Abstract
Background
Chronic alcohol use results in many pathological effects including alcoholic liver disease (ALD). ALD pathogenesis requires endotoxemia. Our previous studies showed that increased intestinal permeability is the major cause of endotoxemia and that this gut leakiness is dependent on alcohol stimulation of inducible nitric oxide synthase (iNOS) in both alcoholic subjects and rodent models of alcoholic steatohepatitis (ASH). The mechanism of the alcohol-induced, iNOS-mediated disruption of the intestinal barrier function is not known. We have recently shown that alcohol stimulates activation of the transcription factor Snail and biomarkers of epithelial mesenchymal transition. Since activated Snail disrupts tight junctional proteins , we hypothesized that activation of Snail by iNOS might be one of the key signaling pathways mediating alcohol stimulated intestinal epithelial cell hyperpermeability.
Methods
We measured intestinal permeability in alcohol-fed C57BL/6 control and iNOS KO mice and measured Snail protein expression in the intestines of these mice. We then examined intestinal epithelial permeability using the Caco-2 cell model of the intestinal barrier ± siRNA inhibition of Snail. We assessed Snail activation by alcohol in Caco-2 cells ± inhibition of iNOS with L-NIL or siRNA. Finally, we assessed Snail activation by alcohol ± inhibition with siRNA for p21-activated kinase (PAK1).
Results
Our data show that chronic alcohol feeding promotes intestinal hyperpermeability in wild type BL/6 but not in iNOS KO mice. Snail protein expression was increased in the intestines of alcohol-treated wild type mice but not in iNOS KO mice. SiRNA inhibition of Snail significantly inhibited alcohol-induced hyperpermeability in Caco-2 cell monolayers. Alcohol stimulation of SnailpS246 activation was blocked by inhibition of iNOS with L-NIL or with siRNA. SiRNA inhibition of PAK1 significantly inhibited alcohol-mediated activation of Snail in Caco-2 cells.
Conclusions
Our data confirmed our prior results and further demonstrated that alcohol-induced gut leakiness in rodents and intestinal epithelial cell monolayers is iNOS dependent. Our data also support a novel role for Snail activation in alcohol-induced, iNOS mediated intestinal hyperpermeability and that PAK1 is responsible for activation of Snail at Ser246 with alcohol stimulation. Identification of these mechanisms for alcohol-induced intestinal hyperpermeability may provide new therapeutic targets for prevention and treatment of alcohol-induced leaky gut, endotoxemia and endotoxin- associated complications of alcoholism such as ALD.
Keywords: alcohol, iNOS, intestinal permeability, Snail, epithelial-mesenchymal transition
Introduction
Chronic alcohol use results in many pathological effects including alcoholic liver disease (ALD) which is the second leading cause of liver transplantation in the U.S. (Grant et al., 1988; Mandayam et al., 2004). Alcohol use is increasing worldwide and is a significant healthcare burden of almost $200 billion annually in the U.S. alone (Rehm et al., 2003). Although alcohol has direct deleterious effects on hepatocytes and causes fatty liver in heavy drinkers and alcohol fed rodents (Purohit et al., 2008a), only 20–30% of alcoholics develop alcoholic steatohepatitis and progressive liver disease leading to cirrhosis and liver failure (Grant et al., 1988; Purohit et al., 2008a). Thus, an additional co factor is required to cause sustained hepatic inflammation and liver cell loss in alcoholics. Indeed, we and others have shown that gut derived endotoxin is the key co-factor and intestinal hyperpermeability [leaky gut] is the major source of endotoxemia in alcoholics and alcohol fed rodents (Bjarnason et al., 1984; Bode et al., 1987; Keshavarzian et al., 1999; Rao et al., 2004).
We have previously shown that alcohol-induced intestinal hyperpermeability is dependent on alcohol activation of iNOS in vitro (Banan et al., 1999; Banan et al., 2000) and in vivo (Tang et al., 2009). However, the mechanism through which iNOS disrupts intestinal barrier integrity after alcohol stimulation has not been clearly established. The aim of the current study was to determine the mechanism of iNOS mediated gut leakiness by alcohol. We chose to study the role of the transcription factor Snail in alcohol-induced disruption of intestinal epithelial cell permeability because: (1) Snail is a redox sensitive transcription factor and thus iNOS may activate Snail (Radisky et al., 2005), (2) We have recently shown that alcohol at physiological concentrations as low as 0.2% (43mM) results in phosphorylation of Snail at Ser246 (SnailpS246) as well as stimulation of nuclear localization of SnailpS246 in the Caco-2 cell line and also in the nontransformed IEC-6 intestinal cell line (Forsyth et al., 2010); (3) Such nuclear localization of SnailpSer246 has been shown to be required for Snail activation as a transcription factor stimulating epithelial mesenchymal transition (EMT) (Yang et al., 2005). EMT is characterized by increased expression of such mesenchymal markers as vimentin as well as decreased expression of epithelial cell markers, especially cell-cell junctional proteins (Thiery and Sleeman, 2006; Acloque et al., 2009). These include proteins of the adherens junction such as E-cadherin as well as tight junctional proteins such as occludin and claudins(Thiery and Sleeman, 2006); (4) We showed that alcohol activation of SnailpS246 is associated with increased expression of vimentin and other markers of EMT (Forsyth et al., 2010) ; and (5) We found that Snail expression was increased in the colons of active alcoholics (Forsyth et al., 2010).
We therefore hypothesized that alcohol activation of iNOS-mediated oxidative stress results in activation of Snail and activated Snail will lead to disruption of tight junctional proteins and gut leakiness. To test this hypothesis, we used both an alcohol fed mouse model of gut leakiness and an alcohol-treated Caco-2 intestinal epithelial cell monolayer in vitro model of intestinal permeability.
MATERIALS AND METHODS
Reagents
Alcohol (ethanol) solutions were made daily (AAPER Alcohol and Chemical Co., Shelbyville, KY). L NIL [L-N6-(1-iminoethyl)-lysine.2 HCL] was from A.G. Scientific (San Diego, CA). FSA [fluorescein 5/6 sulfonic acid, trisodium salt] was from Invitrogen (Carlsbad, CA). Histone H3, total Snail, PAK1, and iNOS Abs were from Cell Signaling Technology, (Danvers, MA); SnailpS246 Ab was from Abcam (Cambridge, MA), while anti-actin Ab was from Sigma (St. Louis, MO). Fluorescent secondary Ab was anti-rabbit Alexa fluor 488 (Invitrogen).
Cell culture
Caco-2 cells (ATCC #CRL2101, human colorectal adenocarcinoma) were cultured at 37°C/5% CO2 in DMEM/10% Fetal Bovine Serum media with 5 mM penicillin-streptomycin (Banan et al., 1999). Cell viability was routinely tested with live/dead assay (Invitrogen) or trypan blue staining and found to be >95% for all experiments.
Treatment of cells with alcohol
Caco-2 cell monolayers were treated with a physiologically relevant dose of alcohol (0.2%, 43mM, about 2–4 drinks). Cells were stimulated once with alcohol (at the indicated concentrations) at the start of each experiment. Alcohol concentrations in media were determined with the alcohol testing kit (Pointe Scientific, Canton, MI). Nuclear extracts were prepared using a nuclear extraction kit (Pierce Biotechnology, Rockford, IL). Signaling experiments were terminated by removal of media and addition of PBS for scrapping, SDS/RIPA buffer for whole cell lysates, or nuclear extract kit buffer.
