Abstract
Objective
Extensive joint hypermobility, lower serum cartilage oligomeric matrix protein (COMP), and early-onset osteoarthritis (OA) are phenotypes of inherited pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED). However, few studies have evaluated the association between articular hypermobility and primary OA. Therefore, we evaluated this association and tested the hypothesis that COMP level is associated with hypermobility in OA and non-OA individuals.
Methods
Two separate cohorts were available for analysis, the extended CARRIAGE family and a subset of the GOGO sib pair cohort. In the CARRIAGE family, we performed hand and knee examinations, hypermobility evaluations (Beighton criteria), and obtained sera for COMP and hyaluronan (HA). COMP and HA, extensive joint radiographic and hypermobility data were also available for the GOGO cohort.
Results
The prevalence of hypermobility was 13% in the CARRIAGE and (5%) in the GOGO cohort. In the CARRIAGE family, hypermobility was associated with a significantly lower prevalence of hand (especially proximal interphalangeal joint) and knee OA, and lower mean serum COMP in both the total cohort and non-hand OA subgroups. These results were further validated in the GOGO subsets without radiographic OA where hypermobility was also associated with a significantly lower mean serum COMP (p<0.01). Serum HA did not differ on the basis of hypermobility in either cohort.
Conclusions
We report an inverse relationship of hypermobility, hand and knee OA, and show that hypermobility is associated with lower serum COMP levels. Genetic variations of the COMP gene may account for some subgroups of benign joint hypermobility.
Keywords: joint hypermobility, osteoarthritis, cartilage oligomeric matrix protein, hyaluronan
Introduction
Osteoarthritis (OA) is a multifactorial complex disorder associated with chronic disability and various risk factors (1), including age, obesity, female gender, muscle weakness, joint malalignment, and genetic predisposition (2). Joint hypermobility due to ligamentous laxity has empirically been regarded to be a risk factor for OA (3), although results of the few studies to date have been conflicting (Table 1). The true prevalence and risk for musculoskeletal disorders associated with hypermobility is unknown.
Table 1.
Summary of previous studies of osteoarthritis and articular hypermobility.
| Study (reference) | Country of origin or ethnic origin | Study design | Number with hypermobility/ Total n (%) | Hypermobility Criteria used | Relationship of joint hypermobility and OA |
|---|---|---|---|---|---|
| Scott et al 1979 (36) | UK | Clinical population; Age-matched OA-control | 9/100(9%) | Beighton score≥4 & left 2nd MCP joint | In general:
|
| Bridges et al 1992 (3) | USA | Clinical observation | 20/130(15%) | Beighton score≥5 | 12 “OA”of 20 hypermobile patients |
| Jonsson et al 1995 (34) | Iceland | Established hand OA patients | 19/100(19%) | Beighton score≥4 |
|
| Jonsson et al 1996 (35) | Iceland | Established thumb base female OA patients | 17/50(34%) | Beighton score≥4 |
|
| Dolan et al 2003 (33) | UK | Postmenopausal community population | 79/716(11%) | Beighton score≥1 |
|
| Kraus et al 2004 (32) | Caucasian (UK & USA) | OA sib-pairs family study | 39/1043(3.7%) | Beighton score≥4 |
|
| Chen et al 2008-CARRIAGE family study | Mixed Africa and Native American (USA) | Extended family-based study | 36/280(12.9%) | Beighton score≥4 |
|
OA = osteoarthritis; “OA” means type of osteoarthritis not reported; COMP = cartilage oligomeric matrix protein CMC1 = carpometacarpal; DIP = distal interphalangeal; PIP = proximal interphalangeal; MCP = metacarpophalangeal
Joint hypermobility, estimated to affect approximately 5% to 25% of the population depending on age, sex, and race (4), is observed as both a lone benign trait and as one manifestation of a variety of severe but rare heritable disorders including Marfan syndrome (1 in 12,000), Ehlers-Danlos syndrome (1 in 5,000), osteogenesis imperfecta (1 in 100,000), pseudoachondroplasia (PSACH, less than 1 in 200,000 in the USA), and some forms of multiple epiphyseal dysplasia (MED, 1 in 10,000) (5, 6). The latter conditions, PSACH and MED, are characterized by prominent joint laxity and variable short stature, short extremities, and early-onset OA, especially in the hips and knees, and are due to mutations in genes coding for cartilage oligomeric matrix protein (COMP) (7). During the ascertainment of the CARRIAGE (CARolinas Region Interaction of Aging Genes and Environment) family, we noticed joint hypermobility in many members. We therefore investigated the association between joint hypermobility and clinical OA phenotypes, and the OA-related serum biomarkers, COMP, and hyaluronan (HA). We then validated our results in a larger family-based study (GOGO).
Methods
Study Populations
The CARRIAGE family study is a prospective family-based longitudinal study of the interactions between aging, genetic susceptibility, and environmental risk pertaining to the development of several age-related chronic diseases including OA, cardiovascular disease, and eye diseases (glaucoma, and macular degeneration). This family came to be studied in the context of health fairs we were requested to conduct at several large family reunions. The extended family described here is of mixed African American and native American ancestry and one of the most extensively pedigreed existing families in the United States comprising nine generations with 3357 pedigreed members, and originating from one founder born in the 1700s (8). Ascertainment of 350 family members was conducted during three family reunions from 2002–2006. Ascertainment included physician-performed examinations for hand OA and joint hypermobility (n=287), and knee OA (n=120). Height and weight were measured, and general medical history ascertained including a query or examination for blue sclerae. The majority of these participants (n=278) also consented to blood sampling. For purposes of these analyses, we excluded 2 participants with known clinical rheumatoid arthritis and subjects younger than 25 years of age (n=5) to avoid potential confounding of the biomarker measures by cartilage growth plate metabolism (9, 10). After these exclusions, a total of 280 participants provided full clinical hand data, a total of 271 had both clinical hand data and biomarker data, and of these, 115 participants also had clinical knee examination data. Written informed consent was obtained from each participant and the study was conducted with the approval of the Duke Institutional Review Board.
