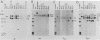Abstract
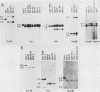
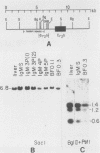
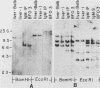
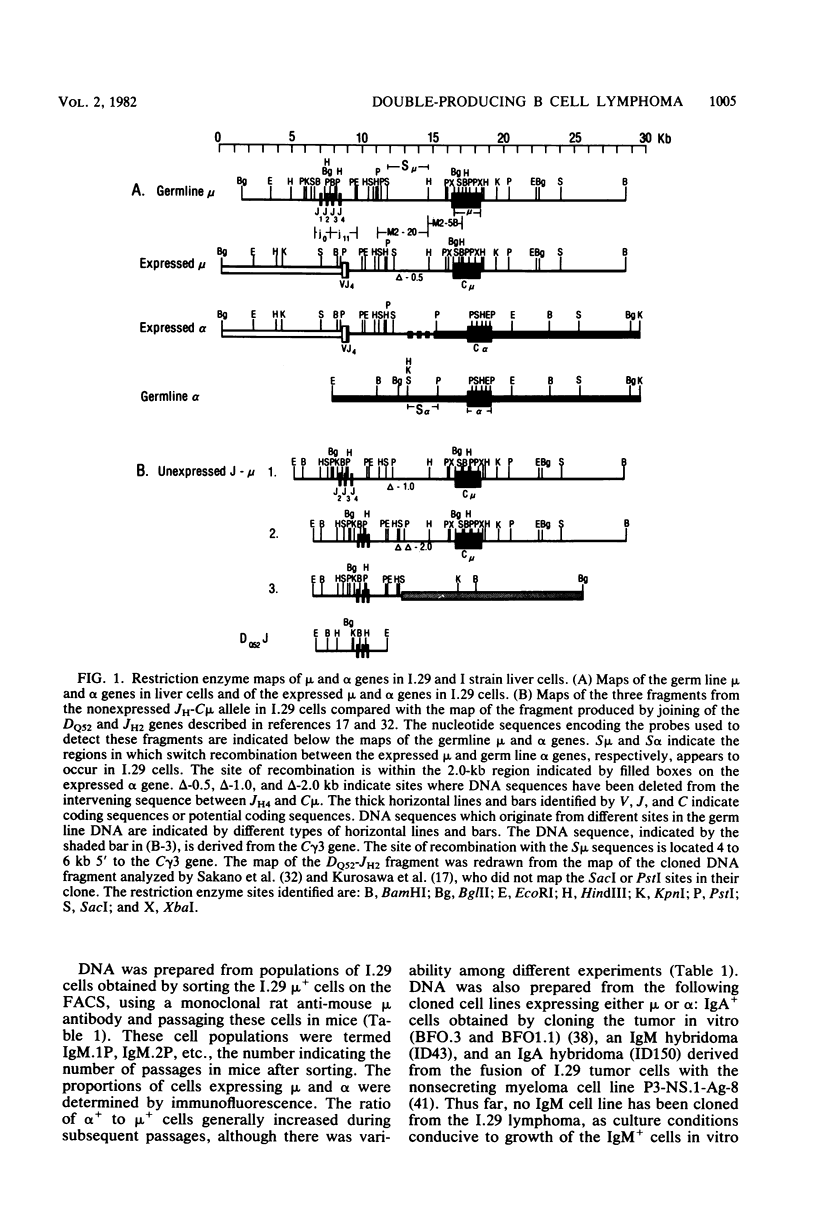
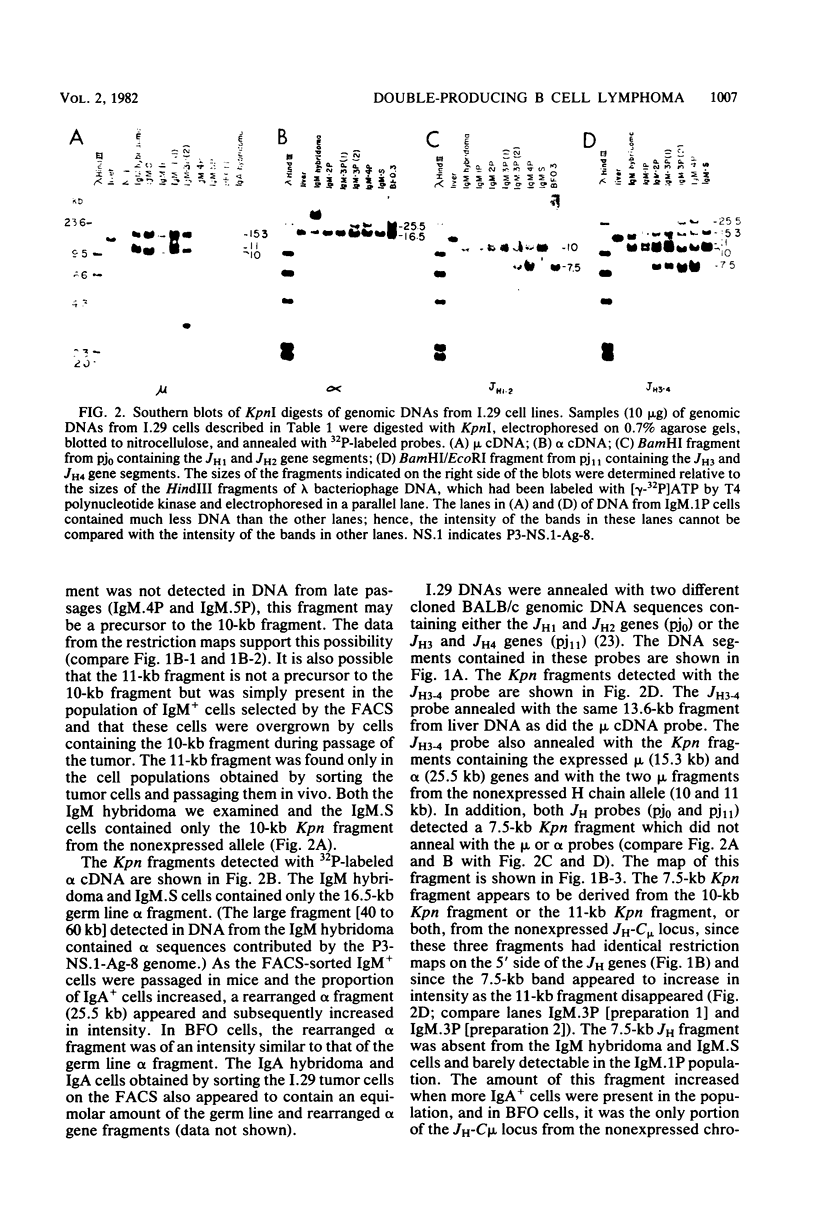
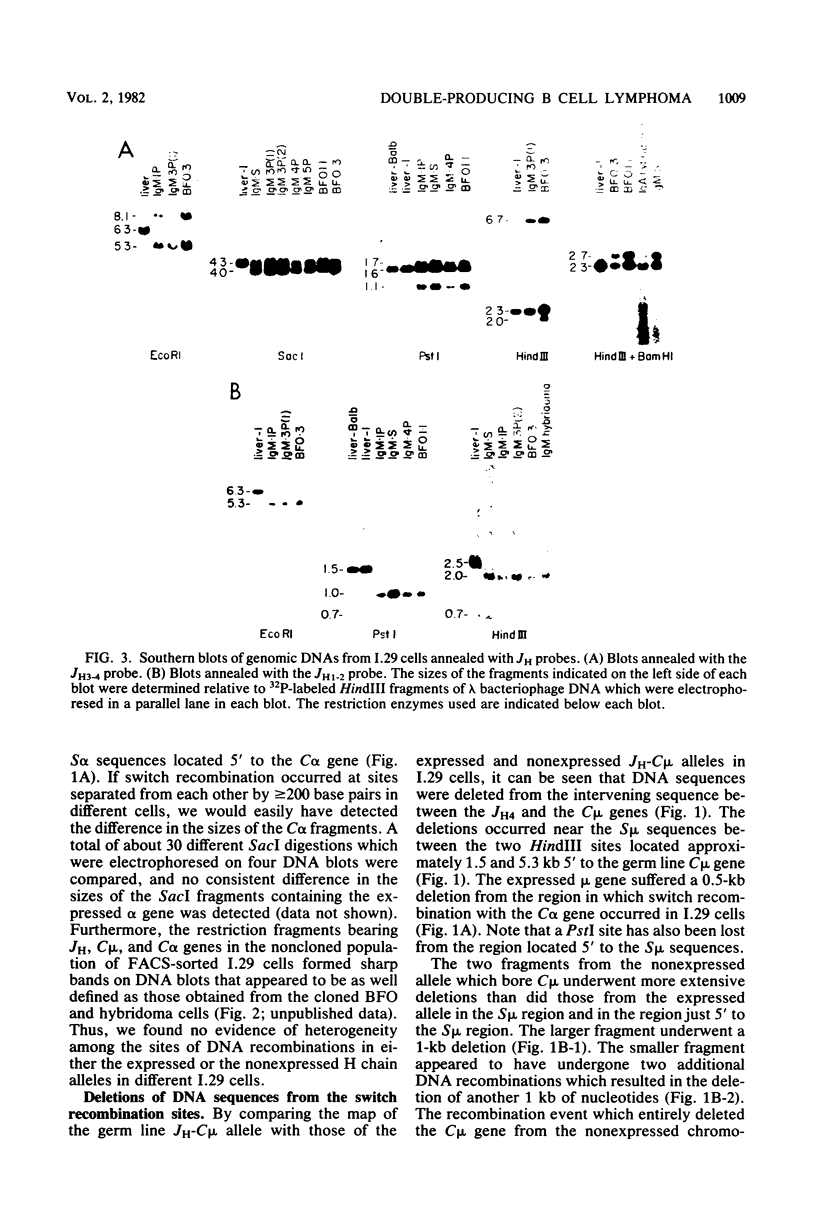
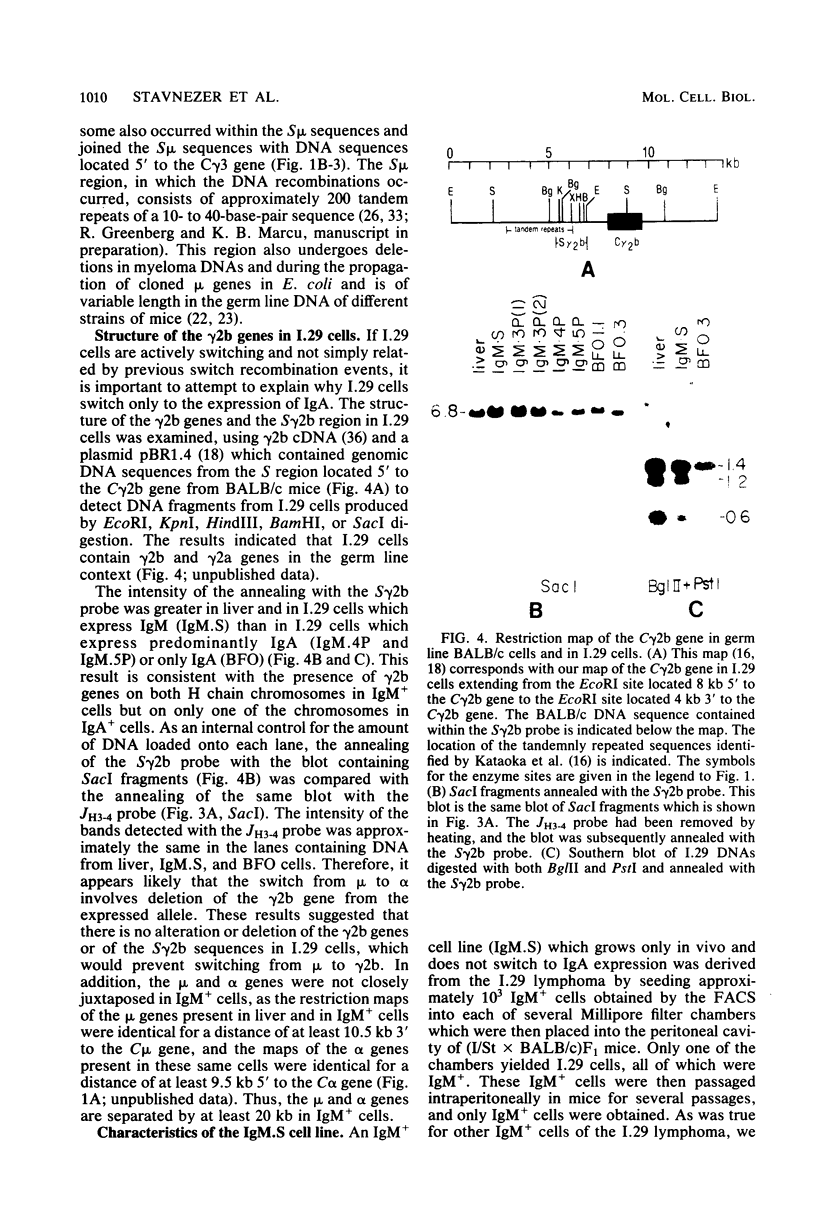
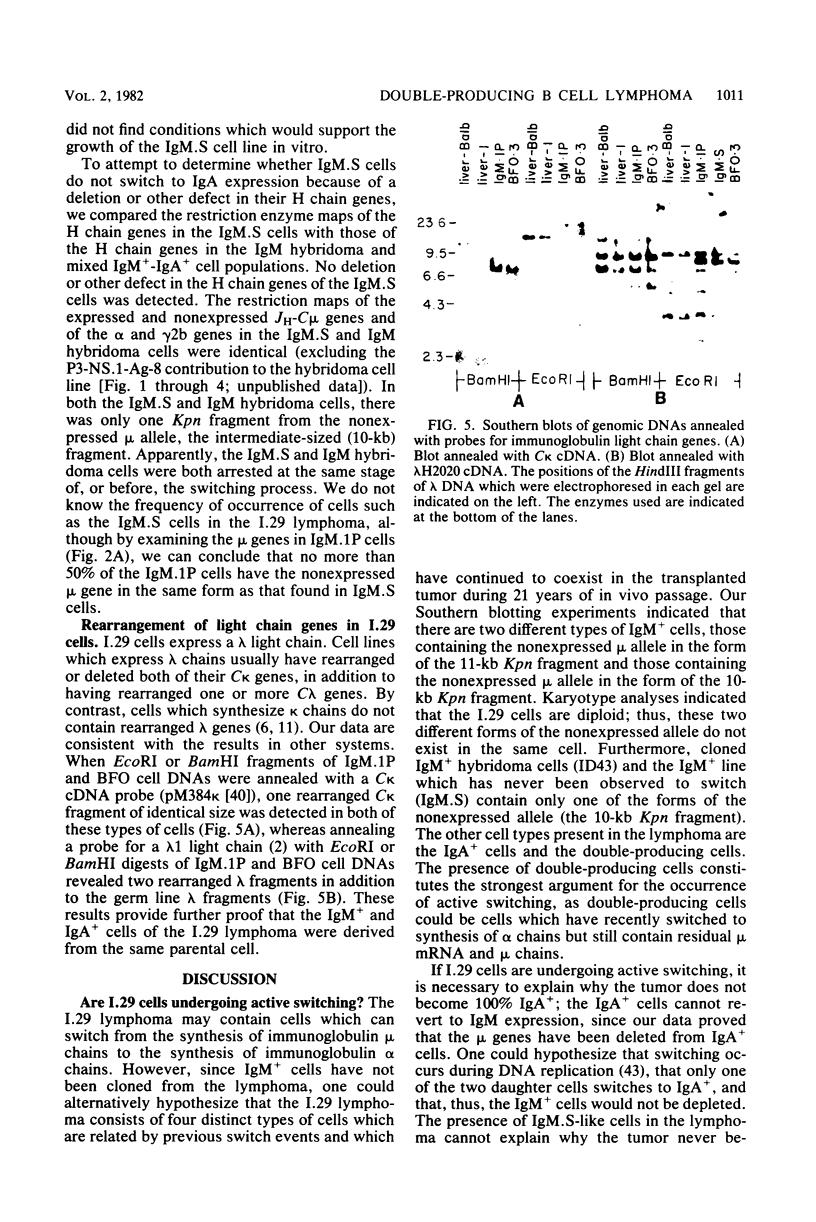
The B cell lymphoma I.29 consists of a mixture of cells expressing membrane-bound immunoglobulin M (IgM) (lambda) and IgA (lambda) of identical idiotypes. Whereas most of the cells express either IgM or IgA alone, 1 to 5% of the cells in this tumor express IgM and IgA simultaneously within the cytoplasm and on the cell membrane (R. Sitia et al., J. Immunol. 127:1388-1394, 1981; R. Sitia, unpublished data). When IgM+ cells are purified from the lymphoma and passaged in mice or cultured, a portion of the cells convert to IgA+. These properties suggest that some cells of the I.29 lymphoma may undergo immunoglobulin heavy chain switching, although it is also possible that the mixed population was derived by a prior switching event in a clone of cells. We performed Southern blotting experiments on genomic DNAs isolated from populations of I.29 cells containing variable proportions of IgM+ and IgA+ cells and on a number of cell lines derived from the lymphoma. The results were consistent with the deletion model for heavy chain switching, as the IgM+ cells contained rearranged mu genes and alpha genes in the germ line configuration on both the expressed and nonexpressed heavy chain chromosomes, whereas the IgA+ cells had deleted both