Summary
Lenalidomide has demonstrated impressive antileukaemic effects in patients with chronic lymphocytic leukaemia (CLL). The mechanism(s) by which it mediates these effects remain unclear. Clinically, CLL patients treated with lenalidomide demonstrate an acute inflammatory reaction, the tumour flare reaction that is suggestive of an immune activation phenomenon. Samples from CLL patients treated with lenalidomide were used to evaluate its effect on the tumour cell and components of its microenvironment (immune cellular and cytokine). Lenalidomide was unable to directly induce apoptosis in CLL cells in vitro, however it modulated costimulatory (CD80, CD83, CD86) surface molecules on CLL cells in vitro and in vivo. Concurrently, we demonstrated that NK cell proliferation was induced by lenalidomide treatment in patients and correlated with clinical response. Cytokine analysis showed increase in levels of TNF-α post-lenalidomide treatment, consistent with acute inflammatory reaction. Furthermore, the basal cytokine profile (high IL-8, MIG, IP-10 and IL-4 levels and low IL-5, MIP1a, MIP1b, IL12/p70) was predictive of clinical response to lenalidomide. Collectively, our correlative studies provide further evidence that the antileukaemic effect of lenalidomide in CLL is mediated not only through modulation of the leukaemic clone but also through elements of the tumour microenvironment.
Keywords: lenalidomide, chronic lymphocytic leukaemia, immune cells, microenvironment, immune activation
B-chronic lymphocytic leukaemia (B-CLL) is an incurable malignant lymphoproliferative disease of mature-appearing B lymphocytes. It is a heterogeneous disease, with clinical presentation that ranges from an indolent phase (Rai stage 0 or 1) to advance stage disease (Rai stage 3 or 4). The indolent phase often does not manifest any symptoms and therefore requires no therapeutic intervention. These patients often have prolonged or near normal survival (median ≥12 years). On the other hand, patients with advance stage disease are usually symptomatic with progressive disease and compromised survival (median 3 years) (Grever et al, 1988; Keating et al, 1998). In patients with advance stage disease primary therapy usually includes chemotherapy, such as fludarabine (Johnson et al, 1996; Rai et al, 2000; Leporrier et al, 2001), chlorambucil or bendamustine. These are often combined with an anti- CD20 monoclonal antibody (rituximab) resulting in enhanced complete and overall response rates (Byrd et al, 2003). All patients eventually relapse and develop resistance to therapy. For patients who relapse after fludarabine-based therapies the US Federal Drug Administration approved treatment is alemtuzumab (a monoclonal antibody against CD52) (Keating et al, 2002). This results in an overall response rate (complete and partial remission) of 33% with only 2% of the patients achieving a complete response (CR) (Keating et al, 2002). These rather dismal clinical outcomes underscore the need to identify new therapeutic targets and new therapeutics in B-CLL. In this regard, it is important to note that all the standard therapeutic approaches have the same basic strategy of directly targeting the tumour cell itself. However, novel strategies are suggested from our developing understanding of B-CLL biology.
It is increasingly clear that the tumour microenvironment plays a critical role in B-CLL growth, survival and resistance to therapy. As the tumour cells are B cells, which normally interact with other immune cells, the immune system is a component of the microenvironment that may potentially mediate both anti-tumour immune responses as well as providing pro-survival support to the leukaemic B cells (and tipped in favour of the latter in leukaemic patients). Cytokines and growth factors directly regulate proliferation, differentiation and death of normal B-lymphocytes, and abnormal cytokine networks clearly exist within the B-CLL microenvironment (Hoffbrand et al, 1993; Reittie et al, 1996; Kay et al, 2002; Orsini & Fao, 2005). Interaction with stroma and the inherent ability of B-CLL cells to secrete pro-survival cytokines (vascular endothelial growth factor [VEGF], tumour necrosis factor-α [TNF-α] and interleukin [IL]-6, which can be detected at high levels in patient serum), and expression of corresponding receptors on B-CLL cells results in the formation of a paracrine/autocrine growth promoting loop (Hoffbrand et al, 1993; Zaninoni et al, 2003; Orsini & Fao, 2005).
In addition to direct effects on the B-CLL cell, abnormal expression of cytokines also modulates T-cell dysfunction in patients, which in turn can support B-CLL survival (Kiaii et al, 2005). For example, CD4+ T cells from leukaemic patients support the survival of B-CLL cells, indicating that non-malignant immune cells are an important component of the microenvironment (Mellstedt & Choudhury, 2006). In fact, B-CLL patients express both quantitative and functional T-cell abnormalities (Johnston & Kay, 2004), and these abnormalities appear to be induced by the B-CLL cell itself through contact-dependent interactions (Gorgun et al, 2005). Consistent with this, B-CLL cells downregulate expression of the CD80 (B7-1) and CD86 (B7-2) costimulatory ligands, which may blunt antitumour T cell activation and possibly induce anergy (Cantwell et al, 1997; Dorfman et al, 1997). Similarly, NK cell dysfunction has been reported in B-CLL patients, including impaired release of cytolytic molecules (Kay & Zarling, 1984; Burton et al, 1989). However, in vitro phytohaemagglutinin stimulation of NK cells from B-CLL patients results in increased TNF-α production, suggesting a partial or reversible functional impairment (Ziegler et al, 1981; Katrinakis et al, 1996).
Thus, elements of the malignant microenvironment may represent important therapeutic targets in B-CLL. However, as the appreciation for the key role of the B-CLL microenvironment is relatively recent, therapeutic strategies to specifically target this microenvironment have been largely unexplored.
Immnunomodulatory drugs (IMiDs) are a new class of antineoplastic agents that include thalidomide and its analog lenalidomide (Singhal et al, 1999; Richardson et al, 2002; List et al, 2005). Although the exact mechanism of antitumour activity remain undefined, these drugs have been shown to modulate the tumour cell microenvironment through downregulation of critical prosurvival cytokines (including IL-6, TNF-α, PDGF and VEGF) and activation of the immune effector cells (both T and NK cells) (Muller et al, 1999; Li et al, 2003; Anderson, 2005; Chang et al, 2006). Our group has previously reported that IMiD-antitumour activity in a xenogenic mouse model of lymphoma was associated with expansion of natural killer (NK) cells, and reversed with NK cell depletion (Hernandez-Ilizaliturri et al, 2005). The ability of the IMiD compounds to modulate the tumour microenvironment (both cellular and cytokine) in experimental preclinical models led us to investigate their antileukaemic effects in clinical trials in B-CLL. We first reported the clinical activity of lenalidomide in patients with relapsed or refractory B-CLL (Chanan-Khan et al, 2006). Significant clinical responses (including molecular complete remission) in heavily pretreated patients were observed despite high-risk features (Sher et al, 2010). These responses were correlated with a tumour flare reaction (TFR) in patients treated with lenalidomide (Chanan- Khan et al, 2011). TFR was manifested as swelling and redness of affected lymph, suggesting that lenalidomide modulates the immune system and inflammation in a profound way. Patients demonstrating TFR were managed by administration of non-steroidal anti-inflammatory drugs. Furthermore, use of steroids in a subset of patients decreased the intensity of the flare (Musial et al, 2006). Effective management of TFR with anti-inflammatory agents or steroids supports the idea that lenalidomide therapy in B-CLL patients induces robust inflammation.
