Abstract
Medicine is an ever changing field and interventional radiology (IR) procedures are becoming increasingly popular because of high efficacy and its minimally invasive nature of the procedure. Management of disease processes in the extra cranial head and neck (ECHN) has always been a challenge due to the complex anatomy of the region. Cross sectional imaging of the ECHN has grown and evolved tremendously and occupies a pivotal and integral position in the clinical management of variety of head and neck pathologies. Advances in angiographic technologies including flat panel detector systems, biplane, and 3-dimensional rotational angiography have consolidated and expanded the role of IR in the management of various ECHN pathologies. The ECHN is at cross roads between the origins of great vessels and the cerebral vasculature. Thorough knowledge of functional and technical aspects of neuroangiography is essential before embarking on head and neck vascular interventions. The vessels of the head and neck can be involved by infectious and inflammatory conditions, get irradiated during radiotherapy and injured due to trauma or iatrogenic cause. The ECHN is also a common site for various hypervascular neoplasms and vascular malformations, which can be treated with endovascular and percutaneous embolization. This pictorial essay provides a review of variety of ECHN pathologies which were managed by various IR procedures using different approaches.
Keywords: Carotid blowout, Embolization, stent graft, sclerotherapy, vascular interventions
Introduction
Interventional Radiology (IR) has evolved rapidly in past two decades with advancement in imaging technology. There has also been significant improvement and development of more sophisticated hardware with the help of which it is possible to target any vascular territory in the body and carry out therapeutic procedures by minimally invasive technique.
The head and neck region is unique in terms of the critical anatomy and vital neurovascular structures involved. It needs detailed knowledge of the head and neck vascular anatomy and special technique to perform vascular IR procedure in extra cranial head and neck (ECHN).[1]
The pathologies where IR can offer minimally invasive treatment include vascular malformations, tumor embolization, and ECHN bleeding.
Vascular malformations
ECHN vascular malformations usually cause cosmetic as well as functional disability.[2] The slow flow type of venous and lymphatic malformations respond very well to percutaneous sclerotherapy with sclerosing agents like sodium tetradecyl sulfate (STS), polidocanol sulfate, or ethanol[1] [Figure 1]. Other abnormalities like plunging ranula, sialocele, and other benign cysts also respond well to percutaneous sclerotherapy.[3]
Figure 1 (A-F).
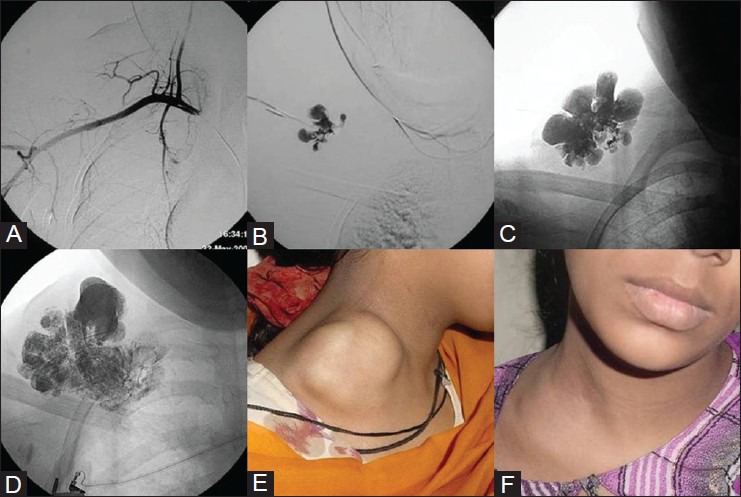
Percutaneous sclerotherapy of venous malformation. A young girl with right supraclavicular compressible swelling. (A) Right subclavian angiogram shows no vascular blush within the lesion. (B) Direct puncture contrast injection confirms venous malformation. (C, D) Images showing intralesional distribution of sclerosant mixture after percutaneous sclerotherapy. (E) Pretreatment, (F) Posttreatment photograph shows regression of the lesion. Courtesy: Dr Uday Limaye, INR Division, KEMH, Mumbai
High flow arteriovenous malformations (AVM) of ECHN present with disfigurement or episodes of bleeding. They need to be managed by multi specialty team where IR has an important role to embolize the abnormal vascularity, which can be followed by surgery to completely remove the lesion.[3,4] When AVM is extensive with involvement of bones and soft tissues, multiple sessions of embolization by transarterial route or direct puncture using liquid embolizing agents like onyx [ev3, Irvine, USA] or n-BCA (n-Butyl-2-Cynoacrylate) glue [Bbraun, Aesculap, Germany] would be required[5] [Figure 2]. In cases where transarterial approach is precluded due to previous surgical ligation of external carotid artery (ECA), percutaneous route is used for embolization. However, transarterial embolization can still be performed by gaining vascular access into one of the superficial ECA branch after surgical dissection [Figure 3].
Figure 2 (A-F).
