Abstract
Most prior research on the neurobiology of addiction has focused on the role of subcortical systems, such as the amygdala, the ventral striatum and mesolimbic dopamine system, in promoting the motivation to seek drugs. Recent evidence indicates that a largely overlooked structure, the insula, plays a crucial part in conscious urges to take drugs. The insula has been highlighted as a region that integrates interoceptive (i.e. bodily) states into conscious feelings and into decision-making processes that involve uncertain risk and reward. Here, we propose a model in which the processing of the interoceptive effects of drug use by the insula contributes to conscious drug urges and to decision-making processes that precipitate relapse.
Evidence for the role of the insula in addiction
Addiction to drugs of abuse is a major public health concern. By itself, cigarette smoking, the most common addictive behavior, is the largest preventable cause of morbidity and mortality in the developed world [1]. Drug addiction is a mental disorder characterized by the compulsive use of drugs that persists despite awareness of negative consequences [2]. Underlying addiction is a set of physiological and psychological processes, such as tolerance, withdrawal, learning, incentive motivation, conscious urges and maladaptive decision making, that have distinct yet complimentary roles in the development and maintenance of addiction.
The insular cortex, or insula, is of particular interest in the study of drug addiction because of its probable role in conscious urges to take drugs. Many functional imaging studies have revealed activation of the insula during drug urges, although none of these studies has focused on the insula specifically. Many of these studies have shown that activity within the insula is correlated with the subjects’ ratings of urge (Table 1). This indicates that the insula has a role in the generation of the conscious feeling of urge. However, these studies merely demonstrate correlation of insula activity with conscious urges; they do not prove a causal role for the insula in conscious urges – a limitation common to many functional imaging studies. Also, although it seems self-evident that conscious urges should have an important role in promoting drug dependence, some authors (e.g. [3–5]) have argued that conscious urges are less important than implicit (i.e. non-conscious) motivational processes for driving ongoing drug use in a dependent individual. Thus, it is necessary to ask the question of whether the insula (and the conscious urges that might depend upon this region) are necessary for maintaining the addiction to drugs of abuse.
Table 1.
Functional imaging studies demonstrating activity in the insula during drug urgesa
| Drug | Insula | OFCb | ACC | DLPFC | Amygdala | VS | HF | Refs |
|---|---|---|---|---|---|---|---|---|
| Cigarettes | L | L | L,R | Lc | No activity | No activity | No activity | [129] |
| Cigarettes | L | R | No activity | Lc | L,R | L,R | L,R | [130] |
| Cigarettes | Lc,Rc | Lc,Rc | L,R | Lc,Rc | L | No activity | No activity | [131] |
| Cigarettes | Lc,Rc | No activity | Lc,Rc | Lc | No activity | No activity | No activity | [115] |
| Cigarettes | L,R | No activity | Lc,Rc | Lc,Rc | No activity | No activity | No activity | [132] |
| Cigarettes | R | L | L | R | No activity | No activity | No activity | [133] |
| Cigarettes | Rc | Rc | Rc | Rc | Rc | Rc | Lc,Rc | [134] |
| Cocaine | L | No activity | R | No activity | L,R | R | No activity | [135] |
| Cocaine | Lc | Lc | No activity | Rc | Lc | No activity | No activity | [136] |
| Cocaine | Lc,Rc | Lc,Rc | L | No activity | L,R | R | No activity | [137] |
| Cocaine | L,Rc | Lc,Rc | No activity | No activity | No activity | No activity | No activity | [138] |
| Cocaine | R | No activity | L | L,R | No activity | No activity | No activity | [139] |
| Cocaine | R | No activity | L,R | L | No activity | No activity | No activity | [140] |
| Alcohol | L,R | Lc,Rc | Lc,R | No activity | No activity | Lc,R | L | [141] |
| Alcohol | L,R | No activity | L | L | No activity | No activity | No activity | [142] |
| Heroin | L | L | L,R | No activity | No activity | No activity | No activity | [143] |
All are studies of cue-induced urges, except Ref. [134], which is a study of abstinence-induced urges.
Abbreviations: ACC, anterior cingulate cortex; DLPFC, dorsolateral prefrontal cortex; HF, hippocampal formation; L, left; OFC, orbitofrontal cortex; R, right; VS, ventral striatum.
Indicates correlation with self-reported urges. Note that activity in the insula is frequently correlated with subjective urges. Also note the paucity of activation in subcortical structures, which indicates that conscious urges mediated by the insula might be dissociable from processes mediated by these subcortical regions.
More recent studies have examined how executive functions mediated by the insula might play a part in addiction. Paulus and colleagues [6–8] have shown that a high level of activity in the insula during a simple decision-making task is associated with relapse to methamphetamine use, indicating that dysfunction of the insula underlies some of the abnormal decision making that leads to continued drug use in the face of negative consequences. The idea that insula dysfunction underlies drug addiction is also supported by a study showing that chronic cocaine users have reduced grey: white matter ratios in the insula [9]. This finding is of note also because of the well-known increase in prevalence of cigarette smoking among schizophrenics, who also have a reduction in insular grey matter [10]. It remains to be seen whether these observed abnormalities in insula structure reflect a strengthening of processes that promote ongoing drug use (e.g. urges), a weakening of processes that prevent ongoing drug use (e.g. decision-making functions that avert relapse) or both.
We explicitly addressed the role of the insula in addiction in a recent study [11] in which we examined the effects of insula lesions in addicted smokers. We examined smoking addiction because of its prevalence and public health importance, in addition to the ready availability of smokers among the population of individuals with stroke. We compared 19 smokers who sustained damage in the insula with 50 smokers who sustained damage in other brain areas, retrospectively assessing changes in their smoking behavior after suffering from brain damage. We found that smokers with brain damage involving the insula were >100 times more likely than smokers with brain damage not involving the insula to undergo a ‘disruption of smoking addiction’, characterized by the ability to quit smoking easily, immediately, without relapse and without a persistence of the urge to smoke. In one case, this disruption of addiction after insula damage was so profound as to lead one patient to proclaim that his ‘body forgot the urge to smoke’. This study was notable because it provided the first evidence in humans that a specific brain region played a crucial part in addiction, although it was limited in that it examined disruption of addiction retrospectively and looked only at a single drug of abuse. More importantly, perhaps, this study drew attention to a brain region that had been largely ignored in the drug addiction literature up until that point.
This finding was quickly corroborated by a study in rats [12] showing that inactivation of the insula by focal injection of the local anesthetic lidocaine disrupts amphetamine-conditioned place preference. In the conditioned place-preference paradigm, the animals were required to choose between entering a white room where amphetamine was delivered or a black room where saline was delivered. Rats initially preferred the black room because a darker environment provides a greater sense of safety from predators. Over time, the animals came to prefer the white (amphetamine-paired) room, presumably because of explicit knowledge about the relationship between a specific action (entering the white room) and its outcome (receiving a dose of amphetamine). Because of the conditions of the experiment, this also meant that the animals had to choose between obtaining a drug under perceived risk and receiving no drug under perceived safety. This preference for the amphetamine-paired environment was then abolished by injection of lidocaine into the insula. Once the lidocaine wore off, the rats once again preferred the amphetamine-paired environment.
The functional imaging data, together with the lesion and inactivation findings in humans and rats, provide evidence that (i) the insula is necessary for the explicit motivation to take drugs (e.g. conscious drug urges), (ii) this function is common across drugs of abuse and (iii) explicit motivation is an important factor in promoting drug addiction. These findings, which in many ways raise more questions than they answer, provide a strong impetus to further explore the functions of the insula in addiction. Doing so requires a theoretical framework that integrates our current understanding of the functions of the insula with our knowledge of the psychological processes that underlie drug addiction.