Measurement of protein expression
Total Snail, SnailpS246, PAK1, iNOS, and β-actin (loading control) protein levels were assayed by western blot. Western blotting and densitometry analysis with Image J software (NIH) was performed with cell or tissue lysates equalized for total protein and cell number as previously described (Forsyth et al., 2002; Forsyth et al., 2007). For animal tissue analysis proximal colon tissue from mice in each of the respective 4 treatment groups was obtained at the time of sacrifice using a Rush IACUC approved experimental protocol and IACUC approved CO2 inhalation euthanasia. Proximal colon tissue was removed and immediately placed in cryovials in liquid NO2 and later transferred to −80°C freezers for storage. Samples for western blotting are prepared by homogenization in lysis buffer and loaded in wells equalized for 30 µg total protein after protein is measured as described (Tang et al., 2009).
Immunofluorescent staining of cells
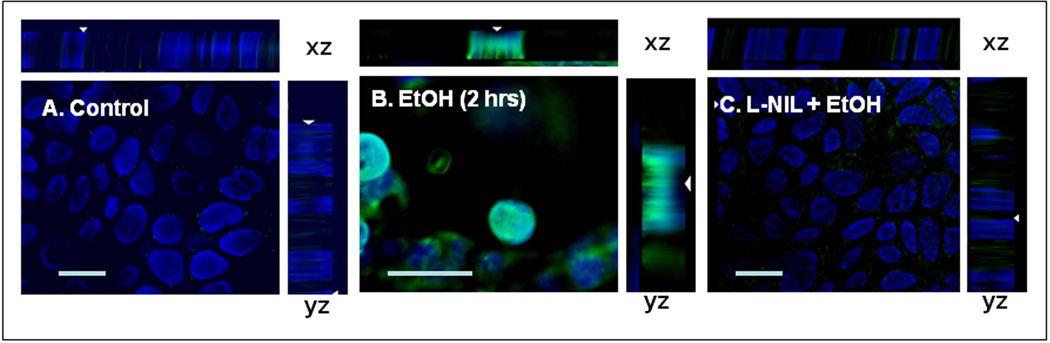
Cells grown on glass coverslips in complete medium in 6-well plates were treated with alcohol (0.2%– 43mM) for the indicated times in three separate experiments. Cells were then washed with PBS, fixed/permeabilized with paraformaldhyde/triton x-100, and stained with primary and fluorescent secondary Abs as described (Forsyth et al., 2007). For the SnailpS246 images (Fig. 4) (20×1µM z- stacks) were taken. All images were taken using a Zeiss Axiovert 100 microscope with Axiovision software (Carl Zeiss Inc, Thornwood, NY) using an oil immersion 40× objective as described (Forsyth et al., 2007) except for panel 4B which was taken using a 63× objective for greater detail. Scale bars in all panels equal 10µm. The plane of focus selected was the one that revealed the greatest overall clarity for any given image. Images are representative from three experiments in triplicate (N=9).
Fig. 4. Immunofluorescent staining of Caco-2 cells reveals iNOS inhibition prevents alcohol stimulated nuclear SnailpS246 localization.
Caco-2 cells grown on glass coverslips in 6-well plates were treated with alcohol (EtOH, 0.2%) for 2h and then fixed and stained with Ab to SnailpS246+FITC 2° Ab. Nuclei were stained with DAPI. Cells for 4C were also pretreated with the iNOS inhibitor L-NIL (200µM, 1h).Images are representative from triplicate coverslips from three separate experiments (N=9). Scale Bar = 10 microns. Images were obtained using Zeiss Axiovision imaging as described in Methods with a 40× oil immersion objective (4A&C) and a 63× objective for greater detail in 4B.
Intestinal epithelial cell monolayer barrier function
Barrier permeability was determined using Caco-2 cells grown to confluence on Type 1 collagen coated 12 mm/0.2µm PET tissue culture plate inserts (Transwell, Corning) as we previously described (Banan et al., 1999; Banan et al., 2000; Forsyth et al., 2007). Permeability of insert Caco-2 monolayers was measured as apical to basolateral flux of the fluorescent marker fluorescein-5-(and- 6)-sulfonic acid trisodium salt (FSA, 478 d) (Invitrogen) or as Transepithelial Electrical Resistance (TER). TER is determined using a dual electrode system designed for cell culture insert analysis (EVOM, World Precision Instruments, Sarasota, FL). Fluorescent FSA signals were quantitated using a fluorescence plate reader and reported as “FSA Clearance” (nmol/cm2/h). Excitation/emission spectra for FSA were: 485 nm/530 nm. Controls and a standard curve were done with each run.
Treatment of cells with siRNA
Caco-2 cells were treated with siRNA using a modification of our previously published methods (Forsyth et al., 2010). Briefly, 105 cells are combined with 11 picomoles of siRNA in 50µl Lipofectamine (Invitrogen, Carlsbad, CA) and 50ul Optimem (Invitrogen), mixed by gently shaking and then plated on Type 1 collagen coated Corning 12mm PET culture inserts (#3460) (Corning Inc, Corning, NY) with addition of another 400ul (upper well) and 1ml (lower well) Optimem. Inserts are used for permeability studies 72–96h after plating, when cells are confluent. PAK1 and iNOS SiRNA were On Target Smartpool from Dharmacon (Lafayette, CO), while control (nontargeting) siRNA was from Santa Cruz Biotechnology (Santa Cruz, CA).
Alcohol diet and intestinal permeability in mice
Male 6–8wk C57BL/6J (control strain) and iNOS (NOS2; specifically # 002609; B6.129P2- Nos2tm1Lau/J; C57BL/6 background strain and C57BL/6J is the Jackson Labs recommended control) whole animal KO mice were purchased from Jackson Labs (Bar harbor, ME). Genotypes of all NOS2 KO mice were verified by PCR using DNA prepared from tail snips. Pair-fed mice were maintained on a fish oil liquid diet with 28% of calories from alcohol or control liquid diet with dextrose matched for calories for 4 weeks as described (N=6 each condition)(Tipoe et al., 2008). The alcohol dose was gradually increased every 2 to 3 days up to a maximum of 28% calories by 2 weeks. Study Day 1 was defined as the first day mice received 28% of calories as alcohol in the liquid diet. Mice received diet ad lib and were weighed daily. Intestinal permeability was measured twice: (i) just prior to alcohol administration; (ii) just before sacrifice. For intestinal permeability testing, mice were maintained in a metabolic cage (for 6 hour urine collection) and gut permeability is measured for each mouse after a 200µl sugar bolus by gavage as we previously described(Farhadi et al., 2006; Keshavarzian et al., 2009a). Each mouse received 1ml of normal saline (s.c.) prior to sugar gavage in order to increase urine output. Measurement of urinary sugars using GC is used to calculate intestinal permeability and is expressed as percent oral dose excreted in the urine as we have described(Keshavarzian et al., 1999; Farhadi et al., 2006; Keshavarzian et al., 2009b). The widely used 6 hour urinary L/M ratio represents small bowel permeability (L = lactulose, M=mannitol) (Hollander, 1999).All animal experiments were conducted under an animal protocol approved by the Rush University Medical Center IACUC.
Data Analysis and Statistical analysis
The data are presented as means ± SE. For protein levels, group means were compared by ANOVA and post-hoc tests since the data were normally distributed. For permeability data in mice, group medians were compared using the nonparametric analyses, Kruskal-Wallis test, because data were not normally distributed. P-values < 0.05 were considered statistically significant. All analyses were done using SPSS (SPSS Inc., Chicago, IL).
