Abstract
Background:
Peroxisome proliferator-activated receptor gamma (PPAR γ) is a transcription factor, which is abundantly expressed in adipose tissue and has a direct link to adiposity. It seems that long-chain polyunsaturated fatty acids (LC-PUFAs) can regulate PPAR γ expression. The purpose of this study was to investigate the effects of n-3LC PUFA supplementation on plasma levels of PPAR γ and thyroid hormones in obesity.
Materials and Methods:
In a randomized double-blind controlled trial, 66 subjects with obesity were assigned to 2 groups. Participants in intervention group consumed omega3 capsules contained 1000 mg n-3 fatty acids (180 mg of eicosapentaenoic acid [EPA] and 120 mg of docosahexaenoic acid [DHA]) and placebo group consumed placebo capsules contained paraffin twice a day for 4 wk. Fasting blood samples and weight measurements were collected at the baseline and at the end of the trial. Plasma PPAR γ and thyroid hormones were measured by ELISA. Data were analyzed using a repeated measure model-two factor for comparing two groups in two times.
Results:
No significant changes were observed in PPAR γ levels between and within the groups after supplementation (P>0.05). N-3LC PUFA supplementation significantly increased T4 levels after 4 wk (P<0.05) but T3 and TSH did not change significantly.
Conclusion:
Our study showed that n-3LC PUFAs supplementation increased T4 levels. However, no significant changes in T3, TSH and PPAR γ plasma levels were observed in obese adults.
Keywords: Docosahexaenoic acid, eicosapentaenoic acid, N-3 LC PUFAs supplementation, obesity, peroxisome proliferator activated receptor gamma, thyrotropin, thyroxin, triiodothyronine
INTRODUCTION
One of the most important issues of the health system in the worldwide is obesity, which is presented as one of the top 10 global health problems by the world health organization.[1] Nowadays, some believe that it is the most perilous disease in the world.[2] In recent years, with increasing prevalence of obesity, an intense interest in clarifying the mechanisms underlying the formation of adipose tissue has been formed.[3] Caloric excess, inactivity, and inherited factors are the main causes of changes in adipose tissue, which leads to alterations in its normal function as a lipid buffer, energy store, and a dynamic endocrine organ that is critical for normal metabolic homeostasis.[4] Peroxisome proliferator-activated receptor γ gene (PPARG) is a candidate gene with a direct link to adiposity.[5]
PPAR γ is a transcription factor, which is abundantly expressed in adipose tissue and has a key role in adipocyte differentiation.[6] These receptors form heterodimers with the retinoid X receptor (RXR), bind to PPAR response elements (PPREs) in the regulatory region of target genes, and modulate their transcription.[7] There are a lot of evidence shows that PPAR γ is a prominent regulator in the formation of fat cells and their ability to function normally in the adults. PPAR γ is induced during adipocyte differentiation and forced expression of PPAR γ in non-adipogenic cells effectively changes them into mature adipocytes. In addition, PPAR γ knockout mice fail to develop adipose tissue.[8]
There is a vast range of naturally compounds seeming to act as ligands for PPARs.[9] It seems that Long-chain polyunsaturated fatty acids (LC-PUFAs) are probable candidates as compounds, which regulate PPAR γ expression.[10] Also, a range of eicosanoids have been shown to be PPAR ligands, which often have more coherence than their parent molecules.[9] Some from animal studies showed that n-3 LCPUFA supplementation can reduce weight gain and diminish body fat, in particular visceral fat, which is related to changes in gene expression.[11] Some ex vivo studies showed that activation of PPAR γ in adipose tissue is a possible cause of apoptosis of large fat cells in visceral and/or sc fat depots from rodents.[12] Some studies showed that treatment of Zucker (fa/fa) rats with thiazolidinedione (a PPAR agonist), increased the number of small adipocytes and decreased the number of large adipocytes.[6] However, fewer studies on humans have been done. Results of these studies imply that PPAR γ gene expression partially could be controlled, by nutritional regulation.[13]
However, the direct effect of various nutrients on PPAR γ gene expression is ambiguous. Recent evidence demonstrated interactions between PPAR and thyroid hormone nuclear receptors (TRs) that regulate some genes involved in lipid oxidation and thermogenesis.[14,15] Experimental studies on animals have shown a correlation between thyroid hormones and weight changes.[16] However, studies on thyroid hormones in obese adults are also inconsistent.[17]
The aim of the present study was to verify the effects of n-3LC PUFA supplementation on plasma levels of PPAR γ and thyroid hormones in obesity.
