Abstract
Opioid receptors have been targeted for the treatment of pain and related disorders for thousands of years, and remain the most widely used analgesics in the clinic. Mu (μ), kappa (κ), and delta (δ) opioid receptors represent the originally classified receptor subtypes, with opioid receptor like-1 (ORL1) being the least characterized. All four receptors are G-protein coupled, and activate inhibitory G-proteins. These receptors form homo- and hetereodimeric complexes, signal to kinase cascades, and scaffold a variety of proteins.
In this review, we discuss classical mechanisms and developments in understanding opioid tolerance, opioid receptor signaling, and highlight advances in opioid molecular pharmacology, behavioral pharmacology, and human genetics. We put into context how opioid receptor signaling leads to the modulation of behavior with the potential for therapeutic intervention. Finally, we conclude that there is a continued need for more translational work on opioid receptors in vivo.
Introduction
Opioids are the most widely used and effective analgesics for the treatment of pain and related disorders. Opiates have been used for thousands of years for the treatment of pain, and in the last century we have made huge strides in the development of opioids derived from naturally occurring opiates within the fields of receptor pharmacology and medicinal chemistry. In addition to pain, opioids are frequently used in the treatment of numerous other disorders including diarrhea, cough, post-operative pain and cancer (Table 1).
Table 1.
Organ system effects of morphine and its surrogates. The actions summarized in this table are observed for all clinically available opioid agonists
| Organ Systems |
Effects | Additional information |
|---|---|---|
| Central Nervous system |
↑ Analgesia | |
| ↑ Euphoria | leading to risk of addiction and abuse | |
| ↑Sedation | ||
| ↓ Rate of respiration | ||
| ↓ Cough reflex | Codeine used for treatment of pathological cough |
|
| ↑ Miosis-Constriction of the pupils | ||
| ↑ Truncal rigidity | Most apparent when using fentanyl, sufentanil, alfentanil |
|
| ↑ Nausea and vomiting | ||
| Peripheral |
Gastrointestinal system |
|
| ↑ Constipation | ||
| ↓ Gastric motility | ||
| ↓ Digestion in the small intestine | ||
| ↓ Peristaltic waves in the colon | ||
| ↑ Constriction of biliary smooth muscle | ||
| ↑ Esophageal reflux | ||
|
Other smooth muscle |
||
| ↑ Depression of renal function | ||
| ↓ Uterine tone | ||
| ↑ Urinary retention | ||
|
Skin |
||
| ↑ Itching and sweating | ||
| ↑ Flushing of the face, neck and thorax | ||
|
Cardiovascular system |
||
| ↓ Blood pressure and heart rate if cardiovascular system is stressed |
||
|
Immune System |
||
| ↓ Formation of rosettes by human lymphocytes | ||
| ↓ Cytotoxic activity of natural killer cells | ||
|
Other |
||
| Behavioral restlessness |
||
Opioid systems are critical in the modulation of pain behavior and antinociception. Opioid peptides and their receptors are expressed throughout the nociceptive neural circuitry in addition to critical regions of the central nervous system included in reward and emotion-related brain structures. To date, four different opioid receptor systems (Mu (μ),Delta (δ),Kappa (κ),opioid receptor like-1 (ORL1) and their genes have been characterized at cellular, molecular, and pharmacological levels (1).
The most commonly used opioids for pain management act on μ opioid receptor (MOR) systems (Figure 1). While μ opioids continue to be some of the most effective analgesics, they are also efficacious mood enhancers and cause activation of central dopamine reward pathways that modulate euphoria. These unwanted side effects have driven researchers at basic and clinical levels to actively pursue other opioid receptors as putative drug targets for pain relief (Table 1).
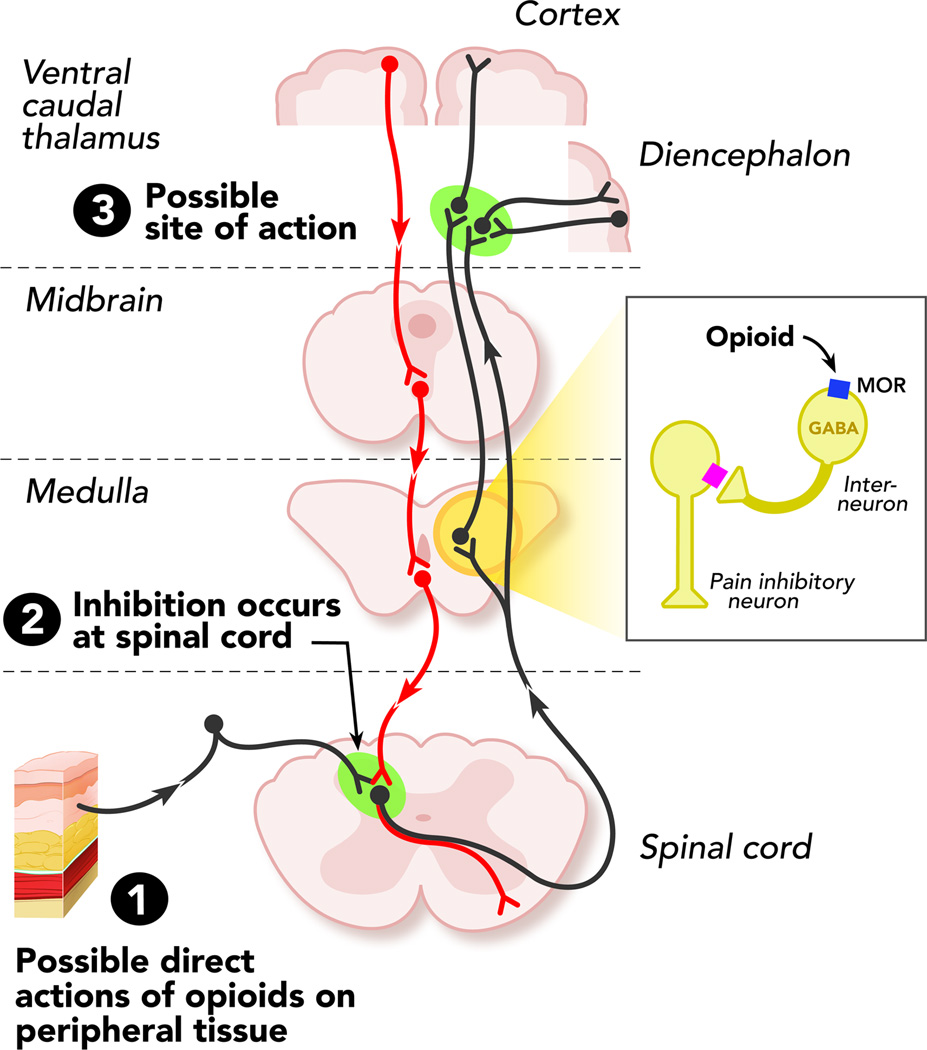
Figure 1. Sites of action of opioid analgesics.
The grey pathway shows the sites of action on the pain transmission pathway from periphery to central nervous system. The red pathway shows the actions on pain modulating neurons in the mid brain and medulla. Abrevations: MOR- μ opioid receptor
The opioid receptor subtypes were pharmacologically and genetically identified over two decades ago (1). From that point on numerous studies have implicated all four opioid receptors in an array of behavioral effects including: analgesia, reward, depression, anxiety, and addiction. In addition, all four receptor subtypes have been characterized at cellular levels with respect to the downstream signal transduction pathways they activate. However, there are fewer studies that have directly linked opioid signal transduction to behavioral events. One of the “holy grails” in opioid pharmacology research has been to identify pathway-specific opiate receptor agonists that could activate antinociceptive signaling, without causing μ agonist-mediated euphorigenic responses, or kappa agonist-mediated dysphoria (2; 3). Understanding the diversity of signaling at opioid receptors and how second messenger activation leads to modulation of pain and reward could reveal novel opioid receptor drug candidates.
In this review we will highlight the current status of in vitro molecular pharmacology at opioid receptors and also discuss many of the recent advances, which connect these molecular studies with opioid behavioral pharmacology. We will discuss the advances in opioid receptor pharmacology and highlight the connections between signaling at opioid receptors, tolerance to opioids, and behavioral responses. The review’s primary aim is to discuss recent efforts in understanding how opioid receptors mediate a diverse array of molecular/cellular responses whilst also modulating behaviors such as analgesia, reward, depression, and anxiety. We summarize the modern advances in opioid receptor signaling to mitogen-activated protein kinases (MAPK) and receptor protein-protein interaction networks and propose that there is a strong potential for selective ligand intervention at opioid receptors to treat a variety of central and peripheral nervous system disorders by using biased ligands and pathway selective pharmacology. Moreover, we will highlight how a greater connection between these advances at the molecular levels to behavioral pharmacology is imperative to fully understand the field of opioid pharmacology.
Opioid tolerance in the clinic
Prior to developing a detailed understanding of the molecular and cellular actions of opioid receptors it is important to consider their general effects, and those observed in daily clinical settings. Different potencies of opiate drug formulations have been effective in the treatment of a variety of acute, chronic and cancer related pain disorders. The clinical utility of opioids continues to be limited by a compromise between efficacy and side effects. The most common side effects of opiates can be divided into peripheral effects (constipation, urinary retention, hives, bronchospasm) and central effects (nausea, sedation, respiratory depression, hypotension, miosis, cough suppression), all of which seriously affect their clinical utility and the patients quality of life (4; 5) (Table 1). There have been many attempts to develop better opioid drugs but this has been largely unsuccessful due to our incomplete understanding about the development of tolerance to the analgesic effects (6).
Opioid tolerance is typically defined in the clinic as the need to increase a dose to maintain the analgesic affects. This increase in dose however, can exacerbate the perpetual problem of the side effects mentioned above. This continual cycle of insufficient analgesia and side effects is among the greatest challenges of using opioids in the clinic. Because of these limitations, opioid tolerance can ultimately lead to low patient compliance and treatment discontinuation. These clinical problems further highlight the continued need for a better understanding of the molecular and pharmacological mechanisms of opioid receptor tolerance, regulation, and signal transduction.
Classical Opioid Receptor Signaling
Opioid receptors are expressed in pain-modulating descending pathways, which include the medulla locus coeruleus, and periaqueductal gray area. They are also expressed in limbic, midbrain, and cortical structures (Figure 1). The activation of opioid receptors at these locations directly inhibit neurons, which in turn inhibit spinal cord pain transmission(4;5) The process by which these receptors engage in disinhibition is mostly understood with respect to analgesia, however research is still active in this area because investigators continue to unravel novel modulatory mechanisms in these opioid circuits.
All four opioid receptors are 7-transmembrane spanning proteins that couple to inhibitory G-proteins. Following activation by an agonist, such as the endogenous μ-opioid peptide endorphin, or exogenous agonists like morphine and fentanyl the Gα and Gβγ subunits dissociate from one another and subsequently act on various intracellular effector pathways (7; 8). Early work in opioid receptor pharmacology demonstrated that guanine nucleotides such as guanosine triphosphate (GTP) modulate agonist binding to opioid receptors in membrane preparations from brain tissue. It was later determined that GTPase activity is stimulated by opioid agonists and endogenous opioid peptides (9). Agonist stimulation of opioid receptors was also shown to inhibit cyclic adenosine monophosphate (AMP) production in a similar way to other types of G-protein coupled receptors (GPCR) (10). Using pertussis toxin to selectively adenosine diphosphate (ADP)-ribosylate the G-protein, the inhibitory function of opioid receptors on cAMP signaling was found to be Gαi dependent (11; 12). Today it is widely accepted that all four opioid receptor types couple to pertussis toxin sensitive G-proteins including Gαi, to cause inhibition of cAMP formation.
The classical and perhaps most important aspect of opioid receptor signal transduction relates to their ability to modulate calcium and potassium ion channels (Figure 2). Following Gαi dissociation from Gβγ, the Gα protein subunit moves on to directly interact with the G-protein gated inward rectifying potassium channel, Kir3. Channel deactivation happens following GTP to guanosine diphosphate (GDP) hydrolysis and Gβγ removal from interaction with the channel (13–15). This process causes cellular hyperpolarization and inhibits tonic neural activity. In several reports, the inhibitory effects of opioids on neural excitability were shown to be mediated by interactions of opioid receptors with G protein-regulated inwardly rectifying potassium channel (Kir3) (16; 17).
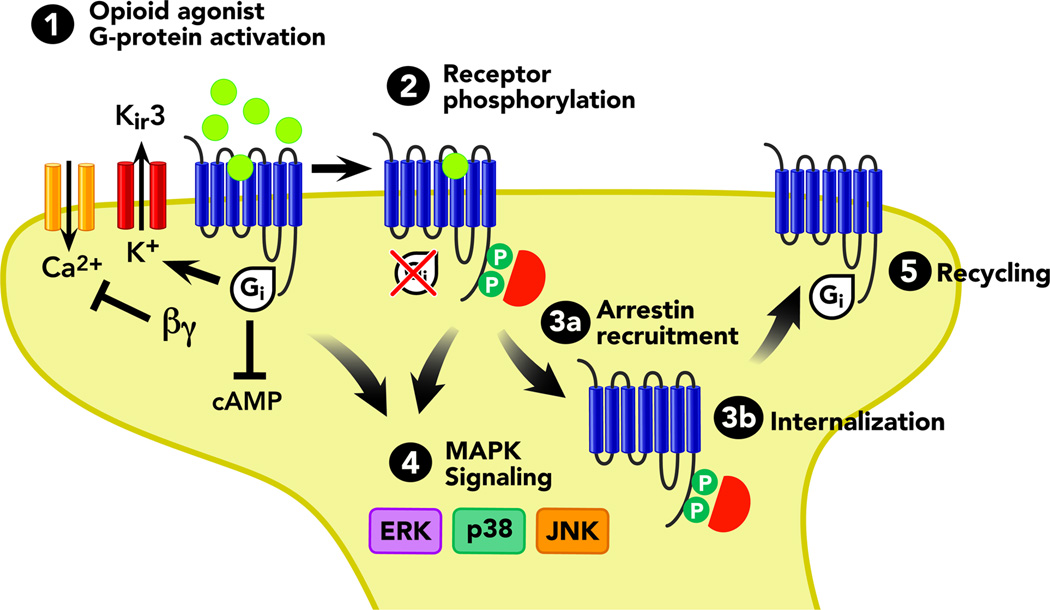
Figure 2. Summary of Opioid Receptor Signaling.
