Abstract
Polycystin-2 functions as a cation-permeable transient receptor potential ion channel in kidney epithelial cells and when mutated results in human autosomal dominant polycystic kidney disease. For further exploration of the in vivo functions of Polycystin-2, this study examined its expression and function during zebrafish embryogenesis. pkd2 mRNA is ubiquitously expressed, and its presence in the larval kidney could be confirmed by reverse transcription–PCR on isolated pronephroi. Immunostaining with anti-zebrafish Polycystin-2 antibody revealed protein expression in motile kidney epithelial cell cilia and intracellular cell membranes. Intracellular localization was segment specific; in the proximal nephron segment, Polycystin-2 was localized to basolateral cell membranes, whereas in the caudal pronephric segment, Polycystin-2 was concentrated in subapical cytoplasmic vesicles. Polycystin-2 also was expressed in muscle cells and in a variety of sensory cells that are associated with mechanotransduction, including cells of the ear, the lateral line organ, and the olfactory placodes. Disruption of Polycystin-2 mRNA expression resulted in pronephric kidney cysts, body axis curvature, organ laterality defects, and hydrocephalus—defects that could be rescued by expression of a human PKD2 mRNA. In-frame deletions in the first extracellular loop and C-terminal phosphofurin acidic cluster sorting protein–1 (PACS-1) binding sites in the cytoplasmic tail caused Polycystin-2 mislocalization to the apical cell surface. Unlike zebrafish intraflagellar transport protein (IFT) mutants, cyst formation was not associated with cilia defects and instead correlated with reduced kidney fluid output, expansion of caudal duct apical cell membranes, and occlusion of the caudal pronephric nephron segment.
Autosomal dominant polycystic kidney (ADPKD) disease is caused primarily by mutations in two genes, Polycystin-1 and Polycystin-2. Polycystin-2 belongs to the transient receptor potential (TRP) channel family (1) and can function either as an intracellular calcium release channel or as a cilia-anchored mechanosensory channel (2–7). Channel activity measurements have shown that Polycystin-2 can function as nonselective cation channel both alone (8) and activated in the presence Polycystin-1 (9). Comparative analysis of Polycystin-2 and homologous TRP channels in a variety of organisms has provided useful insights into the function of Polycystin-2 in diverse cellular contexts as a mediator of sensory signaling (1). The Caenorhabditis elegans homolog pkd2 is localized to sensory neuron cilia, where it plays an essential role in guiding mating behavior (10). The Drosophila pkd2 homolog is localized to the tip of the sperm axoneme, where it functions to guide sperm to the female egg storage chamber as a prerequisite for fertilization (11). Muscle contractility also is impaired in Drosophila pkd2 mutants, suggesting a role for Polycystin-2 in intracellular calcium release (12). Sea urchin Polycystin-2 has been proposed to function together with the Polycystin-1– related membrane protein receptor for egg jelly protein (REJ-1) in sperm to initiate the acrosome reaction as a prelude to fertilization (13). Studies of Polycystin-2 expression and function in mouse knockouts show that disruption of PKD2 results in embryonic kidney cysts, vascular and heart septal defects, and randomized organ laterality (14,15).
In addition to its broad expression in different species, cell types, and organs, Polycystin-2 protein is expressed in several different cell membrane compartments, including apical monocilia and cytoplasmic endoplasmic reticulum (ER)/Golgi membranes, and at the basolateral cell surface (2,3,7,16–18). The presence of Polycystin-2 in these different membrane compartments indicates that its cellular function is likely to be dependent on its subcellular localization. In cultured mammalian epithelial cells, apical monocilia can function as mechanosensitive flow sensors, where cilia bending results in a spreading wave of intracellular calcium release (19). The localization of Polycystin-2 and its interacting partner Polycystin-1 to apical cilia, coupled with the ability of Polycystin-2 antibodies to block flow-induced calcium release (5), has suggested that Polycystin-2 is a cell surface channel responsible for initiating flow-induced calcium signaling. Polycystin-2 also has been localized to motile cilia in the oviduct and the mouse embryonic node (20-22). In both kidney epithelia and smooth muscle cells, evidence also has been found for Polycystin-2 expressed in ER/Golgi membranes acting as an intracellular calcium release channel (4). In cultured cells, localization of Polycystin-2 to intracellular membranes requires a C-terminal sequence that includes a cluster of acidic amino acids (DDSEEDDDED) (2,23). This motif is known to bind phosphofurin acidic cluster sorting protein-1 (PACS-1) proteins, which directs retrograde transport and ER retention of proteins in the secretory pathway (23,24).
The zebrafish has emerged as a useful model of kidney development and function owing to the feasibility of disrupting gene expression in mutants or using antisense oligonucleotides and also to the ease of viewing organ phenotypes in living larvae. A zebrafish PKD2 homolog has been identified in a zebrafish insertional mutagenesis screen (25). Targeting zebrafish pkd2 with translation-blocking morpholino (MO) oligos results in pronephric kidney cyst formation and other phenotypes (25,26). Analysis of potential cellular mechanisms underlying these phenotypes requires knowledge of where Polycystin-2 is expressed in pronephric epithelial cells and how, at the level of whole-organ function, defects in Polycystin-2 might lead to cyst formation. To explore further the function of pkd2, we analyzed zebrafish pkd2 mRNA expression pattern and protein localization during organogenesis. We found that whereas zebrafish pkd2 mRNA is broadly expressed, Polycystin-2 protein is localized to specific membranes in the kidney and other tissues, including sensory organs. Using antisense oligos, we also generated new pkd2 loss-of-function MO “alleles” in zebrafish embryos that create internal in-frame deletions. Examination of these embryos using both physiologic assays of kidney function and ultrastructural analysis provide evidence that cyst formation in embryos that lack functional Polycystin-2 protein correlates with altered cell structure in pronephric duct epithelial cells and changes nephron fluid flow. In-frame internal deletions in Polycystin-2 protein also provide in vivo evidence that specific protein domains are required to retain Polycystin-2 protein in intracellular cell membranes.
Materials and Methods
Zebrafish Lines
Wild-type TL or TÜAB zebrafish were maintained and raised as described previously (27). Dechorionated embryos were kept at 28.5°C in E3 solution with or without 0.003% 1-phenyl-2-thiourea (Sigma, St. Louis, MO) to suppress pigmentation and staged according to somite number or hours postfertilization (hpf) (27).
Cloning Polycystin-2 and Phylogenetic Analysis
Zebrafish Polycystin-2 cDNA was amplified from 48 hpf total RNA by reverse transcription-PCR (RT-PCR) using a primer design that is based on tblastn searches of Sanger Center zebrafish genomic sequence using mammalian PKD1 and PKD2 protein sequence. A full-length clone for Polycystin-2 was acquired by 5′ and 3′ rapid amplification of cDNA ends.
In Situ Hybridization and Immunohistochemistry
In situ hybridization was performed using standard techniques as described previously (28). A zebrafish Polycystin-2 antibody was raised in rabbits using the peptide EKMHHEEVGLGVPDEC coupled to KLH (CoCalico, Philomath, OR). The antibody was affinity-purified against the immunizing peptide using Sulfo-link resin (Pierce, Rockford, IL) and used at 1:400 dilution. Whole-mount immunocytochemistry was performed on embryos that were fixed in methanol: DMSO (80:20; α 6F [Developmental Studies Hybridoma Bank, University of Iowa], acetylated tubulin mAb 13-6-11B, and anti-zebrafish Polycystin-2) or by 4% paraformaldehyde/PBS fixation (96535 anti-mammalian Polycystin-2). Embryos were blocked in 10% normal goat serum and incubated in primary antibody in 1% DMSO/2% normal goat serum/0.1% Tween-20/PBS overnight at 4°C. After washing in incubation medium, secondary antibodies (Alexa 548, Alexa 488; Molecular Probes, Eugene, OR) were used at 1:1000. Washed embryos were cleared in benzyl benzoate:benzyl alcohol and photographed on a Nikon800 fluorescence microscope or on a BioRad Radiance6000 confocal fluorescence microscope. For sections, stained embryos were dehydrated and embedded in JB-4 resin (Polyscience Inc., Warrington, PA) following the manufacturer's instructions and cut at 1 to 4 μm.
