Abstract
Small non-coding microRNAs (miRNAs, miRs) regulate gene expression in virtually all cells, and they have been implicated in cardiovascular disease and aging. In a paper recently published in Nature, miR-34a was identified as an aging-associated apoptotic and overall damaging factor for the heart.
The miRNA canonical but not exclusive mechanism of action involves the targeting of messenger RNAs (mRNAs) by either complementary or semi-complementary binding between a short (8 bp) miRNA “seed sequence” and one or more miRNA binding regions in the mRNA 3′ untranslated region (UTR), thus inhibiting gene expression. An individual miRNA directly targets a plethora of genes and multiple miRNAs can compete for the same mRNA targets, which creates intricate regulatory networks able to fine-tuning molecular pathways affecting biological and pathophysiological functions, including cell death, lifespan and aging.
In a recent paper in Nature, Boon and colleagues elegantly show upregulation of miR-34a in the heart during aging in humans and mice1. Corroborating evidences are derived from work on mice with loss of Ku80. The Ku80 unit has been implicated in multiple processes, including DNA repair, telomere maintenance and apoptosis. Ku80 knockout mice are more prone to cancer and age prematurely2. Interestingly, miR-34a level is increased in the heart of Ku80 heterozygous and homozygous knockout mice. In the latter, inhibition of miR-34a expression by systemic use of a miRNA inhibitor retarded age-dependent cardiac deterioration1.
The other members of miR-34 (b and c) were also overexpressed in the aged heart. However, they are produced as a bicistronic unit from a different chromosome than miR-34a and regulation of this miRNA family is not entirely clear.
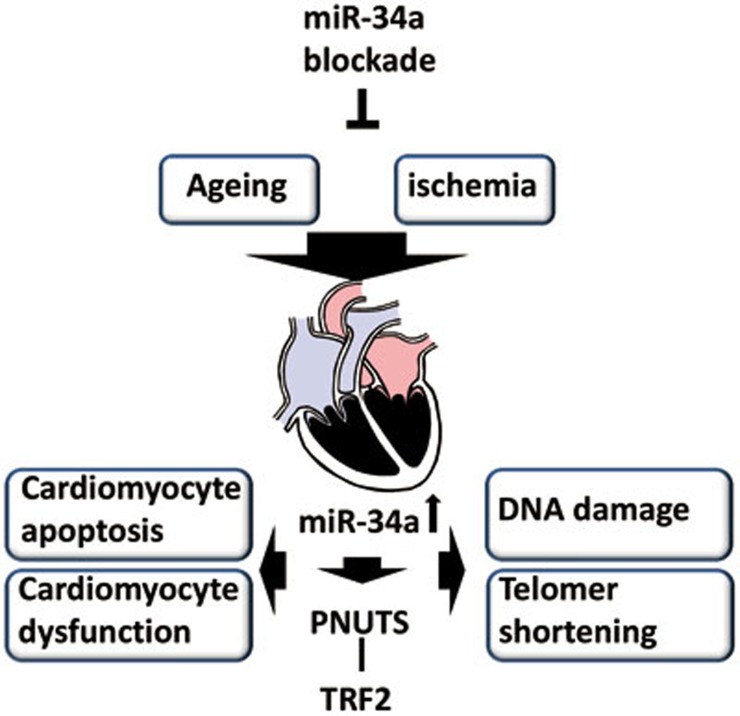
Boon et al. showed that age-associated miR-34a upregulation is more robust than that of miR-34b-c. In the heart, miR-34a localizes mainly in cardiomyocytes, but also in other cardiac cell types where its function is less clear. Cardiac aging is associated with increased apoptosis, fibrosis deposition, hypertrophy and reduced function. All these features could be corrected by systemic use of miR-34a inhibitors1 (Figure 1). Whether this miRNA is more upstream than others in aging processes remains to be determined as other miRNAs involved in cardiac aging have been identified3.
Figure 1.
Schematic diagrams of the effects of microRNA-34 (miR-34) inhibition in the aged or ischemic heart. PNUTS, protein phosphatase 1 nuclear-targeting subunit (miR-34 target gene); TRF2, telomeric protein telomere repeat factor 2.
miR-34 level is elevated in the mouse infarcted heart1,4, where its inhibition proved therapeutically useful by acting on the aforementioned processes and additionally promoting post-ischemic reparative angiogenesis (Figure 1). Similar beneficial effects were evident using a miR-34a knockout model. The myocardial infarct (MI) data by Boon et al.1 are partially in line with Bernardo et al.5, which proved the therapeutic benefit of the full miR-34 family inhibition in both mouse MI and pressure overload via transverse aortic constriction (TAC) models. However, they did not find miR-34a inhibition alone able to gain a therapeutic benefit5. The appeal of miRNA-34a as a therapeutic target is apparent and deserves further translational efforts to tackle ischemic heart disease, especially that associated with aging. It remains to be elucidated whether miR-34a inhibition would produce an even stronger therapeutic effect when MI is induced in spontaneously or prematurely aged mice.
