Abstract
Breast cancer survivors often use clues to convey their concerns to their oncologists. The authors conducted a randomized trial of a communication coaching intervention in which 22 female breast cancer survivors were randomized to the coaching and 22 to treatment as usual. They hypothesized that the intervention would increase breast cancer self-efficacy, improve mood, and reduce fears of recurrence. Through a series of ANCOVAs they found that the intervention led to increases in self-efficacy. Changes in self-efficacy predicted changes in anxiety, depression, and womanhood fears. This coaching intervention shows promise but requires additional studies to establish is efficacy and effectiveness.
Keywords: physician-patient communication, coaching intervention, breast cancer, randomized trial, nursing
The American Cancer Society estimated that more than 2 million women would be breast cancer survivors in 2008 (Jemal et al., 2008). With improved early detection and more effective treatment, breast cancer survival rates continue to rise, making quality-of-life issues such as fear of recurrence a concern for a growing number of women (Avis, Crawford, & Manuel, 2004). Quality-of-life studies for women diagnosed with breast cancer have focused mainly on issues related to immediate survival and adjustment during or immediately following treatment. Additionally, the majority of women studied have been older than age 45 at diagnosis. Few studies have addressed the long-term survivorship issues of women several years after diagnosis, and even fewer have addressed the unique concerns of women diagnosed at age 45 or younger. Studies have indicated that younger women may have different concerns than older women because life tasks are different (Brezden, Phillips, Abdolell, Bunston, & Tannock, 2000; Ferrell, Dow, Leigh, Ly, & Gulasekaram, 1995; Ganz, Rowland, Desmond, Meyerowitz, & Wyatt, 1998). For example, family transitions in the younger age group may relate to occupational changes, rearing children, and caring for parents. Another reason for this differential response may also include the fact that for an older person illness is an anticipated occurrence whereas for younger people it is not (Kornblith et al., 2007). Older people may have more experience with illness either personally or in family members. Younger women may also perceive greater potential of loss because they may be anticipating children, in the middle of raising a family, or building a career (Bloom, Stewart, Chang, & Banks, 2004) .
Survivors often report difficulties with communication with oncologists and general practitioners (Epstein & Street, 2007). Because younger survivors are at increased risk for negative quality-of-life outcomes, it is imperative that interventions be developed to improve communication between younger survivors and health care providers. The purpose of the current study is to compare a coaching intervention that uses a prompt sheet with usual care to improve cancer provider and survivor communication about survivor worries. We have found that survivors who report being unable to discuss their worries and concerns at diagnosis with their provider report less self-efficacy in managing their symptoms, managing worry about recurrence, and asking health care providers or others for help (Ziner, Champion, Sledge, & Bell, under review).
BACKGROUND
Cancer survivors often give only clues to their underlying emotional distress rather than bringing up these issues directly with physicians. Younger and female cancer survivors give more clues inviting informational and emotional support than do older or male survivors; in one study, physicians responded to informational clues 72% of the time versus 28% of the time to emotional clues (Butow, Brown, Cogar, Tattersall, & Dunn, 2002). When the first clues are ignored, survivors tend to use more clues rather than asking directly, which can lengthen the session (Levinson, Gorawara-Bhat, & Lamb, 2000). It is likely that survivors do not think that they can adequately express their concerns directly or are afraid to do so. In the current study, we directly intervened to help survivors present the worries and concerns they have in a structured manner. We hypothesize that improving how survivors communicate with their oncologists would increase survivor self-efficacy to express their concerns directly to manage their cancer (Han et al., 2005; Vries, Mesters, Van de Steeg, & Honing, 2005). Social cognitive theory posits self-efficacy, the belief in one’s capabilities to carry out certain tasks, is an essential determinant of success in completing those tasks (Bandura, 1997). In the current study, we sought to increase survivors’ self-efficacy in managing their cancer by coaching them to express their concerns directly to their oncologists rather than using indirect clues and hints. We hypothesize that self-efficacy will be related to changes in other outcomes.
Research to train health care providers for improving communication skills during survivor visits has revealed mixed findings. A recent review of health care provider communication training in oncology underscores the difficulty of implementing and evaluating such programs (Butler, Degner, Baile, & Landry, 2005). Many programs were of short duration and uptake of communication skills training was low because of the time constraints of busy clinicians. Most programs were not structured to become part of regular ongoing training (Razavi et al., 2003). With the exception of Glimelius, Birgegard, Hoffman, Kvale, and Sjoden (1995), few examined the effect of training on survivor outcomes, measuring only physician or nurse self-reported improvements in communication skills. Most showed that changes in communication skills were short lived. Such programs should continue to be studied, but we are proposing to examine the other side of the communication equation: the survivor.
Another method of improving communication during health care provider visits is to intervene directly with survivors. Research has shown that a question prompt sheet given to cancer survivors prior to a visit increased survivor knowledge of prognosis (Butow, Dunn, Tattersall, & Jones, 1994). In a later study, the same research group intervened with survivors and physicians. Physicians were randomized to respond actively to the survivor’s question asking about treatment issues or to respond passively to their question sheet (Brown, Butow, Dunn, & Tattersall, 2001). When physicians responded passively to the questions on the prompt sheet, survivors were more anxious and sessions were longer. When oncologists responded specifically to the question prompt sheet, survivors reported lower anxiety. In the current study, we extend this research to breast cancer survivors and their concerns.
Many cancer survivors report difficulties communicating with their oncologists about their long-term symptoms, fears, or lifestyle disturbances (Epstein et al., 2007). Communication between cancer survivors and health care providers is important to cancer survivor well-being (Arora, 2003; Butow, Dunn, & Tattersall, 1995). Survivors who report better communication also report better coping with their cancer (Butow et al., 1995), lower anxiety (Takayama, Yamazaki, & Katsumata, 2001), and greater satisfaction with information (Kirmayer, 1994). One study found that a mere 40 seconds of communication reflecting compassion from a physician resulted in lower survivor anxiety and better understanding of cancer information (Fogarty, Curbow, Wingard, McDonnell, & Somerfield, 1999). The current study sought to help survivors communicate in a way that will makes it easier for physicians to provide the empathy many survivors seek, thus increasing survivors’ self-efficacy or confidence in managing their cancer and reducing their anxiety and distress.