The exact mechanism of action of lenalidomide in B-CLL remains unclear, but the association between TFR and clinical response suggests host inflammatory/immune responses are centrally involved. To more clearly define this we have characterized immunological parameters in patients with B-CLL treated with lenalidomide.
Patients and methods
Subjects
All patients treated with lenalidomide on a Phase II clinical trial (Chanan-Khan et al, 2006) were eligible to participate in this correlative study. The clinical trial was approved by the Institutional Review Board and all patients gave written informed consent. Detailed eligibility criteria is described previously (Chanan-Khan et al, 2006), importantly, all patients must have a confirmed diagnosis of B-CLL and have received at least one prior therapy for their disease.
Correlative study design
Peripheral blood samples were obtained from all consenting patients. Samples were obtained at baseline and then after 7 d of treatment with lenalidomide. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque method and an aliquot was cryopreserved for storage. After thawing, PBMCs were cultured in Aim V media (Invitrogen, Carlsbad, CA, USA) and allowed to recover for 1 day prior to analysis or in vitro treatment. The following studies were performed.
Apoptosis and viability
Apoptosis and Viability was analyzed on previously cryopreserved samples after in vitro treatment with various concentrations of lenalidomide 5–100 mm. Only the 100 mm concentration is shown. At various times after lenalidomide treatment (24, 48 or 72 h), cells were harvested and analyzed using reagents from Biosource (Camarillo, CA, USA) according to manufacturers recommendations.
Western blotting
Alterations in Bcl-2 family proteins and phospho-ERK were detected by Western blotting after PBMCs were lysed in 20 mmol/l Tris pH 7·5, 120 mmol/l NaCl, 100 mmol/l NaF, 0·5% Nonidet P40 containing freshly added 0·2 mmol/l sodium orthovanadate, 50 mmol/l beta-glycerolphosphate, 10 mmol/l sodium pyrophosphate, 4 mmol/l phenylmethylsulfonyl fluoride, 2 mmol/l Benzamidine and 10 μg/ml each of leupeptin and aprotonin. Equivalent amounts of protein were loaded in each well as determined by Bradford protein assay (BioRad, Hercules, CA, USA). Antibodies used to detect each protein are as follows: Bcl-2, Bcl-xL, Mcl-1 and pERK from Santa Cruz Biotechnology (Santa Cruz, CA, USA), total ERK from Cell Signaling Technologies, Danver, MA (p44/p42 MAP kinase antibody (#9102) and beta actin from Sigma (St. Louis, MO, USA).
Immune effector cell profile
Peripheral blood was obtained at baseline and then after 7 d of treatment with lenalidomide. Patient samples were analyzed immediately upon harvesting for T, NK and B cell repertoire using flow cytometry with the following antibody panels (BD Biosciences, San Jose, CA, USA) CD3/CD8/CD45 panel (CD8+ T lymphocytes), CD3/CD4/CD45 (CD4+ T lymphocytes), CD16/CD56/CD45 (NK cells) and CD19/CD45 (B lymphocytes). All antibodies were titred and used at saturating concentrations.
Expression of co-stimulatory molecules
Costimulatory molecule expression was determined by flow cytometry. PBMCs obtained from patients were treated in vitro with lenalidomide (50, 100, 200 μmol/l) or vehicle control (dimethyl sulfoxide, DMSO) for 24 and 96 h, and stained for the B cell activation marker CD83 and the costimulatory molecules CD40, CD80 and CD86 using the following panels CD19/HLA-DR/CD40/CD83, CD19/CD80/CD86. To confirm the effect of lenalidomide on expression of costimulatory molecules in vivo, PBMCs were obtained at baseline and then after 7 d of treatment with lenalidomide. Expression of activation marker (CD83) and costimulatory molecules was analyzed on CD19+ gated lymphocytes.
Plasma cytokine profile
Plasma for cytokine and growth factor analysis was separated from whole blood collected in Na EDTA and stored frozen at −80°C until analysis by a Luminex soluble bead array (Luminex Corp., Austin, TX, USA). Capture and detection antibody pairs directed against different noncompeting epitopes of their respective cytokine and recombinant protein standards for human FLT-3, IL-1β, IL-4, IL-5, IL-8, IL-10, IL12p70, IP10, MCP1, MIG, MIP1α, MIP1β, PDGF, SCF, TGF-β, TNF-α, and VEGF were purchased from Invitrogen and used according to the manufacturers instructions. Briefly, samples and standards were diluted in sample buffer and added to wells containing beads coated with capture antibody; samples for TGF-β were first pretreated with 1 mol/l HCl for 10 min and then neutralized with 1·2 mol/l NaOH to extract free TGF-β from latent complexes. The plates were incubated at ambient temperature on a rocker, washed and then incubated with biotinylated detection antibodies to each cytokine. After a second wash, the phycoerythrin-conjugated streptavidin was added to each well and the plates were incubated and washed before acquisition on a Luminex 100. Blank values were subtracted from all readings. Using BeadView Software (Millipore, Billerica, MA, USA) a log regression curve was calculated using the bead mean fluorescence intensity (MFI) values versus concentration of recombinant protein standard. Points deviating from the best-fit line, i.e. below detection limits or above saturation, were excluded from the curve. Sample cytokine concentrations were calculated from their beads MFIs by interpolating the resulting best-fit line.
Statistical analyses of cytokine levels
Cytokines with measurements below the limit of detection were treated as left censored. Differences in pre- and post-measurements below a limit of detection were treated as interval censored. Log-normal regression models with left censoring were fit for pre- and post-biomarker measurements. Normal regression models with interval censoring were fit for differences (post – pre) in biomarker measurements. Model parameters were estimated separately for stable disease (SD) patients and for complete remission/partial remission (CR/PR) to obtain fitted densities. These fitted densities were used to estimate the probability that a SD patient had a higher cytokine level than a CR/PR patient. Tests for differences in cytokine distributions were done using a likelihood ratio test, comparing full versus reduced models.
