Abstract
Understanding how and when the left-right (LR) axis is first established is a fundamental question in developmental biology. A popular model is that the LR axis is established relatively late in embryogenesis, due to the movement of motile cilia and the resultant directed fluid flow during late gastrulation/early neurulation. Yet, a large body of evidence suggests that biophysical, molecular, and bioelectrical asymmetries exist much earlier in development, some as early as the first cell cleavage after fertilization. Alternative models of LR asymmetry have been proposed that accommodate these data, postulating that asymmetry is established due to a chiral cytoskeleton and/or the asymmetric segregation of chromatids. There are some similarities, and many differences, in how these various models postulate the origin and timing of symmetry breaking and amplification, and these events’ linkage to the well-conserved subsequent asymmetric transcriptional cascades. This review examines experimental data that lend strong support to an early origin of LR asymmetry, yet are also consistent with later roles for cilia in the amplification of LR pathways. In this way, we propose that the various models of asymmetry can be unified: early events are needed to initiate LR asymmetry, and later events could be utilized by some species to maintain LR-biases. We also present an alternative hypothesis, which proposes that individual embryos stochastically choose one of several possible pathways with which to establish their LR axis. These two hypotheses are both tractable in appropriate model species; testing them to resolve open questions in the field of LR patterning will reveal interesting new biology of wide relevance to developmental, cell, and evolutionary biology.
Keywords: asymmetry, left-right, laterality, chirality, cytoskeleton, cilia
Introduction
Hidden beneath the bilaterally symmetrical exterior of virtually all vertebrates is an internal body plan with asymmetrically positioned organs. Consistent left-right (LR) asymmetry is a highly conserved feature in amphibians, reptiles, birds, fish and mammals, all of which orient their hearts and visceral organs with the same biases in placement and morphology. Our fascination with asymmetry dates back centuries (Perloff, 2011), and many modern day scientists work to address a fundamental biological question: what are the mechanisms used to reliably establish developmental chirality?
Understanding how consistent LR asymmetry is established in human embryogenesis is particularly important because errors in asymmetry account for a class of birth defects with medical consequences for affected individuals (Casey and Hackett, 2000; Cohen et al., 2007; Hackett, 2002; Peeters and Devriendt, 2006). These patients typically encounter difficulties due to the inability of the vasculature to make proper connections between the heart, lungs and various visceral organs when one or more organs has inappropriate placement (situs). Birth defects of LR asymmetry include situs inversus (the complete reversal of the internal organs), heterotaxia (the lack of concordance between the internal organs), single organ inversions such as dextrocardia (the reversal in position and morphology of the heart), and isomerisms (symmetry of the LR axis, leading to duplication or complete loss of single organs such as the spleen); several of these conditions raise unique challenges for medical treatment and decreased lifespan relative to individuals with normal organ situs (situs solitus).
Importantly, LR asymmetries extend beyond the basic body plan. In many animals, asymmetries are apparent in the structure, circuitry and function of the brain (Guglielmotti and Cristino, 2006; Roussigne et al., 2012), and these asymmetries have been linked to functional asymmetries in a number of behaviors (Facchin et al., 2009; Rogers et al., 2004). In human beings, brain asymmetries may be responsible for the development of language, and individuals with decreased brain lateralization are more likely to have academic difficulties, learning disabilities such as dyslexia, and diseases including schizophrenia (Corballis, 2012; Crow, 2008; Leonard and Eckert, 2008). Other asymmetries in behavioral features such as handedness are fascinating examples of lateralization where the LR bias is strong, but significantly weaker than the bias for asymmetric organ placement (e.g., ~90% right hand dominance versus 99.95% situs solitus) (Corballis, 2009). Interestingly, handedness has been linked to other conditions including susceptibility to intestinal parasites (Uslu et al., 2010), ear dominance and craniofacial asymmetries (Dane et al., 2002), asthma and autoimmune diseases (Krommydas et al., 2004; McManus et al., 1993), and birth weight (James, 2001), among others (reviewed in (McManus, 2005)).
Over several decades of research, additional cryptic asymmetries have been uncovered in humans and other vertebrates. For example, LR biases have been observed in the location of disease and infection in bilateral organs such as the kidney (Schreuder, 2011), the incidence of unilateral polydactyly (Schnall and Smith, 1974), electrophysiological properties of the developing eye (Pai et al., 2012), and the sidedness of external birth defects such as cleft palate (Paulozzi and Lary, 1999). LR biases have also been found for the site of mammary, ovarian, lung and testicular cancers (Wilting and Hagedorn, 2011) and there are LR biases for unfavorable prognoses following colon cancer detection and the likelihood of these cancers to metastasize to the lungs and liver (Benedix et al., 2010; Meguid et al., 2008; Singh et al., 2010). Thus, understanding the generation of asymmetry is not only of fundamental importance for the basic developmental biology of a major body axis, but also has strong biomedical relevance. While important progress has been made, aspects of LR asymmetry with potentially significant impacts on human health await a more complete molecular dissection of LR patterning.
Agreement and Disagreement in the Field
There is general agreement that the establishment of LR asymmetry requires three major phases (Levin and Palmer, 2007; Tabin, 2011). In the first, bilateral symmetry is broken in such a way that the LR axis is consistently oriented relative to the dorsal-ventral and anterior-posterior axes. In the second, LR differences produced in the first step are translated into the differential expression of genes on the left and right sides of the body midline. Finally, in the last step, asymmetric gene expression drives changes in cell behavior, such as migration rates (Lenhart et al., 2013), that result in the asymmetric position and morphology of the heart and visceral organs.
The final step, asymmetric positioning and shape of the internal organs, is highly conserved in all vertebrate species. This remarkable conservation suggests that there is likely to be an evolutionary advantage not just to bilateral asymmetry, but to this consistently-asymmetric packaging of the internal organs. Furthermore, the steps regulating asymmetric gene expression have been well-explored and a consistent picture of left- and right-sided transcriptional cascades has emerged (Levin, 1998; Nakamura and Hamada, 2012; Nakamura et al., 2006). The initial step in the LR asymmetry pathway – termed symmetry breaking – is the one that is most debated. For lack of any other viable class of models, it is generally agreed that a symmetrical embryo must distinguish its left from its right only after the dorsal-ventral and anterior-posterior axes have been determined, by consistent orientation of some subcellular component that is inherently chiral by virtue of its biochemical structure (Brown and Wolpert, 1990). Thus the field is in agreement about the inherently biophysical origin of asymmetry, prior to asymmetric transcriptional events. However, there is considerable debate on three major issues: (1) What is the chiral element that first breaks symmetry? (2) When during embryogenesis does it act? and (3) How conserved among diverse phyla are these mechanisms, and which model systems best represent the “general case”? Question #3 is particularly important because fascinating evolutionary biology issues are highlighted by the use of similar and distinct molecular mechanisms in species with very different body plans, and because biomedical implications of asymmetry research are impacted by the question of whether the mouse is the best model for human laterality.
The predominant model proposes that the movement of cilia in the early neurulating embryo provides chiral fluid flow in a small pocket of tissue, termed the node (mouse), the gastrocoel roof plate (GRP, Xenopus), or Kupffer’s Vesicle (KV, zebrafish) (Figure 1). These motile cilia produce a directional fluid flow in the node with a strong right-to-left current because of the cilia’s biochemical structure and orientation (Basu and Brueckner, 2008; Brueckner, 2001; Tabin, 2006). The cilia model includes three possible mechanisms by which this vertical fluid flow is amplified: the accumulation of extracellular morphogens on the left side of the embryo, asymmetric distribution of nodal vesicular particles (NVPs, small membrane-bound vesicles that transport morphogens such as sonic hedgehog and retinoic acid), or asymmetric detection of fluid flow itself by mechanosensory cilia leading to calcium signaling on one side of the embryo (McGrath and Brueckner, 2003; McGrath et al., 2003; Norris, 2012; Tabin and Vogan, 2003; Tanaka et al., 2005; Yost, 2003).
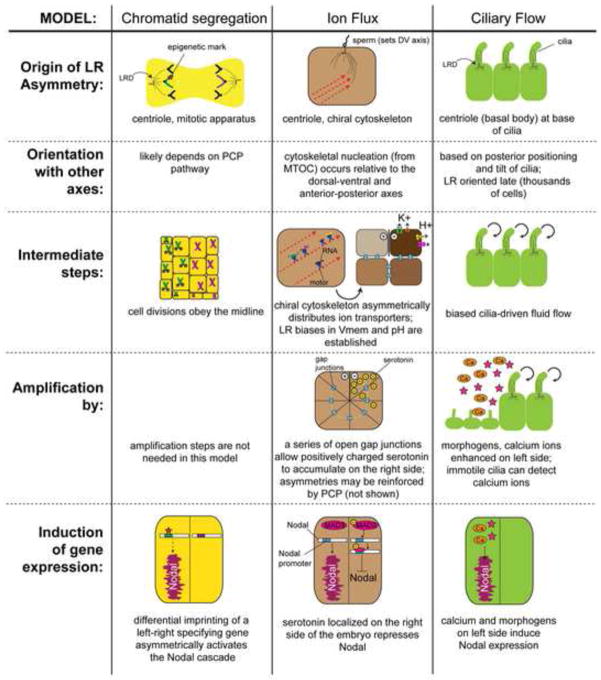
Figure 1. Schematic outlining three major models of LR asymmetry.
For each model, predictions about the origin of asymmetry, the mechanism for aligning the LR axis, the intermediate and amplification steps, and information about how early asymmetries influence asymmetric gene expression are described. Most noteworthy is that all three models agree on an intracellular cytoskeletal origin of asymmetry: the centriole.
Although the cilia model is frequently represented in medical and cell biology textbooks as a definitive and general explanation of embryonic asymmetry, it is really comprised of 2 distinct claims that need to be considered separately: (A) that ciliary motion is the very first step that initiates asymmetry de novo, and (B) that this is a general, well-conserved mechanism working in all vertebrates. In fact, while this model is strongly supported by data in mouse, there are very significant challenges to both the primacy of cilia and their involvement in LR patterning among other vertebrates (Levin, 2006; Vandenberg and Levin, 2010b). While numerous invertebrate phyla orient their LR axis without the benefit of cilia (Okumura et al., 2008; Speder et al., 2007), suggesting a divergence of asymmetry mechanisms in evolutionary history, recent data suggest that a number of intracellular elements are very broadly conserved in LR patterning, including invertebrates and even plants (Levin and Palmer, 2007; Lobikin et al., 2012; Oviedo and Levin, 2007). Together with the known existence of consistent asymmetries long before cilia appear even in species that do drive a nodal flow (Adams et al., 2006; Danilchik et al., 2006; Fukumoto et al., 2005b; Kramer et al., 2002; Levin et al., 2002), an alternative, intracellular model, was proposed (Klar, 1994; Levin and Nascone, 1997). The intracellular model differs from the ciliary model because it proposes A) that the origin of organismic asymmetry is not extracellular cilia but rather cytoplasmic cytoskeletal chirality, B) that the initial LR patterning steps occur extremely early, during the first few cell cleavages in most organisms, and C) that with the possible exception of the mouse, elements of this pathway are broadly conserved. The cilia model has been explicated in a number of excellent recent reviews (Basu and Brueckner, 2008; Norris, 2012; Shiratori and Hamada, 2006). Here, we discuss important features of alternative models, critically evaluate the evidence for each, and attempt to synthesize available data into a consistent picture of LR patterning throughout the tree of life. We end this review with a discussion of two new hypotheses; are the available data best synthesized by a model in which cilia operate as a downstream amplification mechanism in a pathway that uses earlier steps to break symmetry, or do some phyla allow individual embryos to stochastically select which of two alternate pathways are used to determine each individual’s developmental laterality?
Transducing cytoplasmic chirality into multi-cellular asymmetry
It has long been known that single cells use their cytoskeleton to drive consistent chirality (Alpatov, 1946; Frankel, 1991; Heacock and Agranoff, 1977; Nelsen et al., 1989; Xu et al., 2007). How would asymmetric shape or intracellular transport in key embryonic cells determine asymmetric transcription in cell fields? Three classes of non-mutually-exclusive proposals relative to the intracellular model have been made: the ion flux model, the chromatin segregation model, and the planar cell polarity (PCP) model (Figure 1).
