Abstract
Inflammation is associated with preterm premature rupture of membranes (PPROM) and adverse neonatal outcomes. Subchorionic thrombi, with or without inflammation, may also be a significant pathological finding in PPROM. Patterns of inflammation and thrombosis may give insight into mechanisms of adverse neonatal outcomes associated with PPROM. To characterize histologic findings of placentas from pregnancies complicated by PPROM at altitude, 44 placentas were evaluated for gross and histological indicators of inflammation and thrombosis. Student’s t-test (or Mann–Whitney U-test), χ2 analysis (or Fisher’s exact test), mean square contingency and logistic regression were used when appropriate. The prevalence of histologic acute chorioamnionitis (HCA) was 59%. Fetal-derived inflammation (funisitis and chorionic plate vasculitis) was seen at lower frequency (30% and 45%, respectively) and not always in association with HCA. There was a trend for Hispanic women to have higher odds of funisitis (OR = 5.9; P = 0.05). Subchorionic thrombi were seen in 34% of all placentas. The odds of subchorionic thrombi without HCA was 6.3 times greater that the odds of subchorionic thrombi with HCA (P = 0.02). There was no difference in gestational age or rupture-to-delivery interval, with the presence or absence of inflammatory or thrombotic lesions. These findings suggest that PPROM is caused by or can result in fetal inflammation, placental malperfusion, or both, independent of gestational age or rupture-to-delivery interval; maternal ethnicity and altitude may contribute to these findings. Future studies focused on this constellation of PPROM placental findings, genetic polymorphisms and neonatal outcomes are needed.
Keywords: altitude, chorioamnionitis, fetal inflammatory response, funisitis, subchorionic thrombi
Introduction
The developing fetus responds to stress in the intrauterine environment; this adaptation is an important contributor to the predisposition of adult-onset disease. Within the maternal-fetal unit, the placenta has increasingly been recognized as a major programming agent in the development of heart disease, hypertension, Type 2 diabetes, obesity and lung cancer.1–4 Yet, placental studies in the developmental origins of disease have been limited to those pregnancies complicated by intrauterine growth restriction and/or preeclampsia, or otherwise normal term pregnancies. Preterm premature rupture of membranes (PPROM) is associated with a stressful intrauterine environment, but is understudied in its relation to the developmental origins of disease. Intrauterine infection is thought to be a major contributor to PPROM, as well as the main risk factor for adverse neonatal outcomes, including cerebral palsy.5–12 Furthermore, it makes intuitive sense that the hostile fetal environment associated with PPROM may be an important modulator in the programming of adult metabolic syndrome.
Although PPROM accounts for ~30–40% of preterm deliveries,13 the pathological findings in PPROM placentas are not well characterized. Prior studies of placental pathology have focused on preterm birth in the context of other complications.14–19 In those studies that have examined the PPROM subset, definitions of PPROM are not uniform, making generalizability difficult. Additionally, pathological studies of preterm placentas have focused on intrauterine inflammation, primarily chorioamnionitis and fetal vasculitis. However, histologic evidence of inflammation is not present in the majority of these placentas, nor is there necessarily a strict progression from chorioamnionitis to fetal vasculitis, as conventionally imparted.16,20–24 Furthermore, studies of placental pathology in PPROM have been conducted in populations residing at sea level, calling to question whether different patterns exist at altitude.
We postulate that (1) there are unrecognized contributors to PPROM; (2) these non-inflammatory contributors may sometimes be found in association with inflammation; and (3) isolated fetal-derived inflammation may also contribute to PPROM. We further hypothesize that no differences in inflammatory patterns exist – but thrombotic findings may be more apparent – between our high altitude cohort (1600 m/5280 ft) and findings published from sea-level populations. Therefore, the goal of this study was to comprehensively characterize inflammatory and thrombotic histologic findings in placentas from pregnancies complicated by PPROM in a high-altitude population. Uncovering patterns of placental pathology in PPROM may reveal clues to the development of adult-onset disease, give insight into the variation of neonatal outcomes associated with PPROM, and ultimately aid in developing targeted interventions to prevent PPROM and its adverse consequences.
Methods
Placentas from PPROM pregnancies (>24 or <34 weeks at PPROM) were collected from women enrolled in a prospective cohort at the University of Colorado Hospital from July 1, 2010 to June 30, 2012. PPROM was confirmed by standard clinical characteristics of alkaline pH, ferning and pooling of amniotic fluid in the vagina on speculum examination in women >24 weeks or <34 weeks of gestation before the onset of labor. Clinical chorioamnionitis was defined as two or more of the following clinical criteria: maternal fever >38.0°C, maternal tachycardia (>100 beats/min), fetal tachycardia (>160 beats/min), maternal fundal tenderness, maternal leukocytosis (WBC > 15,000) and maternal purulent vaginal discharge odd ratio (OR) amniocentesis with findings consistent with chorioamnionitis.
