Abstract
We developed a stochastic simulation model to evaluate the impact of Escherichia coli O157:H7 (O157) vaccination on key epidemiological outcomes. The model evaluated a reduction in the O157 prevalence in feedlot cattle as well as concentration in cattle feces due to vaccination. The impact of this reduction on outcomes at slaughter/harvest and consumption was evaluated by simulating the relationships between the O157 prevalence and concentration at various points in the ground beef supply chain. The uncertainty and variability associated with the O157 contamination was explicitly modeled in production, slaughter, and consumption modules. Our results show that vaccination can have a significant benefit with respect to relevant outcomes such as (1) the number of human O157 illnesses due to the consumption of ground beef, (2) the number of production lots with high O157 contamination levels, (3) the likelihood of detection by U.S. Department of Agriculture Food Safety and Inspection Service testing, and (4) the probability of multiple illnesses due to ground beef servings from the same lot. These results show that these outcomes are strongly impacted by preharvest vaccination. For example, if the vaccine is used so as to reduce the prevalence of E. coli shedding cattle by 80% and if all U.S. steers and heifers were vaccinated, the expected number of human illnesses from ground beef-associated O157 would be reduced almost 60%. If the vaccine is 60% or 40% effective, the illness rate would be reduced approximately 45% or 40%, respectively. The number of production lots (10,000-lb lots) with high O157 contamination levels (>1000 servings) would be reduced by 96% if all steers and heifers received an 80% effective vaccine regimen. The analysis shows that resulting reduction in the number of shedding animals and the reduced concentration of E. coli on carcasses can combine to reduce human illnesses and cost to beef packers.
Introduction
Approximately 265,000 of the estimated 48 million foodborne illness cases each year are caused by Shiga toxigenic Esherichia coli (STEC), with E. coli serogroup O157:H7 (O157) responsible for 36% and non-O157 serogroups for the remainder (CDC, 2011). Symptoms of STEC infections include severe stomach cramps, bloody diarrhea, and vomiting. If fever develops, it rarely exceeds 101°F (38.5°C). Most people recover within 5–7 days, but some develop severe or life-threatening complications, including hemolytic uremic syndrome. Young children, the elderly, and people who are immunocompromised face higher risk from STEC infections than healthy adults.
For beef cattle producers and the meat industry, O157 contamination creates significant economic burden, legal liability, and public health concern. Ground beef that tests positive for O157 is considered adulterated, so even a low prevalence of contaminated meat produces a major economic risk for packers. Publicity surrounding recalls has also heightened awareness about bacterial contamination among consumers, with 40% saying they are extremely concerned (NCBA, 2010). In practice, reducing O157 contamination requires vigilance along the entire supply chain from farm to fork. Currently, postharvest processes, such as low water activity, chilled storage, and carcass wash procedures are well established and on average work well. For example, the national ground beef prevalence of O157 is about 0.2% (USDA-FSIS, 2009). Yet occasionally the high prevalence of O157 in cattle at the production stage aligns with high O157 carcass presence at the harvest stage, producing high O157 concentration at the consumption stage. The convergence of these outlier events on a single day (an event day) can produce ground beef production lots with an exceptionally high O157 concentration in the final product. Some say a single event day, with its extra testing requirements, quality control interventions, and internal and/or external recalls, can exact a significant economic toll.
Recently, two O157-specific bacterial extract vaccines for use in feedlot cattle have been granted conditional approval by the U.S. Department of Agriculture (USDA). The vaccines do not entirely prevent infections, but preliminary data demonstrated that vaccination reduced the percentage of animals shedding O157 at slaughter (Thomson et al., 2009; Thornton et al., 2009). This phenomenon could potentially decrease the prevalence of contaminated carcasses. The value of preharvest O157 vaccination hinges on three questions: Will vaccination significantly reduce the number of human illnesses and other relevant outcomes resulting from beef contamination with O157? Will those reductions offset the cost of vaccination relative to other interventions? And will the system (farm-to-fork) provide a sufficient signal to cattle feeders to vaccinate? The objective of this article is to provide quantitative analysis of the effect vaccination may have on human health and food safety.
Materials and Methods
Model development
We developed a stochastic simulation model that explicitly evaluates the impact of vaccination in reducing O157 prevalence and concentration in cattle feces. The model includes production, slaughter, and consumption modules, designed to examine four relevant outcomes: (1) the number of human O157 illnesses due to the consumption of ground beef, (2) the number of production lots with high O157 contamination levels, (3) the likelihood of detection by USDA Food Safety and Inspection Service (FSIS) testing, and (4) the probability of multiple illnesses due to ground beef servings from the same lot. We then modeled the impact of preharvest O157 vaccination on these outcomes. Due to space constraints, this article will discuss only the results for outcomes 1 and 2.
Our stochastic model was developed to simulate the O157 prevalence and concentration at various points in the ground beef supply chain starting with feedlots and continuing through various production steps, as the basis for predicting the number of O157 illnesses due to consumption of ground beef from U.S. steers and heifers and imported ground beef. The exact cattle prevalence and other variables are imperfectly known and constantly changing. Limited available studies give a range and statistical distributions that are often not normal (Poisson, Gamma); therefore, the model used these distributions of possible numbers to calculate multiple results. Key modeling assumptions appear in Table 1.
Table 1.
