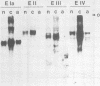
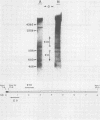
Abstract
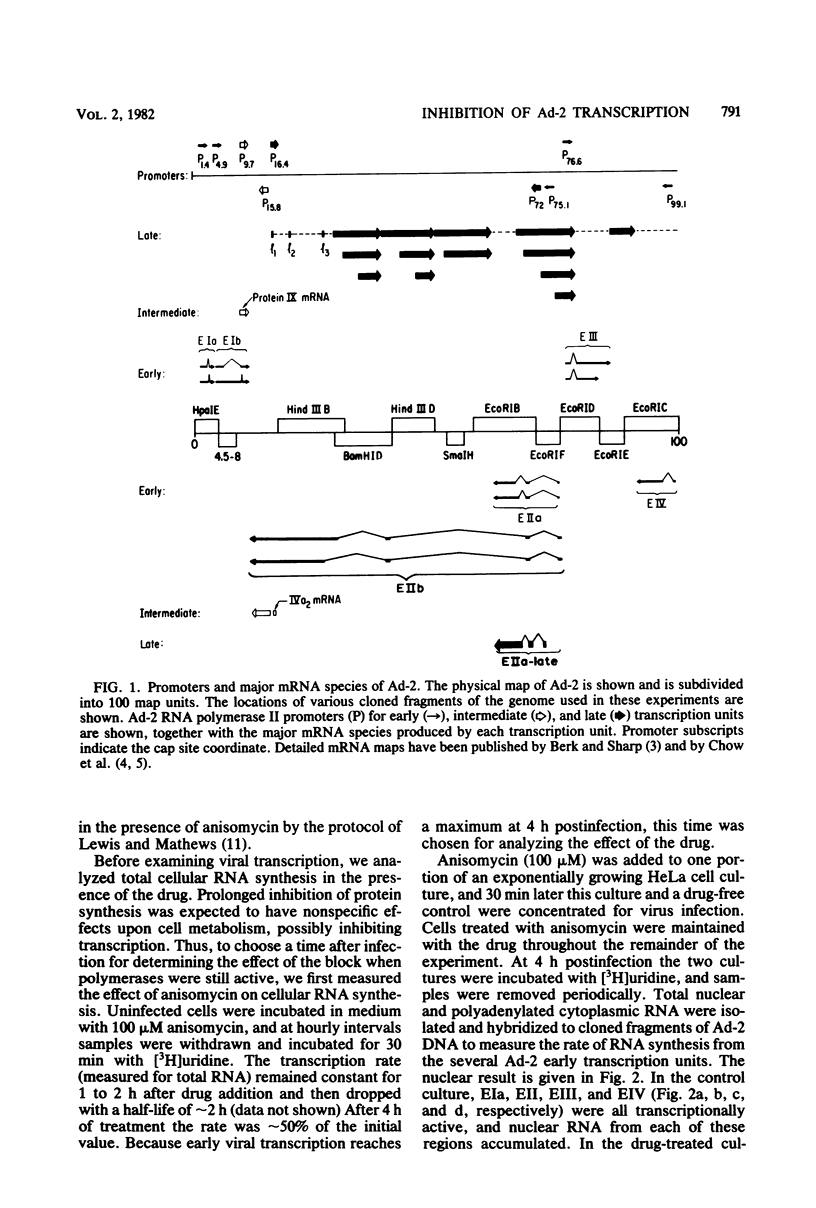
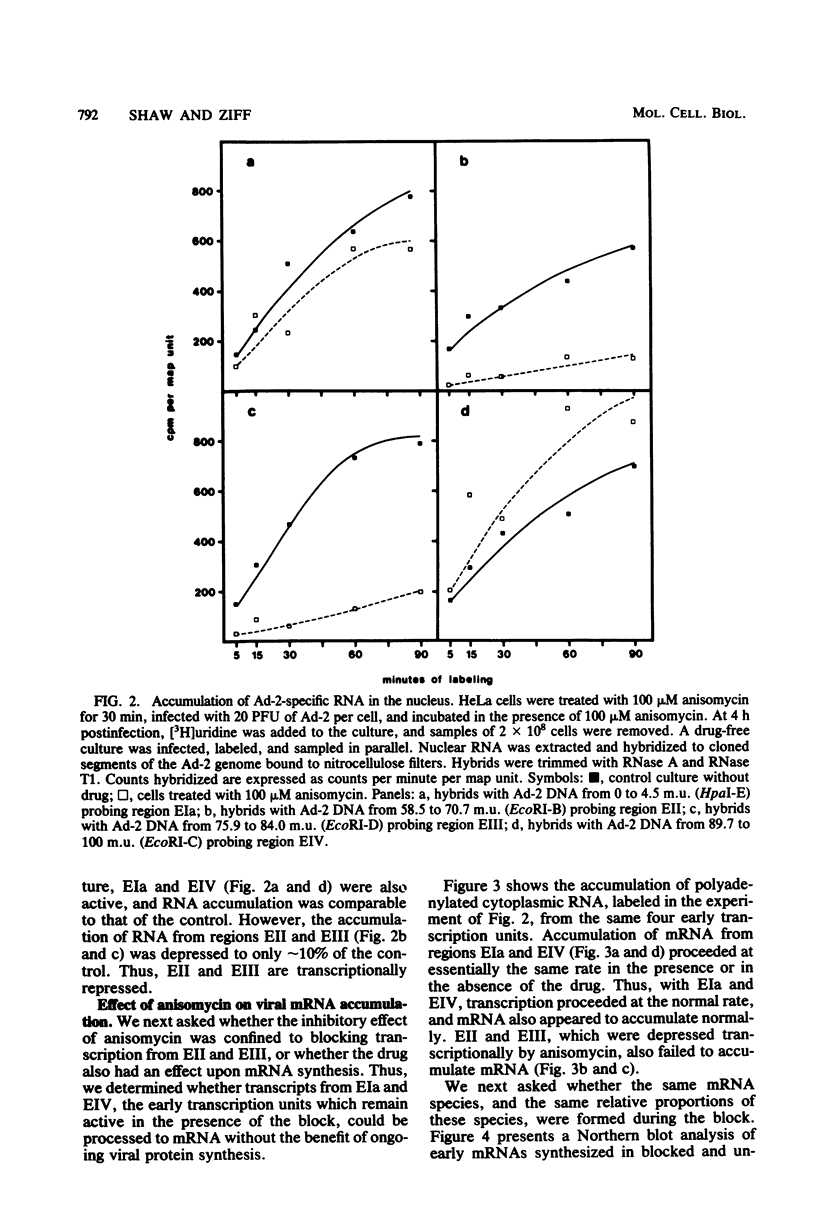
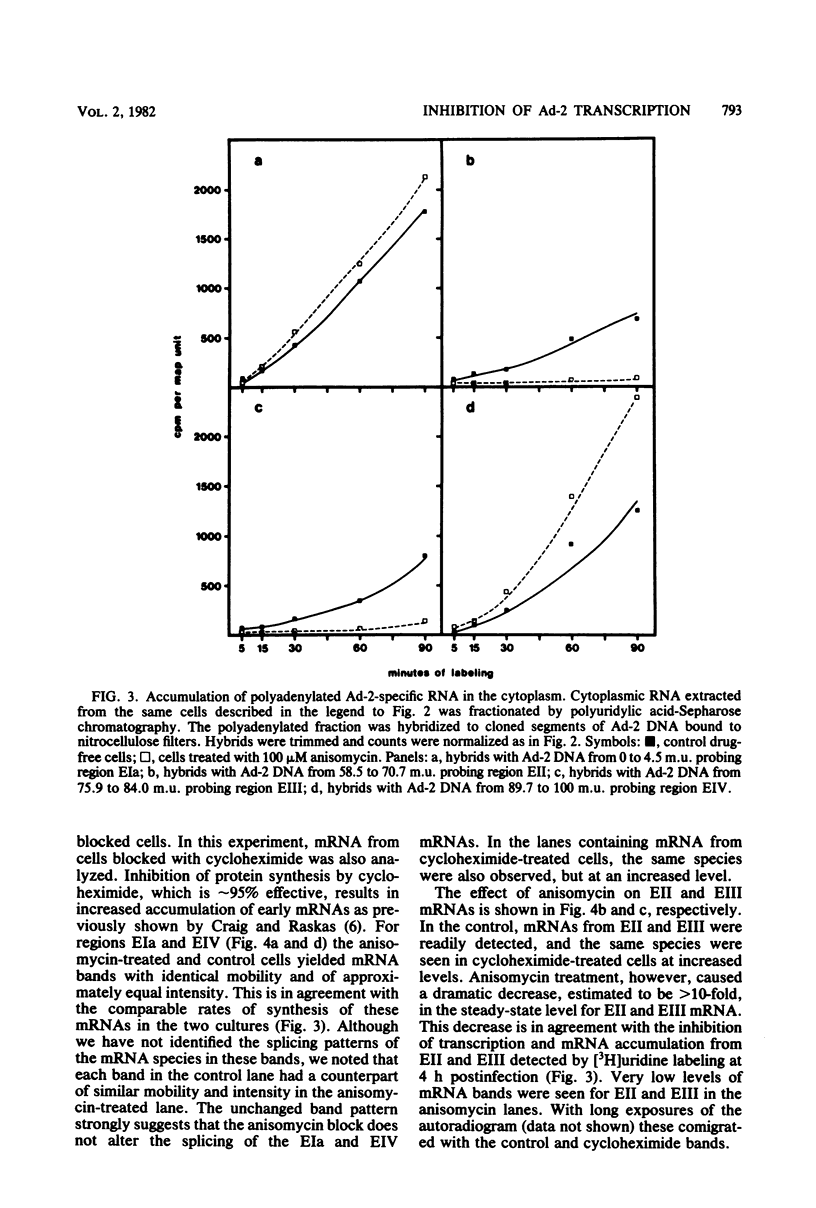
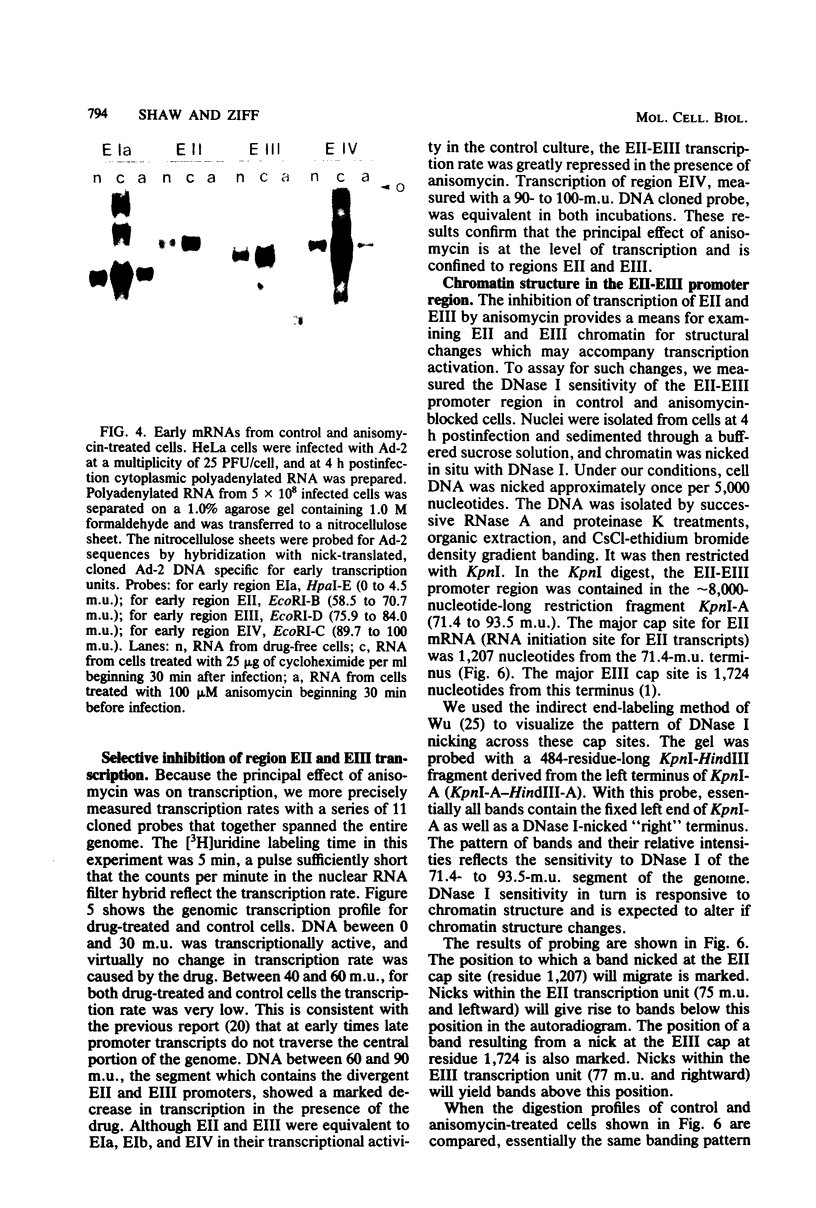
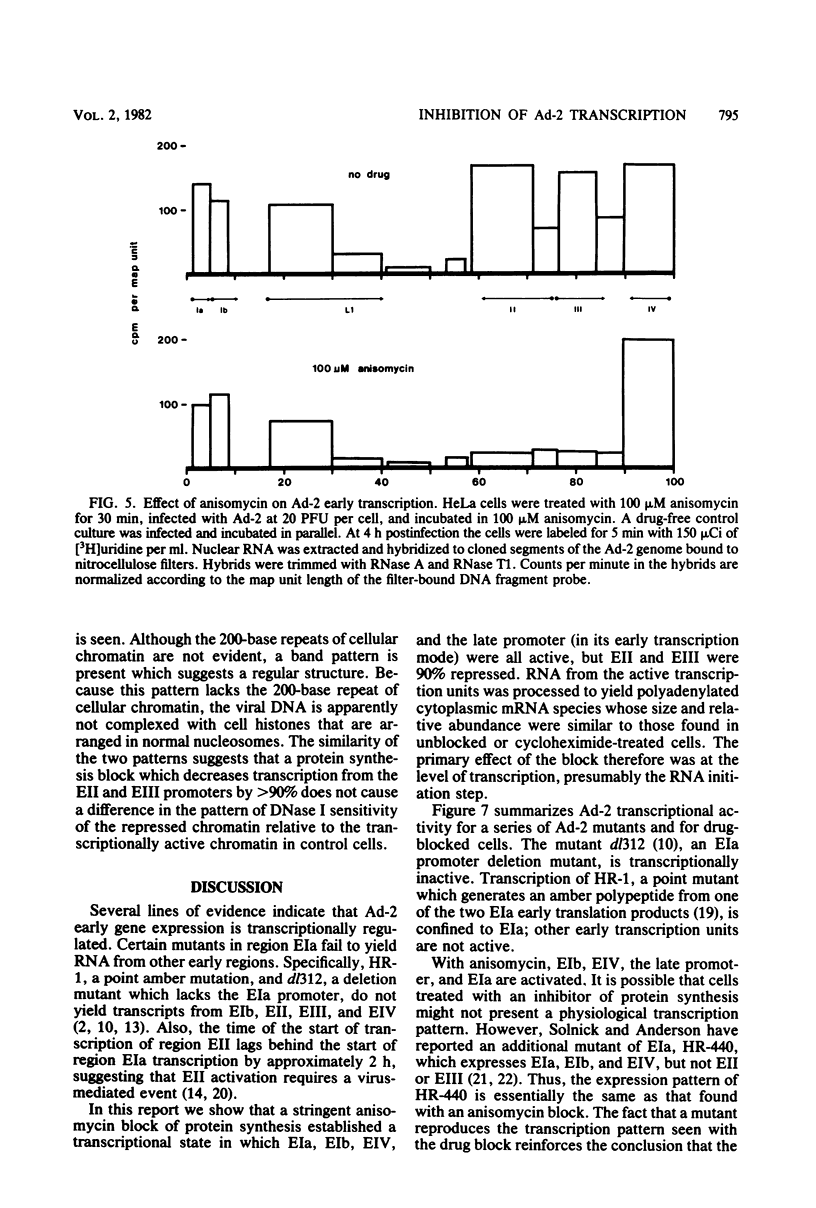
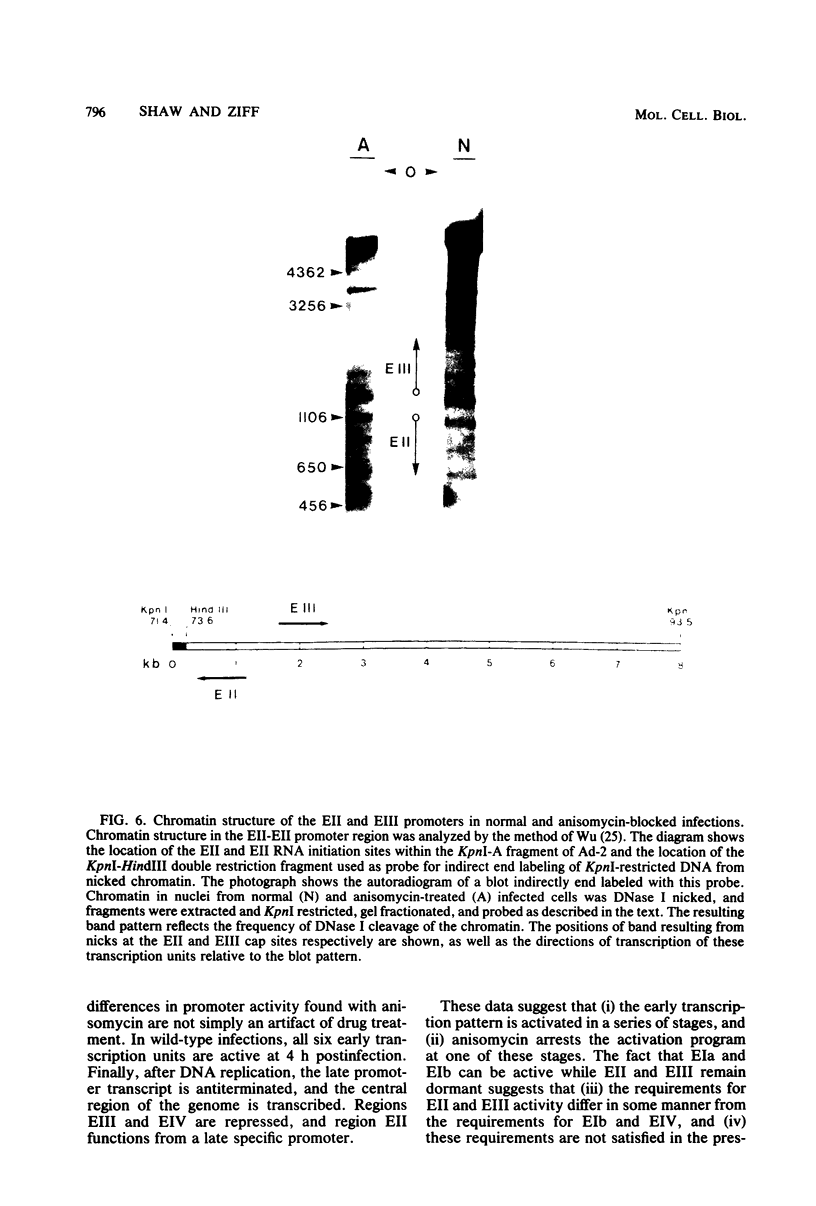
The transcription of adenovirus type 2 genes proceeds through a broad three-phase program. From 1 to 4 h postinfection six early transcription units (EIa, EIb, EII, EIII, EIV, and the promoter-proximal segment of the late transcription unit) are activated. From 4 to 6 h postinfection transcription of the early genes is depressed. After the onset of viral DNA replication at approximately 6 h postinfection, the transcript from the late promoter is antiterminated, and this transcript