MO Antisense Oligonucleotide and mRNA Injections
Wild-type embryos (TUAB) at the one- to two-cell stage were microinjected with 0.1 to 0.25 mM antisense MO oligos (Gene Tools, Philomath, OR) in 200 mM KCL and 0.1% Phenol Red. Final antisense MO oligo concentration in the cytoplasm was estimated to be between 100 and 200 nM.
The sequence of the translation blocking oligonucleotide was Pkd2 MO ATG: 5′-GCTCATCGTGTATTTCTACAGTAAC-3′ the splice donor blocking oligonucleotide sequences were pkd2 MOex3 5′-AATTACTTTCCAGAAGTCCTCCATG-3′, pkd2 MOex5 5′-GATCAACCCGTTACCTGACAATACA-3′, pkd2 MOex12 5′-CAGGTGATGTTTACACTTGGAACTC-3′, pkd2 MOex13 5′-CATCATCATCACCTCCATGACTCCA-3′.
Randomized oligonucleotide (control) was 5′-CCTCTTACCTCAGTTACAATTTATA-3′ and showed no effect on development. mRNA rescue experiments were carried out by co-injecting 10, 30, and 100 pg of in vitro transcribed human PKD2 mRNA (Message Machine kit; Ambion, Austin, TX) with the MO. Human full-length PKD2 template plasmid was a gift from Dr. Leo Tsiokas (University of Oklahoma, Oklahoma City, OK). Nested RT-PCR primers are designed from flanking exon coding sequence to confirm MO oligo efficacy and characterize the altered mRNA splicing products. Amplification of β-actin was performed as a positive control. For MOex3, pkd2ex3F1 TCGTCTTTTGGGTGAGAGCAACA, pkd2ex3R1, CGAATGGTGCCTTGTCCTCATTG, and pkd2ex3F2 TCCTCTTCCTGCTCACCCTCTGC, pkd2ex3R2 TCGTCTCGCAGATCCTCATGGAC. For MOex5, pkd2ex5F1 GGCCCGTTTCTTAACGGCATGTA, pkd2ex5R1 CTCTTGAAGTAGCGCAGCCGATG, pkd2ex5F2 GGCCCGTTTCTTAACGGCATGTA, and pkd2ex5R2, CTGGAGACATAGCGGAGCAGACG For MOex12 and MOex13, pkd2ex9F1 GCACCTTTCAGGCCTGCATTTTC, pkd2ex14R1 GGAGTGGCCTGATGATGATGGTG, pkd2ex9F2 CACGCAGTTCCGGATCATACTGG, and pkd2ex14R2 CCAGCTCGTCCCTAACCAACCTG
Histologic and Ultrastructural Analysis
For histology analysis, embryos were fixed in 1% paraformaldehyde, 1.5% glutaraldehyde, 70 mM NaPO4 (pH 7.2), and 3% sucrose; embedded in glycolmethacrylate (JB-4 resin; Polyscience, Inc.); and sectioned at 4-μm sections. Sections were stained in methylene blue/azure II (28) and mounted with Permount. Embryos were prepared for electron microscopy by previously published protocols (28).
High-Speed Videomicroscopy
1-Phenyl-2-thiourea-treated embryos were put in E3 egg water that contained 40 mM 2,3-butanedione monoxime (Sigma) for 5 min to stop the heart beat and then changed to 20 mM 2,3-butanedione monoxime-containing egg water for observation. The embryos then were analyzed using a ×40/0.55 water immersion lens on a Zeiss Axioplan microscope (Zeiss, Germany) equipped with a high-speed Photron FastCAM-PCI 500 videocamera (Photron, San Diego, CA). Image acquisition of beating cilia was 250 frames per second and 1088 frames total per take by Photron FastCAM version 1.2.0.7 (Photron). Image processing was done using Photoshop 7.0 (Adobe Systems, Mountain View, CA), and movies were compiled in Graphic Converter v.4.5.2 (Lemke Software, Peine, Germany).
Fluorescent Dye Injection and Fluorescence Videomicroscopy
For urine excretion assays, a 5% solution of tetramethylrhodamineconjugated 70-k molecular weight dextran (Molecular Probes) was injected into the common cardinal vein of 3.0- to 3.5-dpf embryos that were anesthetized with 0.2 mg/ml tricaine (3-aminobenzoic acid ethyl ester; Sigma) in egg water; these then were examined using a ×40/0.55 water immersion lens on a Zeiss Axioplan microscope equipped with a MTI SIT68 fluorescence camera. The video was recorded in real time with a Panasonic PV-8400 tape recorder. Digitization was done using SonicMyDVD Version 3.5.2 software (Adaptec, Milpitas, CA); still frames were captured using QuickTime v.6.5.1, and movies were recompiled by Graphic Converter (Lemke Software).
Results
Expression of Zebrafish Polycystin-2 and Immunolocalization in Developing Embryos
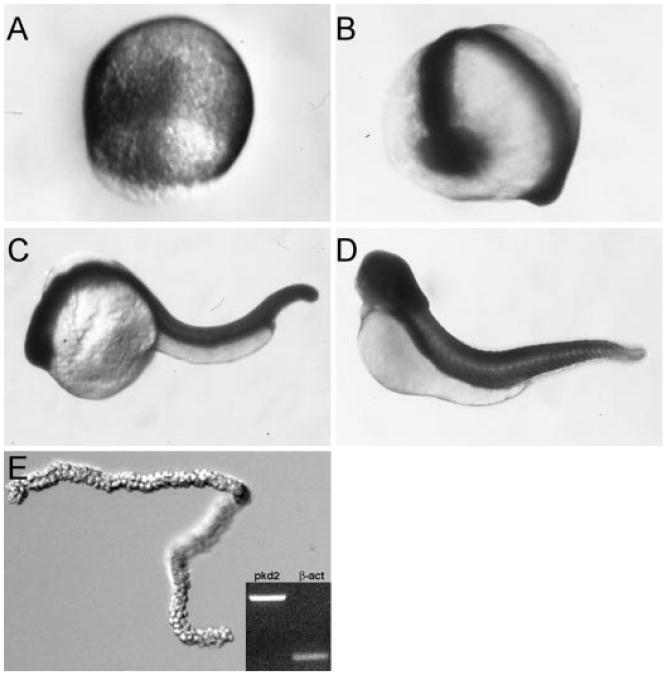
Polycystin-2 has been shown to be widely expressed in mammalian embryos and to function in multiple cell types (3,7,14,16–18,29). To examine pkd2 mRNA expression in zebrafish embryogenesis, we performed whole-mount in situ hybridization on embryos from gastrulation through free-swimming larval stages of development. pkd2 mRNA was ubiquitous and observed at all stages of development by whole-mount in situ hybridization, including gastrulation, somatogenesis, and 48 hpf (Figure 1, A through D), and in early larval stages (72 hpf; data not shown). Expression of pkd2 mRNA in pronephric tubules and ducts was confirmed by RT-PCR on isolated pronephric duct RNA (Figure 1E). RT-PCR also demonstrated that zebrafish pkd2 mRNA is widely expressed in adult tissues, including the brain, eye, heart, gut, spleen, kidney, and gonad (data not shown).
Figure 1.
Expression of pkd2 mRNA during embryogenesis. By whole-mount in situ hybridization, pkd2 is expressed ubiquitously during epiboly (A), at the 18-somite stage (B), at 24 h postfertilization (hpf; C) and at 48 hpf (D). Reverse transcription–PCR (RT-PCR) on RNA from isolated pronephric ducts (E) confirmed expression of pkd2 in pronephroi.