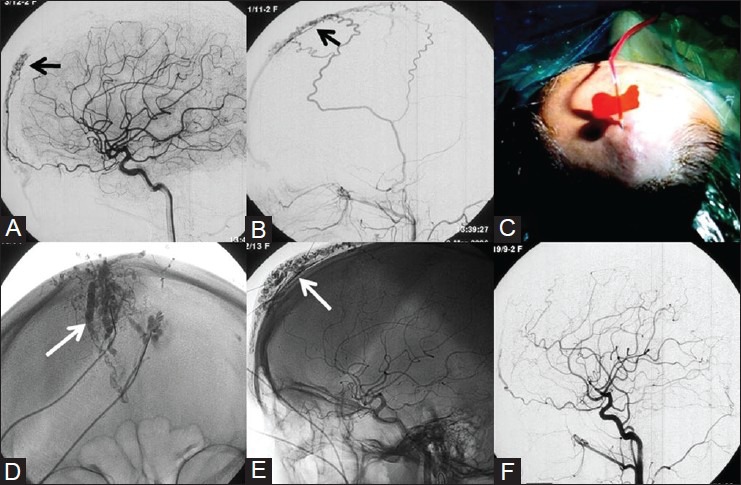
Percutaneous embolization of Scalp AVM – 34-year-old female patient with history of pulsatile swelling on her forehead. DSA images of Left ICA (A) and Left ECA (B) shows forehead AVM with feeders from the supraorbital branch of ophthalmic artery and branches from superficial temporal artery (STA). (C) Photograph showing percutaneous direct puncture of the AVM and glue embolization. (D, E) Radiograph shows glue cast (white arrow) conforming to the AVM. (F) Postemoblization angiogram shows no residual AVM. Courtesy: Dr Uday Limaye, INR Division, KEMH, Mumbai
Figure 3(A-F).
Embolization of Facial AVM. A 24-year-old female presented with facial disfigurement on the right side and bleeding from pinna with previous history of surgery for facial AVM. (A) Reformatted CT angiography, (B) Volume rendered (VR) image shows extensive soft tissue AVM with tortuous dilated vascular channels. DSA images of Right ICA (C) and Vertebral artery (D) show feeders from the ophthalmic artery and cervical branches of the vertebral artery (white arrow). Note the ligated right ECA stump (arrowhead). (E) Surgical dissection of superficial temporal artery was done to gain vascular access (arrow) and embolized using onyx injection (F) Radiograph shows the cast of onyx (dotted arrow) conforming to the AVM feeder that was embolized
Approximately, 75-95% of patients with low flow venous malformation show good results with ethanol or STS[6,7] while poor outcome is seen with diffuse AVM.[8]
Tumor embolization
ECHN tumors need embolization procedure for some highly vascular tumors like juvenile nasopharyngeal angiofibroma (JNA) and glomus tumors either preoperatively or to stop an episode of bleeding. Similarly, other tumors like hypervascular metastases, schwannomas, plasmacytomas, chordomas, and hemangiopericytomas may also need preoperative embolization.[1] Benign vascular lesions like hemangiomas can be effectively treated by embolization and surgery may be avoided [Figure 4].
Figure 4(A-H).
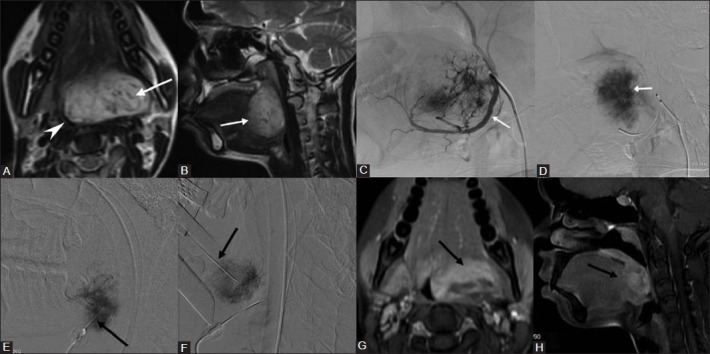
Direct puncture sclerotherapy of tongue base slow flow vascular malformation. A 21-year-old female with a large slow flow vascular malformation of the tongue base presented with dysphagia and difficulty in speech. (A) Axial, (B) Sagittal T2W images shows a hyperintense lesion (arrow) in the base of tongue causing marked narrowing of oropharynx (arrow head). (C) Arterial, (D) Venous phase of Lingual artery (arrow) angiogram shows progressively increasing and persistent vascular blush in the lesion (arrow) – suggestive of slow flow vascular malformation. Direct puncture sclerotherapy was performed through percutaneous (E) under sonographic guidance and transoral (F) route (Black arrow) using STS. Using expandable suspending laryngoscope, the base of tongue can be optimally visualized and direct puncture of the lesion can be performed by transoral route. (G) Axial, (H) Sagittal fat suppressed contrast T1W images done after 6 weeks shows significant reduction in the size and enhancement of residual malformation (arrow) with good clinical improvement in symptoms
Preoperative embolization of ECHN tumors is frequently performed by transarterial route. Preoperative embolization helps to prevent blood loss during surgery and provides a clean field for the surgical resection[1] [Figure 5]. During transarterial tumor embolization, the feeders from ECA are super selectively catheterized using micro catheter to prevent nontarget embolization. Commonly used embolizing materials are particulate embolizing agents like polyvinyl alcohol (PVA) particles, [Cook Incorporated, Bloomington, USA], embospheres [Biosphere Medical SA, Paris, France] in the size range of 100–300 microns. The aim of embolization is to inject the particles in the arteriolar capillary bed of tumor parenchyma, so that collateral circulation does not develop rapidly.[9] Coil embolization of feeding arteries is not routinely performed when open surgical resection is planned. However, as endoscopic resection of skull base tumors is being increasingly performed, proximal coil embolization of the feeding artery (e.g., internal maxillary artery coiling in case of JNA) after particulate embolization of tumor bed provides additional protection from possible avulsion of the vessel and intraoperative bleed.
