Abstract
The history of consumer protection against household poisons presents a key case study of the uniquely American struggle to balance public health and safety with the interests of business. By the late 19th century, package designs, warning labels, and state statutes had formed an uneven patchwork of protective mechanisms against accidental poisonings. As household chemicals proliferated in the early 20th century, physicians concerned with childhood poisonings pressured the federal government to enact legislation mandating warning labels on packaging for these substances. Manufacturers of household chemicals agreed to labeling requirements for caustic poisons but resisted broader regulation. Accidental poisonings of children continued to increase until the enactment of broad labeling and packaging legislation in the 1960s and 1970s. This history suggests that voluntary agreements between government agencies and manufacturers are inadequate to protect consumers against household poisonings and that, in the United States, protective household chemical regulation proceeds in a reactive rather than a precautionary manner.
PUBLIC HEALTH CONCERN over household poisonings has recently reemerged in the United States, amid a sharp rise in rates of pediatric morbidity from accidental ingestion of over-the-counter and prescription pharmaceutical products. This alarming trend has occurred despite decades of efforts to encourage parents to poison-proof their homes. In response, officials from the Centers for Disease Control and Prevention have sought voluntary compliance from manufacturers of over-the-counter medicines to implement new changes in packaging design that deliver medicines in single-dose packages or restrict disbursal of medicines from containers. Centers for Disease Control and Prevention officials and other health advocates have also sought to raise public awareness about these dangers.1
This episode, although reflecting new and specific hazards stemming from increasing sales of over-the-counter and prescription pharmaceuticals, also represents the latest iteration in a longer cycle of technological innovation, advocacy, and cautious regulation to prevent household poisonings in the United States.2 Ever since the mid-19th century, when potentially poisonous chemical products first became directly available to consumers through national mail-order distribution networks, US physicians, pharmacists, legislators, and others have promoted the adoption of package designs, warning labels, and other restrictions to protect consumers against accidental poisonings. Manufacturers have sometimes embraced these efforts and at other times resisted them. Federal, state, and local governments have acted as reluctant brokers in the regulatory process, negotiating a fragile balance between the interests of commerce and the imperatives of human health.
The regulatory histories of food, drugs, and cosmetics in the United States, as well as those of tobacco, alcohol, and motor vehicles, have been well documented.3 Little attention has been paid, however, to the history of consumer protection against household poisons, which are defined here broadly as any chemical products used in the home and believed or known to cause morbidity or mortality from inhalation, ingestion, or exposure when misused. In particular, debates over warning labels and packaging design have been ignored.4 Additionally, the longer history of product safety regulation has sometimes been eclipsed by the explosion in consumer product safety regulation that occurred in the late 1960s and 1970s.5 In this case study, we seek to bring to light this earlier regulatory history with regard to household poisons and to examine the lessons that this history offers for contemporary debates over poison prevention and product packaging.
Just as Harry Marks noted with regard to the history of compulsory drug prescriptions, the history of household poison regulation does not fully support economic regulation theorists’ axiom that “regulation is acquired by the industry and is designed and operated primarily for its benefit.”6 Although the Manufacturing Chemists Association gained control over labeling regulations during the 1930s, this control slipped from its grasp by the 1960s in the face of known product hazards and broad public health advocacy campaigns. Regulation of household poisons thus unfolded not as a proactive strategy of industry but as a reactive cycle characterized by both alliances and conflicts between manufacturers and public health advocates. This cycle involved several stages: (1) a class of products became available for sale to individuals for home use; (2) its hazards became known through publicly reported adverse events (deaths or injuries); (3) manufacturers and inventors introduced technological innovations to limit these hazards or public health advocates campaigned for legal mandates to require warnings, restrictions, or both on the products; and (4) manufacturers resisted regulation, proposing less coercive alternatives such as weak informational regulation (i.e., voluntary warning labels), or judged proposed regulation to favor their interests and participated in shaping its provisions to their advantage. When health advocates in the 1930s tried to bypass these stages by introducing precautionary regulation before products caused deaths and injuries in the home, they failed. Advocates were most successful in securing regulation when armed with clear evidence that a product or class of substances already on the market had killed or seriously harmed children. This evidence often led manufacturers to endorse or soften their resistance to legislative mandates and caused legislators to put aside the antipaternalism and antiregulatory sentiment that “dominates political ideologies,” as Ronald Bayer and James Colgrove have noted with regard to tobacco regulation.7 Although packaging restrictions played a significant role, the warning label served as the chief battleground for regulatory compromises and the principal product of regulatory activity for household chemicals.
LATE 19TH-CENTURY PATCHWORK OF PROTECTIONS
Packaging innovation long preceded regulation as a means of protection against accidental ingestion of poisons in the household or pharmacy. In the late 18th century, apothecaries in Europe and the United States began storing poisons in distinctively shaped bottles to prevent mix-ups with other products. By the mid-19th century, bottles for poisons were often marked with skull and crossbones or red labels emblazoned with the word “poison.”8
State legislatures, the principal locus of regulatory authority during much of the 19th century, passed several laws to make these labels mandatory. The first such statute, enacted in New York State in 1829, specified,
No person is allowed to sell arsenic or prussic acid or any other substance or liquid usually denominated “poison” without endorsing on it the word “Poison” in a conspicuous manner.9
By mid-century, pharmacists and physicians, seeking to solidify their professional status, organized in favor of such laws.10 The American Pharmaceutical Association, founded in 1852, passed a resolution at its second annual meeting stating that
all packages or bottles [of poisonous substances] shall be distinctly labeled with the word “Poison” or a death’s head symbol, conspicuously printed.11
The American Medical Association (AMA), founded in 1847, passed a similar resolution at its 1860 annual meeting and also recommended laws restricting retail sales of poisons such as “morphia, strychnine, arsenic, prussic acid, or corrosive sublimate.”12 In 1868, the American Pharmaceutical Association proposed a uniform state law to restrict the sale of poisons. The drafters based their model act on Great Britain’s 1868 Pharmacy Act, which required anyone selling a stipulated list of poisonous substances to possess a pharmacist’s license and required pharmacists to record these sales.13 Despite stiff resistance to such regulation in the West, where untrained storekeepers often dispensed such substances, 33 states adopted some form of the model pharmacy law between 1870 and 1890.14
Innovations in the design, manufacture, and distribution of containers for household poisons accompanied this regulation. As the country rapidly industrialized during the 1870s, glass-making factories began mass production of irregularly shaped, textured poison bottles with colored glass. These bottles featured pointed rough surfaces designed to warn users of their contents even if handled in the dark.15 Whitall Tatum and Company, a New Jersey–based glassworks, distributed its blue glass medicine bottles and its pointed stopper, embossed with the word “poison,” through its catalog. As did other late 19th-century manufacturers of consumer goods, this company built a national market for its products by taking advantage of expanding railway networks and selling its wares to the growing population of city-dwelling Americans who, as William Leach has noted, had become buyers of “goods made by unknown hands ” rather than self-sufficient producers.16
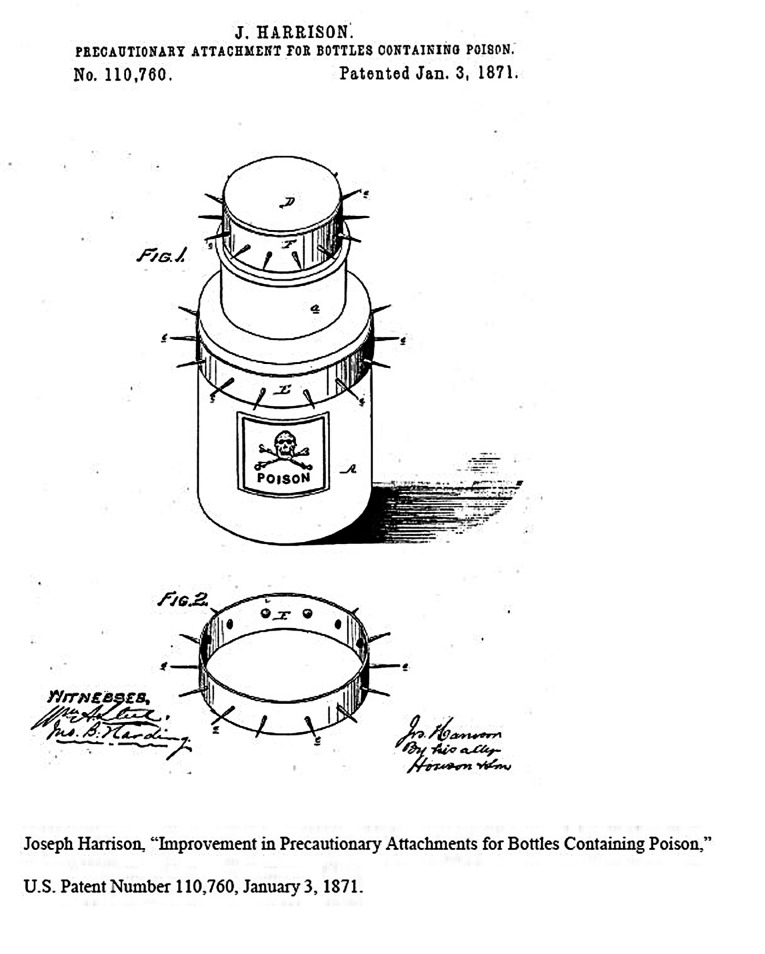
At the same time, inventors began patenting safety attachments and specially designed poison bottles to sell in this mushrooming marketplace. The first such patent, granted in 1871, described
an elastic band, and protuberant points or spikes, which can be fitted to and adjusted on bottles, jars &c., of different sizes, and containing poison, in such a position that no one can obtain the contents of the bottle, &c., without previous warning, by the spikes punching the hand, of the dangerous character of the contents.17 (Figure 1)
FIGURE 1—
Joseph Harrison, “Improvement in Precautionary Attachments for Bottles Containing Poison,” US Patent Number 110,760. Letters patent dated January 3, 1871.