The GOGO (Genetics of Generalized Osteoarthritis) cohort is a large sample of Caucasian sibling pairs and nuclear family members ascertained through a collaborative consortium of seven sites in the USA and UK (11). Full biomarker data, Beighton scores, and hand, knee, and hip radiographic OA data were available for 708 individuals from two of the sites (Duke University, Durham, NC and University of North Carolina at Chapel Hill, NC, USA). There were no individuals in either the CARRIAGE family or the GOGO cohort with excessively short stature or radiographic features (in the case of the GOGO cohort) suggestive of PSACH or MED, nor anyone with blue sclerae suggestive of osteogenesis imperfecta.
Survey of joint symptoms in the CARRIAGE family
Information was obtained on self-reported joint symptoms on the basis of the following question: “Which of the following joints have bothered you in the last year?” The surveyed joint-sites included hands, knees, hips, spine (neck, upper, lower back), ankles, shoulders, elbows, wrists, and big toes.
Definitions of clinical hand and knee OA outcomes in the CARRIAGE family
OA outcomes in the CARRIAGE family are listed and described in Table 2 and included clinical hand OA by modified American College of Rheumatology (ACR) criteria (12), clinical hand OA by GOGO criteria (11, 13), and clinical knee OA by ACR criteria (14). A Non-Hand OA subgroup in the CARRIAGE family was defined on the basis of not meeting the clinical modified hand OA ACR criteria or the clinical hand OA GOGO criteria.
Table 2.
Summary of investigations and definitions.
| Outcome | Definition | CARRIAGE Family N evaluated (280) | GOGO cohort N evaluated (708) |
|---|---|---|---|
| Hand OA - modified* American College of Rheumatology (ACR) criteria |
|
√ (47) | |
| Hand OA - GOGO criteria |
|
√ (52) | |
| Non-Hand OA | Lacks Hand OA by modified* ACR criteria | √ (233) | √ (167) |
| Non-Hand OA | Lacks Hand OA by GOGO criteria | √ (228) | |
| Non-DIP OA | No radiographic OA based on Kellgren Lawrence grade <2 bilaterally in all joints of a group | √ (77) | |
| Non-PIP OA | √ (135) | ||
| Non-CMC1 OA | √ (333) | ||
| Non-Knee OA | No radiographic OA based on Kellgren Lawrence grade <2 in joint group bilaterally | √ (374) | |
| Non-Hip OA | √ (399) | ||
| Non-Knee and Hip OA | √ (251) | ||
| Knee OA - clinical ACR criteria | knee pain and a minimum of 3 of 6 other features:
|
√ (115) | |
| Hypermobility evaluation | Beighton criteria treated as a continuous variable (0–9) or as a dichotomous variable (non-hypermobility <4; hypermobility ≥4) | √ (280) | √ (708) |
| Serum COMP and HA | √ (271) | √ (708) |
We did not utilize the ACR hand symptoms and deformity criteria as part of our definition of hand OA, so we refer to these as modified ACR criteria; COMP=cartilage oligomeric matrix protein; HA=hyaluronan
CARRIAGE family N of 280 is the final number evaluated after excluding 5 under 25 years old and 2 with rheumatoid arthritis
Definitions of non-OA subgroups in the GOGO cohort
The non-OA subgroups were defined using the available clinical data for the hand as well as on the basis of Kellgren Lawrence (15) grade <2 radiographic OA of the hands, knees or hips (Table 2).
Beighton criteria for hypermobility
Hypermobility was determined according to the criteria established by Beighton et al in 1973 (16) that have high inter- and intra-rater reliability (17). Patients were graded on a 0–9 point scale based on their ability to achieve the following: (a) passive dorsiflexion of the fifth finger ≥90°; (b) passive apposition of the thumb to the forearm; (c) hyperextension of the elbow ≥10°; (d) hyperextension of the knee ≥10°; and e) ability to rest the palms flat on the floor with straight knees. Beighton scores were analyzed as continuous traits and as binary traits; for binary trait analyses, patients were considered as exhibiting hypermobility if they scored 4 or more out of 9 points (16).
Biomarker analyses
Serum was isolated, aliquoted and stored within 4 hours of blood collection at −80°C until biomarker analyses were performed. Duplicate serum biomarker assays were performed for each sample, and analyses were repeated as necessary for samples with a >15% coefficient of variation (CV). COMP was measured by an in-house sandwich ELISA method as previously described, using monoclonal antibodies 17C10 (epitope in the EGF-like domain) and 16F12 (epitope in the NH2-terminal domain) against human COMP (18). The minimum detection limit is 120 ng/ml. Intra-assay and inter-assay CVs were < 5.8% and 8.7%, respectively. HA was measured by an enzyme-linked binding protein assay (Corgenix Inc. Westminster, Colorado, USA). The assay uses enzyme-conjugated hyaluronic acid binding protein (HABP) from bovine cartilage to specifically capture HA from human serum. The minimum detection limit is established at 10 ng/ml. Intra-assay and inter-assay CVs were <4.7% and 7.0%, respectively.
Statistical analyses
The Chi-square test was used to compare the prevalence of joint symptoms and prevalence of OA by hypermobility status. The Mann-Whitney U test was used to assess the mean numbers of OA joints according to hypermobility status. The serum biomarker concentrations were logarithmically transformed to meet requirements of normality for parametric statistical analyses. Two-sample t test was used to evaluate for differences in mean biomarker concentrations between the hypermobility and non-hypermobility groups. One-Way ANOVA with the Tukey-Kramer multiple comparison test was used to evaluate the relationship of Beighton scores with concentrations of biomarkers. Generalized Estimating Equations (GEE) were used to control for the dependency due to familial clustering of CARRIAGE family members and GOGO nuclear families (SAS version 9.1, SAS Institute, Cary, NC). For the CARRIAGE family, we classified individuals into eight clusters based on their relationship to eight members descended from the founder of the CARRIAGE family. For the GOGO cohort, individuals were clustered by family. Age adjustment was performed for all analyses and additional adjustment for BMI was performed for all analyses involving prevalence of clinical or radiographic OA. Adjustment for hand OA status was included in the logistic regression analysis of hypermobility and COMP in the CARRIAGE family. Analyses of hypermobility and COMP were performed in the full GOGO sample of patients from two sites (Duke and UNC) and in subgroups without radiographic OA. Significant results were declared based on a two-sided p-value of <0.05.