Key Modeling Assumptions
| 1. All ground beef imported into the United States is destined for mixing with domestic ground beef in portions driven by external economic factors. |
| 2. The ground beef processed in the United States is domestically consumed, i.e., no exports. |
| 3. The Escherichia coli O157:H7 prevalence and concentration in imported ground beef is similar to that in ground beef from non-vaccinated domestic steers and heifers. |
| 4. Neither the mixing of ground beef from cows and bulls with the ground beef from steers and heifers, nor vaccination of cows and bull was considered in the current model. |
| 5. The O157:H7 prevalence in a load of preevisceration beef carcasses is linearly proportional to the fecal prevalence in the feedlot from which the cattle are derived. |
| 6. The risk reduction effect of test-and-hold protocols implemented by the beef industry is not evaluated in this version of the simulation model. |
| 7. The number (colony forming units [CFU]/serving or CFU/325-g sample) of O157:H7 in a portion of ground beef from a production lot is Poisson distributed. |
| 8. The number (CFU/60 pieces of trim) of O157:H7 in an N60 sample from a production lot is Poisson distributed. |
| 9. The simulation model was developed for a yearly time frame, and seasonal variations in parameters were not considered. We assume that parameter estimates represent yearly averages. |
| 10. The annual number of O157:H7 cases due to consumption of ground beef was calculated from the observed human illnesses in the baseline year reported in the Centers for Disease Control and Prevention FoodNet data combined with the accepted but flawed attribution parameter of the human illnesses to ground beef consumption. |
| 11. The current study focuses on the O157:H7 strain. We do not consider other Shiga toxin–producing Escherichia coli strains in this study. |
The parameters and calculations of the three modules are listed in Table 2. The Production Module estimates relevant parameters at the feedlot level, such as O157 prevalence among feedlots and among cattle within O157-positive feedlots, under different vaccination scenarios. Given the limited published data on O157 vaccine efficacy, there is significant uncertainty for the values of the parameters (P10 and E) representing the impact of O157 vaccination on the prevalence and concentration in feces. Therefore, in this article, we modeled these parameters with three scenarios.
Table 2.
Summary of Key Parameters and Their Distribution Used in Model of Escherichia coli 017:H7 Outcomes Analysis
| Parameter description | Data sources | Formula/distribution | Estimated value |
|---|---|---|---|
| Production module | |||
| The mean number of cattle per feedlot (P1) | NAHMS 99 | Deterministic parameter | 5500 |
| Number of steers and heifers slaughtered per year (P2) | USDA | — | 26,000,000 (in baseline year) |
| Among feedlot prevalence of E. coli O157:H7 (P4) | Sargeant et al., 2003 | Beta(71,4) | 95% (90–98%)a |
| Mean prevalence of E. coli O157:H7 in a positive feedlot (P5) | Smith et al., 2001; Worner et al., 2006; Sargeant et al., 2003 | Beta(1914, 21322)/P4 | 14.2% (13.5–15%)a |
| Vaccine effectiveness in reducing E. coli O157:H7 feces prevalence in a feedlot (P10) | Input parameter | — | Set at 80%, 60%, and 40% for this analysis |
| Slaughter module | |||
| Ratio of preevisceration carcass prevalence to fecal prevalence in cattle from the feedlot (S1) | Elders, 2000; Barkocy-Gallagher, 2003; Arthur, 2007 | Custom stochastic distribution from simulation results (Fig. 1) | 2 (0.71–4.34) |
| Effectiveness of generic postevisceration interventions in reducing carcass prevalence of E. coli O157:H7 (S2) | Elders et al.,2000; Arthur et al.,2004; Barkocy Gallagher et al.,2003 | Beta(537, 22) | 96% (94–97%) |
| Indicator for whether the unvaccinated feedlot is contaminated with E. coli O157:H7 (S3u) | — | Binomial (1,P4) | 95%a |
| The prevalence of E. coli O157:H7 in cattle from an unvaccinated feedlot (S4u) | — | Min(1,Exponential (P5)) | 14.2% (0.7–42.5%) |
| Estimated prevalence of E. coli O157:H7 among preevisceration beef carcasses from an unvaccinated feedlot (S5u) | — | (1−(1−min(S1 * S4u,1))2 | 37.6% (2–100%) |
| Number of steers and heifers contributing trim to a 10,000-lb production lot of ground beef trim (S6) | — | 10,000/(carcass weight * percent trim) | 68 |
| Number of E. coli O157:H7–positive carcasses after generic postevisceration intervention from an unvaccinated feedlot contributing trim for the production lot (S7u) | — | Binomial (S6, (S5u) * (1−S2)) | 0.967 (0–4) |
| Indicator for whether the feedlot with vaccinated cattle is contaminated with E. coli O157:H7 (S8v) | — | Binomial (1,P4) | 95%a |
| The prevalence of E. coli O157:H7 in cattle from a vaccinated feedlot (S9v) | Varies with vaccine effectiveness (input parameter) | Binomial (P1 * S4u, (1−P10))/P1) | Depends on input scenario |
| Estimated prevalence of E. coli O157:H7 among preevisceration beef carcasses from an vaccinated feedlot (S10v) | — | 1−(1−min(S1 * S9v,1))2 | 37.6% (2–100%) |