Source. Patent 110,760 data obtained from US Patent and Trademark Office online patent database, http://www.uspto.gov/patents/process/search/index.jsp. Full image of patent obtained through online patent search tool, http://www.pat2pdf.org/pat2pdf/foo.pl. Accessed November 27, 2012.
Inventors in the 1870s and 1880s also devised stoppers with alarm bells on them, fasteners that made it difficult to open bottles, and containers for poisons that would sound electric alarms when opened without deactivation. They directed these inventions mainly toward druggists, but some could be used in the household.18 These inventions inspired state and local legislators to propose bills mandating sales of poisons in specially colored, shaped, or marked bottles.19 These bills encountered effective opposition by pharmacists, who contended that people would reuse the bottles and then mix the poison with a “beverage or harmless household substance” and argued that the laws would unfairly restrict their business practices.20
Around 1900, the wider regulatory tide turned as Progressive reformers sought to expose hidden dangers in the home and industrial workplace. During this era, muckraking journalists cast a spotlight on the dangers of patent medicines.21 These medicines, made available through the same nationwide distribution networks that brought colored poison bottles into the household, ordinarily included no ingredients list on the label. When they did, the information was often vague.22 In one journalistic exposé, Ladies’ Home Journal editor Edward Bok noted the lack of required poison labels on patent medicines sold in the United States. An American woman who buys a soothing syrup for her teething baby will not be apprised that it contains morphine, he noted, but
when the same “soothing syrup” which she may have given to her child is sold in England there is on the wrapper of every bottle these words, printed by the manufacturers of the syrup themselves: “This preparation, containing, among other valuable ingredients, a small amount of morphine, is in accordance with the Pharmacy act hereby labeled POISON.”23
Such articles prompted Congress to add provisions against mislabeling of drugs into a Pure Food bill then under consideration. The resulting 1906 Pure Food and Drug Act made it “unlawful for any person to manufacture within any Territory or the District of Columbia any article of food or drug which is adulterated or misbranded.” 24 This law signaled the federal government’s entry into the regulatory arena as well as the growing power of consumers as a constituency. Although containing no provisions requiring poison labels, it paved the way for future federal legislation requiring labeling of consumer products.25
CONSUMER CHEMICAL REVOLUTION, 1900–1925
By the early 20th century, a new type of chemical was becoming commonplace in the middle-class household, as Americans began to embrace what Nancy Tomes has called “the Gospel of Germs”—the belief that microbes cause disease and must be attacked through vigorous hygienic measures. This ideology stemmed from advances in germ theory, such as Robert Koch’s 1882 discovery of the tuberculosis bacillus. Germ theory focused hygienic concerns, already a preoccupation of mid-19th-century urban public health reformers, more narrowly on cleanliness of the body and the household.26 At the same time, an emergent group of experts, home economists, counseled women to become expert household managers. Their advice included detailed instructions on how to deploy germ-fighting chemicals to protect home and family from the microbial menace.27
Although early home economics manuals included detailed recipes for mixing cleaning chemicals, the early 20th century was marked by the advent of mass consumption—“the production, distribution, and purchase of standardized, brand-named goods aimed at the broadest possible buying public,” according to Lizabeth Cohen.28 Popularly advertised brand-name cleaning products included Naptha Soaps for laundry, Fairy Soap for the face, Gold Dust powder for general household cleaning, Parson’s Ammonia, Old English Floor Wax, Johnson’s Liquid Wax, O-Cedar floor and furniture polishers, and Wright’s Silver Cream.29 As with other national brand name items such as the Kodak camera and Shredded Wheat cereal, the brand name in cleaners came to represent an assurance of quality, reliability, and safety.30 Sales of “drug, toilet and household preparations” for domestic consumption increased from $40 million in 1879 to more than $765 million in 1920, according to census data.31 Per capita expenditures on cleaning and polishing preparations alone increased sevenfold between 1909 and 1929.32 Department stores began opening separate departments for household cleaning items, and chain stores such as F. W. Woolworth also began to stock extensive stores of household products.33
As advertisers assured women that these new prepackaged products would make their lives more convenient and safer, home economists and others in the nascent field of consumer advocacy began sounding alarms about the hidden dangers these goods posed. These advocates warned that cleaning agents could be swallowed by children; that appliances could cause electrocution and fires or release dangerous chemicals; and that gas stoves could explode or asphyxiate families. To mitigate these dangers, consumer advocates sought to educate and advise women about proper use, packaging, and labeling of these products. 34 Good Housekeeping magazine, which embodied this new type of expert consumer advice, directed readers of a 1923 issue to go through their medicine cabinets and “label or relabel the bottles which have become indistinct.” For poisons, the magazine instructed readers “to stick a pin in the cork from top to side or directly through from side to side, so that in touching the cork you will surely feel the pin.” This advice, as did other consumer literature on household safety, placed primary responsibility for protecting against the dangers of household poisons on the female homemaker.35
THE 1920S CRUSADE AGAINST CAUSTIC POISONS
As household cleaning chemicals proliferated, physicians began to report cases of gruesome pediatric injuries from accidental ingestion. Some called for laws to protect children against the dangers of caustic cleaners. Chevalier Jackson of Jefferson Medical College in Philadelphia, known for introducing the bronchoscope into US medical practice, led this crusade.36 In 1910, Jackson secured passage of a Pennsylvania law requiring that lye, an alkaline solution of potassium salts, and other “domestic” poisons be labeled as such by their sellers.37 Over the next decade, 12 other states passed similar legislation, and Jackson lobbied for a federal caustic poisons law.38 The AMA endorsed this effort. Surveying 1400 physicians who treated child poisonings, an AMA committee found 490 cases under treatment.39 In 1926, when legislators introduced bills in both the House and Senate to mandate that caustic poisons such as ammonia, silver nitrate, sodium, and potassium hydroxide be sold with a warning label marked “poison,” the AMA’s survey results supported these efforts.40
In testimony before a Senate Committee considering the caustic poisons bill, Jackson supplied pictures of bottles that looked almost identical to cans of baking powder but contained poisons instead or included the word “poison” in small type or written sideways. His images of injured children and “shrunken, shriveled” child corpses, however, likely made more impact.
Here is the picture of a child of a citizen of Pennsylvania which swallowed a preparation known as “Kleanall” up in the State of Massachusetts. The child’s passage to the stomach was totally obliterated. A person saved her life by putting a tube in the stomach… . When I asked the mother “Why did you let the child have that?” she said, “Why, I did not know that it was poison.” And I got her to bring me a can, and here you can see on this can that I have here, which was bought in the stores, that there is not only no poison label whatever on it, but it says, “Does not injure the finest fabric or the most delicate skin.” Now how could you expect any mother to think that that thing was dangerous?41
This graphic testimony evinced the beginning of a shift in public discourse over who bore responsibility for protecting children against the dangers of hazardous products. By the 1920s, so many new products had been introduced into the middle-class home that, according to public health advocates such as Jackson, the typical homemaker could no longer be held solely responsible for protecting her family from chemical hazards: some responsibility belonged with manufacturers.42
Manufacturers of caustic poisons supported the Caustic Poisons Act; they wanted to have “some uniform regulation that may be followed” rather than inconsistent state statutes, and it sailed through Congress. The law, signed by President Calvin Coolidge in May 1927, specified that mandated warnings be written in gothic capital letters at least as large as the largest type on the label, against a “clear, plain background of a distinctly contrasting color” and include “directions for treatment in case of accidental personal injury.”43 Although injury statistics from this period are unreliable, one survey indicated that a common poisoning-related esophageal injury dropped 50% in three states after enactment of this law.44
The passage of this measure demonstrates both the growing political power of the AMA in the early 20th century and the exceptional willingness of Congress during this era to enact regulatory legislation to protect consumers from dangerous products when child health was involved.45 Over the preceding three decades, Progressive reformers had pushed child health and welfare to the front of the domestic policy agenda. The Children’s Bureau, launched in 1912, had become the first federal social welfare agency. With the political interests of newly enfranchised female voters in mind, in 1921 Congress had passed the Sheppard-Towner Act, granting federal aid to states for maternal, infant, and child health and welfare. Even as the Progressive movement waned during the 1920s, Congress made an exception to its prevailing political ideology of noninterference in commercial matters to enact regulation that protected children from accidental poisoning.46
VOLATILE VOLUNTARISM IN THE 1930S
Five years after the passage of the Caustic Poisons Act, a Senate committee considered the Federal Volatile Poisons Act, a bill drafted by Yale industrial physiologist Yandell Henderson with Senator Hiram Bingham (R–CT). This measure proposed mandatory labels for an enumerated list of substances deemed volatile poisons and enabled the Surgeon General to expand this list at his discretion to include any other substances that he had found
through practical experience or laboratory investigation, to give off, in the course of household consumption, fumes, vapor, or gas dangerous to life or injurious to health.
