Abstract
Hybrids were made between a ouabain-resistant, thioguanine-resistant human lymphoma line able to remove O6-methylguanine from its DNA (Mex+) and human lymphoblastoid lines deficient in this capability (Mex-). The formation of hybrids was confirmed by chromosomal analysis. Hybrid cells had an O6-methylguanine removal capacity per mole of guanine about one third to one half that of the Mex+ parents, i.e., about the same per cell. Cell hybrids removed the same amount of the alkylation adduct 3-methyladenine as did their parents per mole of guanine, i.e., about twice as much per cell. Although the cell hybrids had intermediate resistance to the cytotoxic action of N-methyl-N'-nitro-N-nitrosoguanidine used to induce O6-methylguanine and 3-methyladenine, there is evidence that the ability to remove O6-methylguanine and resistance to the cytotoxic effect of N-methyl-N'-nitro-N-nitrosoguanidine are dissociable characteristics.
Full text
PDF


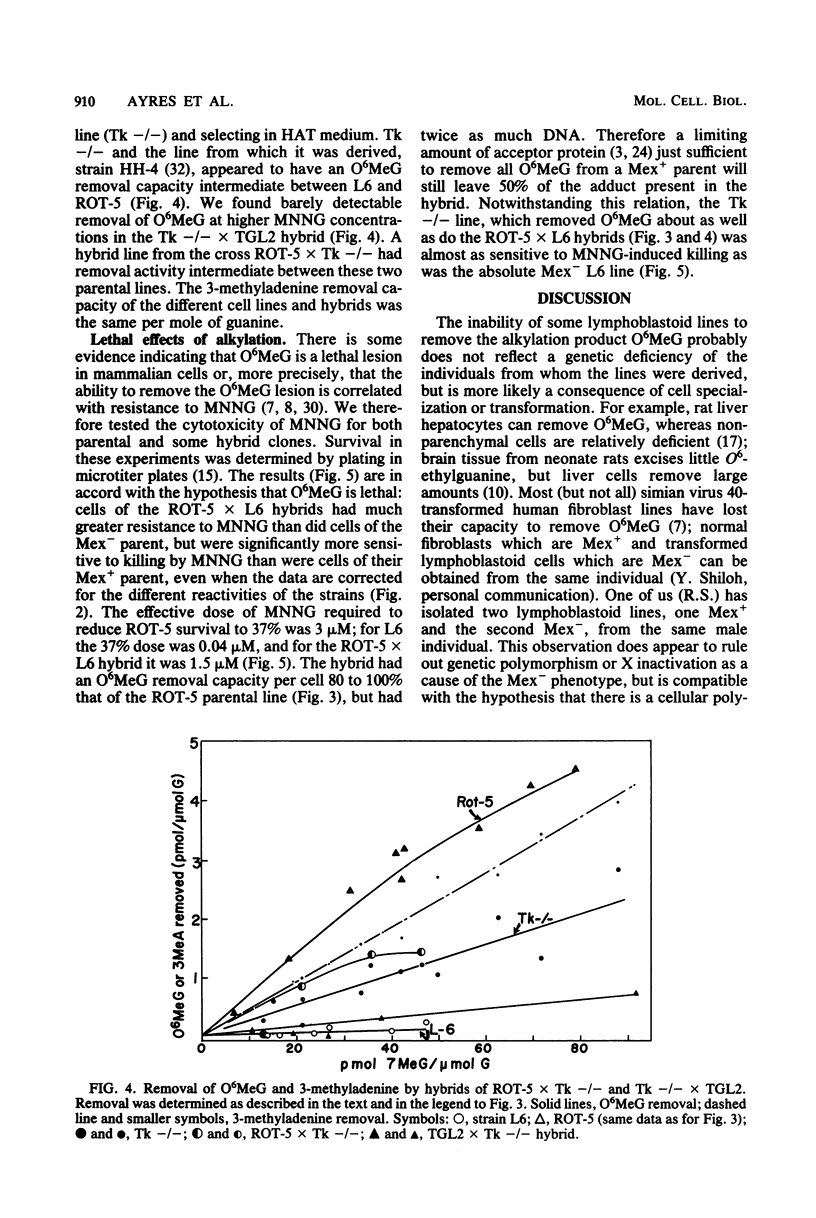
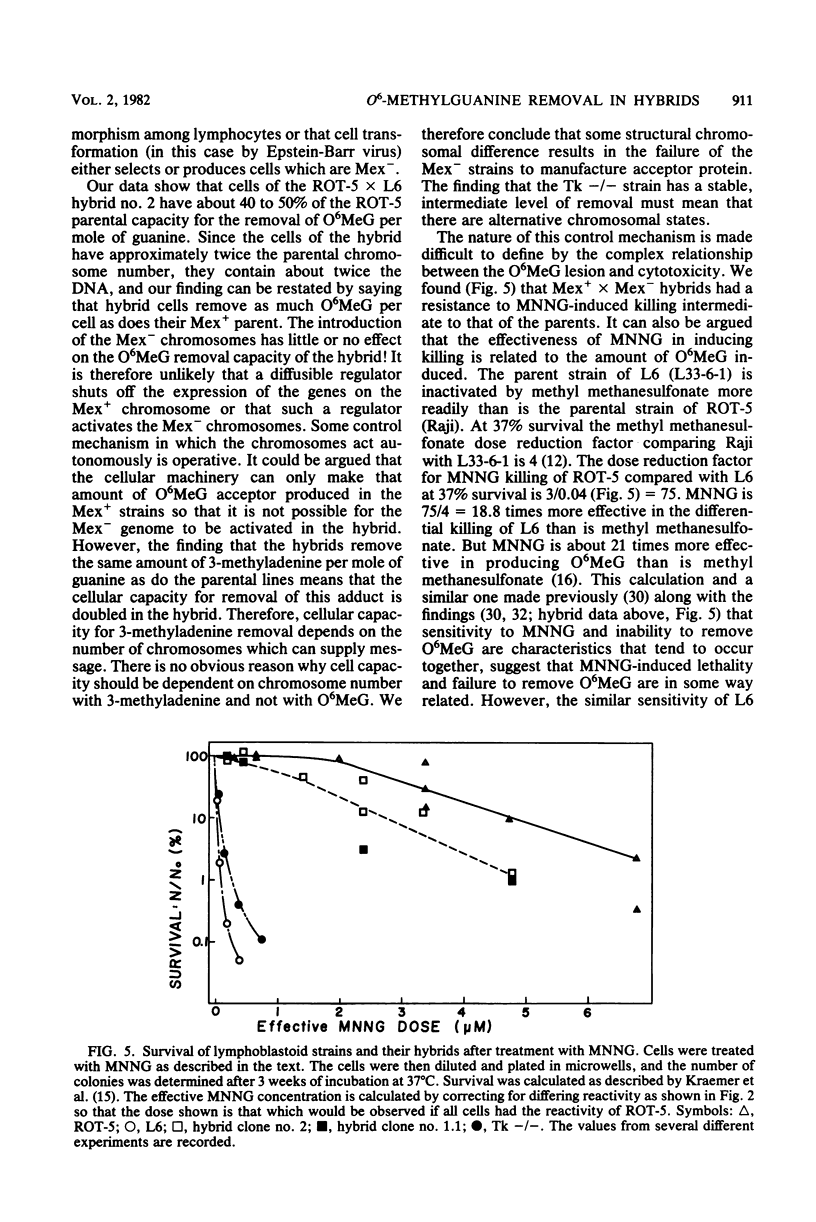


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayres K. N. High cloning efficiency of human lymphoid cells in agarose without feeder layers. J Natl Cancer Inst. 1982 Jun;68(6):919–923. [PubMed] [Google Scholar]
- Baker R. M., Van Voorhis W. C., Spencer L. A. HeLa cell variants that differ in sensitivity to monofunctional alkylating agents, with independence of cytotoxic and mutagenic responses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5249–5253. doi: 10.1073/pnas.76.10.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogden J. M., Eastman A., Bresnick E. A system in mouse liver for the repair of O6-methylguanine lesions in methylated DNA. Nucleic Acids Res. 1981 Jul 10;9(13):3089–3103. doi: 10.1093/nar/9.13.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J. Efficiency of the adaptive response of Escherichia coli to alkylating agents. Nature. 1980 Jul 10;286(5769):176–178. doi: 10.1038/286176a0. [DOI] [PubMed] [Google Scholar]