Anatomy and function of the insula: a historical perspective
The term ‘insula’ was coined by Johann Christian Reil who described an island of cortex (insula is Latin for island) in the depth of the cerebral mantle situated between the banks of the Sylvian fissure. The insula has been divided into various subregions based upon both anatomical connectivity and cytoarchitectonic features [13–17]. The more posterior, granular regions of the insula, which receive inputs from the thalamus, in addition to parietal, occipital and temporal association cortices, have been ascribed a role in somatosensory, vestibular and motor integration. The more anterior, agranular regions, which have reciprocal connections to ‘limbic’ regions, such as the anterior cingulate cortex, the ventromedial prefrontal cortex, the amygdala and the ventral striatum, have been ascribed a role in the integration of autonomic and visceral information into emotional and motivational functions.
The chemoarchitecture of the insula, especially the anterior, agranular insula, supports its role in motivation, emotion and addiction. The agranular insula receives strong dopaminergic innervation [18] and contains a high density of D1 dopamine receptors [19]. Dopaminergic function in the agranular insula might mediate some of the rewarding effects of drugs of abuse in addition to neural plasticity that underlies the development of addiction. The agranular insula also contains a high concentration of endogenous opioids [18] and a high density of μ-opioid receptors [20], which might play a part in pain modulation and the rewarding effects of some drugs of abuse. The agranular insula also contains a high density of type 1 corticotropin-releasing hormone receptors [21], which might have a role in stress-related motivation to take drugs of abuse [22] and to which antagonists have recently been developed [23]. Given the important role that corticotropin-releasing hormones have in alcohol intake [24,25], targeting these receptors can have therapeutic potential for addiction. Thus, the rich chemoarchitecture of the insula provides several potential targets for pharmacological manipulation of its function.
Early lesion studies on the functions of the insula ascribed a role in language [26]. Although scientists of the late 19th and early 20th century included the insula, especially the left anterior insula, as part of the anterior language system because of the relationship between lesions there and symptoms of aphasia, later authors argued that aphasia was secondary to disruption of association fibers surrounding the insula that later became known as the arcuate fasciculus (for review, see Ref. [27]). More recent functional imaging studies point to a role for the left anterior insula in language, in particular the motor aspects of speech production (for review, see Ref. [28]).
Early studies also implicated the insula in gustation. Unpleasant taste experiences, along with gastric sensations, have been described in patients with epileptic seizures arising close to the insula [26,29]. Later anatomical [30] and physiological [31,32] studies have demonstrated that the insula, in particular its opercular aspect, is the primary taste cortex. Lesion studies in humans have shown effects on taste recognition after posterior insula lesions [33,34]. Lesion studies in rodents have demonstrated a role for the agranular insula in working memory for taste [35,36] and in conditioned taste aversion [37,38], in which the animal learns to avoid a previously palatable taste after it becomes associated with a stimulus that causes sickness or malaise. Dopaminergic function within this region has been found to play a particular part in this type of learning [39]. Related to conditioned taste aversion is the experience of disgust. Lesion studies in humans [40] and functional imaging studies [37] have shown the insula to be involved in both the experience of disgust and the recognition of disgust in others.
Similar to its role in taste, the insula is the primary thalamorecipient cortex for general visceral sensation (i.e. sensation arising from the internal organs including the gut, the airway and the cardiovascular system) [41,42]. More recent studies have elaborated on these functions of the insula, and several functional imaging studies have shown that the insula is activated by a variety of visceral stimuli, including changes in cardiovascular function [43,44], stimulation of the gastrointestinal tract [45–48] and stimulation of the upper airway [49]. In addition, electrical stimulation of the insula leads to changes in cardiovascular function [50,51] indicating that this region has visceral motor functions in addition to visceral sensory functions.
Studies in the late 1980s indicated that the insula is linked to the asymbolia for pain in six patients [52], characterized by the lack of an emotional response to painful stimuli. Perhaps this is the first hint that the insula might be involved in the emotional and/or motivational experience of pain. However, this role in emotion was never emphasized. Subsequent studies have emphasized the role the insula has in pain processing, with functional imaging studies indicating a role in representing the sensory or discriminative aspects of pain [53,54] and memory for pain [55].
Given its anatomy and connections, the insula also has a role in the processing of certain forms of somatic sensation. These functions were more recently examined with functional imaging studies, which have shown that this region is activated by thermal stimuli; the activity in the posterior insula contralateral to the side of the thermal stimulus is related to stimulus properties and activity in the right anterior insula (irrespective of the side of stimulation) is related to the conscious perception of the stimulus [56,57]. The insula has also been implicated in the processing of certain kinds of sensual touch [58] and itch [59].
Synthesizing much of this work, Craig [56] tied the myriad sensory functions of the insula under the unifying concept of interoception. Interoception, according to Craig, is the neural mapping of bodily states that have special relevance for the maintenance of homeostatis. Pain, temperature, taste, visceral sensation, inflammation, itch and sensual touch all signal that a change in the state of the body has occurred that will either promote or impede survival. These bodily states all have hedonic value; they are all associated with distinct subjective qualities that are either pleasant or unpleasant. They are distinct from somatic sensations, such as joint-position sense or discriminative touch, which do not possess inherent hedonic value. Using detailed anatomical tracing techniques, Craig and his colleagues have shown that interoceptive states are signaled in the brain through a specialized channel that begins with specific peripheral nerve fibers that synapse on dedicated spinal and brainstem pathways and project to the insula via specific thalamic relays [60,61]. Craig has proposed [56,62] that serial processing of interoceptive information occurs within the insula, with a progression from posterior or dorsal regions to anterior ventral regions, culminating in the right anterior insula where conscious awareness of interoceptive stimuli arises. This has been corroborated by functional imaging evidence showing that both the activity and structure of the right anterior insula are related to the ability to detect one’s heartbeat [43].
Thus, despite several decades of studies on the insula and its functions, it was only in the early 1990s that the notions of conscious emotional experience and feeling began to be attributed to the insula [63]. According to Damasio [64], the right insula, along with right somatosensory cortices, are crucial for subjective emotional feelings because of their role in mapping the bodily states that are elicited by emotions. This was a neurobiological formulation of the ideas of James [65] and was a precursor to the ideas of Craig [56]. Damasio also proposed an ‘as if’ representation of bodily states, in which representations of previously experienced bodily states are evoked in regions such as the insula as if they’ve arisen in the body, even though they have not. This is the recall of interoceptive memory. Damasio’s theory of conscious feeling went further to place the insula within a network of regions that trigger bodily states (e.g. the amygdala and ventromedial prefrontal cortex), map bodily states (e.g. the insula and somatosensory cortices in addition to regions within the brainstem and hypothalamus) and represent the relationship between changes in the bodily state and the objects that elicited them (e.g. the anterior cingulate cortex) [64]. In Damasio’s model, the insula serves an explicit (i.e. available to consciousness) representation of the bodily states that are elicited by emotionally competent stimuli. This representation gives rise to an emotional feeling when it is integrated with representations of objects or events that elicited the bodily states. By this process, feelings are not merely the conscious awareness of sensations arising from the body but complex experiences that lend meaning to objects and events in the world. Since its inception, this model has been substantiated by several functional imaging studies showing that this ‘feeling network’, which includes the insula, is activated by a variety of subjective emotional states including primary emotions such as fear, anger, happiness and sadness (see the meta-analysis in Ref. [66]) and desire states such as hunger [67–69], thirst [70,71], sexual arousal [72–74] and drug urges (Table 1).
The psychology and neurobiology of addiction: a historical perspective
Various models have been applied to the phenomenon of impulsive and compulsive drug use, but most emphasize one of two possibilities. The first attributes drug motivation, especially with drugs that produce physical dependence such as opioids, to the need to alleviate the withdrawal-distress resultant from a previous history of drug use [75]. The other incentive possibility stresses drug-like rather than drug-withdrawal states as the most powerful instigator of drug use and that drug motivation occurs in the absence of withdrawal and independent of any past drug history [76,77]. The view that withdrawal and physical dependence are the prime instigators of drug intake was challenged by an incentive motivational view [76,77] for several reasons. First, the withdrawal view did not explain why drug self-administration gets established in initially non-dependent humans [78] and animals [76,79]. Second, proponents of the incentive view argued that the self-administration of drugs usually occurs when the drug stimulus is still present in the brain rather than when the last drug injection is fully metabolized and the withdrawal condition is fully established [76].
