Abstract
Purpose
We sought to determine the effect of stress-induced senescence on the permeability to albumin of aging endothelial progenitor cells.
Methods
Human umbilical cord blood derived endothelial cells (hCB-ECs) and human aortic endothelial cells (HAECs) were treated with 200 μM H2O2 and permeability to FITC-bovine serum albumin was measured. Some samples were subsequently treated with 100μM 8-pCPT-2'-O-Me-cAMP, a cAMP analog that activates the Epac1-Rap1 pathway. Cell proliferation was measured with the EdU assay. Phase contrast, and immunofluorescence images were taken to observe morphological changes in cells after exposure to H2O2.
Results
hCB-ECs exposed to H2O2 exhibited a significant increase in permeability, but their response differed from the HAECs. Low passage hCB-ECs had a permeability increase of about 82% (p<0.01) compared to aged cells which had a permeability increase of about 37% (p<0.05). This increase in permeability was reduced by treating the cells with 100 μM 8-pCPT-2'-O-Me-cAMP. The younger cells exhibited a significant decrease in proliferation after being subjected to various concentrations of H2O2 whereas the aged cells exhibited a more gradual decrease in the percent of cells in S-phase. These changes also correlated with changes in cell morphology and junction staining. When placed back in the original media, the morphology and permeability of the hCB-ECs returned to the control condition, while the HAECs did not.
Conclusions
The permeability of low and high passage hCB-ECs and HAECs initially increases in response to oxidative stress. hCB-ECs, but not HAECs, were able to recover from the stress 24 hours later. Early passage hCB-ECs were more susceptible to exogenous H2O2 than late passage hCB-ECs. The increase in permeability of hCB-ECs to H2O2 also correlated with decreased cell proliferation and changes in cell junctions.
Keywords: oxidative stress, hydrogen peroxide, endothelial progenitor cell, cell aging
Introduction
The endothelium plays a key role in the regulation and transport of fluid and solutes[1]. An increase in the permeability of the endothelial layer to proteins influences the development and progression of atherosclerosis[1, 2]. Movement across the endothelium can occur through several transport pathways including junctions between endothelial cells (ECs), vesicular transport, and transendothelial channels[3]. There are two primary mechanisms for generating paracellular vascular leaks which would allow macromolecules to travel between ECs, and ultimately cause an inflammation response leading to the development of the atherosclerotic plaque.. The first mechanism is the disruption of tight junctions or adherins junctions. The second mechanism is cytoskeletal contraction, which leads to widened intercellular spaces[4]. It is likely that these mechanisms are not independent and that EC contraction may lead to breaks in EC junctions.
Regions at which atherosclerosis develops are areas of high endothelial cell turnover and high oxidative stress[5]. High cell turnover can lead to replicative senescence, which involves shortening of the telomeres[5]. Eventually these cells stop dividing after reaching a finite number of population doublings. Alternatively, cells experience stress-induced premature senescence, which can be the result of oxidative stress or chromosomal disruption. This type of senescence can be modeled by treatment with hydrogen peroxide (H2O2) [6]. Acute H2O2 treatments of 150μM for 2 to 72 hours have previously been used for human diploid fibroblasts [6]. Additionally, subconfluent human umbilical vein ECs treated with 100μM H2O2 have been shown to survive a 10 day treatment and become senescent [7]. Previous studies have shown that oxidative stress can lead to stress-induced senescence in endothelial progenitor cells and that the senescence can be reversed with an antioxidant scavenger[8–10]. Exogenous H2O2 can be used in vitro to increase permeability in endothelial cells and simulate the leukocyte activation present in regions of disease. Superoxide dismutase conjugated with anti-platelet endothelial cell adhesion molecule has been shown to alleviate the increase in permeability associated with stress-induced senescence[11].
Late-outgrowth endothelial progenitor cells (EPCs) express many of the molecular markers found on large vessel endothelium[12–14]. They have great potential in cardiovascular tissue engineering, making the study of their functional response to replicative and stress-induced senescence important[14–16]. While the origin of these cells is a matter of some dispute [17], ECs that possess the high proliferative potential of late-outgrowth EPCs can be isolated from arterial endothelium[18].
We recently showed that endothelial cells derived from human umbilical cord blood (hCB-ECs) exhibited reduced permeability relative to aortic endothelial cells[19]. As the hCB-ECs underwent additional population doublings, their permeability increased. The age of the cell was asociated with decreased telomerase expression[19]. This increase in permeability correlated with a decrease in tyrosine phosphorylation of occludin, redistribution of tight junction proteins, and an increase in cellular senescence. Treatment of late-passage hCB-ECs with Resveratrol, 8-pCPT-2'-O-Me-cAMP, and Rolipram all decreased the permeability suggesting that the change was mediated through inhibition of phosphodiesterase 4 and activation of the Epac1-Rap1 pathway[19]. There are several advantages to using hCB-ECs as a model for cell aging: 1) they are able to undergo a significantly larger number of cell divisions compared to aortic endothelial cells and 2) the permeability is much lower than the value for aortic endothelial cells and co undergo a wider change in value after treatment with an agonist.
In this study, we examined the effects of both oxidative stress and aging on the permeability of hCB-ECs to albumin. Cell morphology and proliferation were also assessed to determine mechanisms that influence the changes in permeability,
Materials and Methods
Cell Culture
Human cord blood derived endothelial cells (hCB-ECs) were isolated as previously described[20]. Umbilical cord blood was obtained from the Carolina Cord Blood Bank. Prior to receipt, all patient identifiers were removed. The Duke University Institutional Review Board approved the protocol for collection and use of human blood employed in this study.
After collection, blood was diluted 1:1 with Hanks Balanced Salt Solution (HBSS, Invitrogen), placed onto Histopaque 1077 (Sigma), and centrifuged at 740×g for 30 minutes. Buffy coat mononuclear cells were collected and washed three times with “complete EC growth medium,” comprising 8% (vol/vol) fetal bovine serum (FBS) added to Endothelial Basal Media-2 (Cambrex) supplemented with Endothelial Growth Media-2 SingleQuots (containing 2% FBS plus growth factors, Cambrex), and 1% antibiotic/antimycotic solution (Invitrogen). Mononuclear cells were plated on plastic 6 well plates coated with collagen I (rat tail, BD Biosciences) in complete EC growth medium. Medium was exchanged every 24 hours for the first week in culture, to remove non-adherent cells. Colonies of EPC-derived ECs appeared 7–10 days after the initial isolation. The EC colonies were passaged onto collagen I-coated plates and allowed to grow.