Figure 5(A-C).
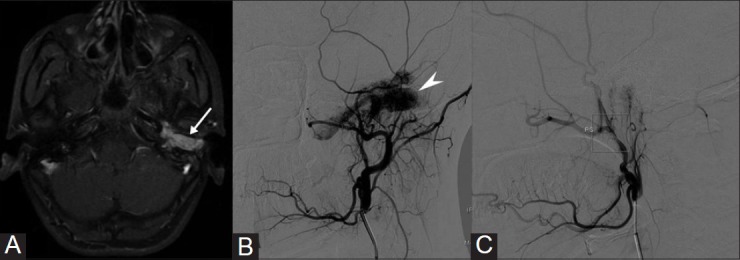
Transarterial angioembolization of Glomus tympanicum. A 16-year-old girl presented with tinnitus and reduced hearing on the left side. (A) Contrast enhanced MR image shows an intensely enhancing mass (white arrow) in the middle ear. (B) Left ECA angiogram shows intense tumor blush (arrow head) predominantly supplied by ascending pharyngeal artery which were embolized using PVA particles. (C) Post embolization ECA angiogram shows significant reduction in the tumor blush
Tumors involving the skull base frequently derive blood supply from ophthalmic or petrous branches of internal carotid artery (ICA). The ICA branches are often difficult to cannulate and there is considerable risk of reflux of the embolic agent with nontarget embolization if transarterial embolization of the ICA feeders are attempted. The territory supplied by ICA can be then be safely embolized by directly puncturing the tumor and injecting liquid embolizing agents like NBCA glue or onyx[10] [Figure 6]. The approach could be either percutaneous, transnasal, or transoral depending on the location of tumor. Though in most cases preoperative embolization is done electively a day or two prior to surgery, it can also be performed intraoperatively by direct puncture of tumor under vision and embolization under fluoroscopic guidance [Figure 7].
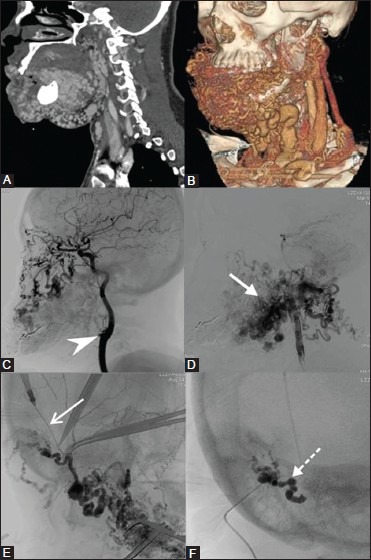
Figure 6(A-F).
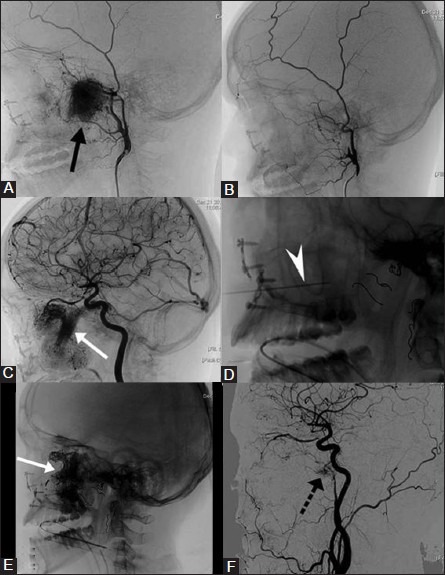
Transarterial and percutaneous embolization of recurrent juvenile nasopharyngeal angiofibroma (JNA). A 16-year-old boy with recurrent JNA was planned for preoperative embolization. (A) Preembolization right ECA angiogram shows intense tumor blush (black arrow) predominantly supplied by the internal maxillary artery (B) Postembolization ECA angiogram shows no tumor blush. (C) ICA angiogram after embolization of the external carotid artery feeders shows extensive tumor blush (arrow) supplied by cavernous and ophthalmic branches of ICA. (D) The tumor territory supplied by ICA was devascularized by direct puncture (arrow head) and glue embolization. Note the coils (dotted arrows) placed in multiple feeding arteries of ECA after embolizing the tumor bed using PVA. (E) Radiograph shows glue cast (white arrow) conforming to the tumor blush supplied by ICA feeders. (F) Postembolization ICA angiogram shows marked reduction in tumor blush with minimal residue (dotted arrow). Estimated blood loss during surgery was less than 500 cc and did not receive blood transfusion
Figure 7(A-C).