Although the evidence that withdrawal and physical dependence are not necessary conditions for drugs to be sought is substantial [76,77], the possibility that withdrawal might be a sufficient condition for the maintenance of drug administration in physically dependent subjects has never been ruled out [80–82]. Thus, more contemporary models of addiction have attempted to reconcile both views by proposing that, although drug seeking might begin through incentive mechanisms, chronic drug use can lead to tolerance to the acute incentive properties of drugs and the primary determinants of continued drug use become the avoidance or termination of drug abstinence [82].
Today, there is a wide scientific consensus that the reinforcing effects of nearly all drugs of abuse are attributed to their ability to stimulate the release of dopamine from neurons arising from the brainstem ventral tegmental area [4,77,82–88]. Historically, the first proposal of a unique relationship between dopamine and reward was provided in 1982 by Wise [89] who implicated dopamine, as opposed to other catecholamines (especially noreadrenaline), in reward processes. The literature from that era clearly demonstrates that the dopaminergic projection to the nucleus accumbens (the mesolimbic dopamine system) is the one that has the most important role in the reward derived from drugs of abuse (e.g. psychostimulants) [77,90]. It was also recognized at the time that the abuse potential of drugs of abuse (e.g. opiates, alcohol, nicotine, caffeine, barbiturates, benzodiazepines, cannabis and phencyclidine) all are linked, one way or another, to this mesolimbic dopamine system. Although these different drugs might act initially on different receptor sites in the brain, ultimately they all act (directly or indirectly) on the mesolimbic dopamine system to exert reward [77,90].
The evidence that blockade of dopamine neurotransmission in the ncleus accumbens interfered with the motivation to seek rewards prompted Wise [89] to propose the ‘anhedonia’ hypothesis that dopamine mediates the pleasure produced by drugs that compulsive drug users seek. However, Wise himself retracted, shortly after, the notion that dopamine blockade reduces pleasure, and he replaced the anhedonia hypothesis with an incentive-based theory of motivation, the ‘psychostimulant’ theory [77]. The key aspect of that theory is that the mesolimbic dopamine system has a key role in mediating the ‘approach’ response elicited by drugs in addition to natural rewards. In other words, a feeling of pleasure is not necessarily experienced when dopamine is released; mesolimbic dopamine strengthens the approach response and motivational arousal elicited by rewards, which are clearly associated with pleasure. Nonetheless, this notion that dopamine is the ‘pleasure’ neurotransmitter of the brain has had an insurmountable appeal; it seems to continue to linger until today in various media reports. Even the more recent functional neuroimaging work in humans that address the reward mechanisms mediated by the nucleus accumbens often discuss the role of dopamine in this region in a manner that is hardly distinguishable from the notions of pleasure.
Later research showed that the process of reward can be further subdivided into (i) a ‘wanting’ component, which makes rewards attractive and wanted and which triggers ‘approach’ and pursuit of the reward; and (ii) a ‘liking’ component, which involves feeling of pleasure [91]. Although there seem to be additional systems in the brain (which remain unidentified) that mediate the liking or pleasure component, the mesolimbic dopamine system is crucial for specifically this wanting component of the reward. With repeated drug use, the mesolimbic dopamine projections to the nucleus accumbens become sensitized and eventually lead to excessive incentive salience attribution to the drugs and drug-related stimuli, which activate this neural circuitry, thus, making them highly attractive and pathologically wanted [4].
Additional research explored other elements of the neural circuitry involved in the motivation to seek drugs, such as the amygdala and its anatomical connections with the ventral striatum [3,92]. Although these early animal studies did implicate the prefrontal cortex in addictive processes [3,92], this role was not emphasized until subsequent human studies came along, which showed a link between abnormalities in the activity of the orbitofrontal region and addiction to cocaine [93–97]. Nonetheless, this earlier work has led to the proposal of several influential models of addiction, indicating that addiction might be related to two processes [84,85,98,99]. One process relates to abnormal activity in the extended amygdala system, thereby resulting in exaggerated processing of the incentive values of drug-related stimuli. The other process relates to abnormal activity of the prefrontal cortex system necessary for inhibiting the substance-seeking action associated with immediate reward.
More than twenty-five years have passed and the original conclusion that manipulations of the mesolimbic dopamine system, and specifically the projection to the ventral striatum (which includes the nucleus accumbens), influence the motivation to seek rewards remains valid [3,87]. Although functional magnetic resonance imaging (fMRI) approaches cannot technically address dopamine, the fact is that the neural regions receiving these dopamine projections (i.e. the ventral striatum, which includes the nucleus accumbens) are now implicated in a variety of reward processes. These include video games, viewing of sexual materials and monetary rewards, to name a few. These findings validate a large body of evidence that employed a variety of elecrophysiological, microdialysis or voltammetric techniques that accumulated over twenty-five years of research and concluded that the mesolimbic dopamine projection to nucleus accumbens plays a key part in reward processes.
In summary, a large literature has examined the neurobiological substrates of addiction with the goal of developing pharmacological and psychological treatments that are targeted at specific neural systems. Studies using animal models have emphasized the role of subcortical systems, such as the amygdala, nucleus accumbens and the mesolimbic dopamine system, in addiction to drugs of abuse (for review, see Refs [4,77,82–85]). These studies have tended to focus on externally observable aspects of addiction (i.e. they are based upon observation of a simple set of drug self-administration behaviors). By contrast, functional imaging studies of human drug abusers, which have tended to focus on the inner experience of subjects as they are exposed to drug-related stimuli (i.e. cue-induced drug urges), have revealed activation in cortical systems including the anterior cingulate cortex, the orbitofrontal and/or ventromedial prefrontal cortex and the insula (Table 1). From the decades of animal and human research on addiction it is clear that both incentives and internal states (e.g. withdrawal) jointly determine the motivation to seek drugs. Incentive stimuli generate motivation in the animal (or human) and instigate approach responses in relation to themselves. However, internal factors associated with deprivation states (such as withdrawal) are viewed as a ‘gate’ that determines how effective the incentive input is in exciting the motivational circuits that ‘pull’ and ‘steer’ the animal (or human) towards the appropriate goal object. Although decades of research have focused on the neural substrates subserving the incentive motivational component of drugs, very little research, even in animals, has addressed the brain mechanisms by which deprivation states, such as drug withdrawal, might interact with the neural circuitry mediating drug incentives. In humans, the importance of these physiological states in modulating the incentive to take drugs and understanding their underlying neural mechanisms have begun to attract attention (for review, see Ref. [7]). The role that we propose for the insula in addiction is consistent with the basic predictions of these influential models of addiction.
The role of interoception in addiction
Although the dopamine system clearly has an important role in addiction to drugs of abuse, drug use does more for the addicted individual than merely providing a means of releasing dopamine in the brain. Drug use involves a complex set of rituals imbued with emotional meaning (both positive and negative) for the addicted individual.
Nearly all drug-use rituals have highly salient and distinctive effects on the body that are likely to contribute to their emotional meaning. For example, snorting cocaine elicits a bitter taste, a harsh sensation in the nose and throat, and increases in heart rate and blood pressure that are mediated by sympathomimetic effects. Alcoholic beverages all have strong tastes and oropharyngeal sensory effects, in addition to autonomic effects. Use of intravenous drugs, such as heroin, involves violating the body envelope with a needle. Cigarette smoking elicits a myriad of effects on the body, including changes in autonomic function that are mediated by the autonomic actions of nicotine and effects on the upper airway that are felt with every puff. Smoking marijuana also has airway sensory and autonomic effects. In addition to the bodily effects of taking drugs, discontinuing the use of many drugs can lead to withdrawal syndromes with characteristic autonomic effects. What ties these bodily effects of drug use and withdrawal together is that they all have interoceptive properties, such that they stimulate sensory pathways that function under normal conditions to signal events that have potential effects on homeostasis.