Subjects with multiple gestation, hypertensive diseases of pregnancy or non-viable pregnancy were excluded. This research was conducted in accordance with the 2004 Declaration of Helsinki, with signed informed consent required for study participation (Colorado Multiple Institutional Review Board study 09–1107).
Demographic and pregnancy data were abstracted from the Perinatal Database of the Department of Obstetrics and Gynecology at the University of Colorado School of Medicine. Data abstraction was performed by a trained research assistant using a standardized protocol and verified by the principal investigator for PPROM subjects (J.A.W.). Ambiguity of clinical data was clarified by a senior maternal-fetal medicine specialist (V.D.W.).
Placenta analysis
Placentas were placed in formalin following delivery and then grossly examined within 72 h to assess for umbilical cord length and insertion, number of vessels, membrane insertion, trimmed weight, appearance of maternal and fetal surfaces, and the parenchyma. A minimum of four formalin-fixed paraffin-embedded blocks were generated, including cross-sections of umbilical cord, free fetal membranes and full-thickness placental parenchyma. At least one 5-μ section from each block was stained with hematoxylin and eosin and then examined by a placental pathologist (M.D.P.) to evaluate for the presence of any microscopic lesions.
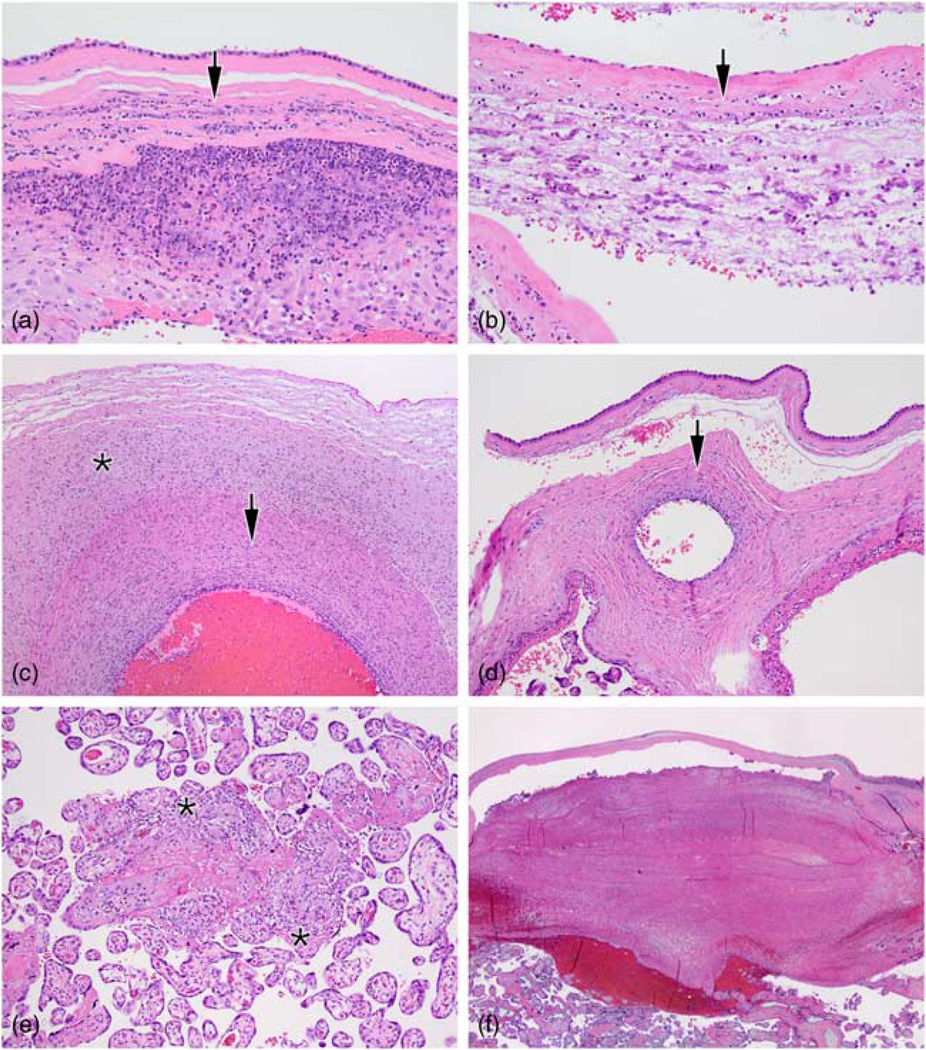
Histologic acute chorioamnionitis (HCA) was defined as the presence of polymorphonuclear cells within the amnion or chorion (Fig. 1a). Fetal-derived inflammation was defined as the presence of an inflammatory infiltrate (polymorphonuclear cells) emanating from the fetal vessels of the umbilical cord (vasculitis or phlebitis) or chorionic plate (chorionic plate vasculitis), or funisitis – fetal inflammatory cells extending into Wharton’s jelly (Fig. 1b and 1c). Maternal-derived inflammation was defined as chronic villitis (maternal lymphocytes infiltrating and destroying villi, Fig. 1d). Additional findings recorded included fetal thrombotic events such as intimal fibrin cushions (remote non-occlusive mural thrombi of stem villous or chorionic plate vessels as evidenced by intramural fibrin aggregates and intimal hyperplasia), hemorrhagic endovasculitis (non-inflammatory necrosis of villous vessels leading to red blood cell extravasation) and intervillous thrombi (leakage of villous capillaries causing slow feto-maternal hemorrhage). Presence of subchorionic thrombi (blood clots below the chorionic plate from maternal malperfusion, Fig. 1e) and placental infarcts (representing cessation of maternal blood flow) were also recorded.