The ion flux model. Driven by both pharmacological and molecular loss-of-function experiments in the frog and chick embryo, this model proposes that existing chiral structures in the embryo’s cytoskeleton (i.e. the MTOC and actin fibers) are oriented relative to the dorsal-ventral and anterior-posterior axes within the first embryonic cleavage (Aw et al., 2008; Danilchik et al., 2006). This chiral cytoskeleton is then responsible for actively directing the asymmetric distribution of proteins including K+ channels and H+ pumps (Qiu et al., 2005); the LR biased placement of these ion transporters leads to consistent differences in the pH and transmembrane voltage on the left and right sides of the embryo (Adams et al., 2006; Morokuma et al., 2008) and bioelectrical gradients drive the asymmetric distribution of small charged molecules such as serotonin through gap junctional paths from left to right in the early embryo (Fukumoto et al., 2003; Fukumoto et al., 2005b; Levin and Mercola, 1998). Serotonin accumulates within the right side of the embryo, where it epigenetically represses the expression of Xnr-1, the Xenopus homologue of the highly conserved left-side marker Nodal (Carneiro et al., 2011). While many of these same components (serotonin, gap junctions, motor proteins, cytoskeletal components, proton pumps, and potassium channels) are now known to function in the establishment of LR asymmetry in many other species including chick, C. elegans, sea urchin, and Ciona, among others (reviewed in (Levin, 2006; Oviedo and Levin, 2007)), the full details are known only in Xenopus and it remains to be discovered exactly how other body plans utilize these building blocks for LR patterning.
The chromatid segregation model. This model proposes that the two chromatids in the single celled embryo are differentially imprinted and segregated during the first cell division, and therefore retain information allowing for the distinction of the left and right sides (Klar, 1994, 2008; Sauer and Klar, 2012a). Exactly how the first two blastomeres are consistently oriented with the dorsal-ventral and anterior-posterior axes (allowing the correctly-imprinted chromatid to go to the correct anatomical side) is as yet unknown, but this process has been shown to be dependent on left-right dynein (LRD) (Armakolas and Klar, 2007; Sauer and Klar, 2012a), a protein that was first implicated in LR asymmetry in the mouse. Most studies examining the role of LRD focus on the immobility of cilia in LRD mutants (McGrath et al., 2003; Schneider and Brueckner, 2000; Supp et al., 1999). Like the cilia model and the ion flux model, the chromatid segregation model assumes that the anterior-posterior and dorsal-ventral axes are established first, likely at the time of fertilization, and that the LR axis is oriented relative to these two axes; the mechanism responsible for orienting the LR axis relative to the other axes is not yet known, but likely to involve aspects of planar cell polarity (discussed in more detail below). Ultimately, the chromatid segregation model proposes that differential imprinting on the chromatids that go to the left and right sides of the embryo influence expression of a yet-to-be characterized LR-specifying gene, such that this gene is only expressed on one side of the midline. Differential chromatin segregation, as well as mother-daughter cell biased segregation of mRNAs and proteins, are sufficient to maintain asymmetry in yeast (Armakolas et al., 2010; Yu et al., 2012) and have recently been shown to play a role in other eukaryotic cells and embryos (Nakano et al., 2011; Tajbakhsh, 2008; Tajbakhsh and Gonzalez, 2009). Indeed, a very similar scheme, first proposed by A. Klar (Klar, 1994, 2008), has now been implicated in C. elegans asymmetry (Nakano et al., 2011). Interestingly, the plausibility of this model is supported by another example of unequal chromosome distribution – that leading to gynandromorphy, which is known to not only manifest with a clear midline separation (reviewed in (Levin, 2006; Levin and Palmer, 2007)) but also consistent LR bias of male and female organs (Mittwoch, 2000, 2001, 2008).
The PCP model. Planar cell polarity (PCP) is a highly conserved intracellular mechanism used to generate concordant orientation of structures such as the Drosophila wing and eye (Maung and Jenny, 2011), the mammalian kidney (Carroll and Das, 2011), the vertebrate limb (Barrow, 2011), and the lung (Yates and Dean, 2011), among others. To establish intracellular asymmetries, the apical and basal surfaces of the cell are first distinguished by a set of proteins that are preferentially expressed on only one surface (Tree et al., 2002). Next, some components of the PCP pathway become asymmetrically localized to one side of the cell due to reciprocal interactions with other PCP proteins (Vichas and Zallen, 2011). The recent emphasis on PCP as a means to generate LR asymmetry has largely focused on the ability of PCP proteins to properly orient cilia in the node (Gray et al., 2011), viewing PCP as a component of the cilia model. Yet because PCP is an ideal system for coordinating large-scale structures with intracellular polarity (Pohl, 2011; Torban et al., 2012; Wang and Nathans, 2007), it has been suggested to be a mechanism for imposing consistent LR axial orientation on tissues as an amplifying mechanism downstream of cytoskeletal cues (Aw and Levin, 2009; Vandenberg and Levin, 2009). This proposal is supported by subsequent data showing that disrupting PCP proteins in embryos without motile cilia at the node (such as chick) (Zhang and Levin, 2009), or in cells that do not contribute to the ciliated node in frog embryos (Vandenberg and Levin, 2012), specifically disrupts LR patterning. Indeed, recent data show that PCP orientation can be downstream of a directionally-biased microtubule-based transport mechanism (Vladar et al., 2012), precisely as predicted by a model in which PCP spreads and coordinates intracellular chirality information.
Data that distinguish among the models of LR asymmetry
a. Do the data that support the cilia model also refute early models?
Some of the strongest data implicating cilia in LR patterning involve experiments where the viscosity of fluid in the node is altered. In the mouse, altering flow at the node perturbs heart situs in 88% of treated animals and asymmetric gene expression in 73% of embryos (Nonaka et al., 2002; Shinohara et al., 2012); proper heart situs was restored when fast leftward flow was applied to cultured embryos. Interestingly, in Xenopus, altered ciliary flow randomizes the LR axis, but the penetrance is much lower than in the mouse, with only 33% of tadpoles displaying randomized situs (of three organs) (Schweickert et al., 2007). Our own repeated attempts to affect LR patterning by altering the viscosity of fluid in the Xenopus GRP have been unsuccessful; moreover, we have also observed that some aspects of the treatment protocol (i.e. high temperature treatment needed to change the viscosity of injected methylcellulose) can themselves non-specifically randomize LR asymmetry, thus confounding results of methylcellulose injection experiments (Vandenberg & Levin, unpublished observations). Therefore, although these quantitative data indicate that disrupting ciliary flow alters LR patterning, the differences observed in the penetrance of LR phenotypes may indicate differences in the relative contributions of cilia to LR patterning in these different species, and future studies definitively examining the role of cilia in Xenopus are still needed.
Other challenges to the early model have arisen following a series of publications that contested an early role for serotonin, gap junctions and an ion transporter (the H+/K+-ATPase, also called ATP4a) (Beyer et al., 2012a; Beyer et al., 2012b; Walentek et al., 2012). These studies provide evidence to suggest that serotonin, connexins, and H+/K+-ATPase have late roles in the LR asymmetry pathway; altering these pathways also influences ciliary parameters including cilia number, cilia length, flow directionality and flow velocity, suggesting that there are roles for serotonin, gap junctions, and ion transporters in the cilia model, or – as previously argued (Levin, 2003b; Levin and Palmer, 2007), that ciliary phenotypes are a byproduct of early events and thus coincident, but not necessarily causal, of LR defects. Regardless of the functional roles of cilia (which are compatible with early events if cilia are an amplifying or parallel, not initiating, step), do these studies challenge a role for serotonin, gap junctions and the H+/K+-ATPase during early cleavage stages?
The first study, focusing on serotonin signaling, used morpholinos to knockdown serotonin receptor R3 in the cells that contribute to the GRP. This treatment randomized pitx2 expression, altered ciliary flow, and disrupted specification of the superficial mesoderm (Beyer et al., 2012a). While these data suggest additional permissive roles for serotonin during later embryogenesis, these experiments also indicate that LR defects could arise due to non-specific effects on superficial mesoderm specification and altered Wnt signaling pathways. Furthermore, these results cannot address early roles of serotonin because morpholinos do not affect maternal serotonin levels, which are high during early cleavage stages and degrade to low concentrations by the stages where the GRP is formed (Fukumoto et al., 2005a; Fukumoto et al., 2005b). Using dominant negative R3 mRNAs, we have confirmed a role for serotonin during early cleavage stages in cells that do not contribute to the ciliated node (Vandenberg et al., 2012b). These results, when considered together with previously published gene-specific dominant negative and pharmacological data (Fukumoto et al., 2003; Fukumoto et al., 2005b), indicate that serotonin is involved in LR patterning long before its role in GRP specification.
Another study, focusing on gap junctional communication, used morpholinos to target connexin-26 and connexin-32, and showed that knockdown of connexin-26, but not connexin-32, on the left side of the embryo randomizes pitx2 expression (Beyer et al., 2012b). Again, morpholinos are unable to address the maternal connexin proteins that function during cleavage stages - the stages that are implicated in the early orientation of the LR axis (Levin and Mercola, 1998). The authors also propose that the asymmetric coupling of early blastomeres observed previously using small- and large-molecular weight dyes (Levin and Mercola, 1998) are artifacts of imaging through whole embryos (Beyer et al., 2012b; Landesman et al., 2000). Not only do sections of these embryos reveal that small molecular weight dyes are transferred to neighboring cells and large molecular weight dyes are not, indicative of selective transport through gap junctions in the early cleavage stage embryo that rules out wholemount imaging artifacts (Figure 2), their model is unable to account for the fact that inducing gap junctional communication with Cx26 mRNA on the ventral side, or blocking it from either the left or right direction on the dorsal side, but not the reverse operations, each randomize asymmetry, demonstrating that it is the non-ubiquitous spatial distribution of gap junctional states in the embryo that are important. Importantly, (Beyer et al., 2012b) show that connexin-26 morpholinos have no effect on ciliary parameters; however, these morpholinos do affect asymmetry gene expression, confirming that the role for gap junctional communication in LR patterning is distinct from cilia and the node.
Figure 2. Sections through early cleavage stage embryos reveal open gap junctions between blastomeres.
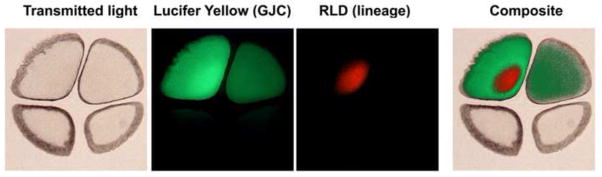
Embryos were co-injected with small and large molecular weight dyes (red labeled rhodamine dextran, 10 kD, and green labeled Lucifer Yellow, ~450 D). The small molecular weight dyes are transferred to neighboring blastomeres via open gap junctions, but large molecular weight dyes do not, ruling out incomplete cleavage or cytoplasmic bridges. Although it has been suggested that previous studies (Levin and Mercola, 1998) reporting these findings were influenced by imaging artifacts of the whole embryo, these sections through the early cleavage stage embryo show the same results – true gap-junctional connectivity.
One more recent paper from the same group focused on the H+/K+-ATPase, which has been shown to play an early role in LR axis specification (Aw et al., 2008; Levin et al., 2002). Knockdown of the H+/K+-ATPase produced randomized expression of pitx2 and alterations in several ciliary parameters (Walentek et al., 2012). However, this study was again based on results obtained with morpholinos, which do not affect maternal H+/K+-ATPase protein present at early cleavage stages (Aw et al., 2008; Levin et al., 2002). Furthermore, the H+/K+-ATPase morphants have diverse and significant phenotypes distinct from LR patterning including shortened anterior-posterior axes, defects of the head, eye and kidney, and reduced pigmentation (Walentek et al., 2012), making it very hard to draw conclusions specifically about LR patterning; these morphologies suggest that the H+/K+-ATPase morpholinos disrupt Wnt signaling – and therefore unsurprisingly alter development of the GRP and disrupt ciliary flow. In any case, a permissive late role for the H+/K+-ATPase in GRP specification does not rule out a specific early role for this ion transporter in LR asymmetry.