The centrality of interoceptive processes in addiction has been well established for the deadliest and most common of the addictions: addiction to cigarette smoking. Several studies have focused on the role of airway stimulation in smoking addiction [100,101]. They have shown, for example, that cigarette smoke has potent airway sensory properties that are due, in large measure, to the effects of nicotine upon vagal, glossopharyngeal and trigeminal afferents in the pharynx and upper airway. These airway sensory effects are experienced as pleasurable and are highly effective at reducing the urge to smoke [102–104]. Importantly, it seems that airway sensory effects of smoking are more effective at reducing urges to smoke than are the pharmacologic effects of nicotine [104–106]. Furthermore, blocking airway sensation significantly reduces the pleasure and satiety obtained from smoking [107,108]. The airway sensory effects of smoking have been extensively researched and manipulated by the tobacco industry to maximize ‘flavor’ and ‘impact’ to promote brand loyalty [109](http://tobaccodocuments.org/rjr/500917506-7534.html; http://tobaccodocuments.org/rjr/505098655-8663.html). The primacy of airway sensation in smoking pleasure and satisfaction might be the reason why nicotine replacement, the current first-line therapy for smoking addiction, is only moderately effective at promoting abstinence [110,111]. This idea has led to the development of novel treatments for smoking addiction, such as denicotinized cigarettes and irritant inhalers, that are designed to simulate the airway effects of smoking without delivering nicotine to the brain. Such treatments have been shown in clinical trials to be effective adjuncts to nicotine replacement for promoting abstinence and preventing relapse, at least in the short term [103]. Importantly, airway sensations stimulated by inhalation of irritant substances are signaled in the insula [49].
Proposed role for the insula in addiction
Evidence points toward a crucial role for the insula in conscious drug urges and in translating interoceptive signals into conscious feelings and behavioral biases during decision making that involves uncertain risk and reward. Although nearly all drugs of abuse exert interoceptive effects that impart distinct subjective qualities to drug-use rituals, very little attention has been paid to the role of the insula in these effects. Thus, the question arises as to whether the insula has a role in representing the interoceptive effects of drug use and whether this representation contributes in some way to the subjective experience of drug use, conscious urges to take drugs and to the decision to take drugs in the face of negative consequences.
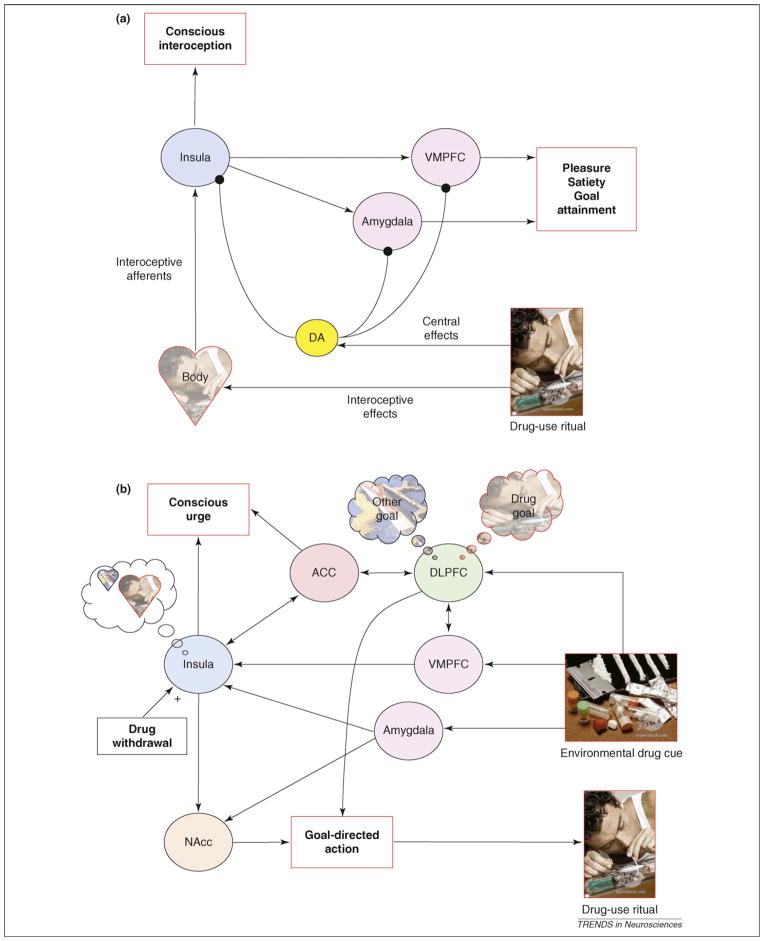
Based on the evidence presented thus far, we propose that the insula is a key neural structure for representing the interoceptive effects of drug use. These include the airway sensory and autonomic effects of cigarette smoking, the taste of alcohol, the sympathomimetic effects of cocaine and the pain of intravenous injection, among others. All of these stimuli engage interoceptive pathways and are transmitted to the brain via specific peripheral, spinal and brainstem pathways that reach the insula via specific interoceptive thalamic relays. The mapping of the interoceptive effects of drug use within the insula constitutes a necessary neural processing step that gives rise to the conscious appreciation of these effects. The subjective pleasure that is derived from the interoceptive effects of drug use, which is different from mere conscious appreciation of these effects, is likely to depend upon downstream regions, but the identities of these regions remain unclear. Nonetheless, regions such as the ventromedial prefrontal cortex (VMPFC) and amygdala have been implicated in processing the reward value of various stimuli, including interoceptive stimuli [112,113], which implies that these regions might play a part in the conscious appreciation of pleasure. This pleasure might be modulated by dopamine release within these areas, which itself is elicited by the direct central nervous system (CNS) effects of the drug (Figure 1a).
Figure 1.
A schematic model of how the interoceptive functions of the insula contribute to the motivation to use drugs. (a) The insula represents the interoceptive effects of drug-use rituals. This gives rise to a specific subjective quality of the drug-use ritual, which includes conscious appreciation of interoceptive effects in addition to pleasure and satiety (i.e. reward). Dopamine (DA) release, stimulated by the central effects of the drug, might modulate the reward derived from the interoceptive effects of drug use and also drives the learning process by which these effects become both pleasurable and desirable. (b) Exposure to environmental cues (e.g. the sight of drug paraphernalia) reactivates representations of the interoceptive effects of the drug-use ritual via the VMPFC and the amygdala. This gives rise to a subjective feeling of conscious urge that is rooted in a memory for these the interoceptive effects. This representation feeds into the nucleus accumbens (NAcc), which plays a part in initiating and invigorating motivated actions or reward seeking. In concert with the dorsolateral prefrontal cortex (DLPFC), which focuses attention and holds representations of specific goals in mind, this process gives rise to a goal-directed action to initiate the specific drug-use ritual, the interoceptive effects of which are currently represented within the insula. The anterior cingulate cortex (ACC) plays a part in conscious feelings of urge both by integrating representations of interoceptive states within the insula with representations of objects in the environment that triggered these states and by monitoring conflict between drug use and other, competing goals. Physiological signals related to drug withdrawal might also modulate these processes via the insula.