Human aortic endothelial cells (HAECs) were obtained from Cambrex/Lonza. HAECs were obtained at passage 3 and, as noted by the supplier, had undergone 17 total population doublings at the time of purchase. HAECs were also cultivated in complete EC growth medium.
The hCB-ECs and HAECs were grown separately in T75 flasks using EBM2 growth media supplemented with penicillin/streptomycin, EGM2 Singlequots Kit, and 10% Fetal Bovine Serum. Media was changed every other day until the time of experiment. Cells were subsequently passaged 1:10 into new T75 flasks upon reaching confluence. Cells at passage 1 are those that were passaged after the isolated hCB-ECs reached confluence. Cells were then subsequently split 1:10. The number of population doublings that occurred prior to each passage was adjusted based upon a 75% attachment rate and calculated according to the formula ln(10)/ln(2)*(4/3) = 4.43 as previously described[21].
Permeability Experiments
Transwell plates (Corning) containing polyester membranes with 0.4μm diameter pores were used for all permeability experiments. The hCB-ECs or HAECs were seeded in the luminal (top) chamber at a density of 105 cells/well in 10% complete FBS media. The abluminal (bottom) chamber also contained 10% complete FBS media. Cells were confluent 1 to 2 days post-plating. The permeability to FITC-bovine serum albumin (BSA) was measured 3 days post-plating. Cells were incubated with serum-free media for 1 hour before the start of the experiment. Treated cells were incubated with 200μM H2O2 1 hour before the start of the experiment. At the start of the experiment, 1mg/mL unlabeled BSA (Sigma) was added to the control abluminal chambers and 1mg/mL unlabeled BSA in 200μM H2O2 was added to the treated abluminal chambers. Similarly, 1mg/mL of FITC-BSA (Sigma) was added to the control luminal chambers and 1mg/mL of FITC-BSA in 200μM H2O2 was added to the treated luminal chambers. Volumes added to the luminal and abluminal chambers ensured no differences in hydrostatic or osmotic pressure. 10μL samples were taken from the abluminal chambers every 20 minutes for 2 hours and diluted in 40μL serum free media in a 96-well plate. The change in hydrostatic pressure due to a decrease in the volume of the abluminal chamber (1.5 Pa) was insufficient to drive a significant fluid flow. Since the albumin concentration did not change with sampling, there was no osmotic pressure difference produced during the experiment. Albumin content of the sample was determined by a fluorimeter using a calibration curve. Permeability coefficients were calculated using the following equation (1) [22].
| (1) |
In the equation above, V1 and V2 are the luminal and abluminal chamber volumes, respectively. C0 and C1 are the concentrations of FITC-BSA in the luminal chamber initially and at time t, respectively. P is the diffusive permeability and Am is the area of the membrane. The slope of the trendline of a plot of the left hand side of equation (1) vs. time was used to calculate the permeability coefficient, which was the total permeability of the cells and the membrane. In order to calculate the permeability of the cells alone, the permeability of the cells and the membrane were presumed to be resistances in series using the equation below.
| (2) |
β-galactosidase Assay
hCB-ECs were seeded in a 96-well plate at a density of 5×103 cells/well. Staining was performed 1 day post-plating. hCB-ECs were incubated with 200μMof H2O2 for 1 hour before fixation with 100% methanol. The senescence β-galactosidase Staining Kit (Cell Signaling Technology) was used to label senescent cells. hCB-ECs in each well were stained with 100μL of a staining solution mixture (pH 6.0) and 10 μL of 2mM Fluorescein Di-β-D-Galactopyranoside (FDG, Invitrogen). The plates were placed in a dry incubator for 24 hours at 37°C. FDG is not fluorescent. However, in the presence of β-galactosidase, fluorescein is cleaved from FDG and the fluorescein fluorescence is proportional to the amount of β-galactosidase present. Solution samples were taken from each well and placed into another 96-well plate for fluorescence detection. A fluorimeter was used to detect fluorescence and fluorescein content was calculated using a calibration curve. hCB-ECs were then washed with PBS and incubated with Hoechst 33342 (Invitrogen) for 30 minutes. A fluorimeter was used to measure Hoechst 33342 intensity. The concentration of FDG was divided by the concentration of Hoechst 33342 to normalize for effects of H2O2 on cell number. Results are reported as normalized β-galactosidase expression.
Proliferation Assays
hCB-ECs were seeded in a 24-well plate at 105 cells/well. For proliferation and live/dead assays and immunofluroescence and phase contrast microscopy, we found that there was no statistical difference in the results between assays done on the plastic wells and the Transwell filters, so plastic wells were used for all experiments. The advantage of plastic wells is that they are inexpensive and easier to image because Transwell filters need to be cut out and mounted onto slides. Assays were performed 48 hours post-plating to ensure cells were confluent. Cells were treated with varying concentrations of H2O2 in 10% complete media for 3 hours immediately before fixing cells. The Click-iT EdU Alexa Fluor 488 Imaging Kit was used to measure the percentage of cells in the S-phase of mitosis. The assay was performed as described in the manufacturer's protocol. The EdU label was incubated for 4 hours. Wells were also incubated with the Hoeschst 33342 stain for 30 minutes to stain all nuclei. A Nikon Eclipse Inverted Microscope was used to image the stained wells. FITC wavelength was used to image all nuclei in cells in the S-phase of mitosis, and DAPI wavelength was used to image all nuclei. The number of EdU labeled cells (FITC) divided by the total number of cells (DAPI) was the percentage of cells in S-phase.
Phase Contrast Morphology Assay
The hCB-ECs or HAECs were seeded in a 6-well plate to ensure a confluent monolayer 1-day post-plating. Cells were then treated with 100 μM H2O2 or 200μM H2O2 for 3 hours or 24 hours. For some hCB-ECs that were exposed to 200 μM H2O2 for 3 hours, the media was subsequently replaced with 10% complete medium to allow the hCB-ECs to recover for 24 hours from the H2O2 treatment. Untreated cells were used as controls. Samples were imaged on Nikon Eclipse Inverted Microscope. ImageJ (NIH) was used to measure cell area.