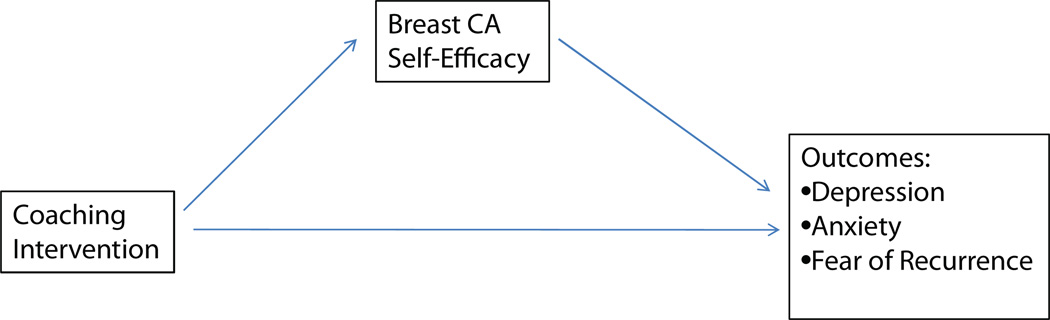
The study premise is that if survivors state their emotional concerns in a direct manner rather than communicating indirectly with clues, their oncologists will be more likely to respond and to provide validation of their concerns. Such actions should lead to increased self-efficacy for survivors in managing their cancer symptoms and ongoing surveillance that may also lead to improvements in other outcomes. Figure 1 shows a graphic of our conceptual model that is very similar to the model proposed by Beckham, Burker, Lytle, Felman, and Costakis (1997) in which changes in self-efficacy are proposed to predict changes in other psychological health and fear of recurrence.
FIGURE 1.
Conceptual model.
METHOD
Design
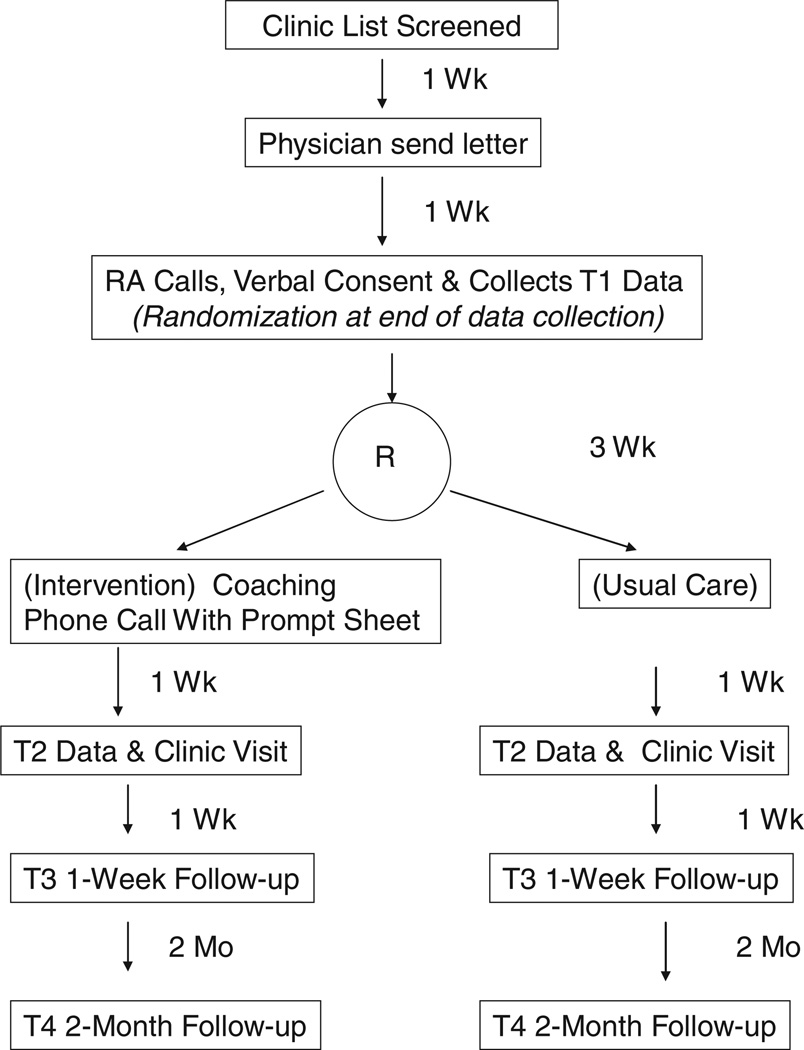
The study was a randomized pilot trial of usual care compared to a worries and concerns prompt sheet and telephone counseling encouraging breast cancer patients to talk to their oncologists about their fears of cancer recurrence as well as anxiety or depression (see Table 2). Participants received the intervention one week prior to the physician visit (Time 1 [T1]) (see Figure 2), the physicians visits took place at T2, the first follow-up assessment took place one week after the physician visit (T3), and the final follow-up assessment occurred 2 months after the physician visit (T4).
TABLE 2.
Question Prompt Sheet
 |
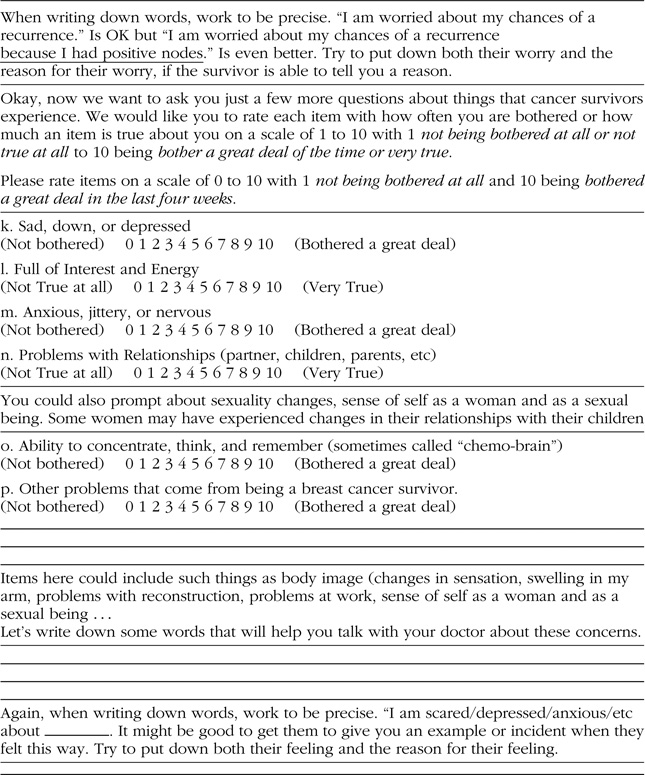 |
FIGURE 2.