Results
Hypermobility and joint symptoms in the CARRIAGE family
Joint hypermobility (Beighton score≥4) was present in 36 (12.9%) of the 280 examined CARRIAGE family participants. The hypermobility group did not differ significantly by age or BMI from the non-hypermobility group (Table 3). The age distribution of the 115 participants in the knee-examined subgroup was similar to the group as a whole with a slightly younger mean age for the hypermobility group, which was not statistically different from the non-hypermobility group (53.5±14.2 years for hypermobile individuals, 58.3±14.7 years for non-hypermobile individuals, p=0.15). The hypermobility group had a female predominance compared with the non-hypermobile group. The prevalence of hand and knee joint symptoms was lower in the hypermobile group, but the prevalence of symptoms in the other joints systems was similar (Table 3).
Table 3.
Demographic characteristics of CARRIAGE family study participants (n=280).
| Hypermobility (N=36) (Beighton score≥4) | Non-hypermobility (N=244) (Beighton score<4) | P Value | |
|---|---|---|---|
| Age, years | 53.27±14.38 | 56.36±14.64 | 0.24 |
| BMI (kg/m2) | 29.67±6.09 | 31.23±6.67 | 0.19 |
| Female % | 83.3% (n=30) | 66.4% (n=162) | |
| Joint self-reported symptoms | N (% of group of 25 total) | N (% of group of 224 total) | |
| Hand symptoms | 1 (4.0%) | 47 (21.0%) | 0.02 |
| Knee symptoms | 5 (20.0%) | 92 (41.1%) | 0.03 |
| Hip symptoms | 5 (20.0%) | 43 (19.2%) | 0.92 |
| Spine symptoms | 12 (48.0%) | 101 (45.1%) | 0.78 |
| Ankle symptoms | 4 (16.0%) | 38 (17.0%) | 0.90 |
| Shoulder symptoms | 7 (28.0%) | 68 (30.4%) | 0.80 |
| Elbow symptoms | 2 (8.0%) | 23 (10.3%) | 0.71 |
| Wrist symptoms | 3 (12.0%) | 30 (13.4%) | 0.84 |
| Big toes | 2 (8%) | 16 (7.1%) | 0.87 |
For evaluation of age and BMI between hypermobility and non-hypermobility groups, P value was generated by unpaired t test
For evaluation of symptoms between hypermobility and non-hypermobility groups, P value was generated by Likelihood ratio Chi-square for joint self-reported symptoms
Hypermobility and Osteoarthritis in the CARRIAGE family
The hypermobility group in the CARRIAGE family had a consistently lower prevalence of hand OA by the modified ACR and GOGO criteria, and a lower prevalence of knee OA by ACR criteria (Table 4). By logistic regression, hypermobility was associated with a decreased likelihood ratio of hand OA in the CARRIAGE family (p=0.02 by modified ACR criteria; p=0.008 by GOGO criteria), which remained significant after age and BMI adjustment (for modified ACR criteria: p=0.024 BMI-adjusted, p=0.043 age-adjusted, and p=0.047 age and BMI adjusted; for GOGO criteria: p=0.009 BMI-adjusted, p=0.018 age-adjusted, and p=0.02 age and BMI adjusted) (Table 4). The hypermobility group demonstrated significantly fewer OA clinically affected PIP joints (p<0.005), and a non-significant but decreased prevalence of OA of DIP and CMC1 joints. Taking all three hand joint groups into consideration, hypermobility was significantly inversely associated with numbers of OA affected joints (Table 4). Hypermobility was also associated with a decreased likelihood ratio of knee OA (p=0.02; p=0.035 BMI-adjusted, p=0.058 age-adjusted, and p=0.068 age and BMI adjusted).
Table 4.
Clinical OA status by hypermobility status in the CARRIAGE family.