The bill required a more extensive label than that mandated under the Caustic Poisons Act. It included
a figure showing a skull-and crossbones and the words “the fumes are poisonous. Do not inhale. Avoid contact with skin. In case of accident send for an inhalator,” and such additional or other warning or direction as the Surgeon General may specify.47
For manufacturers of these chemicals, this language went too far.
A letter to the committee from an executive at American Products Co., maker of a gasoline–carbon tetrachloride product marketed to remove grease stains from clothing, exemplified the industry’s objections to the bill. The executive contended in the letter that
The putting of a skull and crossbones on such a package as this would immediately tend to kill the sale of this item, which would be highly objectionable to us as well as to a great many other manufacturers.
He claimed that his product had “supplied a definite need without any injurious effect to anyone in so far as I know.”48 Numerous makers of antimoth products containing paradichlorobenzene, another substance covered under the bill, similarly objected in letters to the committee that their product was not poisonous to humans or animals (presumably moths constituted an exception).49
Henderson sought to counter these objections with evidence and expertise. The coauthor of an authoritative volume on industrial poison gases, he had also advised the US Bureau of Mines on the hazards of mine gases, served as chief of the medical section of the US War Gas Investigations during World War I and determined how much ventilation was necessary for the Holland Tunnel, establishing a worldwide ventilation standard for automobile tunnels.50 Unlike Jackson, however, Henderson could not shock the committee with pictures of dead and injured children. Most of the compounds addressed in the bill had come onto the market in the preceding decade or were just coming into wide use.51 Instead, Henderson took a precautionary tack, comparing these new products to the gasoline additive tetraethyl lead. When automakers had introduced this product in 1923 in a pure form to be added at the pump, Henderson and other industrial experts had questioned its safety. As a result, manufacturers had suspended production until safety precautions could be developed for its use. They had then reintroduced it in a form that was premixed with gasoline in the factory and agreed to place warnings on gasoline pumps.52 This example, Henderson said, represented “exactly the sort of thing that we are hoping will come about under this law.”53 Instead of waiting until fatalities from volatile household poisons mounted, Henderson proposed that Congress proactively prevent such deaths.54
After his testimony, Henderson wrote to the committee chair to propose an amendment to the bill establishing a system to require chemical manufacturers to submit an application to the Surgeon General and the National Institute of Health to secure approval of a chemical’s safety before they could launch it on the consumer market.55 Under this provision, the Surgeon General would have required labels on any substances found to be “dangerous to life or injurious to health.” This amendment represented a precocious formulation of the precautionary approach later reflected in the 1938 Food, Drug, and Cosmetic Act’s requirement that drug manufacturers obtain safety approval from the Food and Drug Administration (FDA) before introducing a drug into the US market.56
Chemical industry lobbyists vociferously opposed Henderson’s amendment. “Experience has taught us that the use of the poison label as a precautionary warning depends upon its limitation,” W. N. Watson, secretary of the Manufacturing Chemists Association (MCA), the leading chemical industry trade organization, testified in a hearing:
The application of a poison label on relatively harmless products can only result in public indifference to the poison label and the trend will destroy its warning value… . To arbitrarily require by a Federal law that any one of these articles, when used in the household, should carry a poison label, would be tantamount to conviction before trial.57
At the time, the occupational danger posed by inhalation of carbon tetrachloride was already becoming known. In a 1932 syndicated newspaper column, W. A. Evans reported on a case in which two dozen workers had suffered carbon tetrachloride poisoning after using this substance to remove grease stains from felt. He counseled readers that “the room in which [this chemical] is used must be ventilated enough to permit the fumes to escape.” 58 In the hearings, Henderson had also noted that halogen substances such as paradichlorobenzene moth cakes were known to create poisonous gases when they burned and cited instances of occupational poisoning from benzol, cyanogen, and methyl chloride.59
Henderson, however, lacked organized support for his bill. As an industrial safety expert, he should have had backing from the National Safety Council, a well-funded industrial safety advocacy group. However, the proposed legislation protected against dangers in the home, not the workplace, and the council placed home safety as a distant third priority behind industrial and public safety.60 Neither did the AMA become involved, because medical evidence of injuries and deaths from volatile poisons had not yet accumulated. Thus unopposed, the MCA undercut the bill by meeting with the Surgeon General to develop a series of agreements on voluntary labeling of volatile chemicals. This move allayed ambivalent legislators’ concerns. Reluctant to hamper the chemical industry, one of the few growth sectors during the Great Depression, they agreed to drop the bill. The voluntary labeling scheme remained in effect between 1935 and 1952.61
REACTIVE REGULATION IN THE POSTWAR ERA
Less than a year after the defeat of the Volatile Poisons Act, the election of Franklin Delano Roosevelt ushered in a new era of activist government that embraced consumers as a constituency.62 A major piece of New Deal legislation, the 1938 Food, Drug, and Cosmetic Act, improved on the 1906 Pure Food and Drug Act and gave the FDA power to regulate the sale and manufacture of cosmetics. The cosmetic provision gained support after FDA-organized exhibits graphically depicted the eye, skin, and hair injuries that poisonous cosmetics had inflicted on women.63
The development of DDT during World War II and its subsequent marketing for civilian use led to the passage of the 1947 federal Insecticide, Fungicide, and Rodenticide Act. The law mandated proper labeling of this and other agricultural insecticides. Large manufacturers cooperated in drafting the legislation, because they believed standardized regulation of their products would drive out smaller competitors by imposing a regulatory burden only large manufacturers could meet. This act also included requirements that manufacturers and distributors of white powdered insecticides, fungicides, or rodenticides color these common household powders and include on toxic products a red “poison” label with a skull and crossbones that specified an antidote to the poison.64 These coloring provisions were added after the fatal 1942 poisoning of 50 patients at an Oregon State Hospital when a cook accidentally made eggs using the white powdered insecticide sodium fluoride instead of powdered milk.65 Although legislation to require coloring of powdered insecticides was first proposed in 1932, the National Wholesale Grocers Association had fought it, claiming that it made these insecticides unfit for their intended purposes.66 The fatal Oregon incident, the worst in a series of accidental poisonings involving sodium fluoride, had provided the necessary evidence of harm to justify a mandate.67
In 1949, the US Public Health Service’s umbrella agency, the Federal Security Agency, proposed amendments to the Caustic Poison Act that added substances to those requiring warning labels and gave agency administrators discretion to add additional substances to this list. The amendments also included provisions for “distinctive safety containers, distinctive coloring of the article where required and, in the case of poisonous liquids which might intrigue children, bottles with openings sufficiently constricted to make it unlikely that a child would ingest a lethal quantity,” according to a Federal Security Agency medical director. The amendments, however, died in the Senate.68 At the same time the MCA published a warning label manual for its members that replaced its voluntary agreement with the Surgeon General. The Public Health Service reactivated a Chemical Products Agreements Committee to work with the MCA to evaluate voluntary labeling guidelines.69
During this era, accidents had begun to replace communicable diseases as the leading cause of death for children between ages one and four. Home accidents had also begun to far outstrip industrial accidents in frequency.70 As John Burnham noted, pediatricians “found it particularly frustrating that accidents did not seem to be declining as were other causes of children’s deaths.”71 In 1950, the American Academy of Pediatrics formed an Accident Prevention Committee. In addition to studying the problem, the committee focused on physician education, establishment of safer manufacturing standards, and legislation to prevent childhood accidents.72 Unlike Henderson, the pediatricians were able to enlist the National Safety Council as a partner in their efforts. Metropolitan Life Insurance Company and the US Children’s Bureau also lent their support.73
In 1953, the committee conducted a survey of 3000 American Academy of Pediatrics members and found that poisonings constituted 49% of the accidents treated by these physicians.74 Other studies found that inflammable liquids—those targeted by Henderson’s failed 1932 bill—formed a significant proportion of the chemicals being ingested by children (Figure 2).75 To address this problem, the American Academy of Pediatrics created a subcommittee on childhood poisonings. Subcommittee member Louis Gdalman, a pharmacist at St. Luke’s hospital in Chicago, Illinois, who had collected information on the toxicology of more than 9000 consumer products, had started a poison information service at the hospital. This became the first Poison Control Center in the United States and part of the subcommittee’s national poison control program.76 By 1957, 67 Poison Control Centers were operating around the country, and the FDA formed a national clearinghouse to track poison exposures, provide information about product ingredients, and fund development of a standard text on toxicology of these products.77
FIGURE 2—
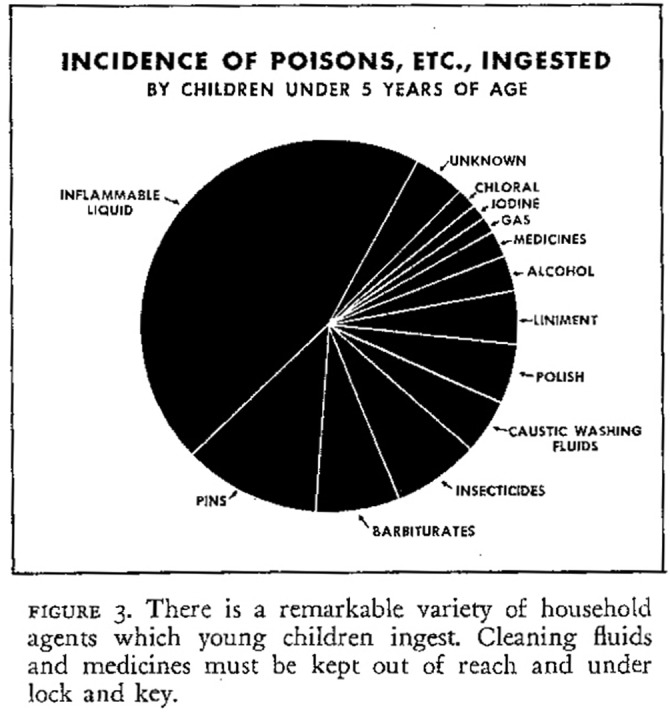
Incidence of poisons, etc., ingested by children younger than 5 years.