Previous studies of mammalian Polycystin-2 protein localization demonstrated expression in intracellular ER/Golgi membranes and in apical, nonmotile cilia of kidney epithelia and mouse node cells (3,18,21,30). To examine the subcellular Polycystin-2 protein distribution in zebrafish, we generated an antipeptide antibody against the N-terminal cytoplasmic zebrafish Polycystin-2 protein sequence EKMHHEEVGLGVPDEC and purified it by antigen affinity chromatography. Whole-mount immunostaining revealed strong expression of Polycystin-2 in the trunk region embryos at 24 and 48 hpf (Figure 2A). Expressing cells included muscle and the pronephric duct. Preincubation with immunizing peptide (Figure 2B) or blocking expression of endogenous Polycystin-2 with a MO oligo targeting the initiator ATG completely abolished anti–Polycystin-2 immunoreactivity (Figure 2C), demonstrating specificity of our antibody. Also, no staining was observed with preimmune serum or in the absence of primary antibody (data not shown). An enlarged view of the anterior segment of a single pronephric duct revealed that Polycystin-2 immunostaining could be seen associated with the basolateral membrane as well as on lumenal cilia (Figure 2D). Cilia that arose from both multiciliated cells and singly ciliated cells were positive for Polycystin-2 (data not shown). In histologic sections, Polycystin-2 also was localized to basolateral cell membranes of pronephric duct cells in the anterior (proximal) nephron segment (Figure 2E). It is interesting that Polycystin-2 subcellular localization in the more posterior pronephric duct shifted to cytoplasmic vesicles that ringed the nucleus and showed a concentration near the apical cell surface (Figure 2F). Basolateral cell membranes of caudal pronephric duct cells were largely negative for Polycystin-2 (Figure 2G). The presence of Polycystin-2 protein in adult zebrafish kidney was assayed by Western blotting. Our anti– Polycystin-2 antibody detected a single protein in lysates of whole adult kidney that migrated at approximately 115 kD (Figure 2H). This result confirms expression of Polycystin-2 in adult kidney and further establishes the specificity of our antibody.
Figure 2.
Immunolocalization of Polycystin-2 in the pronephric kidney. (A) Expression of Polycystin-2 protein in the trunk of a 48-hpf embryo viewed in whole mount is strong in muscle (*) and the pronephric duct (arrowheads). (B) Immunizing peptide preincubation control for antibody staining shows no signal in a similar trunk region (*muscle; arrowheads, pronephric duct). (C) Control for antibody specificity using ATG morpholino (MO) blockade of endogenous protein translation shows no expression in the trunk region (*muscle; arrowheads, pronephric duct). (D) Z-series projection of Polycystin-2 immunofluorescence in anterior pronephric duct shows expression in cilia and associated with basolateral cell membranes and infoldings (arrows) in a whole mount–stained 2.5-d embryo. (E) Cross-sections of the anterior pronephric duct show Polycystin-2 expression associated with basolateral cell membranes (arrows) and apical cilia. (F) Polycystin-2 immunofluorescence in the posterior pronephric duct is punctate and distributed throughout the cells with a concentration of staining near the apical surface. (G) Sections of the posterior pronephric duct in the cloaca region show expression of Polycystin-2 in subapical membrane vesicles and reduced expression in basolateral membranes (arrows). (H) Western blot of adult zebrafish kidney reacted with anti-zebrafish PKD2 antibody reveals a single band migrating at approximately 115 kD.
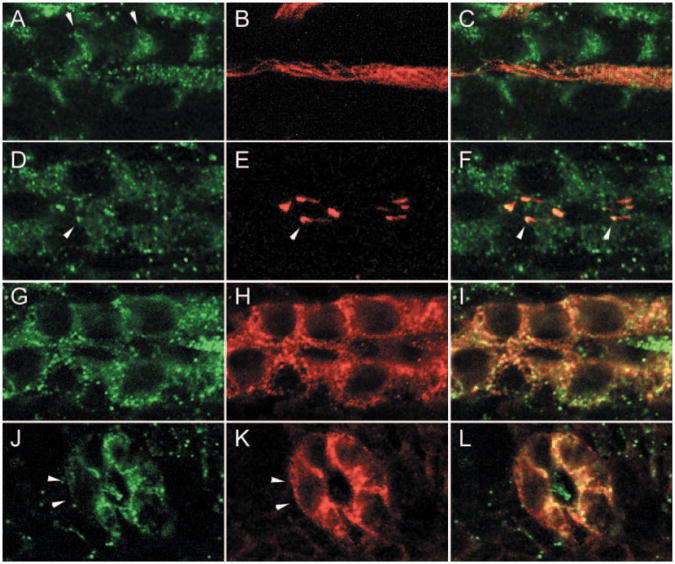
To clarify the subcellular distribution of Polycystin-2 in anterior and posterior nephron segments, we double stained embryos with Polycystin-2 and anti-acetylated tubulin to highlight apical cilia or with anti-NaK ATPase α subunit (α6F) to highlight basolateral membranes. Confocal images of whole-mount stained embryos showed that in the anterior pronephros, Polycystin-2 was associated with basolateral membranes and with bundles of apical cilia that were strongly positive for anti-acetylated tubulin (Figure 3, A through C). In the posterior duct, Polycystin-2 immunostaining was more punctate, suggesting expression on cytoplasmic vesicles (Figure 3D). Polycystin-2 also was associated with single apical cilia and was most strongly localized to the basal portion of cilia and often absent from cilia tips (Figure 3, E and F). The anti-NaK ATPase α1 subunit antibody α6F uniformly labels the basolateral surfaces of pronephric duct cells (Figure 3H). In a tangential optical section of the anterior pronephric duct, Polycystin-2 immunostaining also was associated with the basolateral surface but appeared more punctate compared with α6F staining (Figure 3, G and I). In the posterior duct, confocal images of Polycystin-2 staining in histologic sections showed some areas of co-localization with α6F staining near apical lateral membranes (Figure 3, J through L) but was notably reduced near basal cell surfaces (Figure 3, J and K, arrows). As in anterior segments, lumenal cilia were positive for Polycystin-2 but negative for α6F (Figure 3L). Segment-specific subcellular localization of Polycystin-2 in the pronephric duct raises the possibility that Polycystin-2 may perform distinct functions depending on the cellular context in which it is expressed.
Figure 3.
Nephron segment-specific distribution of Polycystin-2 in basolateral membranes and cilia. (A through C) The anterior pronephric duct. Polycystin-2 (A; green) and acetylated tubulin (B; red) immunofluorescence in confocal sections. (C) Merged image of A and B. Anterior duct Polycystin-2 is present associated with basolateral membranes (arrows in A) and a lumenal bundle of cilia. (D through F) The posterior pronephric duct. Polycystin-2 (D; green) and acetylated tubulin (E; red) immunofluorescence in confocal sections. (F) Merge of D and E shows accumulation of Polycystin-2 in intracellular vesicles and at the base of cilia (arrows). (G through I) The anterior pronephric duct. (G) Tangential confocal section through the basolateral membranes shows a concentration of punctate Polycystin-2 expression in a whole mount-stained 2.5-d embryo. (H) Basolateral membranes of the anterior duct stain uniformly with the monoclonal α6F against the NaK ATPase α1 subunit. (I) Merge of G and H shows that Polycystin-2 and the NaK ATPase show areas of co-localization and some distinct areas of punctate expression. (J through L) The posterior pronephric duct. (J) Cross-section of the duct shows punctate Polycystin-2 immunofluorescence in lateral and apical membranes but absence of expression in basal cell surfaces. A lumenal cilium also is positive for Polycystin-2. (K) NaK ATPase immunofluorescence on basolateral membranes. (L) Merge of J and K.
