Abstract
CYP2A6 is the main nicotine metabolizing enzyme in humans. We investigated the relationships between CYP2A6 genotype, baseline plasma 3HC/COT (a phenotypic marker of CYP2A6 activity), and smoking behaviors in African-American light smokers. Cigarette consumption, age of initiation, and dependence scores did not differ between 3HC/COT quartiles or CYP2A6 genotype groups. Slow metabolizers (both genetic and phenotypic) had significantly higher plasma nicotine levels suggesting cigarette consumption was not reduced to adjust for slower rates of nicotine metabolism. Individuals in the slowest 3HC/COT quartile had higher quit rates with both placebo and nicotine gum treatments (OR 1.85, 95% CI 1.08-3.16, p = 0.03). Similarly, the slowest CYP2A6 genotype group had higher quit rates, although this did not reach significance (OR 1.61, 95% CI 0.95-2.72, p = 0.08). 3HC/COT ratio, and possibly CYP2A6 genotype, may be useful in the future for personalizing the choice of smoking cessation treatment for African-American light smokers.
Keywords: Cytochrome P450 2A6, CYP2A6, nicotine, cotinine, trans-3′-hydroxycotinine, African-Americans, smoking, light smokers
Introduction
While overall smoking rates in North America have declined considerably, there are a growing number of smokers who maintain low levels of cigarette consumption. Light smoking is more prevalent particularly among adolescents, females and some racial/ethnic minority groups. For example, the majority of African-Americans consume ≤10 cigarettes per day (CPD) (1), yet they report high levels of dependence and have difficulty quitting (2, 3). Further, African-Americans have disproportionately higher incidences of tobacco-related illnesses such as lung cancer (4, 5), which may be partly explained by their greater use of mentholated cigarettes with higher nicotine and tar yields (6), deeper inhalation patterns (7), and altered rates of nicotine and nitrosamine metabolism (8, 9).
Nicotine is primarily responsible for the highly addictive properties of tobacco smoke (10). The majority (~80%) of nicotine (NIC) is inactivated to cotinine (COT) (11), and ~90% of this reaction is mediated by the hepatic enzyme cytochrome P450 2A6 (CYP2A6) (12). Cotinine is further metabolized to trans-3′-hydroxycotinine (3HC) primarily by CYP2A6 (13, 14). In addition, CYP2A6 can bioactivate tobacco-specific precarcinogens including (methyl-nitrosamino)-1-(3-pyridyl)-1-butanone (NNK) and N’-nitrosonornicotine (NNN) (15).
The gene encoding CYP2A6 is highly polymorphic, with 36 numbered alleles and numerous single nucleotide polymorphisms (SNPs) identified so far (http://www.cypalleles.ki.se/cyp2a6.htm). Large inter-ethnic and inter-individual variations in CYP2A6 activity have been reported (16). This has been mainly attributed to genetic variations in CYP2A6, although environmental influences (e.g. diet) may also contribute through enzyme induction or inhibition. For example, estrogen has been found to induce CYP2A6 in vitro (17), and females have faster rates of in vivo nicotine metabolism compared to males (18).
Since dependent smokers regulate the amount smoked to maintain plasma and brain nicotine levels (19), variations in CYP2A6 activity is predicted to alter smoking behaviors. Individuals with CYP2A6 alleles encoding enzymes with decreased- or loss-of-function metabolize nicotine at a slower rate (20-22), and have been associated with lower risk of being a smoker in adults (23), lower cigarette consumption (21, 23), reduced smoking intensity (21, 24), and reduced withdrawal symptoms during abstinence (25). In addition, CYP2A6 slow metabolizers achieved higher quit rates in clinical trials (26-28), were more likely to be former smokers in a case-control study (29), and had shorter durations of smoking suggestive of greater quit success (23). However, these studies have been mainly in heavy smoking individuals of European-or Asian-ancestry. There is a paucity of research on the role of CYP2A6 variation and smoking in light smoking populations such as African-Americans. Interestingly, African-Americans have slower rates of nicotine and cotinine metabolism compared to European-Americans (8), and our lab recently identified novel CYP2A6 variants in this population that are associated with lower enzyme activity (22). Thus, variability in CYP2A6 activity may also contribute to the unique smoking patterns observed in this group.
The large variation in CYP2A6 activity and numerous CYP2A6 alleles indicates the need for a phenotype measure for population studies. The 3HC/COT ratio has been validated as a phenotype marker of CYP2A6 activity as the conversion of COT to 3HC is specific to CYP2A6 (13, 14). The ratio of these nicotine metabolites is highly correlated with rates of nicotine clearance (r = 0.70 - 0.95) (14), and has been associated with CYP2A6 genotype in several studies (22, 30, 31). However, these studies have been primarily in heavy smoking individuals of European-ancestry, and it is unknown whether the 3HC/COT ratio derived from baseline ad libitum smoking will also be a good phenotype measure of CYP2A6 activity in African-American light smokers where smoking can be both low and irregular in frequency.
Few smoking cessation clinical trials have focused on African-Americans as treatment studies typically exclude light smokers. A recent study, entitled Kick it at Swope-II (KIS-II), evaluated the efficacy of nicotine replacement therapy (2 mg nicotine gum vs. placebo) and counseling sessions (health education (HE) vs. motivational interviewing (MI)) in 755 African-American light smokers (32). Nicotine gum did not increase quit rates compared to placebo at week 26 follow-up, although those receiving HE were significantly more likely to quit compared to those receiving MI. Thus, further investigations of the biological factors underlying smoking behaviors in light smoking populations are warranted.
Here, we first investigated whether CYP2A6 genotype is related to the 3HC/COT ratio derived from baseline smoking in treatment seeking African-American light smokers enrolled in KIS-II. We then determined whether CYP2A6 variation, as indicated by genotype and the 3HC/COT ratio, is associated with baseline smoking behaviors and treatment outcomes. This is the largest study to date examining the role of CYP2A6 variation in smoking behaviors among African-American light smokers, with the additional benefit of having genetic and phenotype measures available.
Results
Participant characteristics
There was no significant difference in participant characteristics (gender, age, body mass index (BMI), smoking mentholated cigarettes, CPD, exhaled carbon monoxide (CO), baseline plasma NIC and COT, age of regular smoking, or dependence severity) between the total population (n = 755), the subset of participants with 3HC/COT data (n = 646), those with genotype data (n = 588), and those with both genotype and 3HC/COT data (n = 495) (Supplementary table 1).
Variables that influence the 3HC/COT ratio
To examine other factors that may influence the 3HC/COT ratio independent of CYP2A6 genetic variation, an analysis was performed including only CYP2A6*1/*1 individuals (n = 246). The 3HC/COT ratio was significantly higher in females, increased with age, and was higher in those with lower BMI (Table 1). Individuals who smoked mentholated cigarettes also had significantly lower 3HC/COT ratios (p < 0.05). In a multivariate regression model, gender, age and BMI remained independently associated with the 3HC/COT ratio (p < 0.05, Table 1).