Cardiovascular disease is often associated with premature biological aging and shows evidence of cellular senescence characterized by reduced cell proliferation, growth arrest, apoptosis, elevated DNA damage, and telomere shortening and dysfunction. The miR-34 family is highly conserved at sequence and function levels from nematode to mammals (reviewed in6). miR-34 was known to be upregulated during aging and linked with aging-associated pathways, such as DNA damage response, senescence, and cell death (reviewed in6).
Other miRNAs are expressionally changed with aging1 (and reviewed in6). It would be important to study the cardiovascular effects of each of these miRNAs. This could result in the identification of multiple therapeutic targets, which could be used in combination to maximize the benefit especially in the elderly.
Boon and colleagues provide an example of a selected miRNA target gene that is therapeutically valuable. Protein phosphatase 1 nuclear-targeting subunit (PNUTS) is a direct miR-34a target and interacts with the telomeric protein telomere repeat factor 2 (TRF2), which is involved in DNA damage repair and telomere maintenance, thereby regulating telomere length7. PNUTS directly interacts with phosphatase and tensin homolog (PTEN) and sequesters it in the nucleus, thus reducing PTEN-dependent apoptosis8. Adeno-associated viral delivery of PNUTS improved cardiac function after MI in mice1.
Cardiac hypertrophy, apoptosis and fibrosis are not exclusive features of aging and more generally present in the “stressed” heart, including after MI and in response to high arterial blood pressure and activated renin-angiotensin system (which are mimicked by the TAC model). However, the authors provide a more specific although indirect evidence of the link between miR-34a and cardiomyocyte aging by looking at the telomere in vitro. Different from other cells, cardiomyocyte cultures can resist for a few days and do not allow mimic senescence by multiple passaging. It would be important to next clarify in vivo whether telomere length is maintained by miR-34a inhibition in aging mice.
Do aging and/or DNA damage and apoptosis-linked pathways induce miR-34a expression or does miR34-a causes aging and apoptosis? In fact, it would have been important to extensively investigate the mechanisms regulating miR-34a transcription and maturation in order to understand what is underpinning the observed upregulation of mature miR-34a during cardiac aging and stresses. We speculate that the reported positive feedback loop linking p53, miR-34a and SIRT1 plays a role. In fact, p53 can bind to the miR-34 promoter. In turn, miR-34 recapitulates p53 biological effects inhibiting pro-proliferation and anti-apoptotic genes, including BCL2, and silence information regulator 1 (SIRT1)1,9, which limits longevity and promotes post-ischemic angiogenesis and cardiac protection10,11. SIRT1 deacetylates and stabilizes p53, thus eventually controlling miR-34a transcription.
In contrast with the negative cardiac effect observed in mice1, miRNA-34 ensures long-term maintenance of the brain in Drosophila, where the miR-34 target Eip74EF (an ETS domain transcription factor that is a component of steroid hormone signaling pathways) has been shown to be involved in this protective effect12. Hence, it will be important to study the impact of the miRNA-34 family in the mammal brain as well as in other organs.
References
- Boon RA, Iekushi K, Lechner S, et al. Nature. 2013. pp. 107–110. [DOI] [PubMed]
- Gullo C, Au M, Feng G, et al. Biochim Biophys Acta. 2006. pp. 223–234. [DOI] [PubMed]
- Jazbutyte V, Fiedler J, Kneitz S, et al. Age(Dordr)201335747–762. [DOI] [PMC free article] [PubMed]
- Iekushi K, Seeger F, Assmus B, et al. Circulation. 2012. pp. 1765–1773.pp. S1–S7. [DOI] [PubMed]
- Bernardo BC, Gao XM, Winbanks CE, et al. Proc Natl Acad Sci USA. 2012. pp. 17615–17620. [DOI] [PMC free article] [PubMed]
- Inukai S, Slack F.J Mol Biol 2013 Jan 23. doi: 10.1016/j.jmb.2013.01.023 [DOI] [PMC free article] [PubMed]
- Kim H, Lee OH, Xin H, et al. Nat Struct Mol Biol. 2009. pp. 372–379. [DOI] [PubMed]
- Kavela S, Shinde SR, Ratheesh R, et al. Cancer Res. 2013. pp. 205–214. [DOI] [PMC free article] [PubMed]
- Raver-Shapira N, Marciano E, Meiri E, et al. Mol Cell. 2007. pp. 731–743. [DOI] [PubMed]
- Potente M, Ghaeni L, Baldessari D, et al. Genes Dev. 2007. pp. 2644–2658. [DOI] [PMC free article] [PubMed]
- Hsu CP, Zhai P, Yamamoto T, et al. Circulation. 2010. pp. 2170–2182. [DOI] [PMC free article] [PubMed]
- Liu N, Landreh M, Cao K, et al. Nature. 2012. pp. 519–523. [DOI] [PMC free article] [PubMed]