Source. Wheatley, “Accident Prevention and the General Practitioner,” (1951) 445.
In the early 1950s, the American Academy of Pediatrics committee also advocated for new poison labeling legislation.78 As dangers of new household poisons became apparent, states and localities were already adopting such laws. In 1952, after a two-year-old boy and 11 other people died from carbon tetrachloride poisoning in one year, the New York City Board of Health added a section to the city’s Health Code requiring that manufacturers include a warning label on products containing this chemical (one of the substances covered in the failed 1932 Volatile Poisons bill). The provision applied to all cleaning fluids containing carbon tetrachloride and required a conspicuous warning on the front panel of the container.79 Ten states passed similar hazardous chemical labeling laws.80
In 1955, the FDA meanwhile sought revisions to the Caustic Poisons Act. The MCA decided not to oppose these efforts directly but, in the wake of successful negligence lawsuits against chemical manufacturers, instead lobbied for revisions that would better protect its members against liability.81 In 1955, when the organization’s Labels and Precautionary Information Committee learned of the FDA’s plans, it formed a subcommittee to draft its own proposed version of the revisions. The revisions never materialized, but in the following years, federal legislators responded to pediatricians’ concerns about childhood poisonings by drafting several bills mandating labeling of substances not covered by the caustic poisons or insecticide laws. The MCA became involved in the drafting process, and by the time these bills were introduced in Congress, they bore the imprint of MCA lobbyists.82
The resulting federal Hazardous Substances Labeling Act, passed in July 1960, required labeling of any household product deemed to be a “hazardous substance” and encompassed substances that were “toxic, corrosive, irritant, flammable, strong sensitizers, or pressure generating” as well as those that
may cause substantial personal injury or substantial illness during or as a proximate result of any customary or reasonably foreseeable handling or use including reasonably foreseeable ingestion by children.
This act empowered the Secretary of Health, Education, and Welfare to designate which substances required labels and mandated different levels of capital-letter warnings for different chemicals: “POISON” for highly toxic substances; “DANGER” for extremely flammable, corrosive, or toxic substances; and “WARNING” or “CAUTION” on all others. It further mandated that labels include details about the nature of the hazard, what actions should be avoided or followed; instruction on first aid; special instructions for handling, care, or storage; and the statement “Keep Out of the Reach of Children.”83
This law, though mandating warnings, gave chemical manufacturers a wide berth to influence which substances required which words by leaving responsibility for implementation of labeling regulations to the FDA. When the FDA proposed that turpentine would require the “POISON” label with a skull and crossbones, the turpentine industry and its supporters in Congress vigorously protested this designation. The FDA then consulted “a panel of poison specialists” and backed down, agreeing to allow turpentine to carry the less severe “DANGER” label. Only carbon tetrachloride and methyl alcohol remained designated as poisons under the revised regulations.84 MCA officials objected to other regulations—including the placement and type size of the label—and in December 1961 met with FDA officials to reiterate these objections. The FDA agreed to delay implementation of the regulations for three months and to meet with industry scientists “to resolve existing differences on mandatory methodology as set forth in the regulations,” according to MCA committee meeting minutes. The MCA also succeeded in getting the FDA to delay implementation of its requirements for size and placement of labels, to give manufacturers time to redesign the labels and sell existing stocks of products.85 From start to finish, the manufacturers exercised control over the legislation. The act nonetheless encompassed many of the broad protections that Henderson had failed to obtain 30 years earlier.86
Beginning in the late 1950s, pediatricians also initiated a cooperative relationship with a children’s aspirin manufacturer and the FDA to improve packaging on this product following accidental overdoses. According to a leading participant in this effort, this relationship helped “lay the groundwork” for passage of the Poison Prevention Packaging Act (PPPA) in 1970.87 This law, one of many consumer protection measures passed during this “heyday” of consumer activism, gave the secretary of the US Department of Health, Education, and Welfare authority to require “special packaging” of any “household substance” that the secretary deemed hazardous to children.88 In 1972, Congress created the Consumer Product Safety Commission, giving this body responsibility for enforcing the Hazardous Substances Labeling Act, PPPA, and similar statutes.89
Over the next five years, rates of pediatric household poisonings plummeted. By 1981, childhood poisoning death rates had fallen to 25% of their 1961 levels.90 Although the Consumer Product Safety Commission has since wavered in enforcement levels of the Hazardous Substances Labeling Act’s labeling and PPPA’s packaging requirements for household poisons, these statutory requirements have not been rolled back.91
CONCLUSIONS
The history of household poison regulation in the United States represents a definitive rejection of the precautionary approach to consumer health and safety protection.92 Federal legislation to protect consumers with warning labels moved forward only after advocates, backed by organized medicine, marshaled deadly evidence that products sold without sufficient warnings had harmed adults and children. Henderson’s unsuccessful attempt to secure mandated warnings on household chemicals, based on the assumption that chemicals that posed occupational hazards would prove dangerous when used in the home, demonstrated the reluctance of Congress to legislate in a precautionary manner when faced with foreseeable product dangers.
In another vein, it is tempting to view chemical manufacturers’ role in labeling programs as a counterexample to regulatory narratives in which industry has played the role of the obstreperous villain. Indeed, manufacturers in the 1920s endorsed the Caustic Poisons Act, and the MCA sought to influence rather than oppose additional labeling legislation in the late 1950s. However, manufacturers only became cooperative after their products caused undeniable harm, and the MCA’s voluntary labeling program effectively forestalled the enactment of more protective federal legislation for nearly 30 years. During that time, household poisoning deaths from products such as carbon tetrachloride continued to mount. Only after the enactment of comprehensive federal warning label and packaging mandates did such deaths decline. This story thus suggests that voluntary agreements between government and manufacturers over labeling and packaging of poisonous products can be inadequate to reduce accidental injuries and deaths from these products: Mandates are more effective.
Acknowledgments
We received no outside funding for this work but began it under the supportive aegis of the Mailman School’s Center for History and Ethics of Public Health.
We thank David K. Rosner, Ronald H. Lauterstein Professor of Sociomedical Sciences at the Columbia University Mailman School of Public Health, who inspired and encouraged this research. Thanks also go to the librarians at the University of Maryland College Park, who tracked down important materials through interlibrary loan, and the Environmental Working group, which made Manufacturing Chemists Association internal documents available online at http://www.chemicalindustryarchives.org.