Immunofluorescence
hCB-ECs or HAECs were seeded at a density of 50,000 cells/cm2 on 6-well plates. At 2 days post plating, some cells were treated with 200 μM H2O2 for 3 hours. For some samples, the media was subsequently replaced with 10% complete medium to allow the cells to recover from H2O2 exposure for 24 hours. Untreated ECs were used as controls. At each time point, cells were with 3.7% paraformaldehyde at 37°C for 10 minutes and permeabilized with 0.5% Triton-X100 for 10 minutes. Samples were then blocked with PBS/0.02% Tween 20 (Biorad)/10%goat serum (Gibco) for 1 hour at room temperature. The samples were then incubated with an antibody to the mouse-PECAM (1:250 dilution, clone MEM05, Invitrogen) overnight at 4°C. This was followed by appropriate secondary antibody incubation with goat anti-mouse Alexa Fluor 488 (1:250 dilution, Invitrogen) for 1 hour at room temperature. All antibodies were diluted in PBS/10% goat serum. The stains were visualized with a Zeiss 510 Upright Confocal Microscope.
Live/Dead Assay
hCB-ECs or HAECs were seeded at a density of 50,000 cells/cm2 on 6-well plates. Some samples were treated with 200μM H2O2 for 3 hours. Medium in some samples was subsequently replaced with 10% complete medium to allow for a 24-hour recovery period. At the end of each time point, the live dead/assay was performed immediately. A live/dead assay kit (Molecular Probes) was used. Live cells were labeled with fluorescent Calcein and dead cells were labeled with fluorescent Ethidium homodimer. The number of dead cells was calculated as a percentage of the total number of cells using ImageJ (NIH).
Statistical Analysis
Values of n represent the number of experiments that were performed. The experiments represent samples from 2 isolations of hCB-ECs and 1 lot of HAECs. We have previously shown that the effect of the donor upon permeability is not statistically significant[19]. For Fig. 1, a one-way ANOVA was used to determine the effect of cell age on FDG expression. For Fig. 2, a repeated measure ANOVA was performed to compare different data sets. For Fig. 4e, an ANOVA on treatment time was performed to determine the effect of treatment or recovery on cell area. For Fig. 5, a two-factor ANOVA for cell age and treatment was performed to determine the effect of age and treatment on permeability. For Fig. 6, an ANOVA was performed to compare different data sets. For Fig. 7, linear regression was performed to determine goodness of fit. An ANCOVA was then performed to assess differences between the two curves. For Fig. 8, a two-factor ANOVA for cell age and treatment was performed to determine the effect of age and treatment on permeability. Post-hoc Tukey tests were performed when appropriate for additional comparisons. All data presented represent mean ± SE. A p<0.05 was interpreted to denote statistical significance.
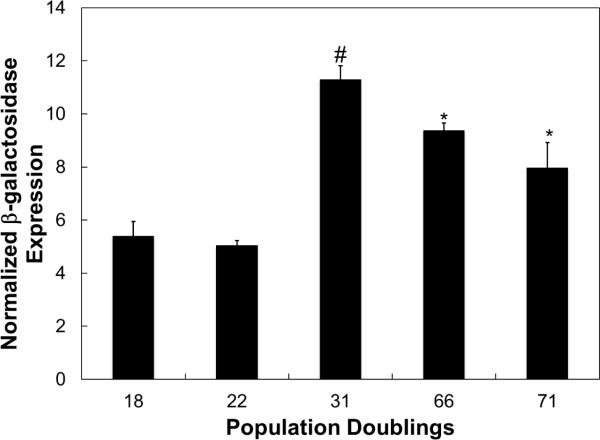
Fig. 1.
β-galactosidase expression in aging hCB-ECs as measured by the fluorescence of the substrate, FDG. FDG intensity was normalized to the DAPI fluorescence per well. β-galactosidase levels increased with increased population doublings. For each experiment, 3 replicates were performed (n=3) *:p<0.05 compared to 18 or 22 population doublings; #:p<0.01 compared to 18 or 22 population doublings.
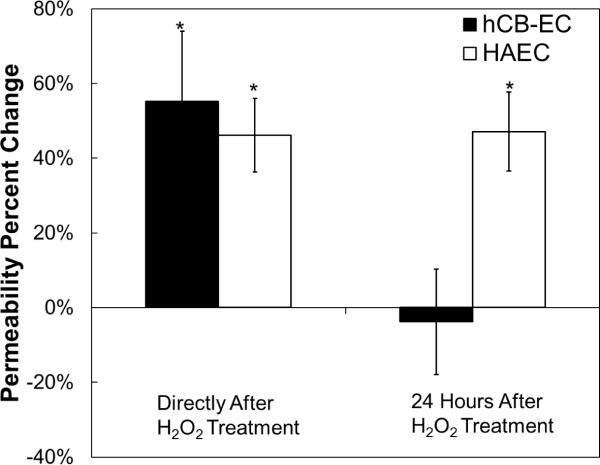
Fig. 2.
Short and long-term effects of a single H2O2 treatment on endothelial permeability to albumin. hCB-ECs and HAECs that underwent fewer than 31 population doublings were incubated with 200 μM H2O2 for 3 hours and albumin permeability was measured over a two hour period either immediately after or 24 hours after H2O2 treatment. Immediately after treatment hCB-EC and HAEC permeability to albumin was elevated. 24 hours after treatment, the HAECs still had an elevated permeability and the hCB-EC permeability was similar to that of the untreated control. For each experiment, 8 replicates were performed (n=3–5) *:p<0.05 compared to untreated control.
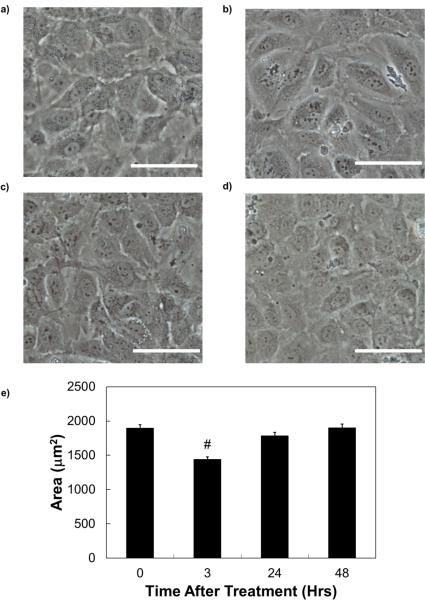
Fig. 4.