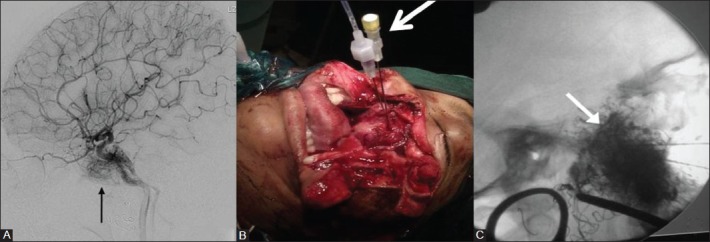
Intraoperative direct puncture embolization of JNA. (A) Post ECA embolization, ICA angiogram shows a small residual tumor blush (arrow), which was not embolized. (B) Due to excessive bleeding during surgery, Intraoperative direct puncture (arrow) and glue embolization was done under fluoroscopic guidance. (C) Radiograph showing glue cast (arrow). Postintraoperative embolization, complete surgical resection was achieved without significant bleed
Extreme care should be taken to prevent the embolizing agent from entering ophthalmic artery or vital circulation of brain. The interventional radiologist should be aware about the potentially dangerous anastomosis between the ECA and ICA and should carefully watch for opening of these collaterals during ECHN embolization.[11]
In situations where internal carotid artery is encased by the tumor, surgical resection would be difficult with high risk of injury to the artery. In such cases a balloon test occlusion (BTO) is performed to assess the ipsilateral cerebral circulation from opposite carotid or vertebra-basilar circulation. If patient tolerates BTO, the involved carotid artery can be safely occluded using coils or detachable balloons[10,11] [Figure 8].
Figure 8(A, B).
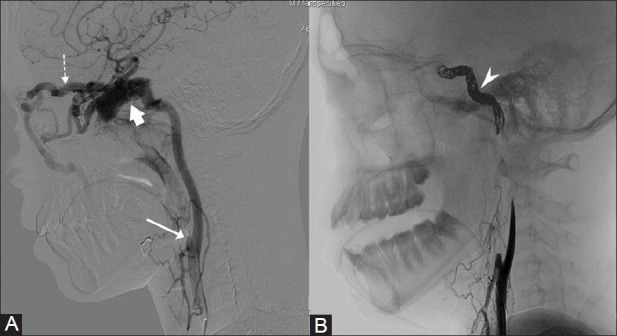
Preoperative ICA occlusion for recurrent JNA. (A) Common carotid angiogram shows ligated external carotid artery (long arrow), with residual tumor being supplied by cavernous branches of ICA (block arrow). There is early shunting of contrast into ophthalmic vein (dotted arrow). (B) Post ICA occlusion, common carotid angiogram shows no tumor blush with coil mass in the ICA. Patient had tolerated the balloon test occlusion (BTO) and intraoperative estimated blood loss was <500 cc
Overall, preoperative embolization is a safe procedure and serious complications occur in less than 2% patients that includes facial numbness, mucosal necrosis, blindness, or cerebrovascular accident.[12,13] Preoperative embolization is not only cost effective, it reduces the morbidity by reducing blood loss and operative time, shortens the hospital stay while reducing the rate of tumor recurrence.[14]
Management of head and neck bleeding
Bleeding in ECHN manifests as epistaxis, hematemesis, or bleeding from an open wound or ulcer.[15] Various causes of bleed include craniofacial injury, infections, tumors, surgery, and vascular abnormalities while medical causes like hypertension and anticoagulation therapy [Figure 9].
Figure 9(A-D).