Over time, as addiction increases, stimuli within the environment that are associated with drug use become powerful incentives, initiating both automatic (i.e. implicit) motivational processes that drive ongoing drug use and relapse in addition to conscious (i.e. explicit) feelings of urge to take drugs. We suggest that the insula has a crucial role in conscious cue-induced urges. It does so by encoding a representation of the interoceptive effects of drug use that become activated when an addicted individual is exposed to environmental drug cues, such as the sight of another person engaging in the drug-use ritual. Cue-induced urges are to be distinguished from withdrawal urges, which are conscious urges that arise from homeostatic processes related to the absence of drug in the body. Withdrawal urges might also depend on the insula and might actually interact with cue-induced urges in ways that we describe later. Other regions that have a role in this process include the VMPFC and the amygdala, which receive information about the presence of drug cues in the environment or about thoughts of drug use and activate internal representations of drug-related bodily states in the insula (Figure 1b). This process might give rise to a distinct subjective feeling of urge that is tied to a memory for the interoceptive effects of the drug-use ritual. This would explain why the urge to smoke a cigarette feels different from the urge to snort cocaine, which itself feels different from the urge to smoke cocaine; each is rooted in distinct interoceptive effects, even though they all have similar direct CNS effects (i.e. facilitation of dopamine release). Upon activation, this internal representation magnifies the incentive value of a specific goal (e.g. smoking a cigarette) that has specific interoceptive effects (e.g. airway sensations) that have been experienced in the past as rewarding. It does so through excitatory projections of the insula to regions that are involved in initiating and invigorating motivated actions, such as the nucleus accumbens [13]. It also focuses attention on this goal by acting in concert with regions that are involved in maintaining specific goals ‘in mind’, such as the dorsolateral prefrontal cortex [114]. Through this process, the thoughts, feelings and actions elicited by drug cues come to be ‘about’ engaging in a specific drug-use ritual. At the same time, several cognitive factors might modulate the intensity of urge, such as the availability of drugs and the potential conflict between taking the drug and competing goals that require abstinence (e.g. avoiding negative consequences or sitting still in a brain scanner). This might depend on neural systems that function in monitoring and resolving conflict, such as the anterior cingulate cortex [115–117] (Figure 1b). Homeostatic disturbances, such as those induced by drug withdrawal, also have the capacity to intensify the activation of the interoceptive representations within the insula, thereby exaggerating further the incentive value of a specific goal.
Both the pleasure obtained from the interoceptive effects of drug use and cue-induced urges are learned emotional states. Many of the interoceptive effects of drug use, such as the airway sensory effects of smoking, are inherently unpleasant sensations that normally act to signal potential damage to the body. With repeated experience of the drug-use ritual, these very same sensations become a source of pleasure and satiety and, in our model, a goal that is brought to mind by exposure to drug cues. We hypothesize that interoceptive effects of drug use gain value through a learning process that involves dopamine-dependent neural plasticity within the insula and related areas, such as the VMPFC and the amygdala. This learning process has a twofold effect: (i) it ‘switches’ the hedonic value of the interoceptive effects of drug use from negative to positive and (ii) it forms associations between representations of these interoceptive effects and representations of objects and events that predict their occurrence. This plasticity, which occurs through biochemical and structural changes within the insula and the VMPFC, leads to the laying down of a highly stable representation of the interoceptive effects of drug use, such that addicted individuals are always at risk of relapse even many years after quitting. Evidence for this plasticity lies in the fact that acute cocaine administration induces immediate-early gene expression in the VMPFC and insula in non-human primates [118] and that chronic cocaine abuse is associated with structural changes in the insula and the VMPFC [9]. Disruption of insula function might reverse this learning process causing the interoceptive effects of drug use to revert to their unlearned, aversive hedonic value and causing drug-associated cues in the environment to lose their ability to evoke memories of the interoceptive effects of drug use. This would explain why one of our patients who suffered from insula damage from a stroke (a patient who smoked more than 2 packs per day for >20 years) reported that his ‘body forgot the urge to smoke’ and that the mere idea of smoking, even in his dreams, became disgusting [11].
Another way in which the interoceptive effects of drug use might contribute to the urge to take drugs is during drug withdrawal. Long-term use of most drugs of abuse results in adaptations in multiple neural, endocrine and visceral systems that are expressed when drug use is discontinued. In the case of alcohol, for example, cessation of long-term use results in tachycardia, hypertension, sweating, tremor and then, if left untreated, delirium, seizures and even death. The physiological state of withdrawal is usually short-lived, lasting days to weeks. Subjectively, withdrawal is associated with dysphoria, along with an intense urge to take the drug. Withdrawal urges might be seen as a subjective need to alleviate the dysphoria [75]. The insula might function to translate the physiological state of withdrawal, with all of its interoceptive components, into subjective dysphoria.
The interoceptive effects of drug use are not only experienced subjectively; they might also contribute to decision-making processes in which the consequences of drug use and abstinence are weighed against each other. Such decision-making processes might be particularly relevant during relapse. When a drug-addicted individual is faced with a stimulus or a thought that elicits a desire to take the drug, this desire, which is represented in the insula in interoceptive terms, must be weighed against explicit knowledge about the aversive medical, legal, economic and social consequences of drug use. We have previously proposed [119] that the VMPFC and insular system has a role in ‘marking’ the potential negative consequences of drug use in interoceptive (i.e. emotional) terms. The contemplation of relapse, then, can be thought of as weighing representations of the pleasurable interoceptive effects of drug use against the negative interoceptive effects of drug use by the VMPFC and insula system. Several factors conspire to tip this process in the favor of drug use: first, because representations of the pleasurable interoceptive effects of drug use within the insula magnify the incentive value of drug use behaviors, they have a strong input to the nucleus accumbens and can, therefore, pull or steer decisions more strongly than representations of the negative effects of drug use. Second, whereas the pleasurable consequences of drug use are immediate and certain, the negative consequences of drug use tend to be delayed and uncertain, which tends to discount their influence on decision making [120]. Third, substance abusers have a ‘myopia’ for the future negative consequences of their actions, which is evidenced by the finding that substance abusers have impairments in decision making under conditions of uncertain risk and reward, similar to patients with VMPFC lesions [121–123]. The end result is a decision-making process that is more likely to lead to relapse than to continued abstinence.
In essence, activation of interoceptive representations through the insula can, on the one hand, sensitize the motivational circuits that pull and steer the animal (or human) towards the appropriate goal object (i.e. the nucleus accumbens and associated mesolimbic dopamine system). On the other hand, the insula activation might impact the prefrontal cortex functions so that it can subvert attention, reasoning, planning and decision-making processes to formulate plans for action to seek and procure drugs [12]. Put differently, these interoceptive representations have the capacity to ‘hijack’ the cognitive resources necessary for exerting inhibitory control to resist drug use [119].
It is important to note that the representation of interoceptive information through the insula might drive an intense motivation not only to seek reward but also to avoid punishment. As mentioned earlier, evidence shows that the insula has a role in the emotional experience of pain. Thus, intensifying the motivation to avoid painful stimuli can be accomplished through excitatory projections from the insula to regions that are involved in initiating and invigorating aversive motivated actions, such as the more posterior regions of the striatum. This formulation can be supported by more recent functional neuroimaging evidence [124–126] indicating that both the insula and more posterior areas of the striatum play a part in aversive motivational learning.
Future directions
Since our initial finding on the effects of insula lesions on smoking addiction, several authors have suggested that the interoceptive functions of the insula might be important for addiction [7,12,127], although they have only discussed these functions in general terms. Here, we propose a specific model in which interoceptive (i.e. bodily) effects of drug-use rituals are encoded by the insula and are integrated into explicit motivational processes that promote addiction, such as conscious urges and the decision to relapse. We also address the role of learning and the dopamine system in these processes.
Much of this model remains to be substantiated through direct experimentation. Future experiments might use functional brain imaging to examine the neural systems that are engaged by the interoceptive effects of drug use because these are distinct from the CNS effects of drug use. Doing so will require tools to isolate the interoceptive effects of drug use from the CNS effects of drug use, such as denicotinized cigarettes and drug analogs and antagonists that do not cross the blood–brain barrier. Such tools can be combined with functional imaging to probe the neural systems that become engaged when addicted and non-addicted individuals are exposed to the interoceptive effects of drug use. These systems might also be probed by animal models that explicitly address the interoceptive effects of drug use. In addition, future experiments might address the neural systems that derive pleasure from the interoceptive effects of drug use, how memories for these interoceptive effects are formed and recalled, and how these processes contribute to conscious urges and the decision to relapse. Additional experiments might examine the role of the CNS effects of drug use (i.e. dopamine release) in modulating these processes.