Cartoon depicting opioid receptor signal transduction and trafficking. In general, all four opioid receptor subtypes, μ, δ, κ, and ORL1 share these common pathways. New research indicates that selective-ligands at each opioid receptor can direct opioid receptors to favor one or more of these signaling events (biased agonism, or ligand directed signaling). Arrows refer to activation steps; T lines refer to blockade or inhibition of function. Abbreviations: α G-protein alphai subunit, arrestin phosphorylation- dependent GPCR scaffold, βγ- G-protein beta-gamma subunit, cAMP- cyclic adenosine monophosphate, ERK ½ - extra-cellular signal- regulated kinase, JNK - c-Jun N-terminal kinase, p38- p38 MAPK, P- phosphorylation
When activated, all four opioid receptors cause a reduction in Ca+2 currents that are sensitive to P/Q-type, N-type, and L-type channel blockers (18). Opioid receptor induced inhibition of calcium conductance is mediated by binding of the dissociated Gβγ subunit directly to the channel. This binding event is thought to reduce voltage activation of channel pore opening (19; 20). Numerous reports have shown that opioid receptors interact with and modulate Ca+2 channels; this has led to the further examination of specific Ca+2 channel subunits that may be involved in opioid receptor modulation. For instance, it was reported that μ-opioid receptor stimulation results in G-protein dependent inhibition of α1A and α1B subunits (21).
It is also clear that the acute administration of opioid agonists reduces Ca+2 content in synaptic vesicles and synaptosomes, with compensatory upregulation of vesicular Ca+2 content during the development of opiate tolerance (22; 23). In addition, since the activation of μ-, δ, and κ opioid receptors inhibits adenylyl cyclase activity the cAMP-dependent Ca+2 influx is also reduced. The evidence for opioid receptors positively coupling to potassium channels while negatively modulating calcium channels has been reported in numerous model systems and cell types. For many years this was thought to be the primary action of opioid receptors in the nervous system. This coupling of opioid receptors to potassium and calcium channels has been demonstrated in a wide range of systems, from neurons in the hippocampus, locus coeruleus, and ventral tegmental area to the dorsal root ganglia, supporting the notion that these channels are highly conserved opioid receptor substrates and represent one of the most important targets for opioid receptor modulation. Newer findings, which we will highlight later in this review (see below, opioid receptor regulation), suggest that while opioid receptors have potent effects on ion channel modulation, they also have slower yet robust effects on other signal transduction pathways.
Molecular Mechanisms of Opioid Tolerance
To date, the molecular and cellular mechanisms mediating the development of tolerance to morphine remain controversial. Traditionally it has been thought that the downregulation of opioid receptors following chronic agonist exposure induces tolerance, as reported in in vitro studies (24; 25). However, recent in vivo studies show that downregulation does not occur consistently with each and every agonist and may not completely explain tolerance. In light of these findings, it has been suggested that MOR proteins are in fact not downregulated but instead may be desensitized and uncoupled from downstream signaling pathways (26). It has been observed that following chronic morphine exposure levels of the second messenger cAMP, are elevated. This elevation in cAMP, however, may not be due to opioid receptor uncoupling from inhibitory G-proteins but instead could likely reflect cellular adaptive changes including the upregulation of adenylyl cyclase, protein kinase A (PKA) and cAMP response element binding protein (CREB) (27). It is this ineffective regulation of cAMP by morphine that is believed by some groups to induce tolerance.
It has also been proposed that the regulation of opioid receptors by endocytosis reduces the development of tolerance and therefore serves a protective role (28; 29). Following endocytosis the cellular response is desensitized to the μ agonist but the receptors can be recycled to the cell surface in an active state, resensitizing the receptor to the agonist. Morphine-activated opioid receptors signal for long periods of time thereby enhancing the production of cAMP, thought to result in tolerance. In vivo studies have shown that facilitation of MOR endocytosis in response to morphine prevents the development of morphine tolerance (28). In addition, it has been shown in vivo that the lack of β-arrestin 2 prevents the desensitization of MOR after chronic morphine treatment and these mice also failed to develop antinociceptive tolerance (30).
Recent studies have identified how ligand-directed responses, more commonly known as biased agonism, are crucial in understanding the complexity of opioid-induced tolerance. The work of Bohn and colleagues showed how β arrestin 1 and β arrestin 2 differentially mediate the regulation of MOR. β arrestins are required for internalization but only β arrestin 2 can rescue morphine-induced MOR internalization whereas both β arrestin 1 and β arrestin 2 can rescue [d-Ala2,N-Me-Phe4,Gly-ol5]enkephalin (DAMGO)-induced MOR internalization (31). These findings suggest that MOR regulation is dependent on the agonist and maybe critical in understanding the mechanism involved in the development of tolerance. Melief et al (2011) further showed how acute analgesic tolerance to morphine is blocked by c-Jun N-terminal kinase (JNK) inhibition but not G protein receptor kinase 3 (GRK-3) knockout. In contrast, using a second class of μ agonists (fentanyl, methadone and oxycodone), acute analgesic tolerance was blocked in GRK-3 knockout but not JNK inhibition (32). Ligand-biased responses are well documented in vitro but less so in vivo, however a recent study addressed biased agonism at DOR in vivo showing that DOR agonists with similar binding and analgesic properties but different internalization potencies lead to the development of differential tolerance at DOR (33). These findings highlight the important implications of ligand-selective responses in GPCR biology (33) and further implicate the need for further work to examine the role and consequences of biased signaling in behavioral models.
Opioid Receptor Regulation
Agonist-induced receptor phosphorylation is believed to be one of the many critical molecular components of opioid tolerance. This process is well established in the GPCR literature and typically occurs following chronic agonist exposure, or sustained release of endogenous opioid peptides. Sustained opioid treatment produces tolerance to the acute effects of the drug and can potentially lead to physical and psychological dependence. As a result of this problem, opioid-receptor trafficking, desensitization, and phosphorylation have been extensively examined (for a detailed review see 34). Here we will highlight the key findings in this area as they connect potential signaling to tolerance mechanisms.
MOR
One common thread between the opioid receptor subtypes is the interesting observation that receptor trafficking and regulation vary depending upon the agonist. For example, morphine is unable to promote receptor internalization in contrast to DAMGO, which causes robust internalization (32; 35; 36). It is thought that morphine tolerance, a major problem in the clinic, is perhaps mediated by these differences in receptor regulatory activity. Several groups are actively working to discern the various mechanisms for the differences in ligand-dependent MOR regulation. The field remains controversial with some groups hypothesizing that MOR internalization does not actually uncouple the receptor from signal transduction pathways, but instead induces recycling of uncoupled receptors to the plasma membrane. Alternatively, the morphine bound receptor, while not internalized may still signal at the cell membrane and because signaling is never attenuated the cellular machinery adapts to produce tolerance. A recent study has shown that morphine acts as a “collateral agonist” to promote receptor-G-protein uncoupling (“jamming”) and JNK activation (see MAPK section), while fentanyl and DAMGO internalize and desensitize normally (35). It is plausible that many processes work together to produce receptor regulation and opioid tolerance, and further study is warranted to continue to decipher these discrepancies.
MORs contain more than 15 serine, threonine, and tyrosine residues that are accessible to protein kinases, which phosphorylate the receptor. All three intracellular loops and the carboxyl terminal tail contain these sites (37). 32P incorporation experiments have been critical to our understanding of MOR receptor phosphorylation and together with site-directed mutagenesis we now have a clear understanding of the key residues involved in MOR phosphorylation. Rat MORs are phosphorylated at Ser375 in the carboxy terminus (38; 39) and treatment with both morphine and DAMGO cause robust phosphorylation of this residue. However, some reports have suggested that morphine and DAMGO induce different degrees of phosphorylation of Ser375 (39), suggesting that Ser375 may not be the only amino-acid residue phosphorylated and responsible for MOR regulation. The highly conserved GPCR “DRY motif” in the second cytoplasmic loop of the μ-opioid receptor has been implicated in regulation of agonist efficacy. Phosphorylation of Tyr166 reduced the efficacy of DAMGO mediated G-protein activation (40). It has also been very recently shown that agonist-selective differences in MOR regulation are in fact determined not only by net incorporation of phosphates into the receptor population as a whole but by individual receptors achieving a critical number of phosphorylated residues (multiphosphorylation) in a specific region of the C-tail (41). Furthermore, this group identified that mulitphosphorylation specifically involves the 375STANT379 motif required for the efficient endocytosis of MOR. These ligand-mediated differences further highlight the ligand-dependent nature of opioid receptor function, and require further study in vivo.
κ opioid receptors (KOR)
KOR trafficking shares some common features with MOR regulation as it is readily phosphorylated, desensitized and internalized. KOR is phosphorylated, desensitized, and internalized by the agonists U50,488 and dynorphin 1–17, but not by other agonists such as etorphine or levorphanol (42; 43). Both dynorphin A and B have been shown to initiate significant receptor internalization in human KORs and three structurally distinct KOR ligands: Salvinorin A, TRK820, and 3FLB were shown to induce KOR internalization with varying rank orders of potency (44). There have been conflicting data in agonist-induced KOR internalization that seems dependent on the cell line, receptor species, and model system used. In Chinese hamster ovary (CHO) cells expressing KOR, the selective KOR agonists U50,488 and U69,593 did not cause robust receptor internalization (45); however in mouse pituitary tumor (AtT20) cells and human embryonic kidney (HEK293) cells U50,488 initiates strong internalization of KOR-GFP receptor proteins (46–48). Despite this, several groups have found consistency in the ability of KOR to become phosphorylated, internalized, and desensitized by its endogenous opioid peptide dynorphin.
δ opioid receptors (DOR)
In contrast to MOR and KOR, DOR were thought to primarily (>90%) exist at intracellular cites (49–51) until recently when mice expressing fluorescently tagged DOR revealed that there was strong membrane localization of DORs in vivo (52). The reasons for this discrepancy between the numerous studies showing intracellular DOR labeling and membrane labeling remain unclear and continue to be controversial. It is plausible that earlier studies using DOR antibodies were flawed due to antibody specificity issues despite the fact that numerous controls were conducted to ensure specificity. DORs tagged with green fluorescent protein, whilst a powerful in vivo tool also require careful interpretation given that GFP is a large protein that may interfere with the typical DOR trafficking machinery. Further investigation is required in both cases, and it is plausible that both concepts are indeed true; the levels of DOR expressed on the cell surface may well be higher than originally hypothesized, yet a large intracellular pool of DOR protein remains. Nevertheless, DOR seems to be a dynamic opioid receptor that can readily traffic in response to agonists. Some reports have shown that chronic morphine treatment promotes movement of DORs to the cell surface in the dorsal horn of the rat spinal cord (49). This effect was dependent on MOR receptor activity, since blocking or deleting MOR genetically (MOR knockout) prevents the effect.
Like MOR and KOR, desensitization of DOR is controlled via phosphorylation, following recruitment of arrestins and sequestration of arrestin-bound receptors (53; 54). Phosphorylation of DOR has been shown with both small molecule organic ligands and peptide treatments. Once again, c-terminal phosphorylation was shown to be critical for opioid receptor regulation. In DOR, the Ser363 residue is the key phosphorylation site (55; 56). This phosphorylation event was shown to be mediated by G- protein coupled receptor kinase 2 (GRK2) (56; 57). Other studies have demonstrated that other amino-acid residues are involved in DOR regulation. For example, Thr353 was found to be important for [D-Ala2, D-Leu5]-Enkephalin (DADLE-mediated down-regulation of DOR, and Leu245 and 246 act as lysosomal targeting motifs that partake in determining agonist-bound DOR localization (58; 59). Furthermore, ligand-specific variability in agonist-dependent DOR phosphorylation has been observed with potential differences between SCN80- and [D-Pen2,5]Enkephalin (DPDPE)-bound conformations recruiting kinases with various efficacies and potencies (60).
Opioid Receptor Like 1
ORL1 receptors (also called nociceptin, or orphaninFQ receptors) are the youngest members of the opioid receptor family and few groups have examined their regulatory properties. Agonist-induced internalization of ORL1 is rapid and concentration dependent (61). Both the endogenous agonist nociceptin and small molecule selective ORL1 agonist Ro646198 promote rapid internalization of ORL1. Agonist challenge also reduces the ability of ORL1 to couple to inhibition of forskolin-stimulated cAMP production, suggesting that ORL1 undergoes similar desensitization mechanisms as compared with the other three opioid receptors subtypes. ORL1 internalization appears to be more rapid than the other opioid receptors with some groups reporting internalization after only two minutes of agonist exposure in CHO cells (61). However, this appears to be dependent on ligand type and cell line expression as ORL1 internalization in human neuroblastoma cells was slower and occurred closer to a thirty minute time point (62). Interestingly, ORL1 receptors were recently demonstrated to co-internalize with N-type Cav2.2 channels following a 30-minute agonist treatment (63). The internalization of the entire signaling complex is not unusual in GPCRs, however the effect in the case of ORL1 is particularly pronounced and it is believed to play a major role in how ORL1 selectively removes N-type calcium channels from the plasma membrane to inhibit calcium influx.
ORL1 receptor regulation, while increasingly studied, is still in the infant stages of understanding when compared to the other three opioid receptor subtypes. To date few site-directed mutagenesis studies have been conducted, and receptor regulation in primary neurons, dorsal root ganglion, or dorsal horn neurons remains unknown. As we move forward in understanding opioid receptor signaling and identify novel opioid receptor targets, ORL1 receptors become likely candidates for the future of opioid pharmacology.
Opioid Receptors and Arrestin Recruitment
Phosphorylation by GRK 2 or 3 of μ,δ, and κ opioid receptors leads to arrestin 2/3 recruitment. Arrestin molecules are key proteins that bind phosphorylated GPCRs to regulate their desensitization, sequestration, sorting and ultimately assist in determining receptor fate. Opioid receptors are regulated by arrestin-2 and arrestin-3 binding (also called β-arrestin 1 and β-arrestin 2, respectively) and this interaction depends on the model system and agonist treatment procedure. Mice lacking arrestin-3 have been shown to have a reduced tolerance to μ-opioids such as morphine, suggesting that MOR regulation requires arrestin-3 (30; 64).