Table 1.
Factors that influence the 3HC/COT in CYP2A6*1/*1 individuals (n = 246)
| Mean 3HC/COT | SD | N | p – value | |
|---|---|---|---|---|
| Univariate analyses | ||||
| Gender | ||||
| Males | 0.37 | 0.19 | 79 | 0.02 |
| Females | 0.46 | 0.32 | 167 | |
| Age 1 | ||||
| 20 – 29 | 0.35* | 0.20 | 22 | 0.002 |
| 30 – 39 | 0.37* | 0.20 | 72 | |
| 40 – 49 | 0.43 | 0.24 | 80 | |
| 50 – 59 | 0.48 | 0.35 | 52 | |
| 60 – 77 | 0.63 | 0.43 | 20 | |
| r | 0.22 | 0.001 | ||
| BMI 2 | ||||
| Low (< 29.7) | 0.46 | 0.3 | 122 | 0.05 |
| High (≥ 29.7) | 0.40 | 0.3 | 122 | |
| r | −0.16 | 0.01 | ||
| Smoke mentholated cigarettes |
||||
| Yes | 0.41 | 0.27 | 190 | 0.02 |
| No | 0.50 | 0.31 | 56 | |
| Multivariate regression analyses 3 | ||||
| Standardized β | 95% CI | p – value | ||
| Gender | 0.15 | 0.005 – 0.044 | 0.01 | |
| Age | 0.24 | 0.001 – 0.002 | < 0.001 | |
| BMI | − 0.16 | − 0.003 – 0.000 | 0.01 | |
| Mentholated cigarettes | − 0.08 | − 0.036 – 0.008 | 0.22 | |
denotes significant difference (p < 0.05) from the oldest age group (60 – 77). r = Spearman’s correlation coefficient between age and 3HC/COT.
BMI was categorized by the median value of 29.7. r = Spearman’s correlation coefficient between BMI and 3HC/COT. BMI data was not available for two participants.
R2 = 0.12. Age and BMI were included as continuous variables.
CYP2A6 genotypes and associations with 3HC/COT
CYP2A6*1B has been associated with faster enzyme activity in European-Americans (33), although no difference was observed previously in individuals of Black-African descent (22). We used a newly revised CYP2A6*1B genotyping assay to account for SNPs occurring at a high frequency in this population that confounded our previous assay (34), and found individuals with CYP2A6*1B had significantly higher 3HC/COT ratios even after controlling for gender, age and BMI as covariates (F(2, 238) = 5.1, p < 0.01, Table 2). To examine the functional impact of other CYP2A6 variants, individuals with CYP2A6*1B were included in the wildtype reference group, as this allows us to compare our results with those reported previously (22), and this genotype was not associated here with altered baseline smoking variables or treatment outcomes.
Table 2.
CYP2A6 genotypes and associated 3HC/COT ratios1
| Allele | Genotype | N | Mean 3HC/COT | SD | % | p – value |
|---|---|---|---|---|---|---|
| CYP2A6*1B | *1/*1 | 169 | 0.40 | 0.29 | 100 | 0.007 |
| *1/*1B | 62 | 0.49 | 0.26 | 123 | ||
| *1B/*1B | 15 | 0.50 | 0.26 | 125 | ||
|
| ||||||
| Reference2 | *1/*1 | 246 | 0.43 | 0.28 | 100 | ------- |
|
| ||||||
| CYP2A6*2 | *1/*2 | 5 | 0.31 | 0.16 | 72 | 0.29 |
| CYP2A6*4 | *1/*4 | 14 | 0.21 | 0.09 | 49 | 0.001 |
| CYP2A6*9 | *1/*9 | 70 | 0.32 | 0.29 | 74 | < 0.001 |
| *9/*9 | 10 | 0.15 | 0.10 | 35 | ||
| CYP2A6*12 | *1/*12 | 4 | 0.23 | 0.11 | 53 | 0.06 |
| CYP2A6*17 | *1/*17 | 49 | 0.26 | 0.15 | 61 | < 0.001 |
| *17/*17 | 5 | 0.06 | 0.03 | 14 | ||
| CYP2A6*20 | *1/*20 | 13 | 0.17 | 0.11 | 40 | < 0.001 |
| *20/*20 | 1 | 0.18 | ----- | 42 | ||
| CYP2A6*23 | *1/*23 | 7 | 0.38 | 0.12 | 88 | 0.56 |
| CYP2A6*24 | *1/*24 | 3 | 0.37 | 0.16 | 86 | 0.50 |
| CYP2A6*25 | *1/*25 | 7 | 0.22 | 0.06 | 51 | 0.10 |
| CYP2A6*26 | *1/*26 | 4 | 0.23 | 0.11 | 54 | 0.09 |
| CYP2A6*27 | *1/*27 | 8 | 0.18 | 0.07 | 42 | 0.001 |
| CYP2A6*28 | *1/*28 | 16 | 0.73 | 0.71 | 170 | 0.007 |
| CYP2A6*35 | *1/*35 | 20 | 0.26 | 0.09 | 61 | < 0.001 |
| *35/*35 | 1 | 0.18 | ---- | 42 | ||
| > 1 variant 3 | ------- | 39 | 0.15 | 0.08 | 35 | < 0.001 |
Univariate analyses including gender, age, and BMI as covariates. Comparisons were made with the CYP2A6*1/*1 individuals as the reference group as was done previously (22). In cases where there is only one individual who is homozygous for the variant, they are combined with the heterozygous variant group for analyses.
Individuals with CYP2A6*1B were included in the reference group.
Compound heterozygotes, such as individuals with CYP2A6*4/*17 genotypes, were grouped as having > 1 variant.