RESULTS
Alcohol-induced intestinal permeability is significantly prevented in iNOS KO mice
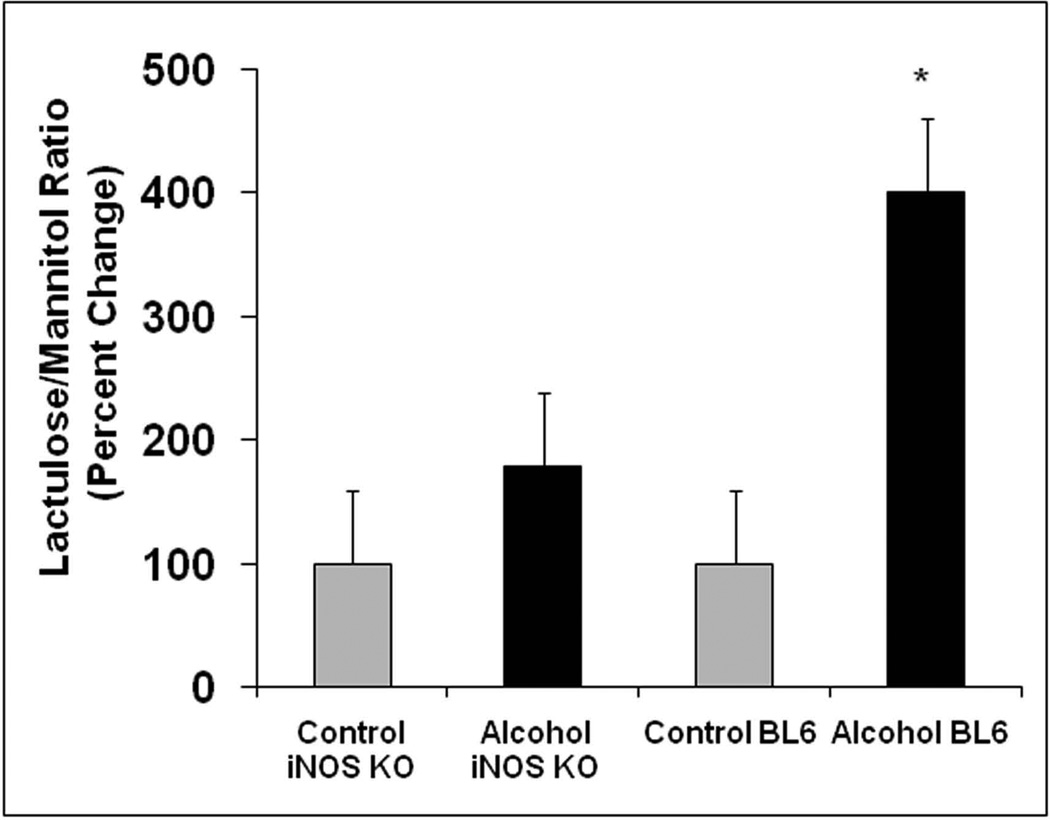
To further investigate the role of iNOS in alcohol- induced intestinal hyperpermeability, we compared the effects of 4 weeks of a daily alcohol containing liquid diet on intestinal permeability in WT BL/6 mice vs. iNOS KO mice [Fig. 1]. As expected, alcohol feeding caused marked disruption of intestinal barrier function in wild type mice with a 300% increase in L/M ratio [P<0.05 alcohol fed WT mice vs dextrose containing liquid pair fed WT mice]. In contrast, alcohol failed to significantly increase the urinary L/M ratio in iNOS KO mice compared to pair fed dextrose control iNOS KO mice. We acknowledge that lack of statistically significant differences between alcohol fed iNOS KO and pair fed iNOS KO could be due to sample size. However, considering that there was also a marked difference in urinary L/M ratio between alcohol fed iNOS KO mice [only 80% alcohol feeding increase in L/M ratio] and alcohol fed wild type mice [300% alcohol feeding increase in L/M ratio] this significant increase in L/M ratio in wild type but not in iNOS KO alcohol fed mice indicates that upregulation of iNOS is the primary mechanism of alcohol to increase the urinary L/M ratio. An increased L/M ratio is thought to represent increased paracellular permeability(Hollander, 1999). These data further support our prior findings establishing that alcohol-induced gut leakiness is iNOS dependent.
Figure 1. Alcohol fed BL/6 mice and not iNOS KO mice exhibit increased intestinal permeability (L/M ratio).
WT BL/6 and iNOS KO mice were fed a liquid diet containing 4.5% alcohol (28% calories) for 4 weeks as described in Methods and then intestinal permeability was determined by gavage of a sugar solution described in Methods followed by analysis of excreted urine sugar levels by GC using 6h urine samples. Data are means ± SE for N=6 mice each condition and are presented as percent change from initial respective control baseline (set as 100%) of the L/M ratio (lactulose/mannitol ratio) with * p<.05 for BL/6 mice given alcohol compared to BL/6 mice without alcohol (300% increase) while the iNOS KO mice showed no significant difference ± alcohol (only 80% increase).
iNOS activation is required for alcohol-induced activation of Snail
We hypothesized that alcohol mediated activation of iNOS might be involved in stimulation of Snail expression/activation by alcohol. To test this hypothesis, we first compared expression of Snail protein in the intestinal tissue from alcohol fed BL/6 and alcohol fed INOS KO mice and then determined the effects of iNOS inhibition in Caco-2 intestinal epithelial cell monolayers.
Snail transcription factor expression is increased in the intestines of alcohol fed wild type [WT] mice but not alcohol fed iNOS KO mice
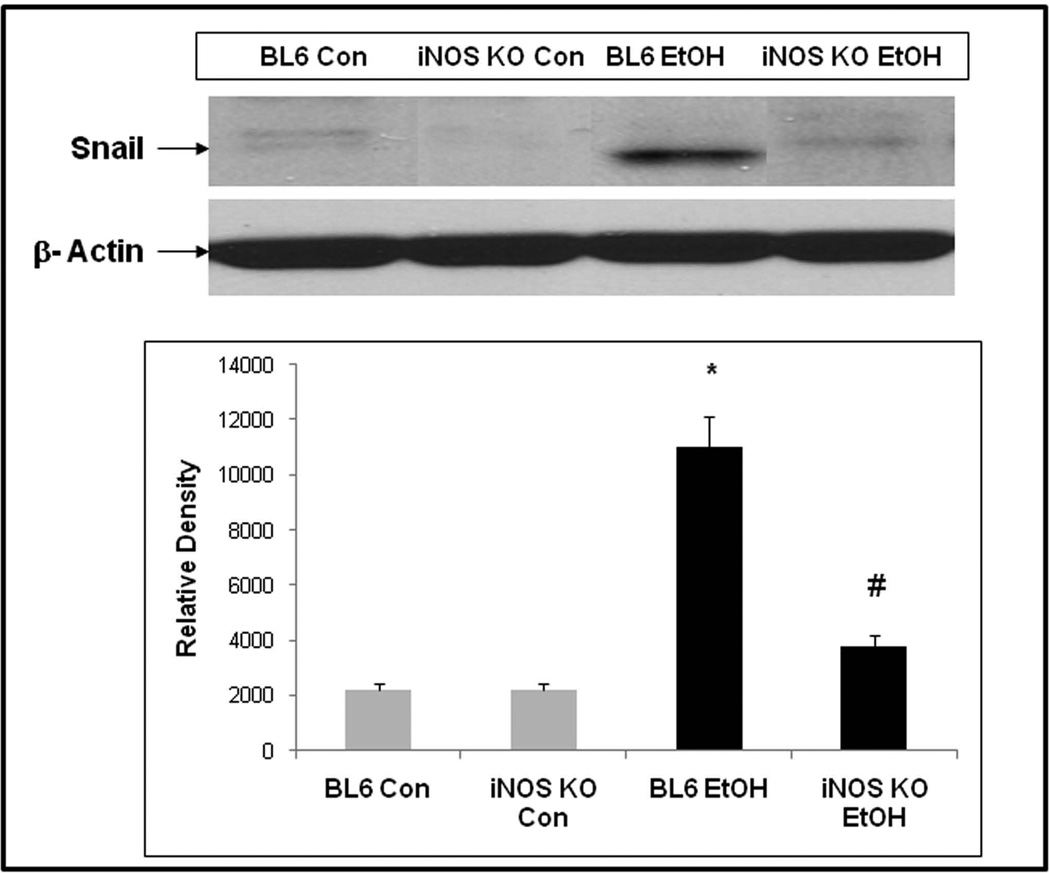
Figure 2 depicts representative western blot data (from N=6 mice, each condition) and shows that Snail protein (30kd) was significantly increased in the intestines proximal colons) of alcohol fed BL/6 mice but not in alcohol fed iNOS KO mice. This finding supports our hypothesis that chronic alcohol feeding increases intestinal Snail protein levels and that this activation requires iNOS.