Using surface plasmon resonance methods, glutathione s-transferase (GST)-pull down assays, and classical immmunoprecipitation methods, the C-terminal tail of DOR, MOR, KOR have been shown to be crucial for arrestin 2/3 binding. C-terminal carboxyl mutant opioid receptors have been widely studied and these serine mutant receptors show decreased agonist-induced receptor internalization and arrestin recruitment. Dominant positive arrestins (such as Arrestin-2-R169E or Arrestin-3-R170E) that bind the non-phosphorylated receptors can rescue serine-mutants MOR, DOR, KOR internalization (48; 65) further implicating arrestin dependence in opioid receptor trafficking. A large majority of studies implicating arrestin have been conducted in heterologous expression systems using overexpressed arrestins and opioid receptor subtypes. These conditions are atypical and do not represent the likely physiological state of opioid receptors and arrestins in vivo, so these data should be interpreted with caution and future studies using in vivo approaches are necessary to further our understanding of arrestin-opioid interactions.
MAPK signaling at opioid receptors
In the discussion above, we highlighted that sustained agonist treatment causes GRK phosphorylation at the carboxyl-terminal domain of opioid receptors activating arrestin-dependent receptor desensitization and internalization (Figure 2). Over the last several years, GPCR research has discovered that the phosphorylated arrestin-bound GPCR complex is not simply inactive, but that it recruits alternate signal transduction cascades, including MAPKs (66). The merging of our prior knowledge regarding opioid receptor phosphorylation, arrestin, and cellular mechanisms of tolerance with an understanding of opioid receptor signaling to MAPKs is becoming more appreciated (Table 2).
Table 2.
A summary of current opioid receptor-dependent signaling
| Receptor | Cascade/Signaling pathway | Model | Reference |
|---|---|---|---|
| Mu Opioid Receptor | ↑ERK 1/2 (GRK3 and arrestin dependent) | In vivo (Murine) | Macey et al. 2006 (71) |
| ↑ERK 1/2 (arestin dependent) | Astrocyctes | Miyatake et al. 2009 (72) | |
| ↓ERK 1/2 (Chronic activation) | Astrocyctes | Ikeda et al. 2010 (76) | |
| ↑JNK 2 (PKC dependent) | In vivo and HEK293 | Melief et al. 2010 (168) | |
| Tan et al. 2009 (97) | |||
| ↑Stat3 Phosphorylation | In vivo (Murine) and CMT- 93 |
Goldsmith et al 2011 (169) | |
| Kappa Opioid Receptor | ↑ERK1/2 | Astrocytes | Belcheva et al. 2005 (170) |
| In vivo | Bruchas et al. 2006 (48) | ||
| McLennan et al. 2008 (171) | |||
| Bruchas et al. 2008 (172) | |||
| Potter et al 2011 (148) | |||
| ↑p38 MAPK (Dependent on GRK3 and arrestin) | Striatal neurons | Bruchas et al. 2006 (173) | |
| Astrocytes | Bruchas et al. 2007 (96) | ||
| In vivo | Xu et al. 2007 (93), | ||
| Bruchas et al. 2011 (150) | |||
| ↑ JNK 1 | In vivo | Melief et al. 2010 (168) | |
| Melief et al. 2011 (32) | |||
| ↑JAK2/STAT3 and IRF2 signaling cascade | PBMCs | Finley et al. 2011 (174) | |
| Delta Opioid Receptor | ↑ERK 1/2 | HEK293 | Eisinger et al. 2009(175) |
| Eisinger et al. 2004 (176) | |||
| Audet et al. 2005 (177) | |||
| ↑ERK 1/2 (Integrin stimulated EGFR mediated) | HEK293 | Eisinger et al. 2008 (79) | |
| ↑ERK 1/2 (Integrin stimulated Trk1 mediated) | NG108-15 | Eisinger et al. 2008 (79) | |
| ↑ PI3K/AKT/ ↓GSK-3β | DOR transfected CHO cells | Olianas et al. 2011a (178) | |
| Rat Nac | |||
| NG108-15 | |||
| NG108-15 | Heiss et al. 2009 (179) | ||
| ↑PI3K/↓GSK-3β (SRC and AMPK dependent) | DOR transfected CHO cells | Olianas et al. 2011b (180) | |
| ↑PI3K (SRC and IGF-1 Dependent) | DOR transfected CHO cells | Olianas et al. 2011c (181) | |
| ↑JNK (AKT dependent Pi3K mediated) | T cells | Shahabi et al. 2006 (89) | |
| ORL-1 | ↑ERK 1/2 | Neuro-2a cells | Harrison et al. 2010 (24) |
| Rats Nac | Chen et al. 2008 (83) | ||
| In vivo (Porcine) | Ross et al. 2005(182) | ||
| ↑p38 MAPK | NG108-15 | Zhang et al. 1999 (98) | |
| ↑JNK | COS7 and NG108-15 | Chan et al. 2000 (183) | |
| In vivo | Ross et al. 2005 (182) | ||
↑= Activation, ↓= Deactivation
Abbreviations: AKT- Serine\threonine protein kinase; CHO- Chinese hamster ovary cells; CMT-93- Mouse rectum carcinoma cells; COS7- Monkey kidney fibroblast cell line ERK1 and 2- Extracellular signal-regulated kinases 1 and 2; HEK293- human embryonic kidney cells; IGF-1- Insulin-like growth factor 1; IRF2- Interferon regulatory factor 2; JAK2- Janus kinase 2; JNK 1 and 2- c-Jun N-terminal kinase; MAPK- p38 mitogen activated protein kinase; NG 108-15- Neuroblastoma glioma hybrid cell line; p38 STAT3- Signal transducer and activator transcription 3; PBMC- Peripheral blood mononuclear cell; PI3K- Phosphoinositide 3-kinase; SRC- Proto-oncogene tyrosine-protein kinase
MAPK pathways are diverse signaling cassettes that govern cellular responses including: cell proliferation, differentiation, apoptosis, transcription factor regulation, channel phosphorylation, and protein scaffolding (67). The MAPK family is composed of 12 to 15 gene products with the most well described forms including extracellular signal-regulated kinases 1 and 2 (ERK1/2), JNK1–3, and p38 (α,β,γ,δ) stress kinase. The MAPKs are distinct in that they have the capacity to respond to a variety of stimuli and transmit a diverse array of intra- and extracellular signals (68). MAPK signaling is regulated by the kinetics of activation, nearby phosphatase activity, and the cellular domain they occupy (67). Initially, ERK MAPKs were shown to require receptor tyrosine kinase transactivation, through epidermal growth factor (EGF) or brain derived neurotrophic factor (BDNF, also called TrkB receptors) (69). Later, reports surfaced that directly linked GPCRs to activation of MAPK signaling pathways, and now most if not all GPCRs have been found to couple to this pathway.
ERK 1/2 signaling at opioid receptors
The most frequently examined opioid-induced MAPK cascade is ERK 1/2. Coscia and colleagues have been crucial in developing our understanding of the relationship between opioid receptors and ERK 1/2 signaling. In one of the initial studies, MOR and KOR stimulation was demonstrated to initiate ERK 1/2 phosphorylation in astrocyte cultures and transfected cell lines (70). The kinetics of ERK 1/2 phosphorylation by MOR and KOR systems vary, yet both receptors can activate ERK 1/2 within 5–10 minutes. MOR mediated ERK 1/2 phosphorylation requires phosphokinase C (PKCɛ) activity and MOR dependent ERK 1/2 signaling requires GRK3 and arrestin in primary neurons, glial cells and heterologous expression systems (71–73). The downstream substrates of MOR-mediated ERK 1/2 have been defined in some cases, and remain unknown in others. In embryonic stem cells, MOR-dependent ERK 1/2 signaling positively modulates and directs neural progenitor cell fate decisions (74; 75). However, in astrocytes chronic morphine can negatively regulate ERK 1/2 signaling by tyrosine kinase pathways to ultimately inhibit neurite outgrowth and synapse formation (76). Most studies use MAPK/ERK (MEK) inhibitors (the proximal upstream kinase) to determine substrates of ERK 1/2 signaling in GPCRs, however few reports have shown direct interaction between μ-opioid-induced ERK and a final substrate. (The in vivo implications of MOR-dependent ERK signaling will be explored in the section opioid signaling and behavior, page 27.) Several groups are now investigating the potential for ligand-specific ERK agonists at opioid receptors.
DOR have also been shown to activate ERK 1/2 through Gβγ and Ras signaling cascades (70) and do not necessarily require receptor internalization or receptor phosphorylation for signaling (77; 78). DOR-mediated ERK signaling was recently found to require integrin signal transduction through transactivation of epidermal growth factor receptor (EGFR) receptor pathways. DOR-mediated EGFR activation also initiated phospholipase C (PLC) signaling to stimulate ERK 1/2 phosphorylation (79). DOR-dependent ERK 1/2 signaling requires further investigation, because coupled with DOR’s critical role in pain and mood regulation, ERK signaling through DOR may reveal a novel mechanism for DOR regulation of neural activity.
KOR-dependent ERK 1/2 phosphorylation occurs in a multi-phase manner with an early period of activity between 5–15 minutes after agonist exposure, and a late phase following 2 hours of agonist treatment. Similar to other GPCRs (80), the bi-phasic ERK 1/2 activation for KOR contains an arrestin-dependent late phase (81) and an arrestin-independent early phase. This group identified Gβγ as a crucial mediator in the early phase ERK 1/2 activation by KOR, and showed that arrestin3 is required for late phase ERK 1/2. KORs activate ERK 1/2 through PI3-kinase, PKCζ, and intracellular calcium (82). Like MOR and DOR however, the substrate for KOR-mediated ERK 1/2 has not been identified, although a recent study suggests that KOR-induced ERK 1/2 also directs stem cell fate towards neural progenitor development. ORL1-receptor dependent ERK 1/2 activation has not been extensively examined, although one group has shown that ORL1 receptor activation does initiate ERK 1/2 phosphorylation (83). The signaling pathways for ORL1-mediated ERK 1/2 phosphorylation in neuronal cell types and in vivo is in active need of further investigation.
c-Jun N-terminal kinase
The JNK pathway is activated by environmental triggers including stress, inflammation, cytokine activation, and neuropathic pain (84; 85). Classically JNK activity can result in transactivation of c-Jun, a component of the activator protein-1 (AP-1) transcription factor complex and JNK phosphorylation is caused by cytokines including Tumor Necrosis Factor (TNF) and interleukin-1β (86). JNK activation has also been implicated in numerous other signaling cascades. JNK is typically activated by Ras-related GTP binding proteins in the Rho family (87). JNK activation by GPCRs and opioid receptors has not been thoroughly examined but has been demonstrated for all the opioid receptor subtypes. Like ERK 1/2, arrestin2 and arrestin3 have been reported to scaffold JNK signaling complexes, and it is believed that arrestin3, has JNK3 specificity although this remains controversial (88). The cellular mechanisms of arrestin-dependent JNK at GPCRs remain unresolved.
Opioid-dependent JNK has been demonstrated by only a few groups. DOR causes protein kinase B (Akt)-dependent JNK phosphorylation through a PI3-kinase mechanism (89), and JNK activity is PI3-kinase independent in others (90). PI3-kinase is required for μ-opioid-dependent JNK activation. In contrast, U50,488-induced (KOR) JNK activation has been shown to be independent of PI3-kinase (90). The substrates and in vivo effects of opioid-induced JNK activation are under active investigation by several groups. KOR (U50,488, dynorphin) agonists activate JNK in a pertussin toxin-sensitive (Gαi) manner (35; 90; 91). U50,488 mediated JNK requires focal adhesion kinases and the GTPase Rac in immune cell types. MOR-induced JNK activation was recently shown to require PKC activity (35).
In two recent studies KOR and MOR-induced JNK phosphorylation by norbinaltorphimine and morphine, were shown to act as “collateral agonists” to cause JNK phosphorylation and initiate uncoupling of the G-protein to block Gαi-mediated transduction (35; 91). The persistent actions of norBNI on KOR-agonist mediated analgesia (21 days) were shown to require JNK, as JNK1 isoform knockout mice show an absence of norbinaltorphimine-dependent 21 day KOR blockade, and selective JNK inhibitors prevented the long-lasting norBNI effect. It was also recently identified that the long duration of action of small molecule KOR antagonists in vivo is determined by their efficacy in activating JNK1. The persistent KOR inactivation by these small molecule collateral agonists did not require sustained JNK phosphorylation (32) implicating intermediate protein(s) or alternate JNK substrates in this process. In contrast, acute morphine tolerance was shown to require JNK2, as JNK2 knockout mice showed an absence of MOR inactivation. How ligand-dependent JNK activation causes receptor uncoupling from Gαi signaling remains unresolved and proteomic biochemical approaches will need to be utilized to identify ligand-dependent protein interactions. Together, this work highlights the remarkable nature of opioid receptor sensitivity to a variety of ligand-stabilizing conformations.
p38 MAPK
The p38 MAPK pathway also plays a key role in environmental stress and inflammation, and is activated by cytokine production (92). In glial cells particularly, p38 MAPK activity is required for an array of cellular responses including: interleukin-6 and interleukin-1β production, inhibitory nitric oxide synthase (iNOS) activity, and TNFα secretion. Activation of p38 MAPK is involved in proliferative and chemotactic responses in some systems and has been shown to play a major role in neuropathic pain responses (93; 94).
Opioid receptor-mediated p38 phosphorylation has been most widely demonstrated for MOR/KOR systems. KOR-induced p38 MAPK has been observed in heterologous expression systems, striatal neurons, astrocytes, and in vivo (2; 3; 8; 93; 95; 96). KOR-mediated p38 MAPK activation requires serine 369 phosphorylation by GRK3 and arrestin3 recruitment (48; 96). μ-opioid receptor internalization has recently been shown to require Rab5 signaling and p38 MAPK. This process seems to be ligand dependent since morphine will not cause p38-dependent receptor internalization, but DAMGO will readily cause internalization (97). Furthermore, μ-opioid receptor cross-regulation of α2A–adrenergic receptors has been shown to require p38 MAPK, and p38 MAPK inhibition blocks DAMGO-induced MOR internalization (97). This cross-activity between MOR and α2A–adrenergic receptors requires arrestin3, suggesting that arrestin3 scaffolding of p38 is likely to be conserved across opioid receptors. To date, there are few studies identifying a role for DOR or ORL1 in mediating p38 phosphorylation. In one report, both ORL1 and DOR were shown to cause p38 phosphorylation through activation of protein kinase A and protein kinase C (98). Opioid-induced p38 has several potential targets including modulation of ion channels and transcription factors. Recently the potassium channel Kir3.1 was demonstrated to become tyrosine phosphorylated via KOR-dependent p38 MAPK Src activation (99). How p38 specifically interacts with various substrates will be an interesting next step and will reveal how such a ubiquitously expressed kinase can selectively modulate the large variety of cellular events.
