90)90185-d. [DOI] [PubMed] [Google Scholar]
- 10.Contreras D, Steriade M. Cellular basis of EEG slow rhythms: a study of dynamic corticothalamic relationships. J Neurosci. 1995;15:604–622. doi: 10.1523/JNEUROSCI.15-01-00604.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunwiddie TV, Basile AS, Palmer MR. Electrophysiological responses to adenosine analogs in rat hippocampus and cerebellum: evidence for mediation by adenosine receptors of the A1 subtype. Life Sci. 1984;34:37–47. doi: 10.1016/0024-3205(84)90328-x. [DOI] [PubMed] [Google Scholar]
- 12.Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- 13.Edeline JM, Dutrieux G, Manunta Y, Hennevin E. Diversity of receptive field changes in auditory cortex during natural sleep. Eur J Neurosci. 2001;14:1865–1880. doi: 10.1046/j.0953-816x.2001.01821.x. [DOI] [PubMed] [Google Scholar]
- 14.Feldmeyer D, Egger V, Lubke J, Sakmann B. Reliable synaptic connections between pairs of excitatory layer 4 neurones within a single 'barrel' of developing rat somatosensory cortex. J Physiol. 1999;521(Pt 1):169–190. doi: 10.1111/j.1469-7793.1999.00169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flagmeyer I, Haas HL, Stevens DR. Adenosine A1 receptor-mediated depression of corticostriatal and thalamostriatal glutamatergic synaptic potentials in vitro. Brain Res. 1997;778:178–185. doi: 10.1016/s0006-8993(97)01060-3. [DOI] [PubMed] [Google Scholar]
- 16.Fredholm BB, IJzerman AP, Jacobson KA, Klotz K-N, Linden J. International Union of Pharmacology. XXV. Nomenclature and Classification of Adenosine Receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 17.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 18.Gil Z, Amitai Y. Properties of convergent thalamocortical and intracortical synaptic potentials in single neurons of neocortex. J Neurosci. 1996;16:6567–6578. doi: 10.1523/JNEUROSCI.16-20-06567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil Z, Connors BW, Amitai Y. Differential regulation of neocortical synapses by neuromodulators and activity. Neuron. 1997;19:679–686. doi: 10.1016/s0896-6273(00)80380-3. [DOI] [PubMed] [Google Scholar]
- 20.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 21.Kidd FL, Isaac JT. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- 22.Kimura M, Saitoh N, Takahashi T. Adenosine A1 receptor-mediated presynaptic inhibition at the calyx of Held of immature rats. J Physiol. 2003 doi: 10.1113/jphysiol.2003.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 24.Lupica CR, Proctor WR, Dunwiddie TV. Presynaptic inhibition of excitatory synaptic transmission by adenosine in rat hippocampus: analysis of unitary EPSP variance measured by whole-cell recording. J Neurosci. 1992;12:3753–3764. doi: 10.1523/JNEUROSCI.12-10-03753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 26.Marchi M, Raiteri L, Risso F, Vallarino A, Bonfanti A, Monopoli A, Ongini E, Raiteri M. Effects of adenosine A1 and A2A receptor activation on the evoked release of glutamate from rat cerebrocortical synaptosomes. Br J Pharmacol. 2002;136:434–440. doi: 10.1038/sj.bjp.0704712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marek GJ, Wright RA, Gewirtz JC, Schoepp DD. A major role for thalamocortical afferents in serotonergic hallucinogen receptor function in the rat neocortex. Neuroscience. 2001;105:379–392. doi: 10.1016/s0306-4522(01)00199-3. [DOI] [PubMed] [Google Scholar]
- 28.Massimini M, Rosanova M, Mariotti M. EEG Slow (~1 Hz) Waves Are Associated With Nonstationarity of Thalamo-Cortical Sensory Processing in the Sleeping Human. J Neurophysiol. 2003;89:1205–1213. doi: 10.1152/jn.00373.2002. [DOI] [PubMed] [Google Scholar]
- 29.Materi LM, Rasmusson DD, Semba K. Inhibition of synaptically evoked cortical acetylcholine release by adenosine: an in vivo microdialysis study in the rat. Neuroscience. 2000;97:219–226. doi: 10.1016/s0306-4522(00)00075-0. [DOI] [PubMed] [Google Scholar]
- 30.McCormick DA, Williamson A. Convergence and divergence of neurotransmitter action in human cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:8098–8102. doi: 10.1073/pnas.86.20.8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. J Physiol. 1998;513:135–148. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliet SH, Poulain DA. Adenosine-induced presynaptic inhibition of IPSCs and EPSCs in rat hypothalamic supraoptic nucleus neurones. J Physiol. 1999;520(Pt 3):815–825. doi: 10.1111/j.1469-7793.1999.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petrescu G, Haulica I. Preliminary data concerning adenosine action upon the evoked potential after electrical stimulation of the vibrissal follicles in the adult rat. Physiologie. 1983;20:229–233. [PubMed] [Google Scholar]
