Summary
Aims
Skeletal muscle is a major metabolic organ and plays important roles in glucose metabolism, insulin sensitivity, and insulin action. Muscle telomere length reflects the myocyte's exposure to harmful environmental factors. Leukocyte telomere length is considered a marker of muscle telomere length and is used in epidemiologic studies to assess associations with ageing-related diseases where muscle physiology is important. However, the extent to which leucocyte telomere length and muscle telomere length are correlated is unknown, as are their relative correlations with glucose and insulin concentrations. The purpose of this study was to determine the extent of these relationships.
Methods
Leucocyte telomere length and muscle telomere length were measured by quantitative real-time PCR in participants from the Malmö Exercise Intervention (MEI; n=27) and the PPP-Botnia studies (n=31). Participants in both studies were free from type 2 diabetes. We assessed the association between leucocyte telomere length, muscle telomere length and metabolic traits using Spearmen correlations and multivariate linear regression. Bland-Altman analysis was used to assess agreement between leucocyte telomere length and muscle telomere length.
Results
In age-, study-, diabetes family history- and sex-adjusted models, leucocyte telomere length and muscle telomere length were positively correlated (r=0.39, 95% CI: 0.15, 0.59). Leucocyte telomere length was inversely associated with 2hr glucose concentrations (r= -0.58, 95% CI: -1.0, -0.16), but there was no correlation between muscle telomere length and 2 hr glucose concentrations (r=0.05, 95% CI: -0.35, 0.46) or between leucocyte telomere length or muscle telomere length with other metabolic traits.
Conclusions
In summary, the current study supports the use of leucocyte telomere length as a proxy for muscle telomere length in epidemiological studies of type 2 diabetes aetiology.
Keywords: Leukocyte telomere length, muscle telomere length, cardiometabolic, type 2 diabetes, skeletal muscle physiology
Introduction
Telomeres, which are the tandem repeats of TTAGGG DNA sequences at the end of human chromosomes, shorten with every cell division and their length is an indicator of the replicative potential of each somatic cell. Telomere attrition is a normal part of ageing and also reflects the cell's exposure to previous intrinsic and extrinsic stressors [1] such as inflammation and oxidative stress, as well as the cell's chromosomal integrity and stability [2]. Critically short telomeres lose functionality and lead to cell death or senescence [3], implicating telomere attrition in ageing-related diseases such as type 2 diabetes mellitus (T2DM).
Although muscle telomere length is relevant to T2DM aetiology, measuring muscle telomere length necessitates biopsies, which are infeasible in large studies; thus, measuring leucocyte telomere length may be a practical alternative [4]. However, it is unknown to what extent leucocyte telomere length and muscle telomere length are correlated, which makes inferences about epidemiological studies of leucocyte telomere length difficult.
The primary purpose of this study was to determine the extent of the relationship between leucocyte telomere length and muscle telomere length. A secondary objective was to assess the relationship between muscle telomere length, leucocyte telomere length, and diabetes-related metabolic traits.
Research Design and Methods
Participants
Fifty-eight non-diabetic participants were selected from the Malmö Exercise Intervention (MEI) study (N=27 men) and the Prevalence, Prediction and Prevention of diabetes (PPP)-Botnia Study (N=15/16 men/women). Two participants from MEI study were using anti-allergy drugs while in PPP-Botnia two participants were using lipid lowering drugs and two participants were using anti-inflammatory drugs. Participants provided written informed consent and the research protocols were approved by the research ethics committees of Helsinki University Central Hospital and Lund University.
Measurements
All participants reported their personal history of T2DM, age, and sex. Blood was drawn from a forearm vein after an overnight fast, from which plasma and buffy coat (for DNA extraction) were separated. Height, weight, and fasting and 2hr glucose and fasting insulin were measured according to methods described previously [5] and muscle biopsies were obtained with a Bergström needle from the right vastrus lateralis [6]. Data for fasting insulin in PPP-Botnia Study were unavailable for one participant.