MATERIALS AND METHODS
Participants and study design
This was a randomized, double-blind, placebo-controlled study in adults with obesity. Sixty six persons were recruited from a specialty and subspecialty clinic of Tabriz University of Medical Science, Tabriz, Iran, by local advertisements. The study was carried out from April to November 2011. By considering 0.05% significant level and 95% power, from other studies, the maximum sample size was calculated 25 in each groups, based on T4 (SD=1.78 for placebo group, SD=2.81 for intervention group and a difference equal 1.4). Taking in to account a drop-out rate of 30% we increased sample size to 33 in each group, which at the end of the study remained 29 and 31 persons in placebo and intervention groups, respectively.
Inclusion criteria were non-smoking, aged 18–45 years with body mass index >30 kg/m2 and not trying to lose weight. Persons should not have endocrine causes of obesity, history of any medical illness, including HIV, diabetes, hepatic, renal or thyroid disease. Additional inclusion criteria were no medication, which would affect their plasma lipid levels, no treatment with blood dilutors, beta blockers, anti-inflammatory drugs and omega 3 or vitamin A supplements for the last 2 months. Women with pregnancy, lactation and menopause were also excluded.
The study protocol was approved by the Medical Ethics Committee of the Tabriz University of Medical Science (code 901). This study also registered in Iranian Registry of Clinical Trials (IRCT138903162017N3). The protocol and aims of the study were fully explained to the participants and all volunteers gave informed consent at the beginning of the study.
Eligible participants were randomly assigned to receive either 1,000 mg of n-3LC PUFA capsules containing 180 mg of EPA and 120 mg of DHA (n=33) or 1,000 mg of paraffin as placebo (n=33) twice a day for 4 weeks. The allocation of intervention or placebo group was concealed from the researchers. Therefore, neither the participants nor the investigators were aware of treatment assignments in this double blind study [Figure 1].
Figure 1.
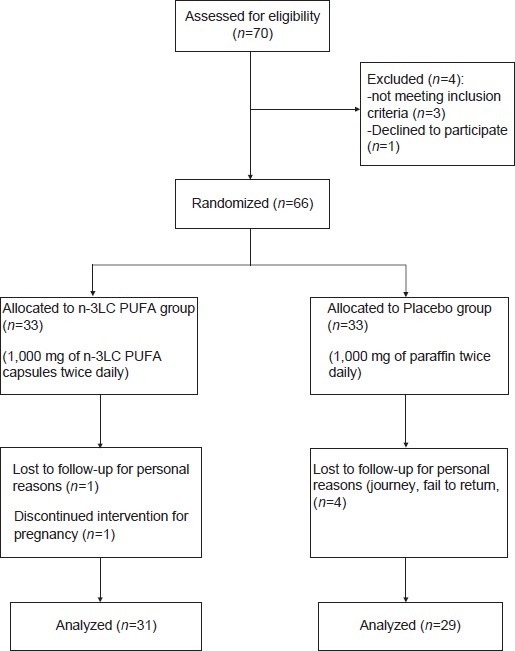
Flowchart of Subject Screening and Enrollment
Placebo was similar in size, color and shape to n-3LC PUFA capsules. The capsules were provided by Zahravi Pharmacy, Tabriz, Iran. At the beginning of the study some variables (gender and BMI) were all matched between two groups. Participants were instructed to consume the supplements with a meal and to maintain their usual dietary habits and physical activity during the study. Consumption of capsules was followed by phone in study duration and if they did not take more than 5 capsules was excluded.