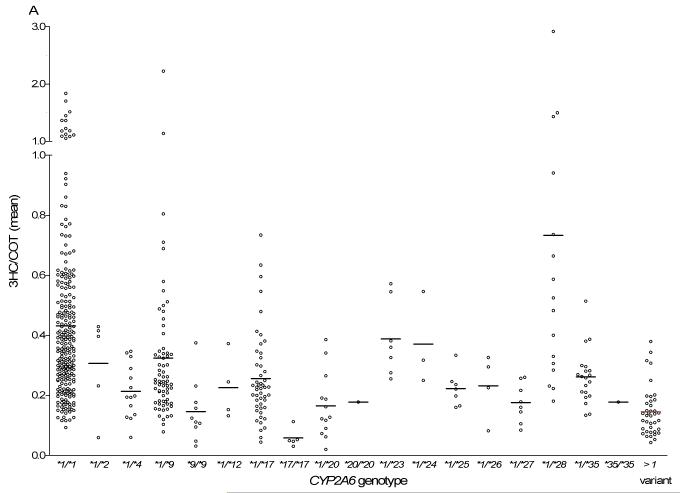
Consistent with previous studies (20, 22), heterozygous individuals with established alleles, CYP2A6*4, *17 and *20, had ~40 – 60% activity remaining, while heterozygous individuals with CYP2A6*9 and *12 had ~50 – 75% activity remaining (Fig. 1A, Table 2). Heterozygous individuals with alleles recently identified in individuals of Black-African descent (CYP2A6*25, *26, *27 and *35) (22) also had ~40 – 60% activity remaining (Fig. 1A, Table 2). Given the low prevalence of these novel variants, the larger size of this current study provides further evidence of their in vivo functional impact. CYP2A6*2, *23, *24 and *28 were associated with 3HC/COT ratios higher than expected (Fig. 1A, Table 2). Thus, in agreement with previous studies in other racial/ethnic groups (30, 31), there is generally a good concordance between CYP2A6 genotype and 3HC/COT as a phenotype measure.
Fig.1.
A) CYP2A6 genotypes and their associated unadjusted 3HC/COT ratios. Each dot represents an individual and the line represents the mean 3HC/COT ratio in each genotype group. The CYP2A6*1/*1 group (n = 246) includes individuals with the CYP2A6*1B allele. The >1 variant group represents compound heterozygote individuals (e.g. CYP2A6*4/*17). B) The unadjusted 3HC/COT ratio was significantly associated with CYP2A6 genotype groupings. Statistical analyses were performed on the log-transformed ratio with gender, age and BMI as covariates. It should be noted that since only 18 individuals were predicted to be poor metabolizers (completely lacking CYP2A6 function due to having two copies of loss-of-function alleles), they were combined with those predicted to have 10-50% activity to form the SM group. C) The 3HC/COT ratio was also adjusted by gender as illustrated in our previous papers (22, 36, 50). Adjustments were made by dividing each ratio by the mean value of their respective gender. For example, a male individual with an adjusted ratio greater than one indicates values that are higher than the mean ratio of all males. NM = normal metabolizers, IM = intermediate metabolizers, SM = slow metabolizers. The 3HC/COT when adjusted for the covariates (gender, BMI, and age) found to be significant in this population, using regression analyses, is as follows (mean ± 95%CI): NM = 0.44 ± 0.03, IM = 0.31 ± 0.05, SM = 0.22 ± 0.03). *** p < 0.001 when compared to the NMs. ## p < 0.01 when compared to IMs. The number of individuals are listed on the x – axis.
Genotype frequencies did not deviate significantly from Hardy-Weinberg equilibrium (p > 0.10). CYP2A6 allele frequencies in this sample of African-Americans did not significantly differ from those reported in Canadian individuals of Black-African descent, with the exception of CYP2A6*28 (Table 3).
Table 3.
CYP2A6 allele frequencies in African-Americans in this population compared to our previous study in individuals of Black-African descent
| African-Americans (n = 1236 alleles) |
Black-African descent1 (n = 560 alleles) |
||
|---|---|---|---|
|
|
|||
| CYP2A6 allele | Frequency (%) | Frequency (%) | p – value |
| CYP2A6*1B | 18.22 | 18.3 | 0.95 |
| CYP2A6*2 | 0.9 | 0.4 | 0.22 |
| CYP2A6*4 | 1.9 | 2.7 | 0.33 |
| CYP2A6*9 | 9.6 | 7.2 | 0.09 |
| CYP2A6*12 | 0.4 | 0.0 | 0.33 |
| CYP2A6*17 | 8.0 | 7.3 | 0.61 |
| CYP2A6*20 | 1.5 | 1.1 | 0.51 |
| CYP2A6*23 | 1.1 | 2.0 | 0.16 |
| CYP2A6*24 | 0.7 | 1.3 | 0.28 |
| CYP2A6*25 | 0.9 | 0.5 | 0.43 |
| CYP2A6*26 | 0.7 | 0.7 | 0.97 |
| CYP2A6*27 | 0.7 | 0.2 | 0.15 |
| CYP2A6*28 | 2.4 | 0.9 | 0.03 |
| CYP2A6*35 | 2.9 | 2.5 | 0.62 |
CYP2A6 genotype grouping strategy
Due to the large number of low prevalence CYP2A6 alleles, participants were categorized by the predicted rate of CYP2A6 activity based on their genotype. This was done according to the grouping strategy used in previous studies (20, 22). Individuals with CYP2A6*28 were excluded from this analysis due to extreme range in 3HC/COT values (Fig. 1A), suggesting some may also have gain-of-function copy number variants which is under current investigation. CYP2A6 gene duplications (CYP2A6*1×2A, *1×2B) leading to increased enzyme function have been described (21, 35). However, these duplications are rare in African-Americans, occurring at < 2% allele frequencies. We have genotyped 343 samples for CYP2A6*1×2A, and 28 samples with high 3HC/COT ratios for CYP2A6*1×2B, but did not find any individuals with these alleles.
Individuals with one copy of the decrease-of-function alleles (CYP2A6*9 and*12) were grouped as IMs (n = 78). Individuals with two copies of the decrease-of-function alleles, one or two copies of loss-of-function alleles (CYP2A6*2, *4, *17, *20, *23, *24,*25, *26, *27 and *35), or one decrease-of-function allele with one loss-of-function allele were grouped as SMs (n = 213). Although the genotype for CYP2A6*2, *23 and *24 were not in agreement with the phenotype measure (3HC/COT), these individuals were categorized as SMs based on previous studies demonstrating their functional impact (20, 22, 36).
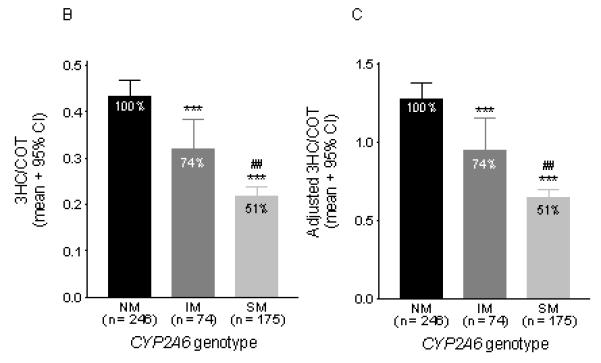
3HC/COT was associated with CYP2A6 genotype
The 3HC/COT ratio was significantly associated with the CYP2A6 genotype groupings (F(2, 492) = 52.6, p < 0.001), and remained significant after controlling for gender, age and BMI as covariates (F(2, 485) = 58.7, p < 0.001). The mean 3HC/COT ratio (± 95% CI) in the CYP2A6 genotype groups were: NM = 0.43 ± 0.04, IM = 0.32 ± 0.07, SM = 0.22 ± 0.02. The 3HC/COT ratio was significantly lower in intermediate and slow metabolizers (IMs and SMs, respectively) compared to the normal metabolizers (NMs) (p < 0.001, Fig. 1B). When we adjusted the ratio for gender (mean ± 95% CI: NM = 1.27 ± 0.10, IM = 0.95 ± 0.20, SM = 0.64 ± 0.06), we observed similar adjusted values as were reported previously in our population of Black-African descent (mean ± 95% CI: NM = 1.18 ± 0.10, IM = 0.77 ± 0.15, SM = 0.51 ± 0.10) (22).
