Abstract
Aims:
The aim of the present study was to evaluate solubility of white mineral trioxide aggregate (WMTA) in an acidic environment.
Materials and Methods:
Twenty-four metal rings were prepared, filled with WMTA and randomly divided into two groups. The samples in groups 1 and 2 were set in synthetic tissue fluid with pH values of 7.4 and 4.4, respectively and then were transferred to beakers containing synthetic tissue fluid with pH values of 7.7 and 4.4. Solubility of WMTA samples were calculated at the 9 experimental intervals. Data was analyzed with two-factor ANOVA and Bonferroni test (P < 0.03).
Results:
The total solubility of WMTA in groups 1 and 2 were −9.1796 ± 1.9158% and −1.1192 ± 2.6236%, (P = 0.028) with weight changes of 9.1574 ± 2.1432% and 7.3276 ± 1.5823%, respectively (P = 0.002). Statistical analysis revealed significant differences between the two groups.
Conclusions:
It was concluded that solubility of WMTA increases in acidic environments and additional therapeutic precautions should be taken to decrease inflammation in endodontic treatment.
Keywords: Acidic environment, mineral trioxide aggregate, pH, solubility
INTRODUCTION
Mineral trioxide aggregate (MTA) was introduced in 1993 to seal the communication pathways between the root canal system and the periodontium. MTA was suggested for the repair of root perforations,[1] as a root-end filling material,[2] and vital pulp therapy.[3] Proper biocompatibility and high sealing ability of MTA made it an appropriate material in endodontics.[1,4,5,6] However, a major drawback for MTA is its long setting time;[7] therefore, environmental factors can influence the properties of MTA during its long setting reaction. When MTA is used as a root-end filling material, as an apical plug or a perforation repair material in necrotic or open apex teeth, it might be exposed to an acidic environment with a pH value of 5 as a result of inflammation or infection.
Researchers have reported a decrease in MTA surface hardness,[8,9,10] greater porosity[9,10,11] and an increase in albumin micro-leakage of MTA samples placed in an acidic environment.[11] Furthermore, it has been demonstrated that diametral tensile strength and push-out bond strength of MTA cement increase in an acidic environment.[12,13]
Solubility of dental materials is an important physical property, which influences other physical properties, including micro-leakage; it also determines how long a material is effective.[14] Therefore, resistance of a material to solubility and dissolution in aqueous environments, including the oral cavity, is very important.[3] The aim of the present study was to evaluate solubility of white mineral trioxide aggregate (WMTA) in synthetic tissue fluid with pH values of 4.4 and 7.4.
MATERIALS AND METHODS
Twenty-four 1-g sachets of WMTA (Angelus Industria de Produtos Odontologicos S/A, Londrina, Brasil) were provided. Each sachet was opened and weighed with an analytic weighing machine (A × 120 Series, Shimadzu, Japan), accurate to ± 0.0001 g, and mixed with 0.33 mL of distilled water. Each sample was placed in metal rings with an inner diameter of 20 mm and a height of 2 mm, which had been pre-weighed to ± 0.0001 g. Twenty-four samples were prepared and randomly divided into two equal groups. The 12 samples in group 1 were soaked in synthetic tissue fluid with a pH of 7.4 using a 2 cm × 2 cm piece of gauze; the 12 samples in group 2 were soaked in synthetic tissue fluid buffered with butyric acid (Merek, Darmastadt, Germany) at a pH value of 4.4 using a 2 cm × 2 cm piece of gauze. The samples were incubated at 37°C and 100% relative humidity for 21 h. The samples were then placed in a vacuum desiccator and subsequently placed in an oven at 105°C for 20 h. Finally, the samples were weighed using an analytic weighing machine accurate to ± 0.0001 g. The dry powder weight was calculated and recorded by subtracting the weight of the ring from the weight of the sample.