- Corsaro C. M., Migeon B. R. Gene expression in euploid human hybrid cells: ouabain resistance is codominant. Somatic Cell Genet. 1978 Sep;4(5):531–540. doi: 10.1007/BF01542924. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Gerald P. S. Induction of mammalian somatic cell hybridization by polyethylene glycol. Methods Cell Biol. 1977;15:325–338. doi: 10.1016/s0091-679x(08)60223-x. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H. Human brain tumour cell strains with deficient host-cell reactivation of N-methyl-N'-nitro-N-nitrosoguanidine-damaged adenovirus 5. Nature. 1979 Jun 28;279(5716):797–799. doi: 10.1038/279797a0. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd, Ziolkowski C. H., Scudiero D. A., Meyer S. A., Lubiniecki A. S., Girardi A. J., Galloway S. M., Bynum G. D. Defective repair of alkylated DNA by human tumour and SV40-transformed human cell strains. Nature. 1980 Dec 25;288(5792):724–727. doi: 10.1038/288724a0. [DOI] [PubMed] [Google Scholar]
- Farber R. A., Davidson R. L. Differences in the order of termination of DNA replication in human chromosomes in peripheral blood lymphocytes and skin fibroblasts from the same individual. Cytogenet Cell Genet. 1977;18(6):349–363. doi: 10.1159/000130781. [DOI] [PubMed] [Google Scholar]
- Goth R., Rajewsky M. F. Persistence of O6-ethylguanine in rat-brain DNA: correlation with nervous system-specific carcinogenesis by ethylnitrosourea. Proc Natl Acad Sci U S A. 1974 Mar;71(3):639–643. doi: 10.1073/pnas.71.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins N. P., Strauss B. S. Differences in the ability of human lymphoblastoid lines to exclude bromodeoxyuridine and in their sensitivity to methyl methanesulfonate and to incorporated [3H]thymidine. Cancer Res. 1979 Feb;39(2 Pt 1):312–320. [PubMed] [Google Scholar]
- Jeggo P. Isolation and characterization of Escherichia coli K-12 mutants unable to induce the adaptive response to simple alkylating agents. J Bacteriol. 1979 Sep;139(3):783–791. doi: 10.1128/jb.139.3.783-791.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett R. H. Cell fusion. Methods Enzymol. 1979;58:345–359. doi: 10.1016/s0076-6879(79)58149-x. [DOI] [PubMed] [Google Scholar]