Phase contrast images of hCB-ECs a) before H2O2 treatment; b) at the end of the 3 hour incubation with H2O2; c) 24 hours after the onset of the incubation with H2O2; d) 48 hours after the onset of the incubation with H2O2; and e) EC area at these time points. There was a change in cell morphology and area after exposure for 3 hours, but cells returned to the morphology of the control condition after 24 and 48 hours. Scale bar = 40μm.
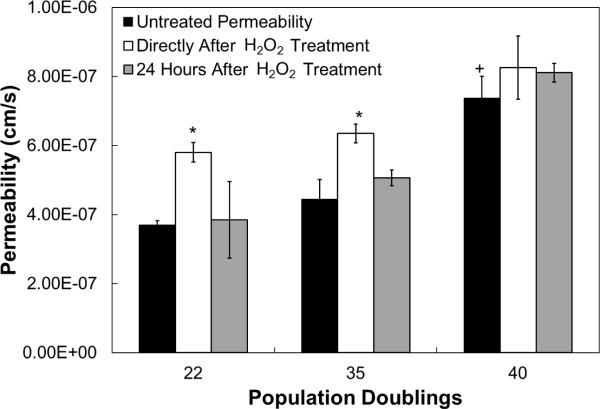
Fig. 5.
Effect of H2O2 treatment and recovery on aging hCB-ECs. hCB-EC permeability increases with increasing population doublings and after H2O2 treatment. The low passage hCB-ECs recover from the stress 24 hours after treatment, while the higher passage hCB-ECs do not. For each experiment, 8 replicates were performed (n=3). *:p<0.05 compared to untreated control of same population doubling; +:p<0.05 compared to 22 population doubling untreated control.
Fig. 6.
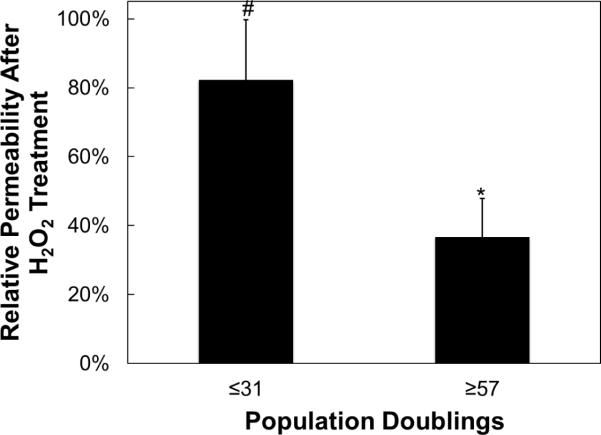
Permeability percent increase due to H2O2 treatment in early passage vs. late passage hCB-ECs. hCB-ECs that underwent 31 or fewer population doublings exhibited an 80% increase in permeability after treatment with H2O2. The hCB-ECs that underwent at least 57 population doublings only had a 37% increase in permeability after treatment with H2O2. The younger hCB-ECs were more affected by the oxidative stress than the aged hCB-ECs. For each experiment, 8 replicates were performed from 3 different donors; *:p<0.05 compared to untreated control; #p<0.01 compared to untreated control.
Fig. 7.
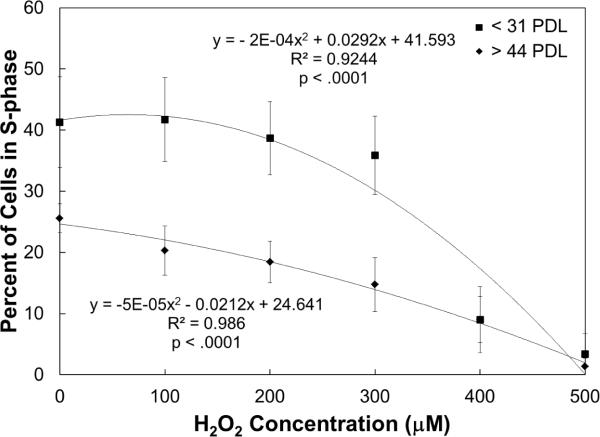
Effect of H2O2 concentration on percent of cells in S-phase. Increasing concentrations of H2O2 decreases the percent of cells in S-phase for all hCB-ECs. The slope is steeper for hCB-ECs with fewer than 31 population doublings than for hCB-ECs that underwent more than 44 population doublings. Low passage hCB-ECs have a higher percent of cells in S-phase than aged hCB-ECs. For each experiment, 2 replicates were performed (n=5)
Fig. 8.
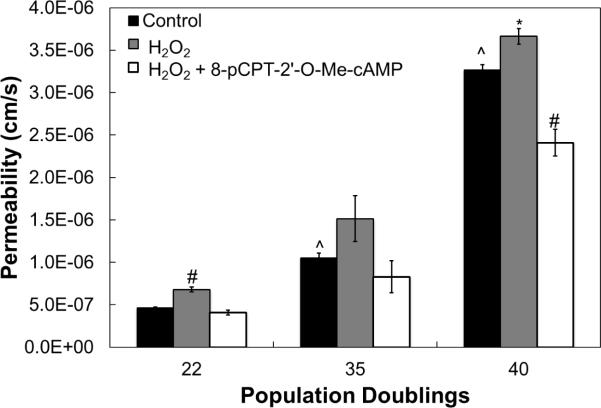
8-pCPT-2'-O-Me-cAMP alleviates H2O2-induced increase in permeability. hCB-EC permeability increases after treatment with H2O2. This increase is mitigated by subsequent treatment with 100μM 8-pCPT-2'-O-Me-cAMP. For each experiment, 8 replicates were performed (n=3) *:p<0.05 compared to untreated control of same population doubling; #:p<0.01 compared to untreated control of same population doubling; ^:p<0.01 compared to 22 population doubling untreated control.