Preparation of the acidic and neutral tissue fluid
Synthetic tissue fluid was prepared by dissolving 1.7 g of KH2 PO4, 11.8 g of Na2 HPO4, 2 g of KCl and 80 g of NaCl in 10 liters of water.[15] The pH of the synthetic tissue fluid in group 1 was adjusted with the use of 0.5 mol of sodium hydroxide at 7.4; in group 2 the pH was adjusted at 4.4 with the use of butyric acid.
Measurement of solubility
In group 1, each specimen was transferred into a beaker containing 50 mL of STF with a pH value of 7.4. A highly accurate Autosampler pipette (Epperdorf automatic pipette) was used to exactly control the volume of STF. The beakers were incubated at 37°C and were retrieved from the incubator at 1-, 2-, 5-, 14-, 21-, 30-, 50- and 78-day intervals according to ISO Specification no. 6876. Then the specimen surfaces were rinsed with distilled water. The rinse water in the beakers was evaporated at a temperature slightly below the boiling point. Then the beakers were desiccated at 75°C and dried. The weight of the remaining dry powder was calculated by subtracting the initial weight of the beakers from the weight of the beakers containing the dry minerals.
In group 2, each specimen was transferred into a beaker containing 50 mL of STF at a pH of 4.4, buffered with butyric acid. The dry material was measured at 1-, 2-, and 5-day intervals. From the 5th day on, the samples were placed in beakers containing STF with a pH value of 7.4; the dry material was measured in a similar manner to that in group 1.
The weight of the dry material in the beakers represented the components dissolved in the STF and the components isolated from the samples. Therefore, in order to calculate the amount of components isolated from the samples the weight of the materials dissolved in 50 mL of the STF fluid was calculated and subtracted from the weight of the desiccated material in the beaker. The solubility at each interval was calculated by the following equation:
![]()
The sum of all the values of all time intervals was reported as the cumulative solubility, with the sum at 78-day interval being reported as the total solubility of the material. At the end of the 78th day all the samples were again placed in a vacuum desiccator for 4 h, followed by a 21-h period in an oven at 105°C. Then the samples were weighed accurate to ± 0.0001 g; weight changes of all the samples were calculated in comparison to baseline weights. Data was analyzed with two-factor ANOVA and Bonferroni test. Statistical significance was defined at P < 0.03.
RESULTS
Tables 1 and 2 summarize daily solubility at the 9 experimental intervals and the total solubility in each group. Means of total MTA solubility in neutral and acidic pH values were −9.1796 ± 1.9158% and −1.1192 ± 2.6236%, respectively, with statistically significant differences between the two groups (P = 0.028). Statistical analysis at each of the 9 time intervals revealed significant differences between the two groups at 5-day and 14-day intervals, with more solubility in the acidic environment compared to that in the neutral environment.
Table 1.
Daily solubility values of mineral trioxide aggregate at the nine experimental intervals in the acidic and neutral environments (mean±SEM) expressed in percentages

Table 2.
Cumulative solubility values of white mineral trioxide aggregate up to 5-day and 9-day intervals (mean±SEM) expressed as percentages

Statistically significant differences were also observed at 50-day and 78-day intervals; solubility in the acidic environment was less than that in the neutral environment (group 1). No significant differences were observed between the two groups at other intervals [Figure 1].
Figure 1.
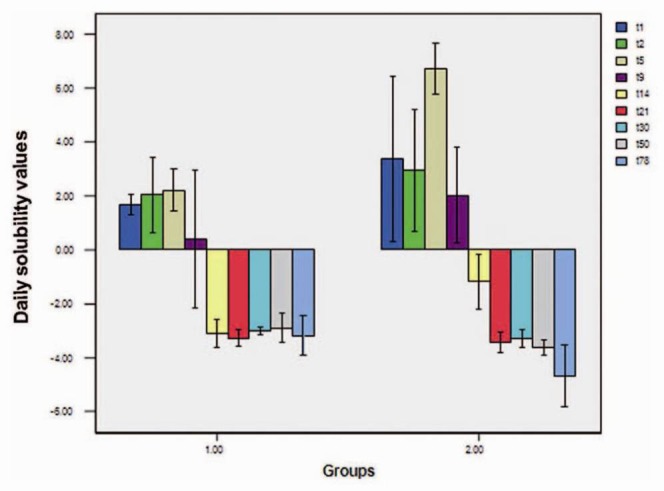
Daily solubility values at 9 experimental intervals
Cumulative solubility value was calculated up to each experimental interval in both groups [Figure 2], with statistically significant differences between the two groups for all intervals except up to 1th and 2th days. Cumulative solubility value up to day 5 added up to 5.8953 ± 0.6708% and 13.0469 ± 1.6160% in groups 1 and 2, respectively, with statistically significant differences between the two groups (P < 0.001) [Table 2].