Results
Effects of Lenalidomide on B-CLL cells
Lenalidomide treatment in vitro does not kill B-CLL cells
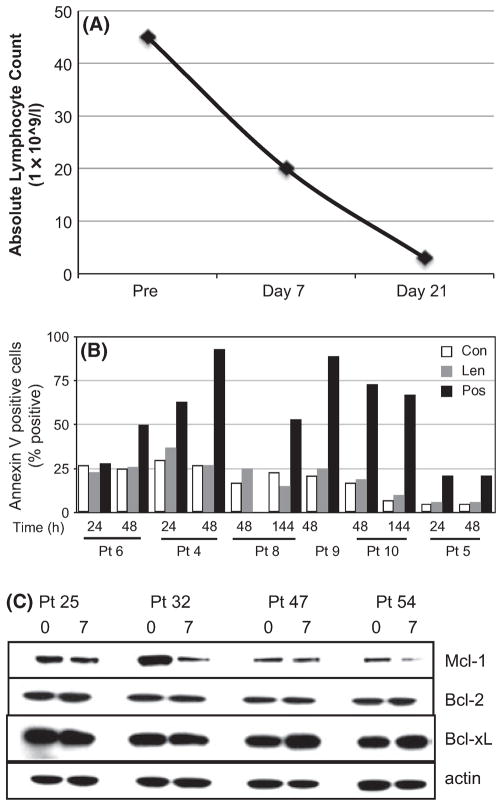
We have previously shown lenalidomide has a potent in vivo anti-leukaemic effect in patients with refractory and relapsed B-CLL manifesting clinically in an overall response rate of 58% (Chanan-Khan et al, 2006). The antileukaemic effect of lenalidomide in patients could be seen as early as 7 d of therapy (Fig 1A). To assess how lenalidomide might mediate its anti-CLL effect, we first determined whether lenalidomide was directly cytotoxic to B-CLL cells in vitro. PBMCs from seven different patients containing >90% B-CLL cells (CD19+/CD5+) were treated with up to 100 μmol/l of lenalidomide. Lenalidomide did not induce apoptosis (annexin V staining) compared to vehicle control at any time point (24, 48 144 h) measured for each patient (Fig 1B). In contrast, fludarabine and cyclophosphamide induced a significant level of apoptosis in the same cell population (positive control).
Fig 1.
Lenalidomide therapy is clinically effective, but does not induce apoptosis of primary B-CLL cells in vitro. (A) Antileukaemic effect [as measured by absolute lymphocyte count in peripheral blood mononuclear cells (PBMCs)] after each course of lenalidomide (Day 7, Day 21) compared to pre-therapy (Pre). (B,C) In vitro treatment of primary tumour cells obtained from B-CLL patients. PBMCs were treated with lenalidomide (Len, 100 μmol/l), vehicle control (Con) or cyclophosphamide and fludarabine (Pos). Apoptosis was measured after the indicated times by flow cytometric staining using annexin V and propidium iodide. (C) PBMCs were isolated from patients prior to therapy (0) or 7 d after therapy (7) and analysed for Bcl-2 family protein levels by Western blotting.
Furthermore, lenalidomide did not affect the apoptotic threshold of B-CLL cells as the expression of the anti-apoptotic proteins Bcl-2 and Bcl-xL were not altered by treatment (Fig 1C). These findings suggest that, unlike traditional chemotherapy, lenalidomide’s mechanism of action in B-CLL is not via direct killing of the leukaemic cells.
Lenalidomide alters B-CLL phenotype through upregulation of costimulatory ligand expression
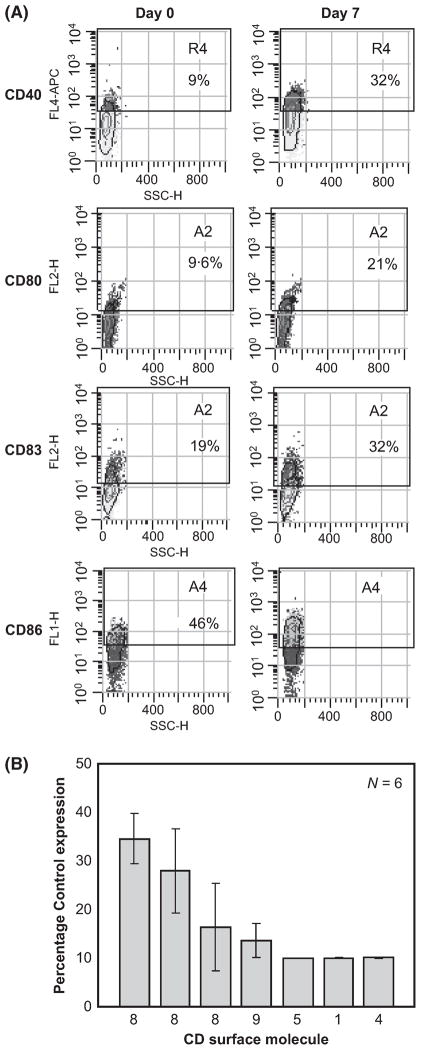
Lenalidomide treatment in B-CLL patients leads to a striking ‘TFR’ observed as an acute inflammation in disease involved lymph nodes (and other areas). In our observation the intensity of the TFR correlated with clinical response (Chanan-Khan et al, 2011). However, the TFR has not been reported in multiple myeloma (another B-cell cancer) patients. This suggested that lenalidomide specifically alters the B-CLL tumour cell phenotype and enhances its immunogenic potential. Thus we investigated lenalidomide-induced changes in expression of costimulatory molecules on B-CLL cells in vitro and in vivo. PBMCs obtained from B-CLL patients were treated in vitro with lenalidomide and stained for the B cell activation marker CD83 and the costimulatory ligands CD40, CD80 and CD86. In vitro treatment with lenalidomide resulted in upregulation of CD80, CD86 and CD40 and the B-cell activation marker CD83, but not HLA-DR (Table I). To assess if this also occurred in vivo, PBMCs were analyzed from patients prior to and after therapy with lenalidomide. Consistent with our in vitro observation, lenalidomide treatment of B-CLL patients resulted in upregulation of CD83 as well as the costimulatory ligands CD40, CD80 and CD86 (Table II, Fig 2A). Intriguingly, the two patients (Patients 27 and 30) with in vivo upregulation of costimulatory molecules showed clinical response to lenalidomide therapy versus no response in the third patient (Patient 25) in whom no change in costimulatory molecules was observed (see Fig 2B).
Table I.
Modulation of surface expression of costimulatory molecules on CLL cells after in vitro lenalidomide treatment.
| Patient
|
26
|
40
|
22
|
21
|
23
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Len | − | + | − | + | − | + | − | + | − | + |
| CD95 | 41 | 30 | 37 | 40 | 29 | 47 | 20 | 26 | 3 | 3 |
| CD83 | 57 | 45 | 38 | 52 | 13 | 27 | 19 | 32 | 6 | 6 |
| CD40 | 21 | 29 | 11 | 18 | 2 | 2 | 4 | 19 | 9 | 32 |
| CD80 | 40 | 21 | 34 | 36 | 10 | 21 | 17 | 20 | 3 | 4 |
| CD86 | 45 | 64 | 4 | 25 | 1 | 2 | 3 | 9 | 30 | 65 |
| HLA-DR | 100 | 93 | 95 | 96 | 80 | 71 | 98 | 100 | 99 | 100 |
| CD59 | 99 | 100 | 99 | 100 | 98 | 98 | 100 | 99 | 90 | 96 |
| MICA/B | 57 | 44 | 59 | 62 | 18 | 26 | 16 | 18 | 2 | 5 |
PBMCs from patients prior to therapy were treated with 100 μmol/l lenalidomide (Len) or vehicle control for 96 h, stained and analysed by flow cytometry. The percentage of positive stained cells versus isotype control was determined after gating the population for viable CD19+ B cells.
The bold values represent antigen expression pattern with significant changes induced by lenalidomide treatment in vitro.
Table II.