Mean telomere length
Isolation of DNA from muscle and blood samples was performed as described previously [7]. Relative leucocyte telomere length and muscle telomere length were quantified by real-time quantitative PCR on an ABI7900HT sequence detection system (Applied biosystems, USA) [8]. Briefly, assays were run in triplicate in 10μl with 5ng template DNA, SYBR Green master mix (Qiagen Laboratories, Sweden), Tel1 (270nM) and of Tel2 (900nM) primers. For single copy gene 36Bμ (300nM) and 36Bd (500nM), primers were used instead of Tel1 and Tel2 (primer sequence are reported in [8]). This quantitative assay determines the amount of telomeric DNA (T) relative to the amount of a single copy control gene (human b-globin) DNA (S), following which the T/S ratio is calculated. The standard curve method was used to calculate an eight point standard curve for each leukocyte sample (0.31–20ng) separately. The coefficient of variance was 0.06-2.75% (telomere assay) and 0.01-1.69% (single copy gene assay) in blood samples and 0.04-0.91% (telomere assay) and 0.02-0.86% (single copy gene assay) in skeletal muscle.
Statistical Analysis
Statistics were calculated using STATA (v8.0, Stata Corporation, College Station, TX, USA). Study-specific z-scores (mean=0; SD=1) were calculated for muscle telomere length and leucocyte telomere length and all comparisons were performed using these values. Student's T-test (or Mann-Whitney U-test for non-normally distributed variables) was used to test differences in continuous variables. The chi-squared test was used to assess differences in frequencies of categorical variables. Data are expressed as mean (SD) for continuous variables and % (number of participants) for categorical variables. We quantified the strength of association between leucocyte telomere length and muscle telomere length with the Spearman correlation coefficient (adjusted for age, sex, diabetes family history, and study) and multivariate linear regression analysis (adjusted for age, sex, diabetes family history, and study). Agreement between the two methods (mean bias) was assessed using Bland–Altman and Pitman's tests. Difference in variance between leucocyte telomere length and muscle telomere length was assessed with the Pitman's test. To assess whether the relation between muscle telomere length and leucocyte telomere length differed by study, sex, or family history of diabetes, pairwise interaction terms (variable* muscle telomere length) were fitted to a linear model where leucocyte telomere length was the dependent variable (all tests P>0.15; data not shown); therefore, data were combined and appropriately adjusted. Finally, we assessed the association of leucocyte telomere length and muscle telomere length with measures related to T2DM (blood glucose, and insulin). Because the study involved only one primary hypothesis test, uncorrected P-values are reported.
Results
Participants in the MEI study were all men, were younger (38.3±4.2 years vs. 50.8±11.3 years; (P≤0.001) and had lower fasting glucose (4.23±0.49 vs. 5.33±0.45 mmol/l; (P≤0.001) than those in the Botnia-PPP study, which included men (n=15) and women (n=16), but they were otherwise comparable (Supplementary table 1).
Relationship of leucocyte telomere length and muscle telomere length (primary test)
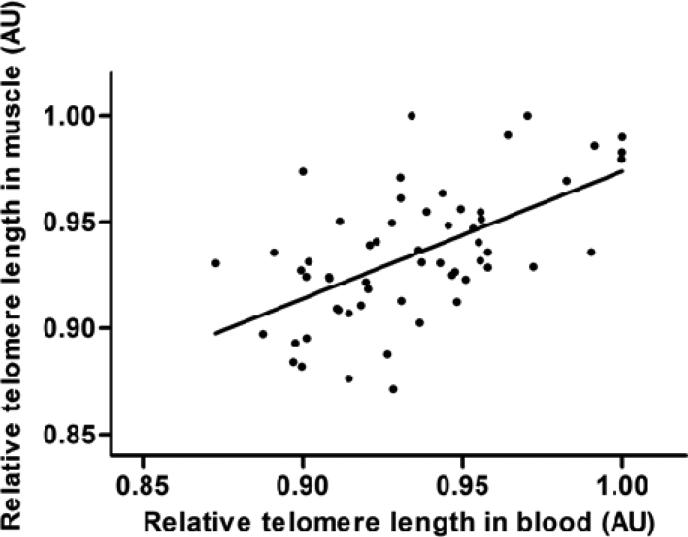
Leucocyte telomere length and muscle telomere length were positively correlated (r=0.39; P=0.003) after adjusting for age, sex, family history of diabetes and study (figure 1). In multivariate linear regression models adjusted for age, sex family history of diabetes and study, one z-score leucocyte telomere length unit was associated with 0.43 (95% CI: 0.15, 0.71) muscle telomere length z-score units.