Fig. 1.
Placental membranes were evaluated for the presence of acute inflammatory cells (arrows) extending through the decidua (the endometrium of the pregnant uterus) into the chorion (the membrane between the fetus and mother) or amnion (the membrane that encloses the fetus) (a, b). Inflammation of the umbilical cord (c) was classified as vasculitis if the inflammatory cells infiltrated through the umbilical cord vessel walls (arrow) or funisitis if they extended into the surrounding Wharton’s jelly (asterisk). In some cases, there was inflammation of the chorionic plate (the fetal side of the placental disc) (d) emanating from fetal vessels and extending toward the amniotic cavity (arrow). Chronic villitis (e) is characterized by maternal lymphocytes encasing and invading the chorionic villi (asterisks). Some placentas showed subchorionic thrombi (f), a laminated clot formed below the chorionic plate because of slow/absent maternal blood flow.
Statistical analysis
Dichotomous variables were compared using χ2 analysis (or Fisher’s exact test) and continuous variables using Student’s t-test or Mann–Whitney U-test when appropriate. Correlation coefficient was assessed using mean square contingency (φ) for dichotomous variables. To assess predictors of placental inflammation or thrombosis, we calculated ORs and 95% confidence intervals (CIs) using logistic regression. Univariate predictors included maternal age, race (Caucasian/non-Caucasian), ethnicity (Caucasian not of Hispanic origin, Caucasian of Hispanic origin, other), diagnosis of clinical chorioamnionitis, nulliparity, cesarean section, gestational age at rupture, gestational age at delivery, and membrane rupture interval from PPROM to delivery, and histologic chorioamnionitis; although smoking during pregnancy, auto-immune disease, and non-gestational diabetes were also predictors of interest, there were no cases within our cohort. Gestational age at delivery was treated as both continuous and trichotomous variables of <28, 28–32 and >32 weeks. To determine independent predictors of placental pathological patterns, we created multivariate models; on multivariate analysis, the model corrected for gestational age at delivery and included all covariates with a P-value of ≤0.10 in the initial analysis. Stata 12.0 (Stata Corp, College Station, TX) was used for all statistical analysis. All tests were two-sided and P<0.05 was considered significant.
Results
Demographics (Table 1)
Table 1.
Clinical characteristics of patients with PPROM
| Parameters | PPROM (n = 44) |
|---|---|
| Maternal age (years, mean ± s.d.) | 30.2 ± 5.6 |
| Race/ethnicity | |
| Caucasian (not of Hispanic heritage) | 16 (36%) |
| Caucasian (of Hispanic heritage) | 21 (48%) |
| Non-Caucasian | 7 (16%) |
| Nulliparity | 10 (23%) |
| Clinical chorioamnionitis | 14 (32%) |
| Gestational age at rupture (weeks, mean ± s.d.) | 30.0 ± 2.7 |
| Gestational age at delivery (weeks, mean ± s.d.) | 31.2 ± 2.7 |
| Rupture interval (days, mean ± s.d.) | 8.5 ± 7.7 |
| Cesarean section | 14 (32%) |
PPROM, preterm premature rupture of membranes; s.d., standard deviation.
Placentas from 44 pregnant women with PPROM were examined. Mean gestational age at membrane rupture was 30.0 ± 2.7 weeks (range 23 3/7–33 4/7 weeks). Mean gestational age at delivery was 31.2 ± 2.7 weeks (range 24 5/7–34 1/7 weeks). Average interval between PPROM and delivery was 8.5 days (range 0–36 days). All pregnant women were treated with antibiotics and betamethasone as per standard of care within our institution. Clinical chorioamnionitis was diagnosed in 32% (n = 14) of all PPROM pregnancies.