Given that insula lesions seem to disrupt the smoking addiction, the question arises as to whether insula lesions also disrupt other forms of addiction. Evidence from rats indicates that insula lesions might also disrupt addiction to amphetamines [12], but future studies might address this question in humans. Furthermore, because there is an overlap between the neural systems that subserve drug motivation and those that subserve natural motivational functions, the question arises as to whether disruption of insula function would also interfere with natural motivated behaviors. Our preliminary results [11] showed that unilateral insula damage that disrupts smoking addiction does not grossly interfere with the motivation to eat. This might be owing to the fact that the motivation to eat, given its crucial importance for survival, is mediated by multiple redundant neural systems. It might also be owing to the fact that the insula is more important for driving behaviors that become pleasurable through learning, as opposed to behaviors such as eating that are innately pleasurable. This raises the question, then, of whether insula damage would interfere with motivational functions that are less essential for survival, such as sex, or for motivational functions related to certain learned pleasures, such as drinking coffee or eating chocolate (i.e. acquired tastes).
The model we present here points to the insula (and the cognitive functions it subserves) as potential targets for therapies for addiction. We have shown in cigarette smokers how damage to the insula can disrupt an addictive behavior. An obvious implication of this finding is that manipulation of insula function might be an effective therapy for addiction. Although surgically lesioning the insula would not be a viable therapy, less invasive manipulations of insula function, such as repetitive transcranial magnetic stimulation or deep brain stimulation, might be effective at disrupting addictive behavior. In addition, the insula, with its complex chemoarchitecture, is rich in targets for pharmacological interventions. Cognitive and behavioral therapies focused on internal representations of the interoceptive effects of drug use that are activated during conscious urges might also be effective treatments for addiction. Sensory ‘replacements’ for drug-use rituals, such as denicotinized cigarettes, are already known to be effective treatments [103,128]. All of these psychological interventions might act synergistically with medications that are targeted at the insula. Additionally, functional imaging techniques, coupled with probes of drug-related interoceptive function, might be used to monitor the activity of the insula to track the progress of therapies for addiction.
Acknowledgments
The research described in this article was supported by a grant from the National Institute on Drug Abuse (NIDA; www.nida.nih.gov) R01 DA023051.
References
- 1.Peto R, et al. Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; 2001. [Google Scholar]
- 3.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 4.Robinson T, Berridge KC. Incentive-sensitization and addicition. Addiction. 2001;96:106–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- 5.Tiffany ST. Is craving the source of compulsive drug use? J Psychopharmacol. 1998;12:23–30. doi: 10.1177/026988119801200104. [DOI] [PubMed] [Google Scholar]
- 6.Ernst M, Paulus M. Neurobiology of decision-making: a selective review from a neurocognitive and clinical persoective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Paulus MP. Decision-making dysfunctions in psychiatry –altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- 8.Paulus MP, Stein MB. An insular view of anxiety. Biol Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 9.Franklin TR, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 10.Crespo-Facorro B. Insular cortex abnormalities in schizophrenia: a structural magnetic resonance imaging study of first-episode patients. Schizophr Res. 2000;46:35–43. doi: 10.1016/s0920-9964(00)00028-1. [DOI] [PubMed] [Google Scholar]
- 11.Naqvi NH, et al. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Contreras M, et al. Inactivation of the interoceptive insula disrupts drug craving and malaise induced by lithium. Science. 2007;318:655–658. doi: 10.1126/science.1145590. [DOI] [PubMed] [Google Scholar]
- 13.Chikama M. Insular cortical projections to functional regions of the striatum correlate with cortical cytoarchitectonic organization in the primate. J Neurosci. 1997;17:9686–9705. doi: 10.1523/JNEUROSCI.17-24-09686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesulam MM. Insula of the old-world monkey. 1 Architectonics in the insulo-orbitotemporal component of the paralimbic brain. J Comp Neurol. 1982;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- 15.Mesulam MM. Insula of the old-world monkey. 3 Efferent cortical output and comments on function. J Comp Neurol. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- 16.Mufson EJ. Insula of the old-world monkey. 2 Afferent cortical input and comments on the claustrum. J Comp Neurol. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- 17.Stefanacci L. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: A retrograde tracing study. J Comp Neurol. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar P. Catecholamine innervation of the human cerebral-cortex as revealed by comparative immunohistochemistry of tyrosine-hydroxylase and dopamine-β-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- 19.Hurd YL. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. J Chem Neuroanat. 2001;22:127–137. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 20.Baumgartner U. High opiate receptor binding potential in the human lateral pain system. Neuroimage. 2006;30:692–699. doi: 10.1016/j.neuroimage.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 21.Sanchez MM. Autoradiographic and in situ hybridization localization of corticotropin-releasing factor 1 and 2 receptors in nonhuman primate brain. J Comp Neurol. 1999;408:365–377. [PubMed] [Google Scholar]
- 22.Contoreggi C. Stress hormone responses to corticotropin-releasing hormone in substance abusers without severe comorbid psychiatric disease. Biol Psychiatry. 2003;54:873–878. doi: 10.1016/s0006-3223(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 23.Habib KE. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommer WH. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala Crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Hansson AC. Region-specific down-regulation of Crhr1 gene expression in alcohol-preferring msP rats following ad lib access to alcohol. Addict Biol. 2007;12:30–34. doi: 10.1111/j.1369-1600.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- 26.Penfield W. The insula – further observations on its function. Brain. 1955;78:445–470. doi: 10.1093/brain/78.4.445. [DOI] [PubMed] [Google Scholar]
- 27.Shuren J. Insula and aphasia. J Neurol. 1993;240:216–218. doi: 10.1007/BF00818707. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann H. The contribution of the insula to motor aspects of speech production: a review and a hypothesis. Brain Lang. 2004;89:320–328. doi: 10.1016/S0093-934X(03)00347-X. [DOI] [PubMed] [Google Scholar]
- 29.Isnard J. Clinical manifestations of insular lobe seizures: a stereo-electroencephalographic study. Epilepsia. 2004;45:1079–1090. doi: 10.1111/j.0013-9580.2004.68903.x. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard TC, et al. Projections of thalamic gustatory and lingual areas in the monkey, Macaca fascicularis. J Comp Neurol. 1986;244:213–228. doi: 10.1002/cne.902440208. [DOI] [PubMed] [Google Scholar]
- 31.Kadohisa M. Neuronal representations of stimuli in the mouth: the primate insular taste cortex, orbitofrontal cortex and amygdala. Chem Senses. 2005;30:401–419. doi: 10.1093/chemse/bji036. [DOI] [PubMed] [Google Scholar]
- 32.Verhagen JV. Primate insular/opercular taste cortex: neuronal representations of the viscosity, fat texture, grittiness, temperature, and taste of foods. J Neurophysiol. 2004;92:1685–1699. doi: 10.1152/jn.00321.2004. [DOI] [PubMed] [Google Scholar]
- 33.Cereda C. Strokes restricted to the insular cortex. Neurology. 2002;59:1950–1955. doi: 10.1212/01.wnl.0000038905.75660.bd. [DOI] [PubMed] [Google Scholar]
- 34.Pritchard TC, et al. Taste perception in patients with insular cortex lesions. Behav Neurosci. 1999;113:663–671. [PubMed] [Google Scholar]