Protein-Protein Networks and Opioid Receptors
In addition to intracellular signaling and receptor modification by phosphorylation, newer biochemical studies strongly suggest that opioid receptors interact with one another, alternate GPCRs, and a whole host of anchoring and membrane protein sets. These interactions are becoming increasingly appreciated as critical to the ultimate functional role of the opioid receptor families. In many ways, the field of protein-protein interactions is at the forefront of opioid receptor molecular pharmacology, as research moves from earlier work in heterologous expression systems to in vivo approaches.
Opioid Dimerization
Numerous reports have demonstrated that GPCRs exist as dynamic protein complexes with large interactions between proteins and other receptor types. Several studies have shown that GPCRs can form dimers and oligomers. This oligomerization includes two varieties: homodimers (same receptor) and heterodimers (different receptor type) (Figure 3). The existence of these GPCR homomers and heteromers has been shown in transfected cell line systems, cell lines, and primary cultures and in some cases in vivo (for review see Rios et al., 2001; Prinster et al., 2005) (100; 101). Despite the fact that GPCR oligomerization remains controversial, it continues to generate interest, as opioid receptor dimers may reveal novel targets for the development of new opioid drugs.
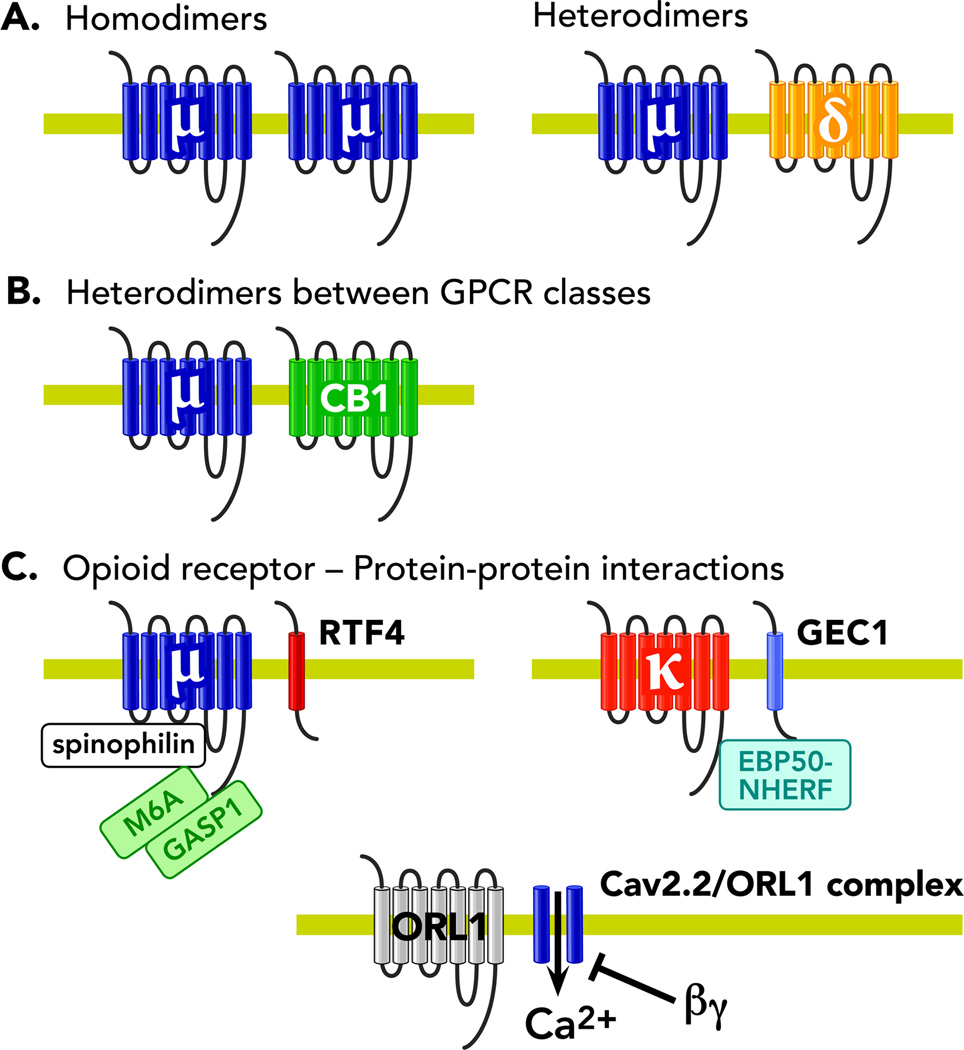
Figure 3. Opioid Dimerization.
A cartoon depicting (A) Opioid receptor homodimers and opioid receptor heterodimers (B) Heterodimers between opioid receptors and other G-Protein coupled receptors (C) Protein-protein interactions involved in opioid receptor signal transduction. Abbreviations: βγ- G-protein beta-gamma subunit, μ- μ opioid receptor, δ- δ opioid receptor, CB1- Cannabinoid receptor type 1, κ- κ opioid receptor, ORL-1- opioid receptor like-1
Devi and colleagues pioneered research into opioid dimerization and originally identified opioid receptor heterodimers (102). They found that δ receptors can exist as homodimers and agonist stimulation causes their dissociation (102; 103). In this seminal work, the authors also found that KOR and DOR form heterodimeric complexes, which appear to alter the trafficking properties of these receptors. They showed how agonist-induced internalization of DOR receptors is substantially reduced in cells expressing DOR/KOR receptors (102). Moreover, it was shown that 6’-guanidinonaltrindole, which selectively targets the KOR/DOR heterodimer, generates a unique signaling entity, giving further evidence for the existence of opioid heterodimers (104). It has been shown that MOR can heterodimerize with ORL1 (105) but the existence of MOR/KOR heterodimers remains more controversial (102; 106). The observation that the antagonism or absence of DOR diminishes the development of morphine tolerance and dependence suggested there may be an interaction between the two receptors, although future biochemical work in vivo is needed to further validate these concepts. Further studies not only identified the existence of MOR/DOR heterodimers but also revealed that MOR/DOR heterodimers have distinct ligand binding and signal transduction properties (107) suggesting that heterodimerization may represent an alternative mechanism for the cell to tune and control second messenger activity.
It was hypothesized that the mechanisms and/or proteins that modulate the level of MOR/DOR complexes are critical in the development of tolerance (108), which in turn inspired research into understanding the events that lead to dimerization. Devi and colleagues recently identified additional signaling proteins, such as RTP4, that partake in opioid receptor oligomer trafficking from the golgi to distribute opioid receptor complexes at the cell membrane (108). In addition, it was found that MOR activation also promotes the formation of complexes between RGS9-2 and Gα subunits. It was shown that pharmacological manipulations were able to disrupt RGS9-2 complexes formed following repeated morphine administration (109). These data provide a better understanding of pharmacological approaches that can be used to improve chronic analgesic responses and tolerance.
Some studies have also shown that opioid receptors can heterodimerize with other classes of GPCR. For example, MOR can interact and potentially heterodimerize with cannabinoid receptor 1 (CB1) (Figure 3) (110; 111). Interactions between MOR and CB1 receptors appear to modulate their effects as evidenced by the administration of delta 9- tetrahydrocannabinol, a cannabinoid CB1R agonist, which can enhance the potency of opioids such as morphine (112). Moreover, the concomitant activation of MOR/CB1R heterodimers leads to significant attenuation of ERK activity compared to the response following the activation of each individual receptor (110).
A number of previous studies have noted interesting functional interactions between the MOR and α2A adrenergic receptor systems (113–115). These reports identified that the presence of α2A receptors is sufficient to potentiate the phosphorylation of MAP kinases in response to morphine whereas the combination of ligands abolishes this effect. The interactions between MOR and α2A receptors provide an alternate mechanism for the control of receptor function and could have profound effects in the development of opioid-adrenergic analgesics. Neurokinin 1 (NK1) and MOR have also been shown to heterodimerize. The interaction between these two receptor types does not alter ligand binding or signal transduction but does change internalization and resensitization (116). Further, substance P (NK1 selective ligand) caused cross phosphorylation and co-internalization of MOR (117). As NK1 and MOR co-exist in the trigeminal dorsal horn it has been suggested that they may functionally interact within a signaling complex in these neurons during nociceptive neurotransmission (117). The functional consequences of opioid receptor oligomerization in vivo is largely unknown, unexplored, and controversial. New technological advances in mouse genetics, and imaging are crucial in resolving these issues. One major area of continued interest is the in vivo demonstration of opioid receptor homo and heterodimerization as well as the development of additional biochemical tools to unequivocally demonstrate that these receptor proteins directly interact with one another.
In addition to receptor-receptor interactions it is increasingly clear that opioid receptors are highly complex systems and that they interact with a whole host of extracellular, intracellular, and membrane proteins. The notion of opioid receptors existing as dynamic signaling complexes sits at the forefront of the future of opioid-based therapeutics. The reasons for this include the notion that different opioid receptor ligands can induce the formation of a diverse array of receptor complexes. Furthermore, it is increasingly appreciated that the opioid receptor’s native environment (i.e. cell type, neural circuit) greatly affects the receptors ability to signal, traffic, and function. The idea of a binary GPCR, as a simple switch mechanism from off to on is becoming widely disregarded as new protein-protein interaction networks, and ligand-dependent properties are increasingly uncovered (32; 33; 35; 69; 96; 118).
Other protein-protein interactions
There are multiple lines of evidence pointing to arrestin molecules as crucial proteins that network and engage opioid receptor signal transduction and orchestrate the interaction of proteins within the cellular milieu. The isolation of other opioid-selective protein-interaction networks has been slow, although more studies are arising examining the many important roles in receptor fate. For one, MOR has been shown to interact with numerous cytoskeletal trafficking proteins most of which participate in membrane protein endocytosis including: GASP-1, spinophilin, glycoprotein M6A, and tamalin (119–121). MOR has also been shown to interact with calmodulin, which is a highly sensitive Ca2+ binding protein implicated in cytoplasmic enzyme activity including adenylyl cyclases and CAM kinases (122). DORs are similar as they also use GASP-1 and glycoprotein M6A for regulating surface trafficking and endocytosis. KORs have been shown to interact with GEC-1 and EBP50-NHERF proteins potentially acting to enhance receptor recovery and recycling rates (123; 124). Given that ORL1 has not been extensively studied, most of our knowledge about its signaling complex centers around the work of the Zamponi group demonstrating ORL1-Cav2.2 complex formation (63; 125). The increasing specificity and affordability of proteomic technologies such as tandem affinity purification (TAP-TAG) approaches (126) will help to further advance our understanding of opioid receptor complexes. Validating protein-protein interactions in vivo continues to be a challenge, but it is expected that with newer mouse genetic tools proteomic dissection of opioid receptor complexes in vivo will become an easier task.
Opioid Signaling and Behavior
μ-Opioid Receptors
The most common behavioral function linked to opioid receptors has been their ability to mediate analgesic effects. Numerous reports have examined how opioid signaling causes opioid-induced analgesia (For recent reviews see Bodnar 2010 (127); Walwyn et al., 2010 (3)). It is generally accepted that MOR signaling to pertussis toxin sensitive Gαi is required for morphine antinociception. Furthermore, as reported in vitro blockade of arrestin3 expression improves morphine-mediated analgesic responses and acts to prevent morphine tolerance over time (128). Spinal cord expression of Gβγ is required for MOR coupling to analgesic responses and is thought to play a key role in how MORs mediate antinociception (129). This is likely to be through modulation of potassium and calcium channels in the dorsal root ganglion, and dorsal horn. Morphine-induced analgesia and tolerance have been linked to numerous signaling pathways including interaction with adenylyl cyclases, AC1, AC8, and AC5 (130; 131).
MOR-dependent behavioral studies linked to MAPK signaling have begun to become more common in the literature. ERK 1/2 phosphorylation has been shown to be upregulated by chronic morphine treatment and in opioid withdrawal (132) and consistent with this idea, MOR-induced ERK 1/2 activity in the periaqueductal gray region acts as a mechanism to counteract morphine tolerance (133). Recently, reports have implicated ERK 1/2 signal transduction in morphine reward and plasticity including place preference and psychomotor sensitization (134; 135). ERK 1/2 activity in the amygdala was also found to mediate anxiety-like behaviors during morphine withdrawal (136). Together, these reports strongly support the concept that ERK 1/2 signaling is an essential mediator of μ-opioid-induced plasticity in the brain and spinal cord.
MOR signaling via other protein kinases and protein-protein interactions to modulate reward and analgesia, such as PKC/PKA and JNK has also been demonstrated. For example, PKC 1 (also called RACK1) is required for morphine reward in mouse models and activation of IRS2-Akt signaling in dopaminergic ventral tegmental neurons is required for the behavioral and cellular actions of μ-opioids, including morphine (137). This same group has demonstrated that morphine action on reward requires the activation of transcription factors include pCREB and DeltaFosB (138). However, we still lack direct information regarding the substrates for these MOR-dependent transcription factors and kinase signaling pathways shown to be required in behaviors like analgesia and reward.
δ Opioid Receptors
Like MOR, DOR signaling research has been primarily focused on mechanisms of opioid analgesia. In addition, DOR research in vivo has been more commonly centered around DOR localization and anatomical characterization with far fewer studies linking DOR signaling with behavioral effects. Ligand-dependent DOR signaling has been an active area of research with reports suggesting that ligand-mediated trafficking govern agonist-induced analgesic tolerance to δ-opioids (33; 139). These studies demonstrated that the DOR agonists SNC80 and ARM390 differ in their ability to cause receptor internalization and downregulation of DOR-mediated Ca+2 channel modulation. DOR antinociception to thermal stimuli requires PLC and PKC activation also determines DOR-α2A synergistic effects in the spinal cord (140). DOR agonists have been increasingly studied for their potential antidepressant and anxiolytic effects in rodent behavioral models (141). However, it is not yet known how or where DOR agonists mediate antidepressant-like behavioral responses.