Expression of Polycystin-2 in Extrarenal Tissues
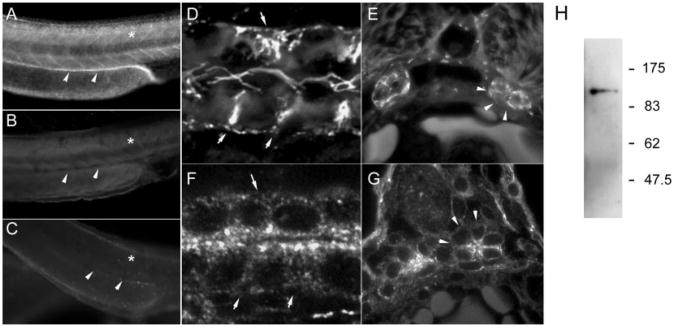
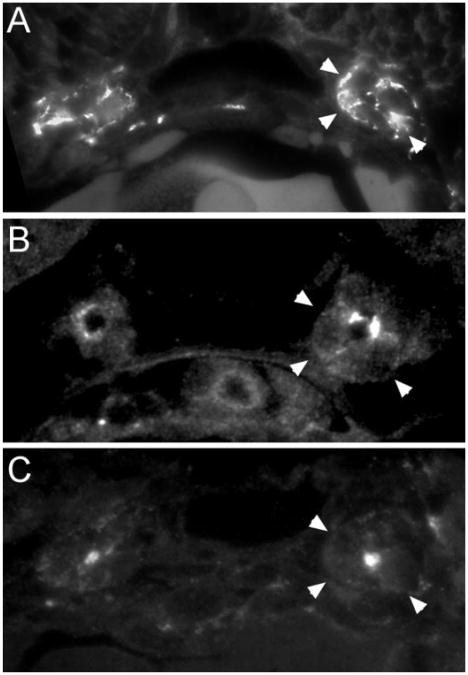
Polycystin-2 expression was observed in several nonkidney tissues, including muscle cells (Figure 4, A and B) and the lining of the brain ventricles (Figure 4C), and in sensory structures, including the olfactory placodes (Figure 4D), the ear (Figure 4E), and the lateral line organs (Figure 4F). In muscle, Polycystin-2 was strongly expressed in cell membranes (Figure 4A) and in a repeating sarcomeric pattern (Figure 4B), suggesting that it may function in sarcoplasmic reticulum membranes. In the brain ventricle, expression was detected in apical cilia (Figure 4C). In sensory organs, Polycystin-2 was expressed in olfactory placodes (Figure 4D), the ear (Figure 4E), and the lateral line organ (Figure 4F). In the lateral line organs, Polycystin-2 was expressed in the apical membrane of ciliated hair cells but not in the cilia themselves (Figure 4F, inset).
Figure 4.
Immunolocalization of Polycystin-2 in nonkidney organs. (A) At 56 hpf, muscle cell membranes of the trunk myotomes are positive for Polycystin-2 in confocal sections. (B) Polycystin-2 expression also is present in repeating, sarcomeric bands on intracellular muscle fibers. (C) Ependymal cell cilia in the brain ventricles are positive for Polycystin-2 (arrows and inset) in histologic sections. (D) The olfactory placode cell membranes are strongly positive for Polycystin-2 (arrow) in histologic sections. (Inset) Confocal section of the olfactory placode showing concentration of Polycystin-2 immunoreactivity in apical membranes of olfactory placode cells. (E) Epithelial cells of the ear show apical staining for Polycystin-2 (Inset) Higher magnification view of ear cells showing Polycystin-2 immunoreactivity in apical membranes and vesicles. (F) Cells of the lateral line organ are strongly positive for Polycystin-2 expression (Inset) Higher magnification view of lateral line organ cells in the central part of this structure showing Polycystin-2 expression.
Disruption of Polycystin-2 Function with Antisense MO Oligos
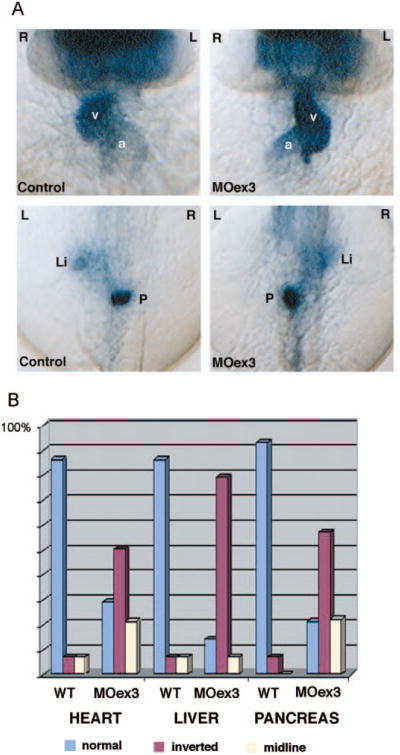
Previous studies demonstrated that loss of Polycystin-2 function in zebrafish results in pronephric cyst formation and defects in left–right asymmetry (25,26). To assess how deletion of specific Polycystin-2 domains may affect the in vivo functions of Polycystin-2, we generated multiple internal deletion alleles of Polycystin-2 by disrupting mRNA splicing with antisense MO oligos targeting exon donor sites. Two different in-frame deletions in the first extracellular loop (Figure 5, A, B and L) were produced by targeting pkd2 exon 3 and exon 5 splice donor sites. Targeting the exon 12 splice donor site resulted in failure to splice intron 12 and read-through to an immediate stop codon in intron 12, producing a predicted C-terminal truncation allele just after a conserved PACS-1 binding motif (Figure 5, A, B and L). Targeting the exon 13 splice site donor resulted in an in-frame deletion of exons 12 and 13 that contain the PACS-binding, ER retention motif (Figure 5, B and L). MO injection effectively eliminated normal pkd2 mRNA for the first 2 d of development; recovery of wild-type message to roughly equal amounts to deleted mRNA was observed by 3 dpf as shown for MOex3 in Figure 5C. All splice donor site–blocking MO-injected embryos exhibited a similar range of phenotypes: Morphant embryos developed dorsal body axis curvature, hydrocephalus, and pronephric kidney cysts by 2.5 d of development (Figure 5, F through K) compared to wild-type (Figure 5, D and E). A MO targeting the pkd2 AUG initiation codon produced a similar pleiotropic phenotype (data not shown). Consistent with previous reports, disruption of zebrafish pkd2 expression also resulted in defects in left-right asymmetry. Heart looping was randomized with roughly 30% normal, 50% inverted, and 20% midline. In situ hybridization using CMLC2 as a marker of the heart chambers and fkd2 and insulin as markers of the liver and pancreas, respectively, revealed that all three organs were abnormally positioned with respect to normal left-right situs (Figure 6).
Figure 5.
