Abstract
Regenerative medicine is a rapidly evolving multidisciplinary, translational research enterprise whose explicit purpose is to advance technologies for the repair and replacement of damaged cells, tissues, and organs. Scientific progress in the field has been steady and expectations for its robust clinical application continue to rise. The major thesis of this review is that the pharmacological sciences will contribute critically to the accelerated translational progress and clinical utility of regenerative medicine technologies. In 2007, we coined the phrase “regenerative pharmacology” to describe the enormous possibilities that could occur at the interface between pharmacology, regenerative medicine, and tissue engineering. The operational definition of regenerative pharmacology is “the application of pharmacological sciences to accelerate, optimize, and characterize (either in vitro or in vivo) the development, maturation, and function of bioengineered and regenerating tissues.” As such, regenerative pharmacology seeks to cure disease through restoration of tissue/organ function. This strategy is distinct from standard pharmacotherapy, which is often limited to the amelioration of symptoms. Our goal here is to get pharmacologists more involved in this field of research by exposing them to the tools, opportunities, challenges, and interdisciplinary expertise that will be required to ensure awareness and galvanize involvement. To this end, we illustrate ways in which the pharmacological sciences can drive future innovations in regenerative medicine and tissue engineering and thus help to revolutionize the discovery of curative therapeutics. Hopefully, the broad foundational knowledge provided herein will spark sustained conversations among experts in diverse fields of scientific research to the benefit of all.
I. Introduction to Regenerative Pharmacology
Historically, small molecule (i.e., compounds of <500–800 mol. wt.) pharmaceutical research and development has focused on compounds with increasingly selective mechanisms of action. This makes sense from a symptom-based approach to the treatment of disease, wherein one wishes to focus on the primary mechanism of action required for drug efficacy while simultaneously limiting off-target effects and minimizing adverse events/side effects. The development requirements for regenerative pharmacology will be much more demanding. In fact, the challenges associated with regenerative pharmacology, that is, curative therapeutics, will in many instances require complex mixtures of compounds [i.e., growth factors such as fibroblast growth factor (FGF), epidermal growth factor (EGF), platelet-derived growth factor, nerve growth factor (NGF), vascular endothelial growth factor (VEGF), insulin-like growth factor (IGF), bone morphogenic proteins (BMPs), etc.] for restoration of tissue/organ function. These latter compounds have significantly higher molecular weights (generally ≈10,000 to >100,000 mol. wt.) than those traditionally developed by the pharmaceutical industry.
In this article, we attempt to pull together a rather vast amount of scientific and technical information from increasingly intersecting interdisciplinary fields of research to emphasize the significant role that pharmacologists can play in developing curative therapeutics. So, what are the potential implications of regenerative pharmacology? Imagine the day when:
Drugs can be targeted to specific nuclei in the brain (e.g., the center affected in Parkinson’s Disease) or any desired region(s) of organs/tissues to exert local therapeutic or healing effects without untoward side effects;
Multiple bioactive compounds can be loaded into a sophisticated drug delivery system(s) that is locally placed to orchestrate a complete functional regenerative response;
One can sufficiently recapitulate the complexity of the internal milieu to permit new functional tissue and organ formation in vitro for subsequent implantation in vivo.
In his recent State of the Union address President Obama alluded to the crucial impact of such efforts on scientific innovation:
“If we want to make the best products, we also have to invest in the best ideas. Every dollar we invested to map the human genome returned $140 to our economy. Today, our scientists are mapping the human brain to unlock the answers to Alzheimer’s; developing drugs to regenerate damaged organs; devising new material to make batteries ten times more powerful. Now is not the time to gut these job-creating investments in science and innovation.” (Read more: http://www.whitehouse.gov/state-of-the-union-2013.)
A major goal of this report is to emphasize that the success of such an effort will be accelerated by the rigorous application of the pharmacological sciences. We currently lack a broad knowledge of the complex pharmacology of mammalian wound healing and functional regeneration. Correction of this knowledge gap demands a global multidisciplinary, collaborative research and effort to stimulate the conversations that must occur at the intersections of pharmacology, biomaterials, biomedical/tissue engineering, nanotechnology, stem cell and developmental biology, etc. We believe that the conceptual framework and scientific foundations, as well as many of the technologies required for success, are already in place or being developed, but the effort is not organized and the necessary conversations are not happening. We hope that the readers of this report will grasp the considerable value of this effort and form the sustained alliances and collaborations required to begin the journey.
However, before launching into a comprehensive discussion of regenerative pharmacology and its central role in the continued development of regenerative medicine technologies, as outlined in Fig. 1, it is important to provide some fundamental background information about the nature of tissue/organ regeneration and the current status of regenerative medicine technologies.
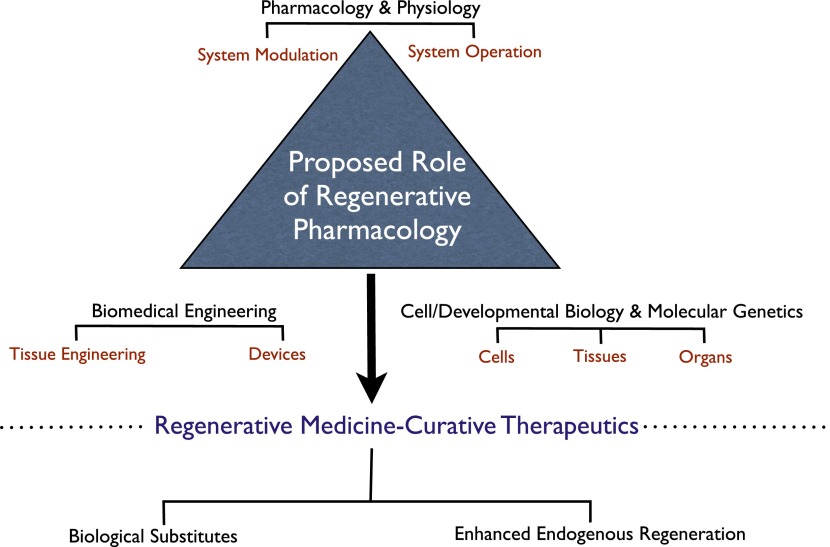
Fig. 1.
Central role of regenerative pharmacology in the development of regenerative medicine technologies and curative therapies. The schematic depicts regenerative pharmacology at the intersection of the classic scientific disciplines traditionally associated with regenerative medicine. Knowledge of biologic system operation (i.e., physiology) leads naturally to system modulation (i.e., pharmacology). This connection underpins traditional small molecule drug therapy, which seeks primarily to ameliorate pathologic symptoms arising from aging and disease. Regenerative pharmacology encompasses a distinct paradigm in that novel technologies arise from contributions to the traditional physiology-pharmacology axis provided by 1) biomedical engineering and 2) an understanding of normal cell and developmental biology and molecular genetics. The synergistic interaction of these disciplines enables the creation of novel technologies to enhance regeneration in vivo or to enable de novo tissue and organ engineering (production of “biological substitutes”) in vitro. The central goal of regenerative pharmacology is to develop potentially curative therapeutics. In this endeavor engineered biologic constructs may serve several purposes. First, they provide tools to determine the etiology of degenerative tissue and organ dysfunction and to identify novel therapeutics. The ability to produce individualized constructs, enabled by induced pluripotent stem cells, will move this approach into the realm of personalized medicine. Advances in miniaturization and the adaptation of engineered biologic systems created by regenerative medicine technologies to high-throughput platforms (i.e., “organs on a chip”) also may usher in a new age in drug development. Finally, the engineered biologic substitutes themselves may serve as therapeutics, capable of reconstituting normal tissue and organ functions when implanted into patients.
A. Regeneration and Regenerative Medicine.
Tissue and organ regeneration occurs throughout the animal kingdom, and this phenomenon has understandably captured the scientific imagination for hundreds of years (Nachtrab and Poss; 2012). There are large disparities in regenerative capacity both between species (e.g., amphibian versus mammalian) and among organs (e.g., liver versus kidney). Exploration of these differences has offered insights regarding the mechanistic basis of regeneration and the diminished or apparently absent regenerative potential in certain systems, including many human tissues (Stocum, 2002; Taub, 2004; Sanchez Alvarado and Tsonis, 2006; Stocum and Cameron, 2011; Baddour et al., 2012). In this scenario, the extensive attention focused on regenerative medicine is understandable given the potential for repair or replacement of old, missing, damaged or diseased cells, tissues, and organs. In fact, regenerative medicine technologies are specifically developed for this purpose. The complexity of endogenous regeneration, the relatively limited mammalian capacity for regeneration, and the vast shortages of donor organs coupled with the seemingly ever-increasing life span of humans have combined to create a huge demand for regenerative medicine.
The goal of regenerative medicine can be concisely codified as the repair and/or replacement of damaged cells, tissues, and organs for functional restoration. It is a global, interdisciplinary effort with a translational research focus on development of therapies for patients afflicted with a variety of age- and disease-related disorders/dysfunction. Regenerative medicine (RM) and its companion field tissue engineering (TE) have provided a variety of current technologies for functional tissue/organ restoration, and these approaches have been described in detail in numerous publications (Freed et al., 2006; Mikos et al., 2006; Grayson et al., 2009; Corona et al., 2010; Atala et al., 2011; Badylak et al., 2012), and thus, only the most salient aspects are discussed herein. Figure 2 provides a general conceptual framework for many aspects of the TE/RM process.
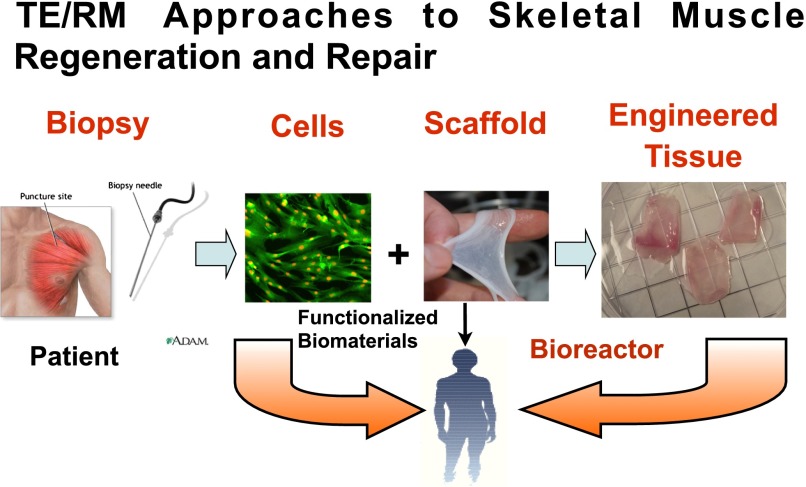
Fig. 2.
Regenerative medicine and tissue engineering approaches to functional tissue restoration, illustrated for striated muscle. Stem or progenitor cells from an appropriate source, in this case a skeletal muscle biopsy, are expanded in culture to provide the requisite starting cells of correct phenotype for the target tissue or organ of interest. Cells may then be injected systemically or applied directly to the site of injury (i.e., cell therapy). Alternatively, the cells may be combined with a scaffold, either naturally derived or synthetic, to yield a tissue engineered construct. Maturation and conditioning of the construct may be achieved by incubation in a bioreactor prior to implantation in the body. For example, a period of exposure to unidirectional stretch improves functionality of skeletal muscle constructs (Moon et al., 2008; Machingal et al., 2011; Corona et al., 2012). As described in the text, functionalized biomaterials may also be directly implanted for tissue or organ restoration.
B. Overview of Current Regenerative Strategies.
Regardless of the precise strategy used for reconstruction, restoration, or repair of the tissue/organ of interest, cells and biomaterials (i.e., scaffolds) provide the basic constituents required for creating new tissue; they represent the “raw materials” from which tissues and organs are built. However, the exact TE/RM approach taken will necessarily depend on the degree of tissue/organ dysfunction. For example, if sufficient tissue/organ viability remains in vivo, then either cells alone (i.e., cell therapy) or scaffolds alone (biomaterial therapy) may be adequate to provide the required regenerative response. Such an approach is feasible at this point in the disease process, as it may still be possible to leverage existing endogenous mechanisms for relatively complete tissue repair and/or restoration of organ function. In contrast, when there is a dearth of viable tissue remaining, as in many cases of traumatic injury and in many congenital and acquired conditions, the degree of end organ dysfunction may be so great that it exceeds the endogenous regenerative capacity of the organ or tissue. In this scenario, any remaining endogenous repair mechanisms will require much greater augmentation via the implementation of TE strategies that produce more fully developed native-like tissue/organ biomimetics (i.e., biologic substitutes), up to and including whole organ replacement—including biomaterial strategies such as whole knee or hip replacements. One method commonly contemplated for creation and maturation of engineered tissues/organs in vitro involves utilization of bioreactor technologies. Bioreactors are laboratory devices that recapitulate relevant aspects of the in vivo physiologic environment such as stretch, flow, compression, etc. By use of this approach, cells may be seeded on a biomaterial/scaffold, placed in a bioreactor, and subjected to appropriate environmental cues that are critical to tissue formation and function. In this fashion, bioreactors may be used to create more advanced three-dimensional (3D) tissue constructs in vitro prior implantation in vivo (see Freed et al., 2006; Goldstein and Christ, 2009; Grayson et al., 2009; Corona et al., 2010; Badylak et al., 2012). Alternatively, bioprinting, which simultaneously deposits cells and materials, can be in complex geometries reminiscent of native tissue architectures and may provide another feasible approach to the creation and assembly of 3D tissues and organs (Boland et al., 2006; Mironov et al., 2009; Jakab et al., 2010; Chang et al., 2011; Marga et al., 2012). The key point here, with respect to the focused aim of this report, is that pharmacology can play an obvious role in all currently contemplated approaches to TE/RM. This point is highlighted in Table 1.
TABLE 1.
Expected applications of pharmacological sciences to the development of TE/RM
| TE/RM Process/Need | Pharmacological Application |
|---|---|
| Functional evaluation of engineered and regenerating tissues | Preclinical assessment and pharmacological characterization of tissue/organ phenotype in vitro and in vivo* |
| Modulation of stem/progenitor cell expansion and differentiation | Screening of growth factor and small molecule libraries; development of improved culture systems (overlap of pharmacology and engineering) ** |
| Targeted cellular delivery of drugs/chemicals to modulate regeneration in vivo | Development of novel drug delivery systems including biomaterials, nanomaterials, and bifunctional compounds that target active agents to specific tissue locations** |
| Biomaterials as reservoirs for bioactive agents and cell delivery vehicles for accelerated tissue formation and function in vitro and in vivo | Development of functionalized “smart” biomaterials** |
| Real-time modulation of tissue formation/regeneration/morphogenesis* | Pharmacological modulation of the entire regenerative process: may incorporate all of the above elements, with the added complexity of replicating the exquisite spatiotemporal regulation characteristic of morphogen gradients in normal development** |
denotes a “passive or dissecting” contribution of regenerative pharmacology; **denotes an “active or directing” role.
C. Status of the Regenerative Medicine Enterprise.
One index of the growing prominence, popularity, and expectations of regenerative medicine is the observation that a Google search for this phrase reveals nearly 6.1 million results (October 15, 2012). A number of substantive national efforts were recently launched to promote a sustained commitment to regenerative medicine. For example, the 2012 Annual Industry Report of the Alliance of Regenerative Medicine (http://alliancerm.org/sites/default/files/ARM-Annual-Industry-Report-2012.pdf) clearly indicates that pharmacology is poised to make a major contribution to the advancement of all major sectors of the regenerative medicine industry. In fact, the top 15 regenerative medicine products are already estimated to have treated 500,000 patients between 1998 and the end of 2011. As described in more detail below, contributions for pharmacology to the development of translational regenerative medicine technologies and therapies can be envisioned for cell-based therapies, as well as the small molecules, biologics, synthetic materials, biomaterials, and scaffolds— all of which are the subject of the Alliance of Regenerative Medicine 2012 Annual Report. Moreover, this same Washington, DC-based nonprofit organization has outlined a national strategy for regenerative medicine (http://www.alliancerm.org/). The overall mission of this organization is to educate key policymakers about the potential of regenerative medicine and, furthermore, to advocate for public policies that establish advantageous environments for funding, regulatory approval, and reimbursement strategies for regenerative medicine technologies/therapies. Such efforts have been aided by the introduction in the United States House of Representatives of the Regenerative Medicine Promotion Act of 2011 (HR 1862).
Another example of the increasing national commitment to regenerative medicine is the Armed Forces Institute of Regenerative Medicine (www.afirm.mil), which was officially formed in March 2008. The Armed Forces Institute of Regenerative Medicine consists of two civilian research consortia working with the U.S. Army Institute of Surgical Research in Fort Sam Houston, TX. Each consortium is a multi-institutional network with a combined total of more than 30 academic and 15 for-profit members. The recent establishment of an National Institutes of Health Center for Regenerative Medicine (www.crm.nih.gov) further bolsters the national effort in this emerging field. The National Institutes of Health also recently published a fact sheet on the past, present, and future of regenerative medicine research and clinical translation (http://report.nih.gov/NIHfactsheets/Pdfs/RegenerativeMedicine(NIBIB).pdf). In short, the present environment provides an excellent opportunity to bring pharmacology to bear in the realm of regenerative medicine and tissue engineering.
With respect to the continued development of regenerative medicine therapies/technologies, we recently noted that: “… the broader clinical use of these groundbreaking technologies awaits improved understanding of endogenous regenerative mechanisms, more detailed knowledge of the boundary conditions that define the current limits for tissue repair and replacement in vivo, and the parallel development of critical enabling technologies (i.e., improved cell source, biomaterials, bioreactors)” (Corona et al., 2010). In fact, as outlined by Parenteau et al. (2012), the opportunity and need for regenerative medicine therapies to drive medical advances is tremendous, and moreover, the interdisciplinary effort that would be required to make this theoretical possibility a reality would be a significant driver of innovation and productivity per se. In addition, investigators have already begun to recognize the importance of the union of traditional pharmacology and regenerative medicine (Stayton et al., 2005; Mooney and Vandenburgh, 2008; Pucéat, 2008; Sakurada et al., 2008; Palatinus et al., 2010; Lee et al., 2011; Jadczyk et al., 2013), and others have begun to use similar terminology to describe this interface (Mozzetta et al., 2009). There is an entire volume devoted to regenerative pharmacology (Christ and Andersson, 2013). In this scenario, regenerative pharmacology is clearly poised to make major contributions to the development of novel therapeutics, and as outlined herein, there are numerous scientific tracks by which pharmacologists can become fully engaged and further accelerate the development of these next-generation clinical therapies. Some representative examples of the spectrum of potential therapeutic possibilities for regenerative pharmacology are presented throughout this document, and for the convenience of the reader, these examples are summarized in Table 2. Nonetheless, the effort remains at a very callow stage at this point.
TABLE 2.
List of diseases and disorders for which regenerative pharmacology approaches are currently being investigated/developed
| Disease, Injury, or Disorder | Section in Document |
|---|---|
| Heart and cardiovascular disease | II.D.1, IV.D.2, VI.A, VI.B |
| Diabetes | II.D.2, IV.E. |
| Genetic diseases | II.D.4 |
| Scar reduction and wound healing | II.D.5 |
| Bladder disease | III.A. |
| Parkinson’s disease | III.B., IV.E.3 |
| Osteoporosis, bone fractures, bone grafting, spinal fusion | IV.B, IV.C. |
| Spinal cord injuries, amyotrophic lateral sclerosis, Alzheimer’s disease, cerebral palsy, macular degeneration | IV.A |
Regenerative medicine can leverage important insights not only from studies of regeneration, as noted above and below, but significant advances can also be derived via improved understanding and application of mechanisms known to be responsible for tissue formation in the first place, that is, from the field of developmental biology. Below we provide a short overview of how understanding the pharmacology of morphogenesis can make important contributions to regenerative medicine.
D. Regenerative Pharmacology and Morphogenesis.
Perhaps the importance of developmental biology (and endogenous regeneration of course) to regenerative pharmacology was intuitively obvious from the outset. That is, chemical processes guide the most fundamental aspects of tissue and organ formation and growth (i.e., morphogenesis) as well as regeneration. The implications of this for regenerative pharmacology are clear, because extracellular signaling molecules known as morphogens modulate the fate, movement, and organization of cells during morphogenesis in both embryos and adults (Wilson et al., 1997; Gurdon et al., 1998, 1999; Gurdon and Bourillot, 2001; Brockes and Kumar, 2008; Wolpert, 2011; Rogers and Schier, 2011; Bentzinger et al., 2012). Commonly studied growth factors, cytokines, and hormones such as the transforming growth factor (TGFβ) superfamily (i.e., TGFβ and BMPs), the fibroblast growth factor (FGF) family, Wnt/β-catenin signaling, retinoic acid, Wnt family members, hedgehog family members, and many others, are known to contribute to morphogenesis through a carefully orchestrated series of events. The activities of these factors are influenced by their respective diffusion profiles, effective concentration gradients, and concentration response relationships, as well as their potential modulation/quenching by the extracellular matrix and other components of the extracellular environment. Without doubt there are many unresolved questions regarding the precise mechanisms by which morphogen gradients guide tissue formation and development. Nonetheless, their impact on gene regulatory networks (Davidson, 2010) is increasingly being appreciated. These considerations form the basis for excellent recent reviews (Rogers and Schier, 2011; Kicheva et al., 2012) and entire volumes (Briscoe et al., 2010). In short, the large size and apparently exquisite distribution requirements of morphogens for normal tissue formation and development indicate that novel drug delivery technologies will be required to ensure that morphogen gradients can be efficiently modulated for curative therapeutics. Certainly, this provides yet another important link to regenerative pharmacology (biomaterials development and drug delivery systems). A more detailed discussion of this point is beyond the focused aim of this report, and the interested reader is referred to the aforementioned references for additional information.
Having reviewed the general characteristics of, and requirements for, tissue and organ regeneration and engineering, the next key question is how exactly can regenerative pharmacology contribute to the development of novel therapeutics?
E. The Relationship of Regenerative Pharmacology to the Disease Process and Development of Novel Therapeutics.
The explicit goal of regenerative pharmacology is to modulate cell, tissue, and organ physiology to accelerate, improve, or enhance functional outcomes (Andersson and Christ, 2007). However, this approach requires a radical change in thinking about the therapeutic development paradigm. Figure 3 outlines the progressive nature of the disease process and contrasts regenerative pharmacology with traditional pharmacotherapy. Most importantly, regenerative pharmacology can be used throughout the life cycle of the disease process, a major distinction from the more traditional pharmacological approaches. That is, the symptomatic treatment of age- or disease-related decrements in tissue or organ function are defined by a therapeutic window in which a sufficient amount of viable tissue must still be present to ensure efficacy. In stark contrast, the uses of regenerative pharmacology range from prophylactic applications through mitigation of reduced function to complete tissue/organ replacement in the advent of end organ failure (Fig. 3). However, as noted above, rigorous application of the pharmacological sciences toward creation of cures for disease requires a major paradigm shift in the discovery and development process for novel therapeutic modalities.
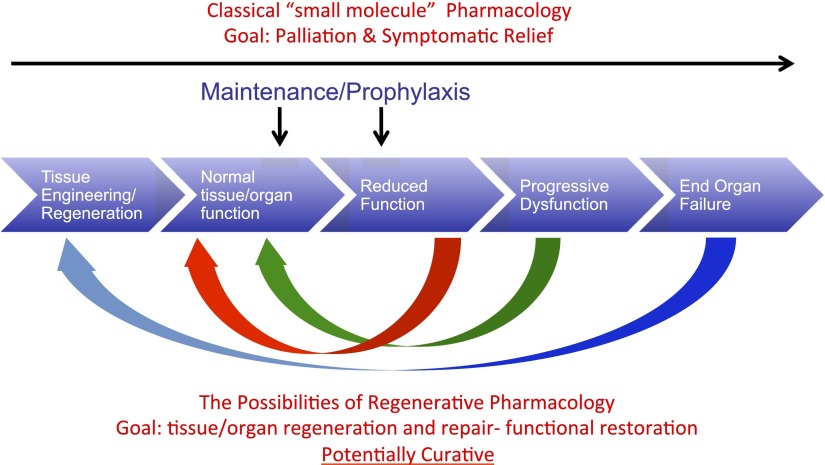
Fig. 3.