CYP2A6 activity and baseline smoking behaviors
The self-reported cigarettes per day (CPD) was not associated with CYP2A6 genotype groups (F(2,585) = 0.26, p = 0.77) or 3HC/COT quartiles (F(3, 642) = 0.25, p = 0.86) (Fig. 2A, B). Exhaled CO levels, a biochemical measure of cigarette smoke exposure, was also not associated with CYP2A6 genotype groups (F(2, 584) = 3.0, p = 0.05) or 3HC/COT quartiles (F(3, 641) = 1.6, p = 0.18) (Fig. 2C, D).
Fig. 2.
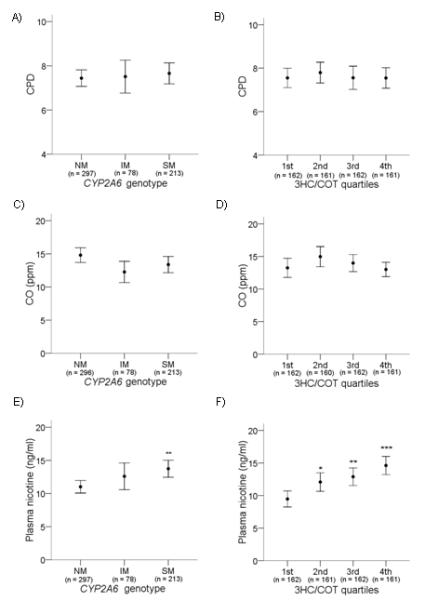
A, B) CYP2A6 genotype and 3HC/COT were not associated with self-reported CPD. C, D) CYP2A6 genotype and 3HC/COT were not associated with expired CO. E) CYP2A6 genotype was associated with nicotine plasma levels. **p < 0.01 when compared to NMs. F) 3HC/COT was associated with nicotine plasma levels. *p < 0.05, **p < 0.01 and *** p < 0.001 when compared to the 1st quartile. Individuals with 3HC/COT values in the 1st quartile have the fastest CYP2A6 activity, while those in the 4th quartile have the slowest CYP2A6 activity. Data are presented as mean ± 95% confidence interval. The number of individuals are listed on the x – axis.
As further evidence that individuals were not altering their cigarette intake to compensate for different rates of nicotine metabolism, as observed in heavier smokers (23), slow CYP2A6 activity was associated with higher plasma nicotine levels. CYP2A6 SMs had significantly higher baseline plasma nicotine levels compared to NMs (F (2, 585) = 6.0, p = 0.003) (Fig. 2E). Similarly, plasma nicotine levels progressively increased by the 3HC/COT quartiles, such that levels are lowest in the 1st quartile (fastest CYP2A6 activity) and highest in the 4th quartile (slowest CYP2A6 activity) (F(3, 642) = 9.8, p < 0.001) (Fig. 2F). Plasma nicotine levels were significantly higher among slow metabolizers in both males and females.
Neither CYP2A6 genotype (F(2, 584) = 0.08, p = 0.92) nor the 3HC/COT quartiles (F(3, 640) = 0.43, p = 0.73) were associated with age of onset of regular smoking. Tobacco dependence was assessed by the Cigarette Dependence Scale (CDS) (37) and the Fagerström Test for Nicotine Dependence (FTND) (38). CYP2A6 genotype was not associated with scores from the CDS (F(2, 585) = 0.33, p = 0.72) or FTND (F(2, 585) = 0.37, p = 0.69). The 3HC/COT quartiles were also not associated with scores from the CDS (F(3, 642) = 0.39, p =0.76) or FTND (F(3, 642) = 0.24, p = 0.87). Gender, age and BMI did not alter any of the relationships listed above when included as a covariate.
CYP2A6 activity and smoking abstinence
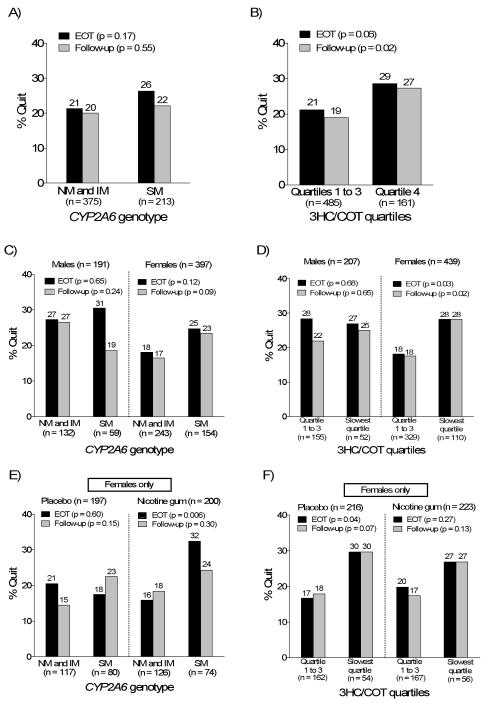
Seven-day point prevalence abstinence, defined as having smoked no cigarettes, not even a puff, for the previous seven days, was assessed at week 8 (end-of-treatment, EOT) and week 26 (follow-up), and verified by CO levels (≤ 10 ppm). Variables previously reported to be predictors of abstinence in this population (counseling, gender, income, age, and BMI) (39), as well as drug treatment, were included in a multiple logistic regression model. Consistent with previous analyses in this population (39), MI counseling and lower income (≤ $1,800) were associated with lower odds of quitting while male gender, older age and higher BMI were associated with greater odds of quitting at both EOT and follow-up (Table 4). CYP2A6 SMs trended towards being significantly more likely to quit smoking at both EOT (p = 0.10) and follow-up (p = 0.08) compared to IMs or NMs combined (Table 4, Fig. 3A). Individuals with the slowest 3HC/COT quartile trended towards having greater odds of quitting at EOT (p = 0.08) and were significantly more likely to quit at follow-up (p = 0.03) (Table 4, Fig. 3B). The effect of CYP2A6 activity on quit success appeared to be more pronounced in females (Fig. 3C, D), and was observed in both the placebo and nicotine gum arms among females (Fig. 3 E, F), but the gender interaction was not significant.