Modulation of surface expression of costimulatory molecules on CLL cells after in vivo lenalidomide treatment.
| 27
|
30
|
25
|
||||
|---|---|---|---|---|---|---|
| Patient | Day 0 | Day 7 | Day 0 | Day 7 | Day 0 | Day 7 |
| CD95 | 33 | 34 | 70 | 77 | 5 | 5 |
| CD83 | 4 | 10 | 6 | 17 | 6 | 5 |
| CD40 | 14 | 25 | 4 | 18 | 9 | 8 |
| CD80 | 1 | 1 | 4 | 15 | 31 | 22 |
| CD86 | 15 | 22 | 2 | 10 | 8 | 7 |
| CD59 | 83 | 96 | 92 | 96 | 97 | 95 |
| DR | 83 | 82 | 34 | 76 | 99 | 99 |
Peripheral blood was collected from patients enrolled on the clinical trial at baseline and then at day 7 of treatment with single agent lenalidomide. Surface expression of costimulatory molecules was assessed by flow cytometry gated on CD19+ B cells.
The bold values represent antigen expression pattern with significant changes induced by lenalidomide treatment in vitro.
Fig 2.
In vivo and In vitro lenalidomide treatment are associated with upregulation of costimulatory molecules on B-CLL cells. PBMCs were isolated from three patients pre- and post- 7 d treatment with lenalidomide and analysed by flow cytometry for CD19+ cells that express the indicated protein. One representative patient sample is shown (A). s The effect of lenalidomide on modulation of the costimulatory molecules was independent of the dose used. B-CLL cells (CD19+) from six patients were treated in vitro with 10μmol/l of lenalidomide for 48 h and surface expression of costimulatory molecules analyzed by flow cytometry (B).
Lenalidomide induces MAPK signalling in B-CLL cells
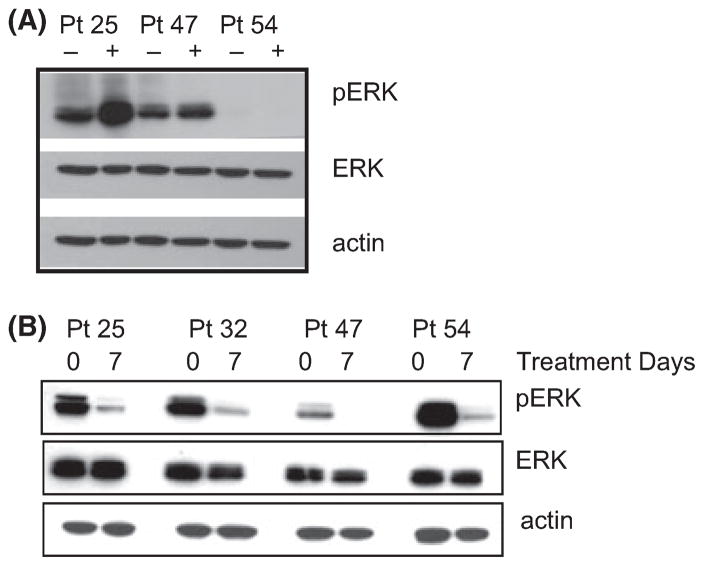
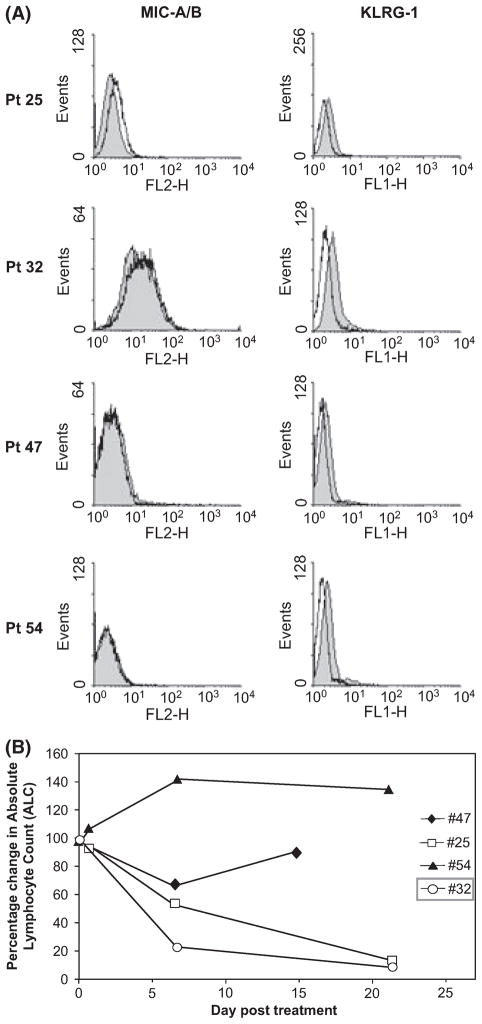
Costimulation pathways involve MAPK/ERK signalling. Therefore, we examined this pathway in B-CLL cells treated in vitro with lenalidomide. In vitro treatment for 24 h triggered MAPK/ERK signalling as measured by increased p-ERK in B-CLL cells (Fig 3A). p-ERK was reduced in PBMCs from patients after 7 d of lenalidomide therapy in vivo,. This is probably because fewer tumour cells were present after lenalidomide therapy (Fig 3B). Together these data demonstrate that lenalidomide modulates expression of costimulatory molecules and induces p-ERK signalling in vitro.
Fig 3.
Lenalidomide modulates ERK signals. (A). PBMCs from Patients 25, 32, 47 and 54) were cultured in dimethyl sulfoxide (−) or 100 μmol/l lenalidomide (+) for 96 h in vitro and proteins were isolated for detection of phospho-ERK (pERK), total ERK (ERK) or actin by Western blot. (B) PBMCs from the same patients in A, either prior to therapy (0) or 7 d after therapy (7), were analysed without any in vitro stimulation for the indicated proteins.
Lenalidomide modulates the host cytokine profile
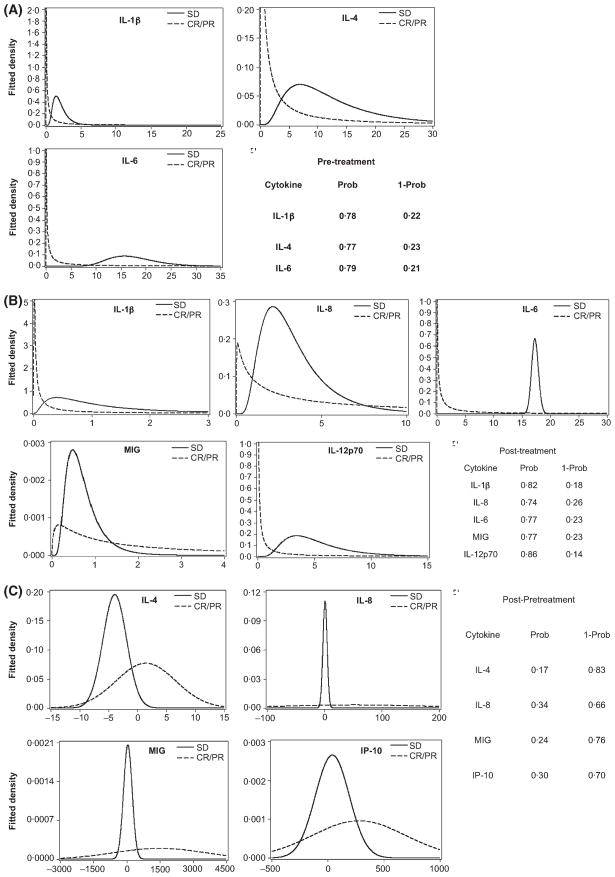
The ability of lenalidomide to upregulate co-stimulatory molecules suggested that lenalidomide might alter immune cell interactions, thereby influencing the cytokine as well as cellular microenvironment. Consistent with this idea, lenalidomide has been reported to modulate cytokine production and T and NK cell activation (Wu et al, 2008; Ramsay & Gribben, 2009; Ramsay et al, 2009). Further support for this idea comes from the tumour flare (Chanan-Khan et al, 2011) associated with lenalidomide therapy in B-CLL, which strongly suggests inflammation and cytokine alterations. We first examined the effect of lenalidomide treatment in patients on a panel of cytokines, comparing patient samples before (day 0) and after (day 7) treatment by Luminex bead assay. To determine if patient outcome correlated with alteration in a particular cytokine, patients were first grouped under the clinical classification of stable disease/progressive disease (SD/PD) or partial remission/complete remission (PR/CR). We then asked if baseline levels (day 0) of the cytokines could differentiate between or predict SD versus PR/CR. For each cytokine measured at each time point (day 0 vs. day 7), the distribution or fitted density for each group was plotted as a function of cytokine measurement (Fig 4).