| OA Definitions | Hypermobility Group N (%) by OA Status | Non-Hypermobility Group N (%) by OA Status | P value | P value (adjusted for age and BMI) |
|---|---|---|---|---|
| Hand: GOGO Criteria (n=280 evaluated) | 1 (2.8%) | 42 (17.2%) | <0.01 | 0.02 |
| GOGO criterion 1 | 1 (2.8%) | 57 (23.4%) | <0.001 | <0.01 |
| GOGO criterion 2 | 5 (13.9%) | 60 (24.6%) | 0.13 | 0.3 |
| GOGO criterion 3 | 4 (11.1%) | 71 (29.1%) | 0.01 | 0.02 |
| Hand: Modified ACR Criteria (n=280 evaluated) | 1 (2.8%) | 36 (14.8%) | 0.02 | 0.05 |
| ACR criterion 1 | 2 (5.6%) | 68 (27.9%) | <0.005 | <0.005 |
| ACR criterion 2 | 2 (5.6%) | 39 (16.0%) | 0.06 | 0.14 |
| Knee: ACR criteria (n=115 evaluated) | 3 (12.0%) | 31 (34.4%) | 0.02 | 0.07 |
| Joint Site Involvement (n=280 evaluated) | mean ± SD # OA Joints | mean ± SD # OA Joints | ||
| DIPs | 0.22 ± 0.63 | 0.60 ± 1.35 | 0.13 | 0.18 |
| PIPs | 0.14 ± 0.49 | 1.01 ± 1.97 | <0.005 | 0.01 |
| CMC1 | 0.028 ± 0.17 | 0.14 ± 0.48 | 0.2 | 0.24 |
| DIPs+PIPs+CMC1 | 0.39 ± 0.99 | 1.75 ± 3.12 | <0.005 | 0.02 |
Hypermobility Group = Beighton score ≥4 (total n=36 in the hand examined group and n=25 in the knee examined group); Non-Hypermobility Group = Beighton score <4 (total n=244 in the hand examined group and n=90 in the knee examined group) GOGO and (modified) ACR criterion given in Table 2
P value by Likelihood ratio Chi-square test for OA definition; p value by Mann-Whitney test for joint sites; DIP = distal interphalangeal; PIP = proximal interphalangeal; CMC1 = carpometacarpal
Hypermobility and biomarkers in the CARRIAGE family
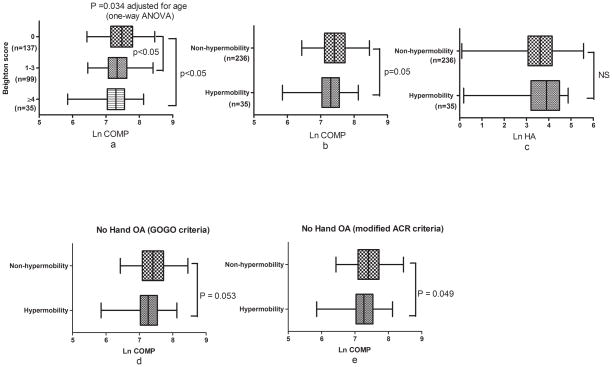
Mean (SD) ln serum COMP decreased significantly with increasing hypermobility by Beighton score: 7.48±0.42 for Beighton score 0; 7.37±0.42 for Beighton score 1–3; 7.29±0.43 for Beighton score≥4 (p=0.034 adjusted for age, Figure 1a). Mean (SD) ln serum COMP was significantly lower in the hypermobility group (7.29±0.43) vs. the non-hypermobility group (7.43±0.42) (p=0.05) (Figure 1b); however, mean ln serum HA did not differ significantly between the two groups (Figure 1c). When adjusted for age and hand OA status (by clinical GOGO or modified hand ACR criteria), mean ln serum COMP was marginally significantly lower in the hypermobility group compared with the non-hypermobility group (by GOGO criteria, p=0.069; by modified hand ACR criteria, p=0.068). To more clearly define the association of hypermobility and serum COMP, independent of hand OA status, we analyzed the relationship in the Non-Hand OA subgroup of the CARRIAGE family. Mean ln serum COMP was also significantly lower in the Non-Hand OA subgroup with hypermobility compared with the members without hypermobility. When hand OA was excluded on the basis of clinical GOGO criteria, mean ln serum COMP levels were 7.26±0.43 in the hypermobility group vs 7.42±0.43 in the non-hypermobility group (p=0.053 adjusted for age); when hand OA was excluded on the basis of the modified ACR criteria, mean ln serum COMP was 7.27±0.42 in the hypermobility group vs 7.42±0.41 in the non-hypermobility group (p=0.049 adjusted for age) (Figure 1d and 1e). When familial clusters were taken into account by the GEE analysis, the inverse association between, hypermobility (using Beighton score as a continuous covariate) and serum COMP level remained highly significant (p=0.0035 age-adjusted). When hypermobility was analyzed as a binary trait (<4 or ≥4), the association was still apparent but less robust (p=0.082 age-adjusted).
Figure 1. The relationship between biomarkers and joint hypermobility in the total cohort (a–c) and Non-Hand OA subgroups (d–e) of the CARRIAGE family.
a) Serum ln COMP (after adjustment for age) and Beighton score separated into 3 groups, no hypermobility (score 0), moderate hypermobility (score 1–3) and high hypermobility (score ≥4); b) Serum ln COMP by hypermobility status; c) Serum ln HA by hypermobility status. Analysis of participants without hand OA was based on d) clinical GOGO criteria (n=228: n=34 with and n=194 without joint hypermobility); or e) modified ACR criteria (n=233: n=34 with and n=199 without joint hypermobility). P value calculated by one-way ANOVA with Tukey multiple comparison (a), or two-sample t test (b–e). Box plots depict mean, 25th and 75th percentiles, and minimum and maximum. Hypermobility status defined by Beighton score <4 (Non-hypermobilty) or ≥4 (Hypermobility). COMP = cartilage oligomeric matrix protein, HA = hyaluronan, NS = non-significant.
Hypermobility and biomarkers in the GOGO cohort
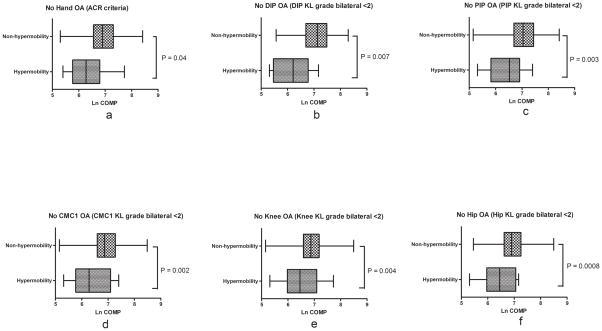
Joint hypermobility was present in 36 (5%) of the 708 GOGO study participants. In agreement with the CARRIAGE family results, mean (±SD) ln serum COMP in the GOGO cohort also decreased significantly with increasing hypermobility by Beighton score: 6.94±0.53 for Beighton score 0; 6.72± 0.53 for Beighton score 1–3; 6.63± 0.54 for Beighton score≥4 (p< 0.0001, adjusted for age). Mean ln serum COMP was consistently lower in the hypermobility group compared with the non-hypermobility group (6.60±0.61 vs 6.91±0.53, p=0.0009; 6.64±0.54 vs 6.90±0.53, p=0.004 adjusted for age). In contrast, before and after controlling for age, mean ln serum HA was similar in the two groups (3.44±0.88 vs 3.63±0.85, p=0.2; 3.63±0.81 vs 3.62±0.80, p=0.9 adjusted for age). To evaluate the possibility that OA itself might cause an apparent diminution in manifestations of hypermobility due to loss of joint range of motion, we repeated the analyses in the individuals without radiographic OA (rOA). For each joint site, mean ln serum COMP was significantly lower in association with joint hypermobility (Figure 2). Results for the 251 individuals lacking both hip and knee rOA were similar, with lower serum COMP in the hypermobile individuals (p=0.002 adjusted for age). In contrast, mean ln serum HA did not differ on the basis of hypermobility status in the non-rOA groups (data not shown). Similar to the CARRIAGE family dataset, the GEE analysis for GOGO data revealed a highly significant inverse association between hypermobility (using Beighton score as a continuous covariate) and serum COMP level (p<0.0001 age-adjusted). When hypermobility was analyzed as a binary trait (<4 or ≥4), the inverse association with serum COMP level was also apparent (p=0.01 age-adjusted).