Table 4.
Logistic regression analyses of predictors of CO-verified quit rates at EOT (week 8) and follow-up (week 26)
| EOT | Follow-up | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Predictor1 | Odds ratio |
95% CI | p | Odds ratio |
95% CI | P |
| A) Genotype, n = 588 | ||||||
| Genotype (SM)2 | 1.54 | 0.92 – 2.57 | 0.10 | 1.61 | 0.95 – 2.72 | 0.08 |
| Drug (placebo) | 0.62 | 0.41 – 0.93 | 0.02 | 0.62 | 0.40 – 0.94 | 0.03 |
| Counseling (MI) | 0.61 | 0.41 – 0.92 | 0.02 | 0.57 | 0.37 – 0.87 | 0.009 |
| Gender (males) | 1.68 | 0.99 – 2.85 | 0.05 | 1.83 | 1.07 – 3.15 | 0.03 |
| Age | 1.04 | 1.02 – 1.06 | < 0.001 | 1.03 | 1.01 – 1.05 | 0.003 |
| BMI | 1.03 | 1.00 – 1.05 | 0.05 | 1.03 | 1.00 – 1.06 | 0.04 |
| Income (≤ $1800) | 0.59 | 0.39 – 0.88 | 0.01 | 0.74 | 0.49 – 1.13 | 0.16 |
| B) 3HC/COT, n = 646 | ||||||
| Quartile (slowest)3 | 1.61 | 0.94 – 2.76 | 0.08 | 1.85 | 1.08 – 3.16 | 0.03 |
| Drug (placebo) | 0.76 | 0.51 – 1.11 | 0.15 | 0.90 | 0.60 – 1.34 | 0.59 |
| Counseling (MI) | 0.72 | 0.49 – 1.06 | 0.10 | 0.56 | 0.37 – 0.83 | 0.005 |
| Gender (males) | 1.59 | 0.98 – 2.58 | 0.06 | 1.33 | 0.80 – 2.22 | 0.28 |
| Age | 1.04 | 1.02 – 1.06 | < 0.001 | 1.03 | 1.01 – 1.05 | 0.002 |
| BMI | 1.01 | 0.99 – 1.04 | 0.32 | 1.02 | 0.99 – 1.05 | 0.14 |
| Income (≤ $1800) | 0.63 | 0.43 – 0.93 | 0.02 | 0.66 | 0.44 – 0.99 | 0.04 |
The odds ratio provided refers to the variable listed in brackets beside each categorical predictor.
The NM and IM group were combined for analyses and compared against the SM group.
Individuals in quartiles 1 to 3 (highest 3HC/COT ratios) were combined for analyses and compared against individuals in the 4th quartile (lowest 3HC/COT ratios).
Fig. 3.
A, B) Association of CYP2A6 genotype and 3HC/COT quartiles with CO-verified quit rates at EOT and follow-up. The NMs and IMs were pooled for analyses and compared to SMs. Individuals with highest 3HC/COT ratios in quartiles 1st to 3rd were pooled for analyses, and compared to individuals with 3HC/COT ratios in the lowest 25th percentile (4th quartile). C, D) Association of CYP2A6 genotype and 3HC/COT quartiles with CO-verified quit rates at EOT and follow-up by gender. E, F) Association of CYP2A6 genotype and 3HC/COT quartiles with CO-verified quit rates at EOT and follow-up by treatment arm. Only data in females is shown for fig. 3E and F. The p-values listed were derived from univariate analyses of quit rates by categories of CYP2A6 activities; the results from the multivariate analysis is presented in Table 4.
Discussion
This is the first study examining the relationship between CYP2A6 activity and smoking behaviors in African-American light smokers. We provided further evidence that the 3HC/COT ratio derived from ab libitum smoking is a good phenotypic measure of CYP2A6 activity in this population as there was a good concordance with CYP2A6 genotypes. Our results also suggest that in this sample of light smokers, cigarette consumption was not lowered to fully compensate for slower rates of nicotine metabolism, and rather, higher plasma nicotine levels were observed in slow metabolizers. The group with the slowest CYP2A6 activity trended towards increased cessation success at EOT which reached significance at follow-up. This suggests that slow CYP2A6 activity, and the resulting higher plasma nicotine levels, may influence some aspect of the addiction process leading to greater cessation.
The 3HC/COT ratio is particularly useful as a phenotypic measure of CYP2A6 activity because COT has a relatively long elimination half-life (~13 – 19 hours) and the level of 3HC is formation-dependent (11). The 3HC/COT ratio, where metabolites were derived from ab libitum smoking, is independent of the time of sampling (40), does not vary widely within individuals over time (40, 41), and has been associated with CYP2A6 genotypes in heavy smoking individuals of European-ancestry (30). Our results and those in our previous study (22, 42) also indicate the ratio has good concordance with CYP2A6 genotype and rates of nicotine metabolism in light smoking populations of Black-African descent. Our observation that the CYP2A6*2, *23, *24 and *28 alleles were associated with 3HC/COT ratios higher than predicted is likely due to other sources of variability in genotype and phenotype, combined with the small number of individuals with these alleles. We estimated that to detect a 50% reduction in 3HC/COT from the wildtype reference group (mean = 0.43) with a power of 0.80, assuming a standard deviation of 0.25, we would need 11 individuals who have the variant of interest. There may also be other unidentified genetic variation, such as gene duplications, and/or individuals may have been exposed to CYP2A6 inducers. Information regarding the use of oral contraceptives, a known CYP2A6 inducer (18), was not collected in this study.
The finding that the 3HC/COT ratio is higher in females is in agreement with previous data (18, 42). Our observation that the 3HC/COT ratio increases with age suggests CYP2A6 activity may be increased among the elderly (31). BMI has also been negatively correlated with the 3HC/COT ratio previously (41, 43), though the relationship between obesity and CYP2A6 activity or nicotine pharmacokinetics have not been well examined. Mentholated cigarettes have been shown to reduce nicotine metabolism in smokers (44). It is notable that mentholated cigarette smokers were younger than non-mentholated cigarette smokers (42.5 vs. 50.0, p < 0.001). Thus, the modest reduction in 3HC/COT among mentholated cigarette smokers may be attributed to an age effect on CYP2A6 activity. Smoking menthol cigarettes did not significantly affect the ratio after controlling for age, gender and BMI. In summary, in addition to CYP2A6 genetic variation, male gender, younger age and lower BMI were also associated with slower CYP2A6 activity.