Fig 4.
Lenalidomide therapy alters the cytokine microenvironment. Plasma cytokine levels were measured as described in the methods. Patients were categorized into Complete Remission/Partial Remission (CR/PR) or stable disease (SD) according to clinical criteria. (A) Pre-treatment distribution of cytokines that differed between CR/PR and SD patient groups. The cytokine measurements (pg/ml) for each population are plotted on the x-axis. (B) Distribution of post-treatment (day 8) cytokines that differed between CR/PR and SD patient groups. (C) Distribution of the change of cytokine levels (post-treatment (day 8) – pre-treatment levels) of cytokines between CR/PR and SD/PD patient groups. Prob, estimated probability that the CR/PR cytokine value is lower than the SD/PD cytokine value; 1-Prob, estimated probability that a PR/CR cytokine value exceeds that of the SD/PD cytokine value.
Lenalidomide modulates natural killer cells
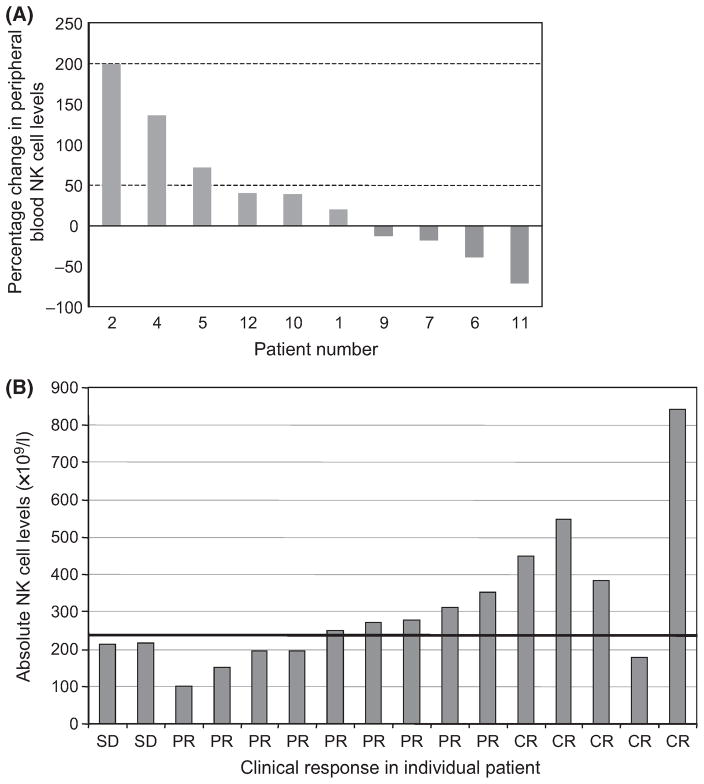
The preceding results as well as the rapid and robust clinical TFR/tumour destruction induced by lenalidomide treatment suggests involvement of the immune system, specifically anti-tumour cellular immunity. Consistent with this, lenalidomide has been reported in vitro to modulate activation and proliferation of T and NK cells (Chang et al, 2006). The rapidity of the TFR (hours) suggests that at least the initial component of this response is mediated by effectors that are non-antigen specific, while the later aspects of the flare (which has a median duration of 14 d) may involve antigen-specific immune effector responses. Given that activated NK cells are known to be capable of robust anti-tumour responses (Pattengale et al, 1982; Belldegrun et al, 1988), we investigated if lenalidomide treatment affected the number of NK cells. Peripheral blood samples at baseline and 7 d post-treatment were obtained from 10 patients. Increases in the NK cell population (Fig 5A) were seen in six patients, ranging from 20 to 199% increase in the absolute number of activated NK (CD16+/CD56+/CD45+) cells. Interestingly, clinical response to lenalidomide correlated with absolute number of pretreatment NK cells. Among the patients who demonstrated a clinical response (PR or CR) all but one had a normal or high baseline NK cell level in the peripheral blood (Fig 5B). Moreover, higher than normal baseline NK cell levels were noted in four of the 5 (80%) patients who achieved CR (Fig 5B).
Fig 5.
Lenalidomide therapy alters NK cells and clinical response correlates with baseline NK levels. (A). Activated NK cells (CD56+/CD45+) were assessed after 7 d of treatment with lenalidomide in patients with B-CLL. Data is represented as the % change of absolute NK numbers compared to pretreatment. (B) Pretreatment levels of peripheral blood NK cells (CD16+/CD56+) at baseline, and correlation with clinical responses (SD-stable disease, PR-partial remission, CR-complete remission).
Lenalidomide-induced expansion of NK cell numbers suggested that B-CLL cells were being induced to upregulate ligands that activate NK cells. MIC-A and MIC-B are the most well characterized activating ligands of NK cells. Therefore, we investigated whether MIC-A or MIC-B was upregulated on B-CLL cells by lenalidomide treatment of PBMCs in vitro. As seen in Fig 6A, B-CLL cells from three of the four patients (Patients 25, 47 and 54) did not express detectable MIC-A/B levels on the surface of their B-CLL cells at day 0 (filled histograms) and in vitro treatment of PBMCs with lenalidomide (open histograms) did not induce MIC-A/B levels in these patients (Patients 47, 54). One patient showed undetectable MIC-A/B staining at baseline, and lenalidomide induced MIC-A/B expression modestly (Patient 25). Finally, Patient 32 showed high levels of MIC-A/B expression at baseline, which was not appreciably altered by lenalidomide treatment in vitro. Similar results were obtained with a MIC-A specific antibody that does not recognize MIC-B (data not shown). KLRG1 staining was used as a control and showed subtle downregulation with in vitro lenalidomide treatment. These findings suggest that induction of MIC A/B expression on B-CLL cells by lenalidomide is not a major cause of the NK expansion seen in patients.