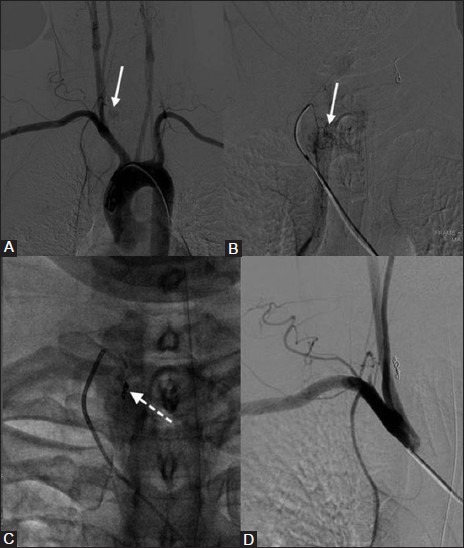
Transarterial embolization for postoperative bleed. (A) DSA image shows abnormal blush (arrow) at the site of tracheotomy site with feeders from the inferior thyroid artery. (B) Selective inferior thyroid angiography shows blush (arrow) more clearly. (C) Inferior thyroid artery feeders were embolized using 300 micon PVA particles followed by coil embolization (dotted arrow). (D) Postembolization check subclavian angiogram shows complete resolution of abnormal blush
Treatment is often variable and multidisciplinary approach is required for successful outcome. Preprocedure computed tomography (CT) angiography helps in identifying the likely source and cause of bleed while also exquisitely depicting the anatomy.[16] Subclavian, carotid, and vertebral artery angiograms are obtained to identify and target the culprit vessel. Endovascular treatment is safe and effective option in the management of these patients.[17]
Bleeding from head and neck malignancy
Endovascular treatment is increasingly being preferred in the management of bleeding from ECHN malignancies as compared with traditional open surgical approach and ligation of involved vessels.[18] Difficult postoperative anatomy, radiation effects, infections preclude safe, and effective surgery in these patients. Angioembolization has been proven to be effective with minimal risk of complications in a few reported series.[19] Goal of endovascular therapy is complete devascularization of the tumor by embolizing the tumor bed. This can be achieved by superselective catheterization of target vessels by microcatheter and embolizing with particulate embolic agents (e.g. PVA/embospheres of size 100-300 micron) [Figure 10].
Figure 10(A-F).
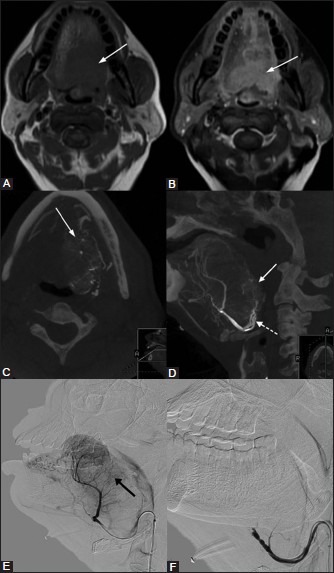
Transarterial embolization for tumor bleed. A 54–year-old male suffering for inoperatble carcinoma tongue presented with persistent tumor bleed in spite of receiving hemostatic radiotherapy. (A) Plain, (B) postcontrast axial T1W images show a large enhancing lesion (white arrow) in the tongue. (C) Axial, (D) Sagittal cone beam CT images of the lingual artery (dotted arrow) angiogram confirms tumor blush (white arrow). (E) Lingual artery angiogram shows tumor blush (black arrow), which was embolized. (F) Postembolization angiogram shows significant reduction in the tumor blush with cessation of the tumor bleed.
Carotid artery blow out
Carotid artery in ECHN is vulnerable to rupture by trauma, infection, malignancy, and iatrogenic injury. Radiotherapy to head and neck malignancy increases the risk of carotid blow out by 7.6–fold.[15] Posttraumatic and iatrogenic vascular injuries can be potentially life threatening though carotid injury is seen in less than 1% of blunt head and neck trauma.[20] Carotid blow out patient usually presents with neck swelling or bleed from nose or mouth which could be sentinel or massive. Carotid blow out can be accurately diagnosed by CT angiography and definitive treatment can be planned by knowing the exact size and location. The blow out can be seen in common carotid, internal, or ECA and imaging would reveal either a pseudoaneurysm or an active bleed.[15,16] The clinical presentation and imaging findings demands the endovascular treatment to be performed on an emergency or semi-emergency basis.
The common carotid and internal carotid artery blow out can be treated by endovascular placement of covered stent [Figures 11 and 12]. This not only achieves immediate hemostasis but also maintains patency of lumen and blood flow to the brain.[15,21,22] Patients requiring stent graft placements need to be administered with antiplatelets before and after the procedure.[15] However, if placement of stent graft becomes technically difficult, the diseased carotid artery can be occluded after confirming adequate cross flow from the contralateral carotid or vertebrobasilar circulation by BTO[15] [Figure 13]. Rarely, in case of uncontrolled massive bleeding after skull base surgery, failed attempt to deploy stent graft mandates immediate occlusion of carotid artery to prevent death from exsanguination[15] [Figure 14].
Figure 11 (A-C).