Endnotes
- 1. S. F. Schillie, N. Shehab, K. E. Thomas, and D. S. Budnitz, “Medication Overdoses Leading to Emergency Department Visits Among Children, ” American Journal of Preventive Medicine 37, no. 3(2009): 181–187. “Accidental Childhood Poisonings Mostly Due to Medicines, ” Science Daily, August 4, 2009, http://www.sciencedaily.com/releases/2009/08/090804191323.htm (accessed April 17, 2012). G. R. Bond, Randall W. Woodward, and Mona Ho, “The Growing Impact of Pediatric Pharmaceutical Poisoning, ” Journal of Pediatrics 160, no. 2 (2012): 265–270.
- 2. “Research and Markets: OTC Pharmaceuticals Market in the United States Is Expected to Increase to a Value of $32.6 Billion by the End of 2015, ” Business Wire, April 23, 2012, http://finance.yahoo.com/news/research-markets-otc-pharmaceuticals-market-171300413.html (accessed April 30, 2012). “Research and Markets: Branded Prescription Pharmaceutical Sales Outlook to 2016, ” Business Wire, March 30, 2012, http://news.yahoo.com/research-markets-branded-prescription-pharmaceutical-sales-outlook-2016-094200385.html (accessed April 30, 2012)
- 3. Young J H, Pure Food: Securing the Federal Food and Drugs Act of 1906 (Princeton, NJ: Princeton University Press, 1989); H. M. Marks, “Revisiting ‘The Origins of Compulsory Drug Prescriptions,’” American Journal of Public Health 85, no. 1 (1995): 109–115; John P. Swann, “FDA and the Practice of Pharmacy: Prescription Drug Regulation Before the Durham-Humphrey Amendment of 1951,” Pharmacy in History 36, no. 2 (1994): 5–70. W. S. Pray, A History of Nonprescription Product Regulation (New York, NY: Pharmaceutical Products Press, 2003). P. Aaron and D. Musto, “Temperance and Prohibition in America: A Historical Overview,” in Alcohol and Public Policy: Beyond the Shadow of Prohibition, ed. Mark H. Moore and Dean R. Gerstein (Washington, DC: National Academy Press, 1981), 127–181. J. Blake, ed., Safeguarding the Public: Historical Aspects of Medicinal Drug Control (Baltimore, MD: Johns Hopkins University Press, 1970). G. Kay, Dying to Be Beautiful: The Fight for Safe Cosmetics (Columbus, OH: Ohio State University Press, 2005). J. Eastman, Styling vs. Safety: The American Automobile Industry and the Development of Automotive Safety, 1900–1966 (Lanham, MD: University Press of America, 1984). M. M. Jones and R. Bayer, “Paternalism and Its Discontents: Motorcycle Helmet Laws, Libertarian Values, and Public Health,” American Journal of Public Health 97, no. 2 (2007): 208–217. F. Chaloupka, M. Wakefield, and C. Czart, “Taxing Tobacco: The Impact of Tobacco Taxes on Cigarette Smoking and Other Tobacco Use,” in Regulating Tobacco, ed. R. L. Rabin and S. D. Sugarman (New York, NY: Oxford University Press, 2001), 11–38. M. Derthick, Up in Smoke: From Legislation to Litigation in Tobacco Politics (Washington, DC: CQ Press, 2012). L. Glickman, Buying Power: A History of Consumer Activism in America (Chicago, IL: University of Chicago Press, 2009)
- 4. This broad definition accounts for the fact that the boundaries for what constitutes a poison and the concept of poisoning have changed over time. As John Burnham has noted, until at least World War II, Americans generally thought of poisons as a narrow category of specific substances; a new conceptualization of poison appeared in the 1950s with the rise in awareness of accidental childhood poisonings in the home, the introduction of a broad array of new chemicals into the consumer marketplace, and the birth of the poison control movement. Now any substance that proved toxic to children could be deemed a household poison. However, the regulatory arena took 20 years to adapt to this conceptual shift—the Poison Prevention Packaging Act (PPPA) being the first legislation to reflect it—and the regulatory history described here generally reflects the earlier conceptualization of poisons as specific substances. See J. C. Burnham, “How the Discovery of Accidental Childhood Poisoning Contributed to the Development of Environmentalism in the United States,” Environmental History Review 19 (1995): 57–81. This article, though addressing the history of childhood poisoning, does not address in depth debates over packaging and labeling. [PubMed]
- 5. L. Cohen, A Consumer’s Republic: The Politics of Mass Consumption in Postwar America (New York, NY: Alfred A. Knopf, 2003), 357. Glickman, Buying Power, 303.
- 6. Marks, “Revisiting ‘The Origins of Compulsory Drug Prescriptions,’” 109. G. J. Stigler, “The Theory of Economic Regulation,” Bell Journal of Economics and Management Science 2, no. 1 (1971):3–21. Also see R. A. Posner, “Theories of Economic Regulation,” Bell Journal 5, no. 2 (1974):335–358. This history partially reflects capture theorists’ contention that industry over time comes to dominate the agencies regulating them (Posner, 341–342), but in this case industry’s power has waxed and waned. K. J. Meier, “The Political Economy of Consumer Protection: An Examination of State Legislation,” Western Political Quarterly 40, no. 2 (1987): 343–359.
- 7. R. Bayer and J. Colgrove, “Children and Bystanders First: The Ethics and Politics of Tobacco Control in the United States, ” in Unfiltered: Conflicts over Tobacco Policy and Public Health, ed. E. A. Feldman and R. Bayer (Cambridge, MA: Harvard University Press, 2004), 8, 15, 17.
- 8. M. Bogard and G. Griffenhagen, History of Drug Containers and Their Labels (Madison, WI: American Institute for the History of Pharmacy, 1999), 91–92. C. Munsey, The Illustrated Guide to Collecting Bottles (New York, NY: Hawthorn Books, 1970), 161.
- 9. M. I. Wilbert, “The Evolution of Laws Regulating the Sale and Use of Poisons, ” Journal of the American Pharmaceutical Association 1, no. 11 (1912): 1259. An 1848 New Hampshire statute required that all sales of arsenic, prussic acid, or corrosive sublimate be recorded. Ohio (1852), Pennsylvania (1860), and Wisconsin (1862) also enacted laws that required sellers of poisons to include both a label on poisons and a record of the poison’s sale. E. Kremers and G. Urdang, Kremers and Urdang’s History of Pharmacy, 4th ed., rev. G. Sonnedecker (Philadelphia, PA: Lippincott, 1976), 216. These laws, especially those that required recording of sales, were likely motivated more by the desire to discourage the use of arsenic and other difficult-to-trace poisons in homicide than by concerns about accidental poisonings. See Deborah Blum, The Poisoner’s Handbook: Murder and the Birth of Forensic Medicine in Jazz Age New York (New York, NY: Penguin Press, 2010), 1–3.
- 10. American Medical Association, 1846-1958 Digest of Official Actions, American Medical Association (Chicago, IL: American Medical Association, 1959), 563–564.
- 11. Bogard and Griffenhagen, History of Drug Containers and Their Labels, 92.
- 12. M. Fishbein, A History of the American Medical Association, 1847 to 1947 (Philadelphia: W. B. Saunders, 1947), 1012. American Medical Association, 1846–1958 Digest of Official Actions, 563.
- 13. E. W. Steib, “Drug Control in Britain, 1850–1914, ” in Safeguarding the Public ed. J. Blake, 20. Also see “The History of the Royal Pharmaceutical Society, ” The Museum of the Royal Pharmaceutical Society of Great Britain, http://www.rpharms.com/museum-pdfs/history-of-the-society.pdf (accessed April 17, 2012)
- 14. Kremers and Urdang, Kremers and Urdang’s History of Pharmacy, 216–217.
- 15. Munsey, The Illustrated Guide to Collecting Bottles, 161–163.
- 16. Ibid, 163. Historic American Buildings Survey, “Industry, ” in Southern New Jersey and the Delaware Bay, Historic Themes and Resources Within the New Jersey Coastal Heritage Trade Route (Washington, DC: US Department of the Interior, National Park Service, 1991), http://www.cr.nps.gov/history/online_books/nj2/chap5.htm (accessed April 17, 2012). Wesley Clair Mitchell, Business Cycles (Berkeley, CA: University of California Press, 1913), 21, quoted and cited in in W. Leach, Land of Desire: Merchants, Power, and the Rise of a New American Culture (New York, NY: Pantheon Books, 1993), 7 (“goods made by…”)
- 17. J. Harrison, “Improvement in Precautionary Attachments for Bottles Containing Poison, ” US Patent Number 110,760, patented January 3, 1871, 2.