Figure 1.
Scatter plot for relative telomere length in blood versus relative telomere length in muscle from the MEI and Botnia-PPP studies (n = 58). Partial Correlation coefficient (p-value): r = 0.39 (0.003), Regression coefficient (95% CI): 0.43 (0.15; 0.71) standardized units of muscle telomere length per standardized unit of leucocyte telomere length, R2 = 0.37. Estimates are adjusted for age, sex and study.
Bland-Altman analysis for muscle telomere length vs. leucocyte telomere length yielded narrow limits of agreement of -0.056 to 0.056, with almost all data points falling within ±2 SD of the mean. Pitman's test showed no evidence of difference in variance between leucocyte telomere length and muscle telomere length (P=0.79) and there was no systematic bias in the relationship between muscle telomere length and leucocyte telomere length across the spectrum of muscle telomere length values (Supplemental Figure 1).
Relationships of leucocyte telomere length and muscle telomere length with T2DM-related traits (secondary tests)
An inverse relationship between leucocyte telomere length and 2hr glucose concentrations was observed (Table 1a), with longer leucocyte telomere length being associated with lower glucose. No statistically significant relationships were observed between muscle telomere length and 2-hr glucose or between muscle telomere length or leucocyte telomere length and any other diabetes-related traits (Tables 1a and 1b).
Table 1a.
Correlation and association between leukocyte telomere length and cardiometabolic risk factors
| n | Partial correlation coefficient (95%; CI) p-value |
Regression coefficient (95%; CI)1 p-value |
|
|---|---|---|---|
| Age (years) | 58 | 0.05 (-0.21; 0.30) 0.72 |
0.002 (-0.006; 0.010) 0.66 |
| BMI (kg/m2) | 58 | 0.07 (-0.19; 0.32) 0.63 |
0.26 (-1.01; 0.54) 0.68 |
| Fasting glucose (mmol/l) | 58 | -0.06 (-0.31; 0.20) 0.66 |
-0.04 (-0.19; 0.12) 0.62 |
| 2h-glucose (mmol/l) | 58 | -0.34 (-0.55; -0.09) 0.013 |
-0.58 (-1.00; -0.16) 0.007 |
| Fasting insulin (mU/l) | 57 | 0.01 (-0.25, 0.27) 0.93 |
0.04 (-1.13; 1.21) 0.95 |
Multivariate linear regression with each cardiometabolic risk factor as the outcome and leukocyte telomere length (z-score) as the main exposure, adjusting for age, sex, diabetes family history and study. The estimates for age are from a model where telomere length was the outcome and age (z-score) the main exposure, adjusting for sex, diabetes family history and study. In theory, statins and anti-inflammatory drugs might affect leucocyte telomere length. In the MEI study none of the participants reported using such drugs, whereas in the PPP-Botnia study two participants reported using lipid-lowering drugs and two participants reported using anti-inflammatory drugs. Controlling for the use of these drugs in analyses had no materially impact on the results of the study.
Table 1b.