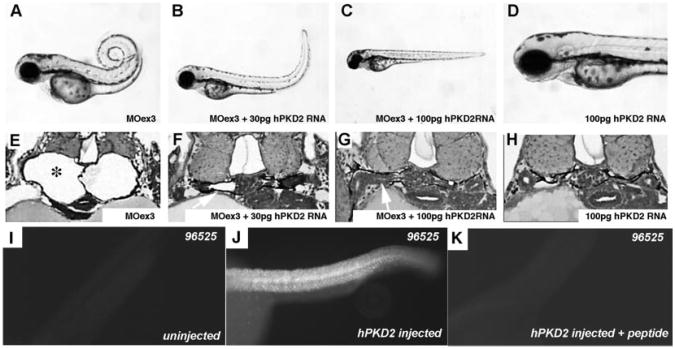
Disruption of pkd2 expression with splice donor antisense MO oligonucleotides. (A) Diagram of Polycystin-2 protein in the cell membrane showing membrane topology and C-terminal motifs associated with Polycystin-2 function. (B) Targeting splice donor sites of exons 3, 5, 12, and 13 with antisense MO oligonucleotides resulted in the production of internal in-frame deletions (exons 3, 5, and 13 MO) and a C-terminal truncation (exon 12 MO). Transmembrane domains of Polycystin-2 are depicted in gray. (C) The efficacy of the injected MO was quantified at 24-, 48-, and 72-h intervals by RT-PCR. Polycystin-2 exon 3 donor MO (MOex3) caused a 39–amino acid in-frame deletion of part of the first transmembrane domain as detected by the presence of a smaller, internally deleted RT-PCR product. Some recovery of normal Polycystin-2 mRNA was observed by 72 hpf. Similar results were observed for all splice donor targeted MO. (D) Control embryos at 72 hpf. (E) Histologic section of normal pronephros at 72 hpf. (F) MOex3-injected embryo showing axis curvature and hydrocephalus (arrow; 97%, 941 of 967 embryos). (G) Histologic section showing cystic pronephric tubules (*) in MOex3-injected embryo. (H) MOex12-injected embryos and section (I) of the cystic pronephros. (J) MOex13-injected embryos showing severe axis curvature and kidney cysts (arrow). (K) Histologic section of embryo in J showing kidney cyst (*). (L) Antisense MO deletions in the Polycystin-2 protein sequence predicted on the basis of nucleotide sequence of RT-PCR products amplified from MO-injected embryos. In-frame deletions are shown in gray for MOex3, MOex5, and MOex13. MOex12 induced a nonsplicing event that resulted in a stop codon immediately after exon 12 in the cDNA (shown in red *). Transmembrane domains (tm) and the PKD1 binding homology domain are highlighted in brown. Membrane targeting motifs in the cytoplasmic C-terminus including the phosphofurin acidic cluster sorting protein–1 (PACS-1)-binding acidic cluster are underlined.
Figure 6.
Polycystin-2 function in left–right asymmetry. (A) Expression of cardiac myosin light chain 2 (cmlc2) in control embryos (top left) demonstrates normal positions of the heart ventricle (v) and atrium (a) relative to the embryo midline. Polycystin-2 MOex3 caused inversion of left–right axes in 50% of injected embryos (top left). Forkhead2 (fkd2) and insulin gene expression revealed similar inversion of liver (li) and pancreas (p) situs, respectively (bottom). (B) Quantification of left–right asymmetry defects. Wild-type embryos (wt) show normal situs in most all cases with a low level of background laterality defects. Polycystin-2 MOex3-injected embryos show a high degree of left–right axis inversion (approximately 50%) as well as midline organ position for the heart, liver, and pancreas.
Pronephric Kidney Cyst Formation and Rescue with Human PKD2 mRNA
To establish specificity of MO effects and to test for conservation of Polycystin-2 function, we determined whether pkd2 MO phenotypes could be rescued by co-injection of a synthetic human PKD2 mRNA. As shown in Figure 7, embryos that were co-injected with pkd2 MOex3 and human PKD2 mRNA were morphologically normal in terms of both kidney structure and axis curvature (Figure 7, C and H). Intermediate amounts of human PKD2 mRNA caused partial rescue of both phenotypes (Figure 7, B and G). Staining injected embryos with a mammalian-specific anti–Polycystin-2 antibody confirmed broad expression of the exogenous rescuing mRNA (Figure 7, I through K). The data indicate that the effects of the splice blocking MO are specific to the PKD2 mRNA and that the human Polycystin-2 protein can functionally replace the zebrafish gene in vivo.
Figure 7.
Rescue of pkd2MOex3 phenotype with human PKD2 mRNA co-injection. (A and E) pkd2 MOex3-injected embryo showing axis curvature and pronephric cysts in cross-section (97%; 617 of 638 injected embryos). (B and F) Co-injection of 30 pg of human PKD2 mRNA with MOex3 MO results in partial rescue of cyst phenotype (arrow in F; 98%, 571 of 583 injected embryos). (C and G) Co-injection with 100 pg of human PKD2 mRNA completely rescues cyst phenotype (arrow in G; 99%, 728 of 733 embryos). (D and H) Injection of human PKD2 mRNA alone has no effect on normal embryos (641 embryos). (I) Polyclonal anti-PKD2 antibody 96526 recognizes mouse and human Polycystin-2 but not zebrafish Polycystin-2 (uninjected wild-type embryo). (J) Embryo injected with human PKD2 mRNA shows broad expression of the exogenous rescuing mRNA. (K) Mouse Polycystin-2 peptide antigen preincubation blocks all 96526 antibody staining, demonstrating specific immunoreactivity of the 96526 antibody.
Internal Polycystin-2 Peptide Deletions Alter Subcellular Localization
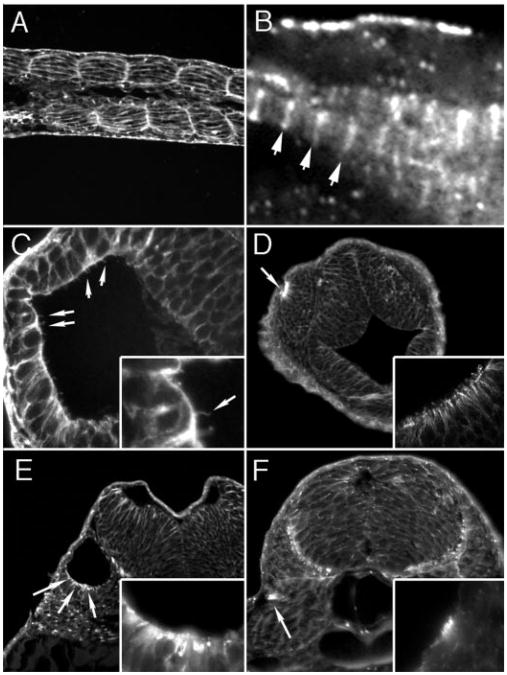
The in-frame internal deletion pkd2 mRNA that we generated by MO-directed missplicing would be predicted to encode proteins with deletions in discrete protein domains. MOex3 and MOex5 would generate 37 and 47 amino acid deletions, respectively, in the Polycystin-2 first extracellular loop while leaving neighboring transmembrane spanning sequences intact. MOex13 would generate a larger, 104 amino acid deletion in the cytoplasmic C-terminus encompassing the conserved PACS binding, ER/Golgi retention motif (23), and potentially other functionally important motifs while leaving the C-terminal EF-hand and putative PKD1-interacting domains (31,32) unaffected. Because deletion of the Polycystin-2 motifs might be expected to result in altered subcellular protein trafficking and localization (2,23), we examined morphant embryos for the localization of the altered Polycystin-2 protein. In contrast to wild-type Polycystin-2 protein, which was concentrated in basolateral cell membranes in anterior duct cells (Figure 8A), Polycystin-2 that lacked either exon 5 sequences of the first extracellular loop or C-terminal exons 12 and 13 was concentrated in apical membranes of pronephric duct cells and strongly reduced or absent from internal and basolateral cell membranes (Figure 8, B and C).
Figure 8.
Apical mislocalization of MO-altered Polycystin-2 proteins. (A) Wild-type Polycystin-2 is concentrated in basolateral membranes of the anterior pronephric ducts (arrows, basolateral cell surface) in histologic sections. (B) MOex5-injected embryo showing a shift in immunoreactivity of altered (in-frame deletion in the first extracellular loop) Polycystin-2 from basal to apical cell membranes. (C) MOex13-injected embryo showing absence of basolateral staining and concentration of Polycystin-2 immunoreactivity in apical and lumenal membranes.
