Abstract
High trait hostility is associated with persistent cigarette smoking. To better understand mechanisms that may account for this association, we examined the effects of acute smoking abstinence and delayed versus immediate smoking reinstatement on responses to a social stressor among 48 low hostile (LH) and 48 high hostile (HH) smokers. Participants completed two laboratory sessions, one before which they had smoked ad lib and one before which they had abstained for the prior 12 hours. During each session, participants completed a stressful speaking task and then smoked immediately after the stressor or after a 15-minute delay. The effect of immediate vs. delayed smoking reinstatement on recovery in negative mood was significantly moderated by hostility. When reinstatement was delayed, HH participants showed significant increases in negative mood over time, whereas LH participants showed little change. When reinstatement was immediate, HH and LH smokers showed similar significant decreases in negative mood. Smoking abstinence did not moderate hostility effects. Cigarette smoking may prevent continuing increases in negative mood following social stress in HH smokers, which may partially explain their low rates of quitting.
Keywords: smoking, hostility, social stress, personality, negative mood
The personality trait of hostility has been conceptualized as a primarily cognitive phenomenon involving cynical attitudes and mistrust of others, although it also refers to a broader construct involving hostile attitudes, angry affect, and aggressive behavior (Miller, Smith, Turner, Guijarro, & Hallet, 1996). Smith's (1994) definition describes hostility as a “devaluation of the worth and motives of others, an expectation that others are likely sources of wrongdoing, a relational view of being in opposition toward others, and a desire to inflict harm or see others harmed “ (p. 26). Although hostility may be associated with aggression, individuals high in hostility do not necessarily display aggressive behaviors. Likewise, although it is correlated with the broader personality dimension of neuroticism as well as trait depression and anxiety, hostility shows even stronger associations with more interpersonally-relevant traits such as agreeableness (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989; Lubin & Van Whitlock, 2002) and trait anger (Han, Nathan, Calhoun, & Butcher, 1995; Smith & Frohm, 1985). Thus, hostility is a sociocognitive trait that is unique from other affectively-valenced personality dimensions.
Cross-sectional studies have consistently found that smokers have higher levels of hostility than non-smokers (Bunde & Suls, 2006). Current smokers report greater hostility than former smokers, and this differences is not accounted for by differences in demographic characteristics or comorbid psychopathology (Kahler, Daughters, et al., 2009). In longitudinal studies, greater hostility has been associated with a lower odds of quitting smoking in a sample of college students followed for 20 years (Lipkus, Barefoot, Williams, & Siegler, 1994), in a community sample of 18–30 year-olds followed for 5 years (Iribarren et al., 2000), and in patients followed for 6 years after coronary angiography (Brummett et al., 2002). Kahler and colleagues (Kahler, Strong, Niaura, & Brown, 2004) found higher trait hostility was associated with poor smoking cessation treatment outcome in smokers with a history of past major depression. A second study found that trait hostility also predicted poor smoking cessation treatment outcomes in a sample of heavy social drinkers, an effect that was more robust than the effects of correlated traits including neuroticism, aggression, and anger (Kahler, Spillane, et al., 2009). Hostility did not predict increases in withdrawal symptoms and negative mood immediately upon quitting smoking in either study but in some analyses predicted slower recovery from withdrawal and mood effects in the one to two weeks after quitting (Kahler, Spillane, et al., 2009; Kahler et al., 2004). Taken together, these findings suggest that hostility warrants further consideration as a potential target for interventions that address risk factors for poor smoking cessation outcome. However, further research on the biobehavioral mechanisms linking hostility and smoking is needed to inform such efforts. In particular, determining how hostility predicts responses to smoking abstinence and responses to smoking following a stressor may shed light on the processes that contribute to continued smoking in high hostile smokers and can guide intervention development for this high-risk group.
Potential Mechanisms Linking Hostility and Persistence of Smoking
The mechanisms accounting for the relationships between hostility and persistence of smoking remain unknown. Among lifetime smokers, hostility was not found to associate significantly with a history of tobacco dependence (Kahler, Daughters, et al., 2009), and in treatment samples, hostility has not been correlated significantly with tobacco dependence severity (Kahler, Spillane, et al., 2009; Kahler et al., 2004). Although some studies have shown that trait anger and neuroticism predict greater abstinence-induced negative affect, withdrawal, and craving (al'Absi, Carr, & Bongard, 2007; Gilbert et al., 1998; Gilbert et al., 2002), the association between hostility and greater nicotine withdrawal has been inconsistent (Kahler, Spillane, et al., 2009; Kahler et al., 2004). Thus, neither greater tobacco dependence nor withdrawal severity appear to explain hostility’s association with persistent smoking. Instead, high rates of smoking among high hostile (HH) persons compared to low hostile (LH) persons may be due to the negative reinforcement properties of nicotine, with reduction in negative affect in response to social interactions being especially relevant. Such a hypothesis is consistent with theoretical models of “self-medication,” which suggest that specific traits predispose individuals to smoke because smoking serves specific adaptive functions for them (Gilbert et al., 1998).
Trait hostility is positively associated with greater interpersonal stress (Benotsch, Christensen, & McKelvey, 1997), greater expression of hostile emotions in social situations (Brummett et al., 1998), and more frequent and intense reports of anger, negative moods, and negative interactions as assessed by diary entries during ambulatory monitoring (Brondolo et al., 2003; Shapiro, Jamner, & Goldstein, 1997). HH participants show greater negative affect in response to experimental provocation than LH participants (Felsten & Hill, 1999; Suarez, Kuhn, Schanberg, Williams, & Zimmermann, 1998), though there have been exceptions (Gallo, Smith, & Kircher, 2000; S. B. Miller et al., 1998). HH individuals also have particularly strong physiological responses to interpersonal stressors. For example, when recalling a self-chosen anger memory, HH participants show a larger and longer-lasting blood pressure response compared to LH participants (Fredrickson et al., 2000). When harassed during problem-solving tasks, HH participants show enhanced blood pressure, heart rate, norepinephrine, testosterone, and cortisol relative to harassed LH participants and nonharassed HH participants (Llabre, Spitzer, Siegel, Saab, & Schneiderman, 2004; Miller et al., 1998; Suarez, Harlan, Peoples, & Williams, 1993; Suarez et al., 1998). Similarly, HH participants show greater physiological arousal and anxiety compared to LH participants when asked to self-disclose personal information about a stressful life event (Christensen et al., 1996; Christensen & Smith, 1993).
That HH individuals experience more frequent and longer-lasting negative affect and physiological arousal than LH individuals suggests they may have greater opportunity to pair smoking with negative affect and to form associations between smoking and the reduction of negative affect. Indeed, compared to LH smokers, HH smokers report more frequent smoking in social situations and to get along better with other people (Kahler et al., 2004). Furthermore, the negatively reinforcing properties of nicotine may be especially potent among individuals high in hostility. For example, among both smokers and nonsmokers high in hostility, nicotine patch has been shown to reduce the frequency of reports of anger and other negative moods compared to placebo patch during a 24-hr monitoring period (Jamner, Shapiro, & Jarvik, 1999); this effect was not found among those low in hostility.