Fig 6.
B-CLL expression of NK-activating ligands. (A) Pre-therapy PBMCs from Patients 25, 32, 47 and 54 were treated with vehicle carrier (filled histograms) or lenalidomide (100μmol/l) for 96 h and analysed by flow cytometry. (B) Reduction of the circulating B-CLL cells of the patients analysed in A. Pt, Patient.
However, given this prominent expansion of NK cells, we next asked whether different baseline levels of MIC-A or MIC-A/B on B-CLL cells predicts patient response to lenalidomide therapy. Therefore, we compared the clinical response (as measured by absolute lymphocyte count) in these four patients (Fig 6B). Patient 32 had elevated MIC-A/B expression on day 0 and responded well to therapy, while two of the patients (Patients 47, 54) with undetectable MIC-A/B showed a poor or delayed response. Lastly, Patient 25, who showed a modest induction of MIC-A/B after in vitro lenalidomide treatment, also manifested a dramatic decrease in leukaemic counts (CD19+). Together these data suggest that high baseline levels of MIC-A and/or MIC-B on B-CLL cells and high NK cell numbers (and/or their expansion) may promote tumour clearance upon lenalidomide therapy.
Discussion
Treatment of B-CLL patients with lenalidomide resulted in significant antileukaemic effects (Chanan-Khan et al, 2006; Ferrajoli et al, 2008; Chen et al, 2010). Interestingly, lenalidomide induced a TFR suggestive of an immune-mediated inflammation (Andritsos et al, 2008; Chanan-Khan et al, 2011). This reaction has now been reported in other B-cell cancers, such as mantle cell lymphoma (Eve & Rule, 2010) and Hodgkin lymphoma (Corazzelli et al, 2010). Utilizing clinical samples from our previously reported clinical trial (Chanan-Khan et al, 2006) we conducted correlative studies to investigate the immunologic effects of lenalidomide in patients with B-CLL. In the current study, we attempted to identify immunological parameters affected by lenalidomide therapy in vivo.
Our in vitro findings indicate that the in vivo antileukaemic activity of lenalidomide is not likely to be due to direct cytotoxicy on B-CLL cells (Chanan-Khan & Porter, 2006). We did not detect induction of B-CLL apoptosis after in vitro treatment with lenalidomide. This observation was also confirmed by Andritsos et al (2008). However, in contrast to the lack of in vitro effect of lenalidomide on viability, we found that lenalidomide-induced expression of costimulatory ligands (CD86, CD80 and CD40) on B-CLL cells both in vitro and in vivo. Upregulation of these ligands are a critical step in engaging an immune response.
The rapid and robust immune response (first evidence of TFR was noted within 24 h) suggests a non-specific, antigenin-dependent effect. Another group had previously reported the importance of NK cells in mediating an anti-lymphoma effect with lenalidomide (Hernandez-Ilizaliturri et al, 2005). Therefore we evaluated the role of NK cells in B-CLL patients treated with lenalidomide. We observed that the number of NK cells in patients’ peripheral circulation appear to be increased by lenalidomide treatment, and pre-treatment levels of NK cells seem to correlate with CR/PR response to therapy. NK cells are known to have significant anti-tumour efficacy. Our data suggest that pretreatment NK cell numbers may be predictive for CR/PR response to lenalidomide therapy. Given that NK cells are activated by ligands, such as MIC-A and MIC-B on target cells, we also found that high baseline expression of MIC-A/B on B-CLL cells correlated with tumour clearance. Our findings suggest that lenalidomide may trigger NK cell recognition and killing of B-CLL cells. Consistent with this idea, a recent report suggests that in vitro treatment with lenalidomide improves NK killing of target cells (Wu et al, 2008).
The prolonged duration of TFR suggests a T cell-dependent antigen-specific inflammatory reaction. Upregulation of the costimulatory ligand expression has the potential to promote T cell activation and subsequent cytokine production. The activation of T cells by lenalidomide and improvement of tumour kill by immune synapse formation has been demonstrated (Ramsay & Gribben, 2009; Ramsay et al, 2009). This is consistent with our observation that recruitment of the immune effector response is the target effect of lenalidomide treatment in B-CLL. The cascade of events that leads to this final response is perhaps initiated by as simple a phenomenon as the upregulation of immune activating co-stimulatory molecules on the surface of the tumour cells. This in turn mobilizes the NK and T-cells, resulting in immediate and persistent inflammatory response that culminates in long-term anti-tumour immunity.
Biochemical evidence of inflammatory/immune reaction was also evaluated. We observed increased TNFα levels in patients after lenalidomide therapy – consistent with induction of an inflammatory response. In contrast to the initial in vitro observations that lenalidomide inhibited TNFα production by lipopolysaccharide (LPS)-stimulated PBMCs, thus suggesting that, while lenalidomide may have direct suppressive effect on TNFα production in vitro, its effect on the cytokine profile in vivo reflects an immune activation response. We further observed that characterization of these in vivo changes in the cytokine profile could be predictive of clinical response. Thus, IL-8, MIG, IP-10 and IL-4 were more likely to be higher in patients achieving a major clinical response such as CR or PR compared to those patients who had no response or achieved SD after lenalidomide therapy, while IL-5, MIP1a, MIP1b, IL12/p70 were more likely to be lower in patients with CR/PR compared to those achieving SD. These parameters will be important to follow and validate in larger studies as potential biomarkers of clinical response to lenalidomide in CLL patients.
To further evaluate if there is a singular focal event that triggers the immunological cascade mediated by lenalidomide, we studied various intracellular signalling pathways and noted induction of MAPK/ERK signalling by lenalidomide in the B-CLL cells. We observed that lenalidomide treatment diminished p-ERK signals at day 7 after treatment compared to day 0. Treatment with lenalidomide in vitro showed slight upregulation of pERK, suggesting that lenalidomide may affect pERK signalling differentially depending upon the complex signals (either in vitro or in vivo) that it may modulate. Lenalidomide is known to modulate signals from LPS stimulation of PBMCs, costimulatory signals in T cells and activation signals in NK cells. However, the implication of ERK in B-CLL cells has not been previously investigated. As ERK is central in many cellular growth and activation signals, the pleiotropic effect of lenalidomide may be explained by its effects on a central integrating signalling molecule such as ERK. Further studies will confirm the role of ERK in lenalidomide-induced TFR.
In summary, the rapid, robust and inflammatory nature of the TFR clearly suggests involvement of the immune system, which is acutely dependent on NK cell function and is then maintained by the rapid recruitment and proliferation of T cells. Our findings suggest that lenalidomide induces specific molecular events that can modify the surface ligand profile of leukaemic cells. This altered tumour cell immunophenotype then induces an acute immune reaction with activation and proliferation of NK cells and subsequent recruitment and proliferation of T cells. Activation of the immune effector cells, and alteration in the immune cytokine milieu disrupts the relationship with the host that supports B-CLL survival in vivo.
Acknowledgments
This research is funded by the Leukemia and Lymphoma Society (LLS Grant No. 6046-08 & 2065-09 ACK).