- Kraemer K. H., Waters H. L., Buchanan J. K. Survival of human lymphoblastoid cells after DNA damage measured by growth in microtiter wells. Mutat Res. 1980 Sep;72(2):285–294. doi: 10.1016/0027-5107(80)90043-3. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Lawley P. D., Thatcher C. J. Methylation of deoxyribonucleic acid in cultured mammalian cells by N-methyl-N'-nitro-N-nitrosoguanidine. The influence of cellular thiol concentrations on the extent of methylation and the 6-oxygen atom of guanine as a site of methylation. Biochem J. 1970 Feb;116(4):693–707. doi: 10.1042/bj1160693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. G., Swenberg J. A. Differential repair of O(6)-methylguanine in DNA of rat hepatocytes and nonparenchymal cells. Nature. 1980 Nov 13;288(5787):185–141. doi: 10.1038/288185a0. [DOI] [PubMed] [Google Scholar]
- Lindahl T. DNA glycosylases, endonucleases for apurinic/apyrimidinic sites, and base excision-repair. Prog Nucleic Acid Res Mol Biol. 1979;22:135–192. doi: 10.1016/s0079-6603(08)60800-4. [DOI] [PubMed] [Google Scholar]
- Loveless A. Possible relevance of O-6 alkylation of deoxyguanosine to the mutagenicity and carcinogenicity of nitrosamines and nitrosamides. Nature. 1969 Jul 12;223(5202):206–207. doi: 10.1038/223206a0. [DOI] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B., Renard A., Verly W. G. Repair of O6-ethylguanine in DNA by a chromatin fraction from rat liver: transfer of the ethyl group to an acceptor protein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6766–6770. doi: 10.1073/pnas.78.11.6766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R., Brésil H., Planche-Martel G., Margison G. P., Pegg A. E. Effect of chronic treatment of rats with dimethylnitrosamine on the removal of O6-methylguanine from DNA. Cancer Res. 1980 Feb;40(2):452–458. [PubMed] [Google Scholar]
- Nicoll J. W., Swann P. F., Pegg A. E. Effect of dimethylnitrosamine on persistence of methylated guanines in rat liver and kidney DNA. Nature. 1975 Mar 20;254(5497):261–262. doi: 10.1038/254261a0. [DOI] [PubMed] [Google Scholar]
- Olsson M., Lindahl T. Repair of alkylated DNA in Escherichia coli. Methyl group transfer from O6-methylguanine to a protein cysteine residue. J Biol Chem. 1980 Nov 25;255(22):10569–10571. [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Formation and metabolism of alkylated nucleosides: possible role in carcinogenesis by nitroso compounds and alkylating agents. Adv Cancer Res. 1977;25:195–269. doi: 10.1016/s0065-230x(08)60635-1. [DOI] [PubMed] [Google Scholar]