Regenerative pharmacology and the disease process. Schematic diagram shows the initiation, development, and progression of tissue and organ dysfunction, leading ultimately to end organ failure. The potential utility of regenerative pharmacology approaches to the maintenance of normal tissue and organ function or the prophylaxis of continued decline is noted. However, the long-term goal is to develop curative pharmacological approaches that address the entire spectrum of tissue and organ function and dysfunction, so that regardless of the particular circumstance, a potentially curative therapy can be developed and applied. As described in detail in the text, regenerative pharmacology represents a significant departure from more traditional approaches that have necessarily focused on palliation and symptomatic relief of pathologic alterations in tissue and organ function.
How might this dramatic shift in pharmacotherapy be achieved? As depicted in Fig. 1, we argue that pharmacology provides a critical lynchpin for the continued advancement of regenerative medicine and the discovery and development of novel curative pharmacotherapeutics. Ultimately, the seamless integration of the pharmacological sciences into regenerative medicine will require the concerted application of both passive and active processes (see Fig. 4). The active approach refers to the use of growth factors and other pharmacological agents to alter cell growth, differentiation, and function. (i.e., "direct,"; enhance, or repress as required; both in vitro and in vivo). The complementary passive approach relies on the use of established pharmacological methods to characterize endogenously regenerated or bioengineered cells and tissues and to dissect the regenerative process. Both of these approaches are currently used in regenerative medicine. However, more systematic application will be required to fully understand regeneration at the levels of molecules, cells, tissues, and organs, and thereby accelerate translational applications.
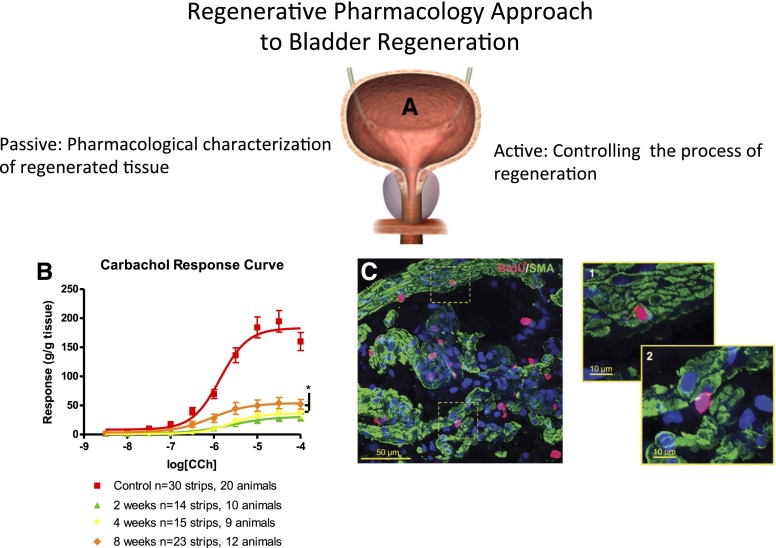
Fig. 4.
Application of pharmacology to bladder regeneration. (A) Representative illustration of the bladder. (B) Representative concentration-response curve data (CRC) for carbachol (CCh)-induced steady-state contractions of isolated bladder strips obtained from regenerating rat bladders at 2, 4, and 8 weeks post-STC (subtotal cystectomy; modified from Burmeister et al., 2010; see for more details). In short, carbachol dose–response curves are from both control animals and at 2, 4, and 8 weeks post-STC. Responses have been normalized to strip weight. Total area under the curve values were 312.8 for controls, 54.65 at 2 weeks, 61.86 at 4 weeks, and 119.7 at 8 weeks post-STC. Maximal steady-state (Emax) values for all STC animals are significantly lower than control tissue (P < 0.001). Emax values at 8 weeks post-STC are significantly higher than 2 and 4 week time points (P < 0.05). As illustrated, the data reveal a time-dependent increase in the magnitude of carbachol-induced contractile response. Note that although the contractile response never fully recovered from the initial injury, the animals were continent (i.e., the bladder emptied normally). Such observations highlight the importance of pharmacology analyses in general and, in this instance, signal transduction mechanisms in particular, in the evaluation of regeneration. Understanding the mechanisms and characteristics of functional recovery will be a key to designing improved therapeutics for bladder and organ regeneration in the future. (C) Colocalization in cells of incorporated BrdU (bromodeoxyuridine), indicative of proliferation, and specific markers for smooth muscle (SMA, smooth muscle actin) in the muscularis propria (MP) of the regenerating bladder of a female rat [the panel was reproduced from Peyton et al. (2012); additional details can be found in the manuscript as well]. Confocal z-stack reconstruction imaging was performed at 600× magnification, where offset pictures are digitally zoomed. The images were obtained from sections 7 days post-STC and reflect the early proliferative response of the rat bladder. BrdU-SMA colabeling was observed within the MP (C-1), but was relatively rare. BrdU-labeled cells within the MP were more commonly observed between smooth muscle cells as well as smooth muscle bundles (C-2).
In this regard, expression of cell- and tissue-specific molecular markers and the presence of characteristic tissue and organ structure and architecture are necessary, but not sufficient, metrics for assessing the potential utility of engineered or regenerating tissues. Clearly, the most important barometer of success for tissue/organ engineering or regeneration technologies is their capacity for functional restoration (i.e., normal physiology). Thus, it is of critical importance that comprehensive physiologic evaluation of engineered and regenerating tissues/organs is embedded in the translational research paradigm.
A key aspect to the development of curative therapeutics will be effective delivery of potentially complex mixtures of high molecular weight compounds in a controllable spatiotemporal fashion. This fact points toward the absolute requirement for vastly improved biomaterials and drug delivery technologies and systems. As such, we devote the next portion of this review to a relatively comprehensive description of biomaterials and how they impact regenerative pharmacology.
F. Drug Delivery Systems/Technologies and Biomaterials.
Advances in research at the intersection of biology, chemistry, and materials science have led to the development of increasingly sophisticated functionalized biomaterials, as well as novel drug delivery systems, as shown in Fig. 5. A comprehensive review of the latest functionalized biomaterials and modern drug delivery systems alone would require a lengthy dedicated report. Moreover, it should be emphasized that extant drug delivery systems and technologies comprise a wide array of mostly application-specific technologies. However, the potential uses of the existing technologies reviewed herein point toward future possibilities.
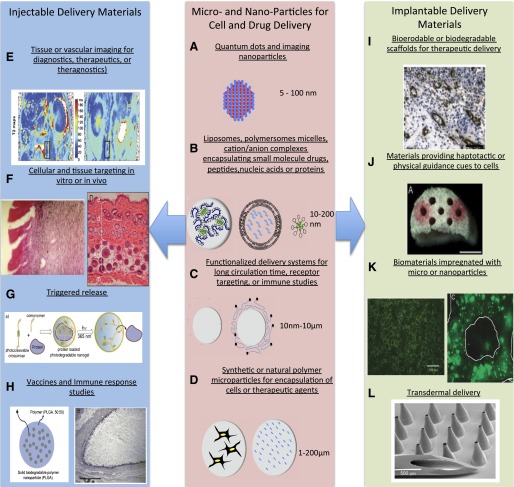
Fig. 5.
Methods to generate functionalized biomaterials for regenerative medicine. Micro- and nanoparticles for cell and drug delivery (center): several micro- and nanoparticle systems are highlighted schematically. (A) Nanoparticles used for imaging modalities include quantum dots (fluorescence) and iron oxide nanoparticles (magnetic resonance imaging). Nanoparticles with hollow centers are can also be loaded with iodine or other image contrast agents. A schematic of the structure of a quantum dot nanoparticle is shown. (B) In addition to contrast agents, small molecule drugs, nucleic acids, peptides, and protein drugs can be loaded into a variety of self-assembling nanoparticle systems that typically range from 10 to 200 nm. Schematics of DNA-polymer complexes, liposomes, and micelles are shown. (C) These nanoparticles can be surface modified with polyethylene glycol (left) to improve pharmacokinetics or can be modified with targeting motifs to improve cellular uptake (right). (D) Larger microscale constructs can also be formed from natural and synthetic polymers for release of therapeutic agents (right) or the delivery of cells (left) to provide cell-based delivery of, for example, insulin in the treatment of diabetes (Opara et al., 2010). Injectable delivery materials (left): the delivery systems described in the center panel have multiple applications to regenerative medicine when delivered either systemically or locally. (E) Shown is the in vivo tracking of implanted scaffolds containing cells loaded with ultrasmall superparamagnetic iron oxide nanoparticles (Reprinted with permission from Harrington et al., 2011). (F) The use of cationic liposomes to deliver DNA encoding for IGF-1 (and Lac-Z for imaging purposes) is shown at left (Reproduced with permission of BENTHAM SCIENCE PUBLISHERS LTD; Jeschke MG, Herndon DN, Baer W, Barrow RE, and Jauch KW (2001) Possibilities of non-viral gene transfer to improve cutaneous wound healing. Curr Gene Ther 1:267–278), whereas the delivery and protection of Wnt proteins for control of hair follicle stem cells to promote dermal thickening and follicle neogenesis in mice is shown at right (Morrell et al., 2008). (G) The ability to not only localize drugs but have the release of their payload triggered by internal [e.g., pH, temperature change, enzymes) or external (temperature, ultrasound, or as shown, light sources (Reprinted with permission from Azagarsamy MA, Alge DL, Radhakrishnan SJ, Tibbitt MW, and Anseth KS (2012) Photocontrolled nanoparticles for on-demand release of proteins. Biomacromolecules 13:2219–2224. Copyright © 2012, American Chemical Society)]. (H) Incorporation of antigens into microparticles or nanoparticles for improved vaccine delivery is shown at left (Reprinted with permission from Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, and Fahmy TM (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33:4957–4964) while the use of biomaterial implants is aiding in elucidating and ultimately minimizing inflammatory responses to implanted materials (Reprinted with permission from Norton LW, Park J, and Babensee JE (2010) Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Control Release 146:341–348). Implantable delivery materials (right), delivery systems shown in the center panel may or may not be part of implantable biomaterial scaffolds as well. (I) One example of this achieving spatiotemporal control over multiple growth factors in which one factor is released rapidly from the scaffold material (e.g., VEGF) and a second growth factor (e.g., platelet-derived growth factor) is released at a slower rate from embedded microparticles to promote angiogenesis or support other aspects of tissue formation (Reprinted with permission from Chen RR, Silva EA, Yuen WW, and Mooney DJ (2007) Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24:258–264). (J) Materials that contain specific topography or pore architecture to simulate native tissue in an increasingly important concept in biomaterial design. As shown, conduits to promote nerve regeneration are advantageous, and the delivery of nerve growth factor from microparticles or the incorporation of extracellular matrix cues such as fibronectin supports these processes (Reprinted with permission from De Laporte L, Huang A, Ducommun MM, Zelivyanska ML, Aviles MO, Adler AF, and Shea LD (2010) Patterned transgene expression in multiple-channel bridges after spinal cord injury. Acta Biomater 6:2889–2897). (K) Methods to incorporate microparticles or nanoparticles into biomaterial scaffolds include incorporation into the matrix of the scaffold (Reprinted with permission from Lee M, Chen TT, Iruela-Arispe ML, Wu BM, and Dunn JC (2007) Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28:1862–1870) or coating onto the scaffold’s pores (Reprinted with permission from Saul JM, Linnes MP, Ratner BD, Giachelli CM, and Pun SH (2007) Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials 28:4705–4716). (L) Another materials-based approach important to the delivery of therapeutics related to regenerative medicine are microneedle patches that overcome diffusion barriers in the skin to allow more efficient, long-term delivery of therapeutics (Reprinted with permission from Davis SP, Martanto W, Allen MG, and Prausnitz MR (2005) Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng 52:909–915).
Most relevant to the focused aims of this review is the utilization of these technologies to 1) overcome the common set of barriers limiting the effectiveness of traditional pharmacotherapy and 2) extend the domain of deliverable therapeutic agents to a wider array of compounds (e.g., large molecular weight growth factors, gene therapies, etc.; see Fig. 5). The first major barrier to a systemically delivered therapeutic is directing the agent to its tissue-level site of action. This involves achieving vascular extravasation or creating technologies that more efficiently deliver the “payload” (e.g., a drug, compound, or gene) from the systemic circulation to within the tissue(s) of interest. Approaches to achieve tissue-level localization include the use of long-circulating nanoparticles that home to sites of high vascular permeability (so-called “leaky vasculature”), delivery via transdermal delivery systems, or (most commonly in regenerative medicine approaches) direct injection or implantation routes. Once the desired agent has been delivered to its tissue target, a second major barrier exists with respect to local diffusion barriers within that tissue. Finally, any requirement for cellular and subcellular targeting specificity (e.g., gene therapy) provides a third major barrier to therapeutic success, that is, the chemical and structural barriers of the cell itself. We address all three of these key issues below.
II. Biomaterials in Regenerative Pharmacology
The field of biomaterials has undergone a transformation from the use of inert substances to the development of materials that are bioactive and can integrate into host tissues. The use of functionalized biomaterials can range from modifications of biomaterials to promote highly selective cell targeting—as in the case of nanoparticulate delivery systems—to the surface modification of implantable materials that promote cell attachment and tissue integration. In both instances, two classes of functionalized biomaterials often used in regenerative medicine applications are 1) particulate (micro- and nanoparticles) for cell and drug delivery and 2) scaffolding systems for tissue engineering approaches that carry or support cellular growth and tissue formation and/or regeneration. This report seeks to emphasize the former, but these two classes of biomaterials are highly interrelated in terms of their potential applications to regenerative medicine. For example, as shown in Fig. 5, I–K, scaffolding systems are often further functionalized by the incorporation of drug delivery systems into the materials. Alternatively, the functionalized biomaterial systems themselves can be drug delivery systems, either through release of exogenous therapeutic agents or through cell-based therapeutic release (Fig. 5, A–H).
A. Particulate Systems for Cell and Drug Delivery
Micro- and nanoparticulate delivery systems owe much of their development to the field of cancer therapeutics. The intent of many of these particulate delivery systems was to provide enhanced systemic delivery of therapeutic agents through improved pharmacokinetics (e.g., longer blood circulation) and pharmacodynamics (e.g., site-directed specificity). Systemic delivery systems offer the advantage of multiple dose administration at well-defined time points. The short half-lives of growth factors and nucleic acids commonly employed in regenerative medicine and tissue engineering suggests that such particulate delivery systems would also be advantageous for these applications because they provide protection from enzymatic degradation and hydrolysis. The ability of these delivery systems to protect therapeutics also makes them useful for inclusion in scaffolding systems.
Understanding each of these systems is important to understanding the potential breadth of their application(s) to tissue regeneration, repair, or replacement using pharmacological approaches. In fact, there are numerous examples of both particulate and implantable biomaterial systems being used for drug delivery applications. The nanoscale particulate systems are mostly based on self-assembly processes. Salient aspects of several of these technologies, which are specifically relevant to regenerative medicine and tissue engineering, are illustrated in Fig. 5.
1. Quantum Dots and Imaging Nanoparticles.
Quantum dots are a crystalline lattice of atoms that act as semiconductors. These materials are gaining increasing usage in cancer studies and regenerative medicine (Fig. 5A). Their popularity as an imaging tool is largely related to their tunability, and applications to medical imaging include fluorescence and near infrared imaging technologies. Quantum dots are fabricated by dissolving an inorganic precursor (e.g., CdO may be used to serve as the Cd component of a CdSe crystal quantum dot) in organic surfactant (e.g., stearic acid) and solvent (e.g., octadecene) at relatively high temperature (e.g., 200°C). After cooling and addition of, e.g., an organophosphorous compound, the second component of the crystal (e.g., Se) may be added at elevated temperature to generate, in the examples above, CdSe nanocrystal quantum dots that are colloidal in nature (Li et al., 2003). Such technologies are critical to nondestructive imaging of engineered and regenerating tissues (theragnostics)—a key aspect to improved regenerative pharmacology approaches (see Fig. 5, E–H). Recently, the use of quantum dots to provide information on pharmacokinetic aspects of nanoparticles (see section II.A.3) was reviewed and indicates the potential for applying these technologies for nondestructive imaging in both pharmacological and tissue engineering realms (Probst et al., 2012).
2. Liposomes, Polymersomes, Micelles, and Cation/Anion Complexes for Encapsulation of Small Molecules, Peptides, Nucleic Acids, or Proteins.
These are also self-assembling systems that are widely used for drug delivery because of the versatility of their payload. The self-assembly processes may be dictated by the materials themselves (liposomes, polymersomes, micelles) or through interaction of the materials with biologic molecules or drugs. An example of the latter is the self-assembly as polymer-DNA complexes (polyplexes) formed through electrostatic interactions of cationic polymers and negatively charged DNA (Fig. 5B). These technologies are critical components of regenerative pharmacology approaches when dealing with labile compounds/agents such as naked DNA and growth factors.
3. Functionalized Delivery Systems for Long Circulation Time, Receptor Targeting, or Vaccine Delivery.
A key aspect to the development of these materials is surface modification. Such modifications may include, for example, grafting of poly(ethylene glycol) to decrease opsonization (Fig. 5C) and improve circulation time. Alternatively, one can include surface coupling of targeting ligands to the carrier (or polyethylene glycol chain as shown at the right) to improve selectivity of cell targeting and enhance cellular uptake. Such systems have been applied to target vascular sites of injury with applications to delivery therapeutics that can promote healing (Shi et al., 2012) or can be used to improve imaging modalities (Yang et al., 2011). It is also important to note that nanoparticle systems are gaining increased emphasis related to vaccine delivery (Fig. 5H) (Reddy et al., 2007; Foster et al., 2010). Given the role of implanted cells in regenerative medicine applications (including some involving autoimmune aspects such as diabetes), the role of nanoparticulate delivery systems for vaccine may be useful for several applications.
4. Synthetic or Natural Polymer Microparticles for Encapsulation of Cells or Therapeutic Agents.
The use of microparticles is also common attributable to the ability to achieve sustained release of individual compounds/agents (Fig. 5D) or, alternatively, differential release profiles for distinct compounds/agents when used in conjunction with different materials or material scaffolds. Such technologies would be absolutely critical, for example, with respect to any attempt to recapitulate the exquisite features of development (morphogenesis) in vivo, as described above. Microcarriers are also an important aspect of regenerative medicine technologies with respect to cell encapsulation for various conditions including the delivery of insulin from islet cells as an approach to treat diabetes (Opara et al., 2010) and other diseases/disorders, and this application is considered in more details in section VI.
The methods for fabrication of nano- and microscale particulates are widely varied and too numerous to describe here. Nonetheless, it is worth noting some important applications in which these carrier systems are finding utility, which serve as a foundation for their inclusion in regenerative pharmacology approaches (Fig. 6). Most U.S. Food and Drug Administration (FDA) approved synthetic and natural polymers have been or can be formed into microscale carriers (∼1–50 μm diameter). When delivered systemically, their dimensions restrict these systems to the vascular compartment, but approaches have been developed to localize delivery of the therapeutic to a specific region of the vasculature. Furthermore, microbubbles carrying therapeutics or imaging contrast agents can be disrupted by external application of ultrasound (Villanueva et al., 2007; Gao et al., 2008) and thereby achieve intracellular delivery of their payload (Barbarese et al., 1995). Delivery to specific sites of vascular injury can be accomplished by the coupling of targeting ligands (e.g., ligands or antibodies that target selectins or cell adhesion molecules upregulated at sites of vascular injury) to the surface of the microcarriers (Omolola Eniola and Hammer, 2005; Banquy et al., 2008), which essentially mimics the behavior of leukocyte rolling and adhesion. Such techniques are also finding applications in improving imaging modalities important to regenerative medicine (see above). Ultrasmall paramagnetic iron oxide nanoparticles (Harrington et al., 2011) or other imaging contrast agents encapsulated in nanocarriers can be used to improve nondestructive imaging modalities (i.e., theragnostics). Specifically, iron oxide is useful for magnetic resonance imaging (Xu et al., 2012a), iodine, or gold nanoparticles for computed tomography (Kao et al., 2003), and as noted above, quantum dots (de Mel et al., 2012) are also being used for imaging modalities in regenerative medicine.
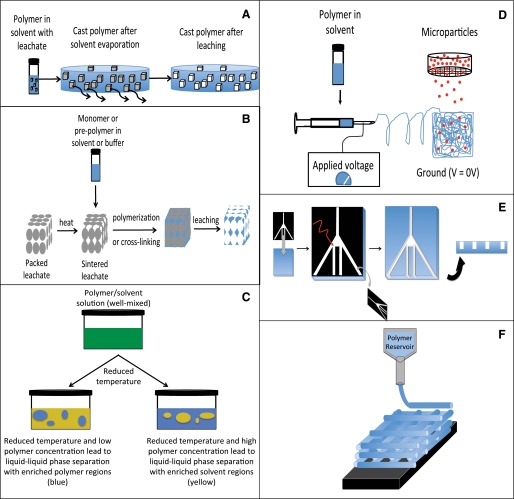
Fig. 6.
Methods to fabricate biomaterial scaffolds for regenerative medicine applications. There are many approaches to fabricating materials. These approaches range from inexpensive and relatively simple to expensive and quite complex. Several commonly used techniques are shown in this schematic. (A) Solvent evaporation/particulate leaching. A particulate (e.g., sodium chloride) that is insoluble in a particular solvent (e.g., chloroform) is cast with a polymer (e.g., PLGA) in solvent. After the solvent is evaporated, the material can be placed into an alternative solvent in which the particulate is soluble but the polymer is not to form the pores. (B) Sintering—particulate leaching that allows formation of interconnected pores of well-defined architecture. In this approach, leachable polymers are packed together and heated (to above their glass transition temperature) to allow partial fusion of the beads and provide a template. After cooling, a second polymer is cast around the sintered bead template to back-fill the empty regions. The polymer used to fabricate the bead template must be selectively soluble in a solvent. As described above, the bead template is then selectively dissolved in an appropriate solvent to yield a highly porous scaffold with interconnected pores (Fukano et al., 2010; Underwood et al., 2011). (C) Phase separation to introduce porosity (Nam and Park, 1999). This approach involves dissolution of a polymer into a solvent. The temperature is raised to one such that the polymer is fully solubilized. By cooling, the solution can phase separate depending on the concentrations of the solvent and the polymer. This phase separation can achieve solvent-rich regions or polymer-rich regions. Removal of the solvent (e.g., by evaporation) can achieve desirable pore architecture within scaffolds. These can be liquid-liquid phase separations, but it is also possible to introduce gaseous materials to achieve “gas foaming” of the desired pore architecture of the material (Riddle and Mooney, 2004). (D) Electrospinning— polymer dissolved in solvent is ejected through a small orifice (typically a needle). An electrical drop is applied between the orifice and collection device and fine nano-fibers are produced. It is also possible to incorporate nano or microparticles into these electrospun scaffolds (Guo et al., 2012). (E) Microfabrication techniques to introduce very high resolution into materials. Typically, such approaches are not used to produce large three-dimensional scaffolds for implantation. However, the techniques allow for very high levels of control over drug delivery or surface topography, allowing investigation of these effects at the individual cell level. (F) Three-dimensional printing/solid free-form fabrication techniques. These methods achieve high levels of dimensional precision for material fabrication at scale that is suitable for implantable scaffold materials. A polymer (in solvent or melt form) is ejected through a small orifice with high precision on a stage with x-y control. A single “layer” is printed and is akin to printing on a piece of paper with a laser printer. By controlling x, y, and z direction resolution, it is possible to fabricate scaffolds with very precise architecture.
As described above for vascular targeting, nanoscale systems (∼10–200 nm) provide the ability to achieve a greater degree of tissue-specific targeting by extravasation. In the application of nanoscale materials to the treatment of cancer, it is recognized that the vasculature of tumors has increased gaps in the endothelium and a reduction in the presence of lymphatic drainage, leading to the so-called enhanced permeation and retention effect. Although reliant on poorly defined vasculature and minimal lymphatic drainage, these principles may have the potential for application to certain aspects of regenerative medicine as well. Targeting the endothelium by conjugating ligands as noted above has also been accomplished with nanoscale carriers (Haun and Hammer, 2008), although not with the same success as with microscale systems (Charoenphol et al., 2010). For nanocarrier systems, the use of appropriate targeting motifs can achieve transcytosis in the case of endothelial or epithelial targeting (Ke et al., 2009) or endocytosis if the carrier is able to extravasate. Taken together, such technologies provide the potential to more selectively deliver therapeutics to target cells while limiting off-target sites (Saul et al., 2006).