In summary, high-resolution studies support a role for cilia movement in asymmetry of mice (Norris, 2012; Shinohara et al., 2012; Yoshiba et al., 2012) and frogs (Blum et al., 2009; Schweickert et al., 2012). No consistent LR asymmetry has been characterized in mouse prior to appearance of the ciliated node; although some asymmetries have been demonstrated in the first few cleavage stages (Roberts et al., 2011; Sun et al., 2011), they have not yet been shown to be consistently biased. The difficulties of early murine embryonic manipulation have precluded serious attempts to test known early mechanisms in the mouse, although it is now known that even 8-cell stage mouse embryo blastomeres are no longer LR-equivalent (suggesting that some degree of LR patterning does occur very early, as in many other species) (Gardner, 2010). Given the major differences in upstream LR patterning steps between mouse and other amniotes such as pig and chick (Gros et al., 2009), it is not clear that mouse is the best model for widely-used asymmetry mechanisms. Another way in which mouse development seems to differ from that of human embryos concerns the LR-bilateral separation of pigmentation patterns in CHILD syndrome that occurs in human beings (Happle, 2002, 2006) but is highly mosaic in mice, even though all of the other important features of this disease are present (Konig et al., 2000). The difference here may be profound precisely because a midline separation resulting from a nondisjunction/inactivation event at very early cleavage stages (as occurs in gynandromorphs (Agate et al., 2003; Levin, 2006)) hints at an extremely early origin of the midline, which may be true in many amniotes but not in mice and is important for early definition of the LR axis. Thus, evidence for cilia as the initiating mechanism in mice does not necessarily apply even to other amniotes, as well as other vertebrates. Regardless of the status of rodents, molecular evidence for early models in mammalian model systems remains one of the major unaddressed opportunities in this field.
b. Challenges to the cilia model, support for the early models
In contrast to the few studies that directly implicate nodal flow in LR patterning (Nonaka et al., 2002; Schweickert et al., 2007; Shinohara et al., 2012), many gaps in the cilia literature raise questions about a causal link between cilia and asymmetry (Levin and Palmer, 2007; Vandenberg and Levin, 2010b). The cilia model is widely thought to be buttressed by the dozens of papers in the mouse model reporting loss of function of ciliary proteins and LR asymmetry defects. However, these studies offer little direct evidence to causally implicate ciliary flow in LR asymmetry because these ‘ciliary’ proteins have non-ciliary functions, and both or either could be responsible for the effect of these mutations and knockdowns on LR patterning (Levin, 2003a; Levin, 2004). In zebrafish, many experimenters target molecular reagents to the KV (the zebrafish equivalent of the node), and when the LR axis is randomized by these treatments, the authors conclude that the ‘ciliary’ protein is required there (Aamar and Dawid, 2010; Ablooglu et al., 2010; Amack et al., 2007; Amack and Yost, 2004; Antic et al., 2010; Bisgrove et al., 2005; Francescatto et al., 2010; Matsui et al., 2011; Schneider et al., 2010; Vick et al., 2009; Wang et al., 2011). However, none of these studies tested the effects of these treatments when specifically targeted to cells that do not contribute to KV, and studies on zebrafish LR patterning almost never include expression or functional data on these molecular targets at pre-KV stages (e.g., cleavage or epiboly timepoints). Thus it is impossible to determine whether these molecular targets specifically affect LR patterning by altering aspects of node function or by an earlier mechanism. Importantly, many studies show less penetrant LR phenotypes when ‘ciliary’ proteins were knocked down specifically at the node compared to when they were knocked down throughout the entire embryo (Table 1), further suggesting that these proteins have important roles in the orientation of the LR axis that can be separated from any explicit actions they might have at the node.
Table 1.
Comparison of LR defect penetrance when constructs are expressed throughout the embryo, compared to the penetrance when targeted only to the node.
| Treatment/mutant | Animal Model | Effect when applied throughout embryo | Effect when directed to node only | Chi Square value, p- value | Reference |
|---|---|---|---|---|---|
| cnpy1 knockdown | zebrafish | 51% with laterality problems 70% with incorrect spaw expression |
41% with laterality problems 42% with incorrect spaw expression |
2.3, p>0.05 12.9, p<0.001 |
(Matsui et al., 2011) |
| Rock2b knockdown | zebrafish | 68% with incorrect spaw expression | 37% with incorrect spaw expression | 31.7, p<0.001 | (Wang et al., 2011) |
| Sox17 knockdown | zebrafish | 58% with incorrect spaw expression 50% with incorrect lefty expression |
44% with incorrect spaw expression 30% with incorrect lefty expression |
1.7, p>0.05 4.7, p<0.05 |
(Aamar and Dawid, 2010) |
| dnah9 knockdown | Xenopus | 55% with incorrect pitx2 expression | 37% with incorrect pitx2 expression | 5.7, p<0.05 | (Vick et al., 2009) |
| dnah5 knockdown | Xenopus | 75% with incorrect pitx2 expression | 63% with incorrect pitx2 expression | 1.3, p>0.05 | (Vick et al., 2009) |
| Tbx16 knockdown | zebrafish | 36% with laterality problems 67% with incorrect spaw expression |
38% with laterality problems 72% with incorrect spaw expression |
sample sizes not provided 0.6, p>0.05 |
(Amack et al., 2007) |
| polaris knockdown | zebrafish | 41% with laterality problems 56% with incorrect lefty expression |
17% with laterality problems 23% with incorrect lefty expression |
15.5, p<0.001 20.7, p<0.001 |
(Bisgrove et al., 2005) |
| pkd2 knockdown | zebrafish | 51% with laterality problems 36% with incorrect lefty expression |
22% with laterality problems 35% with incorrect lefty expression |
21.6, p<0.001 0.0, p>0.05 |
(Bisgrove et al., 2005) |
| ntl knockdown | zebrafish | 100% with incorrect lefty expression | 52% with incorrect lefty expression | 189.0, p<0.001 | (Amack and Yost, 2004) |
Specific examples highlight similar questions about the centrality of the node to the origin of LR asymmetry. The first involves rfx2, a protein involved in ciliogenesis in the zebrafish node. When rfx2 expression is knocked down throughout the zebrafish embryo, randomized organ situs is observed in 35% of embryos (Bisgrove et al., 2012). Yet, when rfx2 expression is restored to the node (i.e. a “rescue” experiment), a significant level of heterotaxia remains (18%), indicating that renewal of this protein in the node does not completely rescue LR patterning. The second example examines the restoration of LRD to the node in LRD mutant mice. Although this rescue does decrease the incidence of inverted heart looping and abnormal pitx2 expression, it cannot completely rescue these defects (Bisgrove et al., 2012). Importantly, many such rescue experiments are not definitive because they utilize gene promoters that are not specific to the node, therefore they may result in expression of the gene of interest prior to the formation of the node, or even in cells that are not destined to be a part of the node. For example, the driving elements used in “node-specific” rescue experiments (Yoshiba et al., 2012), i.e. Nodal, FoxA2, and Pkd2, are all ubiquitously expressed in the embryo at E6.5 (Burtscher and Lickert, 2009; Guillaume and Trudel, 2000; Markowitz et al., 1999; Sasaki and Hogan, 1993; Varlet et al., 1997) and some are present at early cleavage stages (Tang et al., 2009), leaving open the possibility that it is these earlier functions that underlie the functional rescue, not the later expression in the node. Specific knockouts of LRD and other ciliary proteins only at the node, or rescue experiments driving no expression prior to node formation, have not been performed; such genetic manipulations are needed, to provide conclusive evidence as to whether ‘ciliary’ proteins have roles in LR asymmetry that are distinct from their ciliary function at the node. Together, these data suggest that ‘ciliary’ proteins have LR-relevant functions that are distinct from nodal cilia movement, as is clear for several of the major targets in this field such as kinesin3B and LRD (Armakolas and Klar, 2007; Qiu et al., 2005).
We previously compiled a list of molecular genetic reagents that alter LR patterning even when targeted to areas that exclude the GRP in Xenopus embryos (Vandenberg and Levin, 2010b). The list continues to grow and now includes additional examples from the serotonin pathway (Vandenberg et al., 2012b), histone deacetylases (Carneiro et al., 2011), and the PCP pathway (Vandenberg and Levin, 2012). Moreover, there is at least one known example of a ciliary gene, foxj1b, that when knocked down throughout the zebrafish embryo produces LR phenotypes, but when knocked down only in the KV has no effect on LR patterning (Tian et al., 2009). The cilia model also does not accommodate genetic mutants with abnormal or missing cilia but normal LR asymmetry, yet these have been observed (Bangs et al., 2011; Serluca et al., 2009; Valente et al., 2010; Zeng et al., 2010; Zhao and Malicki, 2007), consistent with a model where asymmetry is established by means other than regulated ciliary flow. Thus, much of the data that are widely taken to cement the cilia model do not actually distinguish between the various proposed models for LR patterning. Moreover, aspects of these “ciliary” experiments actually support the early models of LR asymmetry by revealing discordances between ciliary function and LR phenotypes.
The model of cilia as initiators of asymmetry requires that no consistent LR asymmetry appear prior to vortical flow. However, a number of asymmetries (i.e. LR-biased transcription and/or protein expression, asymmetric accumulation of small molecules, asymmetries in physiological measures, etc.) have been detected prior to cilia function even in organisms where ciliated organs exist (Albrieux and Villaz, 2000; Bunney et al., 2003; Fukumoto and Levin, 2005; Kramer et al., 2002; Kramer and Yost, 2002; Ohkawara and Niehrs, 2011; Qiu et al., 2005; Roberts et al., 2011; Stern et al., 1995; Sun et al., 2011), further arguing against cilia as the origin of asymmetry. It is important to keep in mind however that cilia could be important for asymmetry, as amplifiers or a “backup (parallel) pathway”, and not necessarily be the first step.
Recent experiments in frog addressed the relationship between asymmetry and organizer formation, revealing that organizers induced just after the events of early cleavage are not capable of orienting the LR axis properly – a result not compatible with a model of de novo asymmetry establishment by the much later node. UV irradiation prevents the formation of the dorsal-ventral axis in Xenopus embryos by inhibiting cortical rotation (Scharf and Gerhart, 1983; Vincent et al., 1987). Axial patterning is rescued if irradiated embryos are tipped on an angle, physically inducing cortical rotation (Scharf and Gerhart, 1980); patterning can also be restored in later development by injecting components of Spemann’s organizer (i.e. Wnt8, Siamois) or inducing the organizer (via lithium chloride injections) (Engleka and Kessler, 2001; Fan and Sokol, 1997; Kao and Elinson, 1989; Kessler, 1997). The cilia model predicts that proper LR patterning should be observed as long as dorsal-ventral axial patterning is restored prior to the onset of ciliary flow, since ciliary flow is claimed to initiate asymmetry de novo. In contrast, irradiated Xenopus embryos that were rescued at the 1-cell stage via mechanical tipping had normal LR patterning, but irradiated embryos that were rescued at the 32-cell stage (or at gastrula stages) via lithium chloride or Siamois mRNA injections were significantly more likely to have randomized LR asymmetry (Vandenberg and Levin, 2010a). Thus, an organizer that forms just late enough to have missed the early events of the first few cleavages is unable to properly induce LR asymmetry, regardless of its ability to form a normal dorsoanterior axis or any later steps such as ciliogenesis. It has been argued that the incidence of randomized LR asymmetry is low in late-induced organizers (Schweickert et al., 2012), but the rates reported (25–73%) are consistent with the rates of heterotaxia reported for many other molecular genetic reagents that are acknowledged to affect LR patterning [reviewed in (Vandenberg, 2012), see for example (Brizuela et al., 2001; Danilchik et al., 2006; Kramer and Yost, 2002; Ramsdell and Yost, 1999; Yasuhiko et al., 2006)]. These results suggest that timing is critical for orientation of the LR axis in Xenopus: the dorsal-ventral axis must be defined early – within the first cleavage stages – for the LR axis to orient properly, arguing that late events at the GRP are not alone sufficient to initiate normal asymmetry.
The full range of experimental results that cannot be explained by the cilia model, but are consistent with early models of LR axis specification, is summarized in Table 2. Numerous perturbations affect LR patterning when initiated very early but not later in development (Aw et al., 2010; Danilchik et al., 2006; Lobikin et al., 2012; Qiu et al., 2005; Vandenberg et al., 2011), but these have so far been attempted only in externally-developing vertebrates. Timing experiments can be difficult, and it has been argued that drugs can be effective at early stages and ineffective at later stages due to differences in the ability of the chemical to penetrate the embryo at various stages (Blum et al., 2009; Schweickert et al., 2012). Yet, studies using LR-disrupting agents such as low frequency vibrations, which have discrete treatment on/off periods, alter LR patterning when applied early but not when applied later in development (Vandenberg et al., 2011), lending support to the early models.
Table 2.