Correlation and association between muscle telomere length and cardiometabolic risk factors
| n | Partial correlation coefficient (95%; CI) p-value |
Regression coefficient (95%; CI)1 p-value |
|
|---|---|---|---|
| Age (years) | 58 | 0.03 (-0.23; 0.29) 0.84 |
0.001 (-0.009; 0.010) 0.83 |
| BMI (kg/m2) | 58 | -0.12 (-0.37; 0.14) 0.38 |
-0.60 (-1.74; 0.55) 0.30 |
| Fasting glucose (mmol/l) | 58 | 0.20 (-0.06; 0.43) 0.15 |
0.10 (-0.04; 0.24) 0.15 |
| 2h-glucose (mmol/l) | 58 | 0.03 (-0.23; 0.29) 0.84 |
0.05 (-0.35; 0.46) 0.79 |
| Fasting insulin (mU/l) | 57 | 0.05 (-0.21, 0.31) 0.72 |
-0.18 (-1.25; 0.89) 0.74 |
Multivariate linear regression with each cardiometabolic risk factor as the outcome and muscle telomere length (z-score) as the main exposure, adjusting for age, sex, diabetes family history and study. The estimates for age are from a model where telomere length was the outcome and age (z-score) the main exposure, adjusting for sex, diabetes family history and study
Discussion
In this study of young and middle-aged adults, who were free of diabetes, leucocyte telomere length and muscle telomere length were modestly, positively correlated. A statistically significant inverse relationship between leucocyte telomere length and post-challenge glucose concentrations was also observed. No other statistically significant relationships with diabetes-related traits were found, which may in part reflect the low power of this study to detect small effects.
T2DM is a complex ageing-related disorder that results from genetic and environmental risk factors. Cellular senescence contributes to T2DM-related defects in beta-cell function, mitochondrial biogenesis, glycogen synthesis, peripheral insulin sensitivity, and glucose transportation and metabolism [9]. A key aspect of cellular senescence involves skeletal muscle telomere attrition [10]. It is, therefore, logical to hypothesize that telomere attrition in organs that are involved in glucose metabolism and insulin action, including skeletal muscle, liver, adipose tissue, and pancreas, impacts T2DM risk. However, because it is infeasible to collect skeletal muscle biopsies in epidemiologic settings, studies of telomere length and T2DM have generally used leucocyte telomere length as a surrogate marker of telomere length in other tissues [7, 11-13]. For these studies to be appropriately interpreted, it is important to ascertain how strongly correlated leucocyte telomere length and telomere length in other relevant cell types are.
Our observations are in line with previous studies that examined the correlation of leucocyte telomere length with telomere length in other tissues. For example, among new-borns leucocyte telomere length was strongly correlated with telomere length in skin and umbilical artery (r>0.90) [14]. In a small sample of elderly persons (aged 73-95 yrs), leucocyte telomere length was also correlated with skin (r=0.84) and synovial tissue (r=0.73) [15]. That leucocyte telomere length is highly correlated with telomere length in multiple tissues with varying replicative capacity and tissue renewal rates, suggests that telomere length regulation is synchronized across human organs and tissues. The strong correlation of telomere length among tissues from the same donor suggests that telomere length is relatively similar for tissues at birth and that telomeres shorten at roughly similar rates across these tissues [16]. Our results support the premise that leucocyte telomere length is a reasonable marker of the “biological age” of other tissues involved in T2DM aetiology.
At the outset of our study, we hypothesized that muscle telomere length would be more strongly related with glucose and insulin measures than leucocyte telomere length, owing to the important role skeletal muscle plays in systemic glucose disposal. However, our results suggest the opposite is true. It is possible that leucocyte telomere length is more strongly correlated with telomere length in other relevant tissues, such as pancreas, liver, and adipose, and thus provides a strong proxy for telomere length in these tissues, as well as in muscle telomere length. It is also possible that leucocyte telomere length reflects long-term exposure to other diabetogenic factors to a greater extent than muscle telomere length. This is possibly the most plausible explanation, as leukocyte cells divide more rapidly than other human cells, with the shortest lifespan on average [17]; this is partly attributable to high levels of oxidative stress in leukocytes [18], an important pathological factor in T2DM [12].