κ Opioid receptors
Contemporary studies linking kappa-opioid receptor signaling and behavior have been centered around the role of κ-opioids in stress (for recent reviews see Bruchas and Chavkin 2010 (142) and Knoll and Carlezon, 2010 (143)). Stress-induced opioid peptide release has been reported for all of the major opioid systems, and this release causes stress-induced analgesia via action at opioid receptors. In a few crucial reports it was demonstrated that KOR activation following stress can not only increase analgesic responses but can modulate numerous behaviors including reward and depression (144–146). KOR activation of analgesic responses are thought to require Gβγ signal transduction (147) while KOR-induced potentiation of reward and dysphoria are thought to be mediated by more complex events including, but not limited to, MAPK activation (96). Chartoff and colleagues showed that the KOR agonist salvinorin A (salvA) has a biphastic effect on reward. The acute administration of salvA decreased the rewarding impact of intracranial self-stimulation (ICSS), however repeated KOR activation caused a net decrease in the reward potentiating effects of cocaine (148). Both acute and repeated salvA administration increased phosphorylated ERK but only acute salvA increased cFos and only repeated salvA increased CREB (148). These findings provide more information about the effects of KOR activation on the reward related effects of cocaine and will assist in the further dissection of the relationship between activation of KOR and ERK signaling pathways. KOR-mediated p38 MAPK activity has been shown to be required for conditioned place aversion, and swim-stress immobility responses, while cAMP response element (CREB) signaling is critical for prodynorphin gene induction and depression-like behavioral responses (96; 149). It is thought that KOR modulation of dopamine, serotonin, and noradrenergic systems plays a key role in producing the negative behavioral affective responses (142; 143). Reports include KOR-mediated reductions on dopamine release, along with p38-dependent modulation of serotonergic output (96; 147). It was recently shown that KOR-induced p38α MAPK signaling in serotonergic circuitry is required for stress-induced social avoidance, depression-like behaviors, and reinstatement of drug seeking behavior. This report went on to show that KOR-induced p38α MAPK causes a hyposerotonergic state through increased surface serotonin transporter expression (150). The mechanisms and neural circuits in KOR-mediated dysphoria and analgesia are under active investigation by several groups and will greatly assist in both the development of potential antidepressant ligands at KOR in addition to novel analgesics that bypass KOR-mediated dysphoria (151).
Opioids and Genetics
The pathogenesis of addiction involves a series of complex interactions among biological factors including genetic vulnerability and drug-induced alterations in gene expression and proteins. Despite great efforts the progress in finding causal variants underlying drug addiction has been somewhat slow. Numerous case control studies have investigated single nucleotide polymorphisms (SNPs) in opioid receptors genes and their correlation with addiction to opioids. However, these have often produced conflicting results. The most extensively researched example is a polymorphism in OPRM1 (A118G, rs1799971), which results in the replacement of asparagine with aspartic acid at codon 40. Three studies found an association with the variant 118G and opioid dependency (152–154), two studies observed an association with the common allele, 118A, and opioid dependency (155; 156) and nine studies found no overall association with opioid dependency (157–165). This polymorphism highlights the conflicting results obtained from genetics studies of opioid receptor genes and drug dependence.
We have summarized the genetic variants that may contribute to the vulnerability to develop opioid addictions (Table 3). Genetic testing has important clinical applications in the prevention of many diseases but in the field of addiction much work still needs to be done to understand the associations between these genetic variants and addiction related phenotypes.
Table 3.
The association of polymorphisms in opioid receptor genes with opioid addiction and functional differences between these variants
| Receptor (Gene) |
Polymorphism | Synonymous/Non synonymous |
Effects and Associations | Reference |
|---|---|---|---|---|
| Mu Opioid Receptor (OPRM1) |
A118G (rs1799971) |
Non synonymous. (Asn/Asp, Variant lacks the N glycosylation site in OPRM1 extracellular domain) |
118G allele associated with reduced ACTH response to metyrapone | (184) |
| 118G associated with increased endorphin binding affinity and activity. | (185) | |||
| 118G allele reduces agonist-induced receptor signaling efficacy | (186) | |||
| 118G associated with lower OPRM1 expression | (187) | |||
| 118G altered downstream signaling of Erk1/2 and PKA compared to A118 | (188) | |||
| 118G associated with opioid dependency | (152–154) | |||
| 118A associated with opioid dependency | (155) | |||
| 118A associated with opioid and alcohol dependency |
(156) | |||
| No association with heroin dependency | (157–165) | |||
| C17T (rs1799972) | Non synonymous (Ala/Val) |
17T allele associated with cocaine dependence | (189) | |
| TT genotype associated with cocaine and heroin use in African American women |
(190) | |||
| No association with opiate addiction | (152; 155; 162; 164) | |||
| A/G (rs510769) | Intron 1 | G allele and heroin dependence* | (158) | |
| C/T (rs3778151) | Intron 1 | T allele and heroin dependence * | ||
| C/T (rs6473797) | Intron 2 | C allele and heroin dependence* | ||
| *No association after correcting for multiple testing | ||||
| Delta Opioid Receptor (OPRD1) | A/G (rs569356) | Promoter | G allele significantly higher reporter expression. Altered transcription factor binding |
(191) |
| G/T (rs1042114) | Non synonymous (Cys27Phe) |
Cys27 compromised ATP-induced intracellular Ca²+-signaling. Cys27 ↓ HERP |
(192) | |
| Cys27 reduced maturation efficiency and differential subcellular localization |
(193) | |||
| T allele and heroin dependence* | ||||
| C/T rs2236861 | Intron 1 | G allele and heroin dependence* | (158) | |
| A/G rs3766951 | Intron 1 | G allele and heroin dependence* | ||
| A/G rs2236857 | Intron 1 | *No association after correcting for multiple testing | ||
| Kappa Opioid Receptor (OPRK1) | OPRK1 Haplotype | No association between OPRK1 haplotye and opioid dependency | (194) | |
| (195) | ||||
| G36T (rs1051660) | Synonomous | Assocation of the T allele with heroin dependency | (196) | |
| ORL-1 (OPRL1) | G501C | Non synonomous (Lys167Asn) |
167 Asn impairs ERK1/2 activation LDL induced biosynthesis of LOX-1 receptors is genotype dependent. |
(197) |
| A/G (rs6512305) | Intron | Marginal association with opioid dependence | (198) | |
| C206T (rs6090043) |
5´ UTR | Marginal association with opioid dependence | (198) | |
Abbreviations: ACTH- Adrenodorticotrophic hormone; ERK1/2- Extracellular signal-regulated kinases 1 and 2; LDL- Low density lipoproteinPKA- Protein kinase A; ATP- Adenosine triphosphate; LOX-1- Low density lipoprotein-1
Within the last decade it was identified that the gene encoding the MOR undergoes extensive alternative splicing resulting in the generation of multiple versions of this receptor protein. However, correlating these splice variants to pharmacologically defined receptors has proven difficult. The relative contribution to the pharmacological effect of each splice variant could vary from drug to drug and is dependent on their potency and efficacy at a particular site. It has been suggested that the difference in the activation efficacies of various μ-opioids for the receptor splice variants may help explain the subtle but clear differences among various μ-opioids in the clinic. In addition, understanding the functional significance of some of the truncated receptor splice variants will be beneficial as they have been reported to modulate the activity of opioid receptors in other systems (for comprehensive reviews on this topic see Pasternak 2001, 2004) (166; 167).
Conclusions
In this review we discussed a wide array of molecular, cellular, and in vivo studies in opioid receptor pharmacology. We highlighted the traditional G-protein, βγ signaling pathways, regulatory mechanisms, while also discussing the recent advances in the subfields of biochemistry, MAPK signal transduction, genetics, and behavior. It is important to note that we have not attempted to discuss all the fine details regarding the properties of each receptor system. The most common thread in the reports discussed throughout the review is that a large body of our understanding of opioid receptor molecular pharmacology continues to stem from in vitro studies. It is also increasingly clear that the majority of molecular and cellular features of opioid receptors remain disjointed and unconnected to any physiological or behavioral effect, which needs to be the focus of future work in this field.
Many, of the most important observations and discoveries surrounding opioid receptors have relied on in vitro approaches, and they continue to be the starting point for most laboratories in molecular pharmacology. However, given the diverse functionality of the opioid receptor family discussed here, the variety of signaling pathways and interacting proteins our knowledge of how opioid receptors function in animal models and more importantly human populations or disease is limited.
Since their discovery, opioid receptor signaling has been a primary focus of researchers in this field. The major reason for this interest is that it has been widely accepted that a clear understanding of opioid receptor synthesis, cellular localization, trafficking, and pharmacology will lead to novel therapeutics that either directly act on opioid receptors or modulate opioid receptor signaling pathways. With the advent of conditional genetic approaches, receptor tags, antibodies, fluorescent tools, and optogenetic manipulation of neural circuitry, opioid receptor pharmacology is poised for some major breakthroughs in the next decade. It is hopeful that these new molecular and cellular discoveries will lead better opioid analgesics in the clinic with a decreased risk of addiction and tolerance. Furthermore, it is likely that studies at the forefront of molecular and behavioral pharmacology will continue to reveal novel uses for opioids in the treatment of a variety of psychiatric and neurological diseases.
Summary Statement.
This review highlights the recent advances in opioid receptor signaling and discusses their potential for the development of novel opioids in the treatment of pain and neurological disorders.
Funding
Michael R Bruchas -MRB DA025182. Supported by National Institute on Drug Abuse, National Institutes of Health, 6001 Executive Boulevard, Room 5213, Bethesda, MD 20892-9561, U.S.A
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII: Classification of receptors. Pharmacol. Rev. 1996;48:567–592. [PubMed] [Google Scholar]
- 2.Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl.) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walwyn WM, Miotto KA, Evans CJ. Opioid pharmaceuticals and addiction: The issues and research directions seeking solutions. Drug Alcohol Depend. 2010;108:156–165. doi: 10.1016/j.drugalcdep.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahlbeck K. Opioids: A two-faced Janus. Curr Med Res Opin. 2011;27:439–448. doi: 10.1185/03007995.2010.545379. [DOI] [PubMed] [Google Scholar]
- 5.McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, Lau J, Carr D. Management of opioid side effects in cancer-related and chronic noncancer pain: A systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 7.Childers SR, Snyder SH. Guanine nucleotides differentiate agonist and antagonist interactions with opiate receptors. Life Sci. 1978;23:759–761. doi: 10.1016/0024-3205(78)90077-2. [DOI] [PubMed] [Google Scholar]
- 8.Childers SR, Creese I, Snowman AM, Synder SH. Opiate receptor binding affected differentially by opiates and opioid peptides. Eur J Pharmacol. 1979;55:11–18. doi: 10.1016/0014-2999(79)90142-0. [DOI] [PubMed] [Google Scholar]
- 9.Barchfeld CC, Medzihradsky F. Receptor-mediated stimulation of brain GTPase by opiates in normal and dependent rats. Biochem. Biophys Res Commun. 1984;121:641–648. doi: 10.1016/0006-291x(84)90230-4. [DOI] [PubMed] [Google Scholar]
- 10.Minneman KP, Iversen IL. Enkephalin and opiate narcotics increase cyclic GMP accumulation in slices of rat neostriatum. Nature. 1976;262:313–314. doi: 10.1038/262313a0. [DOI] [PubMed] [Google Scholar]
- 11.Hsia JA, Moss J, Hewlett EL, Vaughan M. ADP-ribosylation of adenylate cyclase by pertussis toxin. Effects on inhibitory agonist binding. J Biol Chem. 1984;259:1086–1090. [PubMed] [Google Scholar]
- 12.Taussig R, Iñiguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 13.Wickman K, Clapham DE. Ion channel regulation by G proteins. Physiol Rev. 1995;75:865–885. doi: 10.1152/physrev.1995.75.4.865. [DOI] [PubMed] [Google Scholar]
- 14.Sadja R, Alagem N, Reuveny E. Gating of GIRK channels: Details of an intricate, membrane-delimited signaling complex. Neuron. 2003;39:9–12. doi: 10.1016/s0896-6273(03)00402-1. [DOI] [PubMed] [Google Scholar]