The association between hostility and negative affect is important for smoking cessation. Negative affect has a prominent role in smoking relapse (Kenford et al., 2002; Piasecki, Kenford, Smith, Fiore, & Baker, 1997). Smoking relapses often occur in situations involving negative moods such as anxiety, anger, and depression (Brandon, Tiffany, Obremski, & Baker, 1990; Marlatt & Gordon, 1980; Shiffman, 1982), and affective distress after quitting predicts poor outcome (Burgess et al., 2002; Covey, Glassman, & Stetner, 1990; Ginsberg, Hall, Reus, & Muñoz, 1995; West, Hajek, & Belcher, 1989). HH smokers, compared to LH smokers, may be especially prone to smoking relapse because they frequently experience negative affect and are less capable of alleviating that negative affect without smoking. If this hypothesis is supported, then smoking cessation interventions could be developed to help HH smokers more effectively manage negative moods without smoking.
Study Aims
The purpose of the present study was to enhance understanding of hostility’s association with persistent smoking by examining the effects of smoking and smoking abstinence on responses to a laboratory-based social stressor task in LH and HH smokers. We hypothesized that HH smokers, compared to LH smokers, would show greater increases during the stressor and slower recovery after the stressor in (a) negative mood, (b) urge to smoke, and (c) cardiovascular arousal. We further hypothesized that smoking immediately after the social stress task, compared to smoking after a delay, would facilitate recovery in mood, urge to smoke, and arousal more strongly for HH smokers compared to LH smokers. Such an effect would support the notion that HH smokers are especially prone to smoking relapse because smoking helps them alleviate socially-induced negative affect. Finally, although results from previous clinical trials have indicated that hostility does not predict responses to acute abstinence from smoking, no study has compared the effects of acute smoking abstinence on responses to social stress in LH and HH smokers. A prior study found that the effect of personality on smoking abstinence effects was more prominent during a stressful task (Gilbert et al., 2004). Therefore, we examined whether overnight smoking deprivation, relative to ad lib smoking, would more strongly increase the effects of the social stressor in HH smokers compared to LH smokers.
Method
Participants
Participants were LH (n = 48) and HH (n = 48) smokers recruited from the local community. For inclusion, participants had to: (a) be 18 years of age or older, (b) have smoked cigarettes regularly for at least one year, (c) currently smoke at least 10 cigarettes per day, (d) currently be using no other tobacco products or nicotine replacement, and (e) be able to read English. Individuals were excluded if they were currently dependent on alcohol or drugs other than tobacco or met criteria for current affective disorder.
The study involved recruiting separate groups of LH and HH individuals. Therefore, to be included, subjects had to score either a 5 or lower (LH) or a 10 or higher (HH) on the 17-item version of the Cook-Medley Hostility Scale (Strong, Kahler, Greene, & Schinka, 2005) during a phone screen. Using these cut points ensured substantial differentiation between groups as they correspond closely with the upper (HH; score of > 10) and lower (LH; < 5) thirds of scores from previous community samples (Han, Weed, Calhoun, & Butcher, 1995). LH and HH groups were recruited to be balanced on gender and level of tobacco dependence as assessed by the Fagerström Test for Nicotine Dependence (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). FTND scores of 4 or lower were categorized as low-FTND, while scores of 5 of higher were considered high-FTND. As a result, eight separate blocks were recruited into the study with 12 participants in each block: low-FTND LH females, high-FTND LH females, low-FTND HH females, high-FTND HH females, low-FTND LH males, high-FTND LH males, low-FTND HH males, and high-FTND HH males.
Design
This study utilized a 2 between (LH vs. HH smoker) X 2 within (ad lib smoking vs. 12 hr abstinence prior to the session – to assess the effects of abstinence) X 2 within (immediate vs. delayed smoking – to assess the effects of smoking on recovery from the stressor) mixed factorial design to examine differences between LH and HH smokers on subjective and cardiovascular responses measured before, during, and after a social stressor. The factors were partially crossed with one another. Specifically, all participants completed an experimental session when they had been smoking ad libidum and a session in which they abstained from smoking for at least 12 hours. Half the participants (24 LH and 24 HH) were assigned to the delayed smoking condition on their abstinent day and to the immediate smoking condition on their ad lib day. The other half of participants were assigned to immediate smoking on their abstinent day and delayed smoking on their ad lib day. This partially crossed design allowed us to examine both the main effects and interaction of these two factors.
Procedure
Individuals responding to community advertisements completed an initial phone screen to assess potential eligibility. The FTND and Cook-Medley scale were completed at that screening. Following the phone screen, eligible participants were invited for a baseline session, and if eligible, two additional experimental sessions. At baseline, participants completed an informed consent approved by the Brown University Institutional Review Board. They then completed an alcohol breath analysis (those with positive BrAC were rescheduled) and psychiatric interview to confirm eligibility. They also completed baseline measures of mood, depressive symptoms, smoking characteristics, and recent alcohol and drug use. Finally, participants were introduced to the experimental laboratory and were fitted with a blood pressure cuff for measurement of physiological response. After a 5-minute habituation period, they were instructed that they could smoke freely for the next fifteen minutes, during which time their smoking was videotaped for later assessment of smoking topography.
Assignment to condition and order of experimental sessions
At the end of the baseline session, participants were informed whether they were to smoke ad lib prior to the first experimental session or to abstain from smoking for a minimum of 12 hrs. On the session in which they were assigned to smoking deprivation, participants were instructed not to smoke cigarettes after midnight on the day before that session. All sessions occurred between 12 and 6 pm. Allocation to experimental conditions for the two experimental sessions was achieved by randomly assigning equal number of participants (n = 3) from each of the eight blocks (balanced on gender, hostility, and FTND; see above) to each of four possible condition-orders: (1) deprivation/immediate smoking first followed by ad-lib/delayed smoking second (n = 24); (2) ad-lib/delayed smoking followed by deprivation/immediate smoking (n = 24); (3) deprivation/delayed smoking followed by ad-lib/immediate smoking (n = 24); and (4) ad-lib/immediate smoking followed by deprivation/delayed smoking (n = 24). Thus, order of completion was fully counterbalanced across participants within each of the eight subject blocks. Those who did not complete both sessions successfully were replaced so that we achieved our desired sample size of 96. Overall, 13 out of 109 participants (11.9%) who were eligible following a baseline interview did not complete both experimental sessions. Those not completing the study did not differ significantly from completers on hostility, sex, or level of tobacco dependence.
Experimental sessions
At the outset of experimental sessions, a breath carbon monoxide (CO) reading was obtained. Individuals assigned to the deprivation condition for a given session were required to have a reading of 10 ppm or less. Participants with CO > 10 ppm during their deprived session were able to reschedule that session again within one week; those who do did pass the CO abstinence criterion within a week were dropped from further participation. Following the CO reading, subjects completed self-report and two computerized cognitive measures (not reported here). They were then fitted with a blood pressure cuff for measurement of physiological response. After a 10-minute habituation period, they began the interpersonal stressor, the Debate Task.