Because of the short half-life of nicotine, heavy smokers smoke regularly over the course of the day to maintain sufficient levels in the body to avoid withdrawal symptoms. Accordingly, smokers change their smoking behavior when nicotine yield of cigarettes or rates of nicotine elimination are altered experimentally (45). However, light smokers, including our study population, are not smoking at regular intervals over the course of the day, and thus, their plasma nicotine levels likely fluctuates widely. Accordingly, there was no relationship between cigarette consumption and CYP2A6 activity, and SMs had higher plasma nicotine levels than NMs, indicating a lack of full compensation for altered rates of nicotine metabolism. There is evidence that smoking in chippers (≤ 5 CPD) is under greater stimulus control, i.e. smoking is highly influenced by social and sensory motives and tends to be associated with specific activities, such as after meals or drinking alcohol (46). However, these studies of chippers represent a distinct subset of smokers of European-ancestry, and further investigation into the factors driving light smoking in African-Americans is necessary.
Although we did not observe a relationship between CYP2A6 activity and age of onset of regular smoking, this is a relatively weak measure as it is generally limited by recall bias. Similarly, while we did not see a relationship between CYP2A6 activity and CDS or FTND scores, tobacco dependence is a complex, multi-dimensional construct and there are likely aspects of dependence that were not captured by these scales, such as cravings to smoke. It was recently reported that those with slow CYP2A6 activity may smoke less in response to cravings and were less influenced by smoking-related cues (47). Given the large sample size of our study, it is unlikely that our negative findings result from a lack of statistical power since associations have been reported in smaller studies of heavy smokers of European-descent (23, 25).
Our finding that individuals with slow CYP2A6 activity had greater success at quitting (significant at follow-up) is in agreement with recent findings in clinical trials of heavy smoking individuals of European-ancestry. Individuals with 3HC/COT ratios in the slowest quartile had significantly greater odds of quitting in the placebo arm of one study (26), which was further augmented by treatment with transdermal nicotine patch in another study (27) that was recently replicated (28). The observation of slow CYP2A6 activity and higher quit rates in the placebo arm of this and the previous study suggests greater likelihood of cessation even in the absence of nicotine. This is consistent with the observation that CYP2A6 slow metabolizers were more likely to be former smokers (29) and had shorter smoking durations (23). The higher quit rates among CYP2A6 slow metabolizers were observed primarily in females in this study. Because our sample had disproportionately more females (67%), further replication of this effect in a sample with a larger number of males is necessary.
It is notable that significant associations between CYP2A6 activity and quit rates were observed when individuals were identified by the 3HC/COT quartiles, and only trended towards significance when categorized by CYP2A6 genotypes. Given the large number of participants and the substantial portion of African-Americans defined as genetically slow metabolizers (~36%), our study should have been sufficiently powered. The difference between these two measures may be that the ratio takes into account other sources of variation and/or because of additional unidentified alleles. Large variation in the phenotype is observed among those defined as CYP2A6*1/*1 (i.e. those without identified variants, Fig. 1A), suggesting additional variants may still be present. Thus, CYP2A6 genotypes may have similar utility as 3HC/COT quartiles in predicting smoking cessation in African-American light smokers in the future, particularly as we gain a better understanding of the genetic variations in CYP2A6 in this population.
The results from the current and other recent retrospective studies (26-28) suggest that CYP2A6 activity may have important clinical utility in determining the type of smoking cessation treatment prescribed. For example, individuals with faster CYP2A6 activity might be encouraged to use pharmacotherapy to aid their quit attempts, given their lower quit rates on placebo compared to slower metabolizers. In contrast, slow metabolizers may do well with behavioral therapy alone and/or with nicotine patch treatment but may not benefit greatly from bupropion (26-28). Prospective clinical trials have not yet been performed to directly assess whether the rate of CYP2A6 activity will have utility in the personalization of smoking cessation therapy.
One of the strengths of this study is that we were able to associate CYP2A6 activity with smoking behaviors and treatment outcomes using both genotype and phenotype measures in a large population. A limitation of this study is that it was secondary analyses of a clinical trial designed to test the efficacy of nicotine gum and counseling, and there was an overrepresentation of females. In addition, this treatment-seeking sample of African-American light smokers may not be representative of the general population as they are likely smokers who had difficulty quitting in the past. Further studies are also necessary to determine whether the results are applicable to other racial/ethnic populations, such as Hispanics, where light smoking is also prevalent (48).
In conclusion, this study provides further evidence that the 3HC/COT ratio can serve as a phenotype marker of CYP2A6 activity, and gives new insights into the role of CYP2A6 in light smoking in African-Americans. Confirmation of the validity of the 3HC/COT ratio derived from baseline smoking as a phenotypic marker in light smoking populations will expand its utility for future studies. Secondly, the ability of some individuals to maintain low levels of cigarette consumption contradicts classical theories of tobacco addiction as driven by physical dependence, and further research is needed to examine the biological and psychosocial context underlying light smoking behaviors. There is no safe level of smoking, and a better understanding of the phenomenon will lead to more effective intervention methods for this unique group of smokers
Methods
Study design
A detailed description of the study design and participant recruitment has been described elsewhere (32). Briefly, individuals (n = 755) self-identified as “African-American” or “Black” were randomized into a double-blind, placebo-controlled study at a community health center in Kansas City, Missouri. This study consisted of four treatment arms (n ~189 each) of either placebo or nicotine gum (2 mg), along with either HE or MI counseling. Inclusion criteria included: ≥18 years of age, smoked ≤10 CPD for at least 6 months prior to enrolment, smoked at least 25 of the last 30 days, and interested in quitting smoking in the next 2 weeks.
Drug treatment continued for 8 weeks and the dosing regimen was based on the participant’s baseline cigarette consumption as described previously (32). Six counseling sessions were provided, and all participants were followed for a total of 26 weeks. The research protocol was approved and monitored by the University of Toronto Ethics Review Office and the University of Kansas Human Subjects Committee.
Measurements
Demographic information, smoking history, and severity of nicotine dependence were collected at randomization. Plasma sample was also taken at randomization and the levels of NIC, COT and 3HC from baseline smoking were determined by methods described previously (14).
CYP2A6 genotyping assays
Blood samples for genetic analyses were available from 618 of the 755 participants who consented to genetic testing. Established CYP2A6 alleles (CYP2A6*1B, *2, *4, *9, *12, *17, *20) and novel alleles that were recently identified in a population of Black-African descent (CYP2A6*23, *24, *25, *26, *27, *28, *35) were genotyped using a two-step allele-specific polymerase chain reaction assay as previously described (22, 36, 49, 50). The genotyping assay for CYP2A6*1B was revised (34) because SNPs occurring at a high frequency in this population were found at the location of the primers used in the previous assay. An assay that detects CYP2A6*4A and*4D, as well as the recently identified gene deletions CYP2A6*4E – H, has been reported elsewhere (34).