Results
hCB-EC Characterization
We have previously characterized hCB-ECs with flow cytometry and shown that they were positive for the endothelial-specific CD31 and CD34, and negative for CD14, CD45 and CD115 found on monocytes or hematopoietic cells[19]. We have previously characterized hCB-ECs and found that they expressed von Willebrand factor, CD31 and VE-cadherin[14]. Following exposure to 15 dyne/cm2 for 24 or 48 hours, hCB-ECs aligned with the direction of flow,[14, 23] increased nitric oxide production, and increased mRNA for endothelial cell specific genes sensitive to flow, Kruppel-like factor 2, nitric oxide synthase III, cyclooxygenase 2, and thrombomodulin[14]. The level and organization of actin filaments are similar in hCB-ECs and HAECs as are the associated values of cell stiffness[23].
Senescent Cells Increase with Increasing Population Doublings
To determine whether aging hCB-ECs are more senescent, β-galactosidase expression was measured. β-galactosidase expression increases as the number of senescent cells in a population increases[24]. The concentration of fluorescein reaction product was divided by the concentration of Hoechst 33342 to normalize for effects of H2O2 on cell number. Results are reported as normalized β-galactosidase expression. We found that β-galactosidase expression was greater in hCB-ECs that had undergone more population doublings (p<0.05) (Fig. 1). However, when cells were exposed to 200μM H2O2 for 1 hour, there was no significant change in β-galactosidase expression in hCB-ECs.
Early Passage hCB-ECs and HAECs Respond Differently to H2O2 Treatment
Permeability of hCB-ECs and HAECs that underwent fewer than 31 population doublings was measured over a three-hour period either immediately following a one hour incubation with 200μM H2O2 or 24 hours after treatment with H2O2. Directly after treatment, the permeability of the hCB-ECs to albumin increased by 55%±19% (p<0.05) (Fig. 2). Similarly, the HAEC permeability to albumin increased by 46%±10% (p<0.05) (Fig. 1). When assayed 24 hours after the H2O2 treatment ended, the permeability of the hCB-ECs to albumin was unchanged compared to the untreated control. However, the permeability of the HAECs 24 hours after treatment remained elevated (Fig. 2) relative to the untreated control (p<0.05) and similar to the value obtained three hours after treatment with H2O2. These results show that hCB-ECs are able to recover rapidly from H2O2 exposure.
H2O2 Treatment Alters Cell Contractility
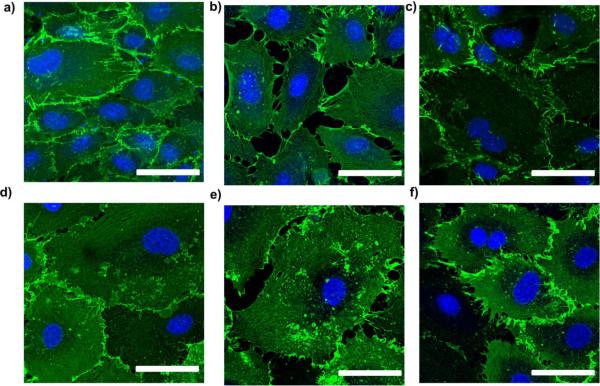
PECAM immunofluorescence was used to assess the integrity of hCB-EC and HAEC cell-cell junctions. For this experiment, ECs that underwent fewer than 31 population doublings were used. For both EC types, the PECAM staining is continuous in the untreated control (Figures 3a and d). After treatment with H2O2, ECs were contracted which allowed gaps to form between cells (Figures 3b and e). This result correlates with the observed increase in permeability for hCB-ECs and HAECs after H2O2 treatment. Following the 24-hour recovery, the hCB-EC junctions appeared continuous and intact (Figure 3c). These results correlate with the ability of the low passage hCB-ECs to recover after treatment with H2O2. However, gaps between cell borders was present in HAECs (Figure 3f) indicating that the HAECs had not fully recovered from the oxidative stress.
Fig. 3.
PECAM immunofluorescence of hCB-ECs (a,b,c) and HAECs (d,e,f) that underwent fewer than 31 population doublings before H2O2 treatment (a, d); at the end of the 3 hour incubation with H2O2 (b, e); 24 hours after the onset of the incubation with H2O2 (c, f). Cells exposed to 200μM H2O2 for 3 hours and then placed back into regular 10% complete media. Scale bar = 50μm.
H2O2 Exposure Alters hCB-EC Morphology
hCB-ECs that underwent 22 population doublings were imaged by phase contrast at various time points after exposure to 200μM H2O2. After 3 hours of exposure to H2O2, there is a change in cell morphology (Fig. 4b) compared to the control condition (Fig. 4a). The H2O2 solution was replaced with media and the cells were imaged at 24 and 48 hours post-plating (Figs. 4c and 4d). At these later times, the cell morphology resembles that of the control condition indicating that the effect of H2O2 may be transient. We analyzed the cell areas using ImageJ. After 3 hours of H2O2 exposure, the cell area decreases. After 24 or 48 hours of recovery, the cell area is close to that of the untreated control (Fig. 4e). These results correlated with an increase in cell density after 3 hours of exposure (data not shown). These changes suggest that the effect of H2O2 may be transient on low passage hCB-ECs.
hCB-EC Permeability Increases with Increasing Population Doublings
Cell age was defined as the number of population doublings the cells underwent since passage 1, the point at which the cells reached confluence after isolation. As we previously observed [19], the in vitro permeability values of hCB-ECs from one isolation was low and increased with increasing cell age (Fig. 5, p<0.05). hCB-ECs at 22 population doublings had an average permeability value of 3.7 ± 0.1 × 10−7 cm/s (n=3), while hCB-ECs at 35 population doublings had an average permeability value of 4.4 ± 0.6 × 10−7 cm/s (n=3), and hCB-ECs at 40 population doublings had an average permeability value of 7.4 ± 0.6 × 10−7 cm/s (n=3, p<0.05). For hCB-ECs that underwent 22 and 35 population doubling conditions (p<0.05), the permeability increased directly after treatment with 200μM H2O2 relative to the untreated control. Subsequent removal of H2O2 allowed the permeability of the hCB-ECs at 22 and 35 population doublings to recover. At 40 population doublings, there was no statistically significant in change of permeability after H2O2 treatment or recovery(Fig. 5). These results indicate that low passage hCB-ECs are able to recover within 24 hours from the H2O2 treatment, and that the permeability of high passage hCB-ECs was not affected significantly by treatment or by recovery. Furthermore, the permeability of hCB-ECs increased with increasing cell age indicating a general trend for hCB-ECs from a single isolation for increases in permeability with increased cell senescence.