Research design.
Recruitment
The project manager for the ongoing study on younger breast cancer survivors (American Cancer Society [ACS] RSGPB-04-089-01PBP) forwarded the names and dates of birth to the cancer center recruitment coordinator. To be eligible for the original study patients had to be (1) female; (2) age 18 to 45 at diagnosis; (3) 3 and 8 years postdiagnosis; (4) Stage I, II, or III invasive breast cancer; (5) treatment included Anthracycline-based chemotherapy or Cytoxan, Methotrexate, and 5-Fluorouracil (CMF); and (6) disease free at the time of enrollment.
Recruitment for the current study had a waiver for Health Insurance Portability and Accountability Act (HIPAA) authorization for recruitment only. Each month clinic appointments for the oncologists included in the study were reviewed for patients referred from the ongoing study. Eligible patients who had an appointment scheduled in the next 6 months were sent a letter by their physician introducing the current study with an option to opt out by calling a toll-free number within 2 weeks. Participants who had not called this number within 2 weeks were recruited by telephone and mailed a written informed consent form.
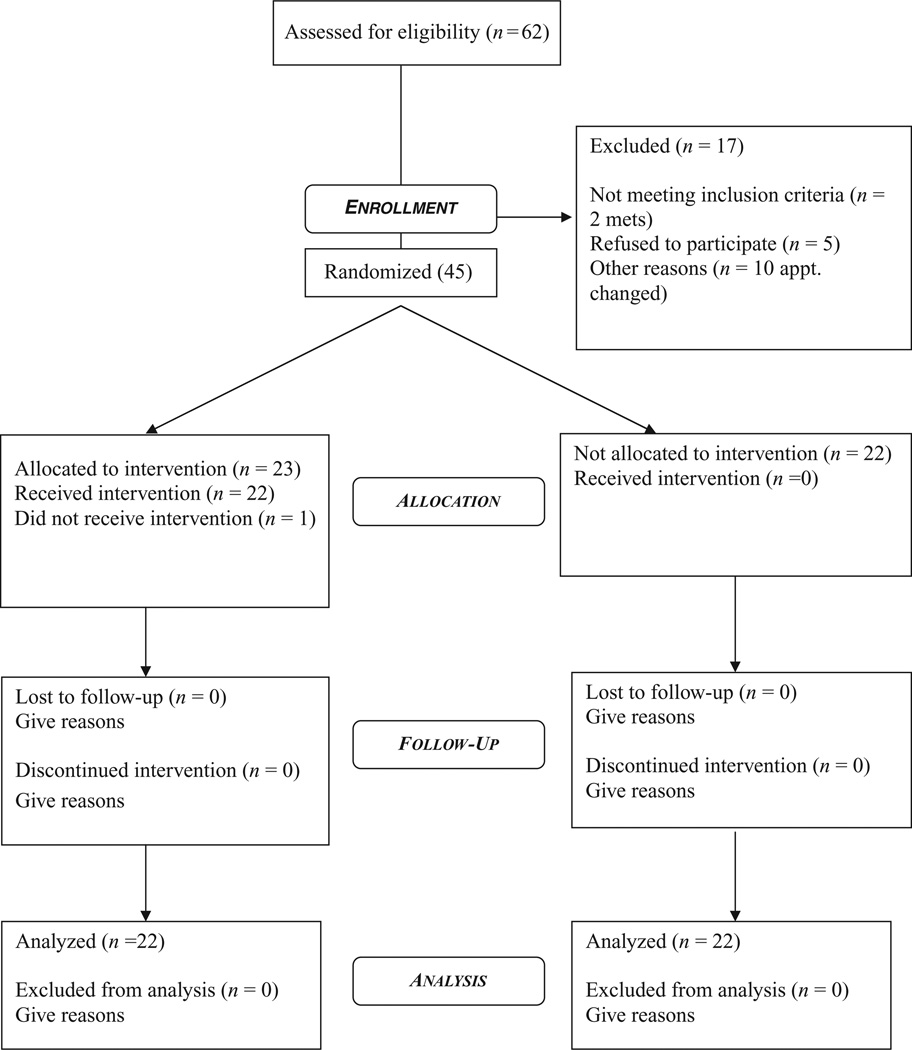
Figure 3 shows the flow of participants through the study using consort guidelines (Moher, Schulz, Altman, & CONSORT Group, 2001). Sixty-seven potential participants’ names were obtained from the project manager. These participants had given permission to be contacted about additional studies. Of these 67, three were found to be ineligible for the current study because they were now in treatment for metastatic breast cancer. We excluded survivors with metastatic disease because, with our small sample, we would be unable to conduct any subanalyses on this group. We also thought that their concerns and questions would likely be very different from survivors with no recurrent disease. Of the remaining 64, eight rescheduled their appointment beyond the enrollment period of the study, five refused due to a lack of time to participate, four could not be reached by telephone, and two had recently been diagnosed with metastatic breast cancer. Enrollment in the study included 45 participants with one participant withdrawing after verbal consent and before written consent. Two women did not complete the final assessment. Thus of the 67 eligible 65% actually participated.
FIGURE 3.
Consort participant flow chart.
Enrollment and Randomization
A research assistant (RA) called the participants, gained verbal consent, explained the written informed consent, and collected baseline (T1) data. At the end of the phone call, survivors were randomized into either the intervention or control group. For survivors who were randomized to the intervention group (prompt plus telephone counseling) an oncology nurse scheduled a telephone call 1 week prior to the visit to counsel the participant on how to fill out the prompt sheet and use it during their next office visit. All survivors, regardless of their randomized group, were asked to bring the signed informed consent to the office visit. An RA, who also had copies of the informed consent document, collected the informed consent, and administered an anxiety scale immediately prior to the clinic visit. One week following the office visit, the patient was contacted by phone to collect outcome data on self-efficacy, depression, anxiety, and fear of recurrence (T3). We collected the final data (T4) at 2 months postoffice visit.