Figure 2. Mean ln COMP by hypermobility status in the Non-OA subgroups of the GOGO cohort.
Analysis of participants without OA of the following specific joint groups: a) hand OA based on ACR criteria (n=167); b) rOA of DIP joints (n=77); c) rOA of PIP joints (n=135); d) rOA of CMC1 joints (n=333); e) rOA of knees (n=374); or f) rOA of hips (n=399). P value (adjusted for age) calculated by two-sample t test with equal variance. Box plots depict mean, 25th and 75th percentiles, and minimum and maximum. Hypermobility status defined by Beighton score: <4 (Non-hypermobilty) and ≥4 (Hypermobility). DIP = distal interphalangeal; PIP = proximal interphalangeal; CMC1 = first carpometacarpal; KL grade = Kellgren-Lawrence grade radiographic OA (rOA); COMP = cartilage oligomeric matrix protein
Discussion
We report evidence for a relationship between serum COMP level and general joint hypermobility. COMP is a 524 KDa homopentameric non-collagenous glycoprotein derived from cartilage and also found in ligaments and tendons (19). Recent in vitro studies have shown that COMP can interact with collagens I, II, IX, fibronectin, and all matrilins (20–23), and that COMP can bind to collagens I, II, and IX with high affinity (24). Interestingly, some autosomal dominant osteochondrodysplasias (PSACH and some MED) are caused by mutations in COMP that interfere with normal extracellular matrix assembly, which is thought to contribute to the development of the patient phenotypes (25, 26). Pronounced hypermobility and low serum COMP are features of these osteochondrodysplasias (27, 28). Low serum COMP may result from retention of mutant COMP within the rough endoplasmic reticulum of chondrocytes and tendon cells (29); but not all the COMP-associated chondrodysplasias appear to be storage diseases (25, 26), so other mechanisms yet to be defined, such as altered COMP protein or RNA synthesis or stability, may account for low serum COMP in these chondrodysplasias. By analogy, genetic variation within the COMP gene might influence both serum COMP levels and ligamentous structure leading to articular hypermobility phenotypes in the CARRIAGE family and GOGO cohort. Of note, Jonsson has recently reported linkage of joint hypermobility (dorsiflexion≥90° of either fifth finger in an Icelandic cohort of 331 subjects) to chromosome 19P 13.3 (LOD score of 3.8), which is within 16Mb of the COMP gene (30). Also, Hakim et al, has reported autosomal dominant inheritance of benign joint hypermobility affecting female twins (31).
Our study also demonstrated that generalized articular hypermobility is inversely associated with clinical hand (PIP) OA and possibly also knee OA. This confirms and extends our previous results in the GOGO cohort showing that hypermobility was associated with a lower prevalence of PIP OA and possibly OA in MCP joints. A strength of this study is that all family members were invited to participate and included, independent of hypermobility status or signs or symptoms of musculoskeletal problems. Although it is possible that the healthier family members may have been more likely to attend the family reunions, we avoided the common selection bias of most other studies related to hypermobility that relied on clinic based populations with a high prevalence of joint symptoms. These family data may therefore be more representative of the general population. Our study showed that after accounting for age, PIP joint and knee OA prevalence was lower in association with joint hypermobility with a similar trend observed for DIP and CMC1 joint OA. In the previously reported study of hypermobility in the GOGO cohort, no conclusions could be drawn regarding hypermobility and DIP joints because study inclusion required OA in at least one DIP in the proband and one sibling (11, 32). No such inclusion criteria were used in the CARRIAGE family study and we saw a trend of fewer OA affected DIP joints in association with hypermobility. It is possible that a larger sample size or radiographic phenotyping might be necessary for further validation of the inverse relationship of hypermobility and OA of DIP, MCP and CMC1 joints.
Our results are also in agreement with a recent community-based study of post-menopausal females showing a reduced risk of radiographic knee OA with joint hypermobility (33). Preliminary data from another study, a cohort of Icelandic subjects (n=1839) with a 31% prevalence of any hypermobility, has also shown a reduction in clinical knee OA in association with hypermobility (Chi square p = 0.04, Dr. Helgi Jonsson personal communication). Moreover, Jonsson et al reported, in two separate studies, less hand interphalangeal joint involvement in association with hypermobility (34, 35). In contrast, some studies have reported a higher prevalence of OA in association with hypermobility. Decades ago, two studies reported a higher prevalence of OA in individuals with joint hypermobility from groups of highly selected patients referred for clinical evaluation (3, 36); however, in one of these studies, different effects were observed for the knee (increased OA) compared with hand (decreased OA) in association with hypermobility (36). Jonsson et al also reported more CMC1 joint OA in association with hypermobility (34). Thus, the type and strength of the effect of hypermobility on OA susceptibility may differ by joint group,
Although several previous studies have emphasized the association of the benign joint hypermobility syndrome with musculoskeletal symptoms (37–39), our study showed that CARRIAGE family members with joint hypermobility had a lower prevalence of self-reported joint symptoms in their hands and knees than participants without hypermobility. Moreover, it has been recognized through studies of children and adolescents, that not all individuals deemed hypermobile have a history of musculoskeletal symptoms and disorders or go on to develop them in their life (40). In agreement with our study, Larsson et al also found a lower prevalence of hand symptoms in instrumentalists with lax fingers performing repetitive fine hand movements compared with their peers with less flexibility (41). The effect of hypermobility on symptoms may be specific to particular joint groups since hypermobility appeared to increase low back symptoms in timpani players (41). Thus, again joint group is one possible factor explaining some of the differences in hypermobility related symptoms and OA risk.