Patterns of inflammation and thrombosis (Table 2)
Table 2.
Patterns of inflammation and thrombosis in PPROM placentas
| Parameters | PPROM (n = 44) |
|---|---|
| HCA | 26 (59%) |
| Chronic villitis | 5 (11%) |
| UC Phlebitis | 11 (25%) |
| UC Vasculitis | 18 (44%) |
| UC Funisitis | 13 (30%) |
| Chorionic plate vaculitis | 20 (45%) |
| Subchorionic thrombi | 15 (34%) |
| Intimal fibrin cushions | 0 |
| Infarct | 1 (2%) |
| HCA + funisitis | 11 (25%) |
| HCA + chorionic plate vasculitis | 17 (39%) |
| HCA + subchorionic thrombi | 5 (11%) |
| Funisitis + subchorionic thrombi | 5 (11%) |
PPROM, preterm premature rupture of membranes; HCA, histologic chorioamnionitis; UC, umbilical cord.
The overall prevalence of HCA was 59% (n = 26). HCA was poorly correlated with clinical chorioamnionitis diagnosis (11/26; 42%; φ = 0.27), although when clinical chorioamnionitis was diagnosed, HCA was often present (11/14; 79%).
Funisitis was seen in 30% of PPROM placentas. Eighty-five percent (11/13) of all PPROM placentas with funisitis also had HCA (φ = 0.34), but clinical chorioamnionitis was poorly correlated with funisitis (φ = 0.09).
Chorionic plate vasculitis was seen in 45% of PPROM placentas (n = 20). Chorionic plate vasculitis typically also had HCA (17/20; 85%; φ = 0.48), although chorionic plate vasculitis had less correlation with clinical chorioamnionitis (9/20; 45%; φ = 0.29). Chorionic plate vasculitis and funisitis were often seen together (φ = 0.30), yet one-third of funisitis cases did not have associated chorionic plate vasculitis.
Chronic villitis was present in only 11% of all PPROM placentas (n = 5), with the majority seen in >32 weeks of gestational age placentas (4/5; 80%). Chronic villitis was uncommonly seen with clinical chorioamnionitis (1/14; 7; φ = −0.09) or HCA (1/26; 4%; φ = −0.29). Chronic villitis was never seen with funisitis or chorionic plate vasculitis.
Subchorionic thrombi were seen in 34% of the placentas (n = 15). Subchorionic thrombi were more frequent in the absence of clinical chorioamnionitis (4/15; 27%; φ = −0.07) or HCA (5/15; 30%; φ = −0.38). Subchorionic thrombi and funisitis were both present in 11% of placentas (n = 5; φ = 0.05). Similarly, subchorionic thrombi and chorionic plate vasculitis were infrequently seen together (n = 8; 18%; φ = 0.11). Only one case of subchorionic thrombi also had chronic villitis.
Inflammation, thrombosis and gestational age (Fig. 2)
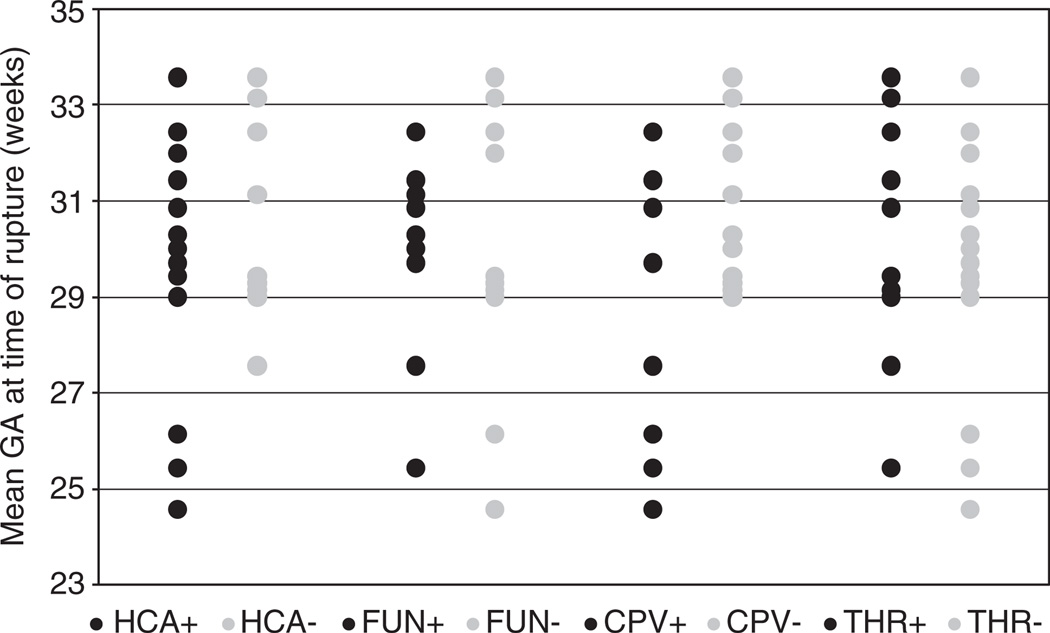
Fig. 2.