Participants Demographics
Younger breast cancer survivors (i.e., diagnosed at age 45 or younger) who have been participating in an ongoing study of breast cancer survivors and who had agreed to be contacted about future studies were invited to participate. Survivors had been recruited for the original study when they were 3 to 8 years postdiagnosis. Breast cancer oncologists at the Indiana University Simon Cancer Center (an NCI designated Comprehensive Cancer Center) were following all survivors. Table 1 shows that the average age of participants was 44.1 and survivors averaged 3.6 years of college. The average age at diagnosis was 38.5, and 95% of the sample was White. All survivors had undergone chemotherapy and currently had no systemic recurrence. Twenty percent were diagnosed at Stage I, 53% at stage II, and 27% at stage II.
TABLE 1.
Sample Demographics
| Control M (SD) |
Intervention M (SD) |
|
|---|---|---|
| Age | 44.0 (4.1) | 44.2 (5.0) |
| Education years | 16.1 (2.7) | 15.1 (2.9) |
| Age at diagnosis | 38.5 (4.3) | 38.5 (5.1) |
| Time since diagnosis | 5.5 (1.6) | 5.6 (1.7) |
| White | 100% | 95% |
t tests were all nonsignificant.
Procedure
Coaching Intervention
The purpose of the coaching intervention was to enable survivors to tell their oncologist worries and concerns that typically go unspoken in oncology consultations with cancer survivors. Table 2 shows the question prompt sheet that was mailed to survivors 2 weeks prior to their oncologist visit. A number of items from the Concerns about Recurrence Scale (CARS; Vickberg, 2003) were used in the sheet to prompt survivors to discuss their fears related to cancer along with questions to prompt them to talk about emotional symptoms or other worries. The coach, who was a trained oncology nurse practitioner, talked with the survivor about their ratings of each item on the sheet. At the end of each of the two sets of items, the coach asked the survivors to put into words what she would like to tell her oncologist about her worries and concerns related to her survivor status. The final step in the intervention was to ask the participant to prioritize what she wanted to talk with her oncologist about and limit her discussion to her top three concerns. The coach then typed the survivors’ concerns onto a Summary Sheet and mailed it to the survivor to use at her upcoming visit. The coach encouraged the survivors to describe their concerns and told them that their oncologist wanted to hear about them. Survivors were instructed to take the Summary Talking Points Sheet to their upcoming visits with their oncologists. At the time of their visit, the RA had another copy of the Summary Talking Points Sheet for the survivor in case she forgot to bring it with her.
Assessment Instruments
Breast Cancer Self-Efficacy
Self-efficacy is the process through which individuals translate knowledge and skills into competent action (Bandura, 1997). Survivors with higher cancer self-efficacy reported less physical impact from their cancer, higher general quality of life, less anxiety and preoccupation with their cancer, a higher fighting spirit, less helpless/hopelessness, and a higher sense of well-being (Merluzzi, Nairn, Hegde, Martinez Sanchez, & Dunn, 2001). Cancer patients’ self-efficacy predicted their quality of life at 4- and 8-month follow-up assessment (Lev, Paul, & Owen, 1999). Self-efficacy affects newly diagnosed breast cancer survivors’ ability to interact with health care providers (Collie et al., 2005). The scale for the current study was a 14-item scale with a 5-point Likert-type responses (1 = strongly disagree, 5 = strongly agree) developed for the larger ongoing breast cancer survivor quality-of-life study. Responses were summed for a total score. Four clinical experts in cancer care, two PhD cancer research experts, and 12 breast cancer survivors reviewed and confirmed the content validity of the scale. Internal consistency reliability was good with a Cronbach’s alpha of .84. The scale consists of items such as “I am able to ask for help when I have problems related to my breast cancer” and “I am able to have a productive work life even though I had breast cancer.” These items measure survivors’ perceived ability (self-efficacy) to manage their lives even though they have been diagnosed and treated for breast cancer.
The Spielberger State-Trait Anxiety Inventory (STAI)–State subscale is a 20-item subscale to assess how people feel “at this moment” (state anxiety) using 4-point Likert-type responses (1 = not at all, 4 = very much so). The subscale is summed for individual subscale scores. Internal consistency for the more transitory S-Anxiety (states) was .93 in our previous study. Concurrent validity with other measures of T-anxiety are high at .73 to .85 (Spielberger, Gorsuch, Vagg, & Jackobs, 1983). Results in breast cancer studies: In a pretest, posttest design study of 123 breast cancer survivor’s alpha was reported as .92 pretest and .94 posttest (Fogarty et al., 1999). In another study of postoperative arm problems in 829 breast cancer survivors, STAI alpha was reported as adequate (Fleissig et al., 2006).
The Center for Epidemiological Studies Depression Scale (CESD; Radloff, 1977) was used to assess depressive symptoms. This is a widely used scale in behavioral cancer research because of its lack of somatic items for depression and its good reliability with a reported Cronbach’s α of .87.
The Concerns about Recurrence Scale (CARS; Vickberg, 2003) was used to assess survivors overall fear of recurrence and worries related to thoughts of a recurrence. The CARS is a two-part instrument developed for breast cancer survivors. The first part consists of four items with a 6-point Likert-type response (1 = don’t think about it, 5 = think about it a lot) assessing overall fear of recurrence; the second part consists of 29 items with a 5-point Likert-type response (1 = worry not at all, 5 = worry all or most of the time) grouped into five subscales that assess the reasons for worry over recurrence. The five subscales assess breast cancer recurrence-related worries about death, health, role, womanhood, and parenting. Internal consistency reliabilities have ranged from .87 to .94, and subscale scores exhibit good discriminate/ convergent validity (e.g., subscales correlate with other measures of distress, but most strongly with assessments of intrusive thoughts).
Statistical Analyses
We first examined the distributions of the variables and found that they did not violate assumptions of normality. We then computed sample demographics for the total sample and compared demographics by group (intervention vs. control) to ensure that random assignment was effective. We used ANCOVA to examine whether there were differences between control and intervention (e.g., T4 self-efficacy) controlling for baseline values of the outcome variable (e.g., baseline self-efficacy). We also calculated effect sizes for the differences using the formula, ES = (Mintervention—Mcontrol) / SDpooled. Unlike R2, which is a measure of variance accounted for, ES can be positive or negative depending on the direction of change. We then conducted posthoc analyses to examine the correlations between T1 self-efficacy and the other outcome measures, measured at T3 and T4. We then conducted a series of ANCOVAs to examine whether the T1 value of self-efficacy and whether changes in self-efficacy from T1 to T4 were associated with outcomes.