- 15.Ippolito DL, Temkin PA, Rogalski SL, Chavkin C. N-terminal tyrosine residues within the potassium channel Kir3 modulate GTPase activity of Galphai. J Biol Chem. 2002;277:32692–32696. doi: 10.1074/jbc.M204407200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torrecilla M, Quillinan N, Williams JT, Wickman K. Pre- and postsynaptic regulation of locus coeruleus neurons after chronic morphine treatment: A study of GIRK-knockout mice. Eur J Neurosci. 2008;28:618–624. doi: 10.1111/j.1460-9568.2008.06348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torrecilla M, Marker CL, Cintora SC, Stoffel M, Williams JT, Wickman K. G-protein-gated potassium channels containing Kir3.2 and Kir3.3 subunits mediate the acute inhibitory effects of opioids on locus ceruleus neurons. J. Neurosci. 2002;22:4328–4334. doi: 10.1523/JNEUROSCI.22-11-04328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rusin KI, Giovannucci DR, Stuenkel EL, Moises HC. Kappa-opioid receptor activation modulates Ca2+ currents and secretion in isolated neuroendocrine nerve terminals. J Neurosci. 1997;17:6565–6574. doi: 10.1523/JNEUROSCI.17-17-06565.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zamponi GW, Snutch TP. Modulating modulation: Crosstalk between regulatory pathways of presynaptic calcium channels. Mol Interv. 2002;2:476–478. doi: 10.1124/mi.2.8.476. [DOI] [PubMed] [Google Scholar]
- 20.Zamponi GW, Snutch TP. Modulation of voltage-dependent calcium channels by G proteins. Curr Opin Neurobiol. 1998;8:351–356. doi: 10.1016/s0959-4388(98)80060-3. [DOI] [PubMed] [Google Scholar]
- 21.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci. U.S.A. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz A, Ruíz F, Flórez J, Pazos A, Hurlé MA. Regulation of dihydropyridine-sensitive Ca++ channels during opioid tolerance and supersensitivity in rats. J Pharmacol Exp Ther. 1995;274:1538–1544. [PubMed] [Google Scholar]
- 23.Díaz A, Flórez J, Pazos A, Hurlé MA. Opioid tolerance and supersensitivity induce regional changes in the autoradiographic density of dihydropyridine-sensitive calcium channels in the rat central nervous system. Pain. 2000;86:227–235. doi: 10.1016/S0304-3959(00)00249-9. [DOI] [PubMed] [Google Scholar]
- 24.Harrison RS, Ruiz-Gómez G, Hill TA, Chow SY, Shepherd NE, Lohman RJ, Abbenante G, Hoang HN, Fairlie DP. Novel Helix-Constrained Nociceptin Derivatives Are Potent Agonists and Antagonists of ERK Phosphorylation and Thermal Analgesia in Mice. J Med Chem. 2010;53:8400–8408. doi: 10.1021/jm101139f. [DOI] [PubMed] [Google Scholar]
- 25.Dang VC, Christie MJ. Mechanisms of rapid opioid receptor desensitization, resensitization and tolerance in brain neurons. Br J Pharmacol. 2011 doi: 10.1111/j.1476-5381.2011.01482.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldhoer M, Bartlett SE, Whistler JL. Opioid receptors. Annu Rev Biochem. 2004:73953–73990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- 27.Nestler EJ. Under Siege: The Brain on Opiates. Neuron. 1996;16:897–900. doi: 10.1016/s0896-6273(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 28.He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 29.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: Implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 30.Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408:720–723. doi: 10.1038/35047086. [DOI] [PubMed] [Google Scholar]
- 31.Groer CE, Schmid CL, Jaeger AM, Bohn LM. Agonist-directed interactions with specific \beta\arrestins determine MU opioid receptor trafficking, ubiquitination, and dephosphorylation. J Biol Chem. 2011;286:1731–1741. doi: 10.1074/jbc.M111.248310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melief EJ, Miyatake M, Carroll FI, Beguin C, Carlezon WA, Cohen BM, Grimwood S, Mitch C, Rorick-Kehn LM, Chavkin C. Duration of action of a broad range of selective kappa opioid receptor antagonists is positively correlated with c-Jun N-Terminal kinase-1 activation. Mol Pharmacol. 2011 doi: 10.1124/mol.111.074195. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pradhan AAA, Walwyn W, Nozaki C, Filliol D, Erbs E, Matifas A, Evans C, Kieffer BL. Ligand-directed trafficking of the δ-opioid receptor in vivo: Two paths toward analgesic tolerance. J Neurosci. 2010;30:16459–16468. doi: 10.1523/JNEUROSCI.3748-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bailey CP, Connor M. Opioids: Cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U.S.A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finn AK, Whistler JL. Endocytosis of the mu opioid receptor reduces tolerance and a cellular hallmark of opiate withdrawal. Neuron. 2001;32:829–839. doi: 10.1016/s0896-6273(01)00517-7. [DOI] [PubMed] [Google Scholar]
- 37.Chavkin C, McLaughlin JP, Celver JP. Regulation of opioid receptor function by chronic agonist exposure: Constitutive activity and desensitization. Mol Pharmacol. 2001;60:20–25. doi: 10.1124/mol.60.1.20. [DOI] [PubMed] [Google Scholar]
- 38.El Kouhen R, Kouhen OM, Law PY, Loh HH. The absence of a direct correlation between the loss of [D-Ala2, MePhe4,Gly5-ol]Enkephalin inhibition of adenylyl cyclase activity and agonist-induced mu-opioid receptor phosphorylation. J Biol Chem. 1999;274:9207–9215. doi: 10.1074/jbc.274.14.9207. [DOI] [PubMed] [Google Scholar]
- 39.Schulz S, Mayer D, Pfeiffer M, Stumm R, Koch T, Höllt V. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. EMBO J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clayton CC, Bruchas MR, Lee ML, Chavkin C. Phosphorylation of the mu-opioid receptor at tyrosine 166 (Tyr3.51) in the DRY motif reduces agonist efficacy. Mol Pharmacol. 2010;77:339–347. doi: 10.1124/mol.109.060558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lau EK, Trester-Zedlitz M, Trinidad JC, Kotowski SJ, Krutchinsky AN, Burlingame AL, von Zastrow M. Quantitative encoding of the effect of a partial agonist on individual opioid receptors by multisite phosphorylation and threshold detection. Sci Signal. 2011;4:ra52. doi: 10.1126/scisignal.2001748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blake AD, Bot G, Li S, Freeman JC, Reisine T. Differential agonist regulation of the human kappa-opioid receptor. J Neurochem. 1997;68:1846–1852. doi: 10.1046/j.1471-4159.1997.68051846.x. [DOI] [PubMed] [Google Scholar]
- 43.Li J-G, Zhang F, Jin X-L, Liu-Chen L-Y. Differential regulation of the human kappa opioid receptor by agonists: Etorphine and levorphanol reduced dynorphin A- and U50,488H–induced internalization and phosphorylation. J Pharmacol Exp Ther. 2003;305:531–540. doi: 10.1124/jpet.102.045559. [DOI] [PubMed] [Google Scholar]
- 44.Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of three distinct kappa ligands (Salvinorin A, TRK-820 and 3FLB) on kappa opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther. 2005;312:220–230. doi: 10.1124/jpet.104.073668. [DOI] [PubMed] [Google Scholar]
- 45.Zhang F, Li J, Li J-G, Liu-Chen L-Y. (−)U50,488H [(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl]benzeneacetamide] induces internalization and down-regulation of the human, but not the rat, kappa-opioid receptor: structural basis for the differential regulation. J Pharmacol Exp Ther. 2002;302:1184–1192. doi: 10.1124/jpet.302.3.1184. [DOI] [PubMed] [Google Scholar]
- 46.McLaughlin JP, Xu M, Mackie K, Chavkin C. Phosphorylation of a carboxyl-terminal serine within the kappa-opioid receptor produces desensitization and internalization. J Biol Chem. 2003;278:34631–34640. doi: 10.1074/jbc.M304022200. [DOI] [PubMed] [Google Scholar]
- 47.McLaughlin JP, Myers LC, Zarek PE, Caron MG, Lefkowitz RJ, Czyzyk TA, Pintar JE, Chavkin C. Prolonged kappa opioid receptor phosphorylation mediated by G-protein receptor kinase underlies sustained analgesic tolerance. J Biol Chem. 2004;279:1810–1818. doi: 10.1074/jbc.M305796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O'Donnell Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: Evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001;440:65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Pickel VM. Preferential cytoplasmic localization of delta-opioid receptors in rat striatal patches: Comparison with plasmalemmal mu-opioid receptors. J Neurosci. 2001;21:3242–3250. doi: 10.1523/JNEUROSCI.21-09-03242.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gendron L, Lucido AL, Mennicken F, O'Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J. Neurosci. 2006;26:953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scherrer G, Imamachi N, Cao Y-Q, Contet C, Mennicken F, O'Donnell D, Kieffer BL, Basbaum AI. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hasbi A, Polastron J, Allouche S, Stanasila L, Massotte D, Jauzac P. Desensitization of the delta-opioid receptor correlates with its phosphorylation in SK-N-BE cells: involvement of a G protein-coupled receptor kinase. J Neurochem. 1998;70:2129–2138. doi: 10.1046/j.1471-4159.1998.70052129.x. [DOI] [PubMed] [Google Scholar]
- 54.Law PY, Kouhen OM, Solberg J, Wang W, Erickson LJ, Loh HH. Deltorphin II-induced rapid desensitization of delta-opioid receptor requires both phosphorylation and internalization of the receptor. J Biol Chem. 2000;275:32057–32065. doi: 10.1074/jbc.M002395200. [DOI] [PubMed] [Google Scholar]
- 55.Bradbury FA, Zelnik JC, Traynor JR. G protein independent phosphorylation and internalization of the delta-opioid receptor. J Neurochem. 2009;109:1526–1535. doi: 10.1111/j.1471-4159.2009.06082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo J, Wu Y, Zhang W, Zhao J, Devi LA, Pei G, Ma L. Identification of G protein-coupled receptor kinase 2 phosphorylation sites responsible for agonist-stimulated delta-opioid receptor phosphorylation. Mol Pharmacol. 2000;58:1050–1056. doi: 10.1124/mol.58.5.1050. [DOI] [PubMed] [Google Scholar]
- 57.Pei G, Kieffer BL, Lefkowitz RJ, Freedman NJ. Agonist-dependent phosphorylation of the mouse delta-opioid receptor: involvement of G protein-coupled receptor kinases but not protein kinase. C Mol Pharmacol. 1995;48:173–177. [PubMed] [Google Scholar]
- 58.Cvejic S, Trapaidze N, Cyr C, Devi LA. Thr353, located within the COOH-terminal tail of the delta opiate receptor, is involved in receptor down-regulation. J Biol Chem. 1996;271:4073–4076. doi: 10.1074/jbc.271.8.4073. [DOI] [PubMed] [Google Scholar]
- 59.Trapaidze N, Keith DE, Cvejic S, Evans CJ, Devi LA. Sequestration of the delta opioid receptor: Role of the C terminus in agonist-mediated internalization. J Biol Chem. 1996;271:29279–29285. doi: 10.1074/jbc.271.46.29279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varga EV, Navratilova E, Stropova D, Jambrosic J, Roeske WR, Yamamura HI. Agonist-specific regulation of the delta-opioid receptor. Life Sci. 2004;76:599–612. doi: 10.1016/j.lfs.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 61.Spampinato S, Baiula M, Calienni M. Agonist-regulated internalization and desensitization of the human nociceptin receptor expressed in CHO cells. Curr Drug Targets. 2007;8:137–146. doi: 10.2174/138945007779315641. [DOI] [PubMed] [Google Scholar]
- 62.Spampinato S, Di Toro R, Qasem AR. Nociceptin-induced internalization of the ORL1 receptor in human neuroblastoma cells. Neuroreport. 2001;12:3159–3163. doi: 10.1097/00001756-200110080-00035. [DOI] [PubMed] [Google Scholar]
- 63.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Fergusson SS, Mezghrani A, Dubei SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nat Neurosci. 2006;9:31–40. doi: 10.1038/nn1605. [DOI] [PubMed] [Google Scholar]
- 64.Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286:2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- 65.Lowe JD, Celver JP, Gurevich VV, Chavkin C. mu-Opioid receptors desensitize less rapidly than delta-opioid receptors due to less efficient activation of arrestin. J Biol Chem. 2002;277:15729–15735. doi: 10.1074/jbc.M200612200. [DOI] [PubMed] [Google Scholar]
- 66.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 67.Raman M, Chen W, Cobb MH. Differential regulation and properties of MAPKs. Oncogene. 2007;26:3100–3112. doi: 10.1038/sj.onc.1210392. [DOI] [PubMed] [Google Scholar]
- 68.Karandikar M, Cobb MH. Scaffolding and protein interactions in MAP kinase modules. Cell Calcium. 1999;26:219–226. doi: 10.1054/ceca.1999.0074. [DOI] [PubMed] [Google Scholar]
- 69.Pierce KL, Lefkowitz RJ. Classical and new roles of beta-arrestins in the regulation of G-protein-coupled receptors. Nat Rev Neurosci. 2001;2:727–733. doi: 10.1038/35094577. [DOI] [PubMed] [Google Scholar]
- 70.Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem. 2006;281:34515–34524. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyatake M, Rubinstein TJ, McLennan GP, Belcheva MM, Coscia CJ. Inhibition of EGF-induced ERK/MAP kinase-mediated astrocyte proliferation by mu opioids: integration of G protein and beta-arrestin 2-dependent pathways. J Neurochem. 2009;110:662–674. doi: 10.1111/j.1471-4159.2009.06156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Groer CE, Tidgewell K, Moyer RA, Harding WW, Rothman RB, Prisinzano, Bohn LM. An opioid agonist that does not induce mu-opioid receptor--arrestin interactions or receptor internalization. Mol Pharmacol. 2007;71:549–557. doi: 10.1124/mol.106.028258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim E, Clark AL, Kiss A, Hahn JW, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu- and kappa-opioids induce the differentiation of embryonic stem cells to neural progenitors. J Biol Chem. 2006;281:33749–33760. doi: 10.1074/jbc.M603862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hahn JW, Jagwani S, Kim E, Rendell VR, HE J, Ezerskiy LA, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J Neurochem. 2010;112:1431–1441. doi: 10.1111/j.1471-4159.2009.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ikeda H, Miyatake M, Koshikawa N, Ochial K, Yamada K, KIss A, Donolin MJ, Panneton WM, Churchill JD, Green M, Siddiqui AM, Leinweber AL, Crews NR, Ezerskiy LA, Rendell VR, Belcheva MM, Coscia CJ. Morphine modulation of thrombospondin levels in astrocytes and its implications for neurite outgrowth and synapse formation. J Biol Chem. 2010;285:38415–38427. doi: 10.1074/jbc.M110.109827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kramer HK, Simon EJ. Mu and delta-opioid receptor agonists induce mitogen-activated protein kinase (MAPK) activation in the absence of receptor internalization. Neuropharmacology. 2000;39:1707–1719. doi: 10.1016/s0028-3908(99)00243-9. [DOI] [PubMed] [Google Scholar]