Figure 2.
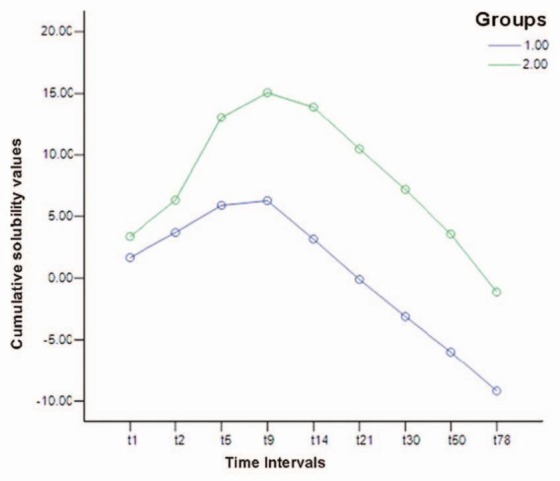
Cumulative solubility values up to 1st, 2nd, 5th, 9th, 14th, 21st, 30th, 50th, 78th days
Regarding the positive solubility values up to day 9, the cumulative solubility values were calculated for both groups up to day 9, compared and statistically analyzed. The results showed greater solubility in the group set and placed in the acidic environment compared to the group placed in the neutral environment (15.0673 ± 2.1235 vs. 6.2740 ± 1.3876) (P = 0.002).
Evaluation of weight changes at the end of the study revealed weight increases in both MTA groups: 9.1574 ± 2.14327% and 7.3276 ± 1.58237% in groups 1 and 2, respectively, with statistically significant differences between the two groups (P = 0.012).
DISCUSSION
In the present study, solubility of WMTA in the buffered STF under neutral and acidic pH values increased in acidic environments. Previous studies have evaluated solubility of MTA in distilled water, which was approximately 3%, less than that determined by ISO specification after 24 h of immersion in distilled water. It has been reported that MTA releases its soluble components in the short- and long-term.[16,17]
In this study, the samples were placed in STF with pH values of 7.4 and 4.4 during setting and thereafter in order to simulate physiologic and pathologic conditions, respectively, to evaluate WMTA solubility. Butyric acid was used to produce an acidic pH in group 2, which is a by-product of metabolism of anaerobic bacteria.[18] Based on the fact that subsequent to therapeutic interventions and elimination of the inflamed tissue, the tissue pH increases to that of a neutral environment the samples in group 2 were placed in the acidic environment for only 5 days.
Studies evaluating the effect of acidic pH on the physical and chemical properties of MTA have shown that MTA is influenced by the pH of the environment.[8,9,10,11,12,13] In the present study, the cumulative solubility of WMTA were greater in the acidic environment compared to that in the neutral environment. Erosion on the surface of cubic crystals, absence of needle-shaped crystal formations, increased porosity and decreased portlandite formation (the main product of MTA hydration, which is calcium hydroxide in crystal form) were observed after MTA was placed in an environment with a pH value of 5. The absence of needle-like crystals in the acidic pH might be attributed to higher surface area and the crystals’ rapid dissolution in the acidic environment. The decrease in the density of portlandite was attributed to dissolution of C3 A, C2 S and C3 S in the acidic environment before participating in the formation of portlandite.[8] Since porosity results in the progression of solubility, the increased MTA solubility in the present study might be attributed to increased porosity and changes in MTA crystalline structure after exposure to an acidic environment.