RESULTS
Survivors’ Concerns
Telephone coaching sessions averaged 25 minutes with a range of 20 to 45 minutes. The majority (90%) of the intervention group participants used the summary/prompt sheet prior to their visit at least once (45%) whereas 15% used the summary prompt sheet four times or more before the visit. Ninety-five percent (95%) had the summary/prompt sheet with them during their visit. More than 80% of the participants rated the summary/prompt sheet as either very helpful (50%) or helpful (31%) for their visit.
In Table 3 are examples of the questions survivors generated to discuss with their oncologists. We grouped them into categories, noted the number of questions survivors asked within that category, and included one example question for each. The categories are listed in order of the frequency of questions in that category. The majority of the questions included concerns about current symptoms, long-term effects of treatment, and recurrence of cancer. Other questions included questions about cognitive changes, current treatment, and risk for other new cancers. A few survivors wanted to tell their oncologist about positive events in their lives.
TABLE 3.
Examples of Questions Survivors Included on Prompt Sheets
| Categories (Frequency) | Examples |
|---|---|
| Symptoms (11) | I have a new hip pain in the last month. It seems to hurt more when I put weight on it, but it does come and go. Should I be worried? |
| Long term side effects (10) | I took the Adriamycin and I worry about the cardiac effects. Are there things that I can do to protect my heart? Or are there things I should know about? For example, I have gotten into caffeine and palpitations. Are there signs I should worry about? |
| Recurrence (8) | Is there anything new that I should know about what I can do to reduce my risk of a recurrence of my breast cancer? |
| Cognitive changes (6) | I am still challenged by my ability to concentrate, think and remember things. Are there any new results about chemo-brain that you have heard about? Are there any new things that we could try to reduce this problem? |
| Current treatment (6) | Is it normal to have foot pain with Aromasin? My foot pain interferes with my quality of life. I am not able to walk on the treadmill as I need to in order to control my weight. The pain also affects my mood. It makes me feel depressed. |
| Help (5) | I continue to have frequent panic attacks related to breast cancer triggers. I tried the consultations with professionals, but I would like your suggestions for self-help things to try. |
| Other cancer (4) | Am I at risk for other cancers? If so, what kinds? |
| Celebrating (2) | Our new baby arrived in March! |
| Testing (2) | Would finding small mets make a difference is my quality of life and remission chances? |
We also examined whether survivors discussed emotional worries and concerns in their questions by conducting a text analysis of emotional language in their questions using the LIWC2007 program (Pennebaker, Booth, & Francis, 2007), which calculates the percentage and frequency of emotion words survivors used in their questions. The survivors in the intervention group wrote 54 questions with the interventionist. We found that 39 of those questions contained affect-oriented words, of which 16 questions contained positive emotions and 30 contained negative emotions such as worry or anxiety (19 questions), anger (3 questions), and sad (7 questions). Seventy-two percent of the questions included an affective component. A total of 21 of the 22 survivors, who received the intervention, wrote questions containing affective content. Twelve survivors wrote questions with positive emotions and 19 with negative emotions with 15 survivors writing questions containing anxious emotions. These results suggest that we accomplished our goal of encouraging survivors to discuss their emotional concerns with their oncologists.
Intervention Analyses
We used ANCOVA to examine differences in outcome by intervention group while adjusting for T1 values. Table 4 shows that only T4 self-efficacy (effect size = 3.17) was different between the two groups (greater for intervention, p = .04) after adjusting for baseline self-efficacy.
TABLE 4.
ANCOVA: Intervention Effects
| Intervention |
Control |
||||||
|---|---|---|---|---|---|---|---|
| N | M | SE | N | M | SE | Effect Size | |
| Time 3 Self-efficacy | 20 | 63.41 | 0.94 | 22 | 61.77 | 0.94 | 1.74 |
| Time 4 Self-efficacy | 17 | 66.86 | 2.2 | 15 | 59.83 | 2.24 | 3.17 |
| Time 3 Depression | 21 | 13.01 | 11.8 | 22 | 13.22 | 12.69 | −0.02 |
| Time 4 Depression | 17 | 11.48 | 12.29 | 15 | 9.26 | 6.54 | 0.24 |
| Time 3 Anxiety | 21 | 32.23 | 2.5 | 21 | 33.91 | 2.5 | −0.67 |
| Time 4 Anxiety | 17 | 31.83 | 2.2 | 15 | 32.59 | 2.8 | −0.30 |
| Fears of Recurrence | |||||||
| Time 3 Health worries | 20 | 21.50 | 1.63 | 22 | 20.2 | 1.63 | 0.80 |
| Time 4 Health worries | 17 | 19.63 | 1.81 | 15 | 21.49 | 1.87 | −1.01 |
| Time 3 Womanhood worries | 21 | 6.28 | 0.84 | 21 | 5.34 | 0.86 | 1.11 |
| Time 4 Womanhood worries | 17 | 6.63 | 0.98 | 15 | 6.5 | 1.01 | 0.13 |
| Time 3 Role worries | 21 | 7.6 | 0.69 | 22 | 7.76 | 0.69 | −0.23 |
| Time 4 Role worries | 17 | 7.51 | 0.92 | 15 | 9.64 | 0.95 | −2.28 |
| Time 3 Death worries | 21 | 4.31 | 0.26 | 22 | 4.61 | 0.26 | −1.15 |
| Time 4 Death worries | 17 | 4.07 | 0.24 | 15 | 4.48 | 0.24 | −1.71 |
| Time 3 Parenting worries | 21 | 2.93 | 0.00 | 16 | 2.93 | 0.00 | — |
| Time 4 Parenting worries | 16 | 2.69 | 0.34 | 15 | 3.28 | 0.35 | −1.71 |
| Time 3 PT Satisfaction | 21 | 478.71 | 38.08 | 22 | 492.36 | 13.99 | −0.52 |
| M = adjusted means | |||||||
Note: Each row is a separate ANCOVA model adjusted for its T1 (baseline) value. Time 3 Parenting Worries are identical to Time 1 Parenting Worries, no ANCOVA analysis can be done. The unadjusted group means are reported.
ES = (Mintervention−Mcontrol)/ SEpooled; PT = Patient.
p ≤ 0.05.