References
- Anderson KC. Lenalidomide and thalidomide: mechanisms of action – similarities and differences. Seminars in Hematology. 2005;42:S3–S8. doi: 10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Andritsos LA, Johnson AJ, Lozanski G, Blum W, Kefauver C, Awan F, Smith LL, Lapalombella R, May SE, Raymond CA, Wang DS, Knight RD, Ruppert AS, Lehman A, Jarjoura D, Chen CS, Byrd JC. Higher doses of lenalidomide are associated with unacceptable toxicity including life-threatening tumor flare in patients with chronic lymphocytic leukemia. Journal of Clinical Oncology. 2008;26:2519–2525. doi: 10.1200/JCO.2007.13.9709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belldegrun A, Uppenkamp I, Rosenberg SA. Anti-tumor reactivity of human lymphokine activated killer (LAK) cells against fresh and cultured preparations of renal cell cancer. Journal of Urology. 1988;139:150–155. doi: 10.1016/s0022-5347(17)42342-1. [DOI] [PubMed] [Google Scholar]
- Burton JD, Weitz CH, Kay NE. Malignant chronic lymphocytic leukemia B cells elaborate soluble factors that down-regulate T cell and NK function. American Journal of Hematology. 1989;30:61–67. doi: 10.1002/ajh.2830300203. [DOI] [PubMed] [Google Scholar]
- Byrd JC, Peterson B, Piro L, Saven A, Vardiman JW, Larson RA, Schiffer C. A phase II study of cladribine treatment for fludarabine refractory B cell chronic lymphocytic leukemia: results from CALGB Study 9211. Leukemia. 2003;17:323–327. doi: 10.1038/sj.leu.2402752. [DOI] [PubMed] [Google Scholar]
- Cantwell M, Hua T, Pappas J, Kipps TJ. Acquired CD40-ligand deficiency in chronic lymphocytic leukemia. Nature Medicine. 1997;3:984–989. doi: 10.1038/nm0997-984. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Porter CW. Immunomodulating drugs for chronic lymphocytic leukaemia. Lancet Oncol. 2006;7:480–488. doi: 10.1016/S1470-2045(06)70723-9. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Musial L, Lawrence D, Padmanabhan S, Takeshita K, Porter CW, Goodrich DW, Bernstein ZP, Wallace P, Spaner D, Mohr A, Byrne C, Hernandez-Ilizaliturri F, Chrystal C, Starostik P, Czuczman MS. Clinical efficacy of lenalidomide in patients with relapsed or refractory chronic lymphocytic leukemia: results of a phase II study. Journal of Clinical Oncology. 2006;24:5343–5349. doi: 10.1200/JCO.2005.05.0401. [DOI] [PubMed] [Google Scholar]
- Chanan-Khan A, Miller KC, Lawrence D, Padmanabhan S, Miller A, Hernandez-Illatazurri F, Czuczman MS, Wallace PK, Zeldis JB, Lee K. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer. 2011;117:2127–2135. doi: 10.1002/cncr.25748. [DOI] [PubMed] [Google Scholar]
- Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, Jagannath S, Dhodapkar MV. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–621. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CI, Bergsagel PL, Paul H, Xu W, Lau A, Dave N, Kukreti V, Wei E, Leung-Hagesteijn C, Li ZH, Brandwein J, Pantoja M, Johnston J, Gibson S, Hernandez T, Spaner D, Trudel S. Single-agent lenalidomide in the treatment of previously untreated chronic lymphocytic leukemia. Journal of Clinical Oncology. 2010;29:1175–1181. doi: 10.1200/JCO.2010.29.8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corazzelli G, De Filippi R, Capobianco G, Frigeri F, De Rosa V, Iaccarino G, Russo F, Arcamone M, Becchimanzi C, Crisci S, Marcacci G, Amoroso B, Lastoria S, Pinto A. Tumor flare reactions and response to lenalidomide in patients with refractory classic Hodgkin lymphoma. American Journal of Hematology. 2010;85:87–90. doi: 10.1002/ajh.21571. [DOI] [PubMed] [Google Scholar]
- Dorfman DM, Schultze JL, Shahsafaei A, Michalak S, Gribben JG, Freeman GJ, Pinkus GS, Nadler LM. In vivo expression of B7-1 and B7-2 by follicular lymphoma cells can prevent induction of T-cell anergy but is insufficient to induce significant T-cell proliferation. Blood. 1997;90:4297–4306. [PubMed] [Google Scholar]
- Eve HE, Rule SA. Lenalidomide-induced tumour flare reaction in mantle cell lymphoma. British Journal of Haematology. 2010;151:410–412. doi: 10.1111/j.1365-2141.2010.08376.x. [DOI] [PubMed] [Google Scholar]
- Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ. Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood. 2008;111:5291–5297. doi: 10.1182/blood-2007-12-130120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorgun G, Holderried TA, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. Journal of Clinical Investigation. 2005;115:1797–1805. doi: 10.1172/JCI24176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grever MR, Kopecky KJ, Coltman CA, Files JC, Greenberg BR, Hutton JJ, Talley R, Von Hoff DD, Balcerzak SP. Fludarabine monophosphate: a potentially useful agent in chronic lymphocytic leukemia. Nouvelle Revue Francaise D Hematologie. 1988;30:457–459. [PubMed] [Google Scholar]
- Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clinical Cancer Research. 2005;11:5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- Hoffbrand AV, Panayiotidis P, Reittie J, Ganeshaguru K. Autocrine and paracrine growth loops in chronic lymphocytic leukemia. Seminars in Hematology. 1993;30:306–317. [PubMed] [Google Scholar]
- Johnson S, Smith AG, Loffler H, Osby E, Juliusson G, Emmerich B, Wyld PJ, Hiddemann W. Multicentre prospective randomised trial of fludarabine versus cyclophosphamide, doxorubicin, and prednisone (CAP) for treatment of advanced-stage chronic lymphocytic leukaemia. The French Cooperative Group on CLL. Lancet. 1996;347:1432–1438. doi: 10.1016/s0140-6736(96)91681-5. [DOI] [PubMed] [Google Scholar]
- Johnston PB, Kay N. Pathogenesis of impaired cellular immune fucntion in CLL. In: Faguet G, editor. Chronic Lymphocytic Leukemia: Molecular Genetics, Biology, Diagnosis, and Management. Human Press; Totowa, New Jersey: 2004. [Google Scholar]
- Katrinakis G, Kyriakou D, Papadaki H, Kalokyri I, Markidou F, Eliopoulos GD. Defective natural killer cell activity in B-cell chronic lymphocytic leukaemia is associated with impaired release of natural killer cytotoxic factor (s) but not of tumour necrosis factor-alpha. Acta Haematologica. 1996;96:16–23. doi: 10.1159/000203709. [DOI] [PubMed] [Google Scholar]
- Kay NE, Zarling JM. Impaired natural killer activity in patients with chronic lymphocytic leukemia is associated with a deficiency of azurophilic cytoplasmic granules in putative NK cells. Blood. 1984;63:305–309. [PubMed] [Google Scholar]