- 35.DeCoteau WE. Short-term memory for food reward magnitude: the role of the prefrontal cortex. Behav Brain Res. 1997;88:239–249. doi: 10.1016/s0166-4328(97)00044-2. [DOI] [PubMed] [Google Scholar]
- 36.Ragozzino ME. The role of the agranular insular cortex in working memory for food reward value and allocentric space in rats. Behav Brain Res. 1999;98:103–112. doi: 10.1016/s0166-4328(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 37.Fresquet N. Insular cortex lesions alter conditioned taste avoidance in rats differentially when using two methods of sucrose delivery. Behav Brain Res. 2004;153:357–365. doi: 10.1016/j.bbr.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Flynn FG, et al. Anatomy of the insula: functional and clinical correlates. Aphasiology. 1999;13:55–78. [Google Scholar]
- 39.Zito KA, et al. The dopamine innervation of the visceral cortex mediates the aversive effects of opiates. Pharmacol Biochem Behav. 1988;30:693–699. doi: 10.1016/0091-3057(88)90086-x. [DOI] [PubMed] [Google Scholar]
- 40.Calder AJ. Impaired recognition and experience of disgust following brain injury. Nat Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- 41.Allen GV. Organization of visceral and limbic connections in the insular cortex of the rat. J Comp Neurol. 1991;311:1–16. doi: 10.1002/cne.903110102. [DOI] [PubMed] [Google Scholar]
- 42.Cechetto DF. Evidence for a viscerotopic sensory representation in the cortex and thalamus in the rat. J Comp Neurol. 1987;262:27–45. doi: 10.1002/cne.902620104. [DOI] [PubMed] [Google Scholar]
- 43.Critchley HD, et al. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZH. Monkey insular cortex neurons respond to baroreceptive and somatosensory convergent inputs. Neuroscience. 1999;94:351–360. doi: 10.1016/s0306-4522(99)00339-5. [DOI] [PubMed] [Google Scholar]
- 45.Aziz Q. Cortical processing of human somatic and visceral sensation. J Neurosci. 2000;20:2657–2663. doi: 10.1523/JNEUROSCI.20-07-02657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hobday DI. A study of the cortical processing of ano-rectal sensation using functional MRI. Brain. 2001;124:361–368. doi: 10.1093/brain/124.2.361. [DOI] [PubMed] [Google Scholar]
- 47.Ladabaum U. Gastric fundic distension activates fronto-limbic structures but not primary somatosensory cortex: A functional magnetic resonance imaging study. Neuroimage. 2007;34:724–732. doi: 10.1016/j.neuroimage.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 48.Wang GJ. Gastric distention activates satiety circuitry in the human brain. Neuroimage. 2008;39:1824–1831. doi: 10.1016/j.neuroimage.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Mazzone SB. Representation of capsaicin-evoked urge-to-cough in the human brain using functional magnetic resonance imaging. Am J Respir Crit Care Med. 2007;176:327–332. doi: 10.1164/rccm.200612-1856OC. [DOI] [PubMed] [Google Scholar]
- 50.Hoffman BL. Stimulation studies of insular cortex of macacamulatta. J Neurophysiol. 1953;16:343–351. doi: 10.1152/jn.1953.16.4.343. [DOI] [PubMed] [Google Scholar]
- 51.Oppenheimer SM. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–1732. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 52.Berthier M, et al. Asymbolia for pain: a sensory-limbic disconnection syndrome. Ann Neurol. 1988;24:41–49. doi: 10.1002/ana.410240109. [DOI] [PubMed] [Google Scholar]
- 53.Hofbauer RK. Cortical representation of the sensory dimension of pain. J Neurophysiol. 2001;86:402–411. doi: 10.1152/jn.2001.86.1.402. [DOI] [PubMed] [Google Scholar]
- 54.Rainville P. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 55.Albanese MC. Memory traces of pain in human cortex. J Neurosci. 2007;27:4612–4620. doi: 10.1523/JNEUROSCI.0695-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Craig AD. How do you feel? Introception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 57.Brooks JCW. fMRI of thermal pain: effects of stimulus laterality and attention. Neuroimage. 2002;15:293–301. doi: 10.1006/nimg.2001.0974. [DOI] [PubMed] [Google Scholar]
- 58.Olausson H. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5:900–904. doi: 10.1038/nn896. [DOI] [PubMed] [Google Scholar]
- 59.Leknes SG. Itch and motivation to scratch: an investigation of the central and peripheral correlates of allergen- and histamine-induced itch in humans. J Neurophysiol. 2007;97:415–422. doi: 10.1152/jn.00070.2006. [DOI] [PubMed] [Google Scholar]
- 60.Blomqvist A. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 2000;123:601–619. doi: 10.1093/brain/123.3.601. [DOI] [PubMed] [Google Scholar]
- 61.Craig AD. Distribution of trigeminothalamic and spinothalamic lamina I terminations in the macaque monkey. J Comp Neurol. 2004;477:119–148. doi: 10.1002/cne.20240. [DOI] [PubMed] [Google Scholar]
- 62.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 63.Damasio AR. Descartes’ Error: Emotion, Reason, and the Human Brain. Grosset/Putnam; 1994. [Google Scholar]
- 64.Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. Harcourt Brace & Company; 1999. [Google Scholar]
- 65.James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- 66.Phan KL. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 67.Del Parigi A. Neuroimaging and obesity – mapping the brain responses to hunger and satiation in humans using positron emission tomography. Ann N Y Acad Sci. 2002;967:389–397. [PubMed] [Google Scholar]
- 68.Pelchat ML, et al. Images of desire: food-craving activation during fMRI. Neuroimage. 2004;23:1486–1493. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 69.Wang GJ, et al. Exposure to appetitive food stimuli markedly activates the human brain. Neuroimage. 2004;21:1790–1797. doi: 10.1016/j.neuroimage.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Egan G. Neural correlates of the emergence of consciousness of thirst. Proc Natl Acad Sci U S A. 2003;100:15241–15246. doi: 10.1073/pnas.2136650100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farrell MJ. Unique, common, and interacting cortical correlates of thirst and pain. Proc Natl Acad Sci U S A. 2006;103:2416–2421. doi: 10.1073/pnas.0511019103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arnow BA. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–1023. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- 73.Karama S, et al. Areas or brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stoleru S, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Arch Sex Behav. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- 75.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 76.Stewart J, et al. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- 77.Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- 78.Zinberg NE, Jacobson RC. The natural history of “chipping”. Am J Psychiatry. 1976;133:37–40. doi: 10.1176/ajp.133.1.37. [DOI] [PubMed] [Google Scholar]
- 79.Bozarth MA, Wise RA. Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science. 1984;224:516–517. doi: 10.1126/science.6324347. [DOI] [PubMed] [Google Scholar]
- 80.O’Brien CP, et al. Classical conditioning in human opioid dependence. In: Goldberg SR, Stolerman IP, editors. Behavioral Analysis of Drug Dependence. Academic Press; 1986. pp. 329–356. [Google Scholar]
- 81.Wikler A. Dynamics of drug dependence: implications of a conditioning theory for research and treatment. Arch Gen Psychiatry. 1973;28:611–616. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 82.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- 83.O’Brien CP, et al. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 84.Everitt BJ, et al. Associative processes in addiction and reward: the role of amygdala and ventral striatal subsystems. In: McGinty JF, editor. Advancing from the Ventral Striatum to the Extended Amygdala. 1999. pp. 412–438. Annals of the New York Academy of Science. [DOI] [PubMed] [Google Scholar]
- 85.Jentsch JD, Taylor JR. Impulsivity resulting from frontostraital dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 86.Berke JD. Addiction, dopamine, and the molecular mechanisms of memory. Neuron. 2000;25:515–532. doi: 10.1016/s0896-6273(00)81056-9. [DOI] [PubMed] [Google Scholar]
- 87.Volkow ND, et al. The addicted human brain viewed in the light of imaging studies: brain circuts and treatment strategies. Neuropharmacology. 2004;47:3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis. Neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wise RA, Bozarth MA. Action of drugs of abuse on brain reward systems: an update with specific attention to opiates. Pharmacol Biochem Behav. 1982;17:239–243. doi: 10.1016/0091-3057(82)90076-4. [DOI] [PubMed] [Google Scholar]