In the present study, total and daily solubility values after day 9 were negative in both groups. Previous studies have evaluated solubility of MTA in distilled water and their numeric values have been positive.[16,17] In the present study, STF was used instead of distilled water. MTA has a different behavior in STF compared to that in distilled water. Exposure of MTA to STF, which contains phosphate ions, results in the formation of hydroxyapatite according to the following reaction:[19]
10Ca+2 + 6 [(PO4)−3] + (OH)−1 →Ca10 (PO4)6 (OH)2
Deposition of hydroxyapatite as an adhering layer on MTA surface and continuation of its deposition inside MTA mass due to its porosity can give rise to changes in the general composition of MTA.[19] Furthermore, NaCl in STF can be incorporated into MTA composition according to the following formula:[20]
(Ca, Mg, Na)10 (PO4, HPO4, CO3)6 (OH)2
When NaCl is present during MTA hydration, more nucleation centers and more numerous needle-like crystals will be observed.[21] In distilled water, hydroxyapatite is not formed in MTA due to the absence of phosphate ions. The negative numeric values in the present study might be attributed to formation of hydroxyapatite and hydration of MTA.
In the present study, the numeric daily and total values of solubility up to day 9 were positive, which is consistent with the results of similar previous studies.[16,17] Sarkar et al. placed MTA samples in STF for 3 days and reported that MTA is soluble in STF and releases its principal cationic components. Calcium, silicate bismuth, iron, aluminum and magnesium were isolated from STF; these elements were traced back to MTA. The most abundant element was calcium because it is highly soluble in biologic fluids.[19]
Evaluation of weight changes at the end of the present study revealed weight gains in the samples in both groups, which was significantly lower in samples set and placed in an environment with a acidic pH, attributable to a higher initial solubility and disturbances in the normal formation of apatite crystals in acidic pH values. Shie et al. reported an increase in the weight of MTA samples placed in physiologic solution, which was attributed to the formation of hydroxyapatite crystals as a result of reaction of MTA components with the physiologic solution.[13] Immersion of MTA in distilled water resulted in a weight gain of 0.2%, which was attributed to the hydration process and entrapment of water in crystalline matrix structure.[22]
According to the results of the present study, placement of WMTA in an inflamed tissue increases its solubility and in cases in which a two-session treatment is possible, placement of some medications, including Ca(OH)2, is recommended to decrease inflammation. In other cases, such as periapical surgery or vital pulp therapy use of MTA with greater thicknesses is recommended. Therefore, further studies are recommended to make changes in MTA formulation in order to increases in its resistance in acidic environments and its efficacy in various tissue conditions.
ACKNOWLEDGMENTS
The authors gratefully thank the Office of Vice Chancellor for Research, Tabriz University of Medical Sciences, for supporting this research study financially. The authors also extend their gratitude to the Dental and Periodontal Research Center, Faculty of Dentistry, Tabriz University of Medical Sciences.