To date, these methods have been largely emphasized in the cancer literature. However, the application of tissue- and cell-targeted carrier systems to the regenerative medicine space is increasing. For example, nonviral delivery of plasmid DNA encoding for IGF-1 (and Lac-Z for imaging purposes) when delivered via direct injection has been effective in improving wound healing responses (Fig. 5F) (Jeschke et al., 2001). Conceptually, nonviral DNA delivery may be advantageous to viral methods by reducing inflammatory response and achieving transient expression attributable to lack of genomic incorporation (Li and Huang, 2007). Liposomal or other nanocarriers may also be effective for the delivery of payloads that are sensitive to degradation such as through proteolysis. For example, Wnt proteins are important in various aspects of stem cell renewal and proliferation processes, but lack known agonists and stability. Wnt proteins, however, have been packaged into liposomes (see Fig. 5F). Delivery of Wnt via liposomes maintains their bioactivity and leads to dermal thickening and hair follicle neogenesis in mice, indicating the importance of their actions on stem cells in the follicle niche (Morrell et al., 2008).
Unlike cancer therapeutics where it is generally desirable to deliver a large payload of chemotherapy drugs to a tumor cell, regenerative medicine approaches typically are considered to benefit from spatiotemporally controlled delivery of therapeutic agents, thereby recapitulating relevant aspects of the carefully orchestrated tissue and organ development process (i.e., morphogenesis; again, see description above). Much effort in the development of such delivery systems involves methods that are crude relative to the exquisite morphogen gradients that guide tissue formation and development but nonetheless quite elegant in terms of polymer chemistry. Numerous release triggers exist, with temperature (Bessa et al., 2010), ultrasound (Borden et al., 2008), and light among the most commonly used methods to allow control over the timing of release. In one example shown (Fig. 5G), photocleavable cross-linkers are used for assembly of the nanoparticle encapsulating a therapeutic protein, which is released upon presentation of the triggering light source (Azagarsamy et al., 2012).
As noted above, systemic biomaterial drug delivery technologies, such as those currently under development for vaccines, are being increasingly applied to regenerative medicine. The use of nanoparticle-based biomaterials for vaccine delivery offers the potential to protect antigens, prolong release, or overcome biologic barriers attributable to the small size of the carrier technologies. Functionalized materials for these approaches take numerous forms. As shown in Fig. 5H, antigens may be encapsulated within FDA-approved polymers such as PLGA formed at diameters of several hundred nanometers up to several micrometers (Demento et al., 2012). In addition, the use of biomaterials as scaffolds for regenerative medicine applications emphasizes the importance of understanding the immunologic response to implanted materials per se. In fact, the selection of a preferred formulation or composition of a biomaterial or drug delivery system may even require the use of sustained release of anti-inflammatories at the site of implantation (Norton et al., 2010) to improve the biologic response to the material and thus facilitate the regenerative process.
As mentioned, overall, there are limited examples of systemically delivered materials for regenerative medicine applications. In general, drawbacks to the systemic delivery of therapeutic agents via functionalized biomaterial drug delivery systems suffer mainly from low accumulation at their site of action. However, progress in nonviral gene delivery and chemotherapy targeting are moving toward more effective compound delivery to target sites. Chemical constituents of the system, diameter and shape of the carrier (Gratton et al., 2008), surface charge (Georgieva et al., 2011), and the presence of targeting motifs (Ng et al., 2009) have been identified as key parameters for more effective systemic delivery of biomaterials and their cargo.
In summary, there are numerous combinations and permutations of functionalized biomaterials and drug delivery systems that can be contemplated for use in regenerative pharmacology to promote tissue and organ regeneration and repair. Below we consider biomaterials that provide novel pharmacological approaches suitable for “building” tissue, that is, tissue engineering.
B. Biomaterials as Scaffolding Systems
Biomaterials are a key component of the tissue engineering paradigm, serving as a provisional matrix for cell infiltration and as depots for the delivery of therapeutic agents (see below). The point has been made that we need to think very differently about biomaterials (Williams, 2009), and without question, scaffolds for future generations of TE/RM technologies are expected to differ considerably from present-day implantable materials. Regardless, important design criteria for these scaffolds include 1) architecture and porosity; 2) mechanical properties and their role in directing cellular response; 3) physical and chemical cues for the promotion of cell attachment, migration, and differentiation; 4) compatibility with cell seeding or infiltration; and 5) degradation profiles suitable for tissue-specific regeneration
Fabrication techniques play a significant role in defining these parameters for more effective scaffolds for TE/RM applications, and numerous approaches have been used to fabricate scaffolds from a variety of biomaterials. Several of the most promising or highly used are shown in Fig. 6. The techniques in use for regenerative pharmacology-based scaffolds differ significantly from the classic biomaterials fabrication techniques. Each technique has advantages and disadvantages, but generally speaking, higher levels of architectural organization are sacrificed for ease and speed of fabrication (Dalton et al., 2009). Porosity is an important aspect of biomaterial scaffolds for tissue engineering to allow cellular infiltration and ultimately optimized tissue regeneration.
1. Controlling Porosity.
Several techniques for customizing the porosity of biomaterials are illustrated in Fig. 6, A–C. One widely used and inexpensive approach to introduce porosity into cells is particulate leaching (Fig. 6A). In this method, a particulate (e.g., sodium chloride) that is insoluble in a particular solvent (e.g., chloroform) is cast with a polymer (e.g., PLGA) in solvent. After the solvent is evaporated, the material can be placed into an alternative solvent in which the particulate is soluble but the polymer is not (e.g., in the case of sodium chloride for PLGA scaffolds, the material can be placed in water; the sodium chloride dissolves quickly, whereas PLGA is insoluble and even with hydrolysis degrades slowly during the leaching process). With particulate leaching, one challenge is to achieve interconnected pores. Pore interconnectivity is important to ensure that cells are able to navigate the scaffolds to repopulate it and ultimately promote optimal tissue formation. An approach not only to ensure pore connectivity but ultimately define it with considerable precision is the concept of sintering (Fig. 6B) (Murphy et al., 2002; Linnes et al., 2007). In this approach, leachable polymers (e.g., polystyrene) are packed together and heated (to above their glass transition temperature) to allow partial fusion of the beads and provide a template. After cooling, a second polymer is cast around the sintered bead template to backfill the empty regions. The polymer used to fabricate the bead template must selectively be soluble in a solvent (that is, soluble in a solvent in which the cast polymer is not). As described above, the bead template is then selectively dissolved in an appropriate solvent to yield a highly porous scaffold with interconnected pores. These physical dimensions have been observed to play a role in regenerative environments, including dermal healing processes (Fukano et al., 2010; Underwood et al., 2011). Another technique to introduce porosity is phase separation (Fig. 6C) (Nam and Park, 1999). This approach involves dissolution of a polymer into a solvent. The temperature is raised to one such that the polymer is fully solubilized (note that this may not require true heating as many polymers are soluble in certain solvents at room temperature or lower). By cooling (or reducing pressure) the solution can phase separate depending on the concentrations of the solvent and the polymer. This phase separation can achieve solvent-rich regions or polymer-rich regions. Removal of the solvent (e.g., by evaporation) can achieve desirable pore architecture within scaffolds. These can be liquid-liquid phase separations, but it is also possible to introduce gaseous materials to achieve “gas foaming” of the desired pore architecture of the material (Riddle and Mooney, 2004).
2. Electrospinning.
The architecture of scaffolds formed by many of the particulate leaching techniques is rudimentary compared with native tissue structure. Electrospinning (Fig. 6D) is a textile fabrication technique that has recently been revived for TE applications. For reviews on this topic see Greiner and Wendorff (2007) or Sill and von Recum (2008). In short, a polymer dissolved in solvent is ejected through a small orifice (typically a needle). An electrical drop is applied between the orifice and collection device (e.g., a flat sheet or spinning mandrel) and fine nanofibers are produced. These electrospun fibers are of critical importance because they can provide topographical cues to cells. This is particularly useful for cells in which cell alignment is important to function such as when creating scaffolds for neural (Wang et al., 2008) or skeletal muscle regeneration (Choi et al., 2008). It is also possible to incorporate nano- or microparticles into these electrospun scaffolds either by using a particle not soluble in the polymer solvent (and spinning the particles with the polymer in solvent) or to add the particles during the spinning process (Guo et al., 2012). Because achieving suitable porosity into electrospun materials is a challenge, this approach has also been combined with the particulate leaching approach described above (Wright et al., 2010). One key advantage of electrospinning techniques is the ability to incorporate cellular cues at the micro- and nanolevel (e.g., topographical cues).
3. Microfabrication.
Microfabrication techniques (Fig. 6E) also allow for incorporation of topographical as well as other key design parameters. Although microfabrication technology is not typically used to produce large three-dimensional scaffolds for implantation, it has numerous applications in allowing better understanding of processes used to direct tissue regeneration. For example, such approaches are also useful for looking at microfluidic effects on cells—an approach difficult to study in vivo or under traditional in vitro cell culture systems. These techniques are used to create so-called “lab/organ-on-a-chip” technologies that allow for the high-throughput testing and screening of pharmacological agents on individual cells (Huh et al., 2010; Ingber and Whitesides, 2012; Neuzil et al., 2012). One approach to microfabrication is to print a photomask, which can be placed over a material surface. During subsequent etching processes, the mask allows control over which areas are, for example, photocross-linked.
4. Three-dimensional Printing.
More recently, three-dimensional (3D) printing or solid free form fabrication (Fig. 6F) has been used for scaffold fabrication, with high levels of spatial resolution for applications in bone, nerve, and cardiovascular tissue engineering as a means to determine optimal scaffold parameters (Boland et al., 2006; Mironov et al., 2009; Jakab et al., 2010; Chang et al., 2011; Marga et al., 2012). With this technique, a polymer (in solvent or melt form) is ejected through a small orifice with high precision on a stage with x-y control. A single “layer” is printed and is akin to printing on a piece of paper with a laser printer. However, the “ink” (polymer) itself is three-dimensional and the “paper” is a stage with z-direction control as well. So, individual layers are printed one on top of the other (so-called layer-by-layer approach). By controlling x-, y-, and z-direction resolution, it is possible to fabricate scaffolds with very precise architecture. It is important to note that certain types of materials are more compatible with this approach. Those that are not compatible may be modified with “fillers” to allow printing, but this may have undesirable effects on resultant material properties or biologic responses. The major drawback to all of these fabrication approaches is that the equipment is highly specialized.
C. Functionalizing Biomaterials
Regardless of the method of scaffold fabrication, there are several properties that are important for biomaterials used for regenerative applications. Two general classes of materials used in regenerative medicine applications are natural and synthetic polymers. One advantage often provided by protein-based natural polymer scaffolds is their ability to promote cell attachment and proliferation through their inherent cell-binding motifs. Collagen (RGD), fibrin (RGD), laminin (YIGSR), and keratin (LDV) all contain three to five amino acid sequences that promote cell binding through integrin or other interactions (Fig. 7Aiii). Because polysaccharide natural polymer scaffolds and synthetic scaffolds lack these integrin-binding sites, it is not uncommon to covalently graft binding motifs into/onto the material or to mix the material with naturally based polymers that contain binding motifs (Connelly et al., 2011; Sapir et al., 2011; Rafat et al., 2012).
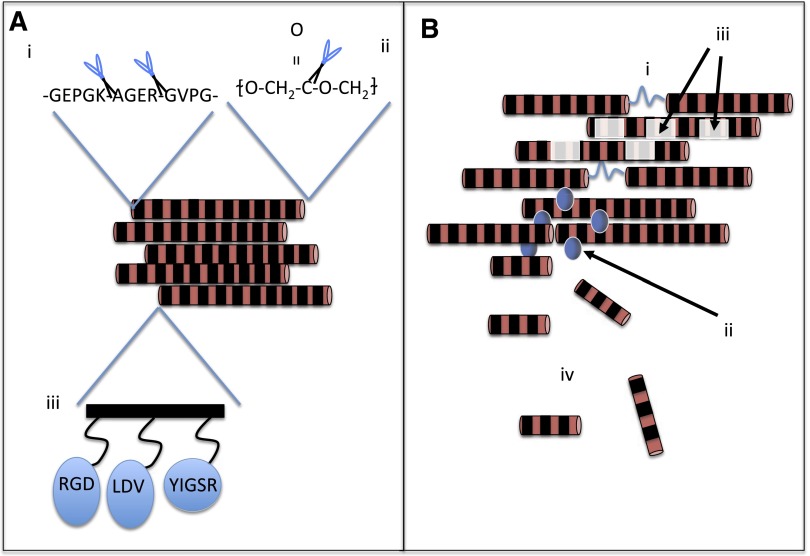
Fig. 7.
Methods to tailor polymeric materials for regenerative medicine applications. Schematic highlights important design parameters for biomaterial scaffold fabrication. (Ai) Proteolytic sequences may be natively inherent (e.g., in natural materials) or engineered into synthetic materials; (Aii) Hydrolytically cleavable sequences may also be a part of the polymer backbone. Synthetic-natural polymer hybrids may allow beneficial aspects of both classes of materials (Xu et al., 2012b); (Aiii) Natural polymers may contain peptidic sequences that promote cell attachment and proliferation through their inherent cell-binding motifs. These amino acid sequences include RGD (e.g., collagen), YIGSR (e.g., laminin), and LDV (e.g., keratin). These sequences may also be grafted into synthetic materials or natural materials that do not contain the sequences inherently (Connelly et al., 2011; Rafat et al., 2012; Sapir et al., 2011). (Bi) Internal bonds that are susceptible to cleavage through internal or external stimuli such as heat, pH, ultrasound, or light (Balmayor et al., 2008; Narayanan et al., 2012; Nelson et al., 2012) allow control over rates of degradation; (Bii) Nano- or microparticles may be also be incorporated into the scaffold (Biondi et al., 2009) and may slowly release their contents (typical for microparticles) or may themselves be released from the material (e.g., nanoparticles). A last important consideration in the material’s degradation is the fashion by which it degrades. These include bulk degradation (Biii) or surface erosion (Biv).
As shown in Fig. 7, it is desirable to have controlled rates of degradation (material aspect) to promote tissue healing (biologic aspect). In the case of natural polymers (Fig. 7Ai), proteolytic sequences are often inherently present. However, such sequences can also be built in to the polymer backbone through a type of synthetic-natural polymer hybrid (David et al., 2012). Traditionally, the more common approach for synthetic materials is to build in hydrolytically cleavable sequences such as ester groups (Fig. 7Aii). There are reports of other synthetic-natural polymer hybrids as well (Xu et al., 2012b) designed to allow functionalization of materials that otherwise lack biologic function.
For both proteolytic and hydrolytically cleavable sequences, the main concept, from a tissue engineering perspective, is to achieve controlled rates of scaffold degradation. However, this property may be useful in not only allowing the scaffold matrix to remain in place for various lengths of time (as desired and designed) but may also be useful in terms of drug delivery. In fact, a number of different materials are known to achieve release of therapeutic agents (small molecule drugs or growth factors) not through diffusion but through degradation of the scaffold material (Saul et al., 2011).
In addition to or instead of hydrolytic or proteolytic “internal triggers,” it may be advantageous to have internal or external triggers such as pH change, temperature change, enzyme, ultrasound or other energy input, or light-triggered degradation (Balmayor et al., 2008; Narayanan et al., 2012; Nelson et al., 2012) (Fig. 7Bi). It is important to note that the opposite of photodegradable linkages (photocross-linkable gels) are an area of active investigation because this allows an in situ solution to gel (sol-gel) transition, potentially allowing more minimally invasive “implantation” or delivery of soft hydrogels to their site of action. Again, these triggering events may promote gel degradation and/or the triggered release of therapeutic agents such as growth factors.
For all of these approaches, a last consideration is the fashion in which the material degrades. Specifically, one can use materials to achieve bulk degradation of the material, or, for example, it is also possible for degradation to occur only at the surface (Fig. 7B, iii and iv. Clearly, the type of degradation has important implications for drug delivery, cell ingrowth, and the regenerative process.
In summary, the scaffold types described above are commonly used with local delivery of therapeutic agents, certain growth factors, and nucleic acids. Biodegradable polymers and hydrogels (many of which are biodegradable) are the most commonly used scaffolding materials for therapeutic delivery. These systems typically elicit minimal and temporary inflammatory responses, can be tailored for favorable degradation profiles, and can achieve sustained release of therapeutics. Drug release profiles can vary from minutes or hours to years and can therefore be used to “jump start” regenerative processes or provide a sustained impact.
D. Examples of Biomaterials Applications to Tissue Engineering/Regenerative Medicine Technologies
In the preceding sections, we mainly described the general desired characteristics of biomaterials for TE/RM applications. Below we provide a few examples of their implementation.
1. Cardiovascular Disease.
Biomaterials are one of the foundations for preventative and curative approaches to cardiovascular disease. Drug-eluting stents are perhaps the best and most well-known example of drug delivery systems within the context of a biomaterial (although not necessarily in a regenerative sense) (Mani et al., 2007; Wessely, 2010). Other examples of biomaterials in cardiovascular applications include pacemakers (in particular, pacing lead wires and their insulators) (Crossley, 2000; Santerre et al., 2005) and tissue engineered blood vessels (Peck et al., 2012). Biomaterials within a regenerative medicine approach are also one of the most promising technologies in achieving functional recovery of heart tissue after myocardial infarction. For example, derivatives of polyurethanes have garnered attention because they have mechanical properties that mimic those of heart tissue —namely, elasticity and strength. These materials can be synthesized to allow biodegradation as heart tissue regenerates (Fujimoto et al., 2007a,b), including controlled rates of degradation (Hong et al., 2010). Advances to date have focused primarily on the mechanical aspects of these materials. However, it is becoming recognized that these systems are compatible with controlled release of important protective or stimulatory molecules such as IGF-1 and hepatocyte growth factor, which may aid in the regenerative process (Nelson et al., 2011), and suggests the next generation of drug delivery in cardiovascular applications beyond drug-eluting stents.
2. Modulation of Stem and Progenitor Cells.
Biomaterials are being used increasingly to direct cell differentiation and behavior. By using poly(acrylamide) gels of varying rigidity, it has been shown that mesenchymal stromal cells (MSC; sometimes referred to as mesenchymal stem cells) can be directed to different lineages ranging from neurons to myoblasts to osteoblasts (Norton et al., 2010). The use of topographical cues can also guide cell behavior. For example, the diameter of electrospun pol(ethersulfone) fibers impacts the attachment, spreading, and differentiation fate of neural stem cells (Gratton et al., 2008). Lastly, and of most importance to regenerative pharmacology, chemical signals released from scaffolds can help to direct cell fate. For example, a growth factor cocktail released from fibrin scaffolds promoted the differentiation of neural progenitor cells toward neuronal and oligodendrocyte phenotypes via heparin-binding methods (Willerth et al., 2008). Moreover, nanoparticulate delivery systems that can achieve endocytosis have recently been used for delivery of proteins involved in the Wnt signaling cascades and affecting cellular proliferation and differentiation (or lack thereof) (Shah et al., 2011).
3. Diabetes.
Hydrogels based on alginate and other polymers have been in use for nearly 30 years to encapsulate insulin-producing pancreatic islet cells (Lim and Sun, 1980). This approach could circumvent the need (or at least serve as a bridge) for the development of an engineered pancreas. However, traditional biomaterial challenges of protein deposition, foreign body response, and fibrous encapsulation have been barriers to achieving the long-term delivery of insulin required for type I diabetes.
Transdermal delivery systems using microneedle technology are an alternative, noncellular approach to insulin delivery As shown in Fig. 5L (Davis et al., 2005), these systems present an array of needles on the microscale that can be attached to a reservoir of drug. The purpose of the microneedles is to allow the drug to bypass the stratum corneum layer of the epidermis, thus overcoming a significant diffusional barrier to drug delivery through the dermal route. These technologies have now reached human trials (Gupta et al., 2009). Reduced pain and inflammation have been reported for these types of delivery devices in which insulin is delivered from a reservoir device. Therefore, these systems may provide an alternative to the current standard of subcutaneous delivery within the context of a reservoir-type material that requires less frequent dosing/application.
4. Treatment of Genetic Diseases.
The primary application of biomaterials for treatment of genetic diseases is in the development of nonviral gene delivery systems for nucleic acids, primarily DNA. Systemically deliverable nanoscale carriers are the primary focus, and many of the barriers described above (extravasation, cellular uptake, subcellular localization) must be overcome to treat genetic diseases. Poly(ethylenimine) has been considered a benchmark for biomaterial-based nonviral gene delivery because it enables high levels of transfection by promoting endosomal escape of DNA. Systems with reduced levels of toxicity and improved transgene expression are being developed through increased understanding of the role of chemical constituents of the delivery vehicle (Liu and Reineke, 2010). Peptidic sequences have been conjugated to various nonviral gene delivery systems to improve cellular uptake (Huang et al., 2010), subcellular transport (Kwon et al., 2008; Moseley et al., 2010), and nuclear localization (Jeon et al., 2007; Moore et al., 2009). Based on safety and toxicity profiles, it is conceivable that these approaches may supplant viral technologies for gene delivery (Li and Huang, 2007). Gene therapy has been of interest in the treatment of cancer for many years, for example to restore mutated tumor suppressor genes such as P53 (Fukushima et al., 2007; Gaspar et al., 2011). Muscular dystrophy also has a complex genetic picture (Kornegay et al., 2012), but canine models (Kornegay et al., 2012) are providing important insights into the disease and potential opportunities for gene therapy through both viral and nonviral methods (Foster et al., 2006; Markert et al., 2008; Wang et al., 2012).
5. Scar Reduction and Wound Healing.
The use of biologically based products to treat burns and skin conditions has reached the clinic, and products such as Alloderm, Apligraf, and Dermagraft are approved for marketing. The ability to provide improved three-dimensional architecture within the context of a biodegradable system that is readily implanted or sutured would augment existing technologies. Several materials, including hyaluronic acid (Scuderi et al., 2008), chitosan (Boucard et al., 2007; Yang et al., 2010), and alginate (Lee et al., 2009) hydrogels as well as calcium hydroxylapatite (Goldberg et al., 2006), have been used to treat skin conditions, including acne, burns, and melanocytic nevi. In addition, several types of therapeutic agents such as antibiotics for prophylaxis (Kim et al., 2008), growth factors to promote healing (Fujihara et al., 2008), and other compounds (Queen et al., 2007) have been incorporated into biologically active materials (Luo et al., 2010) to promote regeneration. Novel technologies, such as synthetic peptides derived from gap junction proteins, have also been introduced to potentially promote healing after biomaterial implantation (Soder et al., 2009). Clearly, methods to properly control the spatiotemporal presentation of molecules that promote improved wound healing are part of the next generation of treatments made possible by delivery systems incorporated into biomaterials.
In the above sections, we attempted to highlight several aspects of biomaterials that are or will play a role in regenerative pharmacology and regenerative medicine. This list is by no means exhaustive, and many of these technologies transcend any one application. Below we describe existing multidisciplinary efforts to establish new experimental models and paradigms for further exploring the potential utility of regenerative pharmacology.
III. Broad Applications of Regenerative Pharmacology
Moving from palliative to curative approaches in complex organs will be a significant challenge. Bladder regeneration and Parkinson’s disease are described below as representative of the biologic complexities that will need to be considered and overcome to achieve curative approaches. First, we will discuss the bladder, because it provides an excellent model system for exploring both the passive (dissecting) and active (directing) components of regenerative pharmacology to future therapeutics (see Table 1). Parkinson’s disease may be amenable to one of several distinct regenerative pharmacology strategies, including drug-, cell-, or gene-based approaches.
A. Bladder Disease
The aim of regenerative medicine/pharmacology is, ideally, to restore normal organ function either by replacing a nonfunctioning organ (end stage disease) or, in the instance when significant viable tissue still remains, improving organ function when it is severely impaired but not yet irreparably damaged/diseased. In the case of the bladder, the physiologic prerequisite is the capacity to store urine at increasing volumes (without increasing intravesical pressure or spontaneous bladder contractions) until complete emptying can be achieved when socially acceptable. Diverse disease etiologies (e.g., neurogenic, congenital, trauma, infections, etc.) compromise the low-pressure, high-volume function (decreased compliance) of the bladder, leading to a number of lower urinary tract symptoms such as urgency, urgency incontinence, frequency, and nocturia. With such diverse etiologies for bladder dysfunction and a large demand (over 50 million people are estimated to have some type of urinary incontinence), many different classes of drugs have been investigated for symptomatic relief. Antimuscarinic drugs (e.g., oxybutynin, tolterodine, solifenacin, darifenacin) are now the first-line therapy for treatment of detrusor overactivity and the overactive bladder syndrome, but lower urinary tract symptoms can also be treated with, e.g., α-adrenoreceptor (AR) blockers alone or in combination with antimuscarinics or with onobotulinum toxin A (Andersson et al., 2009; Mangera et al., 2011).