- 90.Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol. 1988;97:118–132. doi: 10.1037//0021-843x.97.2.118. [DOI] [PubMed] [Google Scholar]
- 91.Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- 92.Everitt BJ, et al. Associative processes in addiction and reward: the role of amygdala and ventral striatal subsystems. Ann N Y Acad Sci. 1999;877:412–438. doi: 10.1111/j.1749-6632.1999.tb09280.x. [DOI] [PubMed] [Google Scholar]
- 93.Stapleton JM, et al. Cerebral glucose utilization in polysubstance abuse. Neuropsychopharmacology. 1995;13:21–31. doi: 10.1016/0893-133X(94)00132-J. [DOI] [PubMed] [Google Scholar]
- 94.Volkow ND, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148:621–626. doi: 10.1176/ajp.148.5.621. [DOI] [PubMed] [Google Scholar]
- 95.London ED, et al. Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex. 2000;10:334–342. doi: 10.1093/cercor/10.3.334. [DOI] [PubMed] [Google Scholar]
- 96.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 97.Childress AR, et al. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Koob GF. The role of the striatopallidal and extended amygdala systems in drug addiction. Ann N Y Acad Sci. 1999;877:445–460. doi: 10.1111/j.1749-6632.1999.tb09282.x. [DOI] [PubMed] [Google Scholar]
- 99.Di Chiara G, et al. Drug addiction as a disorder of associative learning: role of nucleus accumbens shell and extended amygdala dopamine. Ann N Y Acad Sci. 1999;877:461–485. doi: 10.1111/j.1749-6632.1999.tb09283.x. [DOI] [PubMed] [Google Scholar]
- 100.Kou YR, et al. The stimulatory effect of nicotine on vagal pulmonary C-fibers in dogs. Respir Physiol. 1989;76:347–356. doi: 10.1016/0034-5687(89)90075-3. [DOI] [PubMed] [Google Scholar]
- 101.Lee LY, et al. Nicotine is responsible for airway irritation evoked by cigarette smoke inhalation in me. J Appl Physiol. 1993;75:1955–1961. doi: 10.1152/jappl.1993.75.5.1955. [DOI] [PubMed] [Google Scholar]
- 102.Naqvi NH, Bechara A. The airway sensory impact of nicotine contributes to the conditioned reinforcing effects of individual puffs from cigarettes. Pharmacol Biochem Behav. 2005;81:821–829. doi: 10.1016/j.pbb.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Westman EC, et al. Airway sensory replacement combined with nicotine replacement for smoking cessation. A randomized, placebo-controlled trial using a citric acid inhaler. Chest. 1995;107:1358–1364. doi: 10.1378/chest.107.5.1358. [DOI] [PubMed] [Google Scholar]
- 104.Westman EC, et al. Dissociating the nicotine and airway sensory effects of smoking. Pharmacol Biochem Behav. 1996;53:309–315. doi: 10.1016/0091-3057(95)02027-6. [DOI] [PubMed] [Google Scholar]
- 105.Rose JE, et al. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- 106.Johnson MW. Substitutes for tobacco smoking: a behavioral economic analysis of nicotine gum, denicotinized cigarettes, and nicotine-containing cigarettes. Drug Alcohol Depend. 2004;74:253–264. doi: 10.1016/j.drugalcdep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 107.Rose JE, et al. Sensory blockade of smoking satisfaction. Pharmacol Biochem Behav. 1985;23:289–293. doi: 10.1016/0091-3057(85)90572-6. [DOI] [PubMed] [Google Scholar]
- 108.Rose JE, et al. Subjective response to cigarette smoking following airway anesthetization. Addict Behav. 1984;9:211–215. doi: 10.1016/0306-4603(84)90060-1. [DOI] [PubMed] [Google Scholar]
- 109.Carpenter CM. The role of sensory perception in the development and targeting of tobacco products. Addiction. 2007;102:136–147. doi: 10.1111/j.1360-0443.2006.01649.x. [DOI] [PubMed] [Google Scholar]
- 110.Etter JF, Stapleton JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob Control. 2006;15:280–285. doi: 10.1136/tc.2005.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hughes JR. A meta-analysis of the efficacy of over-the-counter, nicotine replacement. Tob Control. 2003;12:21–27. doi: 10.1136/tc.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Baxter MG. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 113.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 114.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 115.Brody AL. Neural substrates of resisting craving during cigarette cue exposure. Biol Psychiatry. 2007;62:642–651. doi: 10.1016/j.biopsych.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Garavan H. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 117.Pochon JB. Functional imaging of decision conflict. J Neurosci. 2008;28:3468–3473. doi: 10.1523/JNEUROSCI.4195-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Porrino LJ, Lyons D. Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex. 2000;10:326–333. doi: 10.1093/cercor/10.3.326. [DOI] [PubMed] [Google Scholar]
- 119.Bechara A. Decision-making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 120.Baker F. Delay discounting in current and never-before cigarette smokers: Similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- 121.Bechara A. Decision-malting deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- 122.Bechara A. Decision-making and addiction (part I): impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 123.Bechara A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- 124.Samanez-Larkin GR. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–323. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Seymour B. Differential encoding of losses and gains in the human striatum. J Neurosci. 2007;27:4826–4831. doi: 10.1523/JNEUROSCI.0400-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Samanez-Larkin GR. Anticipation of monetary gain but not loss in healthy older adults. Nat Neurosci. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gray MA. Interoceptive basis to craving. Neuron. 2007;54:183–186. doi: 10.1016/j.neuron.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buchhalter AR. Tobacco abstinence symptom suppression: the role played by the smoking-related stimuli that are delivered by denicotinized cigarettes. Addiction. 2005;100:550–559. doi: 10.1111/j.1360-0443.2005.01030.x. [DOI] [PubMed] [Google Scholar]
- 129.McBride D. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology. 2006;31:2728–2738. doi: 10.1038/sj.npp.1301075. [DOI] [PubMed] [Google Scholar]
- 130.Franklin TR. Limbic activation to cigarette smoking cues independent of nicotine withdrawal: A perfusion fMRI study. Neuropsychopharmacology. 2007;32:2301–2309. doi: 10.1038/sj.npp.1301371. [DOI] [PubMed] [Google Scholar]
- 131.Brody AL, et al. Brain metabolic changes during cigarette craving. Arch Gen Psychiatry. 2002;59:1162–1172. doi: 10.1001/archpsyc.59.12.1162. [DOI] [PubMed] [Google Scholar]
- 132.McClernon FJ. Abstinence-induced changes in self-report craving correlate with event-related fMRI responses to smoking cues. Neuropsychopharmacology. 2005;30:1940–1947. doi: 10.1038/sj.npp.1300780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee JH. A functional magnetic resonance imaging (fMRI) study of cue-induced smoking craving in virtual environments. Appl Psychophysiol Biofeedback. 2005;30:195–204. doi: 10.1007/s10484-005-6377-z. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z. Neural substrates of abstinence-induced cigarette cravings in chronic smokers. J Neurosci. 2007;27:14035–14040. doi: 10.1523/JNEUROSCI.2966-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kilts CD, et al. The neural correlates of cue-induced craving in cocaine-dependent women. Am J Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 136.Bonson KR, et al. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- 137.Kilts CD. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- 138.Wang GJ, et al. Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci. 1999;64:775–784. doi: 10.1016/s0024-3205(98)00619-5. [DOI] [PubMed] [Google Scholar]
- 139.Garavan H, et al. Cue-induced cocaine craving: neuroanatomical apecificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- 140.Wexler BE, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 141.Myrick H, et al. Differential brain activity in alcoholics and social drinkers to alcohol cues: relationship to craving. Neuropsychopharmacology. 2004;29:393–402. doi: 10.1038/sj.npp.1300295. [DOI] [PubMed] [Google Scholar]
- 142.Tapert SF, et al. fMRI BOLD response to alcohol stimuli in alcohol dependent young women. Addict Behav. 2004;29:33–50. doi: 10.1016/j.addbeh.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 143.Sell LA. Activation of reward circuitry in human opiate addicts. Eur J Neurosci. 1999;11:1042–1048. doi: 10.1046/j.1460-9568.1999.00522.x. [DOI] [PubMed] [Google Scholar]