mu genes and contained one rearranged and one germ line alpha gene. In addition, segments of DNA located within the intervening sequence 5' to the mu gene, near the site of switch recombination, were deleted from both the expressed and the nonexpressed chromosomes. Although mu genes were deleted from both chromosomes in the IgA+ cells, the sites of DNA recombination differed on the two chromosomes. On the expressed chromosome, Smu sequences were recombined with S alpha sequences, whereas on the nonexpressed chromosome, Smu sequences were recombined with S gamma 3 sequences.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abney E. R., Cooper M. D., Kearney J. F., Lawton A. R., Parkhouse R. M. Sequential expression of immunoglobulin on developing mouse B lymphocytes: a systematic survey that suggests a model for the generation of immunoglobulin isotype diversity. J Immunol. 1978 Jun;120(6):2041–2049. [PubMed] [Google Scholar]
- Brack C., Hirama M., Lenhard-Schuller R., Tonegawa S. A complete immunoglobulin gene is created by somatic recombination. Cell. 1978 Sep;15(1):1–14. doi: 10.1016/0092-8674(78)90078-8. [DOI] [PubMed] [Google Scholar]
- Cayre Y., Palladino M. A., Marcu K. B., Stavnezer J. Expression of an antigen receptor on T cells does not require recombination at the immunoglobulin JH-C mu locus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3814–3818. doi: 10.1073/pnas.78.6.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Cooper D., Perry R. P. Rearrangement of immunoglobulin heavy chain genes during B-lymphocyte development as revealed by studies of mouse plasmacytoma cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1422–1426. doi: 10.1073/pnas.77.3.