Blood consists of formed elements including erythrocytes, leucocytes and platelets. Leukocytes are heterogeneous tissues and can be classified into granulocytes (neutrophils, eosinophils and basophils) and agranulocytes (monocytes and lymphocytes) [19]. Telomere length is likely to differ between these different elements, and controlling for the presence of these elements when examining associations between blood telomere length and other factors may improve the precision of the estimates. Cytomegalovirus (CMV) infection can also impact senescence in CD8 T-cells [20] and controlling for CMV infection in analyses of telomere length might also improve the precision of the estimates. However, in this study and most epidemiologic studies information about tissue elements and the frequency of CMV infection is unknown, although the latter is infrequent in the general population [21] and may have minimal impact on telomere study results.
Telomeres may be longer in skeletal muscle than in blood owing to the higher replicative capacity of the latter. However, epidemiological studies typically assess relative telomere length using a PCR-based assay that does not quantify absolute telomere length [8]. Because our study was designed to assess this specific approach, we are unable to examine differences in absolute telomere length between study centers or tissues. Thus, future epidemiological studies might benefit from utilizing.
Our study was restricted to middle-aged white adults and our findings may not generalize to other age and ethnic groups. Moreover, the observed association between leucocyte telomere length and muscle telomere length may be owing to confounding by unmeasured covariates such as inflammation or lifestyle, factors which we could not control for. However, the consistency with which telomere length in other tissues is correlated with leucocyte telomere length, suggests this is unlikely.
In conclusion, the positive correlation and measurement agreement between leucocyte telomere length and muscle telomere length supports the use of leucocyte telomere length as a surrogate marker of telomere attrition in skeletal muscle. To our knowledge, this is the first study to report on this relationship. Our study also sheds light on the relationship between muscle and leukocyte telomere length, which may also improve our mechanistic understanding of diabetes pathophysiology.
Supplementary Material
Acknowledgements
We thank the participants and staff from the Malmö Exercise Intervention (MEI) Study and the Botnia-PPP Study. The current study was funded by Novo Nordisk (PWF and OH), the Swedish Research Council (PWF), the Swedish Heart Lung Foundation (PWF), the Skåne Health Authority (PWF), EXGENESIS (OH), UMAS Fonder (OH), Magn. Bergvalls foundation (OH), Syskonen Svenssons foundation (OH), NuGo and the Wallenberg Foundation. Dr. Qi Sun is supported by a career development award K99HL098459 from the National Heart, Lung, and Blood Institute. The Malmö Exercise Intervention Study was supported by grants from the Swedish Research Council (Linné grant and project grant to Leif Groop) and an equipment grant from the Wallenberg Foundation. The Botnia PPP Intervention study was supported by grants from the Sigrid Juselius Foundation, the Folkhälsan Foundation and the Finnish Ministry of Health.
Abbreviations
- T2DM
Type 2 diabetes mellitus
Footnotes
Author Contributions
Qi Sun (QS), Paul W Franks (PWF) and Ola Hansson (OH) conceived the study and designed analyses. Shafqat Ahmad (SA), Tina Rönn (TR) and Ola Hansson (OH) conducted laboratory analyses. SA, Alexandros Heraclides (AH) and PWF undertook statistical analyses. SA and PWF wrote the manuscript. All authors critiqued the manuscript and provided valuable input on the revisions.
Declaration of Competing Interests: None declared.