Experiments distinguish the main models of LR asymmetry
| Experimental Question: | Cilia model predicts: | Ion Flux model predicts: | Chromatid Segregation model predicts: | Experimental Result: | Explanation: |
|---|---|---|---|---|---|
| Mutations cannot distinguish these models of asymmetry: | |||||
| Should mutation or other molecular disruption of kinesin, dynein, and cytoskeletal proteins randomize LR? | YES | YES | YES | YES | These proteins are implicated in all three models (for ciliary motion or for intracellular transport). |
| Should mutation or other molecular disruption of PCP proteins randomize LR? | YES, if needed for proper cilia placement | YES, if PCP amplifies ion flux derived asymmetry | no prediction | YES | PCP is implicated as a means of amplifying LR information in the cilia & ion flux models. |
| Should mutation or other molecular disruption of PCP, MTOC, or motor proteins far from the node randomize LR? | NO | YES | YES | YES | Targeting embryonic regions outside the node randomizes asymmetry, (Vandenberg and Levin, 2012). |
| Should disruption of PCP, MTOC, or motor proteins at the node cause less penetrant LR phenotypes than disruption throughout the entire embryo? | NO | YES, likely | YES, likely | YES | See Table 1 for examples of reagents that are less effective at randomizing asymmetry when targeted only to the node. |
| Cilia are not required for asymmetry: | |||||
| Should viscosity changes at the node randomize LR? | YES | NO, unless cilia amplify | NO, unless cilia amplify | YES | See: (Nonaka et al., 2002; Schweickert et al., 2007). |
| Should there be mutants with abnormal cilia but normal LR asymmetry? | NO | YES | YES | YES | There are now many examples. For review, see (Vandenberg and Levin, 2010b). |
| Should ciliary parameters (number, length, flow rate) be quantitatively predictive of LR defects? | YES | NO | NO | NO | There is significant overlap in ciliary parameters in wild-type and mutant fish (Vandenberg et al., 2012b) |
| Should vertebrates orient LR asymmetry without cilia? | NO | YES | YES | YES | Pig, Chick: (Gros et al., 2009; Yin et al., 2009) |
| Consistent LR asymmetries are observed prior to node formation or ciliary flow: | |||||
| Should consistent asymmetries in protein localization, bioelectric gradients, or other biochemical properties only be observed after ciliary flow stages? | YES | NO | NO | NO | There are many examples of asymmetries present in the early blastomeres. Reviewed in: (Levin, 2006; Vandenberg and Levin, 2010b) |
| Should the very early blastomeres have functionally equivalent LR identity? | YES | NO | NO | NO | See: (Gardner, 2010; Takano et al., 2007) |
| Timing experiments indicate that asymmetry is established early: | |||||
| Should transient disruption of the cytoskeleton during early cleavage stages alter LR patterning? | NO | YES | YES | YES | See: (Danilchik et al., 2006; Qiu et al., 2005; Vandenberg et al., 2011) |
| Should proper LR patterning be able to be established after the first few cleavage stages? | YES | NO | NO | NO | Late organizers cannot orient the LR axis. See: (Vandenberg and Levin, 2010a) |
| Should left-side tissue explants isolated prior to ciliary flow express asymmetric genes? | NO | YES | no prediction | YES | See: (Vandenberg et al., 2012b) |
| There are highly conserved molecular mechanisms for LR asymmetry across phyla & different body plans: | |||||
| Should the same molecules be implicated in LR patterning in ciliated vertebrates and unciliated animals and plants? | NO, unlikely | YES | YES | YES | Many of the same molecules drive asymmetry in plants and animals that have no cilia as in those that do; see for example (Lobikin et al., 2012; Oviedo and Levin, 2007; Vandenberg and Levin, 2010b) |
| Should single cells be able to orient consistent LR asymmetry without node, cilia, or flow? | NO | YES | YES | YES | See: (Chen et al., 2012b; Heacock and Agranoff, 1977; Wan et al., 2011; Xu et al., 2007) |
Additional data raise questions about ciliary function in mammalian asymmetry. The cilia model does not explain why human monozygotic twins, formed from the splitting of a single embryo within days of fertilization, have significant risks of developing LR patterning defects including situs inversus and dextrocardia, at rates far above background in either dizygotic twins or singletons (AlRais et al., 2011; West et al., 2003). Most importantly, mouse embryos formed from 8-cell embryos that were dissociated and rearranged display specific reversals in the direction of axial rotation (Gardner, 2010), revealing that even in rodents the blastomeres are not LR-equivalent long before cilia appear (a result that is further borne out by transcriptomic analysis (Roberts et al., 2011)). Interestingly, this is the same outcome as seen in the experiments involving micromanipulation of blastomeres in snail (Kuroda et al., 2009) and C. elegans embryos (Wood and Kershaw, 1991) (see below for a discussion of conservation of mechanisms).
Finally, a recent study quantified the number of cilia required to establish LR asymmetry in mouse embryos. In this study, mutant mice with diminished numbers of cilia were examined; the authors concluded that embryos with zero or one rotating node cilium had randomized asymmetric gene expression, but embryos with as few as two cilia were normal (Shinohara et al., 2012). With only a few cilia (2 or more), the flow was reduced yet sufficient for proper LR patterning, irrespective of the location of the cilia within the node. It is difficult to reconcile the claim that mutants like inv exhibit laterality defects because of subtle changes in nodal flow (slower speed but correct direction, (Okada et al., 1999)) with the idea that just 2 cilia, anywhere in the node, are sufficient for normal LR patterning.
In summary, although cilia are likely to contribute to asymmetry (in some species), no data prove that they are the only (or the earliest) mechanism for establishing asymmetry. In contrast, considerable data now support the function of alternative, early mechanisms for LR patterning in a wide range of species.
The Question of Conservation
Because numerous model systems (plants, snails, sea urchins, Ciona, chick, C. elegans, pig, and drosophila) orient their LR axis without ever establishing ciliary flow (Bangs et al., 2011; Bienkowska and Cowan, 2012; Gros et al., 2009; Thompson et al., 2012; Vandenberg and Levin, 2010a), the cilia model implies that early steps of asymmetry are extremely different among phyla, but then converge on the same Nodal-Lefty-Pitx2 cassette. In contrast, the cytoplasmic model is based upon the chirality of a cytoplasmic component that is amplified in different ways in different body plans but is itself ancient and extremely well conserved. We recently reviewed in detail the literature that implicates numerous molecular mechanisms in multiple phyla (Levin, 2006; Levin and Palmer, 2007), and suggests that physiological amplification of cytoskeletal asymmetries is a fundamentally conserved “module” (Okumura et al., 2008; Speder et al., 2007), having been dissected in detail in C. elegans (Chang et al., 2011; Pohl, 2011), snails (Kuroda et al., 2009; Shibazaki et al., 2004), and frog (Aw et al., 2008; Danilchik et al., 2006; Levin et al., 2002).
The deep conservation implied by the ciliary model suggests that all kinds of cells can potentially align a LR axis. Remarkably, a number of recent studies have shown that even single cells in culture, having no access to a multicellular node, ciliary motion, or vortical flow, can establish consistent LR asymmetries of movement, outgrowth, shape change, or morphogenesis (Chen et al., 2012a; Hagmann, 1993; Heacock and Agranoff, 1977; Wan et al., 2011; Xu et al., 2007). What these cells have in common is a well-conserved cytoskeletal structure that is well known to possess chirality (Beisson and Jerka-Dziadosz, 1999; Bell et al., 2008; Frankel, 2000). A recent study directly tested this prediction by probing the functional importance of tubulin in the most widely-separated body plans possible; the same -tubulin mutation recovered originally in an Arabidopsis laterality mutant (Abe et al., 2004; Hashimoto, 2002; Thitamadee et al., 2002) produced specific LR defects in C. elegans, Xenopus, and cultured human neutrophils (Lobikin et al., 2012). In frog, introduction of this mutated protein after the first cleavage or expression in cells that contribute to the GRP both had no effect on laterality, demonstrating that its role in asymmetry occurred during the events immediately following fertilization. Taken together, these data implicate the same molecular mechanism as the foundation of asymmetry in widely divergent phyla (across independent origins of multicellularity) – a result not readily consistent with models in which cilia or completely divergent mechanisms are required to pattern the LR axis.
It is, of course, important to consider which kinds of asymmetry observed in single cells are directly related to asymmetry at the level of the body plan, i.e. the LR axis. The PCP model of LR asymmetry suggests mechanisms by which asymmetries that begin in a small number of cells eventually propagate over a larger field or even an entire organ (Adler et al., 2000; Amonlirdviman et al., 2005; Aw and Levin, 2009). In Xenopus, alterations in the expression of PCP proteins in a small number of cells during early stages of Xenopus development can randomize LR asymmetry in the tadpole (Vandenberg and Levin, 2012); similarly, disruptions in PCP protein expression altered LR patterning in chick embryos (Zhang and Levin, 2009). Yet, not every chiral property of cells must be amplified by LR patterning mechanisms. For example, adult C. elegans display chiral fiber orientation in the basal layer of the cuticle that is not affected by genetic manipulations that alter LR asymmetry of the body plan (Bergmann et al., 1998), and similar discordance between chirality on the cell level and placement of organs has been observed in the human heart (Delhaas et al., 2004). Thus, as future studies examine the relationship between cellular chirality and asymmetry of the LR axis, it will be important to determine which cellular polarization properties amplify to higher levels of biological organization and which are ignored at the level of organ systems.
Getting quantitative: can numbers provide insight into these competing models?
The above discussion illustrates that many questions in the LR field depend crucially on which species is examined. Some data are strong in mouse, but weak in frog, and vice versa. Many mechanisms have not been examined in more than one model system at relevant timepoints, making it important to ask what data are missing from the literature as well as what data exist. We conducted several recent meta-analyses of the LR literature, revealing knowledge gaps that are not widely appreciated. In our first analysis, we found a relatively poor correlation between Nodal gene expression and organ placement (Vandenberg, 2012) - gene expression data vastly over-estimate the effects of various treatments on organ position. Even when this analysis includes additional asymmetric genes (Nodal, Lefty and Pitx2) and three model organisms (fish, mouse and frog), the results overwhelmingly indicate that gene expression overestimates the effects of treatments on organ situs, often by a factor of 20–40% (Figure 3).
Figure 3. Asymmetric gene expression is a poor predictor of asymmetric organ situs.
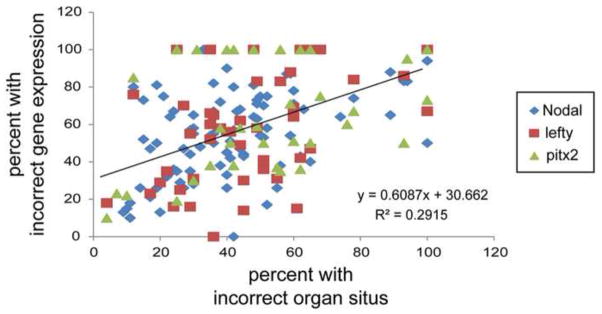
Hundreds of treatments and mutants that were analyzed for both asymmetric expression of Nodal, Lefty or Pitx2 that also reported the effect of treatment on organ situs were examined from the published literature (Vandenberg, 2012). Overwhelmingly, these studies indicate that measures of incorrect gene expression (i.e. a left-sided gene expressed on the right, on both sides, or completely absent) overestimate the effect of a treatment or mutation on organ position. The regression of the data, as indicated on the graph, suggests that ~30% of embryos could have incorrect gene expression, but no problems with organ situs – the definitive readout of embryonic LR asymmetry.
What does this mean for the LR literature? First, studies that only examine asymmetric gene expression are likely to report that treatments are more penetrant than they truly are, considering organ situs is the most relevant endpoint for health outcomes. Thus, if a study reports that 40% of a treated group had abnormal asymmetric gene expression, the effect of that treatment on organ situs could be as low as 0 – 20%. Second, there is missing information on how asymmetric gene expression is translated into asymmetric organ position/morphology: although Nodal is widely accepted as a determinant of organ position, how can it be possible that some embryos have abnormal Nodal expression but normal organ situs? The fact that gene expression overestimates the effects of treatments on organ position suggests a “check-point” between gene expression and organ position, allowing some animals with inappropriate asymmetric gene expression to “correct” this mistake. The nature of this “check-point” is still unknown, but understanding how animals achieve correct target morphology in spite of confounding molecular signals is an important area for future research, particularly because of the tremendous implications for biomedicine and the potential to correct birth defects without surgical interventions (Su et al., 2012; Vandenberg et al., 2012a).