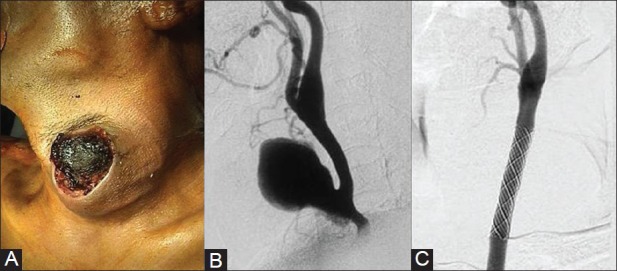
An elderly male presented with a bleeding ulcerated pulsatile swelling in the neck following trauma. (A) Photograph showing ulcerated swelling with blood clot in the neck (B) DSA of Left carotid artery shows a large pseudoaneurysm arising from CCA. (C) Carotid angiogram after stent graft placement shows exclusion of the aneurysm and maintained forward flow within the carotid artery Courtesy: Dr Uday Limaye, INR Division, KEMH, Mumbai
Figure 12 (A-C).
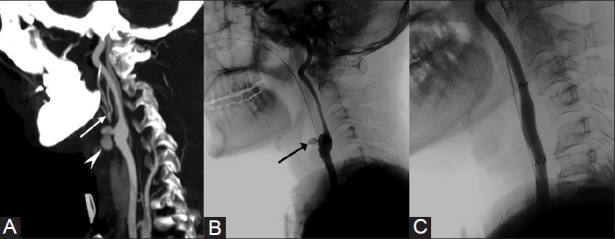
A 54-year-old male patient with history of radiotherapy to metastatic neck nodes presented with recurrent bouts of hematemesis. (A) CT angiogram sagittal reformatted image shows an irregular pseudoaneurysm (arrow head) at the carotid bifurcation with non visualization of ECA origin. Note the branches of ECA (arrow) filled by collateral circulation. (B) DSA of left CCA confirms pseudoaneurysm in the region of carotid bulb (arrow). (C) Angiogram after stent-graft placement shows complete exclusion of pseudoaneurysm from the circulation and maintained distal flow in ICA
Figure 13(A-D).
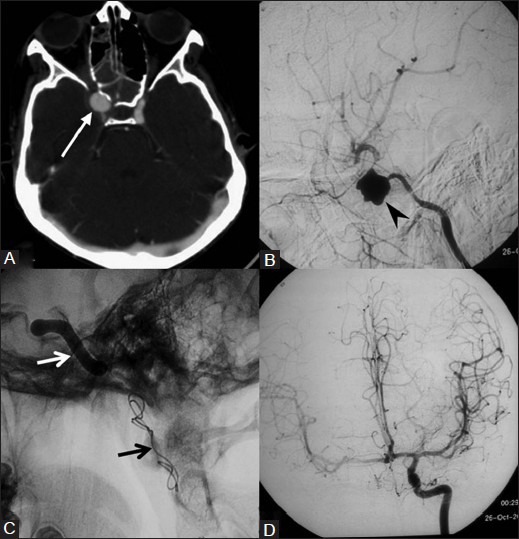
A 12-year-old girl presented with total ophthalmoplegia and epistaxis. CECT of brain (A) and DSA of right ICA (B) show an aneurysm (arrow, arrowhead) in the cavernous portion of ICA. (C) Right ICA occluded using detachable balloon (white arrow) and coils (black arrow) after BTO. (D)Postembolization left carotid angiogram shows opacification of the right cerebral hemisphere branches across the circle of Willis
Figure 14(A-F).
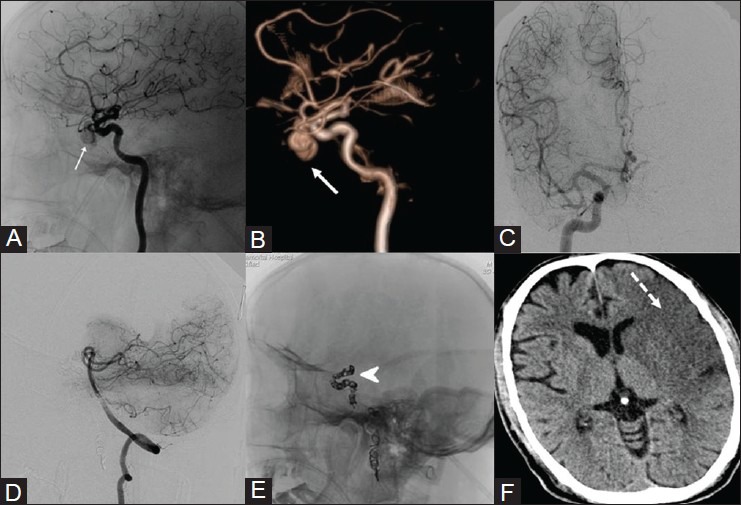
A case of nasoethmoidal carcinoma who had intraoperative injury to the left internal carotid artery during endoscopic surgery. (A) DSA, (B) VR image of left ICA shows a pseudoaneurysm (white arrow) arising from the cavernous portion. (C) Right ICA, (D) vertebral angiogram shows poor cross circulation across the circle of Willis during BTO. In view of torrential bleeding and failed attempt to deploy a covered stent, the left ICA was occluded (E) using coils (arrowhead) to prevent exsanguination. (F) Plain CT brain done after 24 hours shows fully evolved cerebral infarct (dotted arrow)
Blow out of ECA or its branch is usually treated by embolizing the culprit vessel both proximal and distal to the pseudoaneurysm [Figure 15]. The carotid artery can be permanently occluded using coils or balloons and approximately 15-20% would develop cerebral ischemia.[15]
Figure 15(A-D).