| The surface area contaminated with E. coli O157:H7 per contaminated carcass (S11) | Assumption and sampling surface area (Arthur, 2004; Brichta-Harhay, 2007) | Approximate value, may be adjusted to calibrate model similar to 2001 FSIS ground beef RA | 8000 cm2/carcass |
| Slaughter module | |||
| Average carcass weight (S12) | Input parameter | Estimated from NASS 2009 data | 814 lb/carcass |
| Percent of dressed carcass weight processed into ground beef trim per steer or heifer (S13) | Assumption | — | 18% |
| The E. coli O157:H7 average surface concentration on a preevisceration carcass of unvaccinated cattle (S14u,k) | Arthur, 2004; Brichta-Harhay, 2007; Barkocy Gallagher, 2003 | Empirical discrete distribution. | Mean, 7 (0–6) CFU/100 cm2 |
| The surface area contaminated with E. coli O157:H7 per contaminated carcass (S15,k) | Assumption and sampling surface area (Arthur, 2004; Brichta-Harhay, 2007) | Approximate value, may be adjusted to calibrate model similar to 2001 FSIS ground beef RA | 8000 cm2/carcass |
| Total CFU of E. coli O157:H7 on a carcass at preeviceration step (CFU/carcass)S16,k | — | S14u * S15,k/100 | Mean, 565 CFU/carcass |
| Log reduction of E. coli O157:H7 concentration due to generic postevisceration treatments (S17,k) | Arthur et al.,2004, 2001; FSIS risk assessment (Williams et al.,2010) | Lognormal distribution bounded at (0, 3.5) | 1.06 (0.29–2.32) |
| Total CFU of E. coli O157:H7 on a contaminated carcass after generic postevisceration treatment ( CFU/carcass) (S18,k) | — | 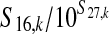 |
Mean, 93 |
| CFU of E. coli 0157:H7 added to a 10,000-lb production lot of trim from a contaminated carcass from unvaccinated feedlot (S19u,k) | 75% of carcass surface area assumed to go towards ground beef trim | 0.75 * S18,k | 82 (0–62) max ∼30,000 |
| Amount of E. coli 0157:H7 in a 10,000-lb production lot from unvaccinated cattle (CFU/production lot) (S21u) | — | 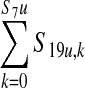 |
63 (0–77) max ∼30,000 |
| Average concentration of E. coli O157:H7 in a 10,000-lb production lot from unvaccinated cattle per gram (S22u) | — | S21u/(10,000 * 454) | 1.42 (0–2) * 10−5 CFU/g |
| Estimated prevalence of E. coli O157:H7 in 325-g ground beef portions in a production lot from unvaccinated cattle (S23u) | — | 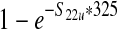 |
0.35% (0–0.5%) |
| Estimated prevalence of E. coli O157:H7 in servings from a production lot from unvaccinated cattle ( S24u) | — | 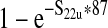 |
0.115% (0–0.15%) |
| Expected number of contaminated servings per production lot from unvaccinated cattle (S25u) | — | 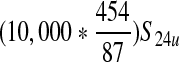 |
60 (0–75) max ∼15,000 |
| Percentage of domestic ground beef in a production lot (S26) | User input | 59% in baseline scenario | |
| Number of E. coli O157:H7–positive carcasses from a vaccinated feed lot in a production lot in which the domestic ground beef trim is from vaccinated cattle (S27v) | — | Binomial (S6 * S26, (S10v) * (1−S2)) | Depends on input scenario |
| Number of E. coli O157:H7–positive carcasses from an unvaccinated (imported) sources in a production lot in which the domestic ground beef trim is from vaccinated cattle (S28) | — | Binomial (S6 * (1−S26), (S5u) * (1−S2)) | Depends on input scenario |
| Total amount of E. coli 0157:H7 in a 10,000-lb production lot from vaccinated cattle (CFU/production lot) S29v | — | 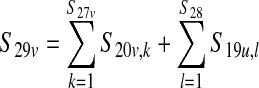 |
Depends on input scenario |
| Slaughter module | |||
| Average concentration of E. coli O157:H7 in a 10,000-lb production lot from vaccinated cattle per gram (S30v) CFU/g | — | S29v/(10,000 * 454) | Depends on input scenario |
| Estimated prevalence of E. coli O157:H7 in 325-g ground beef portions in a production lot from vaccinated cattle (S31v) | — | 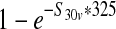 |
Depends on input scenario |
| Estimated prevalence of E. coli O157:H7 in servings from a production lot from vaccinated cattle ( S32v) | — | 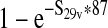 |
Depends on input scenario |
| Expected number of contaminated servings per production lot from vaccinated cattle (S33v) | — | 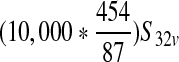 |
Depends on input scenario |
| The annual number of ground beef production lots from vaccinated cattle processed in the United States (S34v ) | — | 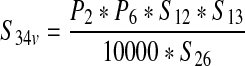 |
Depends on input scenario |
| The annual number of ground beef production lots from vaccinated cattle processed in the United States (S35u) | — | 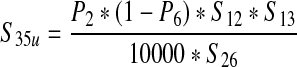 |
Depends on input scenario |
| Indicator for whether the production lot is vaccinated (S36) | — | S36=Binomial(1, f) | Depends on input scenario |
| The number of contaminated servings from a production lot from a slaughter plant where f a fraction of production lots is from vaccinated cattle (S37) | — | 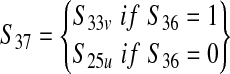 |
Depends on input scenario |
| Indicator for whether the number of contaminated servings from the production lot is greater than 1000 ("hot lot") (S38) | — | 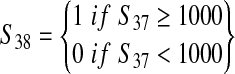 |
Depends on input scenario |
| Prevalence of E. coli 0157:H7–positive 325-g samples from a production lot depending on whether it is vaccinated (S39). Probability of detection by FSIS per visit based on ground beef. | — | 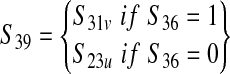 |