Assessments
Blood samples were collected before and at the end of the study after 12 h fasting. Venous blood was drawn into EDTA tubes and Within 1 h after the samples were taken centrifuged, and plasma was frozen at -70°C until analyses were performed. PPAR γ levels in plasma were determined using an ELISA kit (CUSABIO Biotech Co., China) according to the manufacturer’s specifications. Total triiodothyronine (T3), thyroxine (T4) and thyrotropin (TSH) were measured in plasma using an ELISA kit (Monobind Inc, USA).
Weight and height measurements were performed at baseline and on 30th day of the study. Weight was measured without shoes using a carefully calibrated scale (Seca, Germany) and height was measured with use of wall-mounted calibrated meter scales. BMI was calculated from body weight and height (in kg/m2).
The food intakes of participants were collected at the beginning and the end of the study using three 24-h dietary recalls. Regarding the physical activity, all participants were in similar category.
Statistical analyses
All results are expressed as Mean ± SEM. The normality of data was evaluated by Q-Q test. A repeated measure model was used for comparing two groups in two times. All analyses were performed using statistical software Minitab15 and Spss15 for Windows and P value of less than 0.05 was considered statistically significant.
RESULTS
Of all the participants, 60 participants finished the intervention (n=31 in intervention group and n=29 in placebo group). Two of the participants receiving n-3LC PUFA capsules and four participants in placebo group dropped out. Reasons for withdrawal were: Pregnancy, personal reasons and fail to return. Participants demonstrated good compliance with the study design.
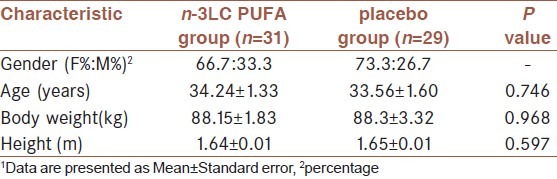
Baseline characteristics of study participants are shown in Table 1. There were no significant differences in the baseline characteristics of two groups.
Table 1.
Baseline characteristics of study participants (n=60)1
Clinical variables at baseline and after 4 weeks of intervention in two groups are shown in Table 2. Mauchly’s test confirmed the Sphericity of covariance matrix. After 4 weeks of supplementation, there were no significant differences between the groups in PPAR γ levels (P=0.972).
Table 2.
Clinical variables at baseline and after 4 weeks of intervention in two groups1
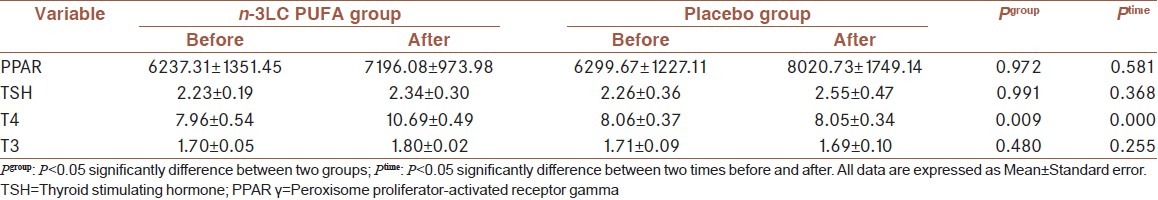
As shown in Table 2, plasma T3, T4 and TSH levels in n-3LC PUFA and placebo groups were 1.80 ± 0.02, 8.06 ± 0.37 and 2.26 ± 0.36, respectively, after supplementation. Among the thyroid hormones, T4 concentration was increased considerably over this period in the n-3LC PUFA group but not in the placebo group (P=0.009). T4 concentration, also, was different from baseline within two groups. However, there were no significant differences between the groups in the change in T3 and TSH. Although, their level was also increased in n-3LC PUFA group but it was not statistically significant.