- 18. W. M. Cateeson, “Improvement in Vessels for Containing Poison, ” US Patent Number 164,265, filed April 15, 1875 and issued June 8, 1875. J. W. Bowles, “Improvement in Poison-Bottles,” US Patent Number 183,117, filed September 16, 1876 and issued October 10, 1876. O. F. Frost, “Alarm for Poison-Receptacles,” US Patent Number 349,641, filed April 3, 1886 and issued September 21, 1886; R. M. Devereaux, “Alarm Bottle Stopper,” US Patent Number 410,730, filed July 26, 1888 and issued September 10, 1889. I. Peters, “Electric Alarm,” US Patent Number 346,587, filed December 16, 1885 and issued August 3, 1886. Munsey, The Illustrated Guide to Collecting Bottles, 161.
- 19. “Sale of Poisons in the District of Columbia, ” 55th Cong., 1st Sess., S. Doc. 15.
- 20. Practice of Pharmacy and Sale of Poisons in the District of Columbia, Et Cetera, 59th Cong., 1st Sess., S. Rep. 820 Amending the Act Regulating Practice of Pharmacy and Sale of Poisons, 59th Cong., 2d Sess., H. Rep. 7580, p. 451 (“beverage or harmless…”). “Regulation for Poison Bottles,” New York Times, January 28, 1899, p. 12. “Against Poison Bottle Bill,” New York Times, April 7, 1899, p. 5. “Governor and the Poison Bottle Bill,” New York Times, April 8, 1899, p. 8. “A New Poison Bottle Bill,” New York Times, January 3, 1900, p. 3. “Pharmacists Oppose Patent Bottle Bill,” New York Times, March 8, 1900, p. 8.
- 21. See S. H. Adams, “Introduction, ” The Great American Fraud, Colliers, October 7, 1905; “Peruna and the Bracers, ” October 28, 1905; “Liquozone,” November 18, 1905; “The Subtle Poisons,” December 2, 1905; “Preying on the Incurables,” January 13, 1906; “The Fundamental Fakes,” February 17, 1906; “The Sure-Cure School,” July 14, 1906; “The Miracle Workers,” August 4, 1906; “The Specialist Humbug,” September 1, 1906; “The Scavengers,” September 22, 1906. Reprinted as S. H. Adams, The Great American Fraud (Chicago, IL: American Medical Association, 1912)
- 22. See J. H. Young, The Toadstool Millionaires: A Social History of Patent Medicines in America before Federal Regulation (Princeton, NJ: Princeton University Press, 1961), 244. Pray, A History of Nonprescription Product Regulation, 9–16.
- 23. E. Bok, “Why ‘Patent Medicines’ Are Dangerous, ” The Ladies’ Home Journal 22, no. 4 (1905), 18.
- 24. A. P. Greeley, The Food and Drugs Act, June 30, 1906: A Study with Text of the Act, Annotated, the Rules and Regulations for the Enforcement of the Act, Food Inspection Decisions and Official Food Standards, (Washington, DC: John Byrne & Co., 1907), 113 (“unlawful for any…). L. S. Goodwin, The Pure Food, Drug, and Drink Crusaders, 1879-1914, (Jefferson, NC: Mcfarland & Co., 1999), 213-240, details the coalition of groups that secured passage of the Pure Food and Drug Act.
- 25. Cohen, A Consumer’s Republic, 21. Cohen refers to the Progressive Era initiatives such as the Pure Food and Drug Act as “the first-wave consumer movement., ” p. 13.
- 26. N. Tomes, The Gospel of Germs: Men, Women, and the Microbe in American Life (Cambridge, MA: Harvard University Press, 1998), 49–61. C. Rosenberg, The Cholera Years, The United States in 1832, 1849, and 1866 (Chicago, IL: University of Chicago Press, 1987), 202–206.
- 27. S. Stage, “Introduction: Home Economics, What’s in a Name?” and N. Tomes, “Spreading the Germ Theory: Sanitary Science and Home Economics, 1880–1930, ” in Rethinking Home Economics: Women and the History of a Profession, eds. S. Stage and V. B. Vincenti (Ithaca, NY: Cornell University, 1997), 1–13, 34–54.
- 28. E. Holt, Encyclopædia of Household Economy (New York, NY: McClure, Phillips, 1903), 293–294. In this manual, Holt included detailed instructions for making preparations at home such as carbolic acid solution and soap, oxalic acid solution (for bleach), and other germicides such as copper and zinc sulphate. Holt placed the responsibility for safe handling of these chemicals entirely on the homemaker, repeatedly instructing the homemaker to mark all containers of these solutions with the word “poison.” Cohen, A Consumer’s Republic, 22.
- 29. J. D. Norris, Advertising and the Transformation of American Society, 1865–1920 (New York, NY: Greenwood Press, 1990), 67–68.
- 30. Ibid., 39.
- 31. Historical Statistics of the United States, Colonial Times to 1970 (Washington, DC: Bureau of the Census, 1975), 284–331, cited in Norris, Advertising and the Transformation of American Society, 68.
- 32. W. H. Lough, High Level Consumption, Its Behavior, Its Consequences (New York, NY: McGraw-Hill, 1935), 236, 241. Using census data, Lough compared consumer spending in selected years between 1909 and 1931. According to his table, consumers spent $6 million in 1909 and $46 million in 1929 on “cleaning and polishing preparations.”.
- 33. Leach, Land of Desire, 136, 270. Our survey of New York Times advertising from 1900 to 1930 indicates a profusion of these products among department store wares. For example, see display ad, New York Times, January 16, 1916, p. RP4. Similar trends to those reported here occurred in expenditures on canned goods, household electricity, and household appliances. J. Dewhurst, America’s Needs and Resources: A New Survey (New York, NY: Twentieth Century Fund, 1955), 180, 702, 704, cited in James R. McGovern, “The American Woman’s Pre-World War I Freedom in Manners and Morals,” Journal of American History 55, no. 2 (1968): 321. Raw population data from U.S. Census Bureau, “No. H.S. 1—Population: 1900 to 2002,” http://www.census.gov/statab/hist/HS-01.pdf (accessed May 1, 2012). Population figures are rounded up to the next million. Per capita estimates are derived from dividing expenditures by population at each time point. The quotient is rounded up to the next cent. See also table in Norris, Advertising and the Transformation of American Society, 84, using most of this data for different calculations.
- 34. J. Tarr and M. Tebeau, “Housewives as Home Safety Managers: The Discovery of the Home as a Place of Risk and Danger, 1850–1940, ” in Accidents in History: Injuries, Fatalities and Social Relations, eds. R. Cooter and B. Luckin (Atlanta, GA: Editions Rodopi, 1997), 196, 204–214.
- 35. “About the Good Housekeeping Research Institute, ” http://www.goodhousekeeping.com/about-good-housekeeping-research-institute (accessed April 19, 2012). “Safety First Around the House,” Good Housekeeping, November 1923, 67, 191. Harvey Wiley, who had led and organized the crusade for the Pure Food and Drug Act of 1906 as the US Department of Agriculture’s chief chemist, joined the magazine’s staff in 1915 after retiring from the US Department of Agriculture and opened the Good Housekeeping Institute as a research laboratory to test these products. See “Harvey W. Wiley: Pioneer Consumer Activist,” FDA Consumer, January–February 2006, http://www.fda.gov/AboutFDA/WhatWeDo/History/CentennialofFDA/HarveyW.Wiley/default.htm (accessed August 22, 2012)
- 36. C. Jackson, Bronchoscopy and Esophagoscopy: A Manual of Peroral Endoscopy and Laryngeal Surgery (Philadelphia, PA: W. B. Saunders, 1922), 18–20. J. Beamis, Jr., “Modern Use of Rigid Bronchoscopy, ” in Interventional Bronchoscopy, ed. C. T. Bollinger and P. N. Mathur (Basel, Switzerland: Karger, 2000), 22–24.
- 37. Lye, in Webster’s Revised Unabridged Dictionary, ed. N. Porter (G. & C. Merriam, 1913), http://machaut.uchicago.edu/websters (accessed April 19, 2012)
- 38. Senate Committee on Interstate Commerce, Hearing on S. 2320, A Bill to Safeguard the Distribution and Sale of Certain Dangerous Caustic or Corrosive Acids, Alkalis, and other Substances in Interstate and Foreign Commerce, 69th Cong. 1st sess., March 5, 1926, 5–7. These other states included Colorado (1925), Florida (1923), Louisiana (1924), Minnesota (1925), Nevada (1925), New Hampshire (1925), New Jersey (1924), Oregon (1925), Pennsylvania, (1923), South Carolina (1922), Vermont (1925), and West Virginia (1925). L. H. Peters, “Diet and Health,” Los Angeles Times, May 17, 1927, p. A7. Also see R. G. Stanfill (retired director, Philadelphia District, U.S. Food and Drug Administration), interview by F. L. Lofsvold, September 12, 1981, History of the US Food and Drug Administration, http://www.fda.gov/downloads/AboutFDA/WhatWeDo/History/OralHistories/SelectedOralHistoryTranscripts/UCM265775.pdf (accessed April 23, 2012)
- 39. Senate Committee on Interstate Commerce, Hearing on S. 2320, 9–11. T. L. Stokes, “Medics Request Bill to Protect, ” The Atlanta Constitution, March 6, 1926.
