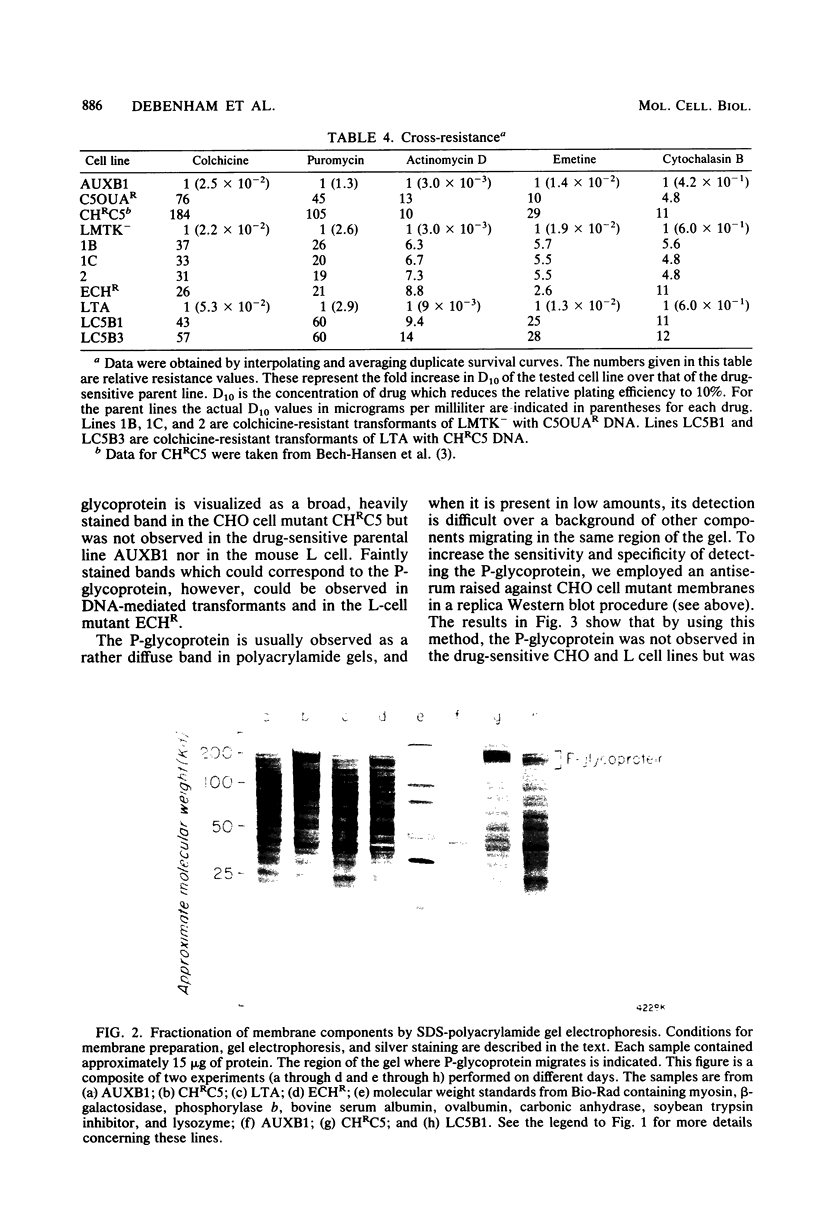
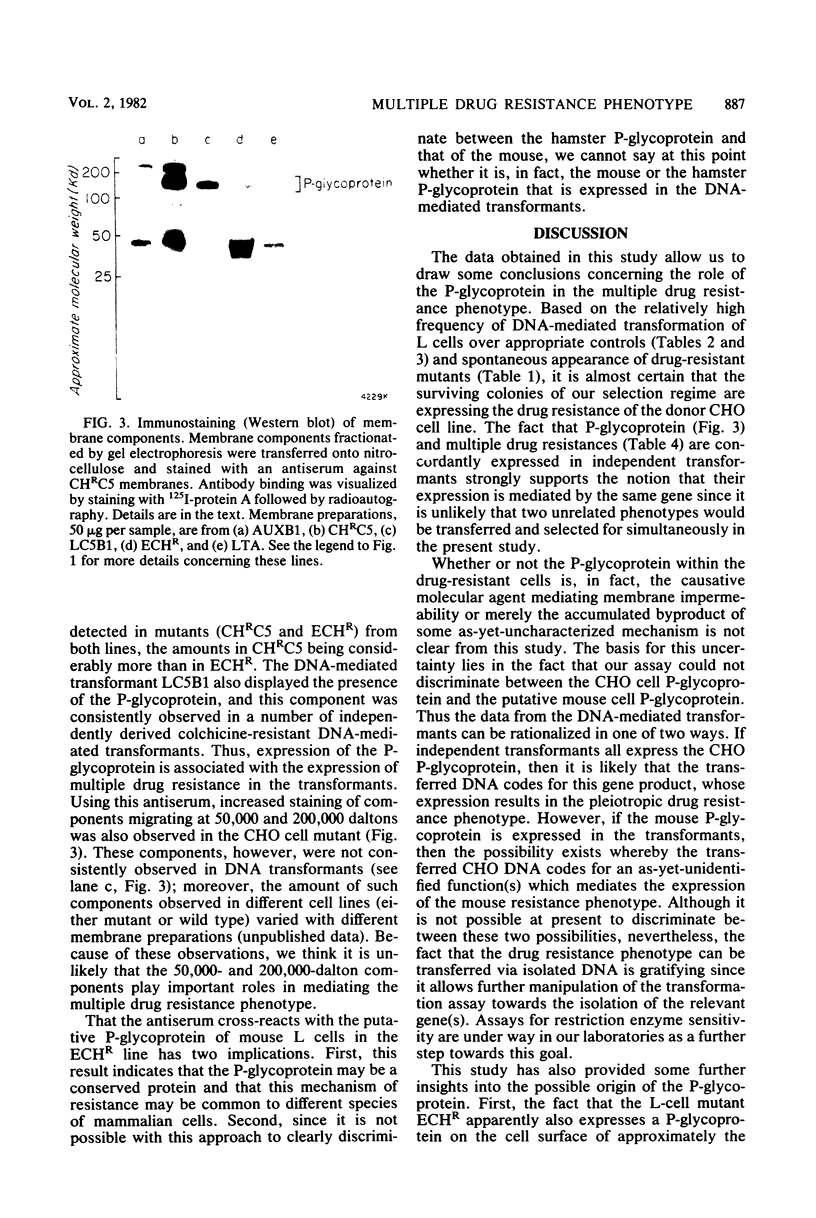
Abstract
Colchicine-resistant Chinese hamster ovary (CHO) cell mutants whose resistance results from reduced drug permeability have been isolated previously in our laboratories. This reduced permeability affects a wide range of unrelated drugs, resulting in the mutants displaying a multiple drug resistance phenotype. A 170,000-dalton cell surface glycoprotein (P-glycoprotein) was identified, and its expression appears to correlate with the degree of resistance. In this study we were able to confer the multiple drug resistance phenotype on sensitive mouse L cells by DNA-mediated gene transfer of DNA obtained from the colchicine-resistant mutants. P-glycoprotein was detected in plasma membranes of these DNA transformants by staining with an antiserum raised against membranes of mutant CHO cells. These results are consistent with a causal relationship between P-glycoprotein expression and the multiple drug resistance phenotype.