Potential Mechanisms of Pronephric Cyst Formation
Because previous studies suggested that the Polycystin-1/2 complex may control cell division (33), we examined pronephric tubules and ducts of morphant embryos for evidence of an increase in cell number. In cross-section, both wild-type and pkd2 morphant ducts were made up of four to five cells; no differences in cell number between wild-type and pkd2 MO-injected embryos at early stages of cyst formation were observed (data not shown). Kidney cyst formation in zebrafish and in mammals also has been associated with a disruption of apical cilia function in tubule epithelial cells (25,34). When cilia were visualized with anti-acetylated tubulin immunofluorescence in pkd2 MO-injected embryos, no differences in cilia length or orientation were observed (data not shown). Cilia in the zebrafish pronephros are motile and contribute to overall fluid movement in the pronephros (34); we therefore asked whether the mechanism of cyst formation might involve a reduction or loss of cilia motility. No defects in pronephric cilia motility were observed by high-speed video microscopy of pkd2MO-injected embryos (data not shown). The data indicate that cyst formation in the pronephros of pkd2 morphants is not likely to be due to an early increase in cell proliferation or a loss of cilia structure or motility.
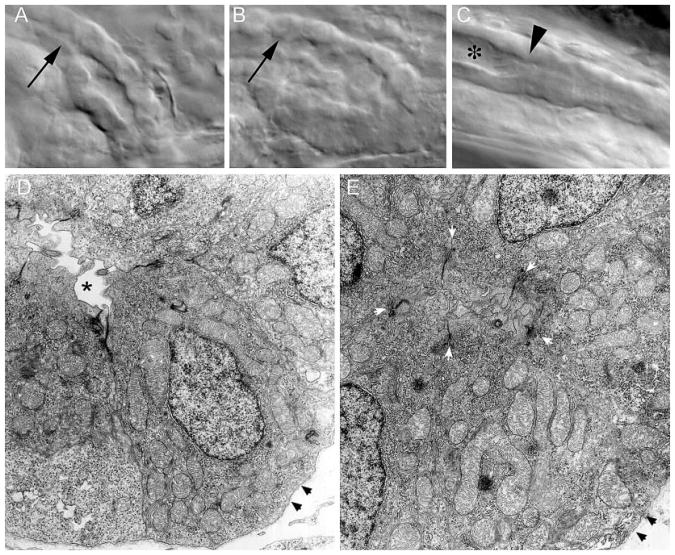
In work on related zebrafish pronephric cyst mutants, we have observed that cyst formation can result from reduced fluid flow or nephron obstruction (34). To establish whether fluid flow might be affected in embryos that lack functional Polycystin-2, we assayed fluid output at the cloaca using rhodamine dextran as a fluid tracer. Vascular injection of fluorescence dextran results in glomerular filtration of this fluid tracer and appearance of a fluorescence “jet” of urine output at the cloaca in wild-type embryos (Figure 9, A and B). Fluorescence fluid output at the cloaca was not observed in any pkd2MO-injected embryos that developed cysts (Figure 9, C and D; n = 5), whereas all wild-type embryos showed fluorescence fluid output at the cloaca (Figure 9, A and B; n = 9). This suggested that nephron fluid flow might be significantly reduced in embryos that lack Polycystin-2 function, possibly as a result of a functional or physical obstruction of the duct. Examination of microscopic images of live fish did in fact suggest that the posterior pronephric duct of morphant embryos may be partially occluded, because it was not possible to visualize the duct lumen structure in these images (Figure 10, B and C) compared with similar images of wild-type embryos (Figure 10A). Further examination of the distal duct ultrastructure by electron microscopy revealed that in wild-type embryos, the caudal pronephric duct lumen is narrow but clearly discernible in all wild-type embryos examined (n = 3; Figure 10D). In contrast, the duct lumen in similar segments of pkd2MO-injected embryos was occluded by the apical cytoplasm of duct epithelial cells (Figure 10E). Limiting apical cell junctions were clearly visible in pkd2MO larvae; however, the apical cytoplasm and apical cell surfaces of duct epithelial cells were seen to be abutting, coalescing around profiles of lumenal cilia (Figure 10E). The results suggest that Polycystin-2 may be required to regulate apical cell cytoskeleton or otherwise maintain lumen patency in the posterior pronephric ducts.
Figure 9.
Reduction in pronephric fluid output in pkd2 morphants. (A and B) Pronephric fluid output detection at the cloaca using rhodamine dextran as a fluid tracer in wild-type embryos at 72 hpf. Appearance of a fluorescence “jet” of urine output at the cloaca is observed (arrowhead in B). (C and D) Polycystin-2 MOex3-injected embryos at 72 hpf show a significant reduction in fluid output at the cloaca (arrowhead in D).
Figure 10.
Structural alterations in the distal pronephric nephron segment of pkd2 morphants. (A) Differential interference contrast images of the cloaca region of the pronephric duct in living, 3-dpf wild-type embryos show a patent lumen extending to the cloaca. (B) Polycystin-2 MOex3-injected embryos show an apparent collapse of the distal duct lumen. (C) Lumenal distension (*) immediately anterior to an area of duct occlusion (arrowhead in C) in a Polycystin-2 MOex3-injected embryo. Cilia beat and length appeared normal in areas of lumenal distension (data not shown). (H) Electron micrograph cross-section of the wild-type distal pronephric duct showing a patent duct lumen (*). For reference, cell basement membrane is denoted with arrowheads. (I) A similar region of the pronephric duct in a Polycystin-2 MOex3-injected embryos appears occluded by extended apical cytoplasm of duct epithelial cells. Five apical adherens junctions of the MOex3 duct epithelial cells can be seen (white arrowheads); however, the apical membranes of these cells abut with opposing cells, occluding the duct lumen. Black arrowheads, basement membrane.
Discussion
Because of the ease of observation, genetic manipulation, and the conservation of gene function, the zebrafish has emerged as a useful model system for studies of vertebrate organogenesis and nephrogenesis. The potential to model disease mechanisms in the zebrafish motivated us to explore the function of Polycystins in the pronephros. We and others find that Polycystin-2 plays a critical role in zebrafish kidney and brain development and in regulating organ situs (25,26). How Polycystin-2 loss of function might cause cyst formation in the pronephros and where Polycystin-2 is expressed in a pronephric epithelial cell were not known. In work presented here, we demonstrate that Polycystin-2 is present in multiple cell membrane systems in the zebrafish kidney, including basolateral cell membranes, intracellular vesicles, and cilia membranes. We provide in vivo evidence that protein domains in the first extracellular loop and C-terminal sequences that include a PACS-binding domain (23) are required to retain Polycystin-2 protein in intracellular membrane systems. Loss of Polycystin-2 function did not lead to defects in cilia function or altered cell proliferation but instead reduced fluid output from the pronephros and altered cell morphology in the most distal segment of the pronephros. Expanded apical cytoplasm and, in a majority of cases, occlusion of the pronephric ducts suggest the possibility of an obstructive mechanism of cyst formation in the zebrafish pronephros.
Polycystin-2 Localization and Function in the Pronephros
Polycystin-2 has been shown to function both as a mechanosensory channel in apical cilia and as an intracellular calcium release channel in intracellular membranes (2–7). In the zebrafish, we find that Polycystin-2 is localized to both cilia and intracellular membranes in kidney epithelial cells, suggesting that it could function in both cell compartments.
Although uniformly present in cilia throughout the pronephric nephron, Polycystin-2 was localized to distinct membranes in proximal versus distal pronephric duct cells. Presence of Polycystin-2 in anterior/proximal duct basolateral cell membranes would be consistent with similar reports of Polycystin-2 localization in mammalian kidney epithelia (2,3). In some of our images, however, Polycystin-2 expression appeared more punctate, suggesting that in the anterior duct, some Polycystin-2 also may be expressed in vesicles that are closely associated with the basolateral membrane as opposed to inserted in the basolateral membrane itself. Subcellular localization of Polycystin-2 in more distal nephron segments in vivo has been less well characterized. Our results show that cells of the distal zebrafish pronephros uniquely express Polycystin-2 in vesicles that are concentrated in the apical cytoplasm and in apical cilia, similar to mouse inner medullary collecting duct cells in culture (35). By electron microscopy, we found that this region of the cytoplasm seems to be rich with small vesicular structures that could contain Polycystin-2 protein. One implication of these results is that Polycystin-2 localization is as much dependent on different protein trafficking systems' functioning in particular cell types as it is on cis-motifs that are present in the protein itself. In addition, it is possible that the some of the differences in Polycystin-2 subcellular localization could reflect a redistribution of Polycystin-2-containing vesicular structures in posterior versus anterior cells. Further studies by immunoelectron microscopy may help to resolve these possibilities.