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleclough C., Perry R. P., Karjalainen K., Weigert M. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981 Apr 2;290(5805):372–378. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- Cory S., Adams J. M. Deletions are associated with somatic rearrangement of immunoglobulin heavy chain genes. Cell. 1980 Jan;19(1):37–51. doi: 10.1016/0092-8674(80)90386-4. [DOI] [PubMed] [Google Scholar]
- Davis M. M., Kim S. K., Hood L. E. DNA sequences mediating class switching in alpha-immunoglobulins. Science. 1980 Sep 19;209(4463):1360–1365. doi: 10.1126/science.6774415. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Hurwitz J. L., Cebra J. J. Successive switching of antibody isotypes expressed within the lines of a B-cell clone. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5424–5428. doi: 10.1073/pnas.77.9.5424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieter P. A., Korsmeyer S. J., Waldmann T. A., Leder P. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981 Apr 2;290(5805):368–372. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- Honjo T., Kataoka T. Organization of immunoglobulin heavy chain genes and allelic deletion model. Proc Natl Acad Sci U S A. 1978 May;75(5):2140–2144. doi: 10.1073/pnas.75.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Kataoka T., Yaoita Y., Shimizu A., Takahashi N., Yamawaki-Kataoka Y., Nikaido T., Nakai S., Obata M., Kawakami T. Organization and reorganization of immunoglobulin heavy-chain genes. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):913–923. doi: 10.1101/sqb.1981.045.01.108. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Hurwitz J. L., Coleclough C., Cebra J. J. CH gene rearrangements in IgM-bearing B cells and in the normal splenic DNA component of hybridomas making different isotypes of antibody. Cell. 1980 Nov;22(2 Pt 2):349–359. doi: 10.1016/0092-8674(80)90345-1. [DOI] [PubMed] [Google Scholar]
- Joho R., Weissman I. L., Early P., Cole J., Hood L. Organization of kappa light chain genes in germ-line and somatic tissue. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1106–1110. doi: 10.1073/pnas.77.2.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka T., Miyata T., Honjo T. Repetitive sequences in class-switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981 Feb;23(2):357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Lang R. B., Stanton L. W., Marcu K. B. On immunoglobulin heavy chain gene switching: two gamma 2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res. 1982 Jan 22;10(2):611–630. doi: 10.1093/nar/10.2.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. R., Kincade P. W., Cooper M. D. Sequential expression of germ line genes in development of immunoglobulin class diversity. Fed Proc. 1975 Jan;34(1):33–39. [PubMed] [Google Scholar]
- Liu C. P., Tucker P. W., Mushinski J. F., Blattner F. R. Mapping of heavy chain genes for mouse immunoglobulins M and D. Science. 1980 Sep 19;209(4463):1348–1353. doi: 10.1126/science.6774414. [DOI] [PubMed] [Google Scholar]