- 40. Ibid.
- 41. Senate Committee on Interstate Commerce, Hearing on S. 2320, 16.
- 42. Ibid., 17–18.
- 43. “The substances covered by the Federal Caustic Poisons Act included: hydrochloric acid, sulfuric acid, nitric acid, carbolic acid (phenol), oxalic acid, any salt of oxalic acid, acetic acid, hypochlorous acid, potassium hydroxide, sodium hydroxide, silver nitrate, and ammonia. It required on all packages of these substances being shipped through interstate commerce ‘the common name of the substances, the name and place of business of the manufacturer, packer, seller, or distributor,’ as well as ‘the word “poison,” running parallel with the main body of reading matter on the label or sticker, on a clear, plain background of a distinctly contrasting color, in uncondensed gothic capital letters, the letters to be not less than twenty-four point size unless there is on the label or sticker no other type so large, in which even the type shall not be smaller than the largest type on the label and sticker’; and ‘directions for treatment in case of accidental personal injury by any dangerous caustic or corrosive substance’ unless the package is being shipped ‘for other than household use.’” Senate, 69th Cong., 2d. Sess., Congressional Record 488–489 (Apr. 2, 1927): 1406–1407.
- 44. The survey indicated that the incidence of esophageal strictures—a narrowing of the esophagus that makes swallowing impossible and can result from accidental ingestion of a caustic poison—had dropped by 50 percent in Arkansas, Florida, and Pennsylvania between 1926 and 1934. The survey’s author attributed this decrease to the Caustic Poisons Act. See H. M. Taylor, “A Preliminary Survey on the Effect Which Lye Legislation Has Had on the Incidence of Esophageal Stricture,” Annals of Otology, Rhinology and Laryngology 44, no. 12 (1935), 1157–1158, cited by G. W. Kernodle, G. Taylor, and W. C. Davison, “Lye Poisoning in Children,” American Journal of Diseases of Children 75, no. 2 (1948), 135–142. [DOI] [PubMed]
- 45. On the increasing authority of physicians and the AMA in the early 20th century, see P. Starr, The Social Transformation of American Medicine (New York, NY: Basic Books, 1982), 79–126.
- 46. Senate Committee on Interstate Commerce, Hearing on S. 2320, p. 12. US House. Children’s Bureau, 62d Cong., 2d sess., 1912, H. Rept. 235, serial 6129, xxxvi–xli. M. Ladd-Taylor, Mother-Work: Women, Child Welfare, and the State, 1900–1930 (Champaign-Urbana, IL: University of Illinois Press, 1994), 135–205. J. S. Lemons, “The Sheppard-Towner Act, Progressivism in the 1920s,” Journal of American History 55, no. 4 (1969): 776–786. The AMA opposed the Sheppard-Towner Act as a potential infringement on private medical practice. This opposition may have been one impetus for the organization to fight for passage of the Federal Caustic Poisons Act, as a demonstration that its members favored child health protection of some sort. [PubMed]
- 47. Volatile Poisons: Hearing Before the Committee on Agriculture and Forestry, United States Senate, Seventy-Second Congress, First Session on S. 3853, A Bill to Regulate Interstate and Foreign Commerce in Poisonous Volatile Substances Intended for Household Consumption, April 19, 1932, (Washington, DC: Government Printing Office, 1932, p. 23 (“through practical experience…); p. 2 (“a figure showing…) The poisons to be regulated by this law included methyl chloride, benzol (benzene), cyanogen, aniline, carbon disulphide, ethylene oxide, formaldehyde, nicotine, nitrobenzol (nitrobenzene), sulphur dioxide, and halogen compounds of hydrocarbons. Hearing, supra, 1-2.
- 48. Ibid., 12–13.
- 49. Ibid., 18–22.
- 50. J. B. West, “Yandell Henderson: April 23, 1873–February 18, 1944, ” Biographical Memoirs, 74(1998): 145–158. [PubMed]
- 51. Senate Committee on Agriculture and Forestry, Volatile Poisons, 19, 36–37. “Uncle Sam’s Home Hints, ” The Washington Post, July 8, 1930, p. 11.
- 52. For more on the tetraethyl lead controversy, see D. Rosner and G. Markowitz, “A ‘Gift of God?’ The Public Health Controversy over Leaded Gasoline during the 1920s, ” American journal of Public Health 75, no. 4 (1985): 344–351. [DOI] [PMC free article] [PubMed]
- 53. Senate Committee on Agriculture and Forestry, Volatile Poisons, 26.
- 54. Henderson had previously expressed his precautionary stance in a letter to the Bureau of Mines’ R. R. Sayers on tetraethyl lead. “In the past, the position taken by the authorities has been that nothing could be prohibited until it was proved to have killed a number of people. I trust that in the future, especially in a matter of this sort, the position will be that a substance like tetraethyl lead can not be introduced for general use until it is proved harmless.” Rosner and Markowitz, “A ‘Gift of God,’” 349.
- 55. Senate Committee on Agriculture and Forestry, Volatile Poisons, p. 43.
- 56. W. F. Janssen, “The Story of the Laws Behind the Labels, ” FDA Consumer, June 1981, http://www.fda.gov/AboutFDA/WhatWeDo/History/Overviews/ucm056044.htm (accessed April 19, 2012)
- 57. Senate Committee on Agriculture and Forestry, Volatile Poisons, 30–34. Watson also cited a US Department of Agriculture bulletin stating that paradichlorobenzene, a halogen that formed the active ingredient in moth cakes, was “nonpoisonous to man” and that carbon tetrachloride was less toxic than gasoline.
- 58. W. A. Evans, “How to Keep Well, ” Chicago Daily Tribune, Dec. 31, 1932, p. 6.
- 59. Senate Committee on Agriculture and Forestry, Volatile Poisons, 24.
- 60. J. Tarr and M. Tebeau, “Housewives as Home Safety Managers, ” 200–203, 214. Tarr and Tebeau noted that the council’s lack of attention to home safety reflected prevalent gender dynamics: It was run by men who concerned themselves more with the safety of the male worker than of the housewife.
- 61. J. D. Kittelton, “Legal Considerations in Drafting Warning Labels, ” Archives of Environmental Health 2 (1961): 263. [PubMed]
- 62. Cohen, A Consumer’s Republic, 31–34.
- 63. G. Kay, “Healthy Public Relations: The FDA’s 1930s Legislative Campaign, ” Bulletin of the History of Medicine 75, no. 3 (2001): 446–487. Also see Kay, Dying to Be Beautiful, 76–105. [DOI] [PubMed]
- 64. C. Bosso, Pesticides and Politics: The Life Cycle of a Public Issue (Pittsburgh, PA: University of Pittsburgh Press, 1997), 45, 53–58. 80th Cong. 1st Sess., Congressional Record 125, 166 (1947)
- 65. US Senate Committee on Commerce, Amending the Insecticide Act: Hearing of Subcommittee of U.S. Senate Committee on Commerce, 78th Cong., 1st Sess. December 6, 1943, 2–4. “Poisoning Laid to Trusty Error, ” Los Angeles Times, November 22, 1942, p. 1.
- 66. “Business World, ” New York Times, January 23, 1932, p. 29.
- 67. “Poison Kills 11 Men at Salvation Army, ” New York Times (AP), November 12, 1940, p. 23. Forney v. Sears, 280 Pac. 56 (Washington, DC, 1929), cited in J. D. Conner and G. A. Burroughs, Manual of Chemical Products Liability: An Analysis of the Law Concerning Liability Arising from the Manufacture and Sale of Chemical Products (Washington, DC: Manufacturing Chemists’ Association and National Agricultural Chemicals Association, 1952), 52–53.