We examined whether cell death was a reason for the inability of the HAECs to recover from the H2O2 treatment. hCB-ECs and HAECs were exposed to 200μM H2O2 for 3 hours. Medium in some samples was subsequently replaced with 10% complete medium to allow for recovery from treatment. Untreated cells were used as controls. For all treatment conditions and both cell types, we found that fewer than 4% of total cells were dead. They percentage of viable cells was not statistically different for each condition and was not statistically different for cell type. Thus cell death is not responsible for the lack of recovery of HAEC permeability 24 hours after treatment.
H2O2 Increases Permeability for hCB-ECs from Several Isolations
To determine the response of aging hCB-ECs to oxidative stress among several different donor sources, hCB-ECs of various population doublings were incubated with 200μM H2O2 for 3 hours and their permeability was compared to that of the untreated controls. Given the variations in the number of cells initially isolated, it is difficult to get all of the isolations at the same population doubling in an experiment, hCB-ECs were pooled into two bins for analysis. Additionally, hCB-ECs that underwent fewer than 31 population doublings generally exhibit lower levels of FDG (Fig. 1). For hCB-ECs of all ages, the permeability increased. However, the permeability percent increase was greater for cells that underwent fewer than 31 population doublings compared with cells (p<0.01) that underwent at least 57 population doublings (p<0.05) (Fig. 6). After exposure to 200μM H2O2 low passage cells had an increase in permeability of 82% compared to untreated cells. In contrast, for aged hCB-ECs permeability values were only 37% greater than the untreated aged cells.
H2O2 Decreases the Percent of hCB-ECs in S-phase of Mitosis
Because cells must break their junctions in order to divide, we examined the effect of increasing H2O2 concentration on cell turnover. All hCB-ECs had an exponential decrease in percent of cells in S-phase with increasing H2O2 concentration. hCB-ECs that underwent fewer than 31 population doublings responded to H2O2 treatment differently than hCB-ECs that underwent at least 44 population doublings (p<0.01) (Fig. 7). hCB-ECs that underwent fewer than 31 population doublings had a higher percentage of cells in S-phase in the control condition compared to cells that have underwent greater than 44 population doublings. A significant drop in the percentage of cells in S-phase did not occur until the younger cells had been treated with 400 μM H2O2. For the aged cells, the drop in percentage of S-phase cells was more gradual. However, for cells of both ages, the percentage of cells in S-phase was equivalent when they were exposed to 400 μM H2O2. The percent of cells in S-phase was less than 5% after cells were exposed to 500μM H2O2.
Effect of H2O2 on Permeability is Decreased After Treating hCB-ECs with 100μM 8-pCPT-2'-O-Me-cAMP
The compound 8-pCPT-2'-O-Me-cAMP is a membrane permeable activator of the exchange factor activated by cAMP, Epac1[25]. Treatment of hCB-ECs of various ages with H2O2 increased the permeability (p<0.05) (Fig. 8). After subsequent treatment with 100μM 8-pCPT-2'-O-Me-cAMP, the permeability was reduced to control levels for cells that have undergone fewer than 31 population doublings (Fig. 8). For cells that underwent at least 35 population doublings, subsequent treatment with 8-pCPT-2'-O-Me-cAMP resulted in permeability values that are lower than the control (Fig. 8). These results indicate that the permeability is increased for all cells exposed to H2O2 and the increase can be moderated with the addition of 8-pCPT-2'-O-Me-cAMP
Discussion
In this study, we found that, unlike aortic endothelial cells and high passage hCB-ECs, low passage hCB-ECs can recover from transient stress induced by H2O2. Increases in hCB-EC permeability induced by exposure to H2O2 can be mitigated by treatment with 8-pCPT-2'-O-Me-cAMP which actives that Epac1-Rap1 pathway. Additionally, changes in cell morphology and cell proliferation correlate with changes in permeability after exposure to stress. In this study, we examined the diffusive permeability, whereas in vivo, albumin and low-density lipoprotein transport across arterial endothelium is likely a combination of diffusive and convective processes[1]. Thus, the measured values in these in vitro experiments may differ from the in vivo values.
The inflammatory response involves a generation of reactive oxygen species, including H2O2[26]. This generation of H2O2 leads to acute inflammation that is associated with endothelial injury[26]. For our studies, we examined the effect of 200μM H2O2 for 3 hours to mimic an acute change reactive oxygen species, after a meal or smoking a cigarette. We performed preliminary experiments to determine the effect of 100μM or 200μM H2O2 on hCB-ECs or HAECs for 24 hours. We examined the cell morphology using phase contrast microscopy and found that the both cell types exhibited significant cell contraction after the short-term exposure to H2O2. If chronic, long-term exposure to H2O2 is to be studied, a lower dose would be necessary.
hCB-ECs and HAECs both had elevated permeability levels directly after exposure to 200μM H2O2. For HAECs and high passage hCB-ECs, this increase in permeability was sustained even 24 hours after the treatment. This result agrees with a previous study which shows that the permeability of bovine pulmonary artery endothelial cells treated with 200μM H2O2 for 20 minutes did not recover 48 hours after treatment[27]. However, the hCB-ECs exhibited a reduction in the permeability after 24 hours to a value that is similar to that of the untreated control.
We examined several possible reasons for the response of aged hCB-ECs to H2O2: junction protein localization, cell morphology, and proliferation. Albumin transport is regulated by tight junction proteins[1]. Because cells must break their junctions in order to divide, an increase in the percent of cells in S-phase may correlate with an increase in permeability. hCB-ECs of all ages exhibited a high percentage of cells in the S-phase, which is higher than those found in the vessel wall. We have previously shown that hCB-Cs co-cultured with vascular smooth muscle cells will reduce the percentage of cells in the S-phase to less than 1%[28]. For future work, this coculture system could then be used to mimic the vessel wall structure and reduce hCB-EC proliferation towards in vivo values. As hCB-ECs were treated with increasing concentrations of hydrogen peroxide, their proliferation decreased. The effect of H2O2 was different for young cells and aged cells (Fig. 5). This is consistent with previous studies showing that low levels of hydrogen peroxide causes cells to enter a state of chronic growth-inhibition[29]. Increasing the concentration of H2O2 led to a more significant drop in percent of cells in S-phase for the low passage cells. In contrast, the aged cells exhibited a more gradual decrease in percent of S-phase cells. This is consistent with the larger change in permeability after exposure to H2O2 for the younger cells compared to the aged cells.