Depends on input scenario |
| Annual probability of detecting E. coli 0157:H7 via FSIS MT43 routine ground beef testing project (S40) | — | S40=1−(E(1−S39))24 | Depends on input scenario |
| Prevalence of E. coli 0157:H7 in trim sample from a production lot based on N60 testing protocol (S41) | 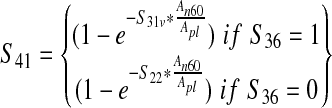 |
Depends on input scenario | |
| S42: Annual probability of detecting E. coli 0157:H7 via routine N60 ground beef trim testing | — | S42=1−(E(1−S41))3 | Depends on input scenario |
| S43: Annual probability of detecting E. coli 0157:H7 via routine FSIS ground beef or trim testing programs | — | S43=1−(1−S42) (1−S40) | Depends on input scenario |
| S44: Number of E. coli O157:H7 illnesses caused by ground beef from a production lot. | — | S44 ∼ Binomial (S37, C8) | Depends on input scenario |
| S45: Indicator variable for multiple illnesses due to consumption of ground beef from the same production lot | — | 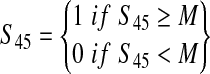 |
Depends on input scenario |
| Consumption module | |||
| Average serving size for ground beef in grams (C1) | 2001 FSIS ground beef risk assessment. CSFII survey data |
Deterministic parameter | 87 g |
| Fraction of E. coli 0157:H7 cases caused by the consumption of ground beef (etiologic fraction C2) | Withee, 2009; Rangel, 2005; Kassenborg, 2004, 2001; FSIS risk assessment | Custom discrete distribution | 26% (15–37%) |
| Number of ground beef servings consumed per year in the United States in baseline per year (C3) | — | Estimated amount of ground beef processed in the United States/C1 | 46927 * 106 servings |
| Baseline prevalence of E. coli 0157:H7 in 325-g ground beef samples based on FSIS testing data (C4) | FSIS ground beef routine verification data for year 2009 | Beta(36,11539) | 0.31% (0.23–0.40%) |
| Baseline prevalence of E. coli 0157:H7 in 87-g ground beef servings based on FSIS testing data (C5) | — | 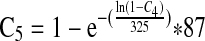 |
0.08% (0.06–0.10%) |
| Baseline number of E. coli O157:H7 contaminated ground beef servings from domestic production based on FSIS sampling (C6) | — | C5* C3 | 39 (29–50) million servings |
| Annual expected number of domestically acquired E. coli O157:H7 illnesses in the United States (C7) | Scallan et al., 2011 | 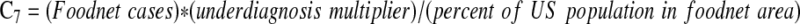 |
76,500 |
| Baseline probability of E. coli O157:H7 illness per contaminated serving of ground beef consumed (C8) | — | 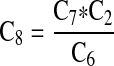 |
5.2 (2.6–8.1) * 10−4 illness per serving |
| Mean number of E. coli O157:H7 contaminated servings from unvaccinated production lots per year (C11u) | — | C11u=E(S35u* S25u) | Depends on input scenario |
| Mean number of E. coli O157:H7 contaminated servings from vaccinated production lots per year (C14v) | — | C14v=E(S34v* S33v) | Depends on input scenario |
| Mean number of E. coli O157:H7 illnesses caused due to consumption of ground beef from feedlot cattle and imported ground beef (C15) | — | C15=E(C11+C14v) | Depends on input scenario |
Two-sided 90% probability interval. The subscripts u and v denote parameters related to unvaccinated cattle and vaccinated cattle respectively. The symbol E denotes expected value of a parameter.
Poisson distribution is a single parameter discrete probability distribution that is used to represent the number of events occurring in an interval of time or space. Given the Poisson distribution, the expected number of events in an interval is proportional to the size of the interval.
Beta distribution is a two-parameter continuous distribution often used to model the uncertainty associated with the probability of an event. The beta(S+1, N-S+1) distribution is used in Bayesian analysis to model the uncertainty in the probability of success of a Binomial process when s successes are observed in N trials.
Binomial distribution is the discrete probability distribution of the number of successes in a sequence of n independent experiments with two possible outcomes in each experiment, each of which yields success with probability p.
NAHMS, National Animal Health Monitoring System; USDA, U.S. Department of Agriculture; FSIS, USDA Food Safety and Inspection Service; CFU, colony-forming units.
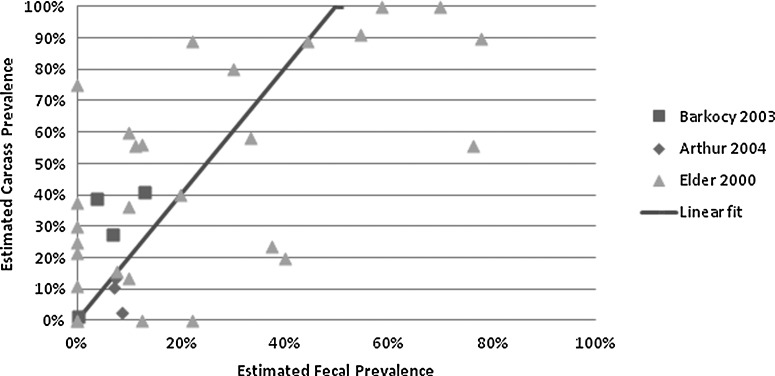
The simulation model was based on the approximated linear relationships between O157 prevalence in feces and on carcasses (Barkocy-Gallagher et al., 2003; Arthur et al., 2004; Elder et al., 2000) (Fig. 1). We fit a linear regression model with intercept equal to zero using data from the three studies (R2=0.68). Uncertainty associated with this parameter was estimated by resampling from the data using @RISK and recalculating the linear coefficient. The final distribution for S1 had a mean of 2 (90% prediction interval [PI], 0.71–4.34). A similar linear model has been used by others and underpins the 2001 FSIS ground beef risk assessment. Similarly, linear models were also utilized to simulate the decreases in O157 concentration due to vaccination or generic slaughter level interventions (Cassin et al., 1998).