The Debate Task required the participant to deliver a speech during a hypothetical situation involving unfair treatment. There were two versions of this task, one involving a debate with a parking enforcement officer over a parking ticket, and one involving a debate with a store manager concerning a false accusation of shoplifting. These two scenarios have been used in prepared speech tasks in a prior study, although without audiotaped provocations that were part of the present method; the speech tasks were shown to increase cardiovascular responding with moderate stability (test-retest correlation) in responding across the two tasks (Kamarck, Debski, & Manuck, 2000). The session in which participants completed these versions was counterbalanced. Participants were informed that they would be given a hypothetical situation in which they had been falsely accused and that their task was to prepare and deliver a 3-minute argument defending themselves while responding to audiotaped challenges that were played through the lab computer. Participants were given 3 minutes to prepare their argument, and they were informed that they would be videotaped during their performance so that their argument could be rated on strength and persuasiveness; they were asked to speak towards a video camera. During the course of the 3-minute task, the participant was interrupted 8 times by an audiotaped voice representing one of the hypothetical individuals in the situation (e.g., the parking enforcement officer); these interruptions were delivered in an intentionally irritating manner. Prior to the stressor, immediately after the stressor and at minutes 5, 10, 15, 20, 25, and 30 after the stressor, participants completed brief measures of mood and craving on the computer. In addition, their blood pressure and heart rate were measured twice within each of those intervals.
Following the stressor and the post-stressor self-report ratings, instructions for participants differed depending on condition assignment. In the immediate smoking condition, participants were immediately told to lift the cover off of a tray that contained their lighter and cigarettes and were informed that they could smoke freely over the next 30 minutes during which they would be monitored. In the delayed condition, participants were instructed after the post-stressor, 5-minute, and 10-minute self-report assessments that they should sit quietly while their cardiovascular activity was monitored. After the 15-minute assessment, they were instructed to lift the cover off of the tray that contained their lighter and cigarettes and were informed that they could smoke freely while they continued to be monitored.
Measures
Hostility
To assess hostility, a brief 17-item version of the Cook-Medley Hostility scale (Cook & Medley, 1954) was administered during a phone screen. This scale is an empirically-derived shortened version of the 50-item true-false Cook-Medley Hostility Scale drawn from the MMPI (Cook & Medley, 1954). This shortened scale correlates strongly with the extended version (r >.93) and taps a unidimensional latent dimension indicative of expectations of a hostile and oppositional interpersonal world (Strong et al., 2005) and has been demonstrated as a reliable and unbiased (i.e., items function similarly across subgroups) measure of hostility.
Baseline session measures
Diagnostic exclusions and lifetime prevalence of key Axis I diagnoses (substance abuse/dependence and major depressive disorder) were determined by the substance use and affective disorders sections of the Structured Clinical Interview for DSM-IV-Non Patient Version (SCID-NP, First, Spitzer, Gibbon, & Williams, 2002). Adequate reliability of the SCID-NP has been shown (Spitzer, Williams, Gibbon, & First, 1989). Level of tobacco dependence was assessed with the FTND (Heatherton et al., 1991), which has shown good internal consistency, a single dimension factor structure, and positive relationships with nicotine intake as assessed by saliva cotinine. Readiness to change smoking was assessed with the contemplation ladder (Biener & Abrams, 1991) and self-efficacy for quitting was assessed using the Temptation Scale, a well-established 9-item scale that includes three subscales for specific smoking temptations, Negative Affect, Positive Affect/Social, and Habit/Craving (Velicer, Diclemente, Rossi, & Prochaska, 1990). Mood state prior to each session was assessed with the Positive and Negative Affect Schedule (Watson, Clark, & Tellegen, 1988). Depressive symptoms were assessed using the Center for Epidemiologic Studies –Depression scale (Radloff, 1977). Alcohol use in the past 30 days was assessed with the Timeline Followback Interview (Sobell & Sobell, 1994), and drug use was assessed with single item ratings of the number of days using marijuana, cocaine, heroin, and other drugs in the past month.
Experimental session measures
At the beginning of each session, nicotine withdrawal symptoms were assessed with the 7-item Minnesota Nicotine Withdrawal Scale (MNWS; (Hughes & Hatsukami, 1986)). At repeated intervals prior to and after the Debate Task, subjects were prompted by the computer to complete craving and mood assessments. All items were assessed using a 0–100 visual analog slider. Urge to smoke was assessed with a single item regarding “urge to smoke RIGHT NOW.” The mood assessment included single-item measures of restlessness, frustration, anger, anxiety, and tension. As we have done previously (Brown, Lejuez, Kahler, & Strong, 2002), the five negative mood ratings were averaged to form a general index of negative mood. Internal consistency of this 5-item scale was very high exceeding an alpha of .90 at all assessments. A visual inspection of results across items indicated a similar pattern for all items, and the combined set of items provided the most reliable index of negative mood. Furthermore, an analysis of a composite of the anger and irritability items yielded the same results as those for the overall negative mood composite.
Cardiovascular responses to the stressor were assessed using a Critikon DINAMAP® Vital Signs Monitor (Model 1846 SX, Critikon, Tampa, FL). Blood pressure and heart rate were assessed at 3, 6, and 9 minutes during the habituation period and averaged to enhance reliability. Likewise, during the Debate Task speech delivery and during each 5 minute assessment block, blood pressure and heart rate were assessed twice and averaged. Given that systolic blood pressure has been the physiological variable most consistently associated with stress-induced differences as a function of hostility in prior research (Christensen & Smith, 1993; Llabre et al., 2004; S. B. Miller et al., 1998; Suarez et al., 1993), it was considered the primary measure of physiological responding in this study.
Smoking topography
At the baseline session and during the free smoking intervals in each experimental session, participants were videotaped. Smoking behavior on these videotapes was coded by trained raters who used a computerized program to record the start and stop time of each puff. The primary variables derived for this study were total number of puffs taken and average puff duration. To standardize the length of smoking time assessed in the immediate and delayed smoking conditions, smoking topography was coded for the first 15 minutes in which smoking was permitted. One-third of tapes were coded by two persons to assess reliability of the ratings. The correlations between the two ratings were high for both total number of puffs (rs = .98, .99, and .89 for baseline, session 1, and session 2) and for mean puff duration (.90, .96, .64).
Data Analysis Plan
We first examined baseline differences between LH and HH smokers. We then examined the effects of smoking deprivation on nicotine withdrawal, negative mood, urge to smoke, and cardiovascular measures (systolic blood pressure was the primary dependent measure) conducted prior the Debate Task; this analysis used a 2 between (LH vs. HH smoker) X 2 within (ad lib smoking vs. 12 hr abstinence prior to the session) repeated measures mixed model analyses as implemented by PROC MIXED in SAS (SAS Institute Inc., 2005). The mixed model analyses allowed for missing data and included all subjects in all analyses. The models covaried sex and level of tobacco dependence (given that these were experimental blocking variables) and included the main effect of hostility on the dependent variable (DV) as well as the hostility X deprivation interaction. We also included age as a covariate (see below). We next examined changes in negative mood, urge to smoke, and systolic blood pressure following the Debate Task using a 2 between (LH vs. HH smoker) X 2 within (ad lib smoking vs. 12 hr abstinence prior to the session) X 2 within (pre vs. post Debate Task) repeated measures mixed model analyses, which also covaried sex, age, and level of tobacco dependence.
Multilevel modeling was then used to test the hypothesis that smoking immediately after the social stress task, compared to smoking after a delay, would facilitate recovery in negative mood, urge to smoke, and systolic blood pressure more strongly among HH smokers compared to LH smokers. These analyses considered only the first four readings after the Debate Task (post-stressor, and 5, 10, and 15 minutes after the stressor). The primary model included the pre-Debate Task value of the respective DV, sex, age, level of tobacco dependence, deprivation condition, delay condition, hostility, and the delay X hostility interaction as predictors of both mean levels and rates of change in the DVs after the Debate Task. In a second step of the analysis, we examined whether deprivation moderated the effects of hostility and the hostility X deprivation interaction on recovery in the DVs. For all analyses, all variables were centered so that main effects and interactions could be interpreted simultaneously.