Roles for Polycystin-2 Domains in Subcellular Localization
Several different protein trafficking motifs in Polycystin-2 now have been described. These motifs include an N-terminal sequence that is required for Polycystin-2 cilia localization (36), an N-terminal GSK-3β phosphorylation site that favors localization in lateral cell membranes similar to E-cadherin and ZO-1 (37), and a C-terminal acidic cluster/PACS-binding domain that promotes retention of Polycystin-2 in the ER/Golgi membranes (23). The exon 12/13 MO deletion allele (MOex13) that we generated would be predicted to encode a protein that lacks the ER retention motif/acidic cluster from the cytoplasmic C-terminus while preserving the more C-terminal sequences. Consistent with results from cell culture studies, this variant protein was detected exclusively in apical cell membranes and/or lumenal cilia of zebrafish anterior pronephric duct cells in vivo, as opposed to the wild-type protein that was evenly distributed between the cilia and basolateral membranes. Because even complete deletion of the cytoplasmic tail of mammalian Polycystin-2 does not abolish its channel activity (38), it is likely that some channel activity may remain associated with the MOex13 Polycystin-2 variant protein as well as with the MOex12 variant that produced a C-terminal truncation (V777X). However, because both MO caused pronephric cyst formation, it is clear that whatever channel activity may remain in these variants is not sufficient for normal Polycystin-2 function, as is the case for similar human PKD2 mutations that are associated with ADPKD (38). Further studies of zebrafish Polycystin-2 point mutants and biophysical studies of channel variants will be required to assess their function critically in the pronephros.
Potential Functions for Polycystin-2 in the Distal Pronephric Duct
The reduction in fluid output and from the pronephros of embryos that lacked Polycystin-2 despite the presence of normally functioning renal cilia prompted us to examine Polycystin-2–expressing nephron segments for any evidence of obstruction to lumenal fluid flow. The ultrastructure of the posterior duct lumen in cystic larvae suggests that the posterior duct is completely occluded by extension of apical cytoplasm of duct cells. One function of pkd2 in zebrafish therefore may be to maintain or specifically regulate the distal pronephric duct lumen diameter. When its expression is disrupted, apical membranes narrow the posterior duct lumen, causing a reduction in fluid flow, which could result indirectly in cyst formation in more anterior, proximal nephron segments. This interpretation is supported by our previous findings that complete occlusion of the posterior pronephric duct in zebrafish larvae by mechanical wounding is sufficient to cause cysts to form rapidly in the anterior nephron segments adjacent to the glomerulus, presumably by a build-up of fluid back pressure (34). In addition, disruption of genes that are required in zebrafish for caudal body axis development can result in failure of distal pronephric duct extension and failed cloacal fusion with the exterior, with subsequent cyst development in anterior, proximal, pronephric nephron segments (39). In this light, cyst development in zebrafish pronephroi could be viewed as similar to human multicystic dysplastic kidney disease or fetal obstructive disorders in which distal obstruction and feedback fluid pressure are sufficient to distend proximal tubules and glomeruli (40,41). Polycystin-2, however, is associated in humans with ADPKD, in which the existence of cysts with seemingly patent connections to the ureter has discounted the idea that obstruction plays a major role in ADPKD (42,43). Although further studies will be required to clarify the relationship of our results to Polycystin-2 function in the mammalian kidney, our studies show that Polycystin-2 in zebrafish is required to maintain lumen patency in the distal portion of the pronephric duct and that a “functional obstruction” is the most likely cause of cyst formation in the pronephros.
How Polycystin-2 might regulate distal pronephric duct lumen size is unclear. One possibility is that Polycystin-2 normally negatively regulates the amount of membrane that is delivered to the apical cell surface and, when absent, allows excess apical membrane insertion. Excess apical membrane insertion in a tubule that is fixed in overall diameter by its basement membrane could result in expansion of the cell apical domain to the point at which it occludes the lumen. Evidence for variation in the amount or rate of apical membrane delivery being part of a mechanism for the formation of epithelial cysts has been demonstrated in Drosophila salivary tubes (44). Alternatively, Polycystin-2 could regulate the structure of the apical cytoskeleton in posterior duct cells. Mammalian Polycystin-2 is known to interact with actin cytoskeleton–associated or regulatory proteins, including hax-1, CD2AP, troponin, and tropomyosin (45–48). Polycystin-2 also has been shown to interact with mDia1, a Rho GTPase effector protein that regulates actin polymerization (49). It is possible that Polycystin-2, acting as a calcium entry channel in response to lumenal flow or as an intracellular release channel in the apical cytoplasm, may play some role in maintaining the rigidity of the subapical actin cytoskeleton.
Potential Roles for Polycystin-2 in Pronephric Cilia
Polycystin-2 in mammalian kidney tubules has been localized to nonmotile, “9 + 0” apical cilia, and evidence from in vitro cell culture studies has shown that nonmotile cilia can act as mechanosensors of fluid flow (5,19). The presence of Polycystin-2 in pronephric cilia, which, unlike “9 + 0” primary cilia on cultured mouse epithelial cells, are “9 + 2” and motile (34), broadens the potential roles for Polycystin-2 in cilia and renal epithelia. Polycystin-2 also has been localized to motile cilia in the female reproductive tract and the mouse embryonic node (20–22), which, taken together with our work, indicates that Polycystin-2 function may not be limited to nonmotile cilia. Although we cannot demonstrate any morphologic defects in pronephric cilia or any difference in cilia beat rate in pkd2 morphants, it remains possible that Polycystin-2 function in motile cilia could be related to mechanosensory signaling. Motile cilia have been shown to relay mechanosensory information, signaling changes in membrane potential in response to increased resistance to beating or initiating or reversing cilia beat in response to touch or pressure (50). The TRP channel TRPV4 is localized to motile cilia in the mammalian oviduct and has been shown to signal changes in cilia beat frequency in response to fluid viscosity changes (20). Motile cilia in the invertebrate statocyst, an organ that is associated with sensing orientation, respond to interference with cilia beat and relay this information as changes in membrane potential (51). It is not known whether Polycystin-2 in motile pronephric cilia could contribute to cilia signaling. However, the presence of Polycystin-2 in multiple types of cilia and ciliated sensory organs in zebrafish larvae suggests that the fish will be a useful model to explore further the in vivo functions of Polycystin-2.
Acknowledgments
This work was supported by National Institutes of Health grants DK53093 and DK54711 to I.A.D. and GM56211 to A.F.S. A.F.S. is an Irma T. Hirschl Trust Career Scientist and an Established Investigator of the American Heart Association. T.O. is supported in part by a PKD foundation grant 69a2r. We thank Mary McKee for assistance with electron microscopy and Jing Zhou, Yiqiang Cai, and Stephan Somlo for Polycystin-2 antibodies.