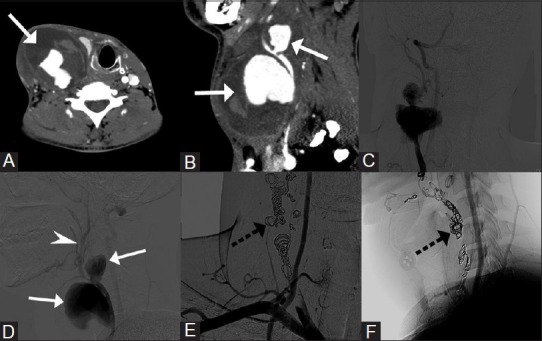
A middle-aged man presented with progressive neck swelling after attempted biopsy. (A) Axial, (B) Sagittal CT angiography images show two partially thrombosed pseudoaneurysm (Arrow) arising from the right carotid artery. (C, D) DSA image shows the pseudoaneurysms (arrow) with no opacification of the ICA and faint distal run-off of ECA branches (arrowhead). Contralateral carotid angiogram revealed good intracranial cross circulation. (E, F) The pseudo aneurysms were treated by coiling (dotted arrow) and occluding ECA as well as CCA. No neurological deficit seen postprocedure
At times, penetrating injuries could result in the formation of complex arterio-venous fistulae, which can be effectively treated by minimally invasive endovascular means using balloons and coils for flow modification[23,24] [Figure 16].
Figure 16 (A-C).
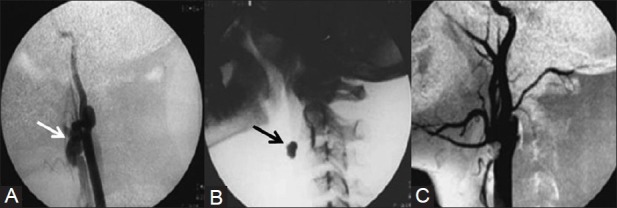
A 15-year-old boy presented with swelling in the left side of the neck with history of injury 3years back. (A) Left carotid angiogram shows fistulous communication (arrow) between ECA and internal jugular vein. Note the poor opacification of rest of ECA (B) Lateral radiograph of neck shows a detachable balloon (arrow) deployed at the site of fistula. (C) Postembolization carotid angiogram shows complete absence of fistula and well opacified ECA branches Courtesy: Dr Uday Limaye, INR Division, KEMH, Mumbai
Overall, IR can provide a wide spectrum of procedures for the effective management of various vascular ailments involving the head and neck region with a minimally invasive approach, where recovery of patient is faster and morbidity significantly less.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Gandhi D, Gemmete JJ, Ansari SA, Gujar SK, Mukherji SK. Interventional Neuroradiology of the Head and Neck. AJNR Am J Neuroradiol. 2008;29:1806–15. doi: 10.3174/ajnr.A1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowe LH, Marchant TC, Rivard DC, Scherbel AJ. Vascular malformation: Classifications and terminology the radiologist needs to know. Semin Rotengenol. 2012;47:106–17. doi: 10.1053/j.ro.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong DC, ter Brugge K. Selected interventional procedures for pediatric head and neck vascular lesions. Neuroimaging Clin N Am. 2000;10:271–92. [PubMed] [Google Scholar]
- 4.Erdmann MW, Jackson JE, Davies DM, Allison DJ. Multidisciplinary approach to the management of head and neck arteriovenous malformations. Ann R Coll Surg Engl. 1995;77:53–9. [PMC free article] [PubMed] [Google Scholar]
- 5.Arat A, Cil BE, Vargel I, Turkbey B, Canyigit M, Peynircioglu B, et al. Embolization of high-flow craniofacial vascular malformations with onyx. AJNR Am J Neuroradiol. 2007;28:1409–14. doi: 10.3174/ajnr.A0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berenguer B, Burrows PE, Zurakowski D, Mulliken JB. Sclerotherapy of craniofacial venous malformations: Complications and results. Plast Reconstr Surg. 1999;104:1–11. [PubMed] [Google Scholar]
- 7.Cabrera J, Cabrera J, Jr, Garcia-Olmedo MA, Redondo P. Treatment of venous malformations with sclerosant in microfoam form. Arch Dermatol. 2003;139:1409–16. doi: 10.1001/archderm.139.11.1409. [DOI] [PubMed] [Google Scholar]
- 8.Ryu CW, Whang SM, Suh DC, Kim SM, Jang YJ, Kim HJ, et al. Percutaneous Direct Puncture Glue Embolization of High-Flow Craniofacial Arteriovenous Lesions: A New Circular Ring Compression device with a beveled edge. AJNR Am J Neuroradiol. 2007;28:528–30. [PMC free article] [PubMed] [Google Scholar]
- 9.Wakhloo AK, Juengling FD, Van Velthoven V, Schumacher M, Hennig J, Schwechheimer K. Extended preoperative polyvinyl alcohol microembolization of intracranial meningiomas: Assessment of two embolization techniques. AJNR Am J Neuroradiol. 1993;14:571–82. [PMC free article] [PubMed] [Google Scholar]