Finally, to examine whether smoking topography differed by hostility group, we conducted mixed model analyses of number of puffs taken and average puff duration in the 15 minute smoking period after the Debate Task. These models controlled for the respective value of the DV at the baseline session as well as sex, age, and tobacco dependence level.
Results
Baseline Differences
Table 1 shows the characteristics of the 48 LH smokers and the 48 HH smokers. By design, both groups were 50% female. HH smokers were significantly younger than LH smokers and were more likely to be non-White. Due to these demographic differences, we re-ran our primary analyses controlling for non-White status and age. Only age was associated with any of the dependent variables examined; we therefore covaried age but not race in all analyses presented. LH and HH smokers were very closely matched on level of tobacco dependence and cigarettes smoked per day. HH smokers scored lower than LH smokers on the Contemplation Ladder, indicating less readiness to quit smoking. They reported significantly higher temptation to smoke in negative affect situations but did not differ significantly from LH smokers on temptation to smoke in positive affect social situations or due to habit and craving. HH smokers reported significantly higher levels of depressive symptoms and negative mood at baseline and were more likely to have a history of major depression than LH participants1. Although HH participants were more likely to have a history of alcohol dependence, they did not differ from LH participants on current alcohol or other drug use.
Table 1.
Demographic, Smoking, Affective, and Substance Use Characteristics of Low Hostile and High Hostile Participants
| Low Hostile (n = 48) |
High Hostile (n = 48) |
p-value | |
|---|---|---|---|
| Variable | % or Mean (SD) |
% or Mean (SD) |
|
| Demographics | |||
| % Female | 50.0 | 50.0 | ns |
| Age | 43.0 (13.2) | 37.4 (12.5) | .04 |
| Race | |||
| % White | 85.4 | 56.3 | .03 |
| % African American | 10.4 | 20.8 | |
| % American Indian | 0.0 | 2.1 | |
| % Asian | 0.0 | 2.1 | |
| % Latino | 0.0 | 10.4 | |
| % >1 Race | 4.2 | 8.3 | |
| Years of education | 13.1 (1.9) | 12.9 (2.2) | ns |
| % Married | 25.0 | 12.5 | ns |
| Smoking Variables | |||
| FTND | 5.3 (1.9) | 5.5 (2.4) | ns |
| Cigarettes per day | 21.0 (6.1) | 22.3 (11.0) | ns |
| Contemplation ladder1 | 4.8 (1.4) | 4.3 (1.3) | .03 |
| Temptation scale- Positive Affect/Social2 | 4.1 (0.8) | 4.3 (0.9) | ns |
| Temptation scale- Negative affect2 | 3.8 (1.1) | 4.5 (0.7) | .0006 |
| Temptation scale- Habit/craving2 | 3.4 (0.9) | 3.7 (1.0) | ns |
| Affective Variables | |||
| Depressive symptoms (CES-D)3 | 6.3 (5.0) | 16.4 (9.7) | <.0001 |
| Negative mood (PANAS)4 | 12.0 (3.1) | 15.0 (5.3) | .001 |
| Positive mood (PANAS)4 | 32.8 (8.9) | 31.9 (9.3) | ns |
| % History of major depression | 29.1 | 50.0 | .03 |
| Substance Use | |||
| Average drinks per week | 4.5 (5.7) | 6.6 (9.5) | ns |
| % Heavy drinking days | 6.9 (11.1) | 8.8 (15.8) | ns |
| % Lifetime alcohol dependence | 16.7 | 41.7 | .007 |
| % Using other drugs currently | 20.8 | 25.0 | ns |
Note: p-values are based on t-test for continuous variables and chi-square tests for categorical variables. FTND= Fagerström Test for Nicotine Dependence
Scores range from 1 (no interest in quitting) to 10 (have quit and will never smoke again).
Scores range from 1 = not tempted at all to 5 = extremely tempted.
Scores range from 0 to 60.
Scores range from 0 to 40.
Depiction of Outcomes
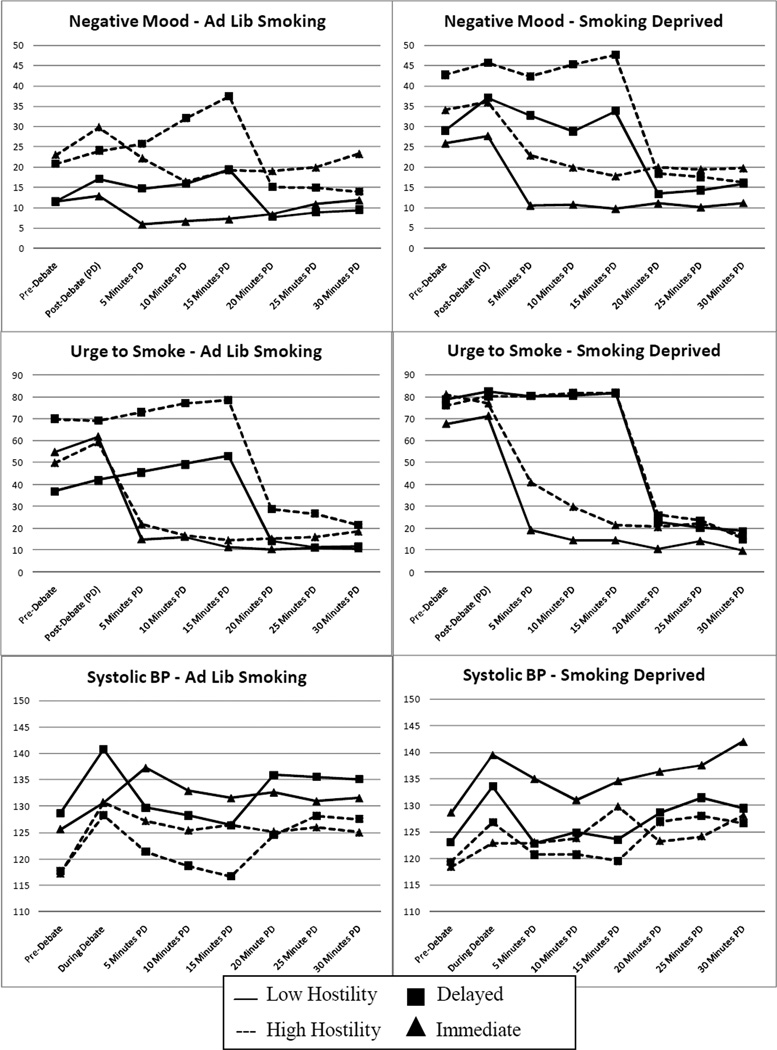
Figure 1 shows the three primary dependent variables (DVs; negative mood, urge to smoke, and systolic blood pressure) prior to the debate task, during or immediately after the debate task, and at five minute intervals following the debate task. The top row of 3 figures shows results on the day participants were smoking ad lib and the bottom row shows results when participants were smoking deprived for at least 12 hours prior to the session. In each figure panel, participants are grouped according to hostility status and according to delayed vs. immediate smoking condition assignment. Participants in the delayed condition were permitted to start smoking after the third interval following the debate task.
Figure 1.