FIG. 1.
Linear estimation of Escherichia coli O157:H7 prevalence in cattle feces and preevisceration carcasses (%) based on publications shown.
The Slaughter Module estimates the O157 prevalence and concentration on beef carcasses and in ground beef components at various processing points. The specific process points modeled include (1) on carcasses preevisceration, (2) on carcasses after generic postevisceration interventions and chilling, (3) in production lots of trim, and (4) in production lots of ground beef. The statistical relationships utilized in this module were derived from data linking the O157 prevalence and concentration at the processing level to the corresponding variables at the feedlot level. The final output of this module is the O157 prevalence in servings of ground beef from 10,000-lb production lots. The variability in the O157 concentration and prevalence in production lots of trim and raw ground beef influence critical outcomes for packers.
Data on O157 contamination in slaughter plants also indicate that there is a high degree of variance in the O157 concentration in a production lot. Consequently, a small fraction of production lots may be contaminated to a high degree (“hot” lots), although the average load per production lot is relatively small. We defined a “hot” lot as one containing more than 1,000 contaminated servings of ground beef. This variability is likely derived from the variance in prevalence and concentration of O157 observed in feedlot cattle (feces samples) and beef carcasses, as well as the presence of some “super shedders” in herds (Jacob et al., 2010; Gyles, 2007; Barkocy-Gallagher et al., 2003). We assumed that testing methods were sensitive enough to detect a single colony-forming unit (CFU) of O157 in a 325-g portion of ground beef, as recommended by the FSIS guidelines (Murphy and Seward, 2004).
The Consumption Module estimates the number of O157 human illnesses attributed to consumption of ground beef from domestic steer and heifer slaughter and imported ground beef (Table 2). The mixing of imported ground beef with ground beef from domestic steer and heifer slaughter was considered (Bosilevac et al., 2006). We estimated that about 40% of the “average” production lot consisted of imported ground beef based upon ground beef imports and total ground beef production from steer and heifer slaughter in the United States.
The first section of the Consumption Module estimates a baseline probability of an O157 illness per contaminated serving of ground beef (C8). In the second section, the Slaughter Module outputs of the number of O157-contaminated servings of ground beef in a production lot (S25u and S33v) and the annual number of production lots from vaccinated and unvaccinated cattle (S34v and S35u) are utilized to estimate the number of O157 illnesses in the current year in which a specific percent of fed cattle have been vaccinated.
To estimate the baseline probability that an O157-contaminated ground beef serving results in an illness, we started with the fraction of O157 illnesses caused due to consumption of ground beef (the etiologic fraction), from outbreak data and epidemiological studies. The annual number of O157 cases due to consumption of ground beef was then calculated based on CDC Foodnet surveillance data for 2009, the baseline year, and the etiologic fraction. The baseline probability that an O157-contaminated ground beef serving results in a human illness was calculated as the ratio of the number of O157 illnesses caused by consumption of ground beef and the estimated number of contaminated ground beef servings.
Scenarios for the impact of O157 vaccination
We analyzed three scenarios for the impact of O157 vaccination. In Scenarios A, B, and C, we assumed vaccine induced prevalence reductions were 80%, 60%, and 40%. Additionally, for scenarios A, B, and C we assumed reduction of 1, 0.3, and 0.3 log10 CFU/carcass reduction in average concentration on resulting carcasses, respectively. To estimate the impact of vaccination under these scenarios, the simulation model was run for 100,000 iterations using @RISK with Monte Carlo Sampling.
Results
Reduction in human illnesses due to vaccination
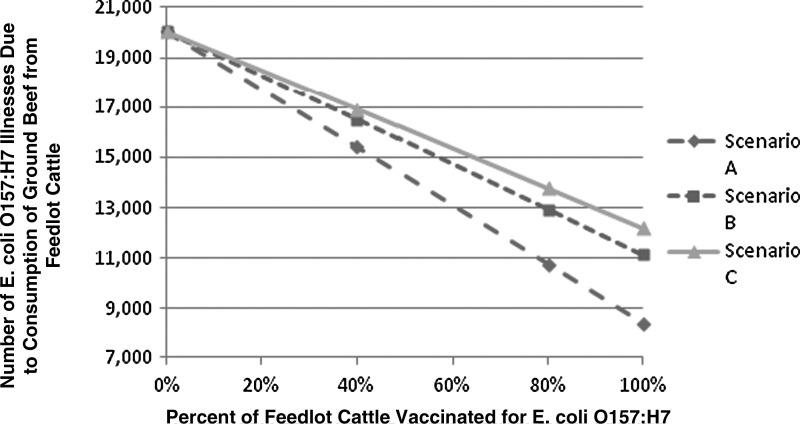
The first outcome evaluated was the value of vaccination with respect to the overall number of human O157 cases prevented. Figure 2 and Table 3 show the relationship between the predicted number of human cases of O157 and the number of feedlot cattle vaccinated (termed “production function” in Withee et al., 2009). The model demonstrated that the number of human illnesses attributed to consumption of O157-contaminated ground beef can be greatly reduced by vaccination. If the vaccine were used so as to be 80% effective, and if all U.S. steers and heifers were vaccinated, the expected number of human illnesses could be reduced by almost 60% (90% PI, 56–60). Even with partial adoption of vaccination in say 50% of feedlots (80% effective), human illnesses would be reduced by nearly 30% (90% PI, 26–32), from approximately 20,057 to 14,231 annual cases on average (Fig. 2). In scenario C, with a 40% effective vaccine, human illnesses would be reduced from approximately 20,057 to 12,187 annual cases with full adoption. These results suggest that, depending on vaccine performance parameters, 2,000–3,300 cattle must be vaccinated to prevent one O157 illness. Note that a portion of the illnesses in these scenarios are associated with imported ground beef and would not be prevented with vaccination of domestic cattle.