Patterns of inflammation and thrombosis in preterm premature rupture of membranes (PPROM) placentas in relation to gestational age. GA, gestational age; HCA, histologic acute chorioamnionitis; FUN, funisitis; CPV, chorionic plate vasculitis; THR, subchorionic thrombi.
There was no difference in the diagnosis of clinical chorioamnionitis with gestational age at PPROM, gestational age at delivery or rupture-to-delivery interval. HCA was equally prevalent in placentas delivered at <28 weeks and 28–32 weeks (88% v. 71%; P = 0.62). However, HCA was less common in placentas delivered >32 weeks (37%; P = 0.02). There was no difference in mean rupture-to-delivery interval between PPROM placentas with and without HCA (7 v. 10 days; P = 0.26).
Funisitis was equally prevalent in placentas delivered at <28 weeks (38%; P = 0.58) and 28–32 weeks (35%; P = 0.51). Although funisitis was seen less frequently in placentas delivered >32 weeks (21%), this finding was not statistically significant (P = 0.34). Chorionic plate vasculitis was more common in placentas delivered <28 weeks of gestational age (88%; P = 0.02) compared with those delivered at 28–32 weeks (41%; P = 0.04) and >32 weeks (31%; P = 0.01). Although gestational age at time of delivery was not significantly associated with funisitis (P = 0.13), the presence of chorionic plate vasculitis was inversely proportional to gestational age at delivery (P = 0.004; φ = −0.42). There was no difference in mean rupture-to-delivery interval between PPROM cases with and without funisitis (9 v. 8 days; P = 0.73), nor chorionic plate vasculitis (8 v. 9 days; P = 0.53).
There was no difference in subchorionic thrombi and gestational age at delivery (P = 0.49), nor was there an association with rupture-to-delivery interval with presence or absence of thrombi (10 v. 8 days; P = 0.32).
Predictors of inflammation and thrombosis
There were no independent predictors of clinical chorioamnionitis or HCA on multivariate analysis. On univariate analysis, HCA was a predictor of funisitis (OR = 5.9; CI = 1.1–31.0; P = 0.04), but was not an independent predictor on multivariate analysis. However, there was a trend for Hispanic women to have higher odds of funisitis compared with non-Hispanic women (OR = 5.4; CI = 1.01–28.2; P = 0.05). Earlier gestational age at rupture and HCA were univariate predictors of chorionic plate vasculitis, but were not independent predictors on multivariate analysis. When adjusted for gestational age at delivery, the odds of subchorionic thrombi without HCA was 6.3 times greater that the odds of subchorionic thrombi with HCA (CI = 1.3–33.3; P = 0.02), regardless of maternal ethnicity.
Discussion
Overall, distinct patterns of placental pathology exist in PPROM. As expected, chorioamnionitis was common in PPROM24–27; at our institution, there was a moderate concordance of HCA with clinical chorioamnionitis. Yet, almost 40% of the PPROM placentas did not have HCA, suggesting a non-inflammatory mechanism for premature rupture. Although fetal-derived inflammation (funisitis and chorionic plate vasculitis) is a central pathological finding in PPROM, we determined that subchorionic thrombi may also be important. These findings suggest that PPROM may be triggered by separate mechanisms (inflammation and/or thrombosis).
Funisitis is recognized as the histologic manifestation of the fetal inflammatory response, and has been associated with adverse neurological outcomes.11,28,29 Funisitis has also been postulated to be the essential indicator of the fetal inflammatory response in preterm, but not term deliveries.30 Our prevalence of PPROM funisitis (30%) is consistent with most studies of PPROM as well as all preterm deliveries.28,30–33 In our cohort, funisitis had higher correlation with HCA than clinical chorioamnionitis. Our data suggest that funisitis – as a marker for the fetal inflammatory response – may not manifest with clinically evident symptoms in the pregnant woman. Additionally, we found a trend for Hispanic women to have higher odds of funisitis compared with non-Hispanic women regardless of maternal age, parity, gestational age, rupture interval or presence of HCA. Although Hispanic infants have been shown to have lower risk of perinatal morbidity and mortality,34 a recent large retrospective cohort study in California found slightly increased risk of spastic or dyskinetic cerebral palsy in normal birth weight Hispanic infants compared with Caucasian infants not of Hispanic origin.35 Interestingly, concentrations of umbilical plasma IL-6 (as a serum correlate of the fetal inflammatory response) was elevated in a predominately Hispanic population, suggesting a genetic component of inflammatory response.36 Funisitis and the fetal inflammatory response could be a mechanistically plausible explanation for this increased risk for cerebral palsy. Although beyond the scope of this study, future exploration into the effect of race/ethnicity, PPROM placental pathology and offspring neurological outcomes may be informative.