Changes in negative affect, urge to smoke, and systolic blood pressure prior to and after the Debate Task. The left-hand panels show responses on the ad lib smoking session. The right-hand panels show responses on the smoking deprived session. Negative affect and urge to smoke were assessed on 0 – 100 scales. Ad lib = participants smoked ad lib prior to the experimental session. Deprived = participants were abstinent from smoking for at least 12 hrs prior to the experimental session. Immediate = smoking was allowed after the post-debate assessment. Delayed = smoking was allowed after the 15 minutes post-debate assessment. Negative mood and urge to smoke were significantly higher and systolic blood pressure was significantly lower on the smoking deprived session compared to the ad lib smoking session. Across sessions, negative mood, urge to smoke, and systolic blood pressure increased significantly following the stressor. For negative mood only, there was a significant interaction between hostility group and immediate vs. delayed smoking; immediate smoking, compared to delayed smoking, was associated with more steep reductions in negative mood for HH participants when compared to LH participants. This effect was not moderated by deprivation condition.
Effects of Smoking Deprivation on Variables Measured Prior to the Debate Task
Repeated measures mixed model analyses, controlling for sex, age, and level of tobacco dependence, indicated that nicotine withdrawal (B = 4.8, SE = 0.6), negative mood (B = 15.9, SE = 2.5), and urge to smoke (B = 22.9, SE = 2.9) were significantly higher prior to completing the Debate Task at the session in which participants were smoking deprived compared to the one in which they were smoking ad lib, ps < .0001. Heart rate prior to the social stressor task was significantly reduced by overnight smoking deprivation (B = −5.0, SE = 1.0, p < .0001), whereas diastolic and systolic blood pressure were not significantly affected. There was a significant main effect of hostility on negative mood (B = 9.0, SE = 3.8, p = .02) and withdrawal symptoms (B = 1.6, SE = 0.8, p = .049), indicating that HH participants reported negative mood prior to the Debate Task across the two experimental sessions, as well as more withdrawal symptoms, the majority of which focus on current negative affect. The hostility X deprivation interaction was nonsignificant. For all other variables, there were no significant main effects of hostility or significant interactions between hostility and smoking deprivation, indicating that smoking deprivation had similar effects in LH and HH smokers prior to the social stressor task.
Initial Responses to the Debate Task
Repeated measures mixed model analyses, controlling for sex, age, and level of tobacco dependence, indicated that negative mood (B = 3.9, SE = 1.2), urge to smoke (B = 3.7, SE = 0.9, and cardiovascular reactivity (heart rate, B = 6.9, SE = 0.7; systolic blood pressure, B = 9.3, SE = 1.2; diastolic blood pressure, B = 8.2, SE = 0.9) were significantly increased as a function of the debate task, all ps < .005. Contrary to our hypotheses, hostility did not significantly moderate the effects of the task on any of these variables. In addition, the effects of hostility on task responding were not significantly moderated by smoking deprivation.
Recovery Following the Debate Task
Multilevel model analyses indicated that HH smokers reported more negative mood overall in the 15 minutes after the stressor (B = 4.4, SE = 1.8, p = .02). Negative mood reduced significantly over the four post-Debate Task ratings (B = −1.4, SE = 0.5, p = .005), but this effect was strongly moderated by delay condition (B = 5.4, SE = 0.9, p <.0001), indicating that the rate of change in negative mood was more steeply negative when participants smoked immediately after the stressor. Finally, there was a significant interaction between hostility and delay on rate of change in negative mood (B = 4.4, SE = 1.8, p = .01), which indicated that the difference in rate of change between immediate smoking and delayed smoking conditions was greater in HH smokers compared to LH smokers. Specifically, similarly rapid reductions in negative mood were seen among LH (B = −3.7, SE = 1.0, p = .0002) and HH smokers (B = −4.6, SE = 1.0, p < .0001) when they smoked immediately after the Debate Task (see Figure 1). By contrast, when smoking was delayed, negative mood in the 15 minutes after the Debate Task continued to escalate in HH smokers (B = 3.0, SE = 1.0, p = .003), while it remained stable in LH smokers (B = −0.4, SE = 1.0, p = .67). In a final step of the analysis, we examined whether the effect of hostility on rate of change in negative mood was moderated by deprivation (i.e., a 3-way interaction between delay, deprivation, and hostility). This effect was nonsignificant.
Analysis of change in urge to smoke indicated that urge to smoke significantly decreased over time (B = −7.2, SE = 0.6, p <. 0001) and that this effect was strongly moderated by delayed vs. immediate smoking (B = 18.2, SE = 1.2, p < .0001). Hostility did not predict mean level of urge to smoke or change in urge to smoke and did not interact significantly with delay condition in predicting change in urge to smoke. Also, the hostility X deprivation X delay interaction did not predict change.
The primary cardiovascular dependent variable, systolic blood pressure, significantly decreased in the 15 minutes after the debate task (B = −1.9, SE = 0.4, p < .0001), an effect that was significantly moderated by smoking delay (B = −2.5, SE = 0.6, p < .0001) such that the rate of decrease in systolic blood pressure was greater when participants were not smoking. The rate of decrease was also significantly moderated by hostility (B = 1.6, SE = 0.7, p = .02), such that HH smokers showed a slower rate of decrease than LH smokers regardless of delay condition. Hostility did not interact with delay in predicting change in systolic blood pressure. Also, the hostility X deprivation X delay interaction did not predict change.2
The secondary cardiovascular reactivity measures, heart rate and diastolic blood pressure, also showed significant decreases after the debate task that were significantly slower in the immediate vs. delayed smoking condition. There were no main or interactive effects of hostility on rate of change in these variables.
Smoking Topography
Repeated measures mixed model analyses, controlling for sex, age, level of tobacco dependence and the respective smoking topography variable at baseline, indicated that total number of puffs (B = 2.3, SE = 0.9, p = .008) but not average puff duration after the Debate Task were significantly higher when participants were smoking deprived compared to when they had smoked ad lib prior to the session. Hostility did not significantly predict smoking topography or the increase in smoking when deprived. Delay condition did not alter topography significantly on either measure.
Discussion
Results of this study indicate that the negatively reinforcing properties of smoking may be one potential mechanism that links hostility and persistence of smoking. Specifically, the effect of immediate vs. delayed smoking on negative mood trajectories following a social stressor differed significantly by hostility group. After a stressor, negative mood among LH smokers remained relatively stable over 15 minutes when they were not able to smoke their cigarettes. By contrast, negative mood continued to increase significantly among HH smokers when they did not smoke immediately after a social stressor. On the session in which smoking occurred immediately after the social stressor, LH and HH smokers showed a similarly rapid decrease in negative mood down to or even below pre-stressor levels. LH and HH smokers showed similar smoking topography regardless of whether smoking was immediate or delayed. The effects of hostility group were independent of the fact that the groups showed large differences in depressive symptoms. Depressive symptoms predicted slower recovery in negative mood after the stressor, but this effect did differ according to whether smoking was immediate or delayed (see Footnote 2).
The ability of smoking to ameliorate negative affect involves multiple processes and may be moderated not only by individual differences, such as hostility, but also by contextual factors (Kassel, Stroud, & Paronis, 2003). Smoking may reduce negative affect simply because it alleviates nicotine abstinence effects (Baker et al., 2004) or because of the specific pharmacological properties of nicotine. In the present study, smoking facilitated recovery of negative mood in HH participants regardless of whether they had been smoking ad lib or had been abstinent from smoking overnight. These results indicate that relief of nicotine abstinence effects did not account for the differential responses of LH vs. HH smokers.