Changes in Breast Cancer Self-Efficacy as a Correlate of Other Outcomes
We had predicted that self-efficacy would mediate the effect of the intervention on other outcomes, thus we examined whether self-efficacy or change in self-efficacy was associated with changes in other outcomes. Table 5 shows the correlations between outcomes variables at T3 and T4 with self-efficacy at T1. Self-efficacy at T1 was correlated with all the T4 outcome variables in the expected direction and with all but anxiety at T3.
TABLE 5.
Correlations Between Self-Efficacy at Time 1 (T1) and Outcome Variables at T3 and T4
| Outcome Variables | T3 | T4 |
|---|---|---|
| Depressive symptoms | −0.34* | −0.59** |
| Health worries | −0.55** | −0.73** |
| Anxiety | −0.20 | −0.49* |
| Womanhood worries | −0.44* | −0.63** |
| Role worries | −0.63** | −0.51** |
| Death worries | −0.51** | −0.47** |
| Parenting worries | −0.44* | −0.33 ~ |
| Satisfaction | 0.51** | 0.41* |
p ≤ .10,
p ≤ 0.05,
p ≤ 0.001.
We then conducted a series of ANCOVAs examining whether self-efficacy at T1 (in one model) or changes in self-efficacy (in a separate model) were associated outcomes after adjusting for the group effect (intervention vs. control). Change in self efficacy was computed as T3−T1. (We did not include the tables, but we did examine change in self-efficacy of T4−T1 and found change in self-efficacy from T1 to T4 was not significant in the models.) Table 6 shows the results of the ANCOVA examining the effects of group and T1 self-efficacy (and group and change in self efficacy) as the two main predictors in the models. Self-efficacy at T1 continued to be a predictor of all outcomes at T3 and T4 except for T3 anxiety. Group continued to be nonsignificant on other outcomes, but change in self efficacy was a predictor of T3 depression (p < 0.05), a strong predictor of T3 anxiety (p < 0.0001), a predictor of womanhood worries (p < 0.05), and a marginal predictor of T4 role worries (p < 0.10).
TABLE 6.
ANCOVA: Group and Change in Self-Efficacy
| T1 |
Change in |
|||||||
|---|---|---|---|---|---|---|---|---|
| Group |
Self-Efficacy |
Group (T1 to T3) |
Self-Efficacy |
|||||
| Outcome Variables | β | SE | β | SE | β | SE | β | SE |
| Time 3 Depression | −1.07 | 3.06 | −0.21 | 0.28 | 1.08 | 2.85 | −0.76 | 0.33* |
| Time 4 Depression | −0.41 | 2.71 | −0.58 | 0.24* | 2.95 | 2.88 | −0.10 | 0.44 |
| Time 3 Anxiety | −2.14 | 3.70 | −0.15 | 0.36 | 0.92 | 3.09 | −1.48 | 0.36*** |
| Time 4 Anxiety | −3.89 | 3.25 | −1.31 | 0.30*** | 0.57 | 4.15 | −0.34 | 0.61 |
| Time 3 Health worries | −0.61 | 2.36 | −0.56 | 0.21* | 0.92 | 2.52 | −0.03 | 0.29 |
| Time 4 Health worries | −3.40 | 2.69 | −0.30 | 0.24 | −2.75 | 2.68 | −0.25 | 0.40 |
| Time 3 Womanhood worries | −0.14 | 1.23 | −0.23 | 0.11* | 0.67 | 1.28 | −0.11 | 0.15 |
| Time 4 Womanhood worries | 0.68 | 1.49 | −0.37 | 0.13** | −0.35 | 1.42 | 0.54 | 0.22* |
| Time 3 Role worries | −0.18 | 0.99 | −0.37 | 0.09*** | 0.59 | 1.17 | 0.15 | 0.14 |
| Time 4 Role worries | −2.13 | 1.36 | −0.34 | 0.11** | −1.57 | 1.47 | 0.39 | 0.22 ~ |
| Time 3 Death Worries | −0.31 | 0.38 | −0.10 | 0.03** | −0.00 | 0.41 | −0.00 | 0.05 |
| Time 4 Death Worries | −0.41 | 0.35 | −0.07 | 0.03* | 0.21 | 0.37 | −0.04 | 0.06 |
| Time 3 Parenting Worries | ||||||||
| Time 4 Parenting worries | 0.59 | 0.52 | −0.00 | 0.04 | 0.72 | 0.52 | 1.03 | 0.31 |
All analyses controlled for T1 values of the dependent variable.
p ≤ .10,
p ≤ .05,
p ≤ .001,
p ≤ .0001.
DISCUSSION
Despite small sample sizes and resulting lower power, our results show that the activation intervention significantly increased breast cancer self-efficacy by T4. The increase was gradual, that is, change appeared at T3 though not significantly, but by T4 the change was significant. We also found that after adjusting for intervention group membership, change in self-efficacy was related to improved depression, very strongly related to improved anxiety at T3 (p = .0002), to increased womanhood worries, and marginally to decreased role worries. T1 self-efficacy was also a strong predictor of other outcomes after adjusting for group. It appears that the main consequence of our intervention was to improve breast cancer self-efficacy. Our results also show that self-efficacy was very strongly related to many other outcomes including anxiety, thus this pilot study shows that this intervention may have potential but requires further examination through a fully powered study.
Our intervention targeted survivors’ reluctance to talk to their oncologists about the full range of their concerns. We found that survivors developed a wide range of questions about symptoms, treatment, and recurrence. More important, we found that all but one survivor’s questions included affective content dealing with positive and negative emotions, in particular anxiety. The 21 questions about symptoms and long-term side effects communicated the worry that survivors felt. Our goal was to encourage survivors to state plainly their worry and distress to their oncologists rather than using clues and hints, which previous research has shown cancer survivors use in their communication. Given that 95% took the question sheet with them into the visit and 80% rated the sheet as helpful, it is likely that greater discussion of these topics took place during the visit. The current study measured communication indirectly by assessing the questions survivors planned to discuss with their oncologists. Future research should examine the communication during the visit to determine if increased conversation about these topics mediates the increase in self-efficacy that we found.
The intervention was an inexpensive one-time telephone call from a breast cancer oncology nurse specialist. The calls were short. The survivors also suggested that the intervention might have been more helpful if received closer to their diagnosis and treatment. We think this intervention could be easily adapted to a Web interface that survivors could access prior to a visit, which may result in improved survivor outcomes because survivors may be more organized for their sessions. As Internet access becomes universally available, this type of intervention could be very cost-effective especially if a follow-up study replicated these results and could show either that length of visits stayed the same or reduced.