There are many other possible factors that might explain the reports of conflicting associations of hypermobility, osteoarthritis and musculoskeletal symptoms. Murray has suggested that hypermobility alone may not account for musculoskeletal syndromes but that other cofactors, such as obesity, sedentary lifestyle, or joint overuse may be important moderators of the symptoms and outcomes of hypermobility (40). Murray has also suggested that a high risk subgroup of children may exist which would be under recognized when the typical Beighton score thresholds used for adults are employed for defining hypermobility. Mechanical joint forces may vary due to ligamentous laxity on a joint specific basis. It is possible that individuals with hypermobility may moderate their activity due to pain or joint instability that may reduce the risk of OA. Finally, hypermobile individuals represent both a phenotypically and genetotypically heterogenous group. In addition to PSACH and MED (COMP mutations), variable degrees of hypermobility are associated with other genetic syndromes including among others, Marfan’s syndrome (fibrillin mutations), and Ehlers Danlos syndromes (mutations of col1A1, col1A2, col3A1, col5A1, col5A2, ADAMTS2, and tenascin XB) (42). The underlying genetic etiology rather than hypermobility, may account for the risk of symptoms and OA, but under diagnosis of these conditions may in part be responsible for the general but erroneous attribution of all hypermobility with risk of OA. These issues deserve to be further explored in future studies.
Although we obtained the same results from two separate cohorts, some shortcomings remain. We are limited due to the cross-sectional nature of our study. Therefore, we cannot completely rule out the possibility that OA masks the manifestations of hypermobility, although OA seldom affects the wrists or 5th MCP joints that contribute to the Beighton score. However, in our study, we believe that the inverse association of hypermobility and OA was not due to waning joint laxity with age and OA because the negative association persisted after adjustment for age. We considered the possibility that lower COMP in the hypermobility group might be a manifestation of younger age and fewer OA affected joints since serum COMP is positively associated with severity of radiographic OA (18, 43). Therefore, we also analyzed the non-OA participants to evaluate the association of hypermobility and the biomarkers, COMP and HA. Serum COMP level was consistently lower in association with hypermobility in both cohorts of non-OA individuals. In contrast, HA levels, a marker of joint tissue turnover of OA (44), were unchanged in association with hypermobility. We thus show that the association of hypermobility with lower prevalence of OA and lower serum COMP, was neither a result of age nor a result of OA masking hypermobility.
To address the possibility that COMP fragmentation was a cause of lower serum COMP with hypermobility, we performed additional sandwich ELISAs, and Western blots (data not shown) on the sera of a test subset of 9 individuals with and without hypermobility, low and high COMP, and OA, using three different monoclonal antibodies to the COMP amino-terminus, middle and carboxy-terminus as described previously (45) (reducing gels for antibodies of 16F12 and 12C4; nonreducing gel for antibody of 17C10). Specifically, we evaluated these sera for evidence of COMP fragments between 50–90 kDa as described previously using polyclonal antibodies against human COMP (46). There were no 50–90 kDa COMP fragments in the sera of any of the test individuals, but COMP fragments of this size were readily discerned in the positive control (EDTA cartilage extract). ELISA assys with combinations of these three anti-COMP antibodies also supported the presence of full-length COMP in the sera (data not shown). Thus, the lower serum COMP level associated with hypermobility was due to absolute lower level of full-length COMP in the sera rather than targeted degradation.
In summary, we report the evidence for a relationship between serum COMP level and general joint hypermobility. In addition, in this extended family of mixed African American and Native American heritage, we have replicated the results from the GOGO Caucasian sib pair cohort, in which we demonstrated that general articular hypermobility was inversely associated with OA of PIP joints. Our study also suggests the possibility of an inverse association of joint hypermobility and knee OA although this result needs to be validated in a larger cohort. We hypothesize that hypermobility may decrease biomechanical strain on joints, which in turn would be protective for hand and knee OA. Hypermobility is a heritable trait associated with lower serum COMP and COMP mutations in some osteochondrodystrophies. The results of our study suggest that genetic variation within the COMP gene may be a candidate to account for benign joint hypermobility, a condition whose etiology has hitherto been unknown. This study also suggests that the extent of hypermobility might serve as a quantitative trait for identifying protective alleles for OA.
Acknowledgments
Funding: NIH/NIA Claude D. Pepper OAIC 2P60 AG11268, the Mary Duke Biddle Foundation, the Trent Foundation, GlaxoSmithKline, and a student grant from the Taiwanese government
We would like to extend our thanks to Dr. William Kraus for funding assistance, to Dr. Vladimir Vilim for the kind gift of the 16F12/17C10 monoclonal antibodies, to Norine Hall, Carol Haynes, and Sarah Nelson for database management, and everyone who made the family reunions possible.