FIG. 2.
Change in the mean number of human illnesses due to Escherichia coli O157:H7 with the number of cattle vaccinated. Scenario A: Vaccine 80% effective, also 1 log10 CFU/carcass reduction in average concentration on resulting carcass. Scenario B: Vaccine 60% effective, also 0.3 log10 CFU/carcass reduction in average concentration on resulting carcass. Scenario C: Vaccine 40% effective, also in 0.3 log10 CFU/carcass reduction in average concentration on resulting carcass.
Table 3.
Predicted Annual Escherichia coli O157:H7 Illnesses per Year Due to Consumption of Ground Beef from Steer and Heifer Slaughter with the Percentage of U.S. Cattle Vaccinated (Adoption Rate)
| |
Mean number of illnesses |
||
|---|---|---|---|
| Vaccine adoption rate | Scenario A | Scenario B | Scenario C |
| 0% | 20057 (10182–30500) | 20057 (10182–30500) | 20057 (10182–30500) |
| 40% | 15396 (7800–23500) | 16486 (8398–25252) | 16909 (8635–25957) |
| 80% | 10736 (5400–16300) | 12916 (6617–19890) | 13761 (7088–21303) |
| 100% | 8405 (4268–12830) | 11130 (5731–17211) | 12187 (6321–18982) |
The intervals shown here are two-sided 95% probability intervals that represent the impact of uncertainty in input parameter as well as variability in the ground beef processing steps. Possible reasons for the relatively wide intervals include the significant uncertainty in the etiologic fraction for O157 illnesses due to ground beef consumption and the significant variability in the number of contaminated servings per production lot (e.g., due to high shedders).
Decrease in contaminated ground beef production lots due to vaccination
Table 4 shows the impact of vaccination, as adoption increases, on the annual number of “hot” production lots. A “hot-lot” was defined as one containing more than 1,000 contaminated servings in a 10,000-lb lot, as calculated for a hypothetical slaughter plant producing 16,000 lots per year. Hot lots or event days may result in an internal recall by the packer. The extra work required to recover, rework, cook, or destroy product is costly. All levels of vaccine efficacy and adoption reduced risk to the packer. Full adoption of an 80% effective vaccine (Scenario A) could reduce the chance of hot lots by 96%.
Table 4.
Change in Predicted Annual Number of “Hot” Production Lots (>1,000 Contaminated Servings) for Slaughter Plants Producing 16,000 Lots per Year with the Percent of Cattle Vaccinated
| Vaccine adoption rate | Scenario A | Scenario B | Scenario C |
|---|---|---|---|
| 0% | 144 (124–163) | 144 (124–163) | 144 (124–163) |
| 40% | 82 (67–97) | 105 (88–122) | 115 (98–133) |
| 80% | 30 (21–39) | 73 (60–88) | 90 (75–106) |
| 100% | 6 (2–10) | 57 (45–69) | 77 (63–91) |
The numbers in the parentheses are two-sided 95% probability intervals.
Discussion
Our results demonstrate that preharvest vaccination against O157 could have a significant benefit for the beef industry by reducing the likelihood of hot lots or event days, and the probability of multiple illnesses due to contaminated ground beef servings. In addition, the model results indicate that a large number of ground-beef-associated human O157 illnesses may be prevented by vaccinating domestic feedlot cattle. Thus, both the packer and the consumer could benefit from routine O157 vaccination of cattle.
In our study, the relationship between the number of human illnesses and vaccinated cattle was quite linear (R2=0.99), similar to the model of Withee et al. (2009). By comparison, we also modeled a reduction in concentration on/in carcasses/feces as well as prevalence of shedders due to vaccination (Fig. 1). Incorporating a reduction in concentration produced an additional impact on epidemiological outcomes.
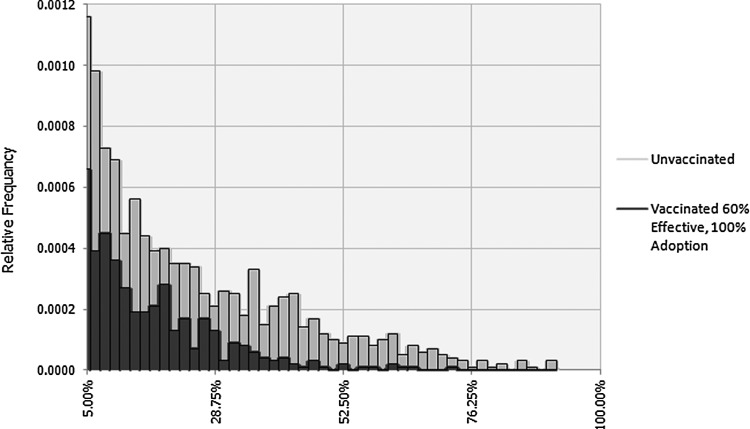
We propose the reason that vaccine is so effective is its impact on outlier events and the tail-ends of the non-normal distributions. Some have speculated about the possibility of “super-shedder” cattle (Jacob et al., 2010; Gyles, 2007). Most packers agree the occurrence of “event days” is sporadic. Figure 3 shows the tail of the histogram (segment of the histogram showing highly contaminated production lots) of simulation results for the prevalence of O157 in 325-g samples by comparing vaccinated and unvaccinated production lots. The x-axis is the prevalence of positive samples expressed as a fraction, and the y-axis is the relative frequency (proxy for probability from simulation results). The graph shows that the probabilities of a very highly contaminated production lot can be reduced considerably with vaccination. This is because vaccination is an independent mitigation measure from postharvest interventions. Vaccination seems to impact the few rare cases where the production lot could be highly contaminated due to various outlier events.