In their study of extremely low gestational age newborns (23–27 weeks of gestation), Hecht et al.16 noted that funisitis did not vary with gestational age, whereas chorionic plate vasculitis was inversely proportional to gestational age. Within our group of slightly older preterm newborns (25–33 weeks of gestation), we saw similar findings. Interestingly, in both studies, funisitis did not necessarily accompany chorionic plate vasculitis or HCA. These data would argue against the concept of funisitis as strictly a progression of inflammation from the chorionic plate after HCA exposure, but support the concept that funisitis may be (in some instances) a response to another unidentified insult, or a de novo fetal inflammatory response.24,36,37 Although the mechanism remains unclear, genetic determinants of inflammation may help to explain these findings.36 Nonetheless, most pathologists agree that these findings most likely reflect sampling error,24,38,39 or likewise represent differences in pathological interpretation.40
Similar to prior studies,17,41 we found that HCA was less prevalent with later gestational age at delivery after PPROM. Yet, rupture-to-delivery interval was not associated with increased prevalence of HCA or fetal inflammation, suggesting that there is no increased inflammation with longer rupture length. In a similar gestational age and birth weight cohort to ours, Ghidini et al.22 showed a lack of relationship between HCA and latency duration in PPROM, even with prolonged (>7 days) rupture interval. Although our cohort is small, we suspect that inflammation, when apparent, is present at time of rupture, given that overall rupture-to-delivery interval did not change inflammatory patterns. Alternatively, these findings may indicate that those women who develop significant placental inflammation may go into labor sooner than those without inflammation.
Distinct clustering of inflammatory patterns v. placental perfusion abnormalities have been found with other mechanisms of preterm delivery such as preterm labor without premature rupture, and preterm preeclampsia, as well as in extremely low gestational age newborns.16,26,32,42 We found a similar prevalence of subchorionic thrombi and funisitis in our cohort. Similar to previous studies, it was uncommon to see inflammation with thrombosis. In fact, the presence of subchorionic thrombi appears to be inversely correlated with inflammation. Despite this inverse correlation, 11% of the PPROM group had both umbilical cord inflammation and subchorionic thrombi present, suggesting a potential relationship between insufficient maternal placental perfusion and the fetal inflammatory response.43,44 Our data suggest that inflammation alone is not the sole trigger of PPROM. Furthermore, indicators of placental perfusion abnormalities such as subchorionic thrombi may be important in the pathogenesis of neonatal outcomes, such as in infants with atypical timing and presentation of periventricular hemorrhagic infarction.42 Our findings highlight the importance of needed studies dissecting the mechanisms of PPROM to properly inform interventions to prevent PPROM or the adverse associated sequelae. Therapeutic targets have traditionally focused on inflammation, but anti-inflammatory agents alone may not be adequate.
This study is unique in exclusively focusing on PPROM, rather than grouping preterm placentas of varying etiologies together. This study also examined the effect of ethnicity in patterns of PPROM placental pathology. A limitation of the study is that placentas were not matched on gestational age from other preterm cohorts. Additionally, maternal systemic vascular and/or autoimmune disease is underrepresented in our population. These factors may indeed predispose to increased risk of PPROM because of global inflammation and/or hypercoagulability that cannot be fully assessed within our present cohort. Finally, our cohort of pregnant women reside and deliver at altitude (1600 m/5280 ft), which may contribute to our increased prevalence of placental perfusion abnormalities (subchorionic thrombi), although our pathological findings differ from very high-altitude series.45 In fact, our thrombotic and inflammatory findings are similar to others cited in this manuscript, all at sea level, suggesting generalizability of our findings. These findings do suggest that PPROM is either caused by or can result in inflammation in the fetal compartment, insufficient maternal placental perfusion or both. Whether a multi-hit phenomenon of fetal inflammation combined with maternal malperfusion may portend poor neonatal outcomes is not known. Furthermore, maternal ethnicity may impart unknown genetic modifiers on placental inflammation, thrombosis, newborn disability and chronic disease. Future studies focused on this constellation of PPROM placental findings, genetic polymorphisms, neonatal outcomes and adult-onset diseases are needed.