References
- 1.Jordan JM, Kraus VB, Hochberg MC. Genetics of osteoarthritis. Curr Rheumatol Rep. 2004;6(1):7–13. doi: 10.1007/s11926-004-0078-0. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 3.Bridges AJ, Smith E, Reid J. Joint hypermobility in adults referred to rheumatology clinics. Ann Rheum Dis. 1992;51(6):793–6. doi: 10.1136/ard.51.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grahame R. Joint hypermobility and genetic collagen disorders: are they related? Arch Dis Child. 1999;80(2):188–91. doi: 10.1136/adc.80.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bravo JF, Wolff C. Clinical study of hereditary disorders of connective tissues in a Chilean population: joint hypermobility syndrome and vascular Ehlers-Danlos syndrome. Arthritis Rheum. 2006;54(2):515–23. doi: 10.1002/art.21557. [DOI] [PubMed] [Google Scholar]
- 6.Briggs MD, Chapman KL. Pseudoachondroplasia and multiple epiphyseal dysplasia: mutation review, molecular interactions, and genotype to phenotype correlations. Hum Mutat. 2002;19(5):465–78. doi: 10.1002/humu.10066. [DOI] [PubMed] [Google Scholar]
- 7.Briggs MD, Hoffman SM, King LM, Olsen AS, Mohrenweiser H, Leroy JG, et al. Pseudoachondroplasia and multiple epiphyseal dysplasia due to mutations in the cartilage oligomeric matrix protein gene. Nat Genet. 1995;10(3):330–6. doi: 10.1038/ng0795-330. [DOI] [PubMed] [Google Scholar]
- 8.Chen HC, Shah S, Stabler TV, Li YJ, Kraus VB. Biomarkers associated with clinical phenotypes of hand osteoarthritis in a large multigenerational family: the CARRIAGE family study. Osteoarthritis Cartilage. 2008 Feb 19; doi: 10.1016/j.joca.2007.12.010. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 9.Urakami T, Manki A, Inoue T, Oda M, Tanaka H, Morishima T. Clinical significance of decreased serum concentration of cartilage oligomeric matrix protein in systemic juvenile idiopathic arthritis. J Rheumatol. 2006;33(5):996–1000. [PubMed] [Google Scholar]
- 10.Thonar E. Serum keratan sulfate concentration as a measure of the catabolism of cartilage proteoglycans. In: Brandt K, editor. Cartilage Changes in Osteoarthritis. Indianapolis: Indiana University School of Medicine; 1990. pp. 105–8. [PubMed] [Google Scholar]
- 11.Kraus VB, Jordan JM, Doherty M, Wilson AG, Moskowitz R, Hochberg M, et al. The Genetics of Generalized Osteoarthritis (GOGO) study: study design and evaluation of osteoarthritis phenotypes. Osteoarthritis Cartilage. 2007;15(2):120–7. doi: 10.1016/j.joca.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Altman R, Alarcon G, Appelrouth D, Bloch D, Borenstein D, Brandt K, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hand. Arthritis Rheum. 1990;33(11):1601–10. doi: 10.1002/art.1780331101. [DOI] [PubMed] [Google Scholar]
- 13.Kraus V, Varju G, Li Y, Martin E, Jordan J, Renner J, et al. Assessment of the usefulness of clinical hand examination for determination of OA affected status for genetic studies. Arthritis Rheum. 2001;44(9 Suppl):528. [Google Scholar]
- 14.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 15.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beighton P, Solomon L, Soskolne CL. Articular mobility in an African population. Ann Rheum Dis. 1973;32(5):413–8. doi: 10.1136/ard.32.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boyle KL, Witt P, Riegger-Krugh C. Intrarater and Interrater Reliability of the Beighton and Horan Joint Mobility Index. J Athl Train. 2003;38(4):281–285. [PMC free article] [PubMed] [Google Scholar]
- 18.Vilim V, Olejarova M, Machacek S, Gatterova J, Kraus VB, Pavelka K. Serum levels of cartilage oligomeric matrix protein (COMP) correlate with radiographic progression of knee osteoarthritis. Osteoarthritis Cartilage. 2002;10(9):707–13. doi: 10.1053/joca.2002.0819. [DOI] [PubMed] [Google Scholar]
- 19.Muller G, Michel A, Altenburg E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect Tissue Res. 1998;39(4):233–44. doi: 10.3109/03008209809021499. [DOI] [PubMed] [Google Scholar]
- 20.Mann HH, Ozbek S, Engel J, Paulsson M, Wagener R. Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J Biol Chem. 2004;279(24):25294–8. doi: 10.1074/jbc.M403778200. [DOI] [PubMed] [Google Scholar]
- 21.Di Cesare PE, Chen FS, Moergelin M, Carlson CS, Leslie MP, Perris R, et al. Matrix-matrix interaction of cartilage oligomeric matrix protein and fibronectin. Matrix Biol. 2002;21(5):461–70. doi: 10.1016/s0945-053x(02)00015-x. [DOI] [PubMed] [Google Scholar]
- 22.Holden P, Meadows RS, Chapman KL, Grant ME, Kadler KE, Briggs MD. Cartilage oligomeric matrix protein interacts with type IX collagen, and disruptions to these interactions identify a pathogenetic mechanism in a bone dysplasia family. J Biol Chem. 2001;276(8):6046–55. doi: 10.1074/jbc.M009507200. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273(32):20397–403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 24.Thur J, Rosenberg K, Nitsche DP, Pihlajamaa T, Ala-Kokko L, Heinegard D, et al. Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J Biol Chem. 2001;276(9):6083–92. doi: 10.1074/jbc.M009512200. [DOI] [PubMed] [Google Scholar]
- 25.Schmitz M, Becker A, Schmitz A, Weirich C, Paulsson M, Zaucke F, et al. Disruption of extracellular matrix structure may cause pseudoachondroplasia phenotypes in the absence of impaired cartilage oligomeric matrix protein secretion. J Biol Chem. 2006;281(43):32587–95. doi: 10.1074/jbc.M601976200. [DOI] [PubMed] [Google Scholar]