FIG. 3.
Tail-end of histogram showing impact of vaccination on number of production lots with high Escherichia coli O157 prevalence (>5%) in 325-g samples. Scenario B: Vaccine 60% effective, 100% adoption.
Given that a substantial portion of ground beef consumed in the United States is imported and to aid in further economic modeling, we modeled ground beef imports and the mixing of domestic and imported ground beef explicitly. This approach is conservative (reducing the observed impact of vaccination) since a smaller proportion of the O157 illnesses are attributed to the consumption of domestic ground beef, reducing the estimates of human illnesses which can be prevented by vaccinating domestic feedlot cattle. Withee et al. (2009) did not consider ground beef imports and attributed a greater number of human illnesses to the consumption of ground beef from domestic cattle slaughter. Unlike Hurd et al. (2010), we assumed a constant probability that contaminated servings of ground beef cause illness, regardless of the specific product streams (e.g., food service) or consumption channel (e.g., retail sale). The differences in risks for various product streams and consumption channels were not considered here, as the focus of the current study was on the impact of vaccination on overall human O157 illnesses due to consumption of all categories of ground beef. Given that only approximately 3% of all raw ground beef is sold directly to the consumer, this is an area for further research (NCBA, 2004).
Significant uncertainty surrounds several input variables associated with slaughter processes, such as the amount of a carcass surface area represented in ground beef trim or the effectiveness of carcass decontamination treatments. Given these uncertainties, the model results for slaughter outcomes are more appropriate for predicting the relative impact of O157 vaccination, rather than for predicting the absolute levels of these outcomes. The results for slaughter outcome measures are representative of average values for a hypothetical large production plant in the United States and are not applicable for any specific slaughter establishment. However, we believe that the modeling approach utilized here would be appropriate for evaluating outcomes for specific slaughter plants, provided that the input parameters are calibrated according to the establishment characteristics.
There is significant uncertainty about the inputs in this model representing the impact of O157 vaccination. Preliminary data indicated that vaccination reduces the O157 prevalence and concentration in feces (p≤0.05 based on sampling at specific times post vaccination as described elsewhere [Thomson et al., 2009; Thornton et al., 2009]).
We modeled these parameters as inputs that users can vary to produce different scenarios to consider the impact of the associated uncertainty. Depending on the nature of the biological processes associated with the functioning of the vaccine, it is possible that the reduction in prevalence is correlated with other variables, such as the reduction in fecal concentration. For example, a large reduction in O157 feces concentration due to vaccination may also be associated with a correspondingly large reduction in O157-measured fecal prevalence. Further studies are required to examine the potential correlation between the reductions in concentration and reductions in prevalence under different vaccine dosage regimens and field conditions. Such studies will also provide the data required to evaluate the impact of vaccination stochastically, that is with correlated probability distributions for reduction in feces concentrations and reduction in prevalence.
This stochastic model based on production, slaughter, and consumption factors demonstrated that preharvest O157 vaccination could reduce human illnesses and decrease contaminated ground beef lots. The analysis shows that vaccine-associated reduction in the number of shedding animals and the reduced concentration of O157 in feces both combine to reduce human illnesses. Thus, the benefits of preharvest O157 vaccination of cattle extend to packers as well as consumers.
Acknowledgments
Dr. Guy Loneragan from Texas Tech University assisted in interpreting data from vaccine trials. Sarah Staples, M.A., E.L.S., assisted with manuscript preparation.
Disclosure Statement
This project was funded by an unrestricted grant to Iowa State University from Pfizer Animal Health (New York, NY). Manuscript preparation was supported by an unrestricted grant from Pfizer Animal Health.