In severe cases refractory to pharmacological treatment, high bladder pressures may develop and lead to upper urinary tract deterioration. Patients that display poorly compliant bladders attributable to structural or neurogenic etiologies are at risk for end stage renal disease and are thus candidates for surgical intervention (Reyblat and Ginsberg, 2008). Augmentation cystoplasty has been performed in bladder diseases arising from many different etiologies, including spinal cord injury, myelomeningocele, interstitial cystitis, idiopathic detrusor overactivity, radiation cystitis, multiple sclerosis, and schistosomiasis. Because transplantation of donor bladders is not an available option, attention turned to regenerative medicine/tissue engineering technologies for this organ. In pioneering studies by Atala et al. (2006), bladder neo-organs were constructed by seeding synthetic scaffolds (collagen or collagen/polyglycolic acid composites) with urothelial cells on the inside and smooth muscle cells on the outside and subsequently implanted into subjects with myelomeningocele. Numerous reports suggested that both animal (Liang and Goss, 1963; Oberpenning et al., 1999; Frederiksen et al., 2004; Burmeister et al., 2010) and human (Sisk and Neu, 1939; Liang, 1962; Tucci and Haralambidis, 1963) bladders have significant regenerative potential after removal of a large portion of the organ (subtotal cystectomy; STC).
Novel pharmacological strategies aimed at harnessing the intrinsic regenerative capacity of the bladder will undoubtedly benefit from an improved basic understanding of de novo bladder regeneration. In this regard, animal models can be used to characterize this compelling regenerative phenomenon, opening up new approaches to regenerative pharmacology. In a multidisciplinary effort to characterize bladder regeneration morphologically, physiologically, and pharmacologically, Burmeister et al. (2010) used a trigone-sparing cystectomy (STC) performed in 12-week-old female rats. By 8 weeks post-STC, the bladder had regrown to a normal size via both computed tomography imaging and in vivo cystometric analysis. Moreover, the bladder displayed urothelial, lamina propria, and detrusor muscle layers, and regained normal thickness upon histologic evaluation. However, there was a decrease in bladder smooth muscle contractility when subjected to cholinergic and electrical stimulation. Specifically, 2 weeks post-STC, cholinergic activation resulted in contractile responses that were ∼20% of normal, noncystectomized controls. There was some functional recovery of detrusor muscle contraction by 8 weeks post-STC, although maximal steady-state contractions remained low (~37% of normal values). In addition, the presence of a response to electrical field stimulation indicated innervation of newly formed tissue. This agrees with an earlier study by Frederiksen et al. (2004) in which whole mount staining of acetylcholinesterase revealed the pattern of nerves in newly formed detrusor.
To put this in proper perspective, despite the observed reduction in smooth muscle contractility, a complete functional rodent bladder regeneration response occurs after surgical removal of 70–80% of the bladder (STC). This is a very different phenomenon than the bladder augmentations that are commonly used to study the impact of various stem cells and biomaterials (i.e., tissue engineering) on bladder regrowth after implantation (see below for more details). In summary, within 8 weeks STC rodents have a regenerated bladder that is both structurally and functionally (with respect to micturition and continence) identical to the native bladder that it replaced (Burmeister et al., 2010). This is true with respect to bladder capacity and bladder wall thickness, as well as the presence of all three bladder wall layers: urothelium, muscularis propria, and lamina propria. To our knowledge, bladder regeneration therefore holds a unique position with respect to its regenerative potential, because there is no other mammalian organ capable of this type of regeneration; this includes the liver, which is the most well studied model of regeneration, but which is more accurately referred to as compensatory hyperplasia (Columbano and Shinozuka, 1996).
A more recent follow up study (Peyton et al., 2012) used fluorescent bromodeoxyuridine (BrdU) labeling to quantify the spatiotemporal characteristics of the proliferative response that mediates the functional regeneration observed by Burmeister et al. (2010), as occurs during the critical first week post-STC. In this study, less than 1% of cells in the bladder wall were labeled with BrdU in control bladders under resting conditions (i.e., no damage), but this percentage increased significantly, by 5- to 8-fold, at all time points post-STC. The spatiotemporal characteristics of the proliferative response were characterized by a significantly higher percentage of BrdU-labeled cells within the urothelium at 1 day than in the muscularis propria (MP) and lamina propria (LP). However, a time-dependent shift at 3 and 5 days post-STC revealed significantly fewer BrdU-labeled cells in the MP than in the LP or urothelium. By 7 days, the percentage of BrdU-labeled cells was similar among urothelium, LP, and MP. STC also caused an apparent increase in immunostaining for Shh, Gli-1, and BMP-4. These studies clearly documented that the early stages of functional bladder regeneration are characterized by time-dependent changes in the location of the proliferating cell population in distinct bladder wall layers and, furthermore, demonstrated time-dependent expression of several evolutionarily conserved developmental signaling proteins during this same 1-week period. This report extends our previous observations (Burmeister et al., 2010) and provides further evidence for the rodent bladder as an excellent model for studying novel aspects of mammalian organ regeneration.
The idea that the bladder tissue formed spontaneously after cystectomy is similar to that which remains was proposed by Frederiksen et al. (2004) who described the pharmacology of regenerating bladder. Fifteen weeks after STC in female rats, transverse strips were excised from the bladder body and were exposed to contractile stimuli, taking into account the proximity of the strip to the trigone. The authors used antagonists of muscarinic receptors (scopolamine) and α1ARs (prazosin) as well as a desensitizing agent of P2X1 receptors (α,β-methylene ATP) to examine the contribution of each receptor type to contractions evoked by electrical field stimulations. Additionally, they used agonists of muscarinic receptors (carbachol), α1-ARs (phenylephrine), and purinergic receptors (α,β-methylene ATP) on separate strips. These authors concluded that although the newly formed bladder smooth muscle is well innervated, it has pharmacological properties similar to the supratrigonal tissue from which it had developed.
Clear species-dependent variations in regenerative capacity of the bladder are to be anticipated (Lin et al., 1989). Furthermore, complete, functional, de novo bladder regeneration may not occur in all species and under all experimental conditions, and thus bladder regeneration has also been studied using implantable tissue engineered grafts in the absence and presence of seeded cells. The rationale for this approach is that provision of a scaffold (biomaterial) and the appropriate cells/stem cells to the site of injury or surgical resection would provide for improved/enhanced bladder regeneration and integration with remaining host tissue. Although this is an exciting strategy, unfortunately there is so little still known about “normal” bladder regeneration that there is no a priori rationale for selecting the most appropriate cell and/or biomaterial for maximizing bladder regeneration. In fact, clinical experience with this technology is limited and not yet ready for wide dissemination, pointing to the need for further investigations into the potential applications of tissue engineering/regenerative medicine applications to bladder reconstruction (Atala et al., 2011). As such, examining the pharmacological performance of regenerated bladders after incorporation of a tissue engineered graft is important to evaluate the proximity to normal bladder function and to develop future approaches based on more detailed mechanistic information. Clearly, more work needs to be done to fully characterize the many different aspects of bladder pharmacology during and after regeneration. Determination of the time course and nature of any changes in, for example, receptor populations, ion channel expression/properties, and second messenger regulation/function would provide important mechanistic insights for the development of more effective pharmacological agents (e.g., growth factors, hormones, cytokines, neurotransmitters, immunomodulators, etc.).
However, therapeutic strategies for bladder regeneration that include implantation of a biomaterial (with or without cells) open the door for the local administration of diverse pharmacological agents to improve the regeneration of the urinary bladder. For example, Kanematsu et al. (2003) analyzed the ability of bladder acellular matrix (BAM) to carry a loaded growth factor (bFGF). They demonstrated sustained release of bFGF from the scaffold both in vivo and in vitro. Moreover, in an augmentation cystectomy model in rats, bFGF promoted angiogenesis and inhibited graft shrinkage in a dose-dependent manner, assayed at 4 weeks postimplantation.
Another proof of concept study evaluated the effects of a distinct factor, vascular endothelial growth factor (VEGF), which has a well-documented role in potentiating angiogenesis. Youssif et al. (2005) demonstrated that delivery of VEGF, again in BAM scaffolds, or by injection, had positive effects early in regeneration, as assessed by both function and histology. The exogenous VEGF increased bladder capacity and decreased residual volume 4 weeks after surgery, enhanced angiogenesis at this time point, and increased smooth muscle content at all time points studied. Furthermore, more nerve growth factor (NGF) positive cells were found up to 8 weeks postsurgery, suggesting that both VEGF and NGF may contribute to bladder regeneration and repair.
The hypothesis that VEGF and NGF synergize to promote bladder regeneration received further supported from Kikuno et al. (2009), who evaluated augmentation techniques in spinal cord injured rats. Eight weeks after initial spinal cord injury, female Sprague-Dawley rats underwent augmentation cystoplasty using BAM with no growth factor or with NGF and VEGF either alone or in combination. They found that at 8 weeks after augmentation surgery, animals that received both growth factors displayed much higher bladder capacity and compliance and increased smooth muscle and nerve content than in any other group. Taken together these studies indicate that both NGF and VEGF contribute strongly to the restoration of bladder function and architecture.
For regenerative pharmacology to fully reestablish bladder function, it seems likely that a specific cocktail of small molecules and growth factors may be required. For example, in addition to the VEGF pathway implicated in the study discussed above, Loai et al. (2010) incorporated VEGF along with hyaluronic acid in a porcine model of augmentation cystoplasty. They showed that using this glycosaminoglycan and growth factor in combination produced the best epithelialization, neovascularization, and smooth muscle regeneration 10 weeks after surgery. It is reasonable to assume that delivering many different growth factors, small molecules, or other compounds may aid in regeneration of the urinary bladder and, furthermore, that active regenerative pharmacology will fill the need to explore these possibilities. The use of cells may facilitate delivery of some factors and also may contribute directly to regeneration. Thus, by further exploring the potential applications of novel biomaterials and drug delivery technologies (as illustrated in Figs. 5–7 and described in the text above), it should be feasible to provide more precise spatiotemporal control over growth factor delivery during bladder regeneration, perhaps decreasing the time course and increasing the efficacy of functional recovery.
B. Parkinson’s Disease
One of the main changes in Parkinson’s disease (PD) is a progressive loss of nigrostriatal dopaminergic neurons. The degeneration of the dopaminergic neurons in the substantia nigra pars compact, which project to the striatum, leads to a reduction of the dopamine (DA) input to this target structure of the nigrostriatal pathway (Dauer and Przedborski, 2003: Lees et al., 2009; Shulman et al., 2011). These modifications in the functional organization of the basal ganglia circuitry lead to the typical motor features of PD (Moore et al., 2005). The precise etiology of PD remains unclear, but pathologic processes such as inflammation, mitochondrial dysfunction, oxidative stress, proapoptotic mechanisms, and accumulation of toxic proteins may play a role (Moore et al., 2005; Shulman et al., 2011).
Treatments of PD focus on symptom relief, neuroprotection, and neurorestoration (Fig. 8). Symptomatic relief can be provided by dopamine substitution therapy and deep-brain stimulation; however, there is an unmet need for the identification of neuroprotective/neurorestorative agents that can modify the progression of the underlying disease processes. Because current treatments aimed at symptomatic relief have numerous limitations (Lees et al., 2009; Hickey and Stacy, 2011), there has been an intense focus on novel therapies, especially those that might provide a definitive cure for the disease. In this regard, stem cell-based therapy offers promise for future treatment of neurodegenerative diseases, including PD. There are two major approaches to stem cell-based therapy for PD, both of which use the cell as the drug delivery vehicle and, therefore, fall under the auspices of regenerative pharmacology. One strategy is aimed at simply replacing the lost cells by transplanting exogenous stem cells, and in this instance clearly the stem cell becomes the direct source of the missing pharmacological agent, that is, dopamine. A second approach is to use the implanted cells as vectors that contain and secrete neuroprotective agents to preserve the surviving neurons or to induce renewal of axonal sprouting; in this latter case we are using pharmacology more indirectly to maintain, restore, or regenerate the endogenous cellular source of the dopamine.
Fig. 8.
Regenerative pharmacology approaches to Parkinson’s disease. Functional analysis of preclinical cell therapy approach to one PD endpoint, namely, bladder dysfunction or overactivity. Top left and right panels show immunofluorescence (red) for superoxide dismutase-2 (SOD-2; A–C, left) and interleukin-6 (IL-6; A–C, right), respectively, in rat brain cells surrounding and in close contact to amniotic fluid derived stem cells (AFS) or BM-MSC (bone marrow-mesenchymal stem cell; both stem cell populations were green fluorescent protein labeled and green) injected into a unilateral nigrostriatal lesion created 2 weeks earlier by stereotactically injecting 8 μg of 6-OHDA into the right median forebrain bundle (MFB; see Soler et al., 2012 for details). The bottom panel shows representative examples of the corresponding cystometric parameter estimates obtained 14 days after sham-injection or injection of AFS cells or BM-MSC, compared with urodynamic responses observed on a healthy control animal; all data are expressed as the mean ± S.E.M. parameters in sham-treated, AFS, BM-MSC, and healthy control groups during follow up (H1, H2). Red bars represent improvement in AFS-injected versus sham-treated rats. Green bars represent improvement in BM-MSC-injected versus sham-treated rats. Of note, the immunofluorescence was no longer detectable 14 days after AFS injection, while the observed recovery was gone by 28 days post-AFS injection. These data highlight two important points. First, and not surprisingly, human stem cell survival in vivo is short lived (<28 days). Second, nonetheless, they can impart significant pharmacological effects and corresponding functional improvement. That is, human AFS cells can temporarily ameliorate bladder dysfunction in a rodent model of Parkinson’s disease. Panels were reproduced from Soler R, Fullhase C, Hanson A, Campeau L, Santos C, and Andersson KE (2012) J Urol; 2012 Apr;187(4):1491–1497.
The effect of various cell sources have been investigated in animal models of PD, as well as in humans, and these include, embryonic stem (ES) cells (Freed et al., 2001; Kim et al., 2002, see Lindvall and Kokaia, 2009), induced pluripotent stem (iPS) cells (Wernig et al., 2008), fetal and adult brain-derived neural stem cells (NSC; Hermann et al., 2006; Schwarz et al., 2006), mesenchymal stem cells (MSC; Hellmann et al., 2006; Cova et al., 2010), and amniotic fluid stem (AFS) cells (Donaldson et al., 2009). In an interesting application of regenerative pharmacology, neural progenitors generated from pluripotent stem cells in culture were induced to give rise to dopaminergic neurons, which hold therapeutic potential for PD. Studer and colleagues recently reported that the application of CHIR99021, a potent GSK-3β inhibitor known to strongly activate canonical WNT signaling, to certain midbrain precursors cells derived from human pluripotent stem cells strongly promotes differentiation to midbrain dopaminergic neurons, precisely the class that degenerates in PD. These neurons survived well and functioned appropriately when grafted into the brain in several animal models, most notably Parkinsonian nonhuman primates (Kriks et al., 2011). Clinical trials using human mesencephalic tissue provided the proof-of-principle for cell replacement in PD patients but also showed clinical limitations (Koch et al., 2009; Lindvall and Kokaia, 2009, 2010; Meyer et al., 2010).
Gene therapy provides another strategy to restore the ability of the brain to deliver dopamine and other agents to the striatum to provide both symptomatic benefit and possibly neuroprotection/neuroregeneration. For this application, genes have thus far been packaged into viral vectors and injected into the brain with the goal of either delivering genes for DA-synthesizing enzymes to the striatum or providing neuroprotection to block or slow ongoing degenerative processes by providing genes for growth factors, antioxidant molecules, or antiapoptotic substances (Muramatsu et al., 2003). Adeno-associated virus (AAV) has been the most commonly used vector because of its ease of use and safety profile (Ozawa et al., 2000; Bankiewicz et al., 2006). This approach is currently being tested in a number of Phase I and II clinical trials (Hickey and Stacy, 2011). Details of some relevant examples are provided below, and furthermore, these strategies and their corresponding pharmacological details are summarized in Fig. 9.
Fig. 9.
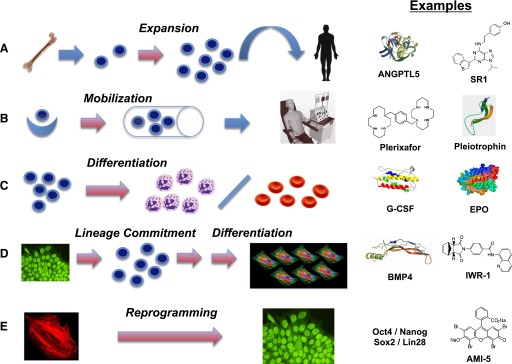
Five strategies by which regenerative pharmacology can affect biology of stem and progenitor cells. Red arrows indicate steps at which a pharmacological agent may be applied. (A) Expansion of lineage-restricted stem or progenitor cells (depicted in cartoon form as blue cells with large nuclei). Exemplified here by isolation of hematopoietic stem cells (HSC, e.g., CD34-positive cells) from human bone marrow and expansion in culture using a cocktail of cytokines/growth factors including angiopoietin-like 5 (AGPTL5) (Drake et al., 2011), prior to infusion into a patient small molecules, such as an aryl hydrocarbon receptor antagonist designated StemRegenin 1 (SR1) (Boitano et al., 2010), likewise can promote HSC expansion in culture. (B) Mobilization of stem cells from an endogenous niche. Exemplified by recruitment of stem cells from tissue niches (blue crescent) to enter the circulation (cylinder depicts a blood vessel) and potentially to undergo expansion prior to further lineage commitment. Plerixafor (Brave et al., 2010) is a small molecule that drives HSC mobilization from bone marrow niches, used in combination with G-CSF. Pleiotrophin (Himburg et al., 2010; Istvanffy et al., 2011) promotes expansion of the HSC pool in vivo. The cells are collected from a donor by apheresis. (C) Differentiation of committed progenitor cells to functional, specialized cells. Exemplified by accelerated maturation of granulocyte progenitors to infection-fighting neutrophils (left) promoted by G-CSF (Frampton et al., 1994) and of erythroid progenitors to red blood cells (right) promoted by erythropoietin (EPO) (Faulds and Sorkin, 1989). (D) Production of specialized cells from pluripotent stem cells (ES or iPS cells) by sequential steps of lineage commitment and terminal differentiation. Pluripotent stem cells are depicted iconically as a cluster with nuclei stained for the pluripotency-associated transcription factor Oct4. Cardiomyocytes (shown iconically by staining for cardiac troponin and other heart-specific proteins) represent a cell type that might be used in tissue engineering and for drug discovery. A key factor in the promotion of commitment to mesodermal and cardiac fates is BMP-4 (Evseenko et al., 2010; Hogan, 1996; Kattman et al., 2011; Murry and Keller, 2008). Small molecule inhibitors of Wnt/beta-catenin signaling, such as IWR-1 (Chen et al., 2009; Willems et al., 2011), drive the generation of cardiomyocytes from human ES cell-derived mesoderm. (E) Production of genetically compatible induced pluripotent stem (iPS) cells from an individual’s own cells. Autologous cells such as skin fibroblasts (shown iconically by staining for F-actin in the cytoskeleton) are reprogrammed to pluripotency by exposure to a set of four transcription factors [e.g., the four identified by the Thomson group—Oct4, Nanog, Sox2, and Lin 28 (Yu et al., 2007)]. The small molecule AMI-5 (Yuan et al., 2011b), an inhibitor of protein arginine methyltransferase (PRMT), enables reprogramming in conjunction with Oct4 alone.
1. Enhancing Striatal Dopamine Effects.
Local production of dopamine in the striatum can be achieved by inducing the expression of enzymes involved in the biosynthetic pathway for dopamine. As pointed out by Hickey and Stacy (2011), the potential benefits are compelling: the ability for selective basal ganglia stimulation by bypassing the need for systemic medications, the avoidance of undesirable side effects induced by indiscriminate dopamine activation, and even the possibility for individualized treatment regimens. For example, one strategy was indicated by in vitro experiments showing that triple transduction with separate AAV vectors expressing tyrosine hydroxylase, l-amino acid decarboxylase (AADC), and GTP cyclohydrolase 1, respectively, increased dopamine production (Shen et al., 2000; see Fig. 8). This study demonstrated that stereotaxic intrastriatal injection of these factors in 6-hydroxydopamine (6-OHDA)-lesioned rats produced sustained behavioral improvement for up to 12 months. Consistent with the rodent study, the same group confirmed and extended their original observations to document that the same triple AAV transduction of striatal cells with dopamine-synthesizing enzymes also produced behavior recovery in a primate model of PD (i.e., 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrinde; MPTP lesion) for up to 10 months (Muramatsu et al., 2002).
A combination of intrastriatal AAV-hAADC gene therapy and administration of the dopamine precursor l-3,4-dihydroxyphenylalanine (l-DOPA) was explored in MPTP-lesioned primates (Bankiewicz et al., 2000). The conversion rate of l-DOPA to dopamine increased after AADC gene transfer, resulting in long-term improvement in clinical rating scores, significantly lowered l-DOPA requirements, and a reduction in l-DOPA-induced side effects. More recently, Muramatsu et al. (2010) evaluated the safety, tolerability, and potential efficacy of AAV vector–mediated gene delivery of AADC into the putamen of six PD patients studied at baseline and at 6 months using multiple measures, including the Unified Parkinson’s Disease Rating Scale, motor state diaries, and positron emission tomography with 6-[18F]fluoro-l-m-tyrosine, a tracer for AADC. Six months after surgery, motor functions improved without apparent changes in the short-duration response to levodopa. Positron emission tomography revealed an increase in 6-[18F]fluoro-l-m-tyrosine activity, which persisted up to 96 weeks.
2. Neuroprotection/Neuroregeneration.
The concept of delivering trophic factors to the central nervous system has evolved since it was first discovered that brain-derived neurotrophic factor or glial cell line–derived neurotrophic factor (GDNF) had a potent protective effect on the survival of midbrain dopaminergic neurons (Lindsay et al., 1991; Lin et al., 1993). BDNF is a member of the same family as the first-described neurotrophin, NGF. GDNF is a member of the transforming growth factor superfamily and has been shown to have both neuroprotective and neuroregenerative effects. Infusion and viral-mediated delivery of GDNF, as well as transplantation of GDNF-producing cells, gave substantial neuroprotection in rodents and primate models of PD induced by either 6-hydroxydopamine or MPTP (Kirik et al., 2004; Ramaswamy et al., 2009; Aron and Klein, 2011). Neurturin (NTN), the naturally occurring analog of GDNF, has also shown protective effects on dopaminergic nigral neurons after 6-OHDA lesioning in rats for up to 6 months (Gasmi et al., 2007a,b). Additionally, MPTP-treated monkeys demonstrated protection of nigral neurons, preservation of dopaminergic striatal innervation, and prevention of motor dysfunction after injection with an AAV-based vector encoding human NTN (Kordower et al., 2006). However, no available treatment has yet proven to have a definitive neuroprotective effect for patients with PD (Schapira and Olanow, 2004; Boll et al., 2011; Seidl and Potashkin, 2011). Clinical trials designed to evaluate the efficacy of GDNF and NTN in patients with PD have so far remained inconclusive (Gill et al., 2003; Nutt et al., 2003; Lang et al., 2006; Bartus et al., 2011).
The glutamic acid decarboxylase gene catalyzes the synthesis of GABA, the major inhibitory neurotransmitter in the brain. Gene transfer of glutamic acid decarboxylase and other methods that modulate production of GABA in the subthalamic nucleus improve basal ganglia function in animal models of PD. LeWitt et al. (2011) used this approach in patients aged 30–75 years who had progressive levodopa-responsive Parkinson’s disease and showed a significant improvements from baseline in Unified Parkinson’s Disease Rating Scale scores compared with the sham-treated group over the 6-month course of the study.
Certainly, some of the newer biomaterial strategies for nonviral gene delivery, as described above, may ultimately be of benefit to the gene-based treatment of PD in the future, as may some of the stem cell-based regenerative pharmacology approaches described below.