Our original meta-analysis revealed that approximately half of all studies implicating cilia in LR asymmetry made no measure of any parameter of cilia morphology or function (Vandenberg, 2012). Thus, in our second meta-analysis, we examined parameters related to cilia including number, length, and flow qualities in wild-type zebrafish, as one would predict that there would be low variability for parameters that are both necessary and sufficient to establish consistent LR asymmetry in this species. Even when controlling for developmental stage, we found significant differences in cilia number, length and flow quality reported for wild-type fish across different studies (Vandenberg et al., 2012b). We also found examples of mutant or treated fish with ciliary parameters that would resemble wild-type/control fish in other experiments (Vandenberg et al., 2012b). These data further question the causative relationship between cilia number, length or flow parameters and LR defects: looking at ciliary parameters does not allow one to assign a specific case to the “LR normal” vs. “LR abnormal” group – a result not at all consistent with ciliary function as the major determinant of LR asymmetry.
We have tried to perform a similar meta-analysis of data in the mouse model, but this type of assessment proved to be very difficult. Few mouse studies have performed quantitative measures of these parameters (Table 3); the existing publications often list large ranges for the measured values and frequently omit statistical analysis (sometimes having to draw conclusions from as few as 2 or 3 embryos (Bisgrove et al., 2012; Ermakov et al., 2009; McGrath et al., 2003; Nonaka et al., 2002; Okada et al., 1999; Shinohara et al., 2012; Supp et al., 1999; Takeda et al., 1999; Watanabe et al., 2003)). Collectively, analysis of available data indicates that accurate and reproducible measurements of ciliary parameters are difficult to obtain, and standardized methods and reporting criteria are needed before a definitive conclusion can be drawn about any causal relationship between cilia parameters and LR asymmetry.
Table 3.
Ciliary parameters identified from published studies of mice.
| Reference | cilia length (μm) | flow rate (um/s) | number of cilia | rotation speed | age of embryos |
|---|---|---|---|---|---|
| (Beckers et al., 2007) | 3.5 ± 0.5 a, n=109 cilia, (unknown n embryos) | E7.5 | |||
| (Nakaya et al., 2005) | 158 +/− 14, n=4 | E7.75 | |||
| (Ermakov et al., 2009) | 4–6 * | E8.5 | |||
| (Bonnafe et al., 2004) | 1.75 ± 0.25, n=3 | Thelier stage 10c (E7–E8) | |||
| (Bonnafe et al., 2004) | 2 ± 0.05, n=3 | Thelier stage 11a (E7–E8) | |||
| (Bonnafe et al., 2004) | 2.7 ± 0.25, n=3 | Thelier stage 11b (E7–E8) | |||
| (Bonnafe et al., 2004) | 3.3 ± 0.25, n=3 | Thelier stage 11c/d (E7–E8) | |||
| (Bonnafe et al., 2004) | 3.5 ± 0.5, n=3 | Thelier stage 12a (E7–E8) | |||
| (Watanabe et al., 2003) | 600 rpm * | ||||
| (Takeda et al., 1999) | 2–4 * | E7.5 | |||
| (Okada et al., 1999) | 20–50 * | 600 rpm * | |||
| (Supp et al., 1999) | 1.5 * | E7.5 | |||
| (Field et al., 2011) | 2.8 ± 0.7 a, (unknown n) | 11 ± 1 Hz a, (unknown n) | 3–4ss | ||
| (Song et al., 2010) | 11 ± 2 Hz a, (unknown n) | E8 | |||
| (McGrath et al., 2003) | 130–210 * | E7.75 – 8 | |||
| (Bisgrove et al., 2012) | 128, 159 n=2 | E8 | |||
| (Buceta et al., 2005) | 14 ± 6, (unknown n) | ||||
| (Nonaka et al., 2002) | 12.9–16.3 (n=2 beads) | E7.75–e8 | |||
| (Shinohara et al., 2012) | 2.2–4 | 2–3.5, n=25 | 59, 78, 80 (n=3) | LHF stage - 2ss | |
| (Tanaka et al., 2005) | 3.5 ± 0.3 b, n=8 | 1–3ss | |||
| (Tanaka et al., 2005) | 3.85 ± 0.9 b, n=10 | 1–3ss |
no additional information is available from these studies, and no basic statistics were performed.
Indicates standard deviation, as provided in the original study;
Indicates SEM, as provided in the original study; if neither standard deviation or SEM is noted, the authors did not provide information about how these values were derived.
Is it possible to unify these models?
Clearly, there are data that support each of the models of LR asymmetry, including some experiments that truly cannot distinguish between them. For example, studies examining mutants or knockdowns of cytoskeletal proteins that have roles in the intracellular cytoskeleton as well as the cilia cannot discriminate between those two cellular aspects. We have compiled a number of experiments that allow the three major models to be distinguished experimentally (Table 2); in many cases, the results of these studies favor one model over the others.
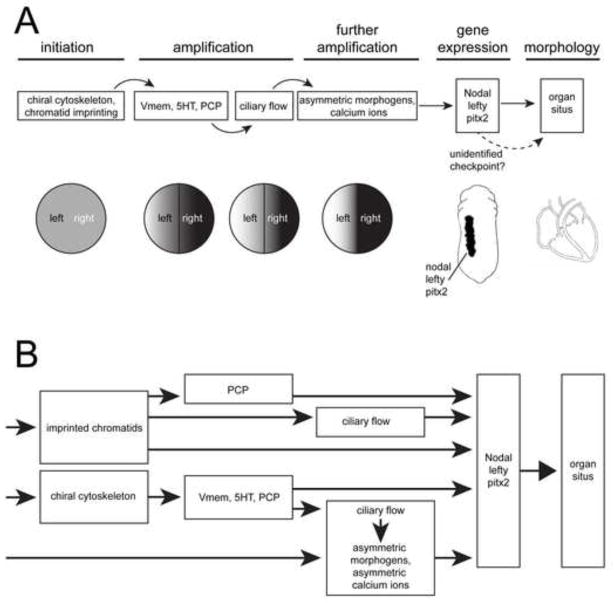
Importantly, many of these results challenge a role for cilia as the initiator of LR asymmetry in several model organisms. However, they do not rule out that cilia could act downstream from other, earlier mechanisms of LR axis orientation. For this reason, it is reasonable to ask whether a unified model of asymmetry can be produced, drawing from both early (ion flux and chromatid segregation) and late (ciliary flow) models. Of course, in each species, there is likely to be only one way (and one developmental time) that LR asymmetry is initiated, and there is strong evidence that this occurs early. Yet, a unified theory of asymmetry could incorporate these early mechanisms together with ciliary flow as an important means by which asymmetry is propagated, amplified, or maintained. Alternatively, ciliary flow could serve as a parallel “check point” for proper LR asymmetry (Figure 4A) functioning to correct defects in previous steps.
Figure 4. Two new models of LR asymmetry pathways.
A) This model proposes that LR asymmetry is initiated during the early cleavage stages of development, but that LR information is then amplified via several mechanisms including the asymmetric distribution of serotonin and the asymmetric motion of cilia to distribute other morphogens and ions. According to this model, LR asymmetry is best established when all mechanisms are working properly. Animals that do not have or utilize cilia for LR patterning (i.e. chick, pig, Drosophila, etc.) depend only on early mechanisms.
B) This model proposes that there are several independent ways that embryos can achieve LR asymmetry. Within a single population, some embryos use one pathway while other embryos use another pathway in a kind of multi-pathway stochasticity. Thus, constructs and pharmaceuticals that target early pathways can only influence LR asymmetry in those embryos that ‘chose’ a pathway that includes early mechanisms; similarly, treatments that alter fluid flow at the node can only influence LR asymmetry in those embryos that ‘chose’ the cilia pathway, resulting in the observed incomplete penetrance observed in most studies.
It is also possible that individual embryos could make a ‘choice’ as to which mechanism they use to initiate the orientation of the LR axis (Figure 4B). In this model, each embryo would stochastically utilize one of several pathways that are each equally capable of orienting the LR axis, and LR patterning in each embryo could only be disrupted by reagents that target the pathway that had been chosen within that embryo. This hypothesis would explain the relatively low penetrance of individual treatments that specifically target early cytoskeletal, ion flux or chromatid segregation endpoints, as well as the low penetrance of treatments that alter ciliary flow in Xenopus embryos, since in any population, a particular reagent would be effective only against those individuals that had chosen that particular pathway for its LR patterning. For example, the highest penetrance of treatments targeting chromatid segregation is expected to be 50% if all embryos choose this pathway (because there is a 50:50 chance of randomly segregating a “left-determining” chromatid to the left blastomere), but the penetrance of treatments targeting chromatid segregation endpoints would be even lower if only a portion of embryos were utilizing this pathway (Sauer and Klar, 2012b).
This model has broader implications for evolutionary developmental biology, since many other examples of variability and robustness in development could potentially involve stochasticity not only with respect to the strength of particular signals (transcript level noise) but also with respect to which of multiple pathways were used in individual organisms. Efforts to address this hypothesis via the disruption of both early and late events have thus far been unsuccessful, as we were repeatedly unable to disrupt LR patterning using the methylcellulose treatments that have been described to target ciliary motion (Vandenberg & Levin, unpublished data). However, future epistasis experiments combining treatments that specifically target early and late events may shine light on this novel hypothesis.
These debates extend far beyond the basic science of building models for developmental biologists; understanding the timing of when the LR axis is oriented, and whether additional amplification steps are necessary for propagation of LR-relevant signaling pathways, has important implications for biomedicine. Pregnant women taking pharmaceuticals that interfere with LR mechanisms could avoid these drugs at very specific periods of gestation to prevent laterality defects in their developing fetuses, but could then return to the treatment as soon as it is safe to do so; this knowledge would allow these patients to have the shortest possible gaps in their treatments. Further, for women that carry fetuses with mutations in LR relevant genes, interventions could be designed for the appropriate period in development. Thus, the question of how and when an embryo orients its LR axis is an important one with implications for biology and medicine.
Conclusion
There are many compelling arguments suggesting that LR asymmetry is established using highly conserved mechanisms among different species (Levin, 2006; Lobikin et al., 2012; Okumura et al., 2008; Vandenberg and Levin, 2010b). In fact, recent studies showing that single cells have chiral properties suggest that asymmetry is an ancient, fundamental property of all cells, and is therefore not unique to vertebrates, the animal kingdom, or even multicellular organisms (Armakolas et al., 2010; Chen et al., 2012a; Hagmann, 1993; Heacock and Agranoff, 1977; Wan et al., 2011; Xu et al., 2007; Yu et al., 2012). It is likely that large-scale asymmetrical patterning pathways have made use of much more ancient cell polarity (Feldman et al., 2007; Marshall, 2011), chirality (Frankel, 2000), or asymmetric cell division (Armakolas et al., 2010) mechanisms. Several models have been proposed for the initiation, orientation and amplification of LR asymmetry, and experimental evidence designed to test these models strongly suggests that the LR axis is established early in development. Yet, this early initiation of the LR axis does not rule out the importance of downstream events, and it is likely that both early and late events must occur with proper coordination for correct laterality to be achieved.
Along with a critique of the latest data in several model species, we have presented sketches of a unified model of LR asymmetry, suggesting that in embryos both early and late events could be necessary for LR patterning. The early events, occurring within the first cleavage stages, are required for the initiation of asymmetry, whereas the later events are required for amplification of LR signals. We have also proposed a novel alternative hypothesis, which postulates that individual embryos use one of several possible mechanisms to initiate asymmetry, orient the LR axis, and amplify LR information. In this model, each embryo makes a stochastic ‘choice’ with respect to which pathway will be active to pattern the LR axis; thus, any given functional perturbation would only affect laterality of a subset of embryos in a population. Testing these two competing hypotheses requires investment in multiple model systems and development of new techniques (such as manipulation of amniote embryos at early cleavage stages), but has the potential to change current paradigms in the field of developmental biology. Most importantly, this information will not only shed new light on the evolutionary re-use of molecular mechanisms in the context of drastic body-plan changes, but also will be invaluable to physicians and scientists concerned with human laterality diseases, and the development of interventions to treat them.
Highlights.
