Abstract
Background: Patients with Turner syndrome (TS) are prone to having metabolic abnormalities, such as obesity, dyslipidemia, hypertension, hyperinsulinemia and type 2 diabetes mellitus, resulting in increased risks of developing atherosclerotic diseases. Objective: To determine the effect of growth hormone (GH) therapy on serum cholesterol levels in prepubertal girls with TS enrolled in the Turner syndrome Research Collaboration (TRC) in Japan. Patients and methods: Eighty-one girls with TS were enrolled in the TRC, and their total cholesterol (TC) levels before GH therapy were compared with reported levels of healthy school-aged Japanese girls. TC levels after 1, 2 and 3 yr of GH treatment were available for 28 of the 81 patients with TS. GH was administered by daily subcutaneous injections, 6 or 7 times/wk, with a weekly dose of 0.35 mg/kg body weight. Results: Baseline TC levels revealed an age-related increase in TS that was in contrast to healthy girls showing unchanged levels. During GH therapy, TC decreased significantly after 1 yr of GH treatment and remained low thereafter. Conclusions: Girls with untreated TS showed an age-related increase in TC that was a striking contrast to healthy girls, who showed unchanged levels. GH therapy in girls with TS brought about a favorable change in TC that indicates the beneficial impact of GH on atherogenic risk.
Keywords: Turner syndrome, growth hormone therapy, serum cholesterol
Introduction
Turner syndrome (TS) is the result of complete or partial X chromosome monosomy in a phenotypic female and is associated with short stature, gonadal dysgenesis and a number of congenital morphological abnormalities, with an approximate incidence of 1 in 2,000–2,500 female live births (1,2,3). As for short stature, it is well established that growth hormone (GH) therapy is effective in increasing adult height (4, 5).
There is, however, no study about the metabolic consequences of GH treatment in Japanese girls with TS. The increased prevalence of coronary heart disease in TS has been attributed to ovarian failure, causing loss of the estrogen effect and premature adiposity (6, 7). There is excess cardiovascular death, and this is thought to be due to ischemic heart disease and coronary artery disease derived from metabolic abnormalities in addition to aortic dissection/rupture due to congenital anomalies (8). This is associated with reduced life expectancy of up to 13 yr and increased overall morbidity and mortality, predominantly as a result of cardiovascular complications (9, 10).
The purpose of this study was to determine the effect of GH therapy on cholesterol levels in prepubertal patients with TS without estrogen therapy enrolled in the Turner syndrome Research Collaboration (TRC) in Japan.
Subjects and Methods
A total of 196 girls with TS were registered in the TRC in Japan from 2000 through 2004. Of them, 105 girls were not formerly treated with GH. Eighty-one of these 105 patients, aged 10.1 ± 3.6 yr (range 2.5–18.5), were prepubertal and had no estrogen replacement. Twenty-eight of these 81 girls, aged 7.6 ± 2.6 yr (range 4.4–12.1), had complete data for TC before and during 1, 2 and 3 yr of GH therapy. The TC values of the 81 patients were compared with those of healthy school-aged Japanese girls extracted from the large amount of nationwide data provided by the Japan Association of Health Service, which carried out a survey from 1993 through 1999 in healthy children, aged 9 to 16 yr, in 19 prefectures out of a total of 47 prefectures in Japan (11). We also evaluated the effect of GH on TC in 28 patients for 3 yr. They showed no pubertal development and had no estrogen replacement during the entire course of the study.
GH was administered by daily subcutaneous injection, 6 or 7 times/wk, with a weekly dose of 0.35 mg/kg body weight.
Responses to GH treatment of TC levels were analyzed by analysis of variance for repeated measures. When the F value was significant (p<0.05), pairs of time periods of treatment were compared by Dunnett’s t-test. Results are presented as means ± SD. A p value less than 0.05 was considered significant.