DISCUSSION
Obesity has become a prevailing epidemic throughout the globe. Effective therapies for obesity become attracting. PUFAs act via nuclear receptors such as PPAR γ and thyroid hormones. In this study, we assessed the effect of EPA and DHA on plasma levels of PPAR γ, and thyroid hormones.
As a result of our study [Table 2], PPAR γ levels had no significant changes after 4 weeks. No published data are available about the effects of n-3LC PUFA supplementation on plasma PPAR γ levels in obesity in vivo. Other studies exist on different cell types and in vitro. For example, our results were similar to the study conducted by MacLaren et al. which reported that EPA supplementation by dose of 100 μM for 24 h, had not significant effects on PPAR γ mRNA levels in cultured bovine endometrial cells.[18] Our result was also in line with the lack of effect of rosiglitazone, a potent activator of PPAR γ, and is also in agreement with previous results obtained in human fat cells.[19]
On the other hand, Li et al. reported that 10 μmol/L EPA and 100 μmol/L DHA increased PPAR γ mRNA levels in HK-2 cells.[20] In the other study, Chambrier et al. showed that 50 μM EPA but not DHA significantly increased PPAR γ mRNA levels in human isolated adipocytes. These authors suggest, therefore, that PPAR γ probably does not regulate the expression of its own gene in human adipocytes.[21] In rodent adipose cell lines, however, activation of PPAR γ with thiazolidinediones appeared to reduce PPAR γ expression,[22] conflicting results from these studies suggesting that there may be species-related differences in the regulation of the PPAR γ gene in adipocytes.
The lack of effect of n-3LC PUFA (synthetic agonist of PPAR) on plasma PPAR in this study might be a consequence of a low level of retinoid X receptor (RXR), the partner of the PPARs to form active heterodimers.[21] These PPAR-RXR heterodimers bind to DNA at direct repeats (DR) in promoters of many genes that regulate gene expression.[23]
We did not assess differential effects of EPA and DHA and this is a limitation of our study. The authors suggest that EPA or DHA may have a different effect on PPAR gene expression and other researches are necessary in the future. However, in this study, EPA and DHA did not induce a significant increase together in PPAR γ levels.
Significant interactions exist between fatty acids and the endocrine system. N-3LC PUFA supplementation had no noticeable effect on plasma T3 and TSH but a significant increase in T4 concentration was observed in our study [Table 2]. Contradictory results are apparent in a study by Souza et al. that 2 groups of rats consumed fish oil or soybean oil as an isocaloric and normolipid diets during lactation. Male offspring received the same diet until weaning at 11 weeks old. Souza et al. suggested similar serum total T3, T4 and TSH between 2 groups of rats.[24]
In Knoop et al. study, animals were fed ad libitum the above basal diet with fish oil or cocoa butter. After 3 weeks of dietary treatment, the findings of the same level of thyroid hormones - T4 and T3 - showed that there was no effect of fish oil supplementation on the thyroid function in rats.[25]
T4 is the most abundant thyroid hormone, accounting for 80%, while T3 accounts for 20% of the thyroid hormones. T4 significant changes in our observation may be due to its high levels in the blood.
N-3LC PUFA can affect thyroid hormones via TRs. TRs are members of the nuclear receptor superfamily and induce T3 transcription. TRs bind to TR receptor (TREs) not only as homodimers but also as heterodimers with other members of the receptor superfamily, such as RXRs. Heterodimerization with RXR dramatically increases the binding of TRs to TREs, the responsiveness of TR to T3, and the transcriptional activation.[26] Recently, it was shown that TRβ competes with PPAR γ for binding to DR1 as a heterodimer with RXR in vitro and in vivo to repress the transcriptional activity of PPAR γ. Thyroid receptors interact with PPARs in part by sharing binding sites and heterodimeric partners such as RXR.[27]
CONCLUSION
In summary, the present study shows that n-3LC PUFAs supplementation in obese adults resulted in higher T4 levels accompanied by no significant changes in T3, TSH and PPAR γ plasma levels. These findings suggest that the increase in thyroid hormone may be one of the mechanisms by which n-3LC PUFAs exert part of their effects in obesity.