Fig. 2. Chronic alcohol feeding results in increased intestinal Snail protein expression in WT BL/6 but not in iNOS KO mice.
WT BL/6 and iNOS KO mice were fed a liquid diet containing 4.5% alcohol (28% calories) for 4 weeks as described in Methods and then levels of Snail (30 kDa) were assessed in proximal colon intestinal tissue by western blotting of tissue lysates equalized for total protein with Ab to total Snail. Upper panel shows representative blots from single mice, histogram densitometry data are means ± SE for all mice (N=6 each condition).* p < 0.05 for alcohol fed BL/6 mice vs. corresponding BL/6 control. # p < 0.05 for alcohol fed BL/6 mice vs. iNOS KO alcohol fed mice. Blots were stripped and reprobed with Ab to actin (42 kDa) to further control for protein loading.
Alcohol-induced Snail transcription factor activation is prevented by iNOS inhibition in Caco-2 cell monolayers
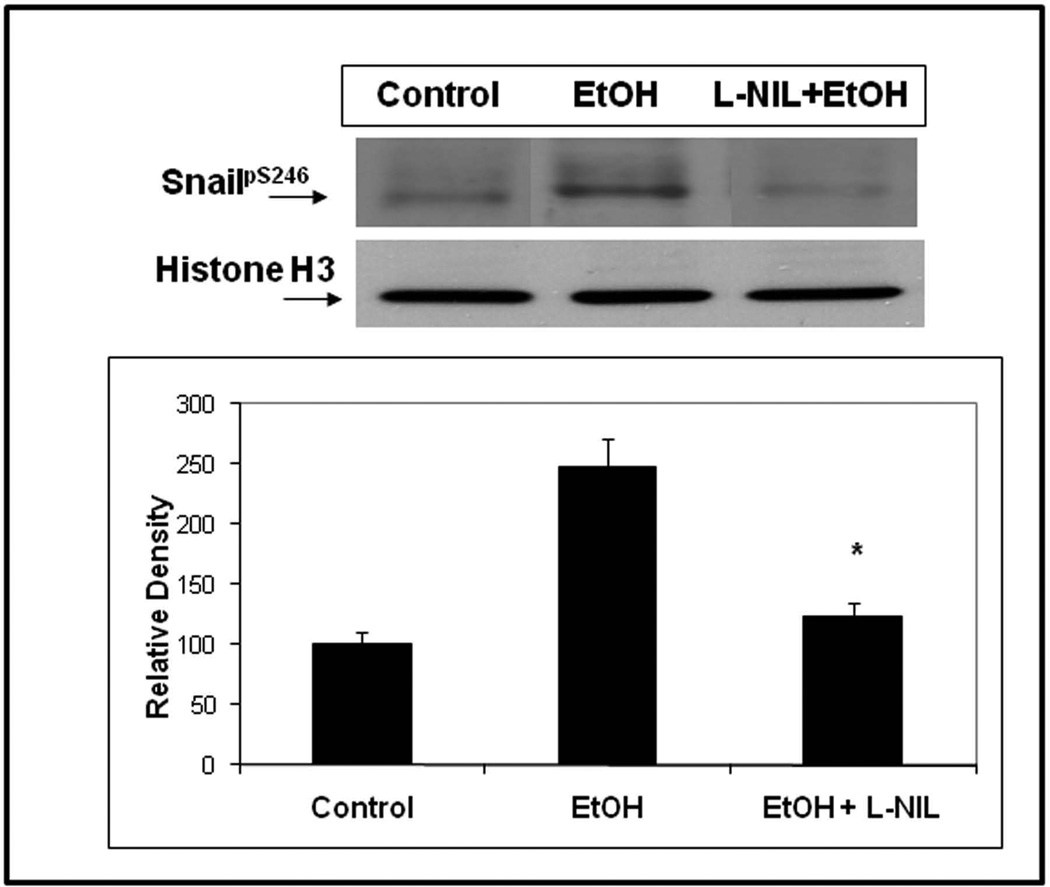
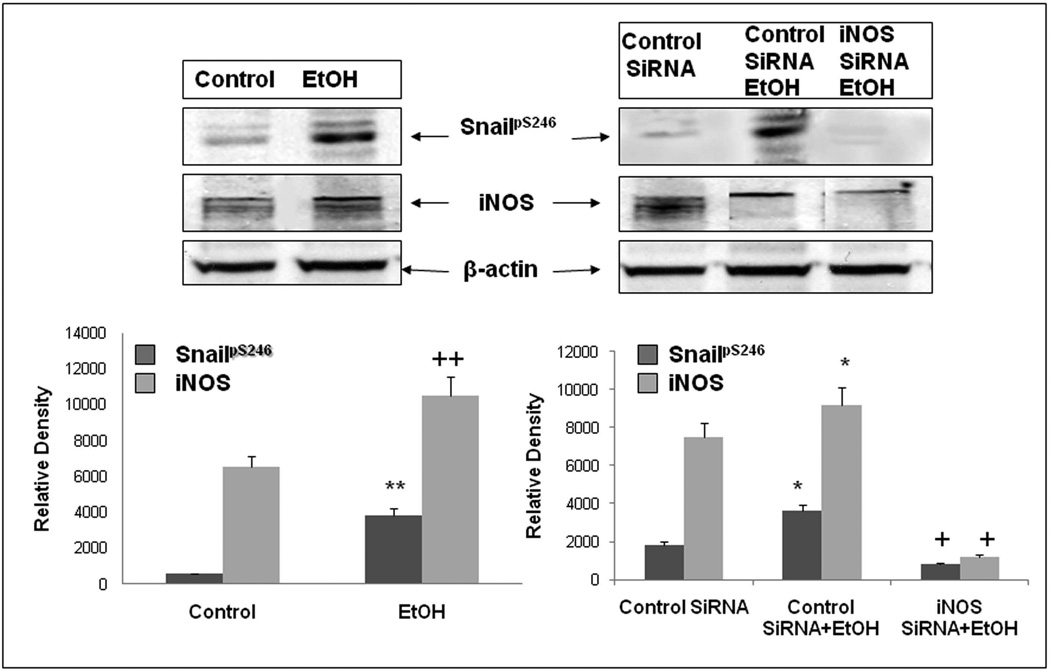
As seen in Fig. 3, treatment of Caco-2 cells with the specific iNOS inhibitor, L-NIL 200µM), significantly blocked alcohol-induced nuclear localization of SnailpS246(p<.05). In addition, we treated Caco-2 cells grown on glass coverslips with alcohol ± L-NIL and performed digital deconvolution of z-stack images using antibody staining for SnailpS246. As seen in Fig. 4, virtually no SnailpS246 is visible in the nucleus of untreated cells (Fig. 4A), while treatment with alcohol resulted in significant nuclear accumulation of SnailpS246 after 2h (Fig. 4B). However, treatment with L-NIL virtually eliminated alcohol-induced nuclear accumulation of SnailpS246 (Fig. 4C), supporting a key role for iNOS signaling in alcohol activation of Snail In intestinal epithelial cells. Finally, to further assess the role of iNOS in alcohol activation of Snail, we pretreated cells with siRNA specific for iNOS or a control (nontargeting) siRNA and found that siRNA specific for iNOS (avg. 65% knockdown) significantly inhibited (p<.05) alcohol-stimulated accumulation of SnailpS246 ;while control siRNA had no significant effect on alcohol stimulated SnailpS246 nuclear accumulation (Fig. 5). Taken together these data strongly support a significant role for iNOS in activation of Snail by alcohol in intestinal epithelial cells.