- 68. J. M. Arena, “Poisoning Accidents and Their Prevention, ” Postgraduate Medicine 11, no. 3 (1952): 245–246 (reprinting a letter from Dr. Erwin E. Nelson, medical director of the Federal Security Agency to Arena on this legislative activity) [PubMed]
- 69. S. J. Hill, “The Manufacturing Chemists’ Association Labeling Program, ” AMA Archives of Industrial Health 12, no. 4 (1955): 378–379. These chemicals included methanol, carbon tetrachloride, similar volatile chlorinated liquid hydrocarbons, carbon disulfide, aniline oils, benzene (benzol), chlorinated naphthalene, chlorinated diphenyl, and chlorinated diphenyl oxides. [PubMed]
- 70. G. M. Wheatley, “Accident Prevention and the General Practitioner, ” Postgraduate Medicine 10, no. 5 (1951): 442–445. Charles B. Scott, “Our National Accident Problem,” American Journal of Public Health 11, no. 2 (1929): 141–143. Donald B. Armstrong and W. Graham Cole, “Study of Home Accidents: Their Public Health Significance,” American Journal of Public Health 31, no. 11 (1941): 1135–1142. The latter article cites 1940 Metropolitan Life Insurance data indicating that 33 000 fatal home accidents such as falls, electrocution, burns, and poisonings occurred in comparison to 17 000 occupational accidents. [DOI] [PubMed]
- 71. Burnham, “How the Discovery of Accidental Childhood Poisoning Contributed to the Development of Environmentalism in the United States, ” 60. [PubMed]
- 72. Arena, “Poisoning Accidents and their Prevention, ” 245. Institute of Medicine, Forging a Poison Prevention and Control System (Washington, DC: National Academies Press, 2004), 81. A. M. Burda and N. M. Burda, “The Nation’s First Poison Control Center: Taking a Stand against Accidental Childhood Poisoning in Chicago,” Veterinary and Human Toxicology 39, no. 2 (1997): 115–119. [PubMed]
- 73. Burnham, “How the Discovery of Accidental Childhood Poisoning Contributed to the Development of Environmentalism in the United States, ” 62. Institute of Medicine, Forging a Poison Prevention and Control System, 81. [PubMed]
- 74. Institute of Medicine, Forging a Poison Prevention and Control System, 81. [DOI] [PubMed]
- 75. Wheatley, “Accident Prevention and the General Practitioner, ” chart, 445. [DOI] [PubMed]
- 76. Institute of Medicine, Forging a Poison Prevention and Control System, 61–63. Burda and Burda, “The Nation’s First Poison Control Center,” 117. J. T. Botticelli and P. G. Pierpaoli, “Louis Gdalman, Pioneer in Hospital Pharmacy Poison Information Services,” American Journal of Hospital Pharmacy 49, no. 6 (1992): 1445–1450. M. Winn, “Poison Center Saves Lives of 374 Children,” Chicago Daily Tribune, November 15, 1954, p. 1. [PubMed]
- 77. “67 Poison Centers Will Trade Data, ” New York Times, June 7, 1957, p. 25. Burda and Burda, “The Nation’s First Poison Control Center, ” 116–117. Institute of Medicine, Forging a Poison Prevention and Control System, 83.
- 78. House Committee on Interstate and Foreign Commerce, Federal Hazardous Substances Labeling Act, Hearing, 86th Cong., 2d Sess., March 14, 1960, 1.
- 79. “12 Carbon Tetrachloride Deaths Force New Cleaning Fluid Label, ” New York Times, July 15, 1952, p. 23. The ordinance required that products containing this chemical be labeled with the following: “Carbon tetrachloride: Danger. Hazardous vapor and liquid. May be fatal if inhaled or swallowed. Do not take internally, do not breathe vapor. Use only with adequate ventilation. Avoid prolonged or repeated contact with skin.”.
- 80. C. L. French, “Report of the Labels and Precautionary Information Committee, Manufacturing Chemists’ Association” (presented to the MCA Board of Directors, March 14, 1961), 2, http://chemicalindustryarchives.org/search/pdfs/cma/19610314_00001313.pdf (accessed April 23, 2012)
- 81. French, “Report of the Labels and Precautionary Information Committee, ” 2. J. D. Conner and G. A. Burroughs, Manual of Chemical Products Liability: An Analysis of the Law Concerning Liability Arising from the Manufacture and Sale of Chemical Products (Washington, DC: Manufacturing Chemists’ Association and National Agricultural Chemicals Association, 1952), 2, 3, 5–6, 42. Simpson v. American Oil Company, 217 N.C. 542, 8 SE.2d 813 (1940). Maize v. Atlantic Refining Company, 352 Pa., 51, 41 A.2d 850 (1945)
- 82. French, “Report of the Labels and Precautionary Information Committee, ” 4. Minutes of the Sixty-Seventh Meeting of the Directors of the Manufacturing Chemists’ Association (February 12, 1957), 439, http://chemicalindustryarchives.org/search/pdfs/cma/19570214_00001623.pdf (accessed April 23, 2012)
- 83. “Statutory Liability: The Federal Hazardous Substances Labeling Act—Sword or Shield?” Food Drug Cosmetic Law Journal 19, no. 8(1964): 419–422. “Safety Labels Required Under New U.S. Law, ” Boston Globe, August 7, 1960, p. A63.
- 84. W. Kornberg, “The South Wins a War: FDA Decides to Drop ‘Poison’ Label on Turpentine for a ‘Danger’ Sign, ” The Washington Post and Times Herald, August 12, 1961, p. A1. “U.S. Announces Rules on Chemical Labels, ” New York Times, August 12, 1961, p. 29.
- 85. Minutes of the Meeting of the Directors of the Manufacturing Chemists’ Association (January 9, 1962), 776–777, http://chemicalindustryarchives.org/search/pdfs/cma/19620109_00001319.pdf (accessed April 24, 2012). Russell Porter, “Hazard Labeling Required on Feb. 1: U.S. Agency Warns Makers of Certain Home Products,” New York Times, January 25, 1962, p. 23. “Begin Label Changes for Aug. 1 Law,” Chicago Daily Tribune, May17, 1962, p. E6.
- 86. J. Van Valkenberg, “How Poison Control Can Save Lives, ” The Hartford Courant, April 8, 1962, p. 1B. G. M. Wheatley, “American Academy of Pediatrics President’s Message: The Federal Hazardous Substances Labeling Act, ” Pediatrics 28, no. 3(1961): 499–500. [PubMed]
- 87. Poison Prevention Packaging Act, 15 US Code 1471–1476 (1970), http://www.cpsc.gov/businfo/pppa.pdf (accessed May 1, 2012). The introduction of children’s aspirin to the market in 1947 led to an astronomical rise in pediatric morbidity and mortality from aspirin poisoning, according to Jay Arena, a leading participant in the pediatricians’ campaign against childhood poisonings. Arena said this led the pediatricians to contact Abe Plough, the leading maker of children’s aspirin, who agreed to test various types of safety caps. At a 1958 meeting with the FDA, Arena said, the manufacturers also agreed to halve the dosage in the aspirin and to try to improve the cap. Arena claimed this meeting “helped to lay the groundwork” for the PPPA. Further analysis of this episode lies beyond the scope of this article, but it serves to further illustrate the trend in cooperation between manufacturers, public health advocates, and government only after products caused child deaths. See J. M. Arena, “The Pediatrician’s Role in the Poison Control Movement and Poison Prevention,” American Journal of Diseases of Children, 137, no. 9 (1983): 870–873.
- 88. R. Adler and R. D. Pittle, “Product Safety: Consumer Product Safety Commission, ” in Changing America: Blueprints for the New Administration, ed. M. Green (New York, NY: Newmarket Press, 1992), 540.
- 89. The PPPA defines a household substance as one covered by the Federal Hazardous Substances Labeling Act; the Food, Drug and Cosmetic Act; or one used as fuel a house’s heating, cooking, or refrigeration system. Cohen, A Consumer’s Republic, 357.
- 90. W. Walton, “An Evaluation of the Poison Prevention Packaging Act, ” Pediatrics 69, no. 3 (1982): 363–370. This study found that the ingestion rate for substances requiring child-resistant closures declined from 5.7 in 1000 children in 1973 to 3.4 in 1000 children in 1978 and that between 1961 and 1981, the death rate due to poisonings of children declined from 2.0 in 100 000 children to 0.5 in 100 000. [PubMed]
- 91. Adler and Pittle, “Product Safety, ” 540–553. Adler and Pittle noted that nine products were exempted from the PPPA’s child-resistant packaging requirements between 1981 and 1991, in keeping with the Reagan and G. H. W. Bush administrations’ anti-regulatory ideologies and that the commissioners under these administrations had “never met a voluntary standard they didn’t like,” p. 552. “Requirements under the Federal Hazardous Substances Act: Labeling and Banning Requirements for Chemicals and Other Hazardous Substances, 15 U.S. C. § 1261 and 16 C.F.R. Part 1500” (Washington, DC: US Consumer Product Safety Commission Office of Compliance, 2002), http://www.cpsc.gov/businfo/regsumfhsa.pdf (accessed April 24, 2012)
- 92. For a recent discussion of the precautionary principle, the idea that underpins this precautionary approach and has shaped European environmental regulation, see N. M. Sachs, “Rescuing the Strong Precautionary Principle from Its Critics, ” University of Illinois Law Review 2011, no. 4 (2011): 1285–1338.