- Samson L., Schwartz J. L. Evidence for an adaptive DNA repair pathway in CHO and human skin fibroblast cell lines. Nature. 1980 Oct 30;287(5785):861–863. doi: 10.1038/287861a0. [DOI] [PubMed] [Google Scholar]
- Sato K., Slesinski R. S., Littlefield J. W. Chemical mutagenesis at the phosphoribosyltransferase locus in cultured human lymphoblasts. Proc Natl Acad Sci U S A. 1972 May;69(5):1244–1248. doi: 10.1073/pnas.69.5.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel P. F. Inducible repair systems and their implications for toxicology. Crit Rev Toxicol. 1981 Mar;8(4):311–362. doi: 10.3109/10408448109089902. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Becker Y. Kinetics of O6-methylguanine repair in human normal and ataxia telangiectasia cell lines and correlation of repair capacity with cellular sensitivity to methylating agents. Cancer Res. 1981 Dec;41(12 Pt 1):5114–5120. [PubMed] [Google Scholar]
- Sklar R., Brady K., Strauss B. Limited capacity for the removal of O6-methylguanine and its regeneration in a human lymphoma line. Carcinogenesis. 1981;2(12):1293–1298. doi: 10.1093/carcin/2.12.1293. [DOI] [PubMed] [Google Scholar]
- Sklar R., Strauss B. Removal of O6-methylguanine from DNA of normal and xeroderma pigmentosum-derived lymphoblastoid lines. Nature. 1981 Jan 29;289(5796):417–420. doi: 10.1038/289417a0. [DOI] [PubMed] [Google Scholar]
- Skopek T. R., Liber H. L., Penman B. W., Thilly W. G. Isolation of a human lymphoblastoid line heterozygous at the thymidine kinase locus: possibility for a rapid human cell mutation assay. Biochem Biophys Res Commun. 1978 Sep 29;84(2):411–416. doi: 10.1016/0006-291x(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Slapikoff S. A., Andon B. M., Thilly W. G. Comparison of toxicity and mutagenicity of methylnitrosourea, methylnitronitrosoguanidine and ICR-191 among human lymphoblast lines. Mutat Res. 1980 May;70(3):365–371. doi: 10.1016/0027-5107(80)90026-3. [DOI] [PubMed] [Google Scholar]