- 10.Valavanis A. Preoperative embolization of the head and neck: Indications, patient selection, goals, and precautions. AJNR Am J Neuroradiol. 1986;7:943–52. [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez Higueras A, Saura Lorente P, García Alonso J, de las Heras García JA. Diagnosis and treatment of head and neck paragangliomas. Uses of angiography and interventional radiology. Acta Otorrinolaringol Esp. 2009;60:53–67. [PubMed] [Google Scholar]
- 12.Gruber A, Bavinzski G, Killer M, Richling B. Preoperative embolization of hypervascular skull base tumors. Minim Invasive Neurosurg. 2000;43:62–71. doi: 10.1055/s-2000-8321. [DOI] [PubMed] [Google Scholar]
- 13.Tseng EY, Narducci CA, Willing SJ, Sillers MJ. Angiographic embolization for epistaxis: A review of 114 cases. Laryngoscope. 1998;108:615–9. doi: 10.1097/00005537-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Ungkanont K, Byers RM, Weber RS, Callender DL, Wolf PF, Goepfert H. Juvenile nasopharyngeal angiofibroma: An update of therapeutic management. Head Neck. 1996;18:60e6. doi: 10.1002/(SICI)1097-0347(199601/02)18:1<60::AID-HED8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 15.Chaloupka JC, Putman CM, Citardi MJ, Ross DA, Sasaki CT. Endovascular therapy for the carotid blowout syndrome in head and neck surgical patients: Diagnostic and managerial considerations. AJNR Am J Neuroradiol. 1996;17:843–52. [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman DN, Hoh BL, Rabinov JD, Pryor JC. CT angiography before embolization for hemorrhage in head and neck cancer. AJNR Am J Neuroradiol. 2003;24:140–2. [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PJ, Kjeldsen AD, Nepper-Rasmussen J. Selective embolization in the treatment of intractable epistaxis. Acta Otolaryngol. 2005;125:293–7. doi: 10.1080/00016480410023029. [DOI] [PubMed] [Google Scholar]
- 18.Morrissey DD, Andersen PE, Nesbit GM, Barnwell SL, Everts EC, Cohen JI. Endovascular management of hemorrhage in patients with head and neck cancer. Arch Otolaryngol Head Neck Surg. 1997;123:15–9. doi: 10.1001/archotol.1997.01900010017002. [DOI] [PubMed] [Google Scholar]
- 19.Sittel C, Gossmann A, Jungehülsing M, Zähringer M. Superselective embolization as palliative treatment of recurrent hemorrhage in advanced carcinoma of the head and neck. Ann Otol Rhinol Laryngol. 2001;110:1126–8. doi: 10.1177/000348940111001208. [DOI] [PubMed] [Google Scholar]
- 20.Miller PR, Fabian TC, Croce MA, Cagiannos C, Williams JS, Vang M, et al. Prospective screening for blunt cerebrovascular injuries: Analysis of diagnostic modalities and outcomes. Ann Surg. 2002;236:386–93. doi: 10.1097/01.SLA.0000027174.01008.A0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang FC, Lirng JF, Luo CB, Guo WY, Teng MM, Tai SK, et al. Carotid blowout syndrome in patients with head-and-neck cancers: Reconstructive management by self-expandable stent-grafts. AJNR Am J Neuroradiol. 2007;28:181–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Dubose J, Recinos G, Teixeira PG, Inaba K, Demetriades D. Endovascular stenting for the treatment of traumatic internal carotid injuries: Expanding experience. J Trauma. 2008;65:1561–6. doi: 10.1097/TA.0b013e31817fd954. [DOI] [PubMed] [Google Scholar]
- 23.Santhosh J, Rao VR, Ravimandalam K, Gupta AK, Unni NM, Rao AS. Endovascular management of carotid cavernous fistulae: Observation on angiographic and clinical results. Acta Neurol Scand. 1993;88:320–6. doi: 10.1111/j.1600-0404.1993.tb05351.x. [DOI] [PubMed] [Google Scholar]
- 24.Gobin YP, Garcia de la Fuente JA, Herbreteau D, Houdart E, Merland JJ. Endovascular treatment of external carotid-jugular fistulae in the parotid region. Neurosurgery. 1993;33:812–6. doi: 10.1227/00006123-199311000-00004. [DOI] [PubMed] [Google Scholar]