- 78.Kramer HK, Onoprishvili I, Andria ML, Hanna K, Sheinkman K, Haddad LB, SImon EJ. Delta opioid activation of the mitogen-activated protein kinase cascade does not require transphosphorylation of receptor tyrosine kinases. BMC Pharmacol. 2002;2:5. doi: 10.1186/1471-2210-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eisinger DA, Ammer H. Delta-opioid receptors activate ERK/MAP kinase via integrin-stimulated receptor tyrosine kinases. Cell. Signal. 2008;20:2324–2331. doi: 10.1016/j.cellsig.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 80.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, Eckhardt AE, Cowan CL, Spurney RF, Luttrell LM, Lefkowitz RJ. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 81.McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Belcheva MM, Clark AL, Haas PD, Sema JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen L-Y, Huang J-X, Yu L-C. Involvement of ORL1 receptor and ERK kinase in the orphanin FQ-induced nociception in the nucleus accumbens of rats. Regulatory Peptides. 2008;151:43–47. doi: 10.1016/j.regpep.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 2007:359–389. doi: 10.1007/978-3-540-33823-9_13. [DOI] [PubMed] [Google Scholar]
- 85.Minden A, Karin M. Regulation and function of the JNK subgroup of MAP kinases. Biochim. Biophys Acta. 1997;1333:F85–F104. doi: 10.1016/s0304-419x(97)00018-8. [DOI] [PubMed] [Google Scholar]
- 86.Baker SJ, Reddy EP. Modulation of life and death by the TNF receptor superfamily. Oncogene. 1998;17:3261–3270. doi: 10.1038/sj.onc.1202568. [DOI] [PubMed] [Google Scholar]
- 87.Nobes C, Hall A. Regulation and function of the Rho subfamily of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 88.Song X, Coffa S, Fu H, Gurevich VV. How does arrestin assemble MAPKs into a signaling complex? J Biol Chem. 2009;284:685–695. doi: 10.1074/jbc.M806124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shahabi NA, McAllen K, Sharp BM. Delta opioid receptors stimulate Akt-dependent phosphorylation of c-jun in T cells. J Pharmacol Exp Ther. 2006;316:933–939. doi: 10.1124/jpet.105.091447. [DOI] [PubMed] [Google Scholar]
- 90.Kam AYF, Chan ASL, Wong YH. Phosphatidylinositol-3 kinase is distinctively required for mu-, but not kappa-opioid receptor-induced activation of c-Jun N-terminal kinase. J Neurochem. 2004;89:391–402. doi: 10.1111/j.1471-4159.2004.02338.x. [DOI] [PubMed] [Google Scholar]
- 91.Bruchas MR, Yang T, Schreiber S, Defino M, Kwan SC, Li S, Chavkin C. Long-acting kappa opioid antagonists disrupt receptor signaling and produce noncompetitive effects by activating c-Jun N-terminal kinase. J Biol Chem. 2007;282:29803–29811. doi: 10.1074/jbc.M705540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu M, Bruchas MR, Ippolito DL, Gendron L, Chavkin C. Sciatic nerve ligation-induced proliferation of spinal cord astrocytes is mediated by kappa opioid activation of p38 mitogen-activated protein kinase. J Neurosci. 2007;27:2570–2581. doi: 10.1523/JNEUROSCI.3728-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watkins LR, Milligan ED, Maier SF. Glial activation: A driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 95.Hahn JW, Jagwani S, Kim E, Rendell VR, He J, Ezerskly LA, Wesselschmidt R, Coscia CJ, Belcheva MM. Mu and kappa opioids modulate mouse embryonic stem cell-derived neural progenitor differentiation via MAP kinases. J Neurochem. 2010;112:1431–1441. doi: 10.1111/j.1471-4159.2009.06479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bruchas MR, Land BB, Aita M, Xu M, Barot SK, Li S, Chavkin C. Stress-induced p38 mitogen-activated protein kinase activation mediates kappa-opioid-dependent dysphoria. J Neurosci. 2007;27:11614–11623. doi: 10.1523/JNEUROSCI.3769-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tan M, Walwyn WM, Evans CJ, Xie C-W. p38 MAPK and beta-arrestin 2 mediate functional interactions between endogenous micro-opioid and alpha2A–adrenergic receptors in neurons. J Biol Chem. 2009;284:6270–6281. doi: 10.1074/jbc.M806742200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang Z, Xin SM, Wu GX, Zhang WB, Ma L, Pei G. Endogenous delta-opioid and ORL1 receptors couple to phosphorylation and activation of p38 MAPK in NG108-15 cells and this is regulated by protein kinase A and protein kinase C. J Neurochem. 1999;73:1502–1509. doi: 10.1046/j.1471-4159.1999.0731502.x. [DOI] [PubMed] [Google Scholar]
- 99.Clayton CC, Xu M, Chavkin C. Tyrosine Phosphorylation of Kir3 following κ-Opioid Receptor Activation of p38 MAPK Causes Heterologous Desensitization. J Biol Chem. 2009;284:31872–31881. doi: 10.1074/jbc.M109.053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rios CD, Jordan BA, Gomes I, Devi LA. G-protein-coupled receptor dimerization: modulation of receptor function. Pharmacol Ther. 2001;92:71–87. doi: 10.1016/s0163-7258(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 101.Prinster SC, Hague C, Hall RA. Heterodimerization of g protein-coupled receptors: specificity and functional significance. Pharmacol. Rev. 2005;57:289–298. doi: 10.1124/pr.57.3.1. [DOI] [PubMed] [Google Scholar]
- 102.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cvejic S, Devi LA. Dimerization of the delta opioid receptor: Implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 104.Waldhoer M, Fong J, Jones RM, Lunzer MM, Sharma SK, Kostenis E, Portoghese PS, Whistler JL. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. Proc Natl Acad Sci U.S.A. 2005;102:9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pan Y-X, Bolan E, Pasternak GW. Dimerization of morphine and orphanin FQ/nociceptin receptors: Generation of a novel opioid receptor subtype. Biochem Biophys Res Commun. 2002;297:659–663. doi: 10.1016/s0006-291x(02)02258-1. [DOI] [PubMed] [Google Scholar]
- 106.Wang D, Sun X, Bohn LM, Sadée W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 107.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Cell surface targeting of mu-delta opioid receptor heterodimers by RTP4. Proc Natl Acad Sci U.S.A. 2008;105:16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Psifogeorgou K, Psigfogeorgou K, Terzi D, Papachatzaki MM, Varidaki A, Ferguson D, Gold SJ, Zachariou V. A unique role of RGS9-2 in the striatum as a positive or negative regulator of opiate analgesia. J Neurosci. 2011;31:5617–5624. doi: 10.1523/JNEUROSCI.4146-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rios C, Gomes I, Devi LA. Mu opioid and CB1 cannabinoid receptor interactions: Reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–395. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. Mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: Electrophysiological and FRET assay analysis. J Pharmacol Sci. 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- 112.Cichewicz DL. Synergistic interactions between cannabinoid and opioid analgesics. Life Sci. 2004;74:1317–1324. doi: 10.1016/j.lfs.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 113.Ossipov MH, Lopez Y, Bian D, Nichols ML, Porreca F. Synergistic antinociceptive interactions of morphine and clonidine in rats with nerve-ligation injury. Anesthesiology. 1997;86:196–204. doi: 10.1097/00000542-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 114.Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A–adrenergic receptors. Mol Pharmacol. 2003;64:1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 115.Drasner K, Fields HL. Synergy between the antinociceptive effects of intrathecal clonidine and systemic morphine in the rat. Pain. 1988;32:309–312. doi: 10.1016/0304-3959(88)90042-5. [DOI] [PubMed] [Google Scholar]
- 116.Pfeiffer M, Koch T, Schröder H, Laugsch M, Höllt V, Schulz S. Heterodimerization of somatostatin and opioid receptors cross-modulates phosphorylation, internalization, and desensitization. J Biol Chem. 2002;277:19762–19772. doi: 10.1074/jbc.M110373200. [DOI] [PubMed] [Google Scholar]
- 117.Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schroder H, Hollt V, Schultz S. Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem. 2003;278:51630–51637. doi: 10.1074/jbc.M307095200. [DOI] [PubMed] [Google Scholar]
- 118.Kenakin TP. Seven transmembrane receptors as nature’s prototype allosteric protein: De-emphasizing the geography of binding. Mol Pharmacol. 2008;74:541–543. doi: 10.1124/mol.108.050062. [DOI] [PubMed] [Google Scholar]
- 119.Milligan G. Opioid receptors and their interacting proteins. Neuromolecular Med. 2005;7:51–59. doi: 10.1385/NMM:7:1-2:051. [DOI] [PubMed] [Google Scholar]
- 120.Whistler JL, Enquist J, Marley A, Fong J, Gladher F, Tsuruda P, Murray SR, Von Zastrow M. Modulation of postendocytic sorting of G protein-coupled receptors. Science. 2002;297:615–620. doi: 10.1126/science.1073308. [DOI] [PubMed] [Google Scholar]
- 121.Charlton JJ, Allen PB, Psifogeorgou K, Chakravarty S, Gomes I, Neve RL, Devi LA, Greengard P, Nestler EJ, Zachariou V. Multiple actions of spinophilin regulate mu opioid receptor function. Neuron. 2008;58:238–247. doi: 10.1016/j.neuron.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang D, Quillan JM, Winans K, Lucas JL, Sadée W. Single nucleotide polymorphisms in the human mu opioid receptor gene alter basal G protein coupling and calmodulin binding. J Biol Chem. 2001;276:34624–34630. doi: 10.1074/jbc.M104083200. [DOI] [PubMed] [Google Scholar]
- 123.Chen C, Wang Y, Huang P, Liu-Chen L-Y. Effects of C-terminal Modifications of GEC1 Protein and \gamma\-Aminobutyric Acid Type A (GABAA) Receptor-associated Protein (GABARAP), Two Microtubule-associated Proteins, on \kappa\ Opioid Receptor Expression. J Biol Chem. 2011;286:15106–15115. doi: 10.1074/jbc.M111.230896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huang P, Steplock D, Weinman EJ, Hall RA, Ding Z, Li J, Wang Y, Liu-Chen LY. kappa Opioid receptor interacts with Na(+)/H(+)-exchanger regulatory factor-1/Ezrin-radixin-moesin-binding phosphoprotein-50 (NHERF-1/EBP50) to stimulate Na(+)/H(+) exchange independent of G(i)/G(o) proteins. J Biol Chem. 2004;279:25002–25009. doi: 10.1074/jbc.M313366200. [DOI] [PubMed] [Google Scholar]
- 125.Evans RM, You H, Hameed S, Altier C, Mezghrani A. Heterodimerization of ORL1 and opioid receptors and its consequences for N-type calcium channel regulation. J Biol Chem. 2010;285:1032–1040. doi: 10.1074/jbc.M109.040634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lyssand JS, Whiting JL, Lee K-S, Kasti R, Wacker JL, Bruchas MR, Miyatake M, Langeberg LK, Chavkin C, Scott JD, Gardner RG, Adams ME, Hague C. Alpha-dystrobrevin-1 recruits alpha-catulin to the alpha1D-adrenergic receptor/dystrophin-associated protein complex signalosome. Proc. Natl Acad Sci U.S.A. 2010;107:21854–21859. doi: 10.1073/pnas.1010819107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bodnar RJ. Endogenous opiates and behavior: 2009. Peptides. 2010;31:2325–2359. doi: 10.1016/j.peptides.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 128.Raehal KM, Bohn LM. The role of beta-arrestin2 in the severity of antinociceptive tolerance and physical dependence induced by different opioid pain therapeutics. Neuropharmacology. 2011;60:58–65. doi: 10.1016/j.neuropharm.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mathews JL, Smrcka AV, Bidlack JM. A novel Gbetagamma-subunit inhibitor selectively modulates mu-opioid-dependent antinociception and attenuates acute morphine-induced antinociceptive tolerance and dependence. J Neurosci. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Li S, Lee ML, Bruchas MR, Chan GC, Storm DR, Chavkin C. Calmodulin-stimulated adenylyl cyclase gene deletion affects morphine responses. Mol Pharmacol. 2006;70:1742–1749. doi: 10.1124/mol.106.025783. [DOI] [PubMed] [Google Scholar]
- 131.Kim KS, Lee KW, Lee KW, Im JY, Yoo JY, Kim SW, Lee JK, Nestler EJ, Han PL. Adenylyl cyclase type 5 (AC5) is an essential mediator of morphine action. Proc Natl Acad Sci U.S.A. 2006;103:3908–3913. doi: 10.1073/pnas.0508812103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asensio VJ, Miralles A, García-Sevilla JA. Stimulation of mitogen-activated protein kinase kinases (MEK1/2) by mu-, delta- and kappa-opioid receptor agonists in the rat brain: regulation by chronic morphine and opioid withdrawal. Eur J Pharmacol. 2006;539:49–56. doi: 10.1016/j.ejphar.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 133.Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009;331:412–418. doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramos-Miguel A, Esteban S, García-Sevilla JA. The time course of unconditioned morphine-induced psychomotor sensitization mirrors the phosphorylation of FADD and MEK/ERK in rat striatum: role of PEA-15 as a FADD-ERK binding partner in striatal plasticity. Eur Neuropsychopharmacol. 2010;20:49–64. doi: 10.1016/j.euroneuro.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 135.Lin X, Wang Q, Ji J, Yu L-C. Role of MEK-ERK pathway in morphine-induced conditioned place preference in ventral tegmental area of rats. J. Neurosci Res. 2010;88:1595–1604. doi: 10.1002/jnr.22326. [DOI] [PubMed] [Google Scholar]
- 136.Hofford RS, Hodgson SR, Roberts KW, Bryant CD, Evans CJ, Eitan S. Extracellular signal-regulated kinase activation in the amygdala mediates elevated plus maze behavior during opioid withdrawal. Behav Pharmacol. 2009;20:576–583. doi: 10.1097/FBP.0b013e32832ec57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- 138.Zachariou V, Bolanos CA, Selley DE, Theobald D, Cassidy MP, Kelz MB, Shaw-Lutchman T, Berton O, Sim-Selley LJ, Dileone RJ, Kumar A, Nestler EJ. An essential role for DeltaFosB in the nucleus accumbens in morphine action. Nat Neurosci. 2006;9:205–211. doi: 10.1038/nn1636. [DOI] [PubMed] [Google Scholar]