IV. Regenerative Pharmacology and Stem Cells
Modulation of the behavior of stem and progenitor cells plays a central role in TE/RM technologies, and is a major target of regenerative pharmacology (Table 3). These cells are able to undergo mitotic division, sometimes very extensively, and also to give rise to more specialized progeny. The defining characteristic of stem cells is the continued maintenance of a pool of unaltered daughter cells that retain this dual potential for proliferation and differentiation, a property known as self-renewal. Stem cells from certain sources, such as the inner cell mass of early stage embryos (ES cells), can give rise to any specialized cell type found in normal development and are designated pluripotent. Other stem cells and progenitors, notably those present throughout life in many adult organs, appear restricted to limited sets of cell lineages. Such cells are termed multipotent or, if constrained to a single fate, unipotent. The restriction of the developmental fate of cells to a particular lineage is called commitment and precedes the acquisition of overt specialized features during cell differentiation (Smith, 2006; Sheridan and Harris, 2009). Whether by recapitulation of normal development or through novel bioengineered pathways for tissue formation and organogenesis (Ingber and Levin, 2007), therapies based on regeneration almost inevitably must engage stem/progenitor cells (Nirmalanandhan and Sittampalam, 2009). Although still in its earliest phase, an era of clinical testing of stem cell therapies has begun (Trounson et al., 2011).
TABLE 3.
Summary of regenerative pharmacology stem cell applications
| Probable Target Cell of Agent | Agent | Reference |
|---|---|---|
| Expansion | ||
| Keratinocytes | Epidermal Growth Factor | Green, 2008 |
| Hematopoetic Stem Cells (HSC) | Angiopoietin-like 5 | Drake et al., 2011 |
| HSC | StemRegenin 1 | Boitano et al., 2010 |
| NSC | Epidermal Growth Factor | Weiss et al., 1996 |
| NSC | Fibroblast Growth Factor-2 | Weiss et al., 1996 |
| Mobilization | ||
| HSC | Pleiotrophin | Deuel et al., 2002 |
| HSC | G-CSF | Frampton et al., 1994 |
| HSC | Plerixafor | Brave et al., 2010 |
| Mesenchymal stem cells (MSC) | LLP2A-alendronate | Guan et al., 2012 |
| Differentiation of endogenous stem/progenitor cells | ||
| Erythroid progenitor cells | Erythropoietin | Faulds and Sorkin, 1989 |
| Myeloid progenitor cells | G-CSF | Frampton et al., 1994 |
| MSC / osteogenic progenitors | BMP-7 | Friedlaender et al., 2001 |
| MSC / osteogenic progenitors | BMP-2 | McKay et al., 2007 |
| MSC / osteogenic progenitors | NELL1 | Zou et al., 2011 |
| MSC / osteogenic progenitors | Wnt3a | Minear et al., 2010 |
| MSC / osteogenic progenitors | WNT pathway agonists | Gwak et al., 2011 |
| MSC / osteogenic progenitors | Antagonists of WNT inhibitors | Agholme and Aspenberg, 2011 |
| Differentiation from pluripotent stem cells (PSC) | ||
| PSC to definitive endoderm | Activin A | D’Amour et al., 2005 |
| PSC to mesoderm & cardiac progenitors | TGF-β family members | Kattman et al., 2011 |
| PSC to cardiomyocytes | FGF-10 | Chan et al., 2010 |
| PSC to cardiomyocytes | Cardiogenol A-D | Wu et al., 2004 |
| PSC to cardiomyocytes | XAV939 | Wang et al., 2011a |
| PSC to cardiomyocytes | “Compound 62” | Shen et al., 2012a |
| Reprogramming to PSC or Lineage-Restricted Cells | ||
| Adult cells to PSC | OCT4/SOX2 / KLF4/c-MYC | Takahashi et al., 2007 |
| Adult cells to PSC | OCT4 SOX2/NANOG/LIN28 | Yu et al., 2007 |
| Adult cells to PSC | HDAC, HMTase inhibitors | Choi and Nam, 2012 |
| Adult cells to PSC | AMI-5 | Yuan et al., 2011b |
| Expansion of PSC | ROCK inhibitors | Watanabe et al., 2007 |
| Pluripotency maintenance of PSC | erythro-9-(2-hydroxy-3-nonyl)adenine | Burton et al., 2010a |
| Pancreatic exocrine to endocrine | Ngn3/Pdx1/MafA | Zhou et al., 2008 |
| Embryonic mesoderm to cardiomyocytes | Baf60c/Gata4/Tbx5 | Takeuchi and Bruneau, 2009 |
| Fibroblasts to cardiomyocytes | Gata4/Tbx5/Mef2c | Ieda et al., 2010 |
| Fibroblasts to neurons | Ascl1/Brn2/Pou3f2/Myt11 | Vierbuchen et al., 2010 |
| Fibroblasts to dopamine neurons | Mash1/Ngn2/Sox2/Nurr1/Pitx3 | Liu et al., 2012 |
| Fibroblasts to hepatocytes | Gata4/Hnfl1α/Foxa3/p19(Arf) inhibitor | Huang et al., 2011 |
| Melanocytes to neural crest | Notch1 | Zabierowski et al., 2011 |
| Fibroblasts to cardiomyocytes | Oct4/Klf4/Sox2/JAK-STAT inhibitor/BMF-4 | Efe et al., 2011 |
HDAC, histone deacetylase; ROCK, Rho-associated protein kinase; JAK-STATE, Janus kinase-signal transducer and activator of transcription.
Pharmacology potentially can enhance stem and progenitor cell-mediated regenerative therapies through at least five distinct strategies A) stem/progenitor cell expansion in culture (Fig. 9A); mobilization of endogenous stem/progenitor cells in situ (Fig. 9B); lineage-specific differentiation of stem/progenitor cells (Fig. 9C); differentiation from pluripotent stem cells (Fig. 9D); and reprogramming to generate pluripotent stem cells and lineage-restricted cells (Fig. 9E). We discuss selected examples of each of these approaches below. In addition, patient-derived or genetically engineered stem cells serve as novel sources for cell-based systems to mimic features of various human illnesses. Such “disease in a dish” models are emerging as powerful tools for drug discovery in regenerative medicine and, more broadly, in essentially any therapeutic arena (Gage, 2010; Walker, 2010). We also introduce this promising area of basic research, which opens up numerous new opportunities for pharmacology.
Growth factors, hormones, and other biologic signaling molecules may exert pharmacological effects to control the expansion, commitment, or differentiation of stem and progenitor cells. As noted earlier in this article, polymeric biomaterials also can exert pharmacological actions on these cells, both as vehicles for delivery of regulatory molecules and as physical substrates that provide functional cues through their mechanical properties (Furth et al., 2007; Keatch et al., 2012). Increasingly, stem cell biology also intersects with small molecule chemistry to expand the tool kit for regenerative pharmacology (Xu et al., 2008).
A. Stem/Progenitor Cell Expansion in Culture
A clinical experiment at Boston’s Peter Bent Brigham Hospital in 1980 marked a pivotal step toward the development of the first cell-based regenerative medicine products. Expansion in vitro of autologous keratinocytes from small remaining areas of skin enabled the rescue of two children who had suffered extensive third-degree burns (Green, 2008). In time this led to a commercialized product (Epicel: cultured epidermal autograft) (De Bie, 2007). Although fibroblasts had long been cultured successfully in the laboratory, the successful expansion of human keratinocytes, the essential covering cells of the skin, was new. Green and colleagues used epidermal growth factor (EGF) along with factors provided by “helper” murine fibroblasts (irradiated to prevent their own growth) to maintain long-term keratinocyte cultures. On the basis of his pioneering studies of transplantation of such expanded epithelial cell populations from skin or cornea, Green concluded that an essential feature for regenerative cell therapy was the presence in the graft of an adequate pool of stem cells (De Luca et al., 2006).
Various additional cell-based therapies now depend on culture conditions that enable substantial multiplication of donor cells. For example, expanded populations of autologous chondrocytes have received regulatory approval for use in cartilage repair (Manfredini et al., 2007). In some cases, animal or human serum may be used as a crude source of the necessary hormones and growth factors, without pharmacological analysis. However, for many cell types, as exemplified by keratinocytes, the use of specific growth factors in a defined, serum-free medium is essential to achieve proliferation of the stem/progenitor cells without premature terminal differentiation. In addition, the elimination of serum from expansion media mitigates regulatory concerns about animal-derived components and potential pathogens.
The best-characterized stem cells used to date in medicine are those of the blood-forming system. These hematopoietic stem cells (HSC) serve as precursors to all the specialized types of blood cells, including erythrocytes, megakaryocytes, and the components of the innate and adaptive immune systems (Bryder et al., 2006; Seita and Weissman, 2010). The presence of HSC and downstream progenitors derived from them in bone marrow and umbilical cord blood provides the basis for stem cell transplantation in the treatment of disorders such as leukemia and aplastic anemia. The cells actually capable of long-term self-renewal and full reconstitution of the hematopoietic system are rare, comprising less than 0.02% of nucleated cells in the bone marrow (Kent et al., 2009; Bonnefoix and Callanan, 2010). Although the HSC displays a remarkable capacity for proliferation in vivo (Cao et al., 2004; Gazit et al., 2008; Notta et al., 2011), only limited success has been achieved in culturing these cells without loss of “stemness.” Recent reviews survey efforts to employ a wide variety of growth factors and cytokines, as well as small molecules, to enable extensive expansion without excessive commitment to differentiation and concomitant loss of self-renewal capacity (Watts et al., 2011; Walasek et al., 2012; Furth et al., 2013). Some agents have been reported to induce up to 1,000-fold expansion of cells expressing CD34, a surface marker characteristic of many hematopoietic stem and progenitor cells. However, the most immature HSC, i.e., those capable of long-term reconstitution of the blood system, still become relatively depleted. A defined culture medium containing a cocktail of protein factors, including angiopoietin-like 5 does enable ~ 20-fold expansion of long-term HSC from human cord blood (Drake et al., 2011). A small molecule purine derivative designated StemRegenin 1 (SR1), which inhibits signaling through a ligand-dependent transcription factor associated with responses to toxic xenobiotics such as dioxin, likewise drives HSC proliferation to a comparable extent (Boitano et al., 2010). Sophisticated automated culturing systems that reduce the exposure of stem cells to inhibitory paracrine signals from more differentiated progeny cells also facilitate expansion of long-term human HSC (Csaszar et al., 2012). Taken together, the studies of the hematopoietic system reveal the challenges faced by regenerative pharmacology in seeking to regulate the complex balance between maintenance of stem cell identity versus commitment to progenitors that lose self-renewal capacity and give rise to large numbers of postmitotic, terminally differentiated cells.
By contrast to HSC, some classes of stem cells present in fetal and adult tissues can proliferate readily in vitro and retain their essential characteristics. For example, in the mid-1990s, Weiss et al. (1996) made the remarkable discovery that stem cells could be isolated from the mammalian central nervous system, not only during fetal development, but also throughout adult life. These neural stem cells (NSC) expand in aggregated spheres when cultured in serum-free, hormonally defined media containing EGF, sometimes further supplemented with fibroblast growth factor 2 (FGF-2). [Note that the name given to a growth factor often is based on the first cell type shown to be responsive, but does not necessarily convey the full range of the factor’s biologic activities.] Multipotent NSC can give rise to neurons, glia, and oligodendrocytes (Gage, 2000; Bergstrom and Forsberg-Nilsson, 2012). The neural stem cells enable life-long neurogenesis in some parts of the brain. Eventually, they may provide a basis to develop curative therapies for devastating neurodegenerative diseases. The use of defined media and bioreactors facilitates the uniform production of clinical grade cell populations (Baghbaderani et al., 2011).
First-in-human clinical studies of neural stem/progenitor cells have begun. For example, StemCells Inc. has initiated four trials of its HuCNS-SC product, although its first program in neuronal ceroid lipofuscinosis (Batten’s disease, a lysosomal storage disorder) was terminated because of insufficient patient enrollment. Possible areas of application for neural stem/progenitors include spinal cord injury, amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease, age-related macular degeneration, and cerebral palsy (Feng and Gao, 2012; Glass et al., 2012; Luan et al., 2012; Politis and Lindvall, 2012; Riley et al., 2012; Sandner et al., 2012).
B. Mobilization of Endogenous Stem Cells
In principle, it would be advantageous to find ways to effect regenerative responses of stem and progenitor cells in situ, without the need to isolate and transplant cells. However, pharmacological manipulation of stem cell proliferation in the body seems a tall order. Many of the growth factors or cytokines that have been found to promote stem cell proliferation and self-renewal act on multiple cell lineages, both during development and in normal tissue turnover. These include stem cell factor, which is the ligand for the c-Kit receptor, and members of the Wnt, Notch, and Hedgehog families of developmentally important signaling factors. Nonetheless, factors that have multiple cellular targets sometimes can exert relatively specific pharmacological effects. For example, pleiotrophin, a heparin-binding cytokine that acts on the receptor protein tyrosine phosphatase-β/ζ has a wide array of cellular targets: it enhances outgrowth of nerve fibers and can regulate cellular proliferation and/or differentiation of ES cells and in representative cell types derived from each of the three embryonic germ layers (Deuel et al., 2002). Even so, administration of recombinant pleiotrophin selectively stimulates the recovery of bone marrow in irradiated mice, accompanied by a 20-fold increase in long-term HSC (Himburg et al., 2010). Thus, it is possible that a pool of specific stem cells (e.g., the HSC) depleted by an insult such as radiation or chemotherapy could be restored by administration of a broadly acting drug without perturbing stem/progenitor cells in unaffected compartments of the body, which may be held in check by other homeostatic mechanisms.
Targeted delivery of a pleiotrophic factor may accomplish a similar end. The Wnt pathway plays a major role in regulating proliferation and differentiation of multiple categories of stem/progenitor cells, including those involved in bone formation. By packaging purified recombinant Wnt3a protein in liposomal vesicles that could be administered locally to skeletal defects in mice, investigators were able to promote more rapid bone regeneration through enhanced proliferation of skeletal progenitors and accelerated differentiation into osteoblasts (Minear et al., 2010).
Mobilization of stem cells from niches and their recruitment to damaged tissue sites also may support regeneration. Several cytokines, including granulocyte colony-stimulating factor (G-CSF; approved by the FDA under the names filgrastim and lenograstim) (Frampton et al., 1994), stimulate HSC to migrate from quiescent niches in the bone marrow and enter into the circulation. This has practical value in the collection of HSC from transplant donors and probably also contributes to recovery of the hematopoietic system from various insults. A small molecule named plerixafor (Mozobil; AMD3100) mobilizes HSC through another mechanism (Brave et al., 2010). It interferes with the action of a chemokine, stromal cell-derived factor-1, at its receptor CXCR4. Although the stromal cell-derived factor-1/CXCR4 axis is widely used to control cell migration in development, inflammation, and other contexts, the pharmacological activity of Plerixafor is sufficiently specific to displace HSC from bone marrow niches without causing unacceptable toxicities. The drug has received marketing approval from both the FDA and the European Committee for Medicinal Products for Human Use.
Regenerative pharmacology approaches can be used to selectively concentrate stem and progenitor cells at sites in need of repair. For stem cells that, unlike HSC, rarely enter into the circulation, transplantation may be best accomplished through grafting of cells embedded in an appropriate biomaterial. The scaffold could be augmented with growth factors and additional extracellular matrix components to help drive proliferation and/or differentiation (Turner et al., 2010). Validation of this approach came from a demonstration that delivery in hyaluronan hydrogels greatly improves retention of HpSC (hepatocyte stem cell) in the liver, promoting more efficient engraftment, proliferation, and vascularization (Turner et al., 2013) (Figs. 5–7). Grafting cells in a hydrogel matrix thus enhances tissue repair and decreases the risk of cells being carried through the bloodstream to become trapped and possibly to survive and even proliferate at distant sites.
A recent report showcases an elegant approach to promote bone regeneration by targeting stem/progenitors to an injured area. MSC are well established as precursors to bone-forming osteoblasts. The number of mesenchymal stem/progenitors in the bone marrow goes down with aging, leading to decreased osteogenesis and potentially contributing to the development of osteoporosis. However, transplantation of exogenous MSC neither leads to long-term engraftment of the marrow nor restores the cellularity of the correct part of the bone structure. To concentrate potentially regenerative cells at the appropriate location, Lane, Lam, and colleagues designed a compound to bridge between a membrane protein present on MSC and the bone surface (Guan et al., 2012). From a peptidomimetic library they selected a high-affinity ligand against integrin α4β1, designated LLP2A, and coupled it to alendronate (Ale), a bone-binding bisphosphonate. The resulting bifunctional reagent (LLP2A-Ale) was assessed for activity in immune-deficient mice grafted with human MSC. The compound stimulated homing and retention of the MSC at the bone surface, where they contributed to increased trabecular bone formation and bone mass over many weeks. Although Ale is used to treat osteoporosis, the bisphosphonate alone, without the targeting peptide, was not effective at the low doses used in these studies. The conjugated compound LLP2A-Ale also could direct endogenous MSC to the bone surface, as judged by stimulation of osteoblast activities. The drug prevented osteopenia (bone loss) with aging after peak bone mineral density had been achieved. Moreover, it curtailed the drastic loss of bone that occurs because of estrogen deprivation after ovariectomy of female mice. The data thus support a novel paradigm for pharmacological direction of stem cells to a target tissue where they can engage in repair and regeneration.
C. Lineage-Specific Differentiation of Stem and Progenitor Cells
Pharmacological administration of individual cytokines or growth factors can drive the production of specific differentiated cell types from multipotent or lineage-restricted stem and progenitor cell populations. The accelerated production of red blood cells after administration of recombinant human erythropoietin (EPO; epoetin) (Faulds and Sorkin, 1989) and of infection-fighting granulocytes after injection of recombinant G-CSF (Frampton et al., 1994) can be considered the first commercially significant examples of regenerative pharmacology. EPO primarily targets lineage-specific erythrocyte progenitors to drive rapid increases in the number of circulating red blood cells to combat anemia or, notoriously, as a performance-enhancing drug for endurance athletes. G-CSF targets committed granulocyte progenitors and speeds the recovery from neutropenia, for example after treatment of cancer patients with cytotoxic chemotherapeutic agents. In both cases the mature blood cells have a limited life span; human erythrocytes survive approximately four months and nonactivated granulocytes less than 1 week. EPO and G-CSF do not permanently alter the balance of stem and progenitor cell compartments in the bone marrow, so their effects are transient.
Pharmacological use of growth factors and cytokines on stem/progenitor cell populations also can have more long-lasting outcomes. United States and European regulatory agencies approved two members of a large family of bone morphogenetic proteins (BMPs) for several orthopedic applications. These proteins induce the proliferation and differentiation of bone precursor cells, likely including MSC. Recombinant human BMP-7, also known as osteogenic protein-1 or eptotermin alfa, delivered via a sponge made of type I collagen, received FDA approval under a Humanitarian Device Exemption for treatment of long-bone fractures that did not heal spontaneously (Friedlaender et al., 2001). The same cytokine, formulated as a putty with collagen and carboxymethylcellulose, is approved for revision spinal fusions. Similarly, a collagen sponge carrying recombinant human BMP-2 (INFUSE Bone Graft, Medtronic Spinal and Biologics, Memphis, TN) has been approved under an Humanitarian Device Exemption for certain fracture repair, bone grafting, and spinal fusion procedures (McKay et al., 2007).
In the United States alone, the market for BMP devices is in the range of $1 billion annually (Burks and Nair, 2010). However, recent critical reviews point out risks of significant and even catastrophic complications that can result from ectopic bone production attendant to BMP therapy (Carragee et al., 2011; Mirza, 2011). Therefore, finding improved agents to promote bone repair remains an important goal for regenerative pharmacology. Simply improving the delivery of BMPs might have significant benefit, as leakage of excess cytokine can result from overloading of collagen sponges. Genetically modified cells represent one possible system to provide BMP locally (Kimelman-Bleich et al., 2011). Osteoconductive materials, permissive for bone development, might synergize with BMPs and potentially reduce side effects. A promising new osteoconductive scaffold material was identified from a combinatorial library of biodegradable poly(β-amino ester)s; screening of a small molecule library revealed a number of "hits" that either promoted or inhibited osteogenic differentiation of MSC (Brey et al., 2010; Brey et al., 2011).
Other protein factors may synergize with or eventually replace BMP-7 or BMP-2 for many orthopedic applications. For example, the growth factor NEL-like molecule-1 (NELL1) acts on osteochondral lineage progenitor cells to promote bone regeneration, while suppressing the competing differentiation pathway to fat cells (adipocytes) (Zhang et al., 2010; Zou et al., 2011; James et al., 2012; Shen et al., 2012b). NELL1, alone or in combination with a BMP, enhances bone formation in a number of in vivo repair models (Li et al., 2011; Siu et al., 2011; Zhang et al., 2011; Zhu et al., 2011).
Although it may appear that there is significant redundancy among the sets of factors that can induce bone regeneration, the optimal choice of pharmacologic agent for a given application may depend on detailed understanding of the physiology of the target tissue and its repair. BMP-2 rapidly and irreversibly induces formation of both cartilage and new bone, but it also can cause unwanted ectopic bone formation (Noel et al., 2004). However, the broadly acting factor Wnt3a, when packaged in liposomes for local delivery, induces a bone-specific pattern of regeneration without ectopic bone formation (Minear et al., 2010). The effect appears consistent with enhancement of normal repair activities carried out by stem/progenitor cells migrating to areas of injury in the periosteum and to the bone marrow cavity. Thus, agents that promote Wnt signaling may have superior characteristics for regenerative pharmacology in orthopedics. Candidate drugs include not only recombinant Wnt3a protein, but also small molecule agonists of Wnt signaling (Gwak et al., 2012) and antagonists of endogenous inhibitors of the Wnt pathway (Agholme and Aspenberg, 2011).
D. Differentiation from Pluripotent Stem Cells
Despite the presence of lineage-restricted stem/progenitor cells in most or all of the body’s tissues, in many circumstances they are not readily accessible and/or may be difficult to propagate in culture. Since the first isolation of human ES cells, 17 years after the initial description of mouse ES cells, these have been touted as a possible source for replacement therapy of any specialized cell type (Odorico et al., 2001; Thomson et al., 1998). The generation of induced pluripotent stem (iPS) cells by genetic reprogramming of somatic cells (Takahashi and Yamanaka, 2006), for which Yamanaka shared the 2012 Nobel Prize in Physiology or Medicine, raised the ante by potentially enabling production of autologous specialized cells of any lineage for any human being (Yu et al., 2007; Nakagawa et al., 2008; Park et al., 2008; Okita and Yamanaka, 2011). A long-term goal would be to develop curative therapies for conditions in which crucial cell populations have been lost through injury or disease, without a need for immunosuppression. Potential clinical targets include such major disease areas as heart failure, liver failure, kidney failure, neurodegeneration, osteoporosis, and diabetes.
There is abundant evidence that ES and iPS cells can be induced to give rise to a wide variety of specialized cells types, and already a significant body of pharmacological data helps to define molecular mechanisms that contribute to differentiation toward various lineages (Atkinson et al., 2013). Nevertheless, achieving efficient, highly specific differentiation of pluripotent stem cells and successfully grafting the resulting stem cell-derived progeny cells to treat human disease pose formidable obstacles of scale, economics, and safety. Detailed discussion of these problems lies beyond the scope of this review. However, at least two other general points appear particularly salient to pharmacological approaches.
First, differentiation from an initial pluripotent stem cell to a desired differentiated cell type in almost all instances will require a series of steps corresponding to distinct milestones in the complex normal morphogenesis of mature tissues and organs. Current strategies generally are based on known developmental sequences. They begin by inducing the pluripotent cells to restrict to one of the three germ layers (ectoderm, mesoderm, and endoderm) that segregate at the gastrulation phase of embryogenesis (Murry and Keller, 2008). In most cases, investigators have next focused on the empirical identification, guided by knowledge of embryonic development, of growth factor/cytokine mixtures required to promote each of the sequential stages toward the desired mature cell type. However, for many cell types only inefficient and/or incomplete differentiation has been achieved to date. Processes that occur over many weeks in the tightly regulated environment of the developing embryo and fetus often cannot be perfectly replicated with cell lines in laboratory culture. 3D methodologies and chemical biology offer powerful new tools that should enhance our ability to derive therapeutic cell types and useful in vitro disease models from stem cells.
The application of bioengineering principles, in particular the development of suspension culture methodologies for the expansion and differentiation of pluripotent stem cells and their derivatives, offers significant opportunities to optimize the production of specialized cell types. For example, a recent report documents an 18-fold increase in definitive endoderm yield using optimized growth factor cocktails and a suspension bioreactor system (Ungrin et al., 2012). However, additional hurdles still must be overcome to generate differentiated cells at high purity and yield.