Acknowledgment
The authors would like to thank Dr Anne Lynch for access to the Perinatal Research Database, Dr David Weitzenkamp for statistical support, Ms Lisa Litzenberger for photo preparation and Ms Whitney Hoover for aid in manuscript preparation.
Financial support
This research was funded by NIH BIRCWH K12 KD HDO57022 (J.A.W.), American Heart Association South-West Affiliates Clinical Research Grant 10CRP3670014 (J.A.W.), Colorado CCTSA Grant 5UL1RR025780 (J.A.W.) and CU Center for Women’s Health Research Junior Researcher Grant (J.A.W.).
Ethical Standards
This research was conducted in accordance with the 2004 Declaration of Helsinki, with signed informed consent required for study participation (Colorado Multiple Institution Review Board study 09–1107).
Footnotes
Conflicts of interest
None.
References
- 1.Thornburg KL, O’Tierney PF, Louey S. Review: the placenta is a programming agent for cardiovascular disease. Placenta. 2010;31(Suppl):S54–S59. doi: 10.1016/j.placenta.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The prenatal origins of lung cancer II. The placenta. Am J Hum Biol. 2010;22:512–516. doi: 10.1002/ajhb.21041. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kajantie E, Thornburg KL, Eriksson JG, Osmond C, Barker DJ. In preeclampsia, the placenta grows slowly along its minor axis. Int J Dev Biol. 2010;54:469–473. doi: 10.1387/ijdb.082833ek. [DOI] [PubMed] [Google Scholar]
- 5.Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- 6.Redline R. Cerebral palsy in term infants: a clinicopathologic analysis of 158 medicolegal case reviews. Pediatr Dev Pathol. 2008:456–464. doi: 10.2350/08-05-0468.1. [DOI] [PubMed] [Google Scholar]
- 7.Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51:749–762. doi: 10.1097/GRF.0b013e318187087c. [DOI] [PubMed] [Google Scholar]
- 8.Nelson KB, Dambrosia JM, Grether JK, Phillips TM. Neonatal cytokines and coagulation factors in children with cerebral palsy. Ann Neurol. 1998;44:665–675. doi: 10.1002/ana.410440413. [DOI] [PubMed] [Google Scholar]
- 9.Nelson KB, Willoughby RE. Infection, inflammation and the risk of cerebral palsy. Curr Opin Neurol. 2000;13:133–139. doi: 10.1097/00019052-200004000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. 2000;284:1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Escobar GJ, Grether JK, et al. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290:2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 13.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 14.Dammann O, Allred EN, Leviton A, et al. Fetal vasculitis in preterm newborns: interrelationships, modifiers, and antecedents. Placenta. 2004;25:788–796. doi: 10.1016/j.placenta.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Hansen AR, Collins MH, Genest D, et al. Very low birthweight placenta: clustering of morphologic characteristics. Pediatr Dev Pathol. 2000;3:431–438. doi: 10.1007/s100249910044. [DOI] [PubMed] [Google Scholar]
- 16.Hecht JL, Allred EN, Kliman HJ, et al. Histological characteristics of singleton placentas delivered before the 28th week of gestation. Pathology. 2008;40:372–376. doi: 10.1080/00313020802035865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillier SL, Martius J, Krohn M, et al. A case-control study of chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 18.Mueller-Heubach E, Rubinstein DN, Schwarz SS. Histologic chorioamnionitis and preterm delivery in different patient populations. Obstet Gynecol. 1990;75:622–626. [PubMed] [Google Scholar]
- 19.Rana A, Sawhney H, Gopalan S, Panigrahi D, Nijhawan R. Abruptio placentae and chorioamnionitis-microbiological and histologic correlation. Acta Obstet Gynecol Scand. 1999;78:363–366. [PubMed] [Google Scholar]
- 20.Guzick DS, Winn K. The association of chorioamnionitis with preterm delivery. Obstet Gynecol. 1985;65:11–16. [PubMed] [Google Scholar]
- 21.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstet Gynecol. 1982;59:539–545. [PubMed] [Google Scholar]
- 22.Ghidini A, Salafia CM, Minior VK. Lack of relationship between histologic chorioamnionitis and duration of the latency period in preterm rupture of membranes. J Matern Fetal Med. 1998;7:238–242. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<238::AID-MFM6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 24.Redline RW. Inflammatory response in acute chorioamnionitis. Semin Fetal Neonatal Med. 2012;17:20–25. doi: 10.1016/j.siny.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73(Pt 1):383–389. [PubMed] [Google Scholar]