The Turner syndrome Research Collaboration (TRC) is an open-label, multicenter, observational study established as a post-marketing research program to evaluate the long-term efficacy and safety of Growject GH products (JCR Pharmaceuticals Co., Ltd., Ashiya, Hyogo, Japan). The TRC complies with the requirements of post-marketing surveillance studies in Japan. As the TRC is a post-marketing research program, data is collected only as provided by the attending physician. JCR Pharmaceuticals Co., Ltd. is responsible to ensure that data collection and data handling comply with Japanese regulatory requirements. Informed consent is required for data collection and agreement to anonymous use of the data.
Results
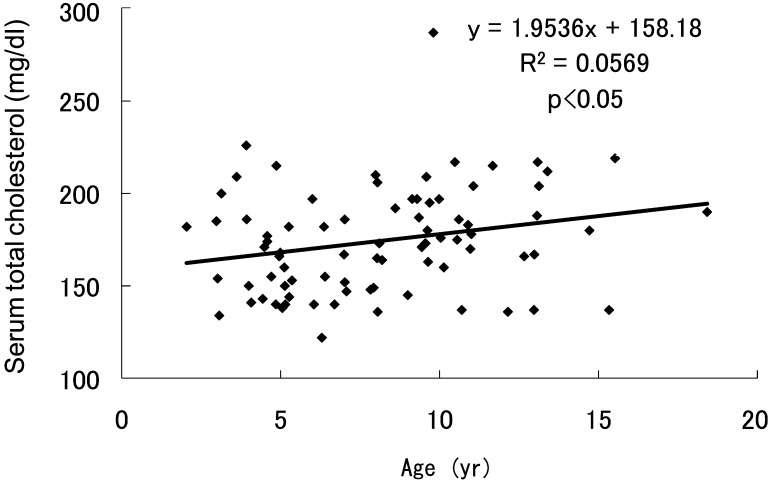
TC levels before GH treatment in the 81 patients with TS revealed a significant age-related increase (Fig. 1) that was in contrast to the values in healthy girls, who showed no significant changes and values of approximately 170 mg/dl (range 167–171) up to 14 yr of age (11).
Fig. 1.
Serum total cholesterol levels of prepubertal girls with Turner syndrome without GH treatment.
The karyotype distribution in the 28 patients with complete data for TC during the study is shown in Table 1. Anthropometric data of this group at baseline and during GH therapy are shown in Table 2. The mean height gain during 3 yr of GH treatment was 17.3 cm, which indicated a significant improvement in the height standard deviation score (HSDS) of 1.2 according to the TS height standard in Japan. There were no significant changes in the body mass index in spite of a linear increase in body weight.
Table 1. Distribution of karyotype (n=28).
| Karyotype | n (%) |
| 45,X | 8 (28.6) |
| 45,X/46, X,I (Xq) | 5 (17.9) |
| 45,X/46,r (X) (p22.3q24) | 2 (7.1) |
| 45,X/46,X,idic (X) (p11) | 2 (7.1) |
| 45,X/47,XXX | 2 (7.1) |
| Others | 9 (32.1) |
Table 2. Anthropometric data of subjects before and after GH treatment (n=28).
| At start | After 1 yr | After 2 yr | After 3 yr | |
| Age (yr) | 7.6 ± 2.6 | 8.5 ± 2.6 | 9.5 ± 2.6 | 10.5 ± 2.7 |
| Height (cm) | 105.7 ± 13.0 | 112.0 ± 12.8* | 117.7 ± 13.0* | 123.0 ± 12.9* |
| HSDS | 0.0 ± 1.0 | 0.6 ± 0.9* | 0.9 ± 0.9* | 1.2 ± 0.9* |
| Body weight (kg) | 19.6 ± 7.2 | 21.8 ± 7.4* | 24.3 ± 7.7* | 28.0 ± 9.3* |
| BMI | 17.0 ± 3.2 | 16.9 ± 3.0 | 17.1 ± 2.2 | 18.0 ± 2.9 |
HSDS, height standard deviation score; BMI, body mass index. *: p<0.01 (vs at start).