A second major concern lies in the relative immaturity of differentiated progeny derived from ES or iPS cells. For example, to generate β-like cells able to maintain normal blood sugar levels and respond to metabolic challenges in vivo, the Baetge team found it necessary to allow human ES cell-derived pancreatic progenitors to mature for more than 3 months after implantation into immune-deficient mice (Kroon et al., 2008). Similar observations come from critical assessment of hepatocytes derived from human pluripotent stem cells (Snykers et al., 2009; Delaforest et al., 2011). Careful comparison of hepatocyte-like cells obtained from human ES cells with authentic adult liver progenitor cells revealed significant differences (Funakoshi et al., 2011). After 3 weeks of differentiation in culture, the ES-derived cells lacked certain key adult features and more nearly resembled human fetal hepatocytes at less than 20 weeks of gestation.
Failure to achieve a normal, adult phenotype by differentiation of pluripotent stem cells in culture appears to be a general problem for many cell fates. A recent study used global transcriptional profiling to assess differentiation to multiple lineages, including representatives of each of the three germ layers (Patterson et al., 2012). In no case was the progeny from ES cells or iPS cells identical to mature tissue-derived cells. Of special concern, the differentiated cells continued to express a subset of genes associated with early embryonic development (e.g., LIN28A, LIN28B, and DPPA4). Overall, they showed characteristics of cells present within the first 6 weeks of human prenatal development. Although the results reinforce the value of ES and iPS cells in understanding human embryology, the immaturity of differentiated progeny derived from pluripotent stem cells may limit their utility in modeling adult human diseases and producing safe cells for regenerative medicine applications.
1. Pharmacological Tools for Differentiation from Pluripotent Stem Cells.
Further application of 3D culture technologies and tissue-specific matrix components, in addition to soluble growth factors and cytokines, have the potential to improve in vitro differentiation from both pluripotent stem cells and lineage-restricted stem/progenitor cells present in fetal and adult tissues (Ott et al., 2008; Baptista et al., 2011; Bratt-Leal et al., 2011; Kraehenbuehl et al., 2011; Leipzig et al., 2011; Mari-Buye and Semino, 2011; Spence et al., 2011; Wang et al., 2011c; Azarin et al., 2012; Cardinale et al., 2012; Purpura et al., 2012). In this arena there exists great potential for regenerative pharmacology to explore synergies among peptidic signaling molecules (growth factors, cytokines), ECM components and synthetic biomaterials, and small molecule pharmaceutical compounds.
Modulation of stem cell survival, growth, and differentiation by small molecules has been validated in concept and represents an area of intense research activity (Ding and Schultz, 2004; Barbaric et al., 2010; Li and Ding, 2010; Zhu et al., 2010; Yuan et al., 2011a; Choi and Nam, 2012; Li et al., 2012; Atkinson et al., 2013). The use of phenotypic screens to identify compounds that can serve as probes for the identification of specific cellular functions associated with differentiation can be viewed as an important application of “chemical genetics” (Sachinidis et al., 2008). Brief mention was already made of applications to Parkinson’s disease. Although there are examples of differentiation modulators for cell types from each of the three embryonic germ layers, for the focused aim of this review, below we provide a more detailed example highlighting potential cardiac applications.
2. Cardiac Lineage as an Example of Regenerative Pharmacology for Guiding Phenotypic Differentiation.
In vertebrates the first functional organ to develop is the heart. Turnover of adult cardiac muscle is extremely limited. In light of the high prevalence of heart disease, replacement of damaged or dead cardiomyocytes stands as an enormous challenge and opportunity for regenerative medicine (Gersh et al., 2009; Bartunek et al., 2010). Human cardiomyocytes also can serve as tools for toxicity testing and drug discovery. Generation of beating cardiomyocytes from pluripotent stem cells has been achieved through several approaches that have been reviewed recently (Rajala et al., 2011; Bernstein, 2012; Zwi-Dantsis and Gepstein, 2012). As with other cell types, the best results for directed differentiation have been achieved using a sequential approach patterned after normal development (Kattman et al., 2011). In this case the first step is induction of mesoderm, followed by progression to cardiac mesoderm, cardiac/cardiovascular progenitors, and cardiomyocytes. The differentiated cells can beat but have a relatively immature phenotype. Key mechanistic steps in the formation of cardiogenic mesoderm and the differentiation of committed progenitors to cardiomyocytes are still poorly understood. Moreover, authentic cardiovascular progenitors must actually give rise to a number of subtypes of cells corresponding to the first heart field, yielding left ventricular cardiomyocytes, and the second heart field, yielding smooth muscle and endothelial cells, sinoatrial nodal and atrioventral nodal cells (involved in pacemaker activity and controlled beating), atrial cardiomyocytes, and right ventricular cardiomyocytes. Recapitulating the full cardiac lineage tree in a controlled, directed manner remains a goal for future studies. Isolation of iPS cell-derived cardiovascular progenitor cells with potential to differentiate to endothelial, smooth muscle, and cardiomyocyte lineages represents an encouraging step in this direction (Nsair et al., 2012). Key steps in lineage specification appear to be determined by surprisingly subtle quantitative changes in signaling by TGF-β family members (Activin/Nodal and BMPs), which poses a significant challenge for effective pharmacology (Kattman et al., 2011). Assessment of temporal changes in chromatin structure provides a powerful new method to identify major regulators of cardiac development and may facilitate the identification of protein factors and small molecules to modulate differentiation (Paige et al., 2012).
Several practical methods have been devised to purify cardiomyocytes generated from pluripotent stem cells. For example, the high level of mitochondrial staining with the dye tetramethylrhodamine methyl ester perchlorate, facilitates enrichment of cardiomyocytes to >99% purity by fluorescence-activated cell sorting (Hattori et al., 2010). A different strategy rests on a genetic trick—linking expression of a drug resistance gene to a promoter expressed exclusively in the cardiac lineage to enable elimination of all undifferentiated stem cells and any cells that have committed to noncardiac fates (Zandstra et al., 2003).
Pharmacological studies have revealed both protein factors and small molecules capable of modulating cardiomyogenesis from pluripotent stem cells. Among members of known families, fibroblast growth factor 10 (FGF-10—a misleading name because, like FGF-7, also known as keratinocyte growth factor, this factor is mitogenic for keratinocytes but not for fibroblasts) appears crucial for normal cardiomyocyte differentiation from ES cells, based on inhibition studies with a neutralizing monoclonal antibody against FGF-10 and the use of inhibitors of the ligand’s cognate receptor protein-tyrosine kinase, FGF receptor-2 (Chan et al., 2010). Screening of a combinatorial compound library led to the discovery of small molecules that could induce cardiomyocyte differentiation from pluripotent stem cells (Wu et al., 2004). Four related diaminopyrimidine compounds, designated Cardiogenol A–D, showed this activity on a mouse embryonal carcinoma cell line, with the most potent having an EC50 of 0.1 μM. Cardiogenol C also induces a cardiomyocyte-like phenotype in multipotent stem cells derived from murine hair follicles (Yau et al., 2011). Studies in that system suggested that the compound enhances Wnt/β-catenin signaling, possibly through downregulation of Kremen1, a receptor for Dickkopf protein, which is known to negatively modulate the Wnt pathway. By contrast, in the ES cell system the administration of an inhibitor of Wnt signaling, XAV939, immediately after the generation of mesoderm progenitor cells strongly enhanced the production of cardiomyocytes (Wang et al., 2011a). XAV939 functions as an inhibitor of Tankyrase, thereby stabilizing Axin and inhibiting Wnt signaling. The apparently contradictory observations on the effects of Cardiogenol C and XAV939 may be reconciled by the precise timing of their administration. Murry and colleagues reported that Wnt/β-catenin signaling has a biphasic role in cardiac lineage differentiation from human pluripotent stem cells; at an early stage it promotes mesoderm induction, while at a later point it limits the production of cardiomyocytes from committed progenitors (Paige et al., 2010).
Yet another library screen led to the discovery of a distinct small molecule that enhanced cardiomyogenesis from ES cells by approximately 3-fold (Shen et al., 2012a). The "hit," designated compound 62 (a 2,6-disubstituted 4-anilinoquianzoline derivative), potently inhibits the tyrosine kinase activity of the EGF receptor (IC50 = 101 nM). However, other EGF receptor inhibitors do not show the same enhancement of cardiac differentiation, suggesting that compound 62 may have another as yet unidentified molecular target that is more specifically associated with the development of the heart.
As noted at several points in this article, regenerative pharmacology can encompass ECM components and other elements of the 3D microenvironment that may complement small molecule signaling modulators. Characterization of a niche for cardiovascular progenitor cells in developing mammalian hearts led investigators to formulate 3D cell culture inserts by electrospinning (see Fig. 6D) and in some experiments to coat them with collagen type IV (Col IV). They observed that Col IV indeed enhanced the expansion of the cardiac progenitor population, most notably when used in the 3D context (Schenke-Layland et al., 2011). In this case the addition of an inhibitor of Wnt/β-catenin signaling (IQ-1) (Miyabayashi et al., 2007) further facilitated expansion of the cardiac progenitors, with the effect additive to that of Col IV (Schenke-Layland et al., 2011). In a related approach, culturing of pluripotent stem cells in a sandwich configuration between layers of Matrigel together with sequential addition of known growth factors (activin A, BMP-4, FGF-2), promoted robust differentiation to cardiomyocytes (Zhang et al., 2012). Matrigel is a commercially available ECM, extracted from a transplantable mouse tumor line, that contains high levels of Col IV and laminin but also a number of less well-defined components including some growth factors (Vukicevic et al., 1992; Kleinman and Martin, 2005). Although much work remains to be done, there is clear evidence that such an approach may eventually yield a variety of novel therapeutics for cardiac disease/dysfunction.
E. Reprogramming to Generate Pluripotent Stem Cells and Lineage-Restricted Cells
Cell differentiation to a great extent reflects epigenetic control—that is, heritable changes in gene expression without alterations in DNA sequence (Ng and Gurdon, 2008). Although the underlying mechanisms remain incompletely understood, packaging of DNA into chromatin and modifications such as DNA methylation clearly play central roles. The understanding that the nucleus of a differentiated vertebrate cell could be reprogrammed to a ground state similar to that of cells in the earliest stages of embryonic development came from Gurdon’s remarkable experiments on nuclear transfer in Xenopus published 50 years ago (Gurdon, 1962; Gurdon et al., 2003) and recognized in 2012 with the Nobel Prize shared with Yamanaka. Gurdon showed that when a nucleus was transplanted from an intestinal cell into the cytoplasm of an enucleated egg, a viable tadpole clone could develop, demonstrating that the somatic cell contained an intact genome. Several decades later Wilmut accomplished the same feat of somatic cell nuclear transfer (SCNT) in a mammal to generate cloned sheep such as the famous "Dolly" (Wilmut et al., 1997).
Yamanaka and Blau (2010) discovered, astonishingly, that the forced expression of four transcription factors that are normally found in early embryonic cells suffices to reprogram differentiated somatic cells to an ES-like state, in much the same way as could be accomplished by SCNT or fusion with pluripotent cells. His group and that of Thomson confirmed that reprogramming of human cells, as with mouse cells, can reset pluripotency (Takahashi et al., 2007; Yu et al., 2007). The implications of these findings for regenerative medicine are enormous. Human iPS cell technology in principle enables the creation of patient-specific cellular therapeutics for any specialized lineage. Perfect histocompatibility matching would presumably obviate any need for immunosuppression (Fairchild, 2009). This assumption has been supported by studies with cloned bovine tissues produced by SCNT (Lanza et al., 2002), but challenged by recent experimental data with murine iPS cells (Zhao et al., 2011). In the case of genetic disorders, it should be possible to correct mutations prior to returning cells to the patient (Xu et al., 2009; Kazuki et al., 2010; Khan et al., 2010; Howden et al., 2011; Wong and Chiu, 2011). For patients with chronic infections such as a hepatitis or immunodeficiency virus, it may be possible to arm cells with protective antiviral genetic elements (Kamata et al., 2010; Rahman et al., 2011).
Taking diabetes as an example, a patient could donate a small sample of skin, hair, or blood for reprogramming to iPS cells. These would be expanded in culture, induced to differentiate to the pancreatic β-cell lineage, and implanted to replace the insulin-producing cells lost because of the disease. In type 1 diabetes, measures might still be required to counter the autoimmune attack that led to destruction of the patient’s original β-cells. For example, triggering antigens might be eliminated or masked by genetic modification. Great basic and practical hurdles remain to be overcome before the iPS cell technology and adjuncts such as the correction of genetic defects can be implemented at a meaningful scale in human medicine. Nevertheless, the overall promise of reprogramming cells to enable autologous therapies for regenerative medicine is spectacular (Nishikawa et al., 2008; Csete, 2010; Okita and Yamanaka, 2011; Ji et al., 2012; Robinton and Daley, 2012).
1. Genetic Reprogramming.
In the first reports of iPS technology, integrating recombinant vectors were used to deliver four genes (OCT4/SOX2/KLF4/c-MYC or OCT4/SOX2/NANOG/LIN28) to fibroblasts or other differentiated cells. The risks of insertional mutagenesis or reactivation of an oncogene (e.g., c-MYC) were perceived as significant safety issues for clinical applications of reprogrammed cells. It quickly became apparent that the expression of the inserted transgenes shuts off relatively early in reprogrammed cells, and endogenous pluripotency genes turn on. Therefore, transient expression of the reprogramming factors should suffice, allowing the use of self-inactivating or deletable viral vectors or nonintegrating viral or plasmid vectors (Okita et al., 2008; Stadtfeld et al., 2008; Chang et al., 2009; Yu et al., 2009). The introduction of the reprogramming transcription factors can also be accomplished in the form of purified recombinant proteins, sometimes with peptide tags to facilitate entry into the cell (Kim et al., 2009; Zhou et al., 2009; Cho et al., 2010; Pan et al., 2010; Zhou and Ding, 2010). Another emerging approach uses synthetic modified mRNA encoding the reprogramming factors to avoid the risk of permanent genetic modification of cells by DNA transduction (Warren et al., 2010).
2. Pharmacological (Chemical) Reprogramming.
Pharmacology already is being applied to the reprogramming process. In fact, much recent attention has focused on the potential of small molecules to make reprogramming more efficient, complete, accurate, and safe and possibly to replace some or even all of the transcription factors now used to reset cells to the pluripotent state (Shi et al., 2008; Ichida et al., 2009; Lyssiotis et al., 2009; Desponts and Ding, 2010; Wang et al., 2011b). Recent review articles from Ding’s laboratory, which has been a major contributor to this field, highlight the rapid progress that has been made (Li and Ding, 2010; Li et al., 2012). In some cases the choice of a target cell type that already expresses some of the four “pluripotency factors” can simplify the reprogramming cocktail. Under favorable circumstances iPS cells can be obtained using either NANOG or OCT4 in combination with small molecules (Theunissen et al., 2011; Yuan et al., 2011b). Studies in ES cells have shown that these two transcription factors are closely associated with the pluripotent phenotype (Chambers, 2004; Kashyap et al., 2009). An important goal will be to produce pluripotent cells that most nearly resemble normal cells of the early embryo, especially in features relevant to the safety and efficacy of future regenerative medicines.
Classes of small molecules that can contribute to reprogramming have been reviewed recently (Sidhu, 2011; Yuan et al., 2011a; Choi and Nam, 2012). Examples include compounds that influence obvious targets for epigenetic regulation such as inhibitors of histone deacetylase and histone methyltransferase (e.g., HMTase G9a) inhibitors, histone demethylase (e.g., LSD1), and inhibitors of DNA methyltransferase (e.g., 5-Aza). Inhibition by a compound designated AMI-5 of a previously unexpected target, methylation of proteins on arginine residues catalyzed by protein arginine methyltransferase, together with inhibition of TGF-β signaling, enable reprogramming to pluripotency by Oct4 as a single genetic factor (Yuan et al., 2011b). Additional compounds that influence cell signaling also can contribute to reprogramming. These include inhibitors of GSK-3, already discussed in other contexts; inhibitors of the Src protein-tyrosine kinases and of the MEK dual-specificity kinase that phosphorylates the tyrosine and threonine residues of ERK kinases required for activation in cellular signal transduction; and an L-type calcium channel agonist.
Attention also has focused on the use of small molecules to facilitate maintenance of pluripotent cells, once lines are established. For example, inhibitors of the Rho-associated protein kinase, a regulator of cell shape and motility acting via modulation of the cytoskeleton, prevents apoptotic death of dissociated ES and iPS cells, which makes their large-scale cultivation significantly easier (Watanabe et al., 2007; Harb et al., 2008; Koyanagi et al., 2008). A more direct effect on pluripotency per se is exerted by erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) and various analogs. These enable long-term growth of ES cells without differentiation, while allowing the rapid reacquisition of differentiation potential once the compound is removed (Burton et al., 2010a). EHNA, therefore, can replace FGF-2, a costly growth factor that is often used to maintain pluripotent human stem cells. EHNA inhibits both cyclic nucleotide phosphodiesterase 2 and adenosine deaminase, but other inhibitors of these enzymes do not have the same biologic effect on pluripotent cells, suggesting that a critical molecular target remains to be determined (Burton et al., 2010b).
3. Transdifferentiation.
Concerns still remain that reprogramming of somatic cells to a pluripotent state by any combination of genetic factors and small molecules may intrinsically be a disruptive process that is likely to induce unpredictable mutations, genetic instability, and epigenetic alterations. This consideration, together with recognition of practical translational advantages, has led a number of groups to explore the possibility of concerted switching of cells from one specialized lineage to another—a process sometimes called transdifferentiation. Viewed broadly, the directed differentiation of pluripotent cells and the direct reprogramming of mature cells to specific lineages may be viewed as complementary technologies for “turning straw into gold,” that is, to produce useful cells for regenerative medicine (Cohen and Melton, 2011).
Initial reports of success with directed transdifferentiation have catalyzed intensive investigation of both genetic and chemical methods for direct reprogramming to a range of specialized cell fates, including candidates for cellular therapeutics. A key paper from Melton’s laboratory reported the conversion of pancreatic enzyme-secreting exocrine cells to insulin-producing endocrine cells by transduction of three transcription factors (Ngn3, Pdx1, and MafA) in the mouse pancreas in vivo (Zhou et al., 2008). Other switches within cell types of related lineage have been reported. For example, embryonic mouse mesodermal cells can be induced to a cardiomyocyte fate in culture or to develop rapidly into cardiomyocytes if injected into the heart, after introduction of three factors, one encoding a cardiac-specific subunit of a chromatin remodeling complex (Baf60c) and two encoding cardiac lineage transcription factors, Gata4 and Tbx 5 (Takeuchi and Bruneau, 2009). Similarly, introduction of Gata4, Tbx5, and a third cardiac transcription factor, Mef2c, converted fibroblasts to functional cardiomyocytes (Ieda et al., 2010).
In experiments focused on neuronal lineages, mouse fibroblasts have been reprogrammed by introduction of three transcription factors (Ascl1, Brn2/Pou3f2, and Myt1l) into neurons that can generate action potentials and form synapses (Vierbuchen et al., 2010). Similarly, genes for five transcription factors, Mash1, Ngn2, Sox2, Nurr1, and Pitx3, induced conversion of fibroblasts into cells resembling dopaminergic (DA) neurons that exhibited characteristic dopamine uptake and electrophysiological profiles (Liu et al., 2012). These DA-like neurons relieved Parkinson disease-like symptoms in a rat model. Thus, induced transdifferentiation may provide yet another regenerative pharmacology strategy to treat PD (see above). Finally, microRNAs involved in terminal differentiation of neural progenitors, augmented by neural transcription factors, promoted transdifferentiation of human fibroblasts to neurons (Yoo et al., 2011). It is noteworthy that fibroblasts, which originate in mesoderm, derive from a different embryonic germ layer than neurons, which normally derive from ectoderm.
In another switch involving cell types representing different germ layers, several groups have reported the generation of induced hepatocyte-like cells from adipose-derived stromal cells or fibroblasts (Lue et al., 2010; Huang et al., 2011; Sekiya and Suzuki, 2011). For example, a combination of the transcription factors Gata4, Hnf1alpha, and Foxa3, together with inactivation of the cell cycle regulator p19(Arf), gave rise to induced hepatocyte-like cells that were able to restore liver function in a mouse genetic model in vivo (Huang et al., 2011).
A related strategy entails selection of lineage-specific stem/progenitor cell populations that can be expanded extensively. In some cases this apparently has been accomplished by relatively minimal changes that appear to reset differentiated cells back to a progenitor stage within the same lineage. For example, mature, pigmented melanocytes were reverted to neural crest stem cells in response to expression of the active intracellular form of Notch1 (Zabierowski et al., 2011). Similarly, astrocytes were converted to neural stem/progenitors (Corti et al., 2012).
A powerful, general means to obtain lineage-committed cells, both mature and expandable stem/progenitor populations, follows from observations that transient exposure to subsets of the four “conventional” pluripotency factors also can mediate cell fate switching across unrelated lineages without the need to isolate stable iPS cells (Nie et al., 2012). Deng and colleagues (Efe et al., 2011) tested this idea in the context of conversion of fibroblasts to cardiomyocytes. Exposure of starting cells to Oct4, Klf4, and Sox2 in the presence of a Janus kinase-signal transducer and activator of transcription inhibitor to limit iPS cell formation was followed by exposure to the cardioinductive growth factor BMP-4 in chemically defined medium. This protocol indeed led to the rapid, efficient production of cardiomyocytes (Efe et al., 2011). The same strategy of transient exposure of a population to the pluripotency factors followed by additional lineage-specific genetic factors and/or inductive signals enabled reprogramming of fibroblasts to neural stem/progenitors capable of both extensive self-renewal and differentiation to neurons, astrocytes, and oligodendrocytes in culture (Kim et al., 2011; Han et al., 2012; Thier et al., 2012). Remarkably, Oct4 alone can induce lineage conversion in cells capable of proliferation, without acquisition of a pluripotent phenotype. Bhatia and colleagues observed that colonies of fibroblasts transduced with Oct4 sometimes express a surface marker found on virtually all blood cells (CD45) (Szabo et al., 2010). They demonstrated that these cells have activated hematopoietic transcriptional programs. Furthermore, the fibroblast-derived cells behaved as multipotent progenitors of the granulocytic, monocytic, megakaryocytic, and erythroid lineages and were able to engraft mice and generate the corresponding mature blood cells.
Rigorous testing of cells obtained by various reprogramming approaches will be required to ascertain the fidelity relative to normal lineage programs, the presence of embryonic and fetal versus adult molecular/genetic signatures, as well as for genomic instability and epigenetic idiosyncrasies. Nonetheless, it seems clear that combinations of genetic and pharmacological manipulation increasingly will enable the directed production of lineage-restricted stem/progenitors and mature specialized cells. As these technologies become robust and cost-effective, they will likely drive the creation of individualized cell-based therapies for multiple genetic disorders and degenerative diseases.
V. Disease Models from Patient-specific Reprogrammed Cells
Going beyond regenerative medicine, the ability to produce cells of essentially any lineage with the genotype of any person, coupled with advances in human genetics and genomics, holds promise to literally transform pharmacology through the creation of new, readily accessible models of human disease. The notion is captured in the phrase “disease-in-a-dish” (Saha and Jaenisch, 2009; Gage, 2010; Walker, 2010). The fundamental assumption is that disease phenotypes can be replicated in specialized cells, produced from iPS cells or by direct reprogramming, that carry the genetic constitution of affected individuals. This appears most straightforward for diseases that result from single mutations with high penetrance, i.e., those that demonstrate simple Mendelian inheritance. A first example came from the identification of motor neuron abnormalities in neural-lineage cells differentiated in vitro from patient-specific iPS cells for human spinal muscular atrophy, an autosomal recessive genetic disorder resulting from mutations that decrease levels of the protein survival motor neuron 1 (Ebert et al., 2009; Ebert and Svendsen, 2010).
The concept of in vitro replication of human disease phenotypes in specialized cells derived from patient-specific iPS cells already has gained at least partial validation for a number of neurologic, hematopoietic, cardiovascular, and metabolic disorders (Unternaehrer and Daley, 2011; Ebert et al., 2012; Maury et al., 2012; Rajamohan et al., 2013). Examples of specific conditions for which patient-specific iPS cells have been obtained and differentiated into disease-relevant cell types include Parkinson’s disease (Byers et al., 2012; Jang et al., 2012) and other neurodegenerative disorders (Ito et al., 2012; Jung et al., 2012), Wilson’s disease (Yi et al., 2012), lysosomal storage disorders (Huang et al., 2012b), and diabetes (Fujikura et al., 2012).