- 139.Pradhan AAA, Becker JAJ, Scherrer G, Tryoen-Toth P, Fillioi D, Matifas A, Massotte D, Gaveriaux-Ruff C, Kieffer BL. In vivo delta opioid receptor internalization controls behavioral effects of agonists. PLoS ONE. 2009;4:e5425. doi: 10.1371/journal.pone.0005425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Overland AC, Kitto KF, Chabot-Doré AJ, Rothwell PE, Fairbanks CA, Stone LS, Wilcox GL. Protein kinase C mediates the synergistic interaction between agonists acting at alpha2-adrenergic and delta-opioid receptors in spinal cord. J Neurosci. 2009;29:13264–13273. doi: 10.1523/JNEUROSCI.1907-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jutkiewicz EM. RB101-mediated protection of endogenous opioids: Potential therapeutic utility? CNS Drug Rev. 2007;13:192–205. doi: 10.1111/j.1527-3458.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010:131444–131455. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010:131456–131473. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. Depressive-like effects of the kappa-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther. 2006;316:440–447. doi: 10.1124/jpet.105.092304. [DOI] [PubMed] [Google Scholar]
- 147.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, Hnasko TS, Palmiter RD, Chavkin C. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U.S.A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated Exposure to the κ-Opioid Receptor Agonist Salvinorin A Modulates Extracellular Signal-Regulated Kinase and Reward Sensitivity. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.021. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bruchas MR, Schindler AG, Shankar H, Messinger DI, Miyatake M, Land BB, Lemos JC, Hagan CE, Neumaier JF, Quintana A, Palmiter RD, Chavkin C. Selective p38α MAPK Deletion in Serotonergic Neurons Produces Stress Resilience in Models of Depression and Addiction. Neuron. 2011;71:498–511. doi: 10.1016/j.neuron.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Muschamp JW, Van’t Veer A, Carlezon WA., Jr Tracking Down the Molecular Substrates of Stress: New Roles for p38α MAPK and Kappa-Opioid Receptors. Neuron. 2011;71:383–385. doi: 10.1016/j.neuron.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kapur S, Sharad S, Singh RA, Gupta AK. A118g polymorphism in mu opioid receptor gene (oprm1): association with opiate addiction in subjects of Indian origin. J Integr Neurosci. 2007;6:511–522. doi: 10.1142/s0219635207001635. [DOI] [PubMed] [Google Scholar]
- 153.Bart G, Heilig M, LaForge KS, Pollak L, Leal SM, Ott J, Kreek MJ. Substantial attributable risk related to a functional mu-opioid receptor gene polymorphism in association with heroin addiction in central Sweden. Mol Psychiatry. 2004;9:547–549. doi: 10.1038/sj.mp.4001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Szeto CY, Tang NL, Lee DT, Stadlin A. Association between mu opioid receptor gene polymorphisms and Chinese heroin addicts. Neuroreport. 2001;12:1103–1106. doi: 10.1097/00001756-200105080-00011. [DOI] [PubMed] [Google Scholar]
- 155.Tan E-C, Tan C-H, Karupathivan U, Yap EPH. Mu opioid receptor gene polymorphisms and heroin dependence in Asian populations. Neuroreport. 2003;14:569–572. doi: 10.1097/00001756-200303240-00008. [DOI] [PubMed] [Google Scholar]
- 156.Schinka JA, Town T, Abdullah L, Crawford FC, Ordorica PI, Francis E, Hughes P, Graves AB, Mortimer JA, Mullan M. A functional polymorphism within the mu-opioid receptor gene and risk for abuse of alcohol and other substances. Mol Psychiatry. 2002;7:224–228. doi: 10.1038/sj.mp.4000951. [DOI] [PubMed] [Google Scholar]
- 157.Nikolov MA, Beltcheva O, Galabova A, Ljubenova A, Jankova E, Gergov G, Russev AA, Lynskey MT, Nelson EC, Nesheva E, Krasteva D, Lazarov P, Mitev VI, Kremensky IM, Kaneva RP, Todorov AA. No evidence of association between 118A>G OPRM1 polymorphism and heroin dependence in a large Bulgarian case-control sample. Drug Alcohol Depend. 2011;117:62–65. doi: 10.1016/j.drugalcdep.2010.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Levran O, Londono D, O’Hara K, Nielsen DA, Peles E, Rotrosen J, Casadonte P, Linzy S, Randesi M, Ott J, Adelson M, Kreek MJ. Genetic susceptibility to heroin addiction; a candidate-gene association study. Genes Brain Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gelernter J, Kranzler H, Cubells J. Genetics of two mu opioid receptor gene (OPRM1) exon I polymorphisms: Population studies, and allele frequencies in alcohol- and drug-dependent subjects. Mol Psychiatry. 1999;4:476–483. doi: 10.1038/sj.mp.4000556. [DOI] [PubMed] [Google Scholar]
- 160.Franke P, Wang T, Nöthen MM, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W. Nonreplication of association between mu-opioid-receptor gene (OPRM1) A118G polymorphism and substance dependence. Am J Med Genet. 2001;105:114–119. [PubMed] [Google Scholar]
- 161.Shi J, Hui L, Xu Y, Wang F, Huang W, Hu G. Sequence variations in the mu-opioid receptor gene (OPRM1) associated with human addiction to heroin. Hum Mutat. 2002;19:459–460. doi: 10.1002/humu.9026. [DOI] [PubMed] [Google Scholar]
- 162.Crowley JJ, Oslin DW, Patkar AA, Gottheil E, DeMaria PA, Jr, O’Brien CP, Berrettini WH, Grice DE. A genetic association study of the mu opioid receptor and severe opioid dependence. Psychiatr Genet. 2003;13:169–173. doi: 10.1097/00041444-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 163.Franke P, Wendel B, Knapp M, Neidt H, Albrecht S, Jahnes E, Propping P, Maier W. Introducing a new recruitment approach to sample collection for genetic association studies in opioid dependence. Eur Psychiatry. 2003;18:18–22. doi: 10.1016/s0924-9338(02)00005-6. [DOI] [PubMed] [Google Scholar]
- 164.Compton P, Geschwind DH, Alarcón M. Association between human mu-opioid receptor gene polymorphism, pain tolerance, and opioid addiction. Am J Med Genet B Neuropsychiatr Genet. 2003;121BM:76–82. doi: 10.1002/ajmg.b.20057. [DOI] [PubMed] [Google Scholar]
- 165.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U.S.A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pasternak GW. Multiple opiate receptors: Déjà vu all over again. Neuropharmacology. 2004;47(Suppl):1312–1323. doi: 10.1016/j.neuropharm.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 167.Pasternak GW. Insights into mu opioid pharmacology the role of mu opioid receptor subtypes. Life Sci. 2001;68:2213–2219. doi: 10.1016/s0024-3205(01)01008-6. [DOI] [PubMed] [Google Scholar]
- 168.Melief EJ, Miyatake M, Bruchas MR, Chavkin C. Ligand-directed c-Jun N-terminal kinase activation disrupts opioid receptor signaling. Proc Natl Acad Sci U.S.A. 2010;107:11608–11613. doi: 10.1073/pnas.1000751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goldsmith JR, Uronis JM, Jobin C. Mu opioid signaling protects against acute murine intestinal injury in a manner involving stat3 signaling. Am J Pathol. 2011;179:673–683. doi: 10.1016/j.ajpath.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.McLennan GP, Kiss A, Miyatake M, Belcheva MM, Chambers KT, Pozek JJ, Mohabbat Y, Moyer RA, Bohn LM, Coscia CJ. Kappa opioids promote the proliferation of astrocytes via Gbetagamma and beta-arrestin 2-dependent MAPK-mediated pathways. J Neurochem. 2008;107:1753–1765. doi: 10.1111/j.1471-4159.2008.05745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bruchas MR, Xu M, Chavkin C. Repeated swim stress induces kappa opioid-mediated activation of extracellular signal-regulated kinase 1/2. Neuroreport. 2008;19:1417–1422. doi: 10.1097/WNR.0b013e32830dd655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18019. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Finley MJ, Steele A, Cornwell WD, Rogers TJ. Transcriptional regulation of the major HIV-1 coreceptor, CXCR4, by the kappa opioid receptor. J Leukoc Biol. 2011;90:111–121. doi: 10.1189/jlb.1010546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Eisinger DA, Ammer H. Down-regulation of c-Cbl by Morphine Accounts for Persistent ERK1/2 Signaling in δ-Opioid Receptor-expressing HEK293 Cells. J Biol Chem. 2009;284:34819–34828. doi: 10.1074/jbc.M109.042937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eisinger DA, Schulz R. Extracellular signal-regulated kinase/mitogen-activated protein kinases block internalization of delta-opioid receptors. J. Pharmacol Exp Ther. 2004;309:776–785. doi: 10.1124/jpet.103.061788. [DOI] [PubMed] [Google Scholar]
- 177.Audet N, Paquin-Gobeil M, Landry-Paquet O, Schiller PW, Piñeyro G. Internalization and Src Activity Regulate the Time Course of ERK Activation by Delta Opioid Receptor Ligands. J Biol Chem. 2005;280:7808–7816. doi: 10.1074/jbc.M411695200. [DOI] [PubMed] [Google Scholar]
- 178.Olianas MC, Dedoni S, Onali P. Regulation of PI3K/Akt signaling by N-desmethylclozapine through activation of δ-opioid receptor. Eur J Pharmacol. 2011;660:341–350. doi: 10.1016/j.ejphar.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 179.Heiss A, Ammer H, Eisinger DA. delta-Opioid receptor-stimulated Akt signaling in neuroblastoma x glioma (NG108-15) hybrid cells involves receptor tyrosine kinase-mediated PI3K activation. Exp Cell Res. 2009;315:2115–2125. doi: 10.1016/j.yexcr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 180.Olianas MC, Dedoni S, Onali P. Signaling pathways mediating phosphorylation and inactivation of glycogen synthase kinase-3β by the recombinant human δ-opioid receptor stably expressed in Chinese hamster ovary cells. Neuropharmacology. 2011;60:1326–1336. doi: 10.1016/j.neuropharm.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 181.Olianas MC, Dedoni S, Onali P. δ-Opioid receptors stimulate GLUT1-mediated glucose uptake through Src- and IGF-1 receptor-dependent activation of PI3-kinase signalling in CHO cells. Br J Pharmacol. 2011;163:624–637. doi: 10.1111/j.1476-5381.2011.01234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ross J, Armstead WM. NOC/oFQ activates ERK and JNK but not p38 MAPK to impair prostaglandin cerebrovasodilation after brain injury. Brain Res. 2005;1054:95–102. doi: 10.1016/j.brainres.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 183.Chan AS, Wong YH. Regulation of c-Jun N-terminal kinase by the ORL(1) receptor through multiple G proteins. J Pharmacol Exp Ther. 2000;295:1094–1100. [PubMed] [Google Scholar]
- 184.Ducat E, Ray B, Bart G, Umemura Y, Varon J, Ho A, Kreek MJ. Mu-opioid receptor A118G polymorphism in healthy volunteers affects hypothalamic-pituitary-adrenal axis adrenocorticotropic hormone stress response to metyrapone. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00313.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: Possible implications for opiate addiction. Proc Natl Acad Sci U.S.A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Oertel BG, Kettner M, Scholich K, Renné C, Roskam B, Geisslinger G, Schmidt PH, Lötsch J. A common human micro-opioid receptor genetic variant diminishes the receptor signaling efficacy in brain regions processing the sensory information of pain. J Biol Chem. 2009;284:6530–6535. doi: 10.1074/jbc.M807030200. [DOI] [PubMed] [Google Scholar]
- 187.Zhang Y, Wang D, Johnson AD, Papp AC, Sadée W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 188.Deb I, Chakraborty J, Gangopadhyay PK, Choudhury SR, Das S. Single-nucleotide polymorphism (A118G) in exon 1 of OPRM1 gene causes alteration in downstream signaling by mu-opioid receptor and may contribute to the genetic risk for addiction. J Neurochem. 2010;112:486–496. doi: 10.1111/j.1471-4159.2009.06472.x. [DOI] [PubMed] [Google Scholar]
- 189.Berretini WH, Hoehe MR, Ferraro TN, Demaria PA, Gottheil E. Human mu opioid receptor gene polymorphisms and vulnerability to substance abuse. Addiction Biology. 1997;2:303–308. doi: 10.1080/13556219772598. [DOI] [PubMed] [Google Scholar]
- 190.Crystal HA, Hamon S, Randesi M, Cook J, Anastos K, Lazar J, Liu C, Pearce L, Golub E, Valcour V, Weber KM, Holman S, Ho A, Kreek MJ. A C17T polymorphism in the mu opiate receptor is associated with quantitative measures of drug use in African American women. Addict Biol. 2010 doi: 10.1111/j.1369-1600.2010.00265.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang H, Gelernter J, Gruen JR, Kranzler HR, Herman AI, Simen AA. Functional impact of a single-nucleotide polymorphism in the OPRD1 promoter region. J Hum Genet. 2010;55:278–284. doi: 10.1038/jhg.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Tuusa JT, Petäjä-Repo UE. Phe27Cys polymorphism of the human delta opioid receptor predisposes cells to compromised calcium signaling. Mol Cell Biochem. 2011;351:173–181. doi: 10.1007/s11010-011-0725-5. [DOI] [PubMed] [Google Scholar]
- 193.Leskelä TT, Markkanen PMH, Alahuhta IA, Tuusa JT, Petäjä-Repo UE. Phe27Cys polymorphism alters the maturation and subcellular localization of the human delta opioid receptor. Traffic. 2009;10:116–129. doi: 10.1111/j.1600-0854.2008.00846.x. [DOI] [PubMed] [Google Scholar]
- 194.Zhang H, Kranzler HR, Yang B-Z, Luo X, Gelernter J. The OPRD1 and OPRK1 loci in alcohol or drug dependence: OPRD1 variation modulates substance dependence risk. Mol Psychiatry. 2008;13:531–543. doi: 10.1038/sj.mp.4002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Gerra G, Leonardi C, Cortese E, D’Amore A, Lucchini A, Strepparola G, Serio G, Farina G, Magnelli F, Zaimovic A, Mancini A, Turci M, Manfredini M, Donnini C. Human kappa opioid receptor gene (OPRK1) polymorphism is associated with opiate addiction. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:771–775. doi: 10.1002/ajmg.b.30510. [DOI] [PubMed] [Google Scholar]
- 196.Yuferov V, Fussell D, LaForge KS, Nielsen DA, Gordon D, Ho A, Leal SM, Ott J, Kreek MJ. Redefinition of the human kappa opioid receptor gene (OPRK1) structure and association of haplotypes with opiate addiction. Pharmacogenetics. 2004;14:793–804. doi: 10.1097/00008571-200412000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Biocca S, Falconi M, Filesi I, Baldini F, Vecchione L, Mango R, Romeo F, Federici G, Desideri A, Novelli G. Functional analysis and molecular dynamics simulation of LOX-1 K167N polymorphism reveal alteration of receptor activity. PLoS ONE. 2009;4:e4648. doi: 10.1371/journal.pone.0004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xuei X, Flury-Wetherill L, Almasy L, Bierut L, Tischfield J, Schuckit M, Nurnberger JI, Jr, Foroud T, Edenberg HJ. Association analysis of genes encoding the nociceptin receptor (OPRL1) and its endogenous ligand (PNOC) with alcohol or illicit drug dependence. Addict Biol. 2008;13:80–87. doi: 10.1111/j.1369-1600.2007.00082.x. [DOI] [PubMed] [Google Scholar]