- Kay NE, Bone ND, Tschumper RC, Howell KH, Geyer SM, Dewald GW, Hanson CA, Jelinek DF. B-CLL cells are capable of synthesis and secretion of both pro- and anti-angiogenic molecules. Leukemia. 2002;16:911–919. doi: 10.1038/sj.leu.2402467. [DOI] [PubMed] [Google Scholar]
- Keating MJ, O’Brien S, Lerner S, Koller C, Beran M, Robertson LE, Freireich EJ, Estey E, Kantarjian H. Long-term follow-up of patients with chronic lymphocytic leukemia (CLL) receiving fludarabine regimens as initial therapy. Blood. 1998;92:1165–1171. [PubMed] [Google Scholar]
- Keating MJ, Flinn I, Jain V, Binet JL, Hillmen P, Byrd J, Albitar M, Brettman L, Santabarbara P, Wacker B, Rai KR. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- Kiaii S, Choudhury A, Mozaffari F, Kimby E, Osterborg A, Mellstedt H. Signaling molecules and cytokine production in T cells of patients with B-cell chronic lymphocytic leukemia (B-CLL): comparison of indolent and progressive disease. Medical Oncology. 2005;22:291–302. doi: 10.1385/MO:22:3:291. [DOI] [PubMed] [Google Scholar]
- Leporrier M, Chevret S, Cazin B, Boudjerra N, Feugier P, Desablens B, Rapp MJ, Jaubert J, Autrand C, Divine M, Dreyfus B, Maloum K, Travade P, Dighiero G, Binet JL, Chastang C. Randomized comparison of fludarabine, CAP, and ChOP in 938 previously untreated stage B and C chronic lymphocytic leukemia patients. Blood. 2001;98:2319–2325. doi: 10.1182/blood.v98.8.2319. [DOI] [PubMed] [Google Scholar]
- Li X, Liu X, Wang J, Wang Z, Jiang W, Reed E, Zhang Y, Liu Y, Li QQ. Thalidomide down-regulates the expression of VEGF and bFGF in cisplatin-resistant human lung carcinoma cells. Anticancer Research. 2003;23:2481–2487. [PubMed] [Google Scholar]
- List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, Heaton R, Knight R, Zeldis JB. Efficacy of lenalidomide in myelodysplastic syndromes. New England Journal of Medicine. 2005;352:549–557. doi: 10.1056/NEJMoa041668. [DOI] [PubMed] [Google Scholar]
- Mellstedt H, Choudhury A. T and B cells in B-chronic lymphocytic leukaemia: faust, Mephistopheles and the pact with the Devil. Cancer Immunology, Immunotherapy. 2006;55:210–220. doi: 10.1007/s00262-005-0675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, Chen Y, Kaplan G, Stirling DI. Amino-substituted thalidomide analogs: potent inhibitors of TNFalpha production. Bioorganic and Medicinal Chemistry Letters. 1999;9:1625–1630. doi: 10.1016/s0960-894x(99)00250-4. [DOI] [PubMed] [Google Scholar]
- Musial L, Miller K, Tonelli A, Manochakian R, Padmanabhan S, Lawrence D, DePaolo D, Coignet J, Chanan-Khan A. Low-dose prednisone decreases the severity but not the frequency of lenalidomide associated Tumor Flare Feaction (TFR) in Chronic Lymphocytic Leukemia (CLL) patients. Blood (Annual ASH Meeting Abstracts); Proceedings of the Annual meeting of the American Society of Hematology; 2006. p. Abstract 4987. [Google Scholar]
- Orsini E, Fao R. Cytokines and soluble molecules in CLL. In: Fauget G, editor. Chronic Lymphocytic Leukemia. Human Press; New Jersey: 2005. pp. 123–142. [Google Scholar]
- Pattengale PK, Gidlund M, Nilsson K, Sundstrom C, Sallstrom J, Simonsson B, Wigzell H. Lysis of fresh human B-lymphocyte-derived leukemia cells by interferon-activated natural killer (NK) cells. International Journal of Cancer. 1982;29:1–7. doi: 10.1002/ijc.2910290102. [DOI] [PubMed] [Google Scholar]
- Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L, Hines J, Threatte GA, Larson RA, Cheson BD, Schiffer CA. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. New England Journal of Medicine. 2000;343:1750–1757. doi: 10.1056/NEJM200012143432402. [DOI] [PubMed] [Google Scholar]
- Ramsay AG, Gribben JG. Immune dysfunction in chronic lymphocytic leukemia T cells and lenalidomide as an immunomodulatory drug. Haematologica. 2009;94:1198–1202. doi: 10.3324/haematol.2009.009274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay AG, Clear AJ, Kelly G, Fatah R, Matthews J, Macdougall F, Lister TA, Lee AM, Calaminici M, Gribben JG. Follicular lymphoma cells induce T-cell immunological synapse dysfunction that can be repaired with lenalidomide: implications for the tumor microenvironment and immunotherapy. Blood. 2009;114:4713–4720. doi: 10.1182/blood-2009-04-217687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reittie JE, Yong KL, Panayiotidis P, Hoffbrand AV. Interleukin-6 inhibits apoptosis and tumour necrosis factor induced proliferation of B-chronic lymphocytic leukaemia. Leukaemia & Lymphoma. 1996;22:83–90. doi: 10.3109/10428199609051732. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Schlossman RL, Weller E, Hideshima T, Mitsiades C, Davies F, LeBlanc R, Catley LP, Doss D, Kelly K, McKenney M, Mechlowicz J, Freeman A, Deocampo R, Rich R, Ryoo JJ, Chauhan D, Balinski K, Zeldis J, Anderson KC. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100:3063–3067. doi: 10.1182/blood-2002-03-0996. [DOI] [PubMed] [Google Scholar]
- Sher T, Miller KC, Lawrence D, Whitworth A, Hernandez-Ilizaliturri F, Czuczman MS, Miller A, Lawrence W, Bilgrami SA, Sood R, Wood MT, Block AW, Lee K, Chanan- Khan AA. Efficacy of lenalidomide in patients with chronic lymphocytic leukemia with high-risk cytogenetics. Leukaemia & Lymphoma. 2010;51:85–88. doi: 10.3109/10428190903406806. [DOI] [PubMed] [Google Scholar]
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. New England Journal of Medicine. 1999;341:1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- Wu L, Adams M, Carter T, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB. lenalidomide enhances natural killer cell and monocyte-mediated antibody-dependent cellular cytotoxicity of rituximab-treated CD20+ tumor cells. Clinical Cancer Research. 2008;14:4650–4657. doi: 10.1158/1078-0432.CCR-07-4405. [DOI] [PubMed] [Google Scholar]
- Zaninoni A, Imperiali FG, Pasquini C, Zanella A, Barcellini W. Cytokine modulation of nuclear factor-kappaB activity in B-chronic lymphocytic leukemia. Experimental Hematology. 2003;31:185–190. doi: 10.1016/s0301-472x(02)01046-9. [DOI] [PubMed] [Google Scholar]
- Ziegler HW, Kay NE, Zarling JM. Deficiency of natural killer cell activity in patients with chronic lymphocytic leukemia. International Journal of Cancer. 1981;27:321–327. doi: 10.1002/ijc.2910270310. [DOI] [PubMed] [Google Scholar]