Fig. 3. The iNOS specific inhibitor L-NIL blocks alcohol-induced activation of SnailpS246.
Caco-2 intestinal epithelial cells grown on culture inserts were assessed for activation of Snail ± 0.2% alcohol (EtOH) treatment for 2h using western blotting of protein equalized nuclear extracts and Ab to SnailpS246.Some cells in lane three were pretreated for 1h with 200µM L-NIL iNOS inhibitor. Blots were stripped and reprobed with Ab to the nuclear protein Histone H3 (17 kDa) to demonstrate equal loading. Blot data are from representative wells from 4 separate experiments in triplicate (N=12). Histogram densitometry data are summarized means ± SE for N=12 for each condition. * p< .05 EtOH+ L-NIL vs. EtOH treatment alone.
Fig. 5. Knocking down iNOS with siRNA prevents EtOH-induced SnailpS246 in Caco-2 cells.
Caco-2 cells grown on inserts were assessed for Snail protein phosphorylation at S246 by western blotting of whole cell lysates with lysis buffer containing 0.2%SDS. Lysates were also assessed for levels of iNOS protein to determine knockdown efficiency and β-actin as a loading control. Some cells were pretreated as described with either control siRNA or siRNA specific for iNOS. Designated cells were treated with 0.2% (43mM) alcohol (EtOH) for 2h. Blots are from representative wells from triplicate wells in one of four separate experiments (N=12). Lower histogram densitometry data are summarized means ± SE for all N=12 wells for each condition for SnailpS246 (dark bars) and iNOS protein (130 kDa) (light bars). **/++ p< .05 for alcohol vs. media treated control. * p< .05 EtOH + control siRNA compared to control siRNA alone treated cells for the respective protein.+ p<.05 iNOS siRNA + EtOH compared to control siRNA + EtOH treated cells.
Alcohol stimulation of SnailpS246 activation requires PAK1
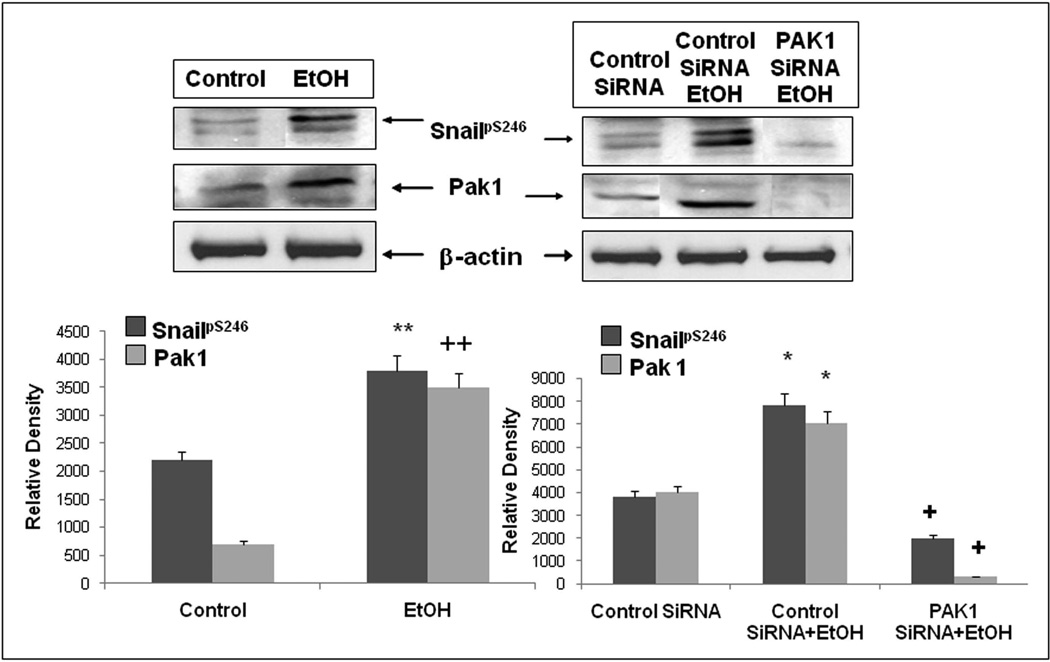
Although other investigators have established that PAK1 mediates phosphorylation (activation) of Snail at Ser246, those studies were carried out in breast cancer and human 293 kidney cell lines (Yang et al., 2005). We therefore wanted to establish whether PAK1 was also mediating alcohol stimulated phosphorylation of SnailpS246 in Caco-2 intestinal epithelial cells. We used siRNA specific for PAK1 or control siRNA and pretreated cells prior to stimulation with 0.2% alcohol for 2h and assessed nuclear SnailpS246 accumulation by western blotting of nuclear extracts for Snail and cytoplasmic fractions for PAK1 expression. As seen in Fig.6, siRNA specific for PAK1 (avg. >80% knockdown) significantly inhibited (p<.05) phosphorylation of Snail at Ser246 in alcohol treated cells. Thus PAK1 is also the principal kinase responsible for Snail activation by alcohol in Caco-2 intestinal epithelial cells.
Figure 6. Inhibition of PAK1 expression with siRNA prevents alcohol-induced activation of SnailpS246.
Caco-2 cells grown on inserts were assessed for Snail phosphorylation at S246 by western blotting of whole cell lysates with lysis buffer containing 0.2%SDS. Lysates were also assessed for levels of PAK1 to determine knockdown efficiency and β-actin as a loading control. Some cells were pretreated as described with either control siRNA or siRNA specific for PAK1. Designated cells were treated with 0.2% (43mM) alcohol (EtOH) for 2h. Data are representative wells from triplicate wells in one of four separate experiments (N=12). Lower densitometry histograms are for means ± SE for all N=12 wells for each condition for SnailpS246 (dark bars) and PAK1 (light bars). **/++ p< .05 for alcohol vs. media treated control. * p< .05 EtOH + control siRNA compared to control siRNA alone treated cells for the respective protein.+ p<.05 PAK1 siRNA + EtOH compared to control siRNA + EtOH treated cells.