- 26.Kramer MS, Chen MF, Roy I, et al. Intra- and interobserver agreement and statistical clustering of placental histopathologic features relevant to preterm birth. Am J Obstet Gynecol. 2006;195:1674–1679. doi: 10.1016/j.ajog.2006.03.095. [DOI] [PubMed] [Google Scholar]
- 27.Aziz N, Cheng YW, Caughey AB. Neonatal outcomes in the setting of preterm premature rupture of membranes complicated by chorioamnionitis. J Matern Fetal Neonatal Med. 2009;22:780–784. doi: 10.3109/14767050902922581. [DOI] [PubMed] [Google Scholar]
- 28.Pacora P, Chaiworapongsa T, Maymon E, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 29.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 30.Kim CJ, Yoon BH, Park SS, Kim MH, Chi JG. Acute funisitis of preterm but not term placentas is associated with severe fetal inflammatory response. Hum Pathol. 2001;32:623–629. doi: 10.1053/hupa.2001.24992. [DOI] [PubMed] [Google Scholar]
- 31.Salafia CM, Vogel CA, Vintzileos AM, et al. Placental pathologic findings in preterm birth. Am J Obstet Gynecol. 1991;165(Pt 1):934–938. doi: 10.1016/0002-9378(91)90443-u. [DOI] [PubMed] [Google Scholar]
- 32.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 33.Germain AM, Carvajal J, Sanchez M, et al. Preterm labor: placental pathology and clinical correlation. Obstet Gynecol. 1999;94:284–289. doi: 10.1016/s0029-7844(99)00324-5. [DOI] [PubMed] [Google Scholar]
- 34.Hessol NA, Fuentes-Afflick E. The perinatal advantage of Mexican-origin Latina women. Ann Epidemiol. 2000;10:516–523. doi: 10.1016/s1047-2797(00)00073-9. [DOI] [PubMed] [Google Scholar]
- 35.Wu YW, Xing G, Fuentes-Afflick E, et al. Racial, ethnic, and socioeconomic disparities in the prevalence of cerebral palsy. Pediatrics. 2011;127:e674–e681. doi: 10.1542/peds.2010-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaiworapongsa T, Romero R, Kim JC, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol. 2002;186:1178–1182. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 37.van Hoeven KH, Anyaegbunam A, Hochster H, et al. Clinical significance of increasing histologic severity of acute inflammation in the fetal membranes and umbilical cord. Pediatr Pathol Lab Med. 1996;16:731–744. [PubMed] [Google Scholar]
- 38.Katzman PJ, Metlay LA. Fetal inflammatory response is often present at early stages of intra-amniotic infection, and its distribution along cord is variable. Pediatr Dev Pathol. 2010;13:265–272. doi: 10.2350/09-02-0604-OA.1. [DOI] [PubMed] [Google Scholar]
- 39.Machin G. Funisitis and chorionic vasculitis: relation to chorioamnionitis, timing and scoring. Fetal Pediatr Pathol. 2011;30:414–430. doi: 10.3109/15513815.2011.618872. [DOI] [PubMed] [Google Scholar]
- 40.Salafia CM, Misra D, Miles JN. Methodologic issues in the study of the relationship between histologic indicators of intraamniotic infection and clinical outcomes. Placenta. 2009;30:988–993. doi: 10.1016/j.placenta.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sebire NJ, Goldin RD, Regan L. Histological chorioamnionitis in relation to clinical presentation at 14–40 weeks of gestation. J Obstet Gynaecol. 2001;21:242–245. doi: 10.1080/01443610120046323. [DOI] [PubMed] [Google Scholar]
- 42.Harteman JC, Nikkels PG, Kwee A, Groenendaal F, de Vries LS. Patterns of placental pathology in preterm infants with a periventricular haemorrhagic infarction: association with time of onset and clinical presentation. Placenta. 2012;33:839–844. doi: 10.1016/j.placenta.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Koeppen M, Eckle T, Eltzschig HK. The hypoxia-inflammation link and potential drug targets. Curr Opin Anaesthesiol. 2011;24:363–369. doi: 10.1097/ACO.0b013e32834873fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kovo M, Schreiber L, Ben-Haroush A, et al. The placental factor in spontaneous preterm labor with and without premature rupture of membranes. J Perinat Med. 2011;39:423–429. doi: 10.1515/jpm.2011.038. [DOI] [PubMed] [Google Scholar]
- 45.Khalid ME, Ali ME, Ali KZ. Full-term birth weight and placental morphology at high and low altitude. Int J Gynaecol Obstet. 1997;57:259–265. doi: 10.1016/s0020-7292(97)00067-2. [DOI] [PubMed] [Google Scholar]