- Maki R., Roeder W., Traunecker A., Sidman C., Wabl M., Raschke W., Tonegawa S. The role of DNA rearrangement and alternative RNA processing in the expression of immunoglobulin delta genes. Cell. 1981 May;24(2):353–365. doi: 10.1016/0092-8674(81)90325-1. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Arnheim N., Banerji J., Penncavage N. A., Seperack P., Lang R., Miesfeld R., Harris L., Greenberg R. Studies on the nature and germ-line stability of DNA sequences flanking the mouse immunoglobulin heavy-chain constant-region genes. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):899–911. doi: 10.1101/sqb.1981.045.01.107. [DOI] [PubMed] [Google Scholar]
- Marcu K. B., Banerji J., Penncavage N. A., Lang R., Arnheim N. 5' flanking region of immunoglobulin heavy chain constant region genes displays length heterogeneity in germlines of inbred mouse strains. Cell. 1980 Nov;22(1 Pt 1):187–196. doi: 10.1016/0092-8674(80)90167-1. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Rogers J., Hunkapiller T., Early P., Nottenburg C., Weissman I., Bazin H., Wall R., Hood L. E. Expression of IgD may use both DNA rearrangement and RNA splicing mechanisms. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1800–1804. doi: 10.1073/pnas.78.3.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt M. N., Francke U. A system of nomenclature for band patterns of mouse chromosomes. Chromosoma. 1973;41(2):145–158. doi: 10.1007/BF00319691. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Obata M., Kataoka T., Nakai S., Yamagishi H., Takahashi N., Yamawaki-Kataoka Y., Nikaido T., Shimizu A., Honjo T. Structure of a rearranged gamma 1 chain gene and its implication to immunoglobulin class-switch mechanism. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2437–2441. doi: 10.1073/pnas.78.4.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno S., Babonits M., Wiener F., Spira J., Klein G., Potter M. Nonrandom chromosome changes involving the Ig gene-carrying chromosomes 12 and 6 in pristane-induced mouse plasmacytomas. Cell. 1979 Dec;18(4):1001–1007. doi: 10.1016/0092-8674(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Parkhouse R. M., Cooper M. D. A model for the differentiation of B lymphocytes with implications for the biological role of IgD. Immunol Rev. 1977;37:105–126. doi: 10.1111/j.1600-065x.1977.tb00247.x. [DOI] [PubMed] [Google Scholar]
- Pernis B., Forni L., Luzzati A. L. Synthesis of multiple immunoglobulin classes by single lymphocytes. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 1):175–183. doi: 10.1101/sqb.1977.041.01.023. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sakano H., Kurosawa Y., Weigert M., Tonegawa S. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981 Apr 16;290(5807):562–565. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Sato H., Boyse E. A., Aoki T., Iritani C., Old L. J. Leukemia-associated transplantation antigens related to murine leukemia virus. The X.1 system: immune response controlled by a locus linked to H-2. J Exp Med. 1973 Sep 1;138(3):593–606. doi: 10.1084/jem.138.3.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Seabright M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma. 1972;36(2):204–210. doi: 10.1007/BF00285214. [DOI] [PubMed] [Google Scholar]