Changes in proliferation are also accompanied by changes in cell morphology[29]. In our studies, we show that all hCB-ECs are growth-arrested after treatment with hydrogen peroxide. Additionally, these changes in proliferation can be correlated with changes in cell morphology and cell area for the low passage hCB-ECs (Fig. 6). The changes in cell morphology are also consistent with cells in an oxidative stress environment, such as those in an atherosclerotic region in vivo[30, 31]. Treatment of aged hCB-ECs with hydrogen peroxide did not have a significant morphological effect on the cells. This may be because the hCB-ECs have already reached a state of replicative senescence and respond less to oxidative stress.
We found that hCB-EC permeability increases in response to H2O2 can be mitigated by treatment with 8-pCPT-2'-O-Me-cAMP. There is evidence showing that H2O2 downregulates intracellular cAMP and can lead to endothelial barrier dysfunction[32]. Filamin translocation from the cell membrane to the cytosol correlated with the decrease in cAMP, and could be prevented by activation of the cAMP-protein kinase pathway[33].
The study of hCB-ECs is important because these cells represent a promising source of endothelium for applications in regenerative medicine[14–16]. EPCs from young animals injected into older animals preferentially localize to sites of endothelial dysfunction and can reverse atherosclerosis in older animals[34]. This result suggests that the development and progression of atherosclerosis is influenced by a decline in progenitor cell adhesion, incorporation into the endothelium and function with age. Further, exogenous addition of hCB-ECs can repair damaged vein grafts[14–16], suggesting that these endothelial cells can restore function when endothelium is damaged.
The results of these studies suggest that the ability of hCB-ECs of various ages to respond to oxidative stress is important in affecting permeability. This is important as the permeability of the endothelium is higher in regions prone to the initiation of atherosclerosis. Additionally, senescent cells are known to be present in areas with atherosclerosis. ECs in these regions undergo more cycles of replication and, due to the local hemodynamics, are more sensitive to extracellular stresses. These environmental conditions can ultimately lead to senescence that increases permeability, furthering the progression of atherosclerosis. The increase in permeability that results from exposure to oxidative stress may be mitigated by activation of the Epac1-Rap1 pathway.
Acknowledgements
The work was supported by National Institute of Health grant HL 88825 (G.A.T.), and a National Science Foundation Graduate Research Fellowship (T.M.C.).
References
- 1.Tarbell JM. Shear stress and the endothelial transport barrier. Cardiovascular Research. 2010 doi: 10.1093/cvr/cvq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen LB, Nordestgaard BG, Stender S, Kjeldsen K. Aortic permeability to LDL as a predictor of aortic cholesterol accumulation in cholesterol-fed rabbits. Arteriosclerosis, Thrombosis, and Vascular Biology. 1992;12:1402–9. doi: 10.1161/01.atv.12.12.1402. [DOI] [PubMed] [Google Scholar]
- 3.Ogunrinade O, Kameya GT, Truskey GA. Effect of fluid shear stress on the permeability of the arterial endothelium. Ann Biomed Eng. 2002;30(4):430–46. doi: 10.1114/1.1467924. [DOI] [PubMed] [Google Scholar]
- 4.Lin Z, Natesan V, Shi H, Dong F, Kawanami D, Mahabeleshwar GH, et al. Kruppel-like factor 2 regulates endothelial barrier function. Arterioscler Thromb Vasc Biol. 2010;30(10):1952–9. doi: 10.1161/ATVBAHA.110.211474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erusalimsky JD, Skene C. Mechanisms of endothelial senescence. Experimental Physiology. 2009;94(3):299–304. doi: 10.1113/expphysiol.2008.043133. [DOI] [PubMed] [Google Scholar]
- 6.Frippiat C, Chen QM, Zdanov S, Magalhaes J-P, Remacle J, Toussaint O. Subcytotoxic H2O2 Stress Triggers a Release of Transforming Growth Factor-β1, Which Induces Biomarkers of Cellular Senescence of Human Diploid Fibroblasts. Journal of Biological Chemistry. 2001;276(4):2531–7. doi: 10.1074/jbc.M006809200. [DOI] [PubMed] [Google Scholar]
- 7.Ota H, Eto M, Kano MR, Ogawa S, Iijima K, Akishita M, et al. Cilostazol Inhibits Oxidative Stress–Induced Premature Senescence Via Upregulation of Sirt1 in Human Endothelial Cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(9):1634–9. doi: 10.1161/ATVBAHA.108.164368. doi:10.1161/atvbaha.108.164368. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Li H, Addabbo F, Zhang F, Pelger E, Patschan D, et al. Adoptive Transfer of Syngeneic Bone Marrow-Derived Cells in Mice with Obesity-Induced Diabetes: Selenoorganic Antioxidant Ebselen Restores Stem Cell Competence. The American Journal of Pathology. 2009;174(2):701–11. doi: 10.2353/ajpath.2009.080606. doi:10.2353/ajpath.2009.080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Brodsky SV, Goligorsky DM, Hampel DJ, Li H, Gross SS, et al. Glycated Collagen I Induces Premature Senescence-Like Phenotypic Changes in Endothelial Cells. Circulation Research. 2002;90(12):1290–8. doi: 10.1161/01.res.0000022161.42655.98. doi:10.1161/01.res.0000022161.42655.98. [DOI] [PubMed] [Google Scholar]
- 10.Brodsky SV, Gealekman O, Chen J, Zhang F, Togashi N, Crabtree M, et al. Prevention and Reversal of Premature Endothelial Cell Senescence and Vasculopathy in Obesity-Induced Diabetes by Ebselen. Circulation Research. 2004;94(3):377–84. doi: 10.1161/01.RES.0000111802.09964.EF. doi:10.1161/01.res.0000111802.09964.ef. [DOI] [PubMed] [Google Scholar]