Smoking also may reduce hostile affect (i.e., anger or frustration) by providing cognitive distraction that allows for "cool" rather than "hot" processing. For example, Ayduk et al. (Ayduk, Mischel, & Downey, 2002) found that having individuals focus on the physical setting of a recalled rejection experience (cool focus) reduced hostile affect and cognition relative to focusing on "hot" aspects such as their physiological and emotional response. HH smokers may be particularly prone to ruminating on negative aspects of social interactions, and distraction may therefore particularly important for them. To test fully pharmacological vs. behavioral effects of smoking on recovery from negative moods in HH smokers would require an experimental design in which smokers would be assigned to smoke either nicotine-containing or denicotinized cigarettes.
The effects of hostility on recovery in negative mood after a social stressor did not extend to urge to smoke. HH smokers reported greater urge to smoke prior to the social stressor, but change in urge to smoke after the stressor was not predicted by hostility. Urge to smoke may have had a ceiling effect on the smoking-deprived session, which might have reduced our ability to demonstrate hostility effects. However, even on the ad lib smoking day, the trajectories in urge to smoke after the stressor in LH and HH smokers appeared similar, showing a slight escalation. One possibility is that the HH smokers in the delayed condition were more likely to ruminate on the Debate Task, which may have limited increases in craving by providing some distraction from thinking about their desire to smoke. Alternatively, the negative mood increases seen in the HH smokers may reflect frustration with not being able to smoke, whereas being prohibited from smoking produced less affective response in LH smokers.
As noted previously, HH individuals have been shown to have greater physiological reactivity to lab-based stressors and slower recovery after the stressors. In the present study, the social stressor task we used did not produce differential responses in LH and HH smokers on immediate cardiovascular responses. The task was a novel one that was designed to avoid the use of confederates and to be administered two times. The level of social stress induced may have been insufficient to show hostility effects on immediate responding, perhaps because the social component of the task was an audiotaped voice rather than an in-vivo confederate, which has been used in most studies showing hostility effects. Nonetheless, the task did reliably increase cardiovascular responding, negative affect, and cigarette craving as intended. Moreover, we did demonstrate that HH smokers showed a slower recovery in blood pressure than LH smokers consistent with prior studies that used different social stressors (Fredrickson et al., 2000; Llabre et al., 2004).
We did not find the hypothesized interactions between hostility and either smoking deprivation or smoking delay on cardiovascular reactivity to and recovery from the stressor. This finding may reflect the paradoxical nature of smoking, in which nicotine reduces anxiety during a stressor and during social interaction but also increases heart rate (Gilbert, Robinson, Chamberlin, & Spielberger, 1989; Gilbert & Spielberger, 1987). Although immediate smoking reduced negative mood in the present study relative to delayed smoking, smoking itself has stimulating effects. For example, smoking abstinence reduced heart rate prior to the stressor. Furthermore, after the stressor, immediate smoking slowed recovery in heart rate and blood pressure relative to delayed smoking. Overall, it appears that effect of smoking on recovery in negative mood in HH smokers is not due to its effects on recovery in cardiovascular arousal.
Results of this study provide further evidence that hostility does not have a unique effect on responses to acute smoking deprivation. Therefore, increased withdrawal symptoms or increased reactivity to stress in the early stages of nicotine withdrawal are not likely to account for hostility’s association with smoking cessation outcome. However, our assessments were limited to acute effects of overnight smoking deprivation. Therefore, we were not able to test recovery from withdrawal over the first several weeks of smoking abstinence, which clinical trials data suggest may be somewhat slower among HH smokers (Kahler, Spillane, et al., 2009; Kahler et al., 2004). Slower recovery in withdrawal among HH smokers may simply reflect a tendency to recover more slowly from stressful experiences in general.
These results have potentially relevant clinical implications. It may be the case that HH smokers are prone to smoking relapse because they have greater difficulty recovering from stress-induced negative mood without smoking. This potential relapse risk factor appears independent of traditional measures of tobacco dependence severity and is not caused or exacerbated by nicotine withdrawal. That HH smokers, compared to LH smokers, reported at baseline being more tempted to smoke in negative affect situations (but not in positive social situations and situations involving habit or craving) further supports this specific connection between smoking and negative affect reduction in HH smokers. Ecological momentary assessment studies have found that prior to negative affect-induced smoking lapses, negative affect shows significant increases in the hours before the lapse (Shiffman & Waters, 2004). HH smokers may be at higher risk for smoking relapse than LH smokers, in part, because their negative affect after a social stressor remains elevated for a longer time, keeping them in an affective state that contributes to smoking lapse risk. This risk for relapse may occur even if they do not explicitly report greater urge to smoke than LH smokers. Incipient negative affect may trigger automatic cognitive processes that support smoking even if greater urge to smoke is not explicitly reported (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004). For example “unconscious” changes in smoking motivation can predict relapse above and beyond subjective indices of craving (e.g., Waters et al., 2003).
Limitations
There were several other limitations to the present study. First, as noted above, the social stressor task did not produce differential initial responding between LH and HH smokers, which may have reduced our ability to detect interactions between hostility and smoking deprivation on responding to a social stressor. Second, we tested only one type of stressor, which was social in nature. It is unclear whether a non-social negative mood induction, for example, would have produced similar results. Third, our measures of urge to smoke were limited. Because urge was assessed so frequently, we relied on a single item rather than on a multi-item assessment that can yield scores related to craving for negative reinforcement vs. positive reinforcement. In addition, we relied solely on self-report measures of craving. Relevant indices that would enrich future research include measures of attentional bias to smoking cues (e.g., the smoking Stroop task) or behavioral economic paradigms that assess the incentive value of smoking (e.g., how much one would be willing to pay for a cigarette).
Conclusions
Given that longitudinal community studies and clinical trials have shown that greater hostility is associated with persistence in smoking, understanding the mechanisms that may account for this effect are crucial to designing intervention strategies that match the motivational basis for smoking in HH smokers. This study suggests that nicotine withdrawal is not a key mechanism, but that negative mood following social stress may be. In the absence of smoking, HH smokers show a continued escalation in negative mood after a social stressor that may predispose them to smoking relapse. Interventions that train HH smokers to recover from negative moods without smoking may be valuable. Integrating cognitive behavioral therapy for depression into smoking cessation treatment would appear to be a logical treatment alternative, but it has not been shown to enhance outcomes in HH smokers (Kahler et al., 2004). This null finding may reflect the fact that that intervention is ineffective at reducing negative moods in the early stages of quitting (Brown et al., 2001; Kahler et al., 2002) or that the intervention is focused on depression rather than the cognitive trait of hostility. Thus, other behavioral intervention approaches, such as those specifically targeting reductions in trait hostility (Davidson, Gidron, Mostofsky, & Trudeau, 2007; Gidron, Davidson, & Bata, 1999) may be required. Finally, laboratory-based studies that differentiate the behavioral from the pharmacological effects of smoking on negative mood after a stressor can inform further the nature of interventions that are likely to be most relevant to HH smokers.
Acknowledgments
This study was supported by grant R21 DA019628 from the National Institute on Drug Abuse to Christopher Kahler. Dr. Monti’s effort was supported in part by a Department of Veterans Affairs Senior Career Research Scientist Award. The authors gratefully acknowledge Dan Belenky, Catherine Costantino, Jennifer Larence, Cheryl Eaton, and Timothy Souza for their assistance on this project.