Survivors in the current study developed their own questions to ask their oncologists. Some of the questions such as those about exercises to improve cognitive functioning after chemotherapy may be beyond the expertise of most oncologists. Recent guidelines for surveillance of breast cancer survivors do not mention recommendations for cognitive problems (Khatcheressian et al., 2006), and we could find no reports of treatments to improve cognitive functioning following chemotherapy. Future coaching interventions may want to focus on what questions are most appropriate for a routine follow-up visit with an oncologist and direct survivors to other resources for concerns that may require other specialists. However, some oncologists may prefer to be the referring person rather than have such a referral take place outside of the oncology consultation.
Our data suggest that the prompt sheet and coaching intervention may have resulted in reduced anxiety, an effect that was mediated through increases in self-efficacy. Anxiety is a source of psychological distress for breast cancer survivors (Bottomley, 1998; Drageset & Lindstrom, 2005). Anxiety and depression remain an ongoing problem for many breast cancer survivors (Payne, Hoffman, Theodoulou, Dosik, & Massie, 1999). Concerns about womanhood, sexuality, and body image are ongoing concerns for survivors especially younger survivors (Fobair et al., 2006). That such a simple intervention may reduce anxiety in breast cancer survivors is encouraging given the sizeable effect size between changes in self-efficacy and reductions in anxiety.
CONCLUSIONS, LIMITATIONS, AND FUTURE DIRECTIONS
Our results found that a short telephone-based intervention may improve breast cancer survivors’ perceived ability to manage the consequences of their disease and its treatment, that is, breast cancer self-efficacy. Although the current study had several limitations—small sample size of survivors and oncologists, select group of highly educated women, and no direct observation of changes in communication—we think our results suggest further study of interventions designed to increase survivors participation in follow-up oncology visits are warranted. Future studies should seek a larger and more representative sample and include direction observation of communication. Some questions survivors wanted to discuss with their oncologists may have been beyond the scope of then oncologists’ expertise; thus, studies of activation interventions may need to provide educational or referral resources to participating oncologists so that they are prepared to respond to these questions.
Acknowledgments
This researched supported by grants from the Oncological Sciences Center and the Regenstrief Center for Healthcare Engineering of Purdue University to Dr. Shields and by American Cancer Society RSGPB-04-089-01PBP to Dr. Champion.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
Cleveland G. Shields, Child Development and Family Studies, Purdue University, West Lafayette, IN, USA.
Kim Wagler Ziner, Indiana University School of Nursing, Indianapolis, IN, USA.
Sara A. Bourff, Indiana University School of Nursing, Indianapolis, IN, USA.
Katherine Schilling, School of Library and Information Sciences, Indiana University, Indianapolis, IN, USA.
Qianqian Zhao, Division of Biostatistics, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
Patrick Monahan, Division of Biostatistics, Department of Medicine, Indiana University School of Medicine, Indianapolis, IN, USA.
George Sledge, Department of Medicine, Division of Hematology/Oncology, Indiana University School of Medicine, Indianapolis, IN, USA.
Victoria Champion, Indiana University School of Nursing, Indianapolis, IN, USA.
REFERENCES
- Arora NK. Interacting with cancer patients: The significance of physicians communication behavior. Social Science & Medicine. 2003;57:791–806. doi: 10.1016/s0277-9536(02)00449-5. [DOI] [PubMed] [Google Scholar]
- Avis NE, Crawford S, Manuel J. Psychosocial problems among younger women with breast cancer. Psycho-Oncology. 2004;13:295–308. doi: 10.1002/pon.744. [DOI] [PubMed] [Google Scholar]
- Bandura A. Self-efficacy: The exercise of control. New York, NY: W. H. Freeman; 1997. [Google Scholar]
- Beckham JC, Burker EJ, Lytle BL, Feldman ME, Costakis MJ. Self-efficacy and adjustment in cancer patients: A preliminary report. Behavioral Medicine (Washington, DC) 1997;23:138–142. doi: 10.1080/08964289709596370. [DOI] [PubMed] [Google Scholar]
- Bloom JR, Stewart SL, Chang S, Banks PJ. Then and now: Quality of life of young breast cancer survivors. Psycho-Oncology. 2004;13:147–160. doi: 10.1002/pon.794. [DOI] [PubMed] [Google Scholar]
- Bottomley A. Anxiety and the adult cancer patient. European Journal of Cancer Care. 1998;7:217–224. doi: 10.1046/j.1365-2354.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: a randomised trial. British Journal of Cancer. 2001;85:1273–1279. doi: 10.1054/bjoc.2001.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler L, Degner L, Baile W, Landry M. Developing communication competency in the context of cancer: A critical interpretive analysis of provider training programs. Psycho-Oncology. 2005;14:861–872. doi: 10.1002/pon.948. [DOI] [PubMed] [Google Scholar]
- Butow PN, Brown RF, Cogar S, Tattersall MH, Dunn SM. Oncologists’ reactions to cancer patients’ verbal cues. Psycho-Oncology. 2002;11:47–58. doi: 10.1002/pon.556. [DOI] [PubMed] [Google Scholar]
- Butow PN, Dunn SM, Tattersall MHN. Communication with cancer patients: Does it matter? Journal of Palliative Care. 1995;11:34–38. [PubMed] [Google Scholar]
- Butow PN, Dunn SM, Tattersall MHN, Jones QJ. Patient participation in the cancer consultation: Evaluation of a question prompt sheet. Annals of Oncology. 1994;5:199–204. doi: 10.1093/oxfordjournals.annonc.a058793. [DOI] [PubMed] [Google Scholar]
- Collie K, Wong P, Tilston J, Butler LD, Turner-Cobb J, Kreshka MA, et al. Self-efficacy, coping, and difficulties interacting with health care professionals among women living with breast cancer in rural communities. Psycho-Oncology. 2005;14:901–912. doi: 10.1002/pon.944. [DOI] [PubMed] [Google Scholar]