Full text
PDF


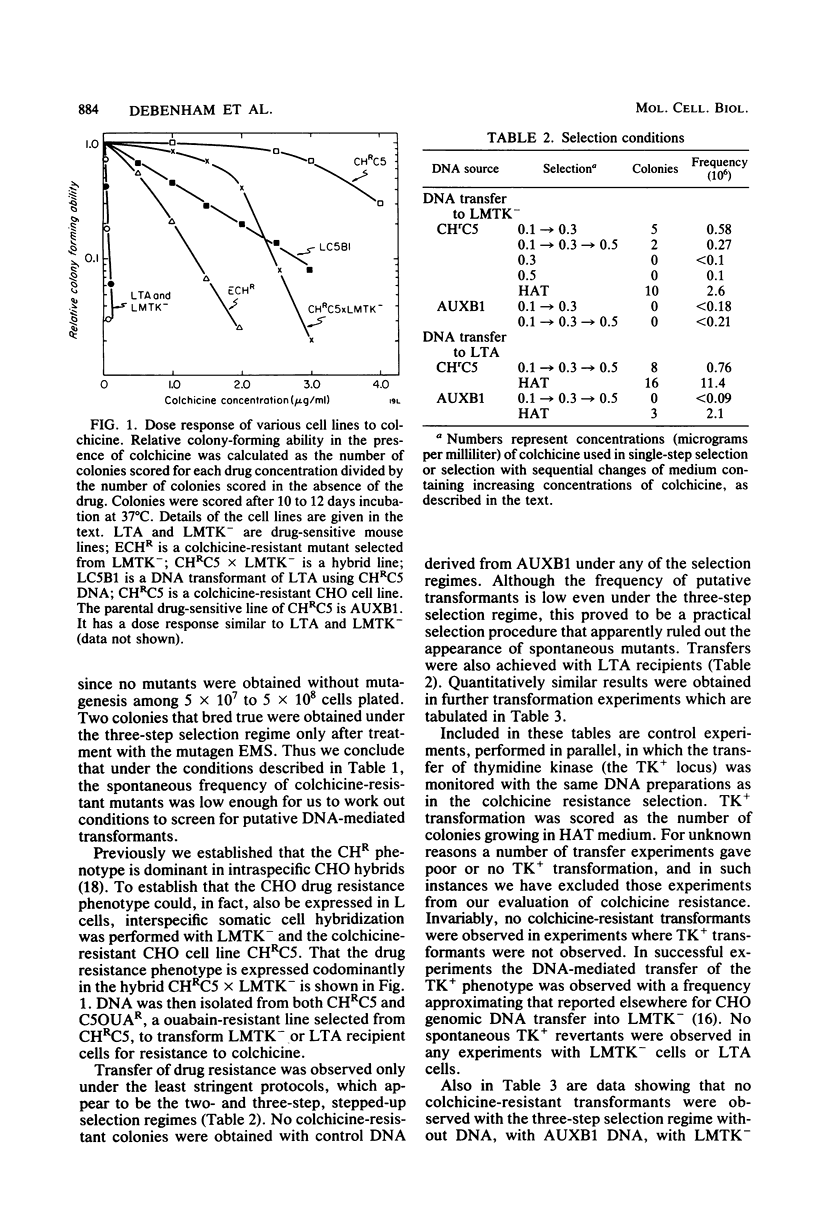
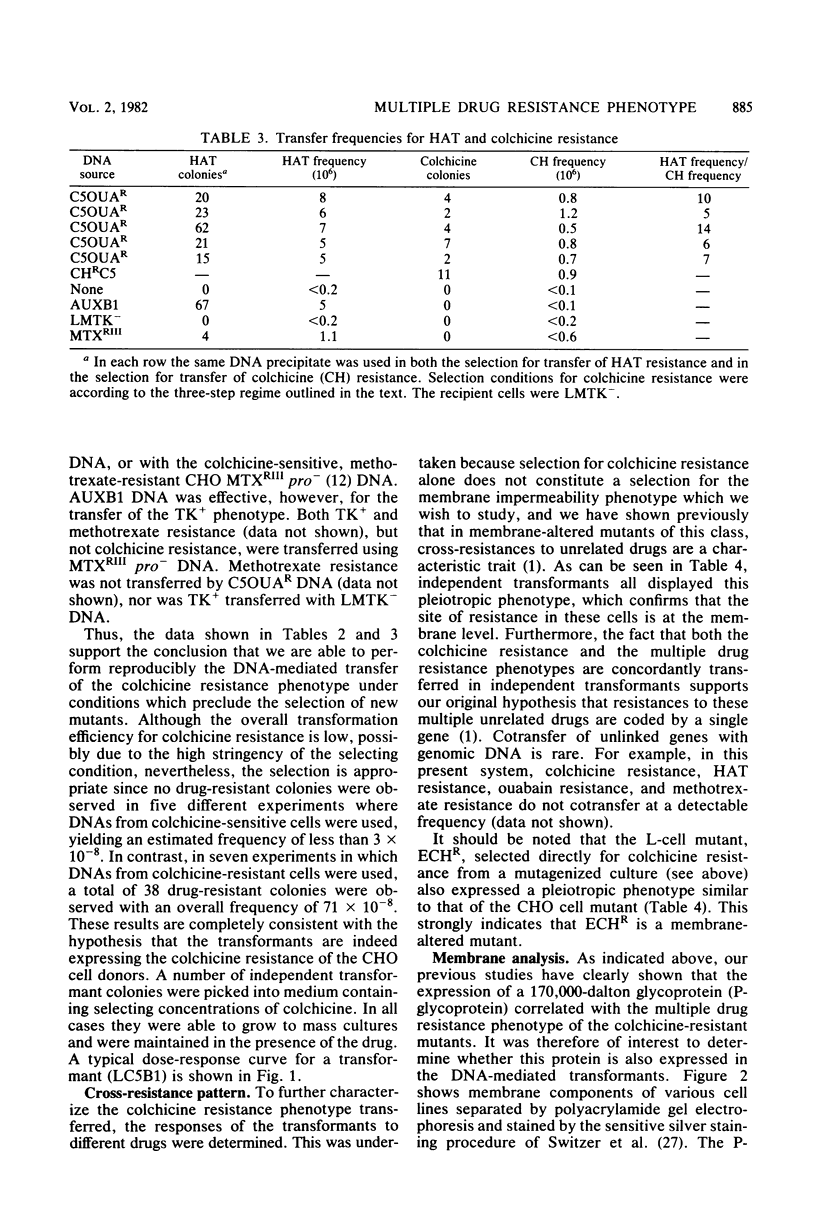


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech-Hansen N. T., Sarangi F., Sutherland D. J., Ling V. Rapid assays for evaluating the drug sensitivity of tumor cells. J Natl Cancer Inst. 1977 Jul;59(1):21–27. doi: 10.1093/jnci/59.1.21. [DOI] [PubMed] [Google Scholar]
- Bech-Hansen N. T., Till J. E., Ling V. Pleiotropic phenotype of colchicine-resistant CHO cells: cross-resistance and collateral sensitivity. J Cell Physiol. 1976 May;88(1):23–31. doi: 10.1002/jcp.1040880104. [DOI] [PubMed] [Google Scholar]
- Beck W. T., Mueller T. J., Tanzer L. R. Altered surface membrane glycoproteins in Vinca alkaloid-resistant human leukemic lymphoblasts. Cancer Res. 1979 Jun;39(6 Pt 1):2070–2076. [PubMed] [Google Scholar]
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- Carlsen S. A., Till J. E., Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Dec 14;455(3):900–912. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Carlsen S. A., Till J. E., Ling V. Modulation of membrane drug permeability in Chinese hamster ovary cells. Biochim Biophys Acta. 1976 Dec 14;455(3):900–912. doi: 10.1016/0005-2736(76)90059-6. [DOI] [PubMed] [Google Scholar]
- Crichley V., Mager D., Bernstein A. Colchicine resistant Friend cells: application to the study of actinomycin D induced erythroid differentiation. J Cell Physiol. 1980 Jan;102(1):63–70. doi: 10.1002/jcp.1041020110. [DOI] [PubMed] [Google Scholar]