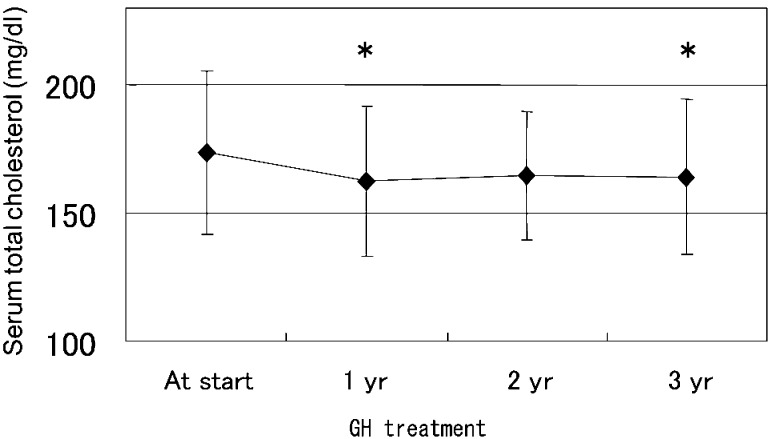
During GH therapy, TC decreased significantly from 173.7 ± 32.2 to 162.3 ± 29.2 mg/dl after 1 yr of treatment and remained low thereafter (Fig. 2). These results were clearly different from the age-related increasing trend of TC at baseline in our TS group.
Fig. 2.
Mean serum total cholesterol levels of girls with Turner syndrome before and during GH treatment. *: p<0.05 (comparison with before GH therapy by Dunnett’s t-test).
Discussion
The present results clearly demonstrated that TC levels in TS tended to rise with chronological age. This result contrasts markedly with the trend in healthy Japanese girls, who showed no significant changes in TC and values of approximately 170 mg/dl (11). The increasing levels of TC with age in prepubertal TS were improved during 3 yr of GH therapy in our subjects, indicating the favorable impact of GH on atherogenic risk.
Adults with TS are at increased risk of ischemic coronary heart disease and cardiovascular death due to metabolic abnormalities, such as hyperlipidemia, hypertension, insulin resistance, type 2 diabetes mellitus and obesity. Epidemiological data indicate that metabolic syndrome is a frequent and cardinal feature in adults with TS (6,7,8).
It has been reported that in children and adolescents with TS, aged 5 to 14 yr, those in the adolescent group (11–14.9 yr) had 25 mg/dl higher levels of TC than in girls before adolescence (5–10.9 yr) (12). Adolescent girls with TS, in addition, had significantly elevated TC levels compared with age-matched normal values (12). Risk factors for arteriosclerotic diseases or a predisposition for the development of such factors may be present early in childhood and adolescence. Ovarian failure, resulting in no significant production of estrogen, which lowers lipid levels and has a cardioprotective effect in girls, may be a potential cause of lipid abnormalities in TS.
On the other hand, a recent study indicated that up to 50% of adults with TS (median age 21 yr) who were receiving some form of estrogen replacement therapy and were euthyroid had hypercholesterolemia (13). In addition, adult women with TS exhibited a distinctly more atherogenic lipid profile of high LDL cholesterol and triglyceride levels than 46XX women with ovarian failure, suggesting that a factor other than sex steroids contributes to a favorable lipid profile (14).
In the natural course, patients with TS are prone to have the abnormalities constituting metabolic syndrome, e.g., obesity, dyslipidemia, hypertension, hyperinsulinemia and type 2 diabetes mellitus, resulting in an increased risk of developing coronary artery disease (6, 7, 15). GH therapy in TS increased lean body mass and decreased adiposity and TC (16, 17), as shown in our study, as well as LDL cholesterol and the atherogenic index (TC/HDL cholesterol) (18). GH may be an additional cardioprotective factor in TS that is independent of estrogen.