- Sitia R., Rubartelli A., Hammerling U. Expression of 2 immunoglobulin isotypes, IgM and IgA, with identical idiotype in the B cell lymphoma I.29. J Immunol. 1981 Oct;127(4):1388–1394. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tada N., Hämmerling U. Secretion of either of a pair of immunoglobulins, IgM or IgX, in somatic hybrid cells derived by fusion of a B-cell lymphoma cell line carrying both immunoglobulin isotypes. Immunogenetics. 1980 Jul;11(1):7–19. doi: 10.1007/BF01567765. [DOI] [PubMed] [Google Scholar]
- Tada N., Kimura S., Binari R., Liu Y., Hämmerling U. New mouse immunoglobulin A heavy chain allotype specificities detected using the hybridoma-derived IgA of I/St mice. Immunogenetics. 1981;13(6):475–481. doi: 10.1007/BF00343715. [DOI] [PubMed] [Google Scholar]
- Wilson R., Miller J., Storb U. Rearrangement of immunoglobulin genes. Biochemistry. 1979 Oct 30;18(22):5013–5021. doi: 10.1021/bi00589a032. [DOI] [PubMed] [Google Scholar]
- Yaoita Y., Honjo T. Deletion of immunoglobulin heavy chain genes from expressed allelic chromosome. Nature. 1980 Aug 28;286(5776):850–853. doi: 10.1038/286850a0. [DOI] [PubMed] [Google Scholar]
- Zasloff M., Ginder G. D., Felsenfeld G. A new method for the purification and identification of covalently closed circular DNA molcules. Nucleic Acids Res. 1978 Apr;5(4):1139–1152. doi: 10.1093/nar/5.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Loo W., Gronowicz E. S., Strober S., Herzenberg L. A. Cell differentiation in the presence of cytochalasin B: studies on the "switch" to IgG secretion after polyclonal B cell activation. J Immunol. 1979 Apr;122(4):1203–1208. [PubMed] [Google Scholar]