Data strongly support a very early, intracellular origin of left-right asymmetry
Cytoskeletal chirality is a highly conserved mechanism of symmetry breakage
Early events amplify via chromatid segregation, PCP, and ciliary flow
We propose that embryos may choose among parallel pathways to set asymmetry
Acknowledgments
The authors would like to thank members of the Levin Laboratory and Tufts Biology Department for many helpful discussions on this topic, Jean-Francois Pare for assistance locating reference materials, and Douglas Blackiston for providing helpful comments on the manuscript. This work was supported by American Heart Association Established Investigator Grant 0740088N and NIH grant R01-GM077425 (to ML), and NRSA grant 1F32GM087107 (to LNV).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Aamar E, Dawid IB. Sox17 and chordin are required for formation of Kupffer’s vesicle and left-right asymmetry determination in zebrafish. Dev Dyn. 2010;239:2980–2988. doi: 10.1002/dvdy.22431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Thitamadee S, Hashimoto T. Microtubule defects and cell morphogenesis in the lefty1lefty2 tubulin mutant of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:211–220. doi: 10.1093/pcp/pch026. [DOI] [PubMed] [Google Scholar]
- Ablooglu AJ, Tkachenko E, Kang J, Shattil SJ. Integrin alphaV is necessary for gastrulation movements that regulate vertebrate body asymmetry. Development. 2010;137:3449–3458. doi: 10.1242/dev.045310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler PN, Taylor J, Charlton J. The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev. 2000;96:197–207. doi: 10.1016/s0925-4773(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci U S A. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrieux M, Villaz M. Bilateral asymmetry of the inositol trisphosphate-mediated calcium signaling in two-cell ascidian embryos. Biol Cell. 2000;92:277–284. doi: 10.1016/s0248-4900(00)01066-2. [DOI] [PubMed] [Google Scholar]
- Alpatov VV. Specific action of optical isomers of mepacrine upon dextral and sinistral strains of Bacillus mycoides Flugge. Nature. 1946;158:838. doi: 10.1038/158838a0. [DOI] [PubMed] [Google Scholar]
- AlRais F, Feldstein VA, Srivastava D, Gosnell K, Moon-Grady AJ. Monochorionic twins discordant for congenital heart disease: a referral center’s experience and possible pathophysiologic mechanisms. Prenat Diagn. 2011;31:978–984. doi: 10.1002/pd.2819. [DOI] [PubMed] [Google Scholar]
- Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev Biol. 2007;310:196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–690. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armakolas A, Klar AJ. Left-right dynein motor implicated in selective chromatid segregation in mouse cells. Science. 2007;315:100–101. doi: 10.1126/science.1129429. [DOI] [PubMed] [Google Scholar]
- Armakolas A, Koutsilieris M, Klar AJ. Discovery of the mitotic selective chromatid segregation phenomenon and its implications for vertebrate development. Curr Opin Cell Biol. 2010;22:81–87. doi: 10.1016/j.ceb.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H, K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol. 2010;346:39–53. doi: 10.1016/j.ydbio.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity? Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs F, Antonio N, Thongnuek P, Welten M, Davey MG, Briscoe J, Tickle C. Generation of mice with functional inactivation of talpid3, a gene first identified in chicken. Development. 2011;138:3261–3272. doi: 10.1242/dev.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrow J. Wnt/planar cell polarity signaling: an important mechanism to coordinate growth and patterning in the limb. Organogenesis. 2011;7:260–266. doi: 10.4161/org.7.4.19049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu B, Brueckner M. Cilia: multifunctional organelles at the center of vertebrate left-right asymmetry. Curr Top Dev Biol. 2008;85:151–174. doi: 10.1016/S0070-2153(08)00806-5. [DOI] [PubMed] [Google Scholar]
- Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc Natl Acad Sci U S A. 2007;104:15765–15770. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisson J, Jerka-Dziadosz M. Polarities of the centriolar structure: morphogenetic consequences. Biol Cell. 1999;91:367–378. [PubMed] [Google Scholar]
- Bell AJ, Satir P, Grimes GW. Mirror-imaged doublets of Tetmemena pustulata: implications for the development of left-right asymmetry. Dev Biol. 2008;314:150–160. doi: 10.1016/j.ydbio.2007.11.020. [DOI] [PubMed] [Google Scholar]
- Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. doi: 10.1007/DCR.0b013e3181c703a4. [DOI] [PubMed] [Google Scholar]
- Bergmann DC, Crew JR, Kramer JM, Wood WB. Cuticle chirality and body handedness in Caenorhabditis elegans. Dev Genet. 1998;23:164–174. doi: 10.1002/(SICI)1520-6408(1998)23:3<164::AID-DVG2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Beyer T, Danilchik M, Thumberger T, Vick P, Tisler M, Schneider I, Bogusch S, Andre P, Ulmer B, Walentek P, Niesler B, Blum M, Schweickert A. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr Biol. 2012a;22:33–39. doi: 10.1016/j.cub.2011.11.027. [DOI] [PubMed] [Google Scholar]
- Beyer T, Thumberger T, Schweickert A, Blum M. Connexin26-mediated transfer of laterality cues in Xenopus. Biology Open. 2012b;1:473–481. doi: 10.1242/bio.2012760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska D, Cowan CR. Centrosomes can initiate a polarity axis from any position within one-cell C. elegans embryos. Curr Biol. 2012;22:583–589. doi: 10.1016/j.cub.2012.01.064. [DOI] [PubMed] [Google Scholar]
- Bisgrove BW, Makova S, Yost HJ, Brueckner M. RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow. Dev Biol. 2012;363:166–178. doi: 10.1016/j.ydbio.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–288. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Blum M, Beyer T, Weber T, Vick P, Andre P, Bitzer E, Schweickert A. Xenopus, an ideal model system to study vertebrate left-right asymmetry. Dev Dyn. 2009;238:1215–1225. doi: 10.1002/dvdy.21855. [DOI] [PubMed] [Google Scholar]
- Bonnafe E, Touka M, AitLounis A, Baas D, Barras E, Ucla C, Moreau A, Flamant F, Dubruille R, Couble P, Collignon J, Durand B, Reith W. The Transcription Factor RFX3 Directs Nodal Cilium Development and Left-Right Asymmetry Specification. Mol Cell Biol. 2004;24:4417–4427. doi: 10.1128/MCB.24.10.4417-4427.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela BJ, Wessely O, De Robertis EM. Overexpression of the Xenopus tight-junction protein claudin causes randomization of the left-right body axis. Dev Biol. 2001;230:217–229. doi: 10.1006/dbio.2000.0116. [DOI] [PubMed] [Google Scholar]
- Brown NA, Wolpert L. The development of handedness in left/right asymmetry. Development. 1990;109:1–9. doi: 10.1242/dev.109.1.1. [DOI] [PubMed] [Google Scholar]
- Brueckner M. Cilia propel the embryo in the right direction. Am J Med Genet. 2001;101:339–344. doi: 10.1002/1096-8628(20010715)101:4<339::aid-ajmg1442>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Buceta J, Ibanes M, Rasskin-Gutman D, Okada Y, Hirokawa N, Izpisua-Belmonte JC. Nodal cilia dynamics and the specification of the left/right axis in early vertebrate embryo development. Biophys J. 2005;89:2199–2209. doi: 10.1529/biophysj.105.063743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M. Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left-right patterning during amphibian embryogenesis. Development. 2003;130:4847–4858. doi: 10.1242/dev.00698. [DOI] [PubMed] [Google Scholar]
- Burtscher I, Lickert H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development. 2009;136:1029–1038. doi: 10.1242/dev.028415. [DOI] [PubMed] [Google Scholar]
- Carneiro K, Donnet C, Rejtar T, Karger BL, Barisone GA, Diaz E, Kortagere S, Lemire JM, Levin M. Histone deacetylase activity is necessary for left-right patterning during vertebrate development. BMC Dev Biol. 2011;11:29. doi: 10.1186/1471-213X-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll TJ, Das A. Planar cell polarity in kidney development and disease. Organogenesis. 2011;7:180–190. doi: 10.4161/org.7.3.18320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B, Hackett BP. Left-right axis malformations in man and mouse. Curr Opin Genet Dev. 2000;10:257–261. doi: 10.1016/s0959-437x(00)00085-x. [DOI] [PubMed] [Google Scholar]
- Chang C, Hsieh YW, Lesch BJ, Bargmann CI, Chuang CF. Microtubule-based localization of a synaptic calcium-signaling complex is required for left-right neuronal asymmetry in C. elegans. Development. 2011;138:3509–3518. doi: 10.1242/dev.069740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Hsu JJ, Zhao X, Guo C, Wong MN, Huang Y, Li Z, Garfinkel A, Ho CM, Tintut Y, Demer LL. Left-right symmetry breaking in tissue morphogenesis via cytoskeletal mechanics. Circulation research. 2012a;110:551–559. doi: 10.1161/CIRCRESAHA.111.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen TH, Hsu JJ, Zhao X, Guo C, Wong MN, Huang Y, Li Z, Garfinkel A, Ho CM, Tintut Y, Demer LL. Left-Right Symmetry Breaking in Tissue Morphogenesis via Cytoskeletal Mechanics. Circ Res. 2012b;110:551–559. doi: 10.1161/CIRCRESAHA.111.255927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS, Anderson RH, Cohen MI, Atz AM, Fogel M, Gruber PJ, Lopez L, Rome JJ, Weinberg PM. Controversies, genetics, diagnostic assessment, and outcomes relating to the heterotaxy syndrome. Cardiol Young. 2007;17(Suppl 2):29–43. doi: 10.1017/S104795110700114X. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The evolution and genetics of cerebral asymmetry. Philos Trans R Soc Lond B Biol Sci. 2009;364:867–879. doi: 10.1098/rstb.2008.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corballis MC. Lateralization of the human brain. Prog Brain Res. 2012;195:103–121. doi: 10.1016/B978-0-444-53860-4.00006-4. [DOI] [PubMed] [Google Scholar]
- Crow TJ. The ‘big bang’ theory of the origin of psychosis and the faculty of language. Schizophr Res. 2008;102:31–52. doi: 10.1016/j.schres.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Dane S, Gumustekin K, Polat P, Uslu C, Akar S, Dastan A. Relations among hand preference, craniofacial asymmetry, and ear advantage in young subjects. Percept Mot Skills. 2002;95:416–422. doi: 10.2466/pms.2002.95.2.416. [DOI] [PubMed] [Google Scholar]
- Danilchik MV, Brown EE, Riegert K. Intrinsic chiral properties of the Xenopus egg cortex: an early indicator of left-right asymmetry? Development. 2006;133:4517–4526. doi: 10.1242/dev.02642. [DOI] [PubMed] [Google Scholar]
- Delhaas T, Decaluwe W, Rubbens M, Kerckhoffs R, Arts T. Cardiac fiber orientation and the left-right asymmetry determining mechanism. Ann NY Acad Sci. 2004;1015:190–201. doi: 10.1196/annals.1302.016. [DOI] [PubMed] [Google Scholar]
- Engleka MJ, Kessler DS. Siamois cooperates with TGFbeta signals to induce the complete function of the Spemann-Mangold organizer. Int J Dev Biol. 2001;45:241–250. [PubMed] [Google Scholar]
- Ermakov A, Stevens JL, Whitehill E, Robson JE, Pieles G, Brooker D, Goggolidou P, Powles-Glover N, Hacker T, Young SR, Dear N, Hirst E, Tymowska-Lalanne Z, Briscoe J, Bhattacharya S, Norris DP. Mouse mutagenesis identifies novel roles for left-right patterning genes in pulmonary, craniofacial, ocular, and limb development. Dev Dyn. 2009;238:581–594. doi: 10.1002/dvdy.21874. [DOI] [PubMed] [Google Scholar]
- Facchin L, Burgess HA, Siddiqi M, Granato M, Halpern ME. Determining the function of zebrafish epithalamic asymmetry. Philos Trans R Soc Lond B Biol Sci. 2009;364:1021–1032. doi: 10.1098/rstb.2008.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MJ, Sokol SY. A role for Siamois in Spemann organizer formation. Development. 1997;124:2581–2589. doi: 10.1242/dev.124.13.2581. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Geimer S, Marshall WF. The mother centriole plays an instructive role in defining cell geometry. PLoS Biol. 2007;5:e149. doi: 10.1371/journal.pbio.0050149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–1142. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francescatto L, Rothschild SC, Myers AL, Tombes RM. The activation of membrane targeted CaMK-II in the zebrafish Kupffer’s vesicle is required for left-right asymmetry. Development. 2010;137:2753–2762. doi: 10.1242/dev.049627. [DOI] [PubMed] [Google Scholar]
- Frankel J. Intracellular handedness in ciliates. CIBA Found Symposium: Biological Asymmetry and Handedness. 1991:73–88. doi: 10.1002/9780470514160.ch6. [DOI] [PubMed] [Google Scholar]