As for GH secretory dynamics in TS, previous studies have reported both normal (1, 3, 19,20,21) and insufficient spontaneous and stimulated GH secretion (22,23,24,25,26). Insufficient secretory dynamics, not deficiency, of GH in TS, which may be due to insufficient estrogen replacement or insufficient secretion of 24-h integrated serum GH, may be an issue to be investigated to clarify which factor contributes to a more favorable lipid profile independent of estrogen effects.
In conclusion, this study demonstrated that increasing levels of TC with age in prepubertal girls with TS improved during 3 yr of GH therapy. The favorable change in TC indicates the beneficial impact of GH on atherogenic risk in these patients.
Acknowledgments
The authors wish to thank all the doctors who have participated in the TRC.
This study was sponsored by JCR Pharmaceuticals Co., Ltd.
References
- 1.Saenger P, Albertsson Wikland K, Conway GS, Davenport M, Gravholt CH, Hintz R, et al. Recommendations for the diagnosis and management of Turner syndrome. J Clin Endocrinol Metab 2001;86: 3061–9 [DOI] [PubMed] [Google Scholar]
- 2.Sybert VP, McCauley E. Turner’s syndrome. New Engl J Med 2004;351: 1227–38 [DOI] [PubMed] [Google Scholar]
- 3.Bondy CA, Turner Syndrome Consensus Study GroupCare of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab 2007;92: 10–25 [DOI] [PubMed] [Google Scholar]
- 4.Stephure DK, The Canadian Growth Hormone Advisory CommitteeImpact of growth hormone supplementation on adult height in Turner syndrome: results of the Canadian randomized controlled trial. J Clin Endocrinol Metab 2005;90: 3360–6 [DOI] [PubMed] [Google Scholar]
- 5.Takano K, Ogawa M, Tanaka T, Tachibana K, Fujita K, Hizuka N, and the members of the committee for the treatment of Turner’s syndrome. Clinical trials of GH treatment in patients with Turner’s syndrome in Japan –a consideration of final height. Eur J Endocrinol 1997;137: 138–45 [DOI] [PubMed] [Google Scholar]
- 6.Gravholt CH, Juul S, Naeraa RW, Hansen J. Morbidity in Turner syndrome. J Clin Epidemiol 1998;51: 147–58 [DOI] [PubMed] [Google Scholar]
- 7.Elsheikh M, Conway GS. The impact of obesity on cardiovascular risk factors in Turner’s syndrome. Clin Endocrinol (Oxf) 1998;49: 447–50 [DOI] [PubMed] [Google Scholar]
- 8.Schoemaker MJ, Swerdlow AJ, Higgins CD, Wright AF, Jacobs PA, United Kingdom Clinical Cytogenetics GroupMortality in women with Turner syndrome in Great Britain: a national cohort study. J Clin Endocrinol Metab 2008;93: 4735–42 [DOI] [PubMed] [Google Scholar]
- 9.Price WH, Clayton JF, Collyer S, De Mey R, Wilson J. Mortality ratios, life expectancy, and causes of death in patients with Turner’s syndrome. J Epidemiol Community Health 1986;40: 97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab 2006;91: 3897–902 [DOI] [PubMed] [Google Scholar]
- 11.Okada T, Murata M, Yamauchi K, Harada K. New criteria of normal serum lipid levels in Japanese children: the nationwide study. Pediatr Int 2002;44: 596–601 [DOI] [PubMed] [Google Scholar]
- 12.Ross JL, Feuillan P, Long LM, Kowal K, Kushner H, Cutler GB., Jr.Lipid abnormalities in Turner syndrome. J Pediatr 1995;126: 242–5 [DOI] [PubMed] [Google Scholar]
- 13.Garden AS, Diver MJ, Fraser WD. Undiagnosed morbidity in adult women with Turner’s syndrome. Clin Endocrinol (Oxf) 1996;45: 589–93 [DOI] [PubMed] [Google Scholar]