- 26.Chen TL, Stevens JW, Cole WG, Hecht JT, Vertel BM. Cell-type specific trafficking of expressed mutant COMP in a cell culture model for PSACH. Matrix Biol. 2004;23(7):433–44. doi: 10.1016/j.matbio.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 27.Mabuchi A, Momohara S, Ohashi H, Takatori Y, Haga N, Nishimura G, et al. Circulating COMP is decreased in pseudoachondroplasia and multiple epiphyseal dysplasia patients carrying COMP mutations. Am J Med Genet A. 2004;129(1):35–8. doi: 10.1002/ajmg.a.30164. [DOI] [PubMed] [Google Scholar]
- 28.Tufan AC, Satiroglu-Tufan NL, Jackson GC, Semerci CN, Solak S, Yagci B. Serum or plasma cartilage oligomeric matrix protein concentration as a diagnostic marker in pseudoachondroplasia: differential diagnosis of a family. Eur J Hum Genet. 2007;15(10):1023–8. doi: 10.1038/sj.ejhg.5201882. [DOI] [PubMed] [Google Scholar]
- 29.Weirich C, Keene DR, Kirsch K, Heil M, Neumann E, Dinser R. Expression of PSACH-associated mutant COMP in tendon fibroblasts leads to increased apoptotic cell death irrespective of the secretory characteristics of mutant COMP. Matrix Biol. 2007;26(4):314–23. doi: 10.1016/j.matbio.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson H, Ingvarsson T, Hauksson V, Petursson H, Kristjansson K, Stefansson K. Linkage analysis for hand hypermobility suggests a susceptibility gene on chromosome 19P. Osteo Cartilage. 2007:C162. [Google Scholar]
- 31.Hakim AJ, Cherkas LF, Grahame R, Spector TD, MacGregor AJ. The genetic epidemiology of joint hypermobility: a population study of female twins. Arthritis Rheum. 2004;50(8):2640–4. doi: 10.1002/art.20376. [DOI] [PubMed] [Google Scholar]
- 32.Kraus VB, Li YJ, Martin ER, Jordan JM, Renner JB, Doherty M, et al. Articular hypermobility is a protective factor for hand osteoarthritis. Arthritis Rheum. 2004;50(7):2178–83. doi: 10.1002/art.20354. [DOI] [PubMed] [Google Scholar]
- 33.Dolan AL, Hart DJ, Doyle DV, Grahame R, Spector TD. The relationship of joint hypermobility, bone mineral density, and osteoarthritis in the general population: the Chingford Study. J Rheumatol. 2003;30(4):799–803. [PubMed] [Google Scholar]
- 34.Jonsson H, Valtysdottir ST. Hypermobility features in patients with hand osteoarthritis. Osteoarthritis Cartilage. 1995;3(1):1–5. doi: 10.1016/s1063-4584(05)80032-9. [DOI] [PubMed] [Google Scholar]
- 35.Jonsson H, Valtysdottir ST, Kjartansson O, Brekkan A. Hypermobility associated with osteoarthritis of the thumb base: a clinical and radiological subset of hand osteoarthritis. Ann Rheum Dis. 1996;55(8):540–3. doi: 10.1136/ard.55.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott D, Bird H, Wright V. Joint laxity leading to osteoarthrosis. Rheumatol Rehabil. 1979;18(3):167–9. doi: 10.1093/rheumatology/18.3.167. [DOI] [PubMed] [Google Scholar]
- 37.Adib N, Davies K, Grahame R, Woo P, Murray KJ. Joint hypermobility syndrome in childhood. A not so benign multisystem disorder? Rheumatology (Oxford) 2005;44(6):744–50. doi: 10.1093/rheumatology/keh557. [DOI] [PubMed] [Google Scholar]
- 38.Remvig L, Jensen DV, Ward RC. Epidemiology of general joint hypermobility and basis for the proposed criteria for benign joint hypermobility syndrome: review of the literature. J Rheumatol. 2007;34(4):804–9. [PubMed] [Google Scholar]
- 39.Ferrell WR, Tennant N, Baxendale RH, Kusel M, Sturrock RD. Musculoskeletal reflex function in the joint hypermobility syndrome. Arthritis Rheum. 2007;57(7):1329–33. doi: 10.1002/art.22992. [DOI] [PubMed] [Google Scholar]
- 40.Murray KJ. Hypermobility disorders in children and adolescents. Best Pract Res Clin Rheumatol. 2006;20(2):329–51. doi: 10.1016/j.berh.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Larsson LG, Baum J, Mudholkar GS, Kollia GD. Benefits and disadvantages of joint hypermobility among musicians. N Engl J Med. 1993;329(15):1079–82. doi: 10.1056/NEJM199310073291504. [DOI] [PubMed] [Google Scholar]
- 42.Wikipedia contributors. Ehlers-Danlos syndrome. [accessed 12 June 2008];Wikipedia, The Free Encyclopedia. 2008 Resource number 216287626. [Google Scholar]
- 43.Kelman A, Lui L, Yao W, Krumme A, Nevitt M, Lane NE. Association of higher levels of serum cartilage oligomeric matrix protein and N-telopeptide crosslinks with the development of radiographic hip osteoarthritis in elderly women. Arthritis Rheum. 2006;54(1):236–43. doi: 10.1002/art.21527. [DOI] [PubMed] [Google Scholar]
- 44.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52(1):105–11. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 45.Vilim V, Lenz ME, Vytasek R, Masuda K, Pavelka K, Kuettner KE, et al. Characterization of monoclonal antibodies recognizing different fragments of cartilage oligomeric matrix protein in human body fluids. Arch Biochem Biophys. 1997;341(1):8–16. doi: 10.1006/abbi.1997.9941. [DOI] [PubMed] [Google Scholar]
- 46.Neidhart M, Hauser N, Paulsson M, DiCesare PE, Michel BA, Hauselmann HJ. Small fragments of cartilage oligomeric matrix protein in synovial fluid and serum as markers for cartilage degradation. Br J Rheumatol. 1997;36(11):1151–60. doi: 10.1093/rheumatology/36.11.1151. [DOI] [PubMed] [Google Scholar]