- Frankel J. Cell polarity in ciliates. In: Drubin DG, editor. Cell Polarity. Oxford University Press; 2000. pp. 78–105. [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema I, Nazarenko D, Levin M. Serotonin is a novel very early signaling mechanism in left-right asymmetry. Developmental Biology. 2003;259:490. [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Levin M. Asymmetric expression of Syndecan-2 in early chick embryogenesis. Gene Expr Patterns. 2005;5:525–528. doi: 10.1016/j.modgep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Gardner RL. Normal bias in the direction of fetal rotation depends on blastomere composition during early cleavage in the mouse. PLoS One. 2010;5:e9610. doi: 10.1371/journal.pone.0009610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RS, Roszko I, Solnica-Krezel L. Planar cell polarity: coordinating morphogenetic cell behaviors with embryonic polarity. Dev Cell. 2011;21:120–133. doi: 10.1016/j.devcel.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–944. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmotti V, Cristino L. The interplay between the pineal complex and the habenular nuclei in lower vertebrates in the context of the evolution of cerebral asymmetry. Brain Res Bull. 2006;69:475–488. doi: 10.1016/j.brainresbull.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Guillaume R, Trudel M. Distinct and common developmental expression patterns of the murine Pkd2 and Pkd1 genes. Mechanisms of development. 2000;93:179–183. doi: 10.1016/s0925-4773(00)00257-4. [DOI] [PubMed] [Google Scholar]
- Hackett BP. Formation and malformation of the vertebrate left-right axis. Curr Mol Med. 2002;2:39–66. doi: 10.2174/1566524023363031. [DOI] [PubMed] [Google Scholar]
- Hagmann J. Pattern formation and handedness in the cytoskeleton of human platelets. Proc Natl Acad Sci U S A. 1993;90:3280–3283. doi: 10.1073/pnas.90.8.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happle R. Dohi Memorial Lecture. New aspects of cutaneous mosaicism. J Dermatol. 2002;29:681–692. doi: 10.1111/j.1346-8138.2002.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Happle R. X-chromosome inactivation: role in skin disease expression. Acta Paediatricia Suppl. 2006;95:9–10. doi: 10.1080/08035320600618775. [DOI] [PubMed] [Google Scholar]
- Hashimoto T. Molecular genetic analysis of left-right handedness in plants. Philos Trans R Soc Lond B Biol Sci. 2002;357:799–808. doi: 10.1098/rstb.2002.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heacock AM, Agranoff BW. Clockwise growth of neurites from retinal explants. Science. 1977;198:64–66. doi: 10.1126/science.897684. [DOI] [PubMed] [Google Scholar]
- James WH. Handedness, birth weight, mortality and Barker’s hypothesis. J Theor Biol. 2001;210:345–346. doi: 10.1006/jtbi.2001.2309. [DOI] [PubMed] [Google Scholar]
- Kao KR, Elinson RP. Dorsalization of mesoderm induction by lithium. Dev Biol. 1989;132:81–90. doi: 10.1016/0012-1606(89)90207-8. [DOI] [PubMed] [Google Scholar]
- Kessler DS. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci U S A. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ. A model for specification of the left-right axis in vertebrates. Trends Genet. 1994;10:392–396. doi: 10.1016/0168-9525(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Klar AJ. Support for the selective chromatid segregation hypothesis advanced for the mechanism of left-right body axis development in mice. Breast Dis. 2008;29:47–56. doi: 10.3233/bd-2008-29106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig A, Happle R, Bornholdt D, Engel H, Grzeschik KH. Mutations in the NSDHL gene, encoding a 3beta-hydroxysteroid dehydrogenase, cause CHILD syndrome. Am J Med Genet. 2000;90:339–346. [PubMed] [Google Scholar]
- Kramer KL, Barnette JE, Yost HJ. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell. 2002;111:981–990. doi: 10.1016/s0092-8674(02)01200-x. [DOI] [PubMed] [Google Scholar]
- Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Developmental Cell. 2002;2:115–124. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- Krommydas GC, Gourgoulianis KI, Raftopoulos V, Kotrotsiou E, Paralikas T, Agorogiannis G, Molyvdas PA. Non-right-handedness and asthma. Allergy. 2004;59:892–893. doi: 10.1111/j.1398-9995.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- Kuroda R, Endo B, Abe M, Shimizu M. Chiral blastomere arrangement dictates zygotic left-right asymmetry pathway in snails. Nature. 2009;462:790–794. doi: 10.1038/nature08597. [DOI] [PubMed] [Google Scholar]
- Landesman Y, Goodenough DA, Paul DL. Gap junctional communication in the early Xenopus embryo. J Cell Biol. 2000;150:929–936. doi: 10.1083/jcb.150.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhart KF, Holtzman NG, Williams JR, Burdine RD. Integration of Nodal and BMP Signals in the Heart Requires FoxH1 to Create Left-Right Differences in Cell Migration Rates That Direct Cardiac Asymmetry. PLoS genetics. 2013;9:e1003109. doi: 10.1371/journal.pgen.1003109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CM, Eckert MA. Asymmetry and dyslexia. Dev Neuropsychol. 2008;33:663–681. doi: 10.1080/87565640802418597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Left-right asymmetry and the chick embryo. Semin Cell Dev Biol. 1998;9:67–76. doi: 10.1006/scdb.1997.0192. [DOI] [PubMed] [Google Scholar]
- Levin M. Hypothesis: motor proteins and ion pumps, not ciliary motion, initiate LR asymmetry. BioEssays. 2003a;25:1002–1010. doi: 10.1002/bies.10339. [DOI] [PubMed] [Google Scholar]
- Levin M. Motor protein control of ion flux is an early step in embryonic left-right asymmetry. Bioessays. 2003b;25:1002–1010. doi: 10.1002/bies.10339. [DOI] [PubMed] [Google Scholar]
- Levin M. The embryonic origins of left-right asymmetry. Crit Rev Oral Biol Med. 2004;15:197–206. doi: 10.1177/154411130401500403. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- Levin M, Nascone N. Two molecular models of initial left-right asymmetry generation. Med Hypotheses. 1997;49:429–435. doi: 10.1016/s0306-9877(97)90092-x. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Lobikin M, Wang G, Xu J, Hsieh YW, Chuang CF, Lemire JM, Levin M. Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms. Proc Natl Acad Sci U S A. 2012;109:12586–12591. doi: 10.1073/pnas.1202659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz GS, Cai Y, Li L, Wu G, Ward LC, Somlo S, D’Agati VD. Polycystin-2 expression is developmentally regulated. The American journal of physiology. 1999;277:F17–25. doi: 10.1152/ajprenal.1999.277.1.F17. [DOI] [PubMed] [Google Scholar]
- Marshall WF. Origins of cellular geometry. BMC biology. 2011;9:57. doi: 10.1186/1741-7007-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui T, Thitamadee S, Murata T, Kakinuma H, Nabetani T, Hirabayashi Y, Hirate Y, Okamoto H, Bessho Y. Canopy1, a positive feedback regulator of FGF signaling, controls progenitor cell clustering during Kupffer’s vesicle organogenesis. Proc Natl Acad Sci U S A. 2011;108:9881–9886. doi: 10.1073/pnas.1017248108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maung SM, Jenny A. Planar cell polarity in Drosophila. Organogenesis. 2011;7:165–179. doi: 10.4161/org.7.3.18143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–392. doi: 10.1016/s0959-437x(03)00091-1. [DOI] [PubMed] [Google Scholar]
- McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- McManus C. Reversed bodies, reversed brains, and (some) reversed behaviors: of zebrafish and men. Dev Cell. 2005;8:796–797. doi: 10.1016/j.devcel.2005.05.006. [DOI] [PubMed] [Google Scholar]
- McManus IC, Bryden MP, Bulmer-Fleming MB. Handedness and autoimmune disease. Lancet. 1993;341:891–892. [PubMed] [Google Scholar]
- Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–2394. doi: 10.1245/s10434-008-0015-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittwoch U. Genetics of sex determination: exceptions that prove the rule. Mol Genet Metab. 2000;71:405–410. doi: 10.1006/mgme.2000.3075. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Genetics of mammalian sex determination: some unloved exceptions. J Exp Zool. 2001;290:484–489. doi: 10.1002/jez.1091. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Different gene expressions on the left and the right: a genotype/phenotype mismatch in need of attention. Ann Hum Genet. 2008;72:2–9. doi: 10.1111/j.1469-1809.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Biochem Biophys. 2008;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Hamada H. Left-right patterning: conserved and divergent mechanisms. Development. 2012;139:3257–3262. doi: 10.1242/dev.061606. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell. 2006;11:495–504. doi: 10.1016/j.devcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Nakano S, Stillman B, Horvitz HR. Replication-Coupled Chromatin Assembly Generates a Neuronal Bilateral Asymmetry in C. elegans. Cell. 2011;147:1525–1536. doi: 10.1016/j.cell.2011.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakaya MA, Biris K, Tsukiyama T, Jaime S, Rawls JA, Yamaguchi TP. Wnt3a links left-right determination with segmentation and anteroposterior axis elongation. Development. 2005;132:5425–5436. doi: 10.1242/dev.02149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelsen EM, Frankel J, Jenkins LM. Non-genic inheritance of cellular handedness. Development. 1989;105:447–456. doi: 10.1242/dev.105.3.447. [DOI] [PubMed] [Google Scholar]
- Nonaka S, Shiratori H, Saijoh H, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–99. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- Norris DP. Cilia, calcium and the basis of left-right asymmetry. BMC Biol. 2012;10:102. doi: 10.1186/1741-7007-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkawara B, Niehrs C. An ATF2-based luciferase reporter to monitor non-canonical Wnt signaling in Xenopus embryos. Dev Dyn. 2011;240:188–194. doi: 10.1002/dvdy.22500. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Okumura T, Utsuno H, Kuroda J, Gittenberger E, Asami T, Matsuno K. The development and evolution of left-right asymmetry in invertebrates: lessons from Drosophila and snails. Dev Dyn. 2008;237:3497–3515. doi: 10.1002/dvdy.21788. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Levin M. Gap junctions provide new links in left-right patterning. Cell. 2007;129:645–647. doi: 10.1016/j.cell.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Pai VP, Vandenberg LN, Blackiston D, Levin M. Neural derived tissues in Xenopus laevis embryos exhibit a consistent physiological left-right asymmetry. Stem Cells International. 2012 doi: 10.1155/2012/353491. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulozzi LJ, Lary JM. Laterality patterns in infants with external birth defects. Teratology. 1999;60:265–271. doi: 10.1002/(SICI)1096-9926(199911)60:5<265::AID-TERA7>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet. 2006;49:349–362. doi: 10.1016/j.ejmg.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Perloff JK. The cardiac malpositions. Am J Cardiol. 2011;108:1352–1361. doi: 10.1016/j.amjcard.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Pohl C. Left-right patterning in the C. elegans embryo: Unique mechanisms and common principles. Commun Integr Biol. 2011;4:34–40. doi: 10.4161/cib.4.1.14144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–189. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF, Yost HJ. Cardiac looping and the vertebrate left-right axis: antagonism of left-sided Vg1 activity by a right-sided ALK2-dependent BMP pathway. Development. 1999;126:5195–5205. doi: 10.1242/dev.126.23.5195. [DOI] [PubMed] [Google Scholar]
- Roberts RM, Katayama M, Magnuson SR, Falduto MT, Torres KE. Transcript profiling of individual twin blastomeres derived by splitting two-cell stage murine embryos. Biol Reprod. 2011;84:487–494. doi: 10.1095/biolreprod.110.086884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers LJ, Zucca P, Vallortigara G. Advantages of having a lateralized brain. Proc Biol Sci. 2004;271(Suppl 6):S420–422. doi: 10.1098/rsbl.2004.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussigne M, Blader P, Wilson SW. Breaking symmetry: the zebrafish as a model for understanding left-right asymmetry in the developing brain. Dev Neurobiol. 2012;72:269–281. doi: 10.1002/dneu.20885. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Hogan BL. Differential expression of multiple fork head related genes during gastrulation and axial pattern formation in the mouse embryo. Development. 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- Sauer S, Klar AJ. Left-right symmetry breaking in mice by left-right dynein may occur via a biased chromatid segregation mechanism, without directly involving the Nodal gene. Frontiers in Oncology. 2012a doi: 10.3389/fonc.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer S, Klar AJ. Left-right symmetry breaking in mice by left-right dynein may occur via a biased chromatid segregation mechanism, without directly involving the Nodal gene. Front Oncol. 2012b;2:166. doi: 10.3389/fonc.2012.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC. Determination of the dorsal-ventral axis in eggs of Xenopus laevis: complete rescue of UV-impaired eggs by oblique orientation before first cleavage. Dev Biol. 1980;79:181–198. doi: 10.1016/0012-1606(80)90082-2. [DOI] [PubMed] [Google Scholar]