References
- 1.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 2.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 3.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- 4.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 5.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 6.Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem. 2002;277:20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- 7.Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet. 2002;11:59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez-Perrett S, Kim K, Ibarra C, Damiano AE, Zotta E, Batelli M, Harris PC, Reisin IL, Arnaout MA, Cantiello HF. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc Natl Acad Sci USA. 2001;98:1182–1187. doi: 10.1073/pnas.98.3.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 10.Barr MM, DeModena J, Braun D, Nguyen CQ, Hall DH, Sternberg PW. The Caenorhabditis elegans autosomal dominant polycystic kidney disease gene homologs lov-1 and pkd-2 act in the same pathway. Curr Biol. 2001;11:1341–1346. doi: 10.1016/s0960-9822(01)00423-7. [DOI] [PubMed] [Google Scholar]
- 11.Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. A flagellar polycystin-2 homolog required for male fertility in Drosophila. Curr Biol. 2003;13:2179–2184. doi: 10.1016/j.cub.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Gao Z, Joseph E, Ruden DM, Lu X. Drosophila Pkd2 is haploid-insufficient for mediating optimal smooth muscle contractility. J Biol Chem. 2004;279:14225–14231. doi: 10.1074/jbc.M312223200. [DOI] [PubMed] [Google Scholar]
- 13.Kierszenbaum AL. Polycystins: What polycystic kidney disease tells us about sperm. Mol Reprod Dev. 2004;67:385–388. doi: 10.1002/mrd.20042. [DOI] [PubMed] [Google Scholar]
- 14.Pennekamp P, Karcher C, Fischer A, Schweickert A, Skry-abin B, Horst J, Blum M, Dworniczak B. The ion channel polycystin-2 is required for left-right axis determination in mice. Curr Biol. 2002;12:938–943. doi: 10.1016/s0960-9822(02)00869-2. [DOI] [PubMed] [Google Scholar]
- 15.Wu G, Markowitz GS, Li L, D'Agati VD, Factor SM, Geng L, Tibara S, Tuchman J, Cai Y, Park JH, van Adelsberg J, Hou H, Kucherlapati R, Edelmann W, Somlo S. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–78. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 16.Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC, Torres VE. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, Cai Y, Chen X, Wu GQ, Geng L, Cleghorn KA, Johnson CM, Somlo S. Vascular expression of polycystin-2. J Am Soc Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- 18.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 19.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 20.Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005;168:869–874. doi: 10.1083/jcb.200409070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 22.Teilmann SC, Byskov AG, Pedersen PA, Wheatley DN, Pazour GJ, Christensen ST. Localization of transient receptor potential ion channels in primary and motile cilia of the female murine reproductive organs. Mol Reprod Dev. 2005;71:444–452. doi: 10.1002/mrd.20312. [DOI] [PubMed] [Google Scholar]
- 23.Kottgen M, Benzing T, Simmen T, Tauber R, Buchholz B, Feliciangeli S, Huber TB, Schermer B, Kramer-Zucker A, Hopker K, Simmen KC, Tschucke CC, Sandford R, Kim E, Thomas G, Walz G. Trafficking of TRPP2 by PACS proteins represents a novel mechanism of ion channel regulation. EMBO J. 2005;24:705–716. doi: 10.1038/sj.emboj.7600566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 25.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–4093. doi: 10.1242/dev.01240. [DOI] [PubMed] [Google Scholar]
- 26.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and polycystin-2 in dorsal forerunner cells and Kupffer's vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 27.Westerfield M. The Zebrafish Book. Eugene: University of Oregon Press; 1995. [Google Scholar]
- 28.Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- 29.Venglarik CJ, Gao Z, Lu X. Evolutionary conservation of Drosophila polycystin-2 as a calcium-activated cation channel. J Am Soc Nephrol. 2004;15:1168–1177. doi: 10.1097/01.asn.0000125616.42669.51. [DOI] [PubMed] [Google Scholar]
- 30.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Wit-man GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 31.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 32.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem. 2004;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 34.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 35.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2006;290:F1320–F1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 36.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 37.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phos-phorylation site which regulates the functional localisation of polycystin-2 in vivo and in vitro. Hum Mol Genet. 2006;15:1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen XZ, Segal Y, Basora N, Guo L, Peng JB, Babakhanlou H, Vassilev PM, Brown EM, Hediger MA, Zhou J. Transport function of the naturally occurring pathogenic poly-cystin-2 mutant, R742X. Biochem Biophys Res Commun. 2001;282:1251–1256. doi: 10.1006/bbrc.2001.4720. [DOI] [PubMed] [Google Scholar]
- 39.Sayer JA, Otto EA, O'Toole J, F, Nurnberg G, Kennedy MA, Becker C, Hennies HC, Helou J, Attanasio M, Fausett BV, Utsch B, Khanna H, Liu Y, Drummond I, Kawakami I, Kusakabe T, Tsuda M, Ma L, Lee H, Larson RG, Allen SJ, Wilkinson CJ, Nigg EA, Shou C, Lillo C, Williams DS, Hoppe B, Kemper MJ, Neuhaus T, Parisi MA, Glass IA, Petry M, Kispert A, Gloy J, Ganner A, Walz G, Zhu X, Goldman D, Nurnberg P, Swaroop A, Leroux MR, Hilde-brandt F. The centrosomal protein nephrocystin-6 is mutated in Joubert syndrome and activates transcription factor ATF4. Nat Genet. 2006;38:674–681. doi: 10.1038/ng1786. [DOI] [PubMed] [Google Scholar]
- 40.Potter EL. Normal and Abnormal Development of the Kidney. Chicago: Year Book Medical Publishers; 1972. [Google Scholar]
- 41.Woolf AS, Price KL, Scambler PJ, Winyard PJ. Evolving concepts in human renal dysplasia. J Am Soc Nephrol. 2004;15:998–1007. doi: 10.1097/01.asn.0000113778.06598.6f. [DOI] [PubMed] [Google Scholar]
- 42.Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polysyctic kidney disease. Annu Rev Med. 2001;52:93–123. doi: 10.1146/annurev.med.52.1.93. [DOI] [PubMed] [Google Scholar]
- 43.Tanner GA, Gretz N, Connors BA, Evan AP, Steinhausen M. Role of obstruction in autosomal dominant polycystic kidney disease in rats. Kidney Int. 1996;50:873–886. doi: 10.1038/ki.1996.387. [DOI] [PubMed] [Google Scholar]
- 44.Myat MM, Andrew DJ. Epithelial tube morphology is determined by the polarized growth and delivery of apical membrane. Cell. 2002;111:879–891. doi: 10.1016/s0092-8674(02)01140-6. [DOI] [PubMed] [Google Scholar]
- 45.Gallagher AR, Cedzich A, Gretz N, Somlo S, Witzgall R. The polycystic kidney disease protein PKD2 interacts with Hax-1, a protein associated with the actin cytoskeleton. Proc Natl Acad Sci U S A. 2000;97:4017–4022. doi: 10.1073/pnas.97.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehtonen S, Ora A, Olkkonen VM, Geng L, Zerial M, Somlo S, Lehtonen E. In vivo interaction of the adapter protein CD2-associated protein with the type 2 polycystic kidney disease protein, polycystin-2. J Biol Chem. 2000;275:32888–32893. doi: 10.1074/jbc.M006624200. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Dai Y, Guo L, Liu Y, Hao C, Wu G, Basora N, Michalak M, Chen XZ. Polycystin-2 associates with tropo-myosin-1, an actin microfilament component. J Mol Biol. 2003;325:949–962. doi: 10.1016/s0022-2836(02)01333-5. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Shen PY, Wu G, Chen XZ. Polycystin-2 interacts with troponin I, an angiogenesis inhibitor. Biochemistry. 2003;42:450–457. doi: 10.1021/bi0267792. [DOI] [PubMed] [Google Scholar]
- 49.Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: Role of mDia1 IN PKD2 localization to mitotic spindles. J Biol Chem. 2004;279:29728–29739. doi: 10.1074/jbc.M400544200. [DOI] [PubMed] [Google Scholar]
- 50.Wiederhold ML. Mechanosensory transduction in “sensory” and “motile” cilia. Annu Rev Biophys Bioeng. 1976;5:39–62. doi: 10.1146/annurev.bb.05.060176.000351. [DOI] [PubMed] [Google Scholar]
- 51.Stommel EW, Stephens RE, Alkon DL. Motile statocyst cilia transmit rather than directly transduce mechanical stimuli. J Cell Biol. 1980;87:652–662. doi: 10.1083/jcb.87.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]