SiRNA inhibition of Snail prevents alcohol-induced intestinal permeability in Caco-2 cells
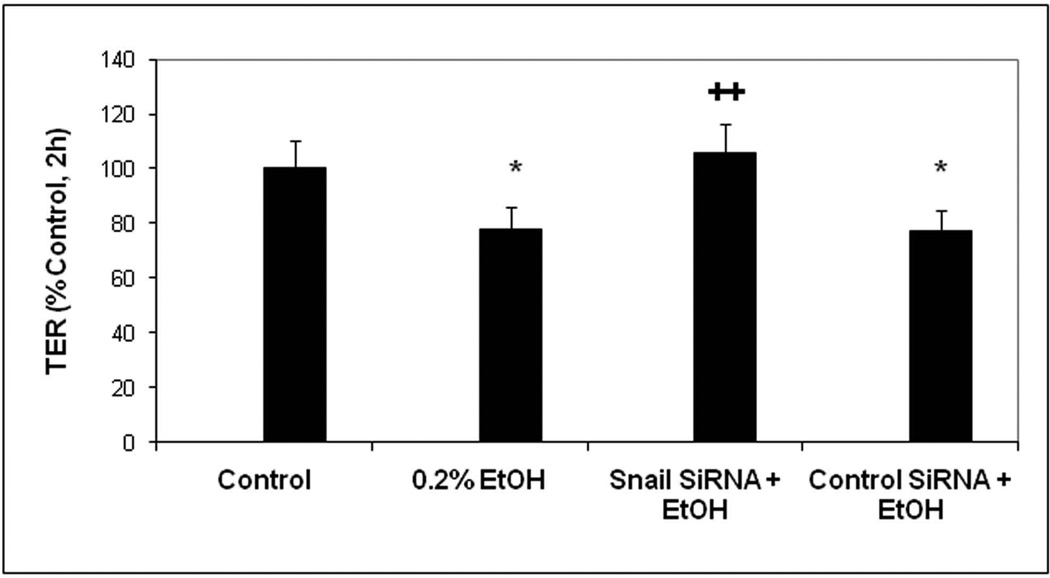
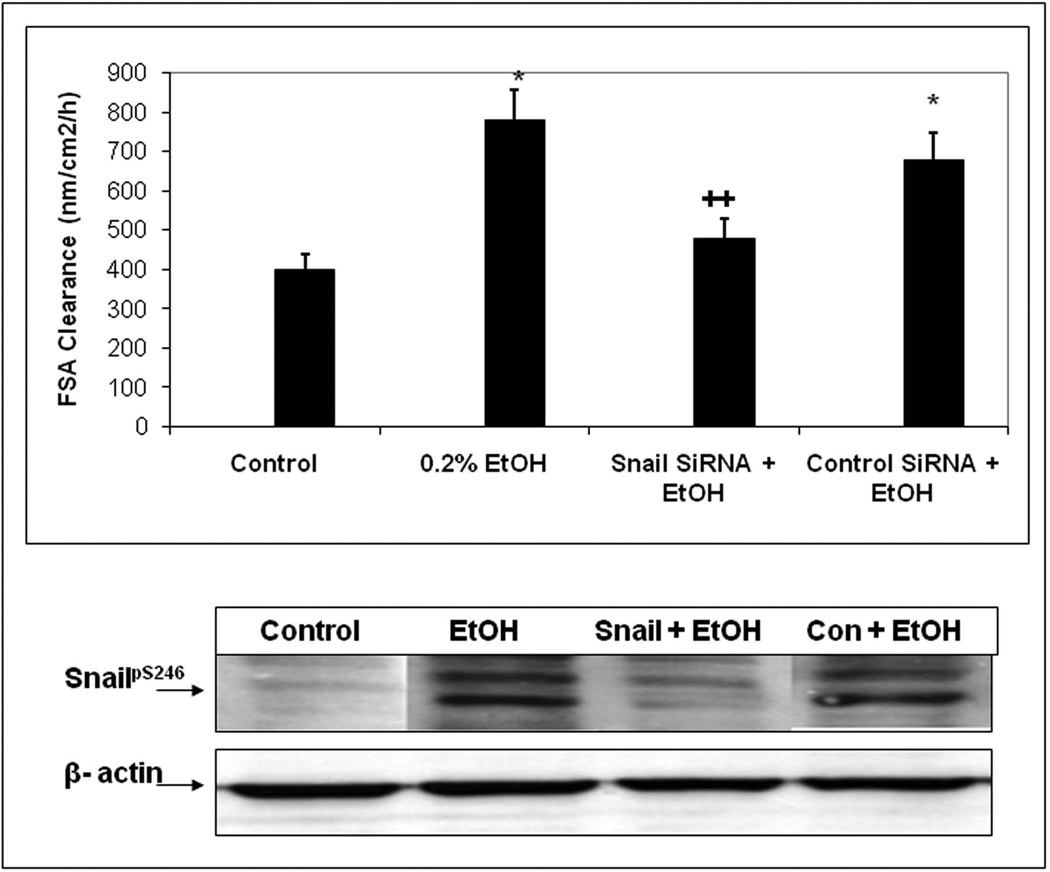
We next wished to determine whether alcohol stimulation of Snail could be playing a role in alcohol induced permeability. To this end, we first measured the effects of 0.2% alcohol over time on Caco-2 cells on inserts Transepithelial Electrical Resistance (TER; Ω×cm2) as % control. In addition, some cells were treated with either a control nontargeting siRNA or siRNA specific for Snail. In separate experiments we assessed Snail knockdown by this method and found that Snail expression was knocked down 70% by Snail-specific siRNA in alcohol treated cells. As seen in Fig. 7A, in alcohol treated cells that were either untreated with siRNA or treated with a control siRNA, the TER declined significantly (p<.05) indicating an increase in permeability. In contrast, cells treated with siRNA specific for Snail showed no significant drop in TER with alcohol treatment. As a second measure of intestinal permeability, we also assessed apical to basal flux of the fluorescent dye FSA (nM/cm2/h) and found that siRNA specific for Snail, but not control siRNA, significantly (p<.05) prevented an alcohol-induced increase in FSA flux across monolayers(Fig. 7B). These findings support a primary role for Snail in alcohol-induced intestinal epithelial cell monolayer hyperpermeability.
Fig. 7. SiRNA knockdown of Snail expression significantly inhibits alcohol-induced permeability in Caco-2 monolayers.
Caco-2 cell monolayers grown on permeable inserts were assessed for permeability at 2h after treatment of some wells with 0.2% alcohol (EtOH).Fig. 7A shows permeability data assessed by TER. Histogram data are means ± SE of triplicate wells in 4 separate experiments (total N=12 for each condition).Fig. 7B (upper) shows permeability at 2h assessed using flux of FSA from the apical to basal chambers as described in Methods. Data are means ± SE of triplicate wells from 4 separate experiments (total N=12 for each condition). Fig. 7B (lower) shows western blotting data from representative wells from each condition in the upper panel assessed for total Snail protein expression with β-actin as a loading control, mean Snail knockdown was >70%. * p< .05 EtOH alone treated cells or EtOH +control siRNA vs. media control cells.++ p<.05 for Snail siRNA + EtOH treated cells vs. control siRNA + EtOH treated cells.
Discussion
Alcohol- induced intestinal hyperpermeability is a major source of endotoxemia in alcoholics and thus plays a key role in the pathogenesis of ALD(Keshavarzian et al., 1999; Bode and Bode, 2005; Purohit et al., 2008b). Therefore, treatments targeted to prevent gut leakiness could be a novel and promising therapeutic approach for prevention and treatment of alcoholic liver disease and even other complications of alcoholism that are associated with endotoxemia and are secondary to inflammation mediated tissue injury and organ dysfunction. However, a comprehensive knowledge of the mechanisms of alcohol-induced gut leakiness is required to identify appropriate target candidates for therapeutic intervention and that was the goal of this study.
We have previously shown that alcohol causes increased intestinal permeability in both Caco- 2 cells in vitro and in a rat model of alcoholic liver disease(Banan et al., 1999; Keshavarzian et al., 2009a) and this alcohol-induced disruption of intestinal barrier integrity in both in vitro and in vivo models is dependent on alcohol activation of NO production by inducible nitric oxide synthase (iNOS, NOS2). For example, we showed that transfected Caco-2 cell monolayers incapable of upregulating iNOS were resistant to alcohol-induced monolayer hyperpermeability and that inhibition of iNOS by its inhibitor L-NIL prevented alcohol-induced increases in FSA flux across monolayers [Banan et al., 2000). Furthermore, treatment of alcohol fed rats with the iNOS inhibitor, L-NIL, prevented gut leakiness induced by chronic alcohol feeding (Banan et al., 2000; Tang et al., 2009). In the present study, we further supported our prior findings that alcohol-induced gut leakiness is iNOS dependent by demonstrating that iNOS KO mice are resistant to alcohol-induced gut leakiness.
The key question then is how alcohol-induced upregulated intestinal epithelial cell iNOS activity disrupts intestinal epithelial barrier function. The current model of intestinal epithelial permeability regulation is based on many studies showing a principal role for the apical junctional complex (AJC) between intestinal epithelial cells (Clayburgh et al., 2004; Laukoetter et al., 2008). The AJC is composed of both the more apical tight junctions (TJ) as well as the lower adherens junctions (AJ) between polarized intestinal epithelial cells. Together these junctions create the AJC and are thought to cooperate to regulate intestinal paracellular permeability to ions and solutes such as endotoxin. Thus, it is reasonable to postulate that alcohol-induced upregulated iNOS must negatively affect these key tight junctional proteins to disrupt the intestinal barrier.