- 34.Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- 35.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Adenosine: A Mediator of the Sleep-Inducing Effects of Prolonged Wakefulness. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter JT, Johnson CK, Agmon A. Diverse types of interneurons generate thalamus-evoked feedforward inhibition in the mouse barrel cortex. J Neurosci. 2001;21:2699–2710. doi: 10.1523/JNEUROSCI.21-08-02699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Porter JT, Nieves D. Presynaptic GABAB receptors modulate thalamic excitation of inhibitory and excitatory neurons in the mouse barrel cortex. J Neurophysiol. 2004;92:2762–2770. doi: 10.1152/jn.00196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prince DA, Stevens CF. Adenosine decreases neurotransmitter release at central synapses. Proc Natl Acad Sci U S A. 1992;89:8586–8590. doi: 10.1073/pnas.89.18.8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rainnie DG, Grunze HC, McCarley RW, Greene RW. Adenosine inhibition of mesopontine cholinergic neurons: implications for EEG arousal. Science. 1994;263:689–692. doi: 10.1126/science.8303279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosanova M, Timofeev I. Neuronal mechanisms mediating the variability of somatosensory evoked potentials during sleep oscillations in cats. J Physiol. 2005;562:569–582. doi: 10.1113/jphysiol.2004.071381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 42.Schurr A, Payne RS, Rigor BM. Protection by MK-801 against hypoxia-, excitotoxin-, and depolarization-induced neuronal damage in vitro. Neurochem Int. 1995;26:519–525. doi: 10.1016/0197-0186(94)00148-n. [DOI] [PubMed] [Google Scholar]
- 43.Shen KZ, Johnson SW. Presynaptic inhibition of synaptic transmission by adenosine in rat subthalamic nucleus in vitro. Neuroscience. 2003;116:99–106. doi: 10.1016/s0306-4522(02)00656-5. [DOI] [PubMed] [Google Scholar]
- 44.Simons DJ, Woolsey TA. Morphology of Golgi-Cox-impregnated barrel neurons in rat SmI cortex. J Comp Neurol. 1984;230:119–132. doi: 10.1002/cne.902300111. [DOI] [PubMed] [Google Scholar]
- 45.Steriade M, Nunez A, Amzica F. Intracellular analysis of relations between the slow (< 1 Hz) neocortical oscillation and other sleep rhythms of the electroencephalogram. J Neurosci. 1993a;13:3266–3283. doi: 10.1523/JNEUROSCI.13-08-03266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steriade M, Nunez A, Amzica F. A novel slow (< 1 Hz) oscillation of neocortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993b;13:3252–3265. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stutzmann GE, Marek GJ, Aghajanian GK. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience. 2001;105:55–69. doi: 10.1016/s0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- 48.Timofeev I, Contreras D, Steriade M. Synaptic responsiveness of cortical and thalamic neurones during various phases of slow sleep oscillation in cat. J Physiol. 1996;494(Pt 1):265–278. doi: 10.1113/jphysiol.1996.sp021489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Timofeev I, Grenier F, Steriade M. Disfacilitation and active inhibition in the neocortex during the natural sleep-wake cycle: An intracellular study. PNAS. 2001;98:1924–1929. doi: 10.1073/pnas.041430398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trussell LO, Jackson MB. Adenosine-Activated Potassium Conductance in Cultured Striatal Neurons. PNAS. 1985;82:4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vazquez E, Sanchez-Prieto J. Presynaptic modulation of glutamate release targets different calcium channels in rat cerebrocortical nerve terminals. Eur J Neurosci. 1997;9:2009–2018. doi: 10.1111/j.1460-9568.1997.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Gupta A, Markram H. Anatomical and functional differentiation of glutamatergic synaptic innervation in the neocortex. J Physiol Paris. 1999;93:305–317. doi: 10.1016/s0928-4257(00)80059-5. [DOI] [PubMed] [Google Scholar]
- 53.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- 54.Zucker RS. Short-term synaptic plasticity. Annu Rev Neurosci. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]