References
- 1.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–83. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 2.Riethman H. Human telomere structure and biology. Annu Rev Genomics Hum Genet. 2008;9:1–19. doi: 10.1146/annurev.genom.8.021506.172017. [DOI] [PubMed] [Google Scholar]
- 3.d'Adda di Fagagna F, Reaper PM, Clay-Farrace L, Fiegler H, Carr P, Von Zglinicki T, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426:194–8. doi: 10.1038/nature02118. [DOI] [PubMed] [Google Scholar]
- 4.Tentolouris N, Nzietchueng R, Cattan V, Poitevin G, Lacolley P, Papazafiropoulou A, et al. White blood cells telomere length is shorter in males with type 2 diabetes and microalbuminuria. Diabetes Care. 2007;30:2909–15. doi: 10.2337/dc07-0633. [DOI] [PubMed] [Google Scholar]
- 5.Isomaa B, Forsen B, Lahti K, Holmstrom N, Waden J, Matintupa O, et al. A family history of diabetes is associated with reduced physical fitness in the Prevalence, Prediction and Prevention of Diabetes (PPP)-Botnia study. Diabetologia. 2010;53:1709–13. doi: 10.1007/s00125-010-1776-y. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson KF, Saltin B, Lindgarde F. Increased skeletal muscle capillary density precedes diabetes development in men with impaired glucose tolerance. A 15-year follow-up. Diabetes. 1994;43:805–8. doi: 10.2337/diab.43.6.805. [DOI] [PubMed] [Google Scholar]
- 7.Adaikalakoteswari A, Balasubramanyam M, Mohan V. Telomere shortening occurs in Asian Indian Type 2 diabetic patients. Diabetic medicine: a journal of the British Diabetic Association. 2005;22:1151–6. doi: 10.1111/j.1464-5491.2005.01574.x. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–81. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- 10.Kadi F, Ponsot E. The biology of satellite cells and telomeres in human skeletal muscle: effects of aging and physical activity. Scandinavian journal of medicine & science in sports. 2010;20:39–48. doi: 10.1111/j.1600-0838.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66:312–9. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic medicine : a journal of the British Diabetic Association. 2011;28:1388–94. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- 13.Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M. Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Translational research: the journal of laboratory and clinical medicine. 2010;155:166–9. doi: 10.1016/j.trsl.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Okuda K, Khan MY, Skurnick J, Kimura M, Aviv H, Aviv A. Telomere attrition of the human abdominal aorta: relationships with age and atherosclerosis. Atherosclerosis. 2000;152:391–8. doi: 10.1016/s0021-9150(99)00482-7. [DOI] [PubMed] [Google Scholar]
- 15.Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U. Telomere length in different tissues of elderly patients. Mech Ageing Dev. 2000;119:89–99. doi: 10.1016/s0047-6374(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 16.Takubo K, Aida J, Izumiyama-Shimomura N, Ishikawa N, Sawabe M, Kurabayashi R, et al. Changes of telomere length with aging. Geriatr Gerontol Int. 2010;10(Suppl 1):S197–206. doi: 10.1111/j.1447-0594.2010.00605.x. [DOI] [PubMed] [Google Scholar]
- 17.Abramson N, Melton B. Leukocytosis: basics of clinical assessment. American family physician. 2000;62:2053–60. [PubMed] [Google Scholar]
- 18.Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5:325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 19.Athens JW. Blood: leukocytes. Annual review of physiology. 1963;25:195–212. doi: 10.1146/annurev.ph.25.030163.001211. [DOI] [PubMed] [Google Scholar]
- 20.van de Berg PJ, Griffiths SJ, Yong SL, Macaulay R, Bemelman FJ, Jackson S, et al. Cytomegalovirus infection reduces telomere length of the circulating T cell pool. Journal of immunology. 2010;184:3417–23. doi: 10.4049/jimmunol.0903442. [DOI] [PubMed] [Google Scholar]
- 21.Dollard SC, Staras SA, Amin MM, Schmid DS, Cannon MJ. National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clinical and vaccine immunology : CVI. 2011;18:1895–9. doi: 10.1128/CVI.05228-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.