The question is how can activated iNOS then disrupt AJC proteins? One possible mechanism is that iNOS (through NO production) activates redox sensitive transcription factor(s) capable of disrupting AJC proteins. Another potential candidate is Snail which is a key transcription factor for EMT (Thiery et al., 2009) and one hallmark of EMT is disruption of AJC proteins (Thiery and Sleeman, 2006). Indeed, EMT is characterized by the loss of epithelial cell markers and the increased expression of mesenchymal markers such as vimentin (Acloque et al., 2009). Probably the most well characterized epithelial cell markers that are lost in EMT are the proteins of the AJC (Thiery and Sleeman, 2006). Several studies have examined the direct effects of Snail expression on transcriptional regulation of AJC proteins. The role of Snail in inducing EMT was originally identified by its ability to repress the expression of the AJ protein E-cadherin in epithelial cells (Batlle et al., 2000; Cano et al., 2000). Importantly, we have recently shown alcohol stimulation results in repression of an E-cadherin reporter in Caco-2 cells (Forsyth et al., 2010).The mechanism for this transcriptional repression by Snail was shown to be Snail binding to a sequence motif in the promoter of E-cadherin called the E-box motif. In two other studies, using none intestinal epithelial cell lines, E boxes were identified in the promoters of occludin and claudins (TJ proteins) and it was shown that Snail expression resulted in repression of occludin and claudins (Ikenouchi et al., 2003; Ohkubo and Ozawa, 2004). More recent studies have further confirmed the ability of Snail to repress E-cadherin, occludin, and claudins expression in other in vivo models (Vincent et al., 2009), It should be noted that the majority of studies examining the role of Snail in EMT have focused either on developmental mechanisms, wound healing and fibrosis, or the role of Snail-EMT in cancer pathogenesis(Thiery et al., 2009) and cancer progression(Thiery, 2002; Kalluri and Weinberg, 2009; Thiery et al., 2009). Our study is the first demonstration for a physiological role for Snail in regulation of intestinal permeability. In the present study, using the Caco-2 cell model of intestinal permeability, we show that siRNA mediated specific inhibition of Snail expression significantly inhibits alcohol-induced intestinal permeability. In addition, we show that chemical or siRNA-mediated inhibition of iNOS prevents alcohol-mediated activation and nuclear localization of SnailpS246 .Our findings are similar to our prior study that showed that physiological levels of alcohol are capable of stimulating Snail expression and nuclear localization and stimulating expression of markers characteristic of EMT in Caco-2 and IEC-6 intestinal cells (Forsyth et al., 2010). Also, our findings that Snail protein levels are increased in the intestinal mucosa of alcohol fed mice agrees with our previous finding that Snail expression was increased in the colonic mucosa of chronic alcoholics and in Caco-2 cells in vitro (Forsyth et al., 2010).
However, a major difference between studies in the literature relative to our own is that most studies examined the effects of stable Snail overexpression and not Snail function under physiological conditions. Only one study examined the effect of Snail on permeability of MDCK cells using an inducible promoter(Carrozzino et al., 2005). Those investigators hypothesized, just as we have, that Snail might be involved in physiological functions such as the regulation of permeability of the AJC. They found that induction of Snail expression did not result in overt EMT but did result in a decrease in TER and increased ion permeability, just as we found for stimulation of Snail with alcohol. However, they did not observe an increase in permeability to uncharged solutes. In contrast, we did find increased FSA flux across intestinal epithelial cell monolayers. Nevertheless, their data clearly supports a role for Snail in regulation of epithelial permeability as does our data. One difference is that they used a different 4-day stimulation of Snail expression followed by a calcium switch model. Our findings now further support a role for Snail regulation of intestinal AJC permeability under even more physiological conditions.
Our findings support our hypothesis that alcohol-induced upregulated iNOS is the mechanism through which alcohol activates Snail. When we examined Snail levels in the intestines of alcohol fed mice, we found increased levels of Snail correlated with the increase in intestinal permeability in WT mice but Snail proteins were not elevated in the intestine of iNOS KO mice who also had no significant difference in intestinal permeability. We also showed that alcohol–induced activation of PAK1 is responsible for alcohol-induced activation of Snail and Snail phosphorylation at Ser246. Our conclusion that iNOS activates Snail and upregulated iNOS activates Snail through activation of PAK- 1 is also supported by several studies. First, upregulation of iNOS is strongly associated with EMT in renal fibrosis (Djamali et al., 2005). Second, nitric oxide is known to promote oxidative stress and several studies have shown that inflammation/oxidative stress can activate Snail mediated EMT(Djamali et al., 2005; Radisky et al., 2005; Zhang et al., 2007; Lopez-Novoa and Nieto, 2009). Third, stimulation of HEK cells with nitric oxide has been shown to activate Rac1 and PAK1(Hou et al., 2004). It should also be noted that Rac1 activation by oxidative stress has been shown to activate Snail mediated EMT and cell transformation in breast cancer cells (Radisky et al., 2005). In those studies the authors show Rac1 activation by NO is first required for downstream binding of activated Rac1 to PAK1 with subsequent PAK1 activation. Fourth, PAK1 has been shown by others to phosphorylate and activate Snail at Ser246(Yang et al., 2005). Lastly, we have also shown that alcohol-mediated permeability is dependent on NF-kB(Banan et al., 2007), and NF-kB is an established activator of iNOS as well as Snail-EMT (Blanco et al., 2004; Julien et al., 2007). However, the results of these studies are indirect and only suggest a key role for iNOS and NO in Snail activation (Thiery et al., 2009; Pautz et al., 2010). To our knowledge, our report is the first to demonstrate directly that iNOS is required for activation of Snail by a specific stimulus, in this case alcohol.
Our data that activation of PAK1 and Snail are a key mechanism of alcohol-induced gut leakiness may further explain the molecular mechanism of alcohol-induced intestinal barrier dysfunction reported by other investigators. For example, the actin cytoskeleton, including contraction of the actomyosin ring, has been associated with regulation of intestinal permeability (Clayburgh et al., 2004). More specifically, previous studies have shown that alcohol increases Caco-2 monolayer permeability by activating MLCK, and that the effect of alcohol on tight junctional proteins is reversible (Ma et al., 1999). Regulation of actin and the actomyosin ring has recently been shown to be one of the key functions for Snail as well as Twist during development in a landmark Nature paper by Martin et al (Martin et al., 2009). Also, PAK1 activation is reported to be involved in regulation of MLC and alcohol-induced increased endothelial permeability(Stockton et al., 2004). Stockton et al showed that alcohol produced a reversible disassembly and displacement of perijunctional actin and myosin filaments from the perijunctional areas. Such changes also characterize Snail mediated EMT(Thiery, 2002). We can therefore speculate that the reversible impact of alcohol on AJC proteins in Caco-2 cells might be due to alcohol-induced activation of PAK1 and then Snail. Studies by others have also shown a role for zinc in preventing alcohol-induced intestinal permeability in both Caco-2 cells and mouse models of ALD (Lambert et al., 2003; Zhong et al., 2010). Though the mechanism is not entirely clear they demonstrated that disassembly of AJC proteins is involved in alcohol-induced permeability in Caco-2 cells and in a mouse chronic alcohol model (Zhong et al., 2010). Such changes could also be induced by Snail activation and EMT (Thiery and Sleeman, 2006).