- 11.Han J, Shuvaev VV, Muzykantov VR. Catalase and Superoxide Dismutase Conjugated with Platelet-Endothelial Cell Adhesion Molecule Antibody Distinctly Alleviate Abnormal Endothelial Permeability Caused by Exogenous Reactive Oxygen Species and Vascular Endothelial Growth Factor. Journal of Pharmacology and Experimental Therapeutics. 2011;338(1):82–91. doi: 10.1124/jpet.111.180620. doi:10.1124/jpet.111.180620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(9):1584–95. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 14.Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of Umbilical Cord Blood-Derived Late Outgrowth Endothelial Progenitor Cells Exposed to Laminar Shear Stress. Tissue Engineering Part A. 2009;15(11):3575–87. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmann KA, Johnson SL, Hebbel RP, Tranquillo RT. Shear Stress Responses Of Adult Blood Outgrowth Endothelial Cells Seeded On Bioartifical Tissue. Tissue Eng. 2011 doi: 10.1089/ten.tea.2011.0055. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown MA, Zhang L, Levering VW, Wu JH, Satterwhite LL, Brian L, et al. Human Umbilical Cord Blood-Derived Endothelial Cells Reendothelialize Vein Grafts and Prevent Thrombosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010 doi: 10.1161/ATVBAHA.110.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tura O, Skinner EM, Robin Barclay G, Samuel K, Gallagher RCJ, Brittan M, et al. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. STEM CELLS. 2012:N/A–N/A. doi: 10.1002/stem.1280. doi:10.1002/stem.1280. [DOI] [PubMed] [Google Scholar]
- 18.Ingram DA, Mead LE, Moore DB, Woodard W, Fenoglio A, Yoder MC. Vessel wall-derived endothelial cells rapidly proliferate because they contain a complete hierarchy of endothelial progenitor cells. Blood. 2005;105(7):2783–6. doi: 10.1182/blood-2004-08-3057. [DOI] [PubMed] [Google Scholar]
- 19.Cheung TM, Ganatra MP, Peters EB, Truskey GA. The Effect of Cellular Senescence on the Albumin Permeability of Blood-Derived Endothelial Cells. American Journal of Physiology - Heart and Circulatory Physiology. 2012;303(11):H1374–83. doi: 10.1152/ajpheart.00182.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, et al. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 21.van der Loo B, Fenton MJ, Erusalimsky JD. Cytochemical detection of a senescence-associated β-galactosidase in endothelial and smooth muscle cells from human and rabbit blood vessels. Exptl Cell Res. 1998;241(2):309–15. doi: 10.1006/excr.1998.4035. [DOI] [PubMed] [Google Scholar]
- 22.Albelda SM, Sampson PM, Haselton FR, McNiff JM, Mueller SN, Williams SK, et al. Permeability characteristics of cultured endothelial cell monolayers. J Appl Physiol. 1988;64(1):308–22. doi: 10.1152/jappl.1988.64.1.308. [DOI] [PubMed] [Google Scholar]
- 23.Cao L, Wu A, Truskey GA. Biomechanical effects of flow and coculture on human aortic and cord blood-derived endothelial cells. Journal of Biomechanics. 2011;44(11):2150–7. doi: 10.1016/j.jbiomech.2011.05.024. doi:10.1016/j.jbiomech.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang N-C, Hu M-L. A fluorimetric method using fluorescein di-β-d-galactopyranoside for quantifying the senescence-associated β-galactosidase activity in human foreskin fibroblast Hs68 cells. Analytical Biochemistry. 2004;325(2):337–43. doi: 10.1016/j.ab.2003.11.012. doi:10.1016/j.ab.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Park S-J, Ahmad F, Philp A, Baar K, Williams T, Luo H, et al. Resveratrol Ameliorates Aging-Related Metabolic Phenotypes by Inhibiting cAMP Phosphodiesterases. Cell. 2012;148(3):421–33. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.J V, Ward P. Mechanisms of endothelial cell injury in acute inflammation. Shock. 1994;2(5):311–9. doi: 10.1097/00024382-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 27.McQuaid KE, Smyth EM, Keenan AK. Evidence for modulation of hydrogen peroxide-induced endothelial barrier dysfunction by nitric oxide in vitro. European Journal of Pharmacology. 1996;307(2):233–41. doi: 10.1016/0014-2999(96)00271-3. [DOI] [PubMed] [Google Scholar]
- 28.Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007;35(3):375–86. doi: 10.1007/s10439-006-9230-5. doi:10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- 29.de Bono DP, Yang WD. Exposure to low concentrations of hydrogen peroxide causes delayed endothelial cell death and inhibits proliferation of surviving cells. Atherosclerosis. 1995;114(2):235–45. doi: 10.1016/0021-9150(94)05488-5. [DOI] [PubMed] [Google Scholar]
- 30.Davies MJ, Woolf N, Rowles PM, Pepper J. Morphology of the endothelium over atherosclerotic plaques in human coronary arteries. British Heart Journal. 1988;60(6):459–64. doi: 10.1136/hrt.60.6.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burrig K. The endothelium of advanced arteriosclerotic plaques in humans. Arteriosclerosis, Thrombosis, and Vascular Biology. 1991;11(6):1678–89. [PubMed] [Google Scholar]
- 32.Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. The Journal of Clinical Investigation. 1993;91(4):1421–8. doi: 10.1172/JCI116346. doi:10.1172/jci116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hastie LE, Patton WF, Hechtman HB, Shepro D. H2O2-induced filamin redistribution in endothelial cells is modulated by the cyclic AMP-dependent protein kinase pathway. Journal of Cellular Physiology. 1997;172(3):373–81. doi: 10.1002/(SICI)1097-4652(199709)172:3<373::AID-JCP11>3.0.CO;2-7. doi:10.1002/(sici)1097-4652(199709)172:3<373::aid-jcp11>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 34.Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, et al. Aging, Progenitor Cell Exhaustion, and Atherosclerosis. Circulation. 2003;108(4):457–63. doi: 10.1161/01.CIR.0000082924.75945.48. doi:10.1161/01.cir.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]