Limitations of the study
There are several limitations to this trial. First, we could not assess differential effects of EPA and DHA in this study and second was short duration of the intervention. Future studies are needed to show long-terms effects of n-3 LCPUFAs on PPAR γ in obesity.
ACKNOWLEDGMENTS
This is a report of a database from thesis entitled “The effect of n-3 fatty acids supplementation on plasma levels of Peroxisome proliferator-activated receptors in obesity”. The authors would like to thank the volunteers who participated in the study.
Footnotes
Source of Support: This study is funded by Research Vice-Chancellor and Nutritional Research Center of Tabriz University of Medical Sciences, Tabriz, Iran
Conflict of Interest: None declared.
REFERENCES
- 1.Farooqi IS, O’Rahilly S. Monogenic human obesity syndromes. Recent Prog Horm Res. 2004;59:409–24. doi: 10.1210/rp.59.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355–61. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–307. [PubMed] [Google Scholar]
- 4.Rajala MW, Scherer PE. Minireview: The adipocyte-At the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–73. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 5.Cecil JE, Watt P, Palmer CN, Hetherington M. Energy balance and food intake: The role of PPARgamma gene polymorphisms. Physiol Behav. 2006;88:227–33. doi: 10.1016/j.physbeh.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Okuno A, Tamemoto H, Tobe K, Ueki K, Mori Y, Iwamoto K, et al. Troglitazone increases the number of small adipocytes without the change of white adipose tissue mass in obese Zucker rats. J Clin Invest. 1998;101:1354–61. doi: 10.1172/JCI1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F, Lavan BE, Gregoire FM. Selective modulators of PPAR-gamma activity: Molecular aspects related to obesity and side-effects. PPAR Res. 2007;2007:32696. doi: 10.1155/2007/32696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–7. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 9.Krey G, Braissant O, L’Horset F, Kalkhoven E, Perroud M, Parker MG, et al. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol Endocrinol. 1997;11:779–91. doi: 10.1210/mend.11.6.0007. [DOI] [PubMed] [Google Scholar]
- 10.Nisoli E, Carruba MO, Tonello C, Macor C, Federspil G, Vettor R. Induction of fatty acid translocase/CD36, peroxisome proliferator-activated receptor-gamma2, leptin, uncoupling proteins 2 and 3, and tumor necrosis factor-alpha gene expression in human subcutaneous fat by lipid infusion. Diabetes. 2000;49:319–24. doi: 10.2337/diabetes.49.3.319. [DOI] [PubMed] [Google Scholar]
- 11.Buckley JD, Howe PR. Anti-obesity effects of long-chain omega-3 polyunsaturated fatty acids. Obes Rev. 2009;10:648–59. doi: 10.1111/j.1467-789X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 12.Adams M, Montague CT, Prins JB, Holder JC, Smith SA, Sanders L, et al. Activators of peroxisome proliferator-activated receptor gamma have depot-specific effects on human preadipocyte differentiation. J Clin Invest. 1997;100:3149–53. doi: 10.1172/JCI119870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal-Puig A, Jimenez-Liñan M, Lowell BB, Hamann A, Hu E, Spiegelman B, Flier JS, et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest. 1996;97:2553–61. doi: 10.1172/JCI118703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu YY, Heymann RS, Moatamed F, Schultz JJ, Sobel D, Brent GA. A mutant thyroid hormone receptor alpha antagonizes peroxisome proliferator-activated receptor alpha signaling in vivo and impairs fatty acid oxidation. Endocrinology. 2007;148:1206–17. doi: 10.1210/en.2006-0836. [DOI] [PubMed] [Google Scholar]