Statistical analyses
The 3HC/COT ratio was not normally distributed and was log-transformed for all statistical analyses. Chi-squared test was used to test for Hardy-Weinberg equilibrium, to examine differences in allele frequencies in this sample compared to previously reported values in a population of Black-African descent, and to test for differences in quit rates by CYP2A6 activity groups. Univariate ANOVA was used to assess differences in 3HC/COT between CYP2A6 genotype groups, and to test the association of CYP2A6 activity with CPD, CO levels, nicotine plasma levels, age of regular smoking, and dependence scores. Univariate ANOVA was also used to examine if differences in baseline variables exists between the total participants and those with 3HC/COT data, genotype data, and both 3HC/COT and genotypes. Bonferroni correction was used for post-hoc analyses. Multiple logistic regression models were used to assess the association of CYP2A6 genotype and 3HC/COT quartiles on quit rates at EOT and follow-up. All statistical analyses of treatment effects were performed on an intent-to-treat basis and those lost during follow-up were counted as smokers.
Supplementary Material
Acknowledgements
We would like to thank Qian Zhou, Ewa Hoffmann, Abbas Assadzadeh and Evan Dorey for their technical assistance with the genotyping assays. This study was supported by Centre for Addictions and Mental Health, NSERC CGS-D Scholarship (MKH), CIHR-funded SPICE and TUSP awards (JCM), CA91912 (JSA), CA90334 (KSO), DA02277, DA12393 and DA020830 (NLB), CIHR MOP86471 and a Canada Research Chair in Pharmacogenetics (RFT).
Footnotes
Conflicts of interest Dr. R.F. Tyndale hold shares in Nicogen Research Inc., a company that is focused on novel smoking cessation treatment approaches. None of the data contained in this manuscript alters or improves any commercial aspect of Nicogen, no Nicogen funds were used in this work, and the manuscript was not reviewed by others affiliated with Nicogen. Dr. R.F. Tyndale has also been a paid consultant for Novartis. Dr. Benowitz is a paid advisor to several pharmaceutical companies that market or are developing smoking cessation medications, and also serves as a paid expert witness in litigation against tobacco companies. Dr. Ahluwalia is a consultant to Pfizer Inc.
Supplementary information is available at http://www.nature.com/cpt
References
- 1.Kandel DB, Chen K. Extent of smoking and nicotine dependence in the United States: 1991-1993. Nicotine & Tobacco Research. 2000;2(3):263–274. doi: 10.1080/14622200050147538. [DOI] [PubMed] [Google Scholar]
- 2.Royce JM, Hymowitz N, Corbett K, Hartwell TD, Orlandi MA. Smoking cessation factors among African Americans and whites. COMMIT Research Group. Am J Public Health. 1993;83(2):220–226. doi: 10.2105/ajph.83.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Smoking Cessation During Previous Year Among Adults -- United States, 1990 and 1991. Morbidity and Mortality Weekly Report. 1993:504–507. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention . In: Tobacco use among U.S. racial/ethnic minority groups — African-Americans, American-Indians and Alaska Natives, Asian-Americans and Pacific Islanders, and Hispanics: a report of the Surgeon General. Department of Health and Human Services, editor. Atlanta: 1998. [PubMed] [Google Scholar]
- 5.Haiman CA, Stram DO, Wilkens LR, Pike MC, Kolonel LN, Henderson BE, et al. Ethnic and Racial Differences in the Smoking-Related Risk of Lung Cancer. N Engl J Med. 2006;354(4):333–342. doi: 10.1056/NEJMoa033250. [DOI] [PubMed] [Google Scholar]
- 6.US Department of Health and Human Services . Tobacco use among U.S. racial/ethnic minority groups: African Americans, American Indians and Alaska Natives, Asian Americans and Pacific Islanders, Hispanics. In: Centers for Disease Control and Prevention: Office on Smoking and Health, editor. A report of the Surgeon General, 1998. US Government Printing Office; 1998. Publication No S/N 017-001-00527-4. [Google Scholar]
- 7.Clark PI, Gautam S, Gerson LW. Effect of Menthol Cigarettes on Biochemical Markers of Smoke Exposure Among Black and White Smokers. Chest. 1996;110(5):1194–1198. doi: 10.1378/chest.110.5.1194. [DOI] [PubMed] [Google Scholar]
- 8.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., III Ethnic Differences in N-Glucuronidation of Nicotine and Cotinine. J Pharmacol Exp Ther. 1999;291(3):1196–1203. [PubMed] [Google Scholar]
- 9.Muscat JE, Djordjevic MV, Colosimo S, Stellman SD, Jr., R.J. Racial differences in exposure and glucuronidation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) Cancer. 2005;103(7):1420–1426. doi: 10.1002/cncr.20953. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Nicotine addiction. Prim Care. 1999;26(3):611–631. doi: 10.1016/s0095-4543(05)70120-2. [DOI] [PubMed] [Google Scholar]
- 11.Benowitz NL, Jacob P., 3rd Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clinical Pharmacology & Therapeutics. 1994;56:483–493. doi: 10.1038/clpt.1994.169. [DOI] [PubMed] [Google Scholar]
- 12.Messina ES, Tyndale RF, Sellers EM. A Major Role for CYP2A6 in Nicotine C-Oxidation by Human Liver Microsomes. J Pharmacol Exp Ther. 1997;282(3):1608–1614. [PubMed] [Google Scholar]
- 13.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3′-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277(2):1010–1015. [PubMed] [Google Scholar]
- 14.Dempsey D, Tutka P, Jacob P, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics. 2004;76(1):64. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Yamazaki H, Inui Y, Yun C-H, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13(10):1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- 16.Mwenifumbo JC, Tyndale RF. Genetic variability in CYP2A6 and the pharmacokinetics of nicotine. Pharmacogenomics. 2007;8(10):1385–1402. doi: 10.2217/14622416.8.10.1385. [DOI] [PubMed] [Google Scholar]
- 17.Higashi E, Fukami T, Itoh M, Kyo S, Inoue M, Yokoi T, et al. Human CYP2A6 Is Induced by Estrogen via Estrogen Receptor. Drug Metab Dispos. 2007;35(10):1935–1941. doi: 10.1124/dmd.107.016568. [DOI] [PubMed] [Google Scholar]
- 18.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;79(5):480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 19.McMorrow MJ, Foxx RM. Nicotine’s role in smoking: an analysis of nicotine regulation. Psychol Bull. 1983;93(2):302–327. [PubMed] [Google Scholar]