- Dano K. Experimentally developed cellular resistance to daunomycin. Resistance mechanisms, the daunomycin-pump and cross resistance to adriamycin, vincristine and vinblastine. Acta Pathol Microbiol Scand Suppl. 1976;(256 Suppl):3–80. [PubMed] [Google Scholar]
- Elliott E. M., Ling V. Selection and characterization of Chinese hamster ovary cell mutants resistant to melphalan (L-phenylalanine mustard). Cancer Res. 1981 Feb;41(2):393–400. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kessel D., Bosmann H. B. On the characteristics of actinomycin D resistance in L5178Y cells. Cancer Res. 1970 Nov;30(11):2695–2701. [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- Ling V., Baker R. M. Dominance of colchicine resistance in hybrid CHO cells. Somatic Cell Genet. 1978 Mar;4(2):193–200. doi: 10.1007/BF01538984. [DOI] [PubMed] [Google Scholar]
- Ling V. Drug resistance and membrane alteration in mutants of mammalian cells. Can J Genet Cytol. 1975 Dec;17(4):503–515. doi: 10.1139/g75-064. [DOI] [PubMed] [Google Scholar]
- Ling V., Thompson L. H. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. J Cell Physiol. 1974 Feb;83(1):103–116. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell. 1974 Jul;2(3):173–182. doi: 10.1016/0092-8674(74)90091-9. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Ling V. Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability. J Biol Chem. 1979 Dec 25;254(24):12701–12705. [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- See Y. P., Carlsen S. A., Till J. E., Ling V. Increased drug permeability in Chinese hamster ovary cells in the presence of cyanide. Biochim Biophys Acta. 1974 Dec 10;373(2):242–252. doi: 10.1016/0005-2736(74)90148-5. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]