Footnotes
Previous studies have indicated that the effect of hostility on smoking cessation outcome is independent from depressive symptoms and a history of major depression. However, given that LH and HH smokers differed on these characteristics, as would be expected, analyses were repeated covarying the effects of depressive symptoms and major depression history on responses to abstinence, responses to the social stressor, and recovery after the social stressor. In no case, did controlling for depressive symptoms or depression history render significant effects of hostility on responding nonsignificant. Thus, significant effects of hostility observed were independent from effects of depressive symptoms and major depression history.
Given the large baseline differences between LH and HH smokers on depressive symptoms, post hoc analyses were conducted to determine whether depressive symptoms would have a similar effect on recovery from the stressor as hostility did. In these analyses, CES-D scores replaced hostility group in the model. Greater CES-D scores were associated with significantly (p = .008) slower recovery in negative mood (as well as urge to smoke, p = .02, but not blood pressure) after the stressor. However, immediate vs. delayed smoking did not interact significantly with CES-D scores in predicting rate of change in either negative mood or urge to smoke after the stressor. Thus, the effects of depressive symptoms on recovery from the stressor were distinct from the effects of hostility.
Contributor Information
Christopher W. Kahler, Center for Alcohol and Addiction Studies, Brown University
Adam M. Leventhal, Center for Alcohol and Addiction Studies, Brown University
Suzanne M. Colby, Center for Alcohol and Addiction Studies, Brown University
Chad J. Gwaltney, Center for Alcohol and Addiction Studies, Brown University
Thomas W. Kamarck, University of Pittsburgh
Peter M. Monti, Providence VA Medical Center and the Center for Alcohol and Addiction Studies, Brown University
References
- al'Absi M, Carr SB, Bongard S. Anger and psychobiological changes during smoking abstinence and in response to acute stress: prediction of smoking relapse. International Journal of Psychophysiology. 2007;66:109–115. doi: 10.1016/j.ijpsycho.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayduk O, Mischel W, Downey G. Attentional mechanisms linking rejection to hostile reactivity: the role of "hot" versus "cool" focus. Psychological Science. 2002;13:443–448. doi: 10.1111/1467-9280.00478. [DOI] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychology Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB., Jr The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Benotsch EG, Christensen AJ, McKelvey L. Hostility, social support, and ambulatory cardiovascular activity. Journal of Behavioral Medicine. 1997;20:163–176. doi: 10.1023/a:1025530711432. [DOI] [PubMed] [Google Scholar]
- Biener L, Abrams DB. The contemplation ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, Baker TB. Postcessation cigarette use: The process of relapse. Addictive Behaviors. 1990;15:105–114. doi: 10.1016/0306-4603(90)90013-n. [DOI] [PubMed] [Google Scholar]
- Brondolo E, Rieppi R, Erickson SA, Bagiella E, Shapiro PA, McKinley P, et al. Hostility, interpersonal interactions, and ambulatory blood pressure. Psychosomatic Medicine. 2003;65:1003–1011. doi: 10.1097/01.psy.0000097329.53585.a1. [DOI] [PubMed] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, et al. Cognitive-behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69:471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Jorunal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Brummett BH, Babyak MA, Mark DC, Williams RB, Siegler IC, Clapp-Channing N, et al. Predictors of smoking cessation in patients with a diagnosis of coronary artery disease. Journal of Cardiopulmonary Rehabilitation. 2002;22:143–147. doi: 10.1097/00008483-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Brummett BH, Maynard KE, Babyak MA, Haney TL, Siegler IC, Helms MJ, et al. Measures of hostility as predictors of facial affect during social interaction: evidence for construct validity. Annals of Behavioral Medicine. 1998;20:168–173. doi: 10.1007/BF02884957. [DOI] [PubMed] [Google Scholar]
- Bunde J, Suls J. A quantitative analysis of the relationship between the Cook-Medley Hostility Scale and traditional coronary artery disease risk factors. Health Psychology. 2006;25:493–500. doi: 10.1037/0278-6133.25.4.493. [DOI] [PubMed] [Google Scholar]
- Burgess ES, Brown RA, Kahler CW, Niaura R, Abrams DB, Goldstein MG, et al. Patterns of change in depressive symptoms during smoking cessation: Who's at risk for relapse? Journal of Consulting and Clinical Psychology. 2002;70:356–361. doi: 10.1037//0022-006X.70.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen AJ, Edwards DL, Wiebe JS, Benotsch EG, McKelvey L, Andrews M, et al. Effect of verbal self-disclosure on natural killer cell activity: moderating influence of cynical hostility. Psychosomatic Medicine. 1996;58:150–155. doi: 10.1097/00006842-199603000-00009. [DOI] [PubMed] [Google Scholar]
- Christensen AJ, Smith TW. Cynical hostility and cardiovascular reactivity during self-disclosure. Psychosomatic Medicine. 1993;55:193–202. doi: 10.1097/00006842-199303000-00008. [DOI] [PubMed] [Google Scholar]
- Cook WW, Medley DM. Proposed Hostility and Pharasaic-Virtue scales for the MMPI. Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Depression and depressive symptoms in smoking cessation. Comprehensive Psychiatry. 1990 Jul-Aug;31(4):350–354. doi: 10.1016/0010-440x(90)90042-q. [DOI] [PubMed] [Google Scholar]
- Davidson KW, Gidron Y, Mostofsky E, Trudeau KJ. Hospitalization cost offset of a hostility intervention for coronary heart disease patients. Nicotine & Tobacco Research. 2007;75:657–662. doi: 10.1037/0022-006X.75.4.657. [DOI] [PubMed] [Google Scholar]
- Felsten G, Hill V. Aggression Questionnaire hostility scale predicts anger in response to mistreatment. Behaviour Research and Therapy. 1999;37:87–97. doi: 10.1016/s0005-7967(98)00104-1. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fredrickson BL, Maynard KE, Helms MJ, Haney TL, Siegler IC, Barefoot JC. Hostility predicts magnitude and duration of blood pressure response to anger. Journal of Behavioral Medicine. 2000;23:229–243. doi: 10.1023/a:1005596208324. [DOI] [PubMed] [Google Scholar]
- Gallo LC, Smith TW, Kircher JC. Cardiovascular and electrodermal responses to support and provocation: interpersonal methods in the study of psychophysiological reactivity. Psychophysiology. 2000;37(3):289–301. [PubMed] [Google Scholar]
- Gidron Y, Davidson K, Bata I. The short-term effects of a hostility-reduction intervention on male coronary heart disease patients. Health Psychology. 1999;18:416–420. doi: 10.1037//0278-6133.18.4.416. [DOI] [PubMed] [Google Scholar]
- Gilbert D, McClernon J, Rabinovich N, Sugai C, Plath L, Asgaard G, et al. Effects of quitting smoking on EEG activation and attention last for more than 31 days and are more severe with stress, dependence, DRD2 A1 allele, and depressive traits. Nicotine & Tobacco Research. 2004;6:249–267. doi: 10.1080/14622200410001676305. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Jensen RA, Meliska CJ. Effects of smoking abstinence on mood and craving in men: influences of negative-affect-related personality traits, habitual nicotine intake and repeated measurements. Personality and Individual Differences. 1998;25:399–423. [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, et al. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology. 2002;70:142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Robinson JH, Chamberlin CL, Spielberger CD. Effects of smoking/nicotine on heart rate, anxiety, and lateralization of EEG during a stressful movie. Psychophysiology. 1989;26:311–320. doi: 10.1111/j.1469-8986.1989.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, Spielberger CD. Effects of smoking on heart rate, anxiety, and feelings of success during social interaction. Journal of Behavioral Medicine. 1987;10:629–638. doi: 10.1007/BF00846659. [DOI] [PubMed] [Google Scholar]