Footnotes
Source of Support: Tabriz Faculty of Dentistry, Tabriz University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541–4. doi: 10.1016/S0099-2399(06)81282-3. [DOI] [PubMed] [Google Scholar]
- 2.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 3.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 4.Gorduysus M, Avcu N, Gorduysus O, Pekel A, Baran Y, Avcu F, et al. Cytotoxic effects of four different endodontic materials in human periodontal ligament fibroblasts. J Endod. 2007;33:1450–4. doi: 10.1016/j.joen.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Huang TH, Yang CC, Ding SJ, Yeng M, Kao CT, Chou MY. Inflammatory cytokines reaction elicited by root-end filling materials. J Biomed Mater Res B Appl Biomater. 2005;73:123–8. doi: 10.1002/jbm.b.30182. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimine Y, Ono M, Akamine A. In vitro comparison of the biocompatibility of mineral trioxide aggregate, 4META/MMA-TBB resin, and intermediate restorative material as root-end-filling materials. J Endod. 2007;33:1066–9. doi: 10.1016/j.joen.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Torabinejad M, Hong CU, McDonald F, Pitt Ford TR. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349–53. doi: 10.1016/S0099-2399(06)80967-2. [DOI] [PubMed] [Google Scholar]
- 8.Lee YL, Lee BS, Lin FH, Yun Lin A, Lan WH, Lin CP. Effects of physiological environments on the hydration behavior of mineral trioxide aggregate. Biomaterials. 2004;25:787–93. doi: 10.1016/s0142-9612(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 9.Namazikhah MS, Nekoofar MH, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, et al. The effect of pH on surface hardness and microstructure of mineral trioxide aggregate. Int Endod J. 2008;41:108–16. doi: 10.1111/j.1365-2591.2007.01325.x. [DOI] [PubMed] [Google Scholar]
- 10.Giuliani V, Nieri M, Pace R, Pagavino G. Effects of pH on surface hardness and microstructure of mineral trioxide aggregate and Aureoseal: An in vitro study. J Endod. 2010;36:1883–6. doi: 10.1016/j.joen.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Saghiri MA, Lotfi M, Saghiri AM, Vosoughhosseini S, Fatemi A, Shiezadeh V, et al. Effect of pH on sealing ability of white mineral trioxide aggregate as a root-end filling material. J Endod. 2008;34:1226–9. doi: 10.1016/j.joen.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Shokouhinejad N, Nekoofar MH, Iravani A, Kharrazifard MJ, Dummer PM. Effect of acidic environment on the push-out bond strength of mineral trioxide aggregate. J Endod. 2010;36:871–4. doi: 10.1016/j.joen.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 13.Shie MY, Huang TH, Kao CT, Huang CH, Ding SJ. The effect of a physiologic solution pH on properties of white mineral trioxide aggregate. J Endod. 2009;35:98–101. doi: 10.1016/j.joen.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan AE, Goldberg F, Artaza LP, de Silvio A, Macchi RL. Disintegration of endodontic cements in water. J Endod. 1997;23:439–41. doi: 10.1016/S0099-2399(97)80298-1. [DOI] [PubMed] [Google Scholar]
- 15.Rahimi S, Shahi S, Lotfi M, Yavari HR, Charehjoo ME. Comparison of microleakage with three different thicknesses of mineral trioxide aggregate as root-end filling material. J Oral Sci. 2008;50:273–7. doi: 10.2334/josnusd.50.273. [DOI] [PubMed] [Google Scholar]
- 16.Fridland M, Rosado R. MTA solubility: A long term study. J Endod. 2005;31:376–9. doi: 10.1097/01.don.0000140566.97319.3e. [DOI] [PubMed] [Google Scholar]
- 17.Fridland M, Rosado R. Mineral trioxide aggregate (MTA) solubility and porosity with different water-to-powder ratios. J Endod. 2003;29:814–7. doi: 10.1097/00004770-200312000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka JI, Takano N, Unozawa H, Shigematsu S, Kishino Y, Yonezu H, et al. A rapid diagnosis of anaerobic infection in the oro-maxillary region by gas-liquid chromatography. Bull Tokyo Dent Coll. 1990;31:155–62. [PubMed] [Google Scholar]
- 19.Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I. Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod. 2005;31:97–100. doi: 10.1097/01.don.0000133155.04468.41. [DOI] [PubMed] [Google Scholar]
- 20.LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991;15:1–201. [PubMed] [Google Scholar]
- 21.Lee YL, Lin FH, Wang WH, Ritchie HH, Lan WH, Lin CP. Effects of EDTA on the hydration mechanism of mineral trioxide aggregate. J Dent Res. 2007;86:534–8. doi: 10.1177/154405910708600609. [DOI] [PubMed] [Google Scholar]
- 22.Bodanezi A, Carvalho N, Silva D, Bernardineli N, Bramante CM, Garcia RB, et al. Immediate and delayed solubility of mineral trioxide aggregate and Portland cement. J Appl Oral Sci. 2008;16:127–31. doi: 10.1590/S1678-77572008000200009. [DOI] [PMC free article] [PubMed] [Google Scholar]