In summary, our data further demonstrate that alcohol-induced gut leakiness in rodents and intestinal epithelial cell monolayers is iNOS dependent. Our data also support a novel physiological role for Snail in regulation of AJC permeability. We found a key role for Snail activation in alcohol induced, iNOS-mediated intestinal hyperpermeability in both an in vitro Caco-2 monolayer model and in an in vivo rodent model of alcohol-induced gut leakiness. These data represent the first demonstration of iNOS mediated signaling in activation of Snail as well as the first demonstration for a physiological role for Snail in regulation of intestinal permeability. Identification of these mechanisms for alcohol-stimulated intestinal permeability may provide new therapeutic targets for prevention and treatment of alcohol-induced endotoxemia and complications of alcoholism associated with endotoxemia like ALD. Further studies are needed to determine how activated Snail in intestinal epithelial cells causes intestinal leakiness and to see if alcohol-induced activation of Snail disrupts barrier integrity by repressing expression of AJC proteins (Thiery and Sleeman, 2006; Thiery et al., 2009) or by regulating levels of AJC proteins by post-transcriptional mechanisms such as endocytosis of AJC proteins(Wu et al., 2007; Wu and McClay, 2007).
Acknowledgments
Support: This work was supported in part by NIH funding #AA013745 (A.K.) and an unrestricted research gift from Mrs. and Mr. Larry Field (to AK).
References
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, Fields JZ. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447–460. doi: 10.1016/j.alcohol.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- Blanco AM, Pascual M, Valles SL, Guerri C. Ethanol-induced iNOS and COX-2 expression in cultured astrocytes via NF-kappa B. Neuroreport. 2004;15:681–685. doi: 10.1097/00001756-200403220-00021. [DOI] [PubMed] [Google Scholar]
- Bode C, Bode JC. Activation of the innate immune system and alcoholic liver disease: effects of ethanol per se or enhanced intestinal translocation of bacterial toxins induced by ethanol? Alcohol Clin Exp Res. 2005;29:166S–171S. doi: 10.1097/01.alc.0000189280.19073.28. [DOI] [PubMed] [Google Scholar]
- Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- Carrozzino F, Soulie P, Huber D, Mensi N, Orci L, Cano A, Feraille E, Montesano R. Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am J Physiol Cell Physiol. 2005;289:C1002–C1014. doi: 10.1152/ajpcell.00175.2005. [DOI] [PubMed] [Google Scholar]
- Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]
- Djamali A, Reese S, Yracheta J, Oberley T, Hullett D, Becker B. Epithelial-to-mesenchymal transition and oxidative stress in chronic allograft nephropathy. Am J Transplant. 2005;5:500–509. doi: 10.1111/j.1600-6143.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836:63–68. doi: 10.1016/j.jchromb.2006.03.046. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Forsyth CB, Tang Y, Shaikh M, Zhang L, Keshavarzian A. Alcohol Stimulates Activation of Snail, Epidermal Growth Factor Receptor Signaling, and Biomarkers of Epithelial-Mesenchymal Transition in Colon and Breast Cancer Cells. Alcohol Clin Exp Res. 2010;34:19–31. doi: 10.1111/j.1530-0277.2009.01061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Banan A, Farhadi A, Fields JZ, Tang Y, Shaikh M, Zhang LJ, Engen PA, Keshavarzian A. Regulation of oxidant-induced intestinal permeability by metalloprotease-dependent epidermal growth factor receptor signaling. J Pharmacol Exp Ther. 2007;321:84–97. doi: 10.1124/jpet.106.113019. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12–25. doi: 10.1055/s-2008-1040525. [DOI] [PubMed] [Google Scholar]
- Hollander D. Intestinal permeability, leaky gut, and intestinal disorders. Curr Gastroenterol Rep. 1999;1:410–416. doi: 10.1007/s11894-999-0023-5. [DOI] [PubMed] [Google Scholar]
- Hou Y, Ye RD, Browning DD. Activation of the small GTPase Rac1 by cGMP-dependent protein kinase. Cell Signal. 2004;16:1061–1069. doi: 10.1016/j.cellsig.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. J Cell Sci. 2003;116:1959–1967. doi: 10.1242/jcs.00389. [DOI] [PubMed] [Google Scholar]
- Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119:1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009a;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009b;50:538–547. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol. 2008;14:401–407. doi: 10.3748/wjg.14.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO Mol Med. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma TY, Nguyen D, Bui V, Nguyen H, Hoa N. Ethanol modulation of intestinal epithelial tight junction barrier. Am J Physiol. 1999;276:G965–G974. doi: 10.1152/ajpgi.1999.276.4.G965. [DOI] [PubMed] [Google Scholar]
- Mandayam S, Jamal MM, Morgan TR. Epidemiology of alcoholic liver disease. Semin Liver Dis. 2004;24:217–232. doi: 10.1055/s-2004-832936. [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF. Pulsed contractions of an actin-myosin network drive apical constriction. Nature. 2009;457:495–499. doi: 10.1038/nature07522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkubo T, Ozawa M. The transcription factor Snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci. 2004;117:1675–1685. doi: 10.1242/jcs.01004. [DOI] [PubMed] [Google Scholar]
- Pautz A, Art J, Hahn S, Nowag S, Voss C, Kleinert H. Regulation of the expression of inducible nitric oxide synthase. Nitric Oxide. 2010 doi: 10.1016/j.niox.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008a;42:349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C, Brenner DA, Choudhry MA, Hamilton F, Kang YJ, Keshavarzian A, Rao R, Sartor RB, Swanson C, Turner JR. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: Summary of a symposium. Alcohol. 2008b doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Nieto MA, Werb Z, Bissell MJ. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;286:G881–G884. doi: 10.1152/ajpgi.00006.2004. [DOI] [PubMed] [Google Scholar]
- Rehm J, Room R, Monteiro M, Gmel G, Graham K, Rehn N, Sempos CT, Jernigan D. Alcohol as a risk factor for global burden of disease. Eur Addict Res. 2003;9:157–164. doi: 10.1159/000072222. [DOI] [PubMed] [Google Scholar]
- Stockton RA, Schaefer E, Schwartz MA. p21-activated kinase regulates endothelial permeability through modulation of contractility. J Biol Chem. 2004;279:46621–46630. doi: 10.1074/jbc.M408877200. [DOI] [PubMed] [Google Scholar]
- Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- Tipoe GL, Liong EC, Casey CA, Donohue TM, Jr, Eagon PK, So H, Leung TM, Fogt F, Nanji AA. A voluntary oral ethanol-feeding rat model associated with necroinflammatory liver injury. Alcohol Clin Exp Res. 2008;32:669–682. doi: 10.1111/j.1530-0277.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- Vincent T, Neve EP, Johnson JR, Kukalev A, Rojo F, Albanell J, Pietras K, Virtanen I, Philipson L, Leopold PL, Crystal RG, de Herreros AG, Moustakas A, Pettersson RF, Fuxe J. A SNAIL1-SMAD3/4 transcriptional repressor complex promotes TGF-beta mediated epithelial-mesenchymal transition. Nat Cell Biol. 2009 doi: 10.1038/ncb1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, McClay DR. The Snail repressor is required for PMC ingression in the sea urchin embryo. Development. 2007;134:1061–1070. doi: 10.1242/dev.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu SY, Ferkowicz M, McClay DR. Ingression of primary mesenchyme cells of the sea urchin embryo: a precisely timed epithelial mesenchymal transition. Birth Defects Res C Embryo Today. 2007;81:241–252. doi: 10.1002/bdrc.20113. [DOI] [PubMed] [Google Scholar]
- Yang Z, Rayala S, Nguyen D, Vadlamudi RK, Chen S, Kumar R. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- Zhang A, Jia Z, Guo X, Yang T. Aldosterone induces epithelial-mesenchymal transition via ROS of mitochondrial origin. Am J Physiol Renal Physiol. 2007;293:F723–F731. doi: 10.1152/ajprenal.00480.2006. [DOI] [PubMed] [Google Scholar]
- Zhong W, McClain CJ, Cave M, Kang YJ, Zhou Z. The role of zinc deficiency in alcohol-induced intestinal barrier dysfunction. Am J Physiol Gastrointest Liver Physiol. 2010;298:G625–G633. doi: 10.1152/ajpgi.00350.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]