- 14.Van PL, Bakalov VK, Bondy CA. Monosomy for the X-chromosome is associated with an atherogenic lipid profile. J Clin Endocrinol Metab 2006;91: 2867–70 [DOI] [PubMed] [Google Scholar]
- 15.Ostberg JE, Thomas EL, Hamilton G, Attar MJH, Bell JD, Conway GS. Excess visceral and hepatic adipose tissue in Turner syndrome determined by magnetic resonance imaging: estrogen deficiency associated with hepatic adipose content. J Clin Endocrinol Metab 2005;90: 2631–5 [DOI] [PubMed] [Google Scholar]
- 16.Leger J, Carel C, Legrand I, Paulsen A, Hassan M, Czernichow P. Magnetic resonance imaging evaluation of adipose tissue and muscle tissue mass in children with growth hormone (GH) deficiency, Turner’s syndrome, and intrauterine growth retardation during the first year of treatment with GH. J Clin Endocrinol Metab 1994;78: 904–9 [DOI] [PubMed] [Google Scholar]
- 17.Gravholt CH, Naeraa RW, Brixen K, Kastrup KW, Mosekilde L, Jorgensen JOL, et al. Short-term growth hormone treatment in girls with Turner syndrome decreases fat mass and insulin sensitivity: a randomized, double-blind, placebo-controlled, crossover study. Pediatrics 2002;110: 889–96 [DOI] [PubMed] [Google Scholar]
- 18.Van Pareren YK, de Muinck Keizer-Schrama SMPF, Stijnen T, Sas TCJ, Drop SLS and the Dutch Advisory Group on Growth Hormone. Effect of discontinuation of long-term growth hormone treatment on carbohydrate metabolism and risk factors for cardiovascular disease in girls with Turner syndrome. J Clin Endocrinol Metab 2002;87: 5442–8 [DOI] [PubMed] [Google Scholar]
- 19.Saenger P. Turner’s syndrome. N Engl J Med 1996;335: 1749–54 [DOI] [PubMed] [Google Scholar]
- 20.Ranke MB, Blum WF, Haug F, Rosendahl W, Attanasio A, Enders H, et al. Growth hormone somatomedin levels and growth regulation in Turner’s syndrome. Acta Endocrinol 1987;116: 305–13 [DOI] [PubMed] [Google Scholar]
- 21.Wit JM, Massarano AA, Kamp GA, Hindmarsh PC, van Es A, Brook CG, et al. Growth hormone secretion in patients with Turner’s syndrome as determined by time series analysis. Acta Endocrinol 1992;127: 7–12 [DOI] [PubMed] [Google Scholar]
- 22.Kohno H, Honda S. Low urinary growth hormone values in patients with Turner’s syndrome. J Clin Endocrinol Metab 1992;74: 619–22 [DOI] [PubMed] [Google Scholar]
- 23.Gravholt CH, Naeraa RW, Brixen K, Kastrup KW, Mosekilde L, Jorgensen JOL, et al. Short-term growth hormone treatment in girls with Turner syndrome decreases fat mass and insulin sensitivity: a randomized, double-blind, placebo-controlled, crossover study. Pediatrics 2002;110: 889–96 [DOI] [PubMed] [Google Scholar]
- 24.Pirazzoli P, Mazzanti L, Bergamaschi R, Perri A, Scarano E, Nanni S, et al. Reduced spontaneous growth hormonhe secretionb in patients with Turner’s syndrome. Acta Paediatr 1999;88: 610–3 [DOI] [PubMed] [Google Scholar]
- 25.Gicquel C, Gaston V, Cabrol S, Bouc YL. Assessment of Turner’s syndrome by molecular analysisi of the X chromosome in growth-retarded girls. J Clin Endocrinol Metab 1998;83: 1472–6 [DOI] [PubMed] [Google Scholar]
- 26.Schmitt K, Haeusler G, Blumel P, Plochl E, Frisch H. Short- and long-term (final height) growth responses to growth hormone (GH) therapy in patients with Turner syndrome: correlation of growth response to stimulated GH levels, spontaneous GH secretion, and karyotype. Horm Res 1997;47: 67–72 [DOI] [PubMed] [Google Scholar]