- Ginsberg D, Hall SM, Reus VI, Muñoz RF. Mood and depression diagnosis in smoking cessation. Experimental and Clinical Psychopharmacology. 1995;3(4):389–395. [Google Scholar]
- Han K, Nathan CW, Calhoun RF, Butcher JN. Psychometric characteristics of the MMPI-2 Cook-Medley hostility scale. Journal of Personality Assessment. 1995;65:567–585. doi: 10.1207/s15327752jpa6503_15. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986 Mar;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Iribarren C, Sidney S, Bild DE, Liu K, Markovitz JH, Roseman JM, et al. Association of hostility with coronary artery calcification in young adults: the CARDIA study. Coronary Artery Risk Development in Young Adults. Jama. 2000;283:2546–2551. doi: 10.1001/jama.283.19.2546. [DOI] [PubMed] [Google Scholar]
- Jamner LD, Shapiro D, Jarvik ME. Nicotine reduces the frequency of anger reports in smokers and nonsmokers with high but not low hostility: an ambulatory study. Experimental and Clinical Psychopharmacology. 1999;7:454–463. doi: 10.1037//1064-1297.7.4.454. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Brown RA, Ramsey SE, Niaura R, Abrams DB, Goldstein MG, et al. Negative mood, depressive symptoms, and major depression after smoking cessation treatment in smokers with a history of major depressive disorder. Journal of Abnormal Psychology. 2002;111:670–675. doi: 10.1037//0021-843x.111.4.670. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Daughters SB, Leventhal AM, Rogers ML, Clark MA, Colby SM, et al. Personality, psychiatric disorders, and smoking in middle-aged adults. Nicotine & Tobacco Research. 2009;11:833–841. doi: 10.1093/ntr/ntp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Leventhal AM, Strong DR, Brown RA, Monti PM. Hostility and smoking cessation treatment outcome in heavy social drinkers. Psychology of Addictive Behaviors. 2009;23:67–76. doi: 10.1037/a0012655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahler CW, Strong DR, Niaura R, Brown RA. Hostility in smokers with past major depressive disorder: Relation to smoking patterns, reasons for quitting, and cessation outcomes. Nicotine & Tobacco Research. 2004;6:809–818. doi: 10.1080/1462220042000282546. [DOI] [PubMed] [Google Scholar]
- Kamarck TW, Debski TT, Manuck SB. Enhancing the laboratory-to-life generalizability of cardiovascular reactivity using multiple occasions of measurement. Psychophysiology. 2000;37(4):533–542. [PubMed] [Google Scholar]
- Kassel JD, Stroud LR, Paronis CA. Smoking, stress, and negative affect: correlation, causation, and context across stages of smoking. Psychological Bulletin. 2003;129:270–304. doi: 10.1037/0033-2909.129.2.270. [DOI] [PubMed] [Google Scholar]
- Kenford SL, Smith SS, Wetter DW, Jorenby DE, Fiore MC, Baker TB. Predicting relapse back to smoking: contrasting affective and physical models of dependence. Journal of Consulting and Clinical Psychology. 2002;70:216–227. [PubMed] [Google Scholar]
- Lipkus IM, Barefoot JC, Williams RB, Siegler IC. Personality measures as predictors of smoking initiation and cessation in the UNC Alumni Heart Study. Health Psychology. 1994;13:149–155. doi: 10.1037//0278-6133.13.2.149. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer S, Siegel S, Saab PG, Schneiderman N. Applying latent growth curve modeling to the investigation of individual differences in cardiovascular recovery from stress. Psychosomatic Medicine. 2004;66:29–41. doi: 10.1097/01.psy.0000107886.51781.9c. [DOI] [PubMed] [Google Scholar]
- Lubin B, Van Whitlock R. Development of a measure that integrates positive and negative affect and personality: the Comprehensive Personality and Affect Scales. Journal of Clinical Psychology. 2002;58:1135–1156. doi: 10.1002/jclp.10042. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Gordon JR. Determinants of relapse: Implications for the maintenance of behavior change. In: Davidson PO, Davidson SM, editors. Behavioral Medicine: Changing Health Lifestyles. New York: Brunner/Mazel; 1980. pp. 410–452. [Google Scholar]
- Miller SB, Friese M, Dolgoy L, Sita A, Lavoie K, Campbell T. Hostility, sodium consumption, and cardiovascular response to interpersonal stress. Psychosomatic Medicine. 1998;60:71–77. doi: 10.1097/00006842-199801000-00016. [DOI] [PubMed] [Google Scholar]
- Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychological Bulletin. 1996;119:322–348. doi: 10.1037/0033-2909.119.2.322. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Kenford SL, Smith SS, Fiore MC, Baker TB. Listening to nicotine: Negative affect and the smoking withdrawal conundrum. Psychological Science. 1997;8:184–189. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977 Summer;1:385–401. [Google Scholar]
- SAS Institute Inc. SAS 9.1.3 for Windows [Computer software] Cary, NC: SAS; 2005. [Google Scholar]
- Shapiro D, Jamner LD, Goldstein IB. Daily mood states and ambulatory blood pressure. Psychophysiology. 1997;34(4):399–405. doi: 10.1111/j.1469-8986.1997.tb02383.x. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Relapse following smoking cessation: A situational analysis. Journal of Consulting and Clinical Psychology. 1982;50(1):71–86. doi: 10.1037//0022-006x.50.1.71. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Waters AJ. Negative affect and smoking `lapses: a prospective analysis. Journal of Consulting and Clinical Psychology. 2004;72:192–201. doi: 10.1037/0022-006X.72.2.192. [DOI] [PubMed] [Google Scholar]
- Smith TW. Concepts and methods in the study of anger, hostility and health. In: Siegman AW, Smith TW, editors. Anger, hostility and the heart. Hillsdale, NJ: Erlbaum; 1994. pp. 23–42. [Google Scholar]
- Smith TW, Frohm KD. What's so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychology. 1985;4:503–520. doi: 10.1037//0278-6133.4.6.503. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline followback users' guide. Toronto, Canada: Addiction Research Foundation; 1994. [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. User's guide for the Structured Clinical Interview for DSM-III-R. New York: New York Psychiatric Institute; 1989. [Google Scholar]
- Strong DR, Kahler CW, Greene RL, Schinka J. Isolating a primary dimension within the Cook-Medley hostility scale: A Rasch analysis. Personality and Individual Differences. 2005;39(1):21–33. [Google Scholar]
- Suarez EC, Harlan E, Peoples MC, Williams RB., Jr Cardiovascular and emotional responses in women: the role of hostility and harassment. Health Psychology. 1993;12(6):459–468. doi: 10.1037//0278-6133.12.6.459. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams RB, Jr, Zimmermann EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: the role of interpersonal challenge. Psychosomatic Medicine. 1998;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addictive Behaviors. 1990;15:271–283. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- West RJ, Hajek P, Belcher M. Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychological Medicine. 1989;19:981–985. doi: 10.1017/s0033291700005705. [DOI] [PubMed] [Google Scholar]