- 15.De Lange P, Feola A, Ragni M, Senese R, Moreno M, Lombardi A, et al. Differential 3,5,3’-triiodothyronine-mediated regulation of uncoupling protein 3 transcription: Role of Fatty acids. Endocrinology. 2007;148:4064–72. doi: 10.1210/en.2007-0206. [DOI] [PubMed] [Google Scholar]
- 16.Koritschoner NP, Alvarez-Dolado M, Kurz SM, Heikenwälder MF, Hacker C, Vogel F, et al. Thyroid hormone regulates the obesity gene tub. EMBO Rep. 2001;2:499–504. doi: 10.1093/embo-reports/kve107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reinehr T, Andler W. Thyroid hormones before and after weight loss in obesity. Arch Dis Child. 2002;87:320–3. doi: 10.1136/adc.87.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLaren LA, Guzeloglu A, Michel F, Thatcher WW. Peroxisome proliferator-activated receptor (PPAR) expression in cultured bovine endometrial cells and response to omega-3 fatty acid, growth hormone and agonist stimulation in relation to series 2 prostaglandin production. Domest Anim Endocrinol. 2006;30:155–69. doi: 10.1016/j.domaniend.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Rieusset J, Auwerx J, Vidal H. Regulation of gene expression by activation of the peroxisome proliferator-activated receptor gamma with rosiglitazone (BRL 49653) in human adipocytes. Biochem Biophys Res Commun. 1999;265:265–71. doi: 10.1006/bbrc.1999.1657. [DOI] [PubMed] [Google Scholar]
- 20.Li H, Ruan XZ, Powis SH, Fernando R, Mon WY, Wheeler DC, et al. EPA and DHA reduce LPS-induced inflammation responses in HK-2 cells: Evidence for a PPAR-gamma-dependent mechanism. Kidney Int. 2005;67:867–74. doi: 10.1111/j.1523-1755.2005.00151.x. [DOI] [PubMed] [Google Scholar]
- 21.Chambrier C, Bastard JP, Rieusset J, Chevillotte E, Bonnefont-Rousselot D, Therond P, et al. Eicosapentaenoic acid induces mRNA expression of peroxisome proliferator-activated receptor gamma. Obes Res. 2002;10:518–25. doi: 10.1038/oby.2002.70. [DOI] [PubMed] [Google Scholar]
- 22.Camp HS, Whitton AL, Tafuri SR. PPARgamma activators down-regulate the expression of PPARgamma in 3T3-L1 adipocytes. FEBS Lett. 1999;447:186–90. doi: 10.1016/s0014-5793(99)00268-9. [DOI] [PubMed] [Google Scholar]
- 23.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 24.Souza LL, Nunes MO, Paula GS, Cordeiro A, Penha-Pinto V, Neto JF, et al. Effects of dietary fish oil on thyroid hormone signaling in the liver. J Nutr Biochem. 2010;21:935–40. doi: 10.1016/j.jnutbio.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Knopp J, Klimes L, Brtko J, Sebokova E, Bohov P, Hromadova M. Nuclear 3,5,3’triiodothyronine receptors and malic enzyme activity in liver of rats fed fish oil or cocoa butter. J Nutr Biochem. 1992;3:587–93. [Google Scholar]
- 26.Zhang XK, Kahl M. Regulation of retinoid and thyroid hormone action through homodimeric and heterodimeric receptors. Trends Endocrinol Metab. 1993;4:156–62. doi: 10.1016/1043-2760(93)90105-n. [DOI] [PubMed] [Google Scholar]
- 27.Araki O, Ying H, Furuya F, Zhu X, Cheng SY. Thyroid hormone receptor beta mutants: Dominant negative regulators of peroxisome proliferator-activated receptor gamma action. Proc Natl Acad Sci U S A. 2005;102:16251–6. doi: 10.1073/pnas.0508556102. [DOI] [PMC free article] [PubMed] [Google Scholar]