- Scharf SR, Gerhart JC. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983;99:75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- Schnall BS, Smith DW. Nonrandom laterality of malformations in paired structures. J Pediatr. 1974;85:509–511. doi: 10.1016/s0022-3476(74)80454-3. [DOI] [PubMed] [Google Scholar]
- Schneider H, Brueckner M. Of mice and men: dissecting the genetic pathway that controls left-right asymmetry in mice and humans. Am J Med Genet. 2000;97:258–270. [PubMed] [Google Scholar]
- Schneider I, Schneider PN, Derry SW, Lin S, Barton LJ, Westfall T, Slusarski DC. Zebrafish Nkd1 promotes Dvl degradation and is required for left-right patterning. Dev Biol. 2010;348:22–33. doi: 10.1016/j.ydbio.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder MF. Unilateral anomalies of kidney development: why is left not right? Kidney Int. 2011;80:740–745. doi: 10.1038/ki.2011.204. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Walentek P, Thumberger T, Danilchik M. Linking early determinants and cilia-driven leftward flow in left-right axis specification of Xenopus laevis: a theoretical approach. Differentiation. 2012;83:S67–77. doi: 10.1016/j.diff.2011.11.005. [DOI] [PubMed] [Google Scholar]
- Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–66. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Xu B, Okabe N, Baker K, Lin SY, Sullivan-Brown J, Konieczkowski DJ, Jaffe KM, Bradner JM, Fishman MC, Burdine RD. Mutations in zebrafish leucine-rich repeat-containing six-like affect cilia motility and result in pronephric cysts, but have variable effects on left-right patterning. Development. 2009;136:1621–1631. doi: 10.1242/dev.020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibazaki Y, Shimizu M, Kuroda R. Body handedness is directed by genetically determined cytoskeletal dynamics in the early embryo. Curr Biol. 2004;14:1462–1467. doi: 10.1016/j.cub.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, Reith W, Durand B, Shiratori H, Hamada H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat Commun. 2012;3:622. doi: 10.1038/ncomms1624. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Hamada H. The left-right axis in the mouse: From origin to morphology. Development. 2006;133:2095–2104. doi: 10.1242/dev.02384. [DOI] [PubMed] [Google Scholar]
- Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The reduction in colorectal cancer mortality after colonoscopy varies by site of the cancer. Gastroenterology. 2010;139:1128–1137. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speder P, Petzoldt A, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr Opin Genet Dev. 2007;17:351–358. doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Stern C, Yu R, Kakizuka A, Kintner C, Mathews L, Vale W, Evans R, Umesono K. Activin and its receptors during gastrulation and the later phases of mesoderm development in the chick embryo. Developmental Biology. 1995;172:192–205. doi: 10.1006/dbio.1995.0015. [DOI] [PubMed] [Google Scholar]
- Su CY, Bay SN, Mariani LE, Hillman MJ, Caspary T. Temporal deletion of Arl13b reveals that a mispatterned neural tube corrects cell fate over time. Development. 2012;139:4062–4071. doi: 10.1242/dev.082321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JH, Zhang Y, Yin BY, Li JX, Liu GS, Xu W, Tang S. Differential expression of Axin1, Cdc25c and Cdkn2d mRNA in 2-cell stage mouse blastomeres. Zygote. 2011:1–6. doi: 10.1017/S0967199411000347. [DOI] [PubMed] [Google Scholar]
- Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Dev Suppl. 1999;126:5495–5504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabin CJ. The key to left-right asymmetry. Cell. 2006;127:27–32. doi: 10.1016/j.cell.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Tabin CJ. Establishing robust left-right asymmetry in the vertebrate embryo. Dev Cell. 2011;20:e2. [Google Scholar]
- Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S. Stem cell identity and template DNA strand segregation. Curr Opin Cell Biol. 2008;20:716–722. doi: 10.1016/j.ceb.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Gonzalez C. Biased segregation of DNA and centrosomes: moving together or drifting apart? Nat Rev Mol Cell Biol. 2009;10:804–810. doi: 10.1038/nrm2784. [DOI] [PubMed] [Google Scholar]
- Takano K, Ito Y, Obata S, Oinuma T, Komazaki S, Nakamura H, Asashima M. Heart formation and left-right asymmetry in separated right and left embryos of a newt. Int J Dev Biol. 2007;51:265–272. doi: 10.1387/ijdb.072270kt. [DOI] [PubMed] [Google Scholar]
- Takeda S, Yonekawa H, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left-right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–836. doi: 10.1083/jcb.145.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–177. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, Wang X, Bodeau J, Tuch BB, Siddiqui A, Lao K, Surani MA. mRNA-Seq whole-transcriptome analysis of a single cell. Nature methods. 2009;6:377–382. doi: 10.1038/nmeth.1315. [DOI] [PubMed] [Google Scholar]
- Thitamadee S, Tuchihara K, Hashimoto T. Microtubule basis for left-handed helical growth in Arabidopsis. Nature. 2002;417:193–196. doi: 10.1038/417193a. [DOI] [PubMed] [Google Scholar]
- Thompson H, Shaw MK, Dawe HR, Shimeld SM. The formation and positioning of cilia in Ciona intestinalis embryos in relation to the generation and evolution of chordate left-right asymmetry. Developmental biology. 2012 doi: 10.1016/j.ydbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Tian T, Zhao L, Zhang M, Zhao X, Meng A. Both foxj1a and foxj1b are implicated in left-right asymmetric development in zebrafish embryos. Biochem Biophys Res Commun. 2009;380:537–542. [Google Scholar]
- Torban E, Iliescu A, Gros P. An expanding role of vangl proteins in embryonic development. Curr Top Dev Biol. 2012;101:237–261. doi: 10.1016/B978-0-12-394592-1.00005-3. [DOI] [PubMed] [Google Scholar]
- Tree DR, Ma D, Axelrod JD. A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol. 2002;13:217–224. doi: 10.1016/s1084-9521(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Uslu H, Dane S, Uyanik MH, Ayyildiz A. Relationships between intestinal parasitosis and handedness. Laterality. 2010;15:465–474. doi: 10.1080/13576500903049316. [DOI] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, Adams M, Szymanska K, Mazzotta A, Lee JE, Tolentino JC, Swistun D, Salpietro CD, Fede C, Gabriel S, Russ C, Cibulskis K, Sougnez C, Hildebrandt F, Otto EA, Held S, Diplas BH, Davis EE, Mikula M, Strom CM, Ben-Zeev B, Lev D, Sagie TL, Michelson M, Yaron Y, Krause A, Boltshauser E, Elkhartoufi N, Roume J, Shalev S, Munnich A, Saunier S, Inglehearn C, Saad A, Alkindy A, Thomas S, Vekemans M, Dallapiccola B, Katsanis N, Johnson CA, Attie-Bitach T, Gleeson JG. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN. Laterality defects are influenced by timing of treatments and animal model. Differentiation. 2012;83:26–37. doi: 10.1016/j.diff.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Adams DS, Levin M. Normalized shape and location of perturbed craniofacial structures in the Xenopus tadpole reveal an innate ability to achieve correct morphology. Developmental Dynamics. 2012a;241:863–878. doi: 10.1002/dvdy.23770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Lemire JM, Levin M. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis Model Mech. 2012b doi: 10.1242/dmm.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Perspectives and open problems in the early phases of left-right patterning. Semin Cell Dev Biol. 2009;20:456–463. doi: 10.1016/j.semcdb.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Consistent left-right asymmetry cannot be established by late organizers in Xenopus unless the late organizer is a conjoined twin. Development. 2010a;137:1095–1105. doi: 10.1242/dev.041798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Far from solved: a perspective on what we know about early mechanisms of left-right asymmetry. Dev Dyn. 2010b;239:3131–3146. doi: 10.1002/dvdy.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Levin M. Polarity proteins are required for left-right axis orientation and twin-twin instruction. Genesis. 2012;50:219–234. doi: 10.1002/dvg.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Pennarola BW, Levin M. Low frequency vibrations disrupt left-right patterning in the Xenopus embryo. PLoS One. 2011;6:e23306. doi: 10.1371/journal.pone.0023306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varlet I, Collignon J, Robertson EJ. nodal expression in the primitive endoderm is required for specification of the anterior axis during mouse gastrulation. Development. 1997;124:1033–1044. doi: 10.1242/dev.124.5.1033. [DOI] [PubMed] [Google Scholar]
- Vichas A, Zallen JA. Translating cell polarity into tissue elongation. Semin Cell Dev Biol. 2011;22:858–864. doi: 10.1016/j.semcdb.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol. 2009;331:281–291. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Scharf SR, Gerhart JC. Subcortical rotation in Xenopus eggs: a preliminary study of its mechanochemical basis. Cell Motility & the Cytoskeleton. 1987;8:143–154. doi: 10.1002/cm.970080206. [DOI] [PubMed] [Google Scholar]
- Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Current biology: CB. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walentek P, Beyer T, Thumberger T, Schweickert A, Blum M. ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Reports. 2012 doi: 10.1016/j.celrep.2012.1003.1005. In press. [DOI] [PubMed] [Google Scholar]
- Wan LQ, Ronaldson K, Park M, Taylor G, Zhang Y, Gimble JM, Vunjak-Novakovic G. Micropatterned mammalian cells exhibit phenotype-specific left-right asymmetry. Proc Natl Acad Sci U S A. 2011;108:12295–12300. doi: 10.1073/pnas.1103834108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Cadwallader AB, Jang DS, Tsang M, Yost HJ, Amack JD. The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer’s vesicle in zebrafish. Development. 2011;138:45–54. doi: 10.1242/dev.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130:1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- West PM, Love DR, Stapleton PM, Winship IM. Paternal uniparental disomy in monozygotic twins discordant for hemihypertrophy. J Med Genet. 2003;40:223–226. doi: 10.1136/jmg.40.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilting J, Hagedorn M. Left-right asymmetry in embryonic development and breast cancer: common molecular determinants? Current medicinal chemistry. 2011;18:5519–5527. doi: 10.2174/092986711798347252. [DOI] [PubMed] [Google Scholar]
- Wood WB, Kershaw D. Handed asymmetry, handedness reversal and mechanisms of cell fate determination in nematode embryos. CIBA Found Symposium: Biological Asymmetry and Handedness. 1991:143–159. doi: 10.1002/9780470514160.ch9. discussion 159–164. [DOI] [PubMed] [Google Scholar]
- Xu J, Van Keymeulen A, Wakida NM, Carlton P, Berns MW, Bourne HR. Polarity reveals intrinsic cell chirality. Proc Natl Acad Sci U S A. 2007;104:9296–9300. doi: 10.1073/pnas.0703153104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhiko Y, Shiokawa K, Mochizuki T, Asashima M, Yokoyama T. Isolation and characterization of Xenopus laevis homologs of the mouse inv gene and functional analysis of the conserved calmodulin binding sites. Cell Res. 2006;16:337–346. doi: 10.1038/sj.cr.7310044. [DOI] [PubMed] [Google Scholar]
- Yates LL, Dean CH. Planar polarity: A new player in both lung development and disease. Organogenesis. 2011;7:209–216. doi: 10.4161/org.7.3.18462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, Tickle C. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–664. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–231. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost HJ. Left-right asymmetry: nodal cilia make and catch a wave. Curr Biol. 2003;13:R808–809. doi: 10.1016/j.cub.2003.09.051. [DOI] [PubMed] [Google Scholar]
- Yu C, Bonaduce MJ, Klar AJ. Defining the Epigenetic Mechanism of Asymmetric Cell Division of Schizosaccharomyces japonicus Yeast. Genetics. 2012 doi: 10.1534/genetics.112.146233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Hoover AN, Liu A. PCP effector gene Inturned is an important regulator of cilia formation and embryonic development in mammals. Dev Biol. 2010;339:418–428. doi: 10.1016/j.ydbio.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Levin M. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis. 2009;47:719–728. doi: 10.1002/dvg.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Malicki J. Genetic defects of pronephric cilia in zebrafish. Mech Dev. 2007;124:605–616. doi: 10.1016/j.mod.2007.04.004. [DOI] [PubMed] [Google Scholar]