- Drageset S, Lindstrom TC. Coping with a possible breast cancer diagnosis: Demographic factors and social support. Journal of Advanced Nursing. 2005;51:217–226. doi: 10.1111/j.1365-2648.2005.03495.x. [DOI] [PubMed] [Google Scholar]
- Epstein RM, Street RL. Patient-centered communication in cancer care: promoting healing and reducing suffering (NIH Publ. No. 07-6225 ed.) Bethesda, MD: National Cancer Institute; 2007. [Google Scholar]
- Ferrell BR, Dow KH, Leigh S, Ly J, Gulasekaram P. Quality of life in long-term cancer survivors. Oncology Nursing Forum. 1995;22:915–922. [PubMed] [Google Scholar]
- Fleissig A, Fallowfield LJ, Langridge CI, Johnson L, Newcombe RG, Dixon JM, et al. Post-operative arm morbidity and quality of life. Results of the ALMANAC randomised trial comparing sentinel node biopsy with standard axillary treatment in the management of patients with early breast cancer. Breast Cancer Research and Treatment. 2006;95:279–293. doi: 10.1007/s10549-005-9025-7. [DOI] [PubMed] [Google Scholar]
- Fobair P, Stewart SL, Chang S, D’Onofrio C, Banks PJ, Bloom JR. Body image and sexual problems in young women with breast cancer. Psycho-Oncology. 2006;15:579–594. doi: 10.1002/pon.991. [DOI] [PubMed] [Google Scholar]
- Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? Journal of Clinical Oncology. 1999;17:371–379. doi: 10.1200/JCO.1999.17.1.371. [DOI] [PubMed] [Google Scholar]
- Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: Understanding women’s health-related quality of life and sexual functioning. Journal of Clinical Oncology. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- Glimelius B, Birgegard G, Hoffman K, Kvale G, Sjoden PO. Information to and communication with cancer patients: improvements and psychosocial correlates in a comprehensive care program for patients and their relatives. Patient Education & Counseling. 1995;25:171–182. doi: 10.1016/0738-3991(94)00655-6. [DOI] [PubMed] [Google Scholar]
- Han WT, Collie K, Koopman C, Azarow J, Classen C, Morrow GR, et al. Breast cancer and problems with medical interactions: Relationships with traumatic stress, emotional self-efficacy, and social support. Psycho-Oncology. 2005;14:318–330. doi: 10.1002/pon.852. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Khatcheressian JL, Wolff AC, Smith TJ, Grunfeld E, Muss HB, Vogel VG, et al. American Society of Clinical Oncology 2006 update of the breast cancer follow-up and management guidelines in the adjuvant setting. Journal of Clinical Oncology. 2006;24:5091–5097. doi: 10.1200/JCO.2006.08.8575. [DOI] [PubMed] [Google Scholar]
- Kirmayer LJ. Improvisation and authority in illness meaning. Culture, Medicine & Psychiatry. 1994;18:183–214. doi: 10.1007/BF01379449. [DOI] [PubMed] [Google Scholar]
- Kornblith AB, Powell M, Regan MM, Bennett S, Krasner C, Moy B, et al. Long-term psychosocial adjustment of older vs younger survivors of breast and endometrial cancer. Psycho-Oncology. 2007;16:895–903. doi: 10.1002/pon.1146. [DOI] [PubMed] [Google Scholar]
- Lev EL, Paul D, Owen SV. Age, self-efficacy, and change in patients’ adjustment to cancer. Cancer. 1999;7:170–176. doi: 10.1046/j.1523-5394.1999.74004.x. [DOI] [PubMed] [Google Scholar]
- Levinson W, Gorawara-Bhat R, Lamb J. A study of patient clues and physician responses in primary care and surgical settings. Journal of the American Medical Association. 2000;284:1021–1027. doi: 10.1001/jama.284.8.1021. [DOI] [PubMed] [Google Scholar]
- Merluzzi TV, Nairn RC, Hegde K, Martinez Sanchez MA, Dunn L. Self-efficacy for coping with cancer: revision of the Cancer Behavior Inventory (version 2.0) Psycho-Oncology. 2001;10:206–217. doi: 10.1002/pon.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Schulz KF, Altman DG CONSORT Group (Consolidated Standards of Reporting Trials) The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomized trials. Annals of Internal Medicine. 2001;134:657–662. doi: 10.7326/0003-4819-134-8-200104170-00011. [DOI] [PubMed] [Google Scholar]
- Payne DK, Hoffman RG, Theodoulou M, Dosik M, Massie MJ. Screening for anxiety and depression in women with breast cancer psychiatry and medical oncology gear up for managed care. Psychosomatics. 1999;40:64–69. doi: 10.1016/s0033-3182(99)71273-9. [DOI] [PubMed] [Google Scholar]
- Pennebaker JW, Booth RJ, Francis ME. Linguistic inquiry and word count: LIWC 2007. Austin, TX: LIWC; 2007. Available at www.liwc.net. [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- Razavi D, Merckaert I, Marchal S, Libert Y, Conradt S, Boniver J, et al. How to optimize physicians’ communication skills in cancer care: Results of a randomized study assessing the usefulness of posttraining consolidation workshops. Journal of Clinical Oncology. 2003;21:3141–3149. doi: 10.1200/JCO.2003.08.031. [DOI] [PubMed] [Google Scholar]
- Spielberger C, Gorsuch RLR, Vagg P, Jackobs G. State-Trait Anxiety Inventory, Form Y. Redwood City, CA: MindGarden; 1983. [Google Scholar]
- Takayama T, Yamazaki Y, Katsumata N. Relationship between out-patients’ perceptions of physicians’ communication styles and patients’ anxiety levels in a Japanese oncology setting. Social Science & Medicine. 2001;53:1335–1350. doi: 10.1016/s0277-9536(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Vickberg SM. The Concerns About Recurrence Scale (CARS): A systematic measure of women’s fears about the possibility of breast cancer recurrence. Annals of Behavioral Medicine. 2003;25:16–24. doi: 10.1207/S15324796ABM2501_03. [DOI] [PubMed] [Google Scholar]
- Vries Hd, Mesters I, Van de Steeg H, Honing C. The general public’s information needs and perceptions regarding hereditary cancer: An application of the integrated change model. Patient Education and Counseling. 2005;56:154–165. doi: 10.1016/j.pec.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Ziner KW, Champion VL, Sledge GW, Bell C. Predicting fear of breast cancer recurrence. doi: 10.1188/12.ONF.287-295. (under review) [DOI] [PMC free article] [PubMed] [Google Scholar]