- 20.Benowitz NL, Swan GE, Jacob P, Lessov-Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine[ast] Clin Pharmacol Ther. 2006;80(5):457. doi: 10.1016/j.clpt.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, et al. Duplications and Defects in the CYP2A6 Gene: Identification, Genotyping, and In Vivo Effects on Smoking. Mol Pharmacol. 2000;58(4):747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 22.Mwenifumbo JC, Al Koudsi N, Ho MK, Zhou Q, Hoffmann EB, Sellers EM, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Human Mutation. 2008;29(5):679–688. doi: 10.1002/humu.20698. [DOI] [PubMed] [Google Scholar]
- 23.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine and Tobacco Research. 2007;9(4):511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 25.Kubota T, Nakajima-Taniguchi C, Fukuda T, Funamoto M, Maeda M, Tange E, et al. CYP2A6 polymorphisms are associated with nicotine dependence and influence withdrawal symptoms in smoking cessation. Pharmacogenomics J. 2006 doi: 10.1038/sj.tpj.6500348. [DOI] [PubMed] [Google Scholar]
- 26.Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward Personalized Therapy for Smoking Cessation: A Randomized Placebo-controlled Trial of Bupropion. Clin Pharmacol Ther. 2008 doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 27.Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz NL, Lerman C. Nicotine Metabolism and Response to Transdermal Nicotine. Pharmacology, Biochemistry and Behavior. doi: 10.1016/j.pbb.2008.10.016. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gu DF, Hinks LJ, Morton NE, Day INM. The use of long PCR to confirm three common alleles at the CYP2A6 locus and the relationship between genotype and smoking habit. Annals of Human Genetics. 2000;64(5):383–390. doi: 10.1046/j.1469-1809.2000.6450383.x. [DOI] [PubMed] [Google Scholar]
- 30.Malaiyandi V, Goodz S, Sellers EM, Tyndale RF. CYP2A6 genotype, phenotype and the use of nicotine metabolites as biomarkers during ad libitum smoking. Cancer Epidemiol Biomarkers Prev. 2006;15(10):1812–1819. doi: 10.1158/1055-9965.EPI-05-0723. [DOI] [PubMed] [Google Scholar]
- 31.Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks LJ, Day INM, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clinical Pharmacology & Therapeutics. 2006;80(4):319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2×2 factorial design. Addiction. 2006;101(6):883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- 33.Mwenifumbo JC, Lessov-Schlaggar CN, Zhou Q, Krasnow RE, Swan GE, Benowitz NL, et al. Identification of Novel CYP2A6[ast]1B Variants: The CYP2A6[ast]1B Allele is Associated With Faster In Vivo Nicotine Metabolism. Clin Pharmacol Ther. 2007;83(1):115. doi: 10.1038/sj.clpt.6100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mwenifumbo JC, Sellers EM, Tyndale RF. Novel CYP2A6 gene deletion and conversion polymorphisms in a population of Black-African descent. 2008. Submitted. [DOI] [PMC free article] [PubMed]
- 35.Fukami T, Nakajima M, Yamanaka H, Fukushima Y, McLeod HL, Yokoi T. A Novel Duplication Type of CYP2A6 Gene in African-American Population. Drug Metab Dispos. 2007;35(4):515–520. doi: 10.1124/dmd.106.013557. [DOI] [PubMed] [Google Scholar]
- 36.Ho MK, Mwenifumbo JC, Zhao B, Gillam EMJ, RF T. A novel CYP2A6 allele, CYP2A6*23, impairs enzyme function in vitro and in vivo and decreases smoking in a population of Black African descent. Pharmacogenetics and Genomics. 2008;18(1):67–75. doi: 10.1097/FPC.0b013e3282f3606e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etter J-F, Le Houezec J, Perneger TV. A Self-Administered Questionnaire to Measure Dependence on Cigarettes: The Cigarette Dependence Scale. Neuropsychopharmacology. 2003;28(2):359. doi: 10.1038/sj.npp.1300030. [DOI] [PubMed] [Google Scholar]
- 38.Heatherton TF, Kozlowski LT, Frecker RC, KO. F. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 39.Nollen NL, Mayo MS, Sanderson Cox L, Okuyemi KS, Choi WS, Kaur H, et al. Predictors of Quitting Among African American Light Smokers Enrolled in a Randomized, Placebo-Controlled Trial. Journal of General Internal Medicine. 2006;21(6):590–595. doi: 10.1111/j.1525-1497.2006.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lea RA, Dickson S, Benowitz NL. Within-subject variation of the salivary 3HC/COT ratio in regular daily smokers: prospects for estimating CYP2A6 enzyme activity in large-scale surveys of nicotine metabolic rate. J Anal Toxicol. 2006;30(6):386–389. doi: 10.1093/jat/30.6.386. [DOI] [PubMed] [Google Scholar]
- 41.Mooney ME, Li Z.-z., Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the Nicotine Metabolite Ratio in ad Libitum and Reducing Smokers. Cancer Epidemiol Biomarkers Prev. 2008;17(6):1396–1400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mwenifumbo JC, Sellers EM, Tyndale RF. Nicotine metabolism and CYP2A6 activity in a population of black African descent: Impact of gender and light smoking. Drug and Alcohol Dependence. 2007;89(1):24. doi: 10.1016/j.drugalcdep.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Swan GE, Lessov-Schlaggar CN, Bergen AW, He Yungang, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenetics and Genomics. 2008 doi: 10.1097/FPC.0b013e32832a404f. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benowitz NL, Herrera B, Jacob P., III Mentholated Cigarette Smoking Inhibits Nicotine Metabolism. J Pharmacol Exp Ther. 2004;310(3):1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- 45.Scherer G. Smoking behavior and compensation: a review of the literature. Psychopharmacology. 1999;145:1–20. doi: 10.1007/s002130051027. [DOI] [PubMed] [Google Scholar]
- 46.Shiffman S, Paty J. Smoking patterns and dependence: contrasting chippers and heavy smokers. J Abnorm Psychol. 2006;115(3):509–523. doi: 10.1037/0021-843X.115.3.509. [DOI] [PubMed] [Google Scholar]
- 47.Piper ME, McCarthy DE, Bolt DM, Smith SS, Lerman C, Benowitz N, et al. Assessing dimensions of nicotine dependence: An evaluation of the Nicotine Dependence Syndrome Scale (NDSS) and the Wisconsin Inventory of Smoking Dependence Motives (WISDM) Nicotine & Tobacco Research. 2008;10(6):1009–1020. doi: 10.1080/14622200802097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Office of Applied Studies, Substance Abuse and Mental Health Services Administration [cited Aug 11, 2008];National Survey on Drug Use and Health: Past month cigarette use among racial and ethnic groups. 2006 Available from: http://www.oas.samhsa.gov/2k6/raceCigs/raceCigs.htm.
- 49.Goodz SD, Tyndale RF. Genotyping human CYP2A6 variants. Methods Enzymol. 2002;357:59–69. doi: 10.1016/s0076-6879(02)57666-7. [DOI] [PubMed] [Google Scholar]
- 50.Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, Sellers EM, Tyndale RF. A novel CYP2A6 allele (CYP2A6*35) containng an amino acid substitution (N438Y) is associated with lower CYP2A6 activity in vivo. 2008. Submitted. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.