dominates viral RNA synthesis. The early activation period also proceeds through a series of stages; early regions EIa and EIV are activated first, followed by early region EII. We show that in the presence of anisomycin, a stringent inhibitor of protein synthesis, nuclear RNA and cytoplasmic polyadenylated RNA from regions EIa and EIV accumulate at normal rates, whereas RNAs from regions EII and EIII do not accumulate. We also show that failure to accumulate RNAs from regions EII and EIII is due to reduction of the rate of transcription by greater than 90%. We conclude that the regulation of the promoters for early regions EII and EIII is distinct from the mechanism that operates on the other early transcription units. The promoters for early regions EII and EIII diverge and lie approximately 500 nucleotides apart. We examined the structure of viral chromatin in this region early during infection by mild DNase I digestion in isolated nuclei and indirect end labeling with a DNA probe near these promoters. In control, drug-free cells where EII and EIII are transcribed and in anisomycin-treated cells where EII and EIII are not transcribed we detect the same regular DNase I pattern. We suggest that regulation of EII and EIII is not mediated through a change in gross chromatin structure, but rather by a viral effector, possibly a product of region EIa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Lewis J. B., Broker T. R. RNA transcription and splicing at early and intermediate times after adenovirus-2 infection. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):401–414. doi: 10.1101/sqb.1980.044.01.044. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Fraser N., Ziff E., Weber J., Wilson M., Darnell J. E. The initiation sites for RNA transcription in Ad2 DNA. Cell. 1977 Nov;12(3):733–739. doi: 10.1016/0092-8674(77)90273-2. [DOI] [PubMed] [Google Scholar]
- Fraser N., Ziff E. RNA structures near poly(A) of adenovirus-2 late messenger RNAs. J Mol Biol. 1978 Sep 5;124(1):27–31. doi: 10.1016/0022-2836(78)90145-6. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Mathews M. B. Control of adenovirus early gene expression: a class of immediate early products. Cell. 1980 Aug;21(1):303–313. doi: 10.1016/0092-8674(80)90138-5. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Processing of late adenovirus nuclear RNA to mRNA. Kinetics of formation of intermediates and demonstration that all events are nuclear. J Mol Biol. 1979 Jun 5;130(4):493–506. doi: 10.1016/0022-2836(79)90436-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Wilson M. C. Regulation of adenovirus-2 gene expression at the level of transcriptional termination and RNA processing. Nature. 1981 Mar 12;290(5802):113–118. doi: 10.1038/290113a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Monstein H. J., Akusjärvi G., Philipson L. Adenovirus early gene products may control viral mRNA accumulation and translation in vivo. Cell. 1981 Feb;23(2):485–496. doi: 10.1016/0092-8674(81)90144-6. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw A. R., Ziff E. B. Transcripts from the adenovirus-2 major late promoter yield a single early family of 3' coterminal mRNAs and five late families. Cell. 1980 Dec;22(3):905–916. doi: 10.1016/0092-8674(80)90568-1. [DOI] [PubMed] [Google Scholar]
- Solnick D. An adenovirus mutant defective in splicing RNA from early region 1A. Nature. 1981 Jun 11;291(5815):508–510. doi: 10.1038/291508a0. [DOI] [PubMed] [Google Scholar]
- Solnick D., Anderson M. A. Transformation-deficient adenovirus mutant defective in expression of region 1A but not region 1B. J Virol. 1982 Apr;42(1):106–113. doi: 10.1128/jvi.42.1.106-113.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. J., McGrogan M., Raskas H. J. Regulation of the appearance of cytoplasmic RNAs from region 1 of the adenovirus 2 genome. J Mol Biol. 1978 Dec 15;126(3):395–414. doi: 10.1016/0022-2836(78)90048-7. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Ziff E. B. Transcription and RNA processing by the DNA tumour viruses. Nature. 1980 Oct 9;287(5782):491–499. doi: 10.1038/287491a0. [DOI] [PubMed] [Google Scholar]