References
- Arthur TM. Bosilevac JM. Nou X. Shackelford SD. Wheeler TL. Kent MP. Jaroni D. Pauling B. Allen DM. Koohmaraie M. Escherichia coli O157 prevalence and enumeration of aerobic bacteria, Enterobacteriaceae, and Escherichia coli O157 at various steps in commercial beef processing plants. J Food Prot. 2004;67:658–665. doi: 10.4315/0362-028x-67.4.658. [DOI] [PubMed] [Google Scholar]
- Barkocy-Gallagher GA. Arthur TM. Rivera-Betancourt M. Nou X. Shackelford SD. Wheeler TL. Koohmaraie M. Seasonal prevalence of Shiga toxin-producing Escherichia coli, including O157:H7 and non-O157 serotypes, and Salmonella in commercial beef processing plants. J Food Prot. 2003;66:1978–1986. doi: 10.4315/0362-028x-66.11.1978. [DOI] [PubMed] [Google Scholar]
- Bosilevac JM. Guerini MN. Brichta-Harhay DM. Arthur TM. Koohmaraie M. Microbiological characterization of imported and domestic boneless beef trim used for ground beef. J Food Prot. 2007;70:440–449. doi: 10.4315/0362-028x-70.2.440. [DOI] [PubMed] [Google Scholar]
- Cassin MH. Lammerding AM. Todd EC. Ross W. McColl RS. Quantitative risk assessment for Escherichia coli O157:H7 in ground beef hamburgers. Int J Food Microbiol. 1998;41:21–44. doi: 10.1016/s0168-1605(98)00028-2. [DOI] [PubMed] [Google Scholar]
- [CDC] Centers for Disease Control and Prevention. Division of Foodborne, Bacterial, and Mycotic Diseases. National Center for Zoonotic, Vector-Borne, and Enteric Diseases. 2011. www.cdc.gov/ecoli/general/index.html#how_common. [Sep 6;2012 ]. www.cdc.gov/ecoli/general/index.html#how_common
- [CDC] Centers for Disease Control and Prevention. FoodNet Reports. 2011. http://www.cdc.gov/foodnet/data/reports.html. [Aug 19;2012 ]. http://www.cdc.gov/foodnet/data/reports.html
- Elder RO. Keen JE. Siragusa GR. Barkocy-Gallagher GA. Koohmaraie M. Laegreid WW. Correlation of enterohemorrhagic Escherichia coli O157 prevalence in feces, hides, and carcasses of beef cattle during processing. Proc Natl Acad Sci USA. 2000;97:2999–3003. doi: 10.1073/pnas.060024897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [FSIS] Food Safety Inspection Service. FSIS Risk Assessment of Escherichia coli O157:H7 in ground beef. 2001. www.fsis.usda.gov/ophs/ecolrisk/pubmeet/sld001.htm. [Jan 16;2012 ]. www.fsis.usda.gov/ophs/ecolrisk/pubmeet/sld001.htm
- Gyles CL. Shiga toxin–producing Escherichia coli: An overview. J Anim Sci. 2007;85:E45–E62. doi: 10.2527/jas.2006-508. [DOI] [PubMed] [Google Scholar]
- Hurd HS. Vaughn M. Holtkamp DJ. Dickson JS. Warnick L. Quantitative risk from fluoroquinolone-resistant Salmonella and Campylobacter due to treatment of dairy heifers with enrofloxacin for bovine respiratory disease. Foodborne Pathog Dis. 2010;7:1305–1322. doi: 10.1089/fpd.2010.0550. [DOI] [PubMed] [Google Scholar]
- Jacob ME. Renter DG. Nagaraja TG. Animal- and truckload-level associations between Escherichia coli O157:H7 in feces and on hides at harvest and contamination of preevisceration beef carcasses. J Food Prot. 2010;73:1030–1037. doi: 10.4315/0362-028x-73.6.1030. [DOI] [PubMed] [Google Scholar]
- Murphy RY. Seward RA. Process control and sampling for Escherichia coli O157:H7 in beef trimmings. J Food Prot. 2004;67:1755–1759. doi: 10.4315/0362-028x-67.8.1755. [DOI] [PubMed] [Google Scholar]
- [NCBA] National Cattlemen's Beef Association. Consumer perceptions of beef safety: Research overview. 2010. www.beefresearch.org/CMDocs/BeefResearch/Market%20Research/Reports/Research%20Report%20Consumer%20Perceptions%20of%20Beef%20Safety%20100510.pdf. [Aug 24;2012 ]. www.beefresearch.org/CMDocs/BeefResearch/Market%20Research/Reports/Research%20Report%20Consumer%20Perceptions%20of%20Beef%20Safety%20100510.pdf
- [NCBA] National Cattlemen's Beef Association. Usage and Volumetric Assessment of Beef in Foodservice. 2004. www.beefusa.org/UDocs/MA102-BeefFoodserviceVolumetricFY2007.pdf. [Aug 24;2012 ]. www.beefusa.org/UDocs/MA102-BeefFoodserviceVolumetricFY2007.pdf
- Thomson DU. Loneragan GH. Thornton AB. Lechtenberg KF. Emery DA. Burkhardt DT. Nagaraja TG. Use of a siderophore receptor and porin proteins-based vaccine to control the burden of Escherichia coli O157:H7 in feedlot cattle. Foodborne Pathog Dis. 2009;6:871–877. doi: 10.1089/fpd.2009.0290. [DOI] [PubMed] [Google Scholar]
- Thornton AB. Thomson DU. Loneragan GH. Fox JT. Burkhardt DT. Emery DA. Nagaraja TG. Effects of a siderophore receptor and porin proteins-based vaccination on fecal shedding of Esherichia coli O157:H7 in experimentally inoculated cattle. J Food Prot. 2009;72:866–869. doi: 10.4315/0362-028x-72.4.866. [DOI] [PubMed] [Google Scholar]
- [USDA-FSIS] U.S. Department of Agriculture Food Safety and Inspection Service. Microbiological results of raw ground beef products analyzed for Escherichia coli O157:H7, summarized by calendar year. USDA-FSIS baseline data. 2009. www.fsis.usda.gov/Science/Ecoli_O157_Summary_Tables/index.asp. [Sep 6;2012 ]. www.fsis.usda.gov/Science/Ecoli_O157_Summary_Tables/index.asp
- Williams BJ. Burson DE. Gerlach BM. Van DeWalle AF. Thippareddi H. Multiple antimicrobial interventions for the control of Escherichia coli O157:H7 in very small beef processing facilities. Nebraka Beef Cattle Reports, Paper 597. Lincoln: University of Nebraska–Lincoln. 2010. http://digitalcommons.unl.edu/animalscinbcr/597. [Aug 24;2012 ]. http://digitalcommons.unl.edu/animalscinbcr/597
- Withee J. Williams M. Disney T. Schlosser W. Bauer N. Ebel E. Streamlined analysis for evaluating the use of preharvest interventions intended to prevent Escherichia coli O157:H7 illness in humans. Foodborne Pathog Dis. 2009;6:817–825. doi: 10.1089/fpd.2008.0255. [DOI] [PubMed] [Google Scholar]