A comprehensive review of specific abnormal phenotypes modeled via the disease-in-a-dish approach lies beyond the scope of this article. However, one example can be presented briefly to highlight the potential to generate predictive in vitro models for disorders in which the underlying genetics and biology are relatively complex. Rett syndrome (RTT) exemplifies autism spectrum disorders, a set of neurodevelopmental diseases characterized by behavioral phenotypes such as impaired social interaction and repetitive behaviors. RTT results from mutations in the MECP2 gene, encoding methyl-CpG binding protein 2. This X-linked gene is inactivated randomly in females, so that heterozygous individuals display mosaic expression of an abnormal protein (or complete absence of the protein in about 50% of cells, in the rarer case of null alleles). RTT patient-specific iPS cells have been isolated and shown to undergo X-chromosome inactivation in the course of differentiation to yield functional neurons (Marchetto et al., 2010a; Ananiev et al., 2011; Cheung et al., 2011). Of great importance, the RTT iPS cell-derived neurons showed a number of abnormalities compared with normal controls, such as reduced numbers of synapses and spine density and functional deficits in calcium signaling and electrophysiology (Marchetto et al., 2010a). The studies pointed to a developmental window during which deficits might be corrected. Similar studies with glutaminergic neurons derived from iPS cells obtained from a mouse model of RTT also showed electrophysiological abnormalities, possibly resulting from abnormalities in sodium channel function (Farra et al., 2012). The ability to identify the earliest stages of neurodevelopmental disorders (Marchetto et al., 2010b), some forms of diabetes that typically arise in adolescents and young adults (MODY) (Vaxillaire and Froguel, 2006), and other diseases likely to be traceable to genetically determined events during prenatal development, represents a clear opportunity to apply iPS cell-based assays to search for pharmacological interventions.
What about major diseases that generally become manifest later in life? Common diseases, such as cardiovascular disorders and atherosclerosis, psychiatric disorders, metabolic disorders (e.g., diabetes and obesity), and inflammatory diseases (e.g., asthma, rheumatoid arthritis) also have strong genetic components, although it is rare that a single genetic variation (polymorphism) will strictly predict an individual’s susceptibility. These common disorders are viewed best as quantitative traits in which genotype helps determine extremes of phenotype that can be classified as pathologic (Plomin et al., 2009). In many instances it appears reasonable to anticipate that key aspects of the genetically determined phenotype can be replicated in cultured cells derived from patient-specific stem cells. This should enable screening assays to identify compounds that reverse the disease-like “symptoms” in vitro, with high expectation that effective molecules will target pathways directly relevant to genetically defined subsets of many of the most prevalent human diseases.
VI. Stem Cells as a Tool to Provide Therapeutic Agents
A. Overview of Cells as Therapeutic Delivery Vehicles
As briefly noted in section II above (see Fig. 5D), microcarriers are also an important aspect of regenerative medicine technologies with respect to cell encapsulation for various conditions in which cells can be used to produce therapeutic agents. In this regard, cells are well known to be “factories” for a vast range of “natural” drugs and that they might be considered among the best (and most complex) “drug delivery microcarriers” known to man. Cells can produce therapeutic agents that range from small molecules (e.g., cAMP or hormones) to peptides to polypeptides to higher order protein structures. The resulting “therapeutics” modulate numerous functions, including vasodilation, endocrine functions, inflammation (pro- and anti-), cell division, cell migration, and cell differentiation. For example, one of the hallmarks of leukocyte function is to home to sites of injury via chemical cues, and it is thought that regenerative aspects associated with marrow-derived stem cells may be attributable to (in part) their immunomodulatory capabilities (Caplan, 2001).
Making use of cells to produce therapeutic agents per se is certainly not a new concept. Before the era of high-throughput drug screening and molecular modeling of drug-receptor interactions, many medications were initially isolated from plant sources (e.g., paclitaxel)—indicating their production by cells (Cragg and Newman, 2013). Similarly, the biochemical industry produces important therapeutics from penicillin (Ligon, 2004) to insulin (Ladisch and Kohlmann, 1992) through the use of microbial, plant, and mammalian cultures both with and without recombinant DNA technology. Although these technologies lead to the production of therapeutic agents through more traditional pharmaceutical manufacturing methods (reactors, separation trains, and related quality control measures), the concept of using cells in situ to produce pharmaceutical agents is a more recent development. Examples of this approach range from the production of growth factors from cells within biomaterial scaffolds that take up viral or nonviral particles (Saul et al., 2007; De Laporte et al., 2010) to injecting cells with viral gene delivery particles in situ (Barton-Davis et al., 1998) to genetically engineering cells before implantation (Edwards et al., 2005). Each of these approaches ultimately leads to cell-based production of growth factors. Typically nonviral methods and non-genome DNA delivery from viruses leads to transient expression of the therapeutic, whereas nucleic acid delivery from viruses that incorporates into the genome can lead to long-term expression. The temporal need for the therapeutic agent must therefore be considered when choosing the construct used to deliver the nucleic acids. Conceptually, cellular biochemical pathways might be used to produce drugs ranging from small molecules to proteins, although the methods to optimize delivery in situ pose as a future challenge.
The use of cells to produce a therapeutic agent, though, is clearly not dependent on genetic manipulation of the cells; certain cell types naturally produce a needed therapeutic. The prototype for materials-based approaches to using cells as factories for production of therapeutic agents is the encapsulation of Islet cells for the production of insulin. Alginate encapsulation of pancreatic Islet cells provides a means to achieve a glucose-responsive system that can respond to the physiologic state in a natural way. Furthermore, such approaches have used components that prevent immunologic response, allowing the use of allogeneic or xenogeneic cell sources to be considered (Opara et al., 2010).
One challenge associated with such approaches to using cells to produce pharmacological agents is that this approach implies the use of these cells in their nonnative state. For example, the encapsulation of pancreatic Islets in alginate (a material derived from algae) is not reflective of the native state of the Islet cells in the pancreas where these cells rest on a specific protein-based extracellular matrix. Specifically, it might be expected that the three-dimensional conformation of Islets or any other cell type interacting with a material would differ from its native state, potentially affecting biochemical synthesis of the therapeutic agents. Approaches to better replicate the native state to improve materials for cell-mediated delivery of “native therapeutics” are increasingly investigated, that is, methods for improved recapitulation of native architecture for fabrication of de novo tissues. This has been recognized for some time with the development of small diameter vascular grafts in which proper flow conditions and cell-cell interactions promote proper endothelial cell phenotype for the production of vasodilators such as nitric oxide and proper regulation of cell surface markers of endothelial cell health such as selectins. Recently, we demonstrated the importance of the architecture in a tissue engineered ovary through more native-like cell-cell interactions. Through the proper orientation of granulosa and theca cells in an alginate material, more physiologically relevant levels of estrogen and progesterone were produced (Sittadjody et al., 2013).
B. Mesenchymal Stem Cells as a Therapeutic Delivery Vehicle
Stem cells provide the cellular building blocks of new tissue formation, and moreover, pharmacology can be used to guide this process. However, some stem cell populations, notably mesenchymal stem cells (MSC), also have other unique characteristics that make them useful for direct provision of chemicals or other therapeutic agents for pharmacological modulation of tissue and organ regeneration (Porada and Alemeida-Porada, 2010), as they are well known to elaborate a host of bioactive molecules and factors (Caplan, 2013). Related to these properties, there are two applications we will consider herein: 1) the use of MSC as "factories" to deliver trophic factors—immunosuppressive and anti-inflammatory factors that leverage the cells’ intrinsic ability to home to damaged tissues and tumors; and 2) their use as cellular delivery machines for focal regenerative pharmacology, by local production of factors that modulate regeneration, repair, and restoration of tissue and organ function (Mooney and Vandenburgh, 2008). These are considered below.
1. MSCs as Factories for Trophic Factors.
There is good evidence that MSCs are able to distribute and then lodge/engraft within multiple tissues in the body and release trophic factors that trigger the tissue’s own endogenous repair pathways, and in some cases (e.g., bone, fat, cartilage) provide a source of tissue-specific cells (Pretheeban et al., 2012; Caplan, 2013). However, the potential of MSCs to mediate repair is often observed in the absence of any evidence of sustained engraftment of the transplanted cell in the damaged organ. Rather, the injected MSCs home to the injured area, in particular to hypoxic, apoptotic, or inflamed regions, and release trophic factors that hasten endogenous repair by producing tissue protection, enhancing angiogenesis, inhibiting fibrosis and apoptosis, and stimulating recruitment, retention, proliferation, and differentiation of tissue-residing stem cells (Joyce et al., 2010). In short, a significant body of evidence now indicates that MSCs can stimulate regeneration and repair (Caplan, 2013), and thus are likely to play important roles in promoting tissue recovery of, for example, the myocardium (Brink and Cohen; 2013; Shim et al., 2013; Williams et al., 2013; Zhao and Huang, 2013), the central nervous system (Joyce et al., 2010; Huang et al., 2012b; Kramer et al., 2012), and the liver (Krishna et al., 2011). Studies on the injured heart, in particular, have provided evidence that many of the beneficial effects of MSCs in the repair/regeneration of the damaged myocardium, may be caused by promotion of angiogenesis (Huang et al., 2009). MSCs appear to secrete vascular VEGF and bFGF upon contacting the injured myocardium, which stimulates the formation of new vessels and increases capillary density to increase/restore blood flow to an infarcted region (Li et al., 2009). In addition to these paracrine and trophic activities, it would seem that MSCs have properties that help not only to reduce existing damage but promote the healing process (Porada and Almeida Porada, 2010). Thus in the liver, it has been shown that MSCs can enhance fibrous matrix degradation, likely through the induction of matrix metalloproteinases. Also in the heart, MSCs may release paracrine factors that attenuate fibroblast proliferation and inhibit collagen synthesis/deposition, apparently by stimulating cardiac fibroblasts to secrete matrix metalloproteinases. Taken together, these studies clearly emphasize the intrinsic pharmacological properties of MSCs to modulate tissue/organ regeneration and repair.
2. Mesenchymal Stem Cells as Delivery Machines.
In addition to MSCs, several other cell systems have recently emerged as biologic drug carriers, such as carrier erythrocytes, bacterial ghosts, and genetically engineered stem and dendritic cells (Gutierrez Millan et al., 2012). However, adult MSCs have been widely studied because they are easy to isolate from different tissues (not only from bone marrow) and to differentiate into cells of various organs (Chiu and Rao, 2011; Dhara et al., 2011; Caplan, 2013). These properties, together with their hypoimmunogenicity, make them good candidates either for tissue regeneration or as vehicles in gene therapy.
It is possible either to augment the natural MSC production of specific proteins and to enable the cells to express proteins outside of their native repertoire, which greatly broadens the spectrum of diseases for which these cells may provide therapeutic benefit. For example, with respect to novel treatments for arrhythmias, human bone marrow MSCs, which express connexins and can form functional gap junctions in the heart (Brink and Cohen, 2013), can be gene modified to express a desired ion channel (the “funny current” or HCN channel responsible for pacing), and then can be focally implanted to provide a “biological” pacemaker.
In addition, MSCs can be readily transduced with all of the major clinically prevalent viral vector systems, including those based upon adenovirus, the murine retroviruses, lentiviruses, and AAV (Porada and Almeida-Porada, 2010), to efficiently produce a wide range of cytoplasmic, membrane-bound, and secreted protein products. This ease of transduction coupled with the ability to subsequently select and expand only the gene-modified cells in vitro to generate adequate cell numbers for transplantation, combine to make MSCs one of the most promising stem cell populations for use in gene therapy studies and trials. The ability of MSCs to migrate to a target tissue in vivo suggests the potential use of hMSCs as a cellular delivery system for a variety of bioactive molecules, and recent studies indicate that small interference RNA and microRNA are also among these, as they are able to cross gap junction channels (Brink et al., 2012). Use of genetically recombinant stem cells and biomimetic nanostructured scaffolds for the development of novel biomimetic drug delivery systems has received widespread attention as a promising strategy for wound treatment, in which multipotent stem cells, encoded with plasmid DNA coding for polypeptides, are used both as the cellular therapeutic medium as well as the vehicle for the delivery of functional genes to the wound site (Peng et al., 2012).
The majority of studies using gene-modified stem cells have been undertaken with the purpose of enhancing the natural abilities of stem cells to mediate repair within various tissues; however, MSCs have the ability to accumulate at the site of not only tissue/organ damage/inflammation but may also locate to cancer tissue when administered in vivo (Studeny et al., 2002, 2004; Hall et al., 2007). The MSCs seem to have the ability to “sense” the forming tumor, migrate to the tumor, and contribute to the newly forming tumor stroma (Hall et al., 2007). This property is now recognized as a powerful and unique means of selectively delivering anticancer gene products to tumor cells in vivo (Studeny et al., 2002, 2004; Hall et al., 2007; Hu et al., 2012). Analogous strategies may be applicable to tissue and organ regeneration as well.
VII. Summary and Future Directions
There has been an explosion of information and a parallel increase in technology development since we first attempted to bring the new field of regenerative pharmacology to the attention of pharmacologists (Andersson and Christ). However, our overall goal for doing so remains unaltered, that is, to get pharmacologists more involved in this growing field of research by exposing them to the tools, opportunities, challenges and expertise that will be required to increase awareness and spur excitement within the pharmacological community. Our overall goal was to provide sufficient detail from each of the critical intersecting fields of research to emphasize the necessity for multidisciplinary collaboration and hope that one outcome of this report is that it will indeed generate the required conversations among all of the stakeholders. In short, we believe that the field of regenerative medicine and its companion field, tissue engineering, will benefit tremendously from the more rigorous application of pharmacological sciences. That is, despite the unequivocal success and enormous potential of regenerative medicine and tissue engineering technologies, a greater focus on evaluation of functional outcomes and endpoints is still required. Even from a macroscopic perspective it is clear that a more extensive characterization of basic pharmacokinetic and pharmacodynamics principles is required. Specifically, this should include, among others, assessment of excitation-contraction coupling mechanisms, more rigorous analysis of concentration-response curve data using standard pharmacological analyses/methods, estimation of receptor affinity, receptor subtypes, intrinsic activity, efficacy, potency, etc. In fact, a greater emphasis on the pharmacology and physiology of various regenerative medicine and tissue engineering approaches is critical to increase understanding of tissue/organ regeneration and repair processes and therefore is a necessary prerequisite to increasing the rate of technology development and eventual clinical translation. To this end we have attempted to unite, in a single report, the salient features of diverse fields of research—ranging from materials chemistry and functionalized biomaterials to stem cells, organ/tissue regeneration, wound healing, and development biology—in the hope that providing all of this information at this time would provide the foundation for future interactions and discussions. This report represents the first leg in a long journey.
Abbreviations
- 3D
three dimensional
- 6-OHDA
6-hydroxydopamine
- AADC
l-amino acid decarboxylase
- AAV
adeno-associated virus
- AFS
amniotic fluid cells
- Ale
alendronate
- AR
adrenoreceptor
- BAM
bladder acellular matrix
- BMPs
bone morphogenic proteins
- BrdU
bromodeoxyuridine
- CHIR99021
6-(2-(4-(2,4-dichlorophenyl)-5-(4-methyl-1H-imidazol-2-yl)-pyrimidin-2-ylamino)ethyl-amino)-nicotinonitrile
- Col IV
collagen type IV
- DA
dopamine
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine
- EPO
erythropoietin
- ES
embryonic stem
- FDA
Food and Drug Administration
- FGF
fibroblast growth factor
- G-CSF
granulocyte colony-stimulating factor
- GDNF
glial cell line–derived neurotrophic factor
- GSK
glycogen synthase kinase 3
- HSC
hematopoietic stem cells
- IGF
insulin-like growth factor
- iPS
induced pluripotent stem
- IQ-1
2-[(4-acetylphenyl)diazenyl]-2-(3,3-dimethyl-2,4-dihydro-1H-isoquinolin-1-yl)acetamide; molecular formula C21H22N4O2
- l-DOPA, l-3
4-dihydroxyphenylalanine
- LP
lamina propria
- MP
muscularis propria
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyrinde
- MSC
mesenchymal stromal cells
- NEL
a protein strongly expressed in neural tissue encoding epidermal growth factor-like domain
- NELL1
NEL-like molecule-1
- NGF
nerve growth factor
- NSC
neural stem cells
- NTN
neurturin
- PD
Parkinson’s disease
- PLGA
poly(lactic-co-glycolic acid)
- RM
regenerative medicine
- RTT
Rett syndrome
- SCNT
somatic cell nuclear transfer
- STC
subtotal cystectomy
- TE
tissue engineering
- TGF
transforming growth factor
- VEGF
vascular endothelial growth factor
- XAV939
C14H11F3N2OS
Authorship Contributions
Participated in research design: Christ, Saul, Furth, Andersson.
Wrote or contributed to the writing of the manuscript: Christ, Saul, Furth, Andersson.
Footnotes
This work was supported in part by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grants DK081832 and DK097806].
References
- Agholme F, Aspenberg P. (2011) Wnt signaling and orthopedics, an overview. Acta Orthop 82:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananiev G, Williams EC, Li H, Chang Q. (2011) Isogenic pairs of wild type and mutant induced pluripotent stem cell (iPSC) lines from Rett syndrome patients as in vitro disease model. PLoS ONE 6:e25255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson KE, Chapple CR, Cardozo L, Cruz F, Hashim H, Michel MC, Tannenbaum C, Wein AJ. (2009) Pharmacological treatment of overactive bladder: report from the International Consultation on Incontinence. Curr Opin Urol 19:380–394 [DOI] [PubMed] [Google Scholar]
- Andersson KE, Christ GJ. (2007) Regenerative pharmacology: the future is now. Mol Interv 7:79–86 [DOI] [PubMed] [Google Scholar]
- Aron L, Klein R. (2011) Repairing the parkinsonian brain with neurotrophic factors. Trends Neurosci 34:88–100 [DOI] [PubMed] [Google Scholar]
- Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. (2006) Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 367:1241–1246 [DOI] [PubMed] [Google Scholar]
- Atala A, Lanza R, Thompson TA, Nerem R. (2011) Principles of Regenerative Medicine, Academic Press, New York [Google Scholar]
- Atkinson SP, Lako M, Armstrong L. (2013) Potential for pharmacological manipulation of human embryonic stem cells. Br J Pharmacol 169:269–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azagarsamy MA, Alge DL, Radhakrishnan SJ, Tibbitt MW, Anseth KS. (2012) Photocontrolled nanoparticles for on-demand release of proteins. Biomacromolecules 13:2219–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarin SM, Lian X, Larson EA, Popelka HM, de Pablo JJ, Palecek SP. (2012) Modulation of Wnt/β-catenin signaling in human embryonic stem cells using a 3-D microwell array. Biomaterials 33:2041–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddour JA, Sousounis K, Tsonis PA. (2012) Organ repair and regeneration: an overview. Birth Defects Res C Embryo Today 96:1–29 [DOI] [PubMed] [Google Scholar]
- Badylak SF, Weiss DJ, Caplan A, Macchiarini P. (2012) Engineered whole organs and complex tissues. Lancet 379:943–952 [DOI] [PubMed] [Google Scholar]
- Baghbaderani BA, Mukhida K, Hong M, Mendez I, Behie LA. (2011) A review of bioreactor protocols for human neural precursor cell expansion in preparation for clinical trials. Curr Stem Cell Res Ther 6:229–254 [DOI] [PubMed] [Google Scholar]
- Balmayor ER, Tuzlakoglu K, Marques AP, Azevedo HS, Reis RL. (2008) A novel enzymatically-mediated drug delivery carrier for bone tissue engineering applications: combining biodegradable starch-based microparticles and differentiation agents. J Mater Sci Mater Med 19:1617–1623 [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Eberling JL, Kohutnicka M, Jagust W, Pivirotto P, Bringas J, Cunningham J, Budinger TF, Harvey-White J. (2000) Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol 164:2–14 [DOI] [PubMed] [Google Scholar]
- Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, Bringas J, Herscovitch P, Carson RE, Eckelman W, Reutter B, et al. (2006) Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol Ther 14:564–570 [DOI] [PubMed] [Google Scholar]
- Banquy X, Leclair G, Rabanel JM, Argaw A, Bouchard JF, Hildgen P, Giasson S. (2008) Selectins ligand decorated drug carriers for activated endothelial cell targeting. Bioconjug Chem 19:2030–2039 [DOI] [PubMed] [Google Scholar]
- Baptista PM, Siddiqui MM, Lozier G, Rodriguez SR, Atala A, Soker S. (2011) The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology 53:604–617 [DOI] [PubMed] [Google Scholar]
- Barbarese E, Ho SY, D’Arrigo JS, Simon RH. (1995) Internalization of microbubbles by tumor cells in vivo and in vitro. J Neurooncol 26:25–34 [DOI] [PubMed] [Google Scholar]
- Barbaric I, Gokhale PJ, Jones M, Glen A, Baker D, Andrews PW. (2010) Novel regulators of stem cell fates identified by a multivariate phenotype screen of small compounds on human embryonic stem cell colonies. Stem Cell Res (Amst) 5:104–119 [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. (1998) Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95:15603–15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartunek J, Vanderheyden M, Hill J, Terzic A. (2010) Cells as biologics for cardiac repair in ischaemic heart failure. Heart 96:792–800 [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J, Johnson EMJ, Jr, Olanow CW, Mufson EJ, Kordower JH. (2011) Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord 26:27–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX, Rudnicki MA. (2012) Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström T, Forsberg-Nilsson K. (2012) Neural stem cells: brain building blocks and beyond. Ups J Med Sci 117:132–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein HS. (2012) Cardiac repair and restoration using human embryonic stem cells. Regen Med 7:697–712 [DOI] [PubMed] [Google Scholar]
- Bertram T, Christ GJ, Andersson KE, Aboushwareb T, Fuellhase C, Soler R, Wagner BJ, Jain D, Ludlow JW, Payne R, et al. (2008) Total urinary bladder regeneration with restoration of native structure and pharmacological response. FASEB J 22:581 [Google Scholar]
- Bessa PC, Machado R, Nürnberger S, Dopler D, Banerjee A, Cunha AM, Rodríguez-Cabello JC, Redl H, van Griensven M, Reis RL, et al. (2010) Thermoresponsive self-assembled elastin-based nanoparticles for delivery of BMPs. J Control Release 142:312–318 [DOI] [PubMed] [Google Scholar]
- Biondi M, Indolfi L, Ungaro F, Quaglia F, La Rotonda MI, Netti PA. (2009) Bioactivated collagen-based scaffolds embedding protein-releasing biodegradable microspheres: tuning of protein release kinetics. J Mater Sci Mater Med 20:2117–2128 [DOI] [PubMed] [Google Scholar]
- Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, et al. (2010) Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329:1345–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland T, Xu T, Damon B, Cui X. (2006) Application of inkjet printing to tissue engineering. Biotechnol J 1:910–917 [DOI] [PubMed] [Google Scholar]
- Boll MC, Alcaraz-Zubeldia M, Rios C. (2011) Medical management of Parkinson’s disease: focus on neuroprotection. Curr Neuropharmacol 9:350–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnefoix T, Callanan M. (2010) Accurate hematopoietic stem cell frequency estimates by fitting multicell Poisson models substituting to the single-hit Poisson model in limiting dilution transplantation assays. Blood 116:2472–2475 [DOI] [PubMed] [Google Scholar]
- Borden MA, Zhang H, Gillies RJ, Dayton PA, Ferrara KW. (2008) A stimulus-responsive contrast agent for ultrasound molecular imaging. Biomaterials 29:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucard N, Viton C, Agay D, Mari E, Roger T, Chancerelle Y, Domard A. (2007) The use of physical hydrogels of chitosan for skin regeneration following third-degree burns. Biomaterials 28:3478–3488 [DOI] [PubMed] [Google Scholar]
- Bratt-Leal AM, Carpenedo RL, Ungrin MD, Zandstra PW, McDevitt TC. (2011) Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32:48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brave M, Farrell A, Ching Lin S, Ocheltree T, Pope Miksinski S, Lee SL, Saber H, Fourie J, Tornoe C, Booth B, et al. (2010) FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 78:282–288 [DOI] [PubMed] [Google Scholar]
- Brey DM, Chung C, Hankenson KD, Garino JP, Burdick JA. (2010) Identification of osteoconductive and biodegradable polymers from a combinatorial polymer library. J Biomed Mater Res A 93:807–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brey DM, Motlekar NA, Diamond SL, Mauck RL, Garino JP, Burdick JA. (2011) High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol Bioeng 108:163–174 [DOI] [PubMed] [Google Scholar]
- Brink PR, Cohen IS. (2013) Gap junction–mediated therapies to eliminate cardiac arrhythmias, in Regenenerative Pharmacology (Christ G, Andersson K-E, eds, ed) pp 237–251, Cambridge University Press, New York [Google Scholar]
- Brink PR, Valiunas V, Gordon C, Rosen MR, Cohen IS. (2012) Can gap junctions deliver? Biochim Biophys Acta 1818:2076–2081 [DOI] [PubMed] [Google Scholar]
- Briscoe J, Lawrence PA, Vincent J-P. (2010) Generation and Interpretation of Morphogen Gradients, Cold Spring Harbor Laboratory Press, New York [Google Scholar]
- Brockes JP, Kumar A. (2008) Comparative aspects of animal regeneration. Annu Rev Cell Dev Biol 24:525–549 [DOI] [PubMed] [Google Scholar]
- Bryder D, Rossi DJ, Weissman IL. (2006) Hematopoietic stem cells: the paradigmatic tissue-specific stem cell. Am J Pathol 169:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burks MV, Nair L. (2010) Long-term effects of bone morphogenetic protein- based treatments in humans. J Long Term Eff Med Implants 20:277–293 [DOI] [PubMed] [Google Scholar]
- Burmeister D, Aboushwareb T, Tan J, Link K, Andersson KE, Christ G. (2010) Early stages of in situ bladder regeneration in a rodent model. Tissue Eng Part A 16:2541–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton P, Adams DR, Abraham A, Allcock RW, Jiang Z, McCahill A, Gilmour J, McAbney J, Kane NM, Baillie GS, et al. (2010a) Identification and characterization of small-molecule ligands that maintain pluripotency of human embryonic stem cells. Biochem Soc Trans 38:1058–1061 [DOI] [PubMed] [Google Scholar]
- Burton P, Adams DR, Abraham A, Allcock RW, Jiang Z, McCahill A, Gilmour J, McAbney J, Kaupisch A, Kane NM, et al. (2010b) Erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) blocks differentiation and maintains the expression of pluripotency markers in human embryonic stem cells. Biochem J 432:575–584 [DOI] [PubMed] [Google Scholar]
- Byers B, Lee HL, Reijo Pera R. (2012) Modeling Parkinson’s disease using induced pluripotent stem cells. Curr Neurol Neurosci Rep 12:237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao YA, Wagers AJ, Beilhack A, Dusich J, Bachmann MH, Negrin RS, Weissman IL, Contag CH. (2004) Shifting foci of hematopoiesis during reconstitution from single stem cells. Proc Natl Acad Sci USA 101:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. (2009) Why are MSCs therapeutic? New data: new insight. J Pathol 217:318–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. (2013) Adult mesenchymal stem cells and the NO pathways. Proc Natl Acad Sci USA 110:2695–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale V, Wang Y, Carpino G, Mendel G, Alpini G, Gaudio E, Reid LM, Alvaro D. (2012) The biliary tree–a reservoir of multipotent stem cells. Nat Rev Gastroenterol Hepatol 9:231–240 [DOI] [PubMed] [Google Scholar]
- Carragee EJ, Hurwitz EL, Weiner BK. (2011) A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J 11:471–491 [DOI] [PubMed] [Google Scholar]
- Chambers I. (2004) The molecular basis of pluripotency in mouse embryonic stem cells. Cloning Stem Cells 6:386–391 [DOI] [PubMed] [Google Scholar]
- Chan SS, Li HJ, Hsueh YC, Lee DS, Chen JH, Hwang SM, Chen CY, Shih E, Hsieh PC. (2010) Fibroblast growth factor-10 promotes cardiomyocyte differentiation from embryonic and induced pluripotent stem cells. PLoS ONE 5:e14414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Boland ED, Williams SK, Hoying JB. (2011) Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 98:160–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CW, Lai YS, Pawlik KM, Liu K, Sun CW, Li C, Schoeb TR, Townes TM. (2009) Polycistronic lentiviral vector for “hit and run” reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells 27:1042–1049 [DOI] [PubMed] [Google Scholar]
- Charoenphol P, Huang RB, Eniola-Adefeso O. (2010) Potential role of size and hemodynamics in the efficacy of vascular-targeted spherical drug carriers. Biomaterials 31:1392–1402 [DOI] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, et al. (2009) Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol 5:100–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RR, Silva EA, Yuen WW, Mooney DJ. (2007) Spatio-temporal VEGF and PDGF delivery patterns blood vessel formation and maturation. Pharm Res 24:258–264 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Horvath LM, Grafodatskaya D, Pasceri P, Weksberg R, Hotta A, Carrel L, Ellis J. (2011) Isolation of MECP2-null Rett Syndrome patient hiPS cells and isogenic controls through X-chromosome inactivation. Hum Mol Genet 20:2103–2115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Rao MS. (2011) Cell-based therapy for neural disorders—anticipating challenges. Neurotherapeutics 8:744–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HJ, Lee CS, Kwon YW, Paek JS, Lee SH, Hur J, Lee EJ, Roh TY, Chu IS, Leem SH, et al. (2010) Induction of pluripotent stem cells from adult somatic cells by protein-based reprogramming without genetic manipulation. Blood 116:386–395 [DOI] [PubMed] [Google Scholar]
- Choi JS, Lee SJ, Christ GJ, Atala A, Yoo JJ. (2008) The influence of electrospun aligned poly(epsilon-caprolactone)/collagen nanofiber meshes on the formation of self-aligned skeletal muscle myotubes. Biomaterials 29:2899–2906 [DOI] [PubMed] [Google Scholar]
- Choi Y, Nam TG. (2012) Chemical biology in stem cell research. Arch Pharm Res 35:281–297 [DOI] [PubMed] [Google Scholar]
- Christ GJ, Andersson KE, editors (2013) Regenerative Pharmacology, Cambridge University Press, New York [Google Scholar]
- Cohen DE, Melton D. (2011) Turning straw into gold: directing cell fate for regenerative medicine. Nat Rev Genet 12:243–252 [DOI] [PubMed] [Google Scholar]
- Columbano A, Shinozuka H. (1996) Liver regeneration versus direct hyperplasia. FASEB J 10:1118–1128 [DOI] [PubMed] [Google Scholar]
- Connelly JT, Petrie TA, García AJ, Levenston ME. (2011) Fibronectin- and collagen-mimetic ligands regulate bone marrow stromal cell chondrogenesis in three-dimensional hydrogels. Eur Cell Mater 22:168–176, discussion 176–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona BT, Machingal MA, Criswell T, Vadhavkar V, Dannahower AC, Bergman C, Zhao W, Christ GJ. (2012) Further development of a tissue engineered muscle repair (TEMR) construct in vitro for enhanced functional recovery following implantation in vivo in a murine model of volumetric muscle loss (VML) injury. Tissue Eng Part A 18:1213–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona BT, Ward CL, Harrison BS, Christ GJ. (2010) Regenerative medicine: basic concepts, current status, and future applications. J Investig Med 58:849–858 [DOI] [PubMed] [Google Scholar]
- Corti S, Nizzardo M, Simone C, Falcone M, Donadoni C, Salani S, Rizzo F, Nardini M, Riboldi G, Magri F, et al. (2012) Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp Cell Res 318:1528–1541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cova L, Armentero MT, Zennaro E, Calzarossa C, Bossolasco P, Busca G, Lambertenghi Deliliers G, Polli E, Nappi G, Silani V, et al. (2010) Multiple neurogenic and neurorescue effects of human mesenchymal stem cell after transplantation in an experimental model of Parkinson’s disease. Brain Res 1311:12–27 [DOI] [PubMed] [Google Scholar]
- Cragg GM, Newman DJ. (2013) Natural products: A continuing source of novel drug leads. Biochim Biophys Acta 1830:3670–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley GH. (2000) Cardiac pacing leads. Cardiol Clin 18:95–112, viii–ix viii–ix. [DOI] [PubMed] [Google Scholar]
- Csaszar E, Kirouac DC, Yu M, Wang W, Qiao W, Cooke MP, Boitano AE, Ito C, Zandstra PW. (2012) Rapid expansion of human hematopoietic stem cells by automated control of inhibitory feedback signaling. Cell Stem Cell 10:218–229 [DOI] [PubMed] [Google Scholar]
- Csete M. (2010) Translational prospects for human induced pluripotent stem cells. Regen Med 5:509–519 [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol 23:1534–1541 [DOI] [PubMed] [Google Scholar]
- Dalton PD, Woodfield T, Hutmacher DW. (2009) Snapshot: Polymer scaffolds for tissue engineering. Biomaterials 30:701–702 [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. (2003) Parkinson’s disease: mechanisms and models. Neuron 39:889–909 [DOI] [PubMed] [Google Scholar]
- David G, Cristea M, Balhui C, Timpu D, Doroftei F, Simionescu BC. (2012) Effect of cross-linking methods on structure and properties of poly(ε-caprolactone) stabilized hydrogels containing biopolymers. Biomacromolecules 13:2263–2272 [DOI] [PubMed] [Google Scholar]
- Davidson EH. (2010) Emerging properties of animal gene regulatory networks. Nature 468:911–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SP, Martanto W, Allen MG, Prausnitz MR. (2005) Hollow metal microneedles for insulin delivery to diabetic rats. IEEE Trans Biomed Eng 52:909–915 [DOI] [PubMed] [Google Scholar]
- De Bie C. (2007) Genzyme: 15 years of cell and gene therapy research. Regen Med 2:95–97 [DOI] [PubMed] [Google Scholar]
- De Luca M, Pellegrini G, Green H. (2006) Regeneration of squamous epithelia from stem cells of cultured grafts. Regen Med 1:45–57 [DOI] [PubMed] [Google Scholar]
- de Mel A, Oh JT, Ramesh B, Seifalian AM. (2012) Biofunctionalized quantum dots for live monitoring of stem cells: applications in regenerative medicine. Regen Med 7:335–347 [DOI] [PubMed] [Google Scholar]
- DeLaForest A, Nagaoka M, Si-Tayeb K, Noto FK, Konopka G, Battle MA, Duncan SA. (2011) HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development 138:4143–4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Laporte L, Huang A, Ducommun MM, Zelivyanska ML, Aviles MO, Adler AF, Shea LD. (2010) Patterned transgene expression in multiple-channel bridges after spinal cord injury. Acta Biomater 6:2889–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM, Fahmy TM. (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomaterials 33:4957–4964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desponts C, Ding S. (2010) Using small molecules to improve generation of induced pluripotent stem cells from somatic cells. Methods Mol Biol 636:207–218 [DOI] [PubMed] [Google Scholar]
- Deuel TF, Zhang N, Yeh HJ, Silos-Santiago I, Wang ZY. (2002) Pleiotrophin: a cytokine with diverse functions and a novel signaling pathway. Arch Biochem Biophys 397:162–171 [DOI] [PubMed] [Google Scholar]
- Dhara SK, Majumder A, Dodla MC, Stice SL. (2011) Nonviral gene delivery in neural progenitors derived from human pluripotent stem cells. Methods Mol Biol 767:343–354 [DOI] [PubMed] [Google Scholar]
- Ding S, Schultz PG. (2004) A role for chemistry in stem cell biology. Nat Biotechnol 22:833–840 [DOI] [PubMed] [Google Scholar]
- DiSandro MJ, Baskin LS, Li YW, Werb Z, Cunha GR. (1997) Development and regenerative ability of bladder in the transgenic epidermal growth factor receptor gene knockout mouse. J Urol 158:1058–1065 [DOI] [PubMed] [Google Scholar]
- Donaldson AE, Cai J, Yang MI, Iacovitti L. (2009) Human amniotic fluid stem cells do not differentiate into dopamine neurons in vitro or after transplantation in vivo. Stem Cells Dev 18:1003–1012 [DOI] [PubMed] [Google Scholar]
- Drake AC, Khoury M, Leskov I, Iliopoulou BP, Fragoso M, Lodish H, Chen J. (2011) Human CD34+ CD133+ hematopoietic stem cells cultured with growth factors including Angptl5 efficiently engraft adult NOD-SCID Il2rγ-/- (NSG) mice. PLoS ONE 6:e18382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Liang P, Wu JC. (2012) Induced pluripotent stem cells as a disease modeling and drug screening platform. J Cardiovasc Pharmacol 60:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Svendsen CN. (2010) Stem cell model of spinal muscular atrophy. Arch Neurol 67:665–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert AD, Yu J, Rose FF, Jr, Mattis VB, Lorson CL, Thomson JA, Svendsen CN. (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457:277–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards PC, Ruggiero S, Fantasia J, Burakoff R, Moorji SM, Paric E, Razzano P, Grande DA, Mason JM. (2005) Sonic hedgehog gene-enhanced tissue engineering for bone regeneration. Gene Ther 12:75–86 [DOI] [PubMed] [Google Scholar]
- Efe JA, Hilcove S, Kim J, Zhou H, Ouyang K, Wang G, Chen J, Ding S. (2011) Conversion of mouse fibroblasts into cardiomyocytes using a direct reprogramming strategy. Nat Cell Biol 13:215–222 [DOI] [PubMed] [Google Scholar]
- Evseenko D, Zhu Y, Schenke-Layland K, Kuo J, Latour B, Ge S, Scholes J, Dravid G, Li X, MacLellan WR, et al. (2010) Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc Natl Acad Sci USA 107:13742–13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild PJ. (2009) Transplantation tolerance in an age of induced pluripotency. Curr Opin Organ Transplant 14:321–325 [DOI] [PubMed] [Google Scholar]
- Farra N, Zhang WB, Pasceri P, Eubanks JH, Salter MW, Ellis J. (2012) Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations. Mol Psychiatry 17:1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulds D, Sorkin EM. (1989) Epoetin (recombinant human erythropoietin). A review of its pharmacodynamic and pharmacokinetic properties and therapeutic potential in anaemia and the stimulation of erythropoiesis. Drugs 38:863–899 [DOI] [PubMed] [Google Scholar]
- Feng Z, Gao F. (2012) Stem cell challenges in the treatment of neurodegenerative disease. CNS Neurosci Ther 18:142–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster K, Foster H, Dickson JG. (2006) Gene therapy progress and prospects: Duchenne muscular dystrophy. Gene Ther 13:1677–1685 [DOI] [PubMed] [Google Scholar]
- Foster S, Duvall CL, Crownover EF, Hoffman AS, Stayton PS. (2010) Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconjug Chem 21:2205–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frampton JE, Lee CR, Faulds D. (1994) Filgrastim. A review of its pharmacological properties and therapeutic efficacy in neutropenia. Drugs 48:731–760 [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Arner A, Malmquist U, Scott RS, Uvelius B. (2004) Nerve induced responses and force-velocity relations of regenerated detrusor muscle after subtotal cystectomy in the rat. Neurourol Urodyn 23:159–165 [DOI] [PubMed] [Google Scholar]
- Freed CR, Greene PE, Breeze RE, Tsai WY, DuMouchel W, Kao R, Dillon S, Winfield H, Culver S, Trojanowski JQ, et al. (2001) Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N Engl J Med 344:710–719 [DOI] [PubMed] [Google Scholar]
- Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, Holmes JW, Radisic M, Sefton MV, Kaplan D, Vunjak-Novakovic G. (2006) Advanced tools for tissue engineering: scaffolds, bioreactors, and signaling. Tissue Eng 12:3285–3305 [DOI] [PubMed] [Google Scholar]
- Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am 83-A(Pt 2, Suppl 1)S151–S158 [PMC free article] [PubMed] [Google Scholar]
- Fujihara Y, Koyama H, Ohba M, Tabata Y, Fujihara H, Yonehara Y, Takato T. (2008) Controlled delivery of bFGF to recipient bed enhances the vascularization and viability of an ischemic skin flap. Wound Repair Regen 16:125–131 [DOI] [PubMed] [Google Scholar]
- Fujikura J, Nakao K, Sone M, Noguchi M, Mori E, Naito M, Taura D, Harada-Shiba M, Kishimoto I, Watanabe A, et al. (2012) Induced pluripotent stem cells generated from diabetic patients with mitochondrial DNA A3243G mutation. Diabetologia 55:1689–1698 [DOI] [PubMed] [Google Scholar]
- Fujimoto KL, Guan J, Oshima H, Sakai T, Wagner WR. (2007a) In vivo evaluation of a porous, elastic, biodegradable patch for reconstructive cardiac procedures. Ann Thorac Surg 83:648–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto KL, Tobita K, Merryman WD, Guan J, Momoi N, Stolz DB, Sacks MS, Keller BB, Wagner WR. (2007b) An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J Am Coll Cardiol 49:2292–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukano Y, Usui ML, Underwood RA, Isenhath S, Marshall AJ, Hauch KD, Ratner BD, Olerud JE, Fleckman P. (2010) Epidermal and dermal integration into sphere-templated porous poly(2-hydroxyethyl methacrylate) implants in mice. J Biomed Mater Res A 94:1172–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M, Hattori Y, Yoshizawa T, Maitani Y. (2007) Combination of non-viral connexin 43 gene therapy and docetaxel inhibits the growth of human prostate cancer in mice. Int J Oncol 30:225–231 [PubMed] [Google Scholar]
- Funakoshi N, Duret C, Pascussi JM, Blanc P, Maurel P, Daujat-Chavanieu M, Gerbal-Chaloin S. (2011) Comparison of hepatic-like cell production from human embryonic stem cells and adult liver progenitor cells: CAR transduction activates a battery of detoxification genes. Stem Cell Rev 7:518–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth ME, Atala A, Van Dyke ME. (2007) Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 28:5068–5073 [DOI] [PubMed] [Google Scholar]
- Furth ME, Childers MK, Reid LM. (2013) Stem and progenitor cells in regenerative pharmacology, in Regenerative Pharmacology (Christ GJ, Andersson KE, eds, ed) pp 75–126, Cambridge University Press, New York [Google Scholar]
- Gage F. (2010) The promise and the challenge of modelling human disease in a dish. EMBO Mol Med 2:77–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. (2000) Mammalian neural stem cells. Science 287:1433–1438 [DOI] [PubMed] [Google Scholar]
- Gao Z, Kennedy AM, Christensen DA, Rapoport NY. (2008) Drug-loaded nano/microbubbles for combining ultrasonography and targeted chemotherapy. Ultrasonics 48:260–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, Cunningham JJ, Printz MA, Kordower JH, Bartus RT. (2007a) AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiol Dis 27:67–76 [DOI] [PubMed] [Google Scholar]
- Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, Ketchum ET, Bartus RT. (2007b) Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Mol Ther 15:62–68 [DOI] [PubMed] [Google Scholar]
- Gaspar VM, Correia IJ, Sousa A, Silva F, Paquete CM, Queiroz JA, Sousa F. (2011) Nanoparticle mediated delivery of pure P53 supercoiled plasmid DNA for gene therapy. J Control Release 156:212–222 [DOI] [PubMed] [Google Scholar]
- Gazit R, Weissman IL, Rossi DJ. (2008) Hematopoietic stem cells and the aging hematopoietic system. Semin Hematol 45:218–224 [DOI] [PubMed] [Google Scholar]
- Georgieva JV, Kalicharan D, Couraud PO, Romero IA, Weksler B, Hoekstra D, Zuhorn IS. (2011) Surface characteristics of nanoparticles determine their intracellular fate in and processing by human blood-brain barrier endothelial cells in vitro. Mol Ther 19:318–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. (2009) Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc 84:876–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, Brooks DJ, Svendsen CN, Heywood P. (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med 9:589–595 [DOI] [PubMed] [Google Scholar]
- Glass JD, Boulis NM, Johe K, Rutkove SB, Federici T, Polak M, Kelly C, Feldman EL. (2012) Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: results of a phase I trial in 12 patients. Stem Cells 30:1144–1151 [DOI] [PubMed] [Google Scholar]
- Goldberg DJ, Amin S, Hussain M. (2006) Acne scar correction using calcium hydroxylapatite in a carrier-based gel. J Cosmet Laser Ther 8:134–136 [DOI] [PubMed] [Google Scholar]
- Goldstein AS, Christ G. (2009) Functional tissue engineering requires bioreactor strategies. Tissue Eng Part A 15:739–740 [DOI] [PubMed] [Google Scholar]
- Gratton SE, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM. (2008) The effect of particle design on cellular internalization pathways. Proc Natl Acad Sci USA 105:11613–11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson WL, Martens TP, Eng GM, Radisic M, Vunjak-Novakovic G. (2009) Biomimetic approach to tissue engineering. Semin Cell Dev Biol 20:665–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. (2008) The birth of therapy with cultured cells. Bioessays 30:897–903 [DOI] [PubMed] [Google Scholar]
- Greiner A, Wendorff JH. (2007) Electrospinning: a fascinating method for the preparation of ultrathin fibers. Angew Chem Int Ed Engl 46:5670–5703 [DOI] [PubMed] [Google Scholar]
- Guan M, Yao W, Liu R, Lam KS, Nolta J, Jia J, Panganiban B, Meng L, Zhou P, Shahnazari M, et al. (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat Med 18:456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez Millán C, Colino Gandarillas CI, Sayalero Marinero ML, Lanao JM. (2012) Cell-based drug-delivery platforms. Ther Deliv 3:25–41 [DOI] [PubMed] [Google Scholar]
- Guo X, Elliott CG, Li Z, Xu Y, Hamilton DW, Guan J. (2012) Creating 3D angiogenic growth factor gradients in fibrous constructs to guide fast angiogenesis. Biomacromolecules 13:3262–3271 [DOI] [PubMed] [Google Scholar]
- Gupta J, Felner EI, Prausnitz MR. (2009) Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther 11:329–337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB. (1962) The developmental capacity of nuclei taken from intestinal epithelium cells of feeding tadpoles. J Embryol Exp Morphol 10:622–640 [PubMed] [Google Scholar]
- Gurdon JB, Bourillot PY. (2001) Morphogen gradient interpretation. Nature 413:797–803 [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Byrne JA, Simonsson S. (2003) Nuclear reprogramming and stem cell creation. Proc Natl Acad Sci USA 100 (Suppl 1):11819–11822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon JB, Dyson S, St Johnston D. (1998) Cells’ perception of position in a concentration gradient. Cell 95:159–162 [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Standley H, Dyson S, Butler K, Langon T, Ryan K, Stennard F, Shimizu K, Zorn A. (1999) Single cells can sense their position in a morphogen gradient. Development 126:5309–5317 [DOI] [PubMed] [Google Scholar]
- Gwak J, Hwang SG, Park HS, Choi SR, Park SH, Kim H, Ha NC, Bae SJ, Han JK, Kim DE, et al. (2012) Small molecule-based disruption of the Axin/β-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res 22:237–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B, Dembinski J, Sasser AK, Studeny M, Andreeff M, Marini F. (2007) Mesenchymal stem cells in cancer: tumor-associated fibroblasts and cell-based delivery vehicles. Int J Hematol 86:8–16 [DOI] [PubMed] [Google Scholar]
- Han DW, Tapia N, Hermann A, Hemmer K, Höing S, Araúzo-Bravo MJ, Zaehres H, Wu G, Frank S, Moritz S, et al. (2012) Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 10:465–472 [DOI] [PubMed] [Google Scholar]
- Harb N, Archer TK, Sato N. (2008) The Rho-Rock-Myosin signaling axis determines cell-cell integrity of self-renewing pluripotent stem cells. PLoS ONE 3:e3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington JK, Chahboune H, Criscione JM, Li AY, Hibino N, Yi T, Villalona GA, Kobsa S, Meijas D, Duncan DR, et al. (2011) Determining the fate of seeded cells in venous tissue-engineered vascular grafts using serial MRI. FASEB J 25:4150–4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori F, Chen H, Yamashita H, Tohyama S, Satoh YS, Yuasa S, Li W, Yamakawa H, Tanaka T, Onitsuka T, et al. (2010) Nongenetic method for purifying stem cell-derived cardiomyocytes. Nat Methods 7:61–66 [DOI] [PubMed] [Google Scholar]
- Haun JB, Hammer DA. (2008) Quantifying nanoparticle adhesion mediated by specific molecular interactions. Langmuir 24:8821–8832 [DOI] [PubMed] [Google Scholar]
- Hellmann MA, Panet H, Barhum Y, Melamed E, Offen D. (2006) Increased survival and migration of engrafted mesenchymal bone marrow stem cells in 6-hydroxydopamine-lesioned rodents. Neurosci Lett 395:124–128 [DOI] [PubMed] [Google Scholar]
- Hermann A, Maisel M, Wegner F, Liebau S, Kim DW, Gerlach M, Schwarz J, Kim KS, Storch A. (2006) Multipotent neural stem cells from the adult tegmentum with dopaminergic potential develop essential properties of functional neurons. Stem Cells 24:949–964 [DOI] [PubMed] [Google Scholar]
- Hickey P, Stacy M. (2011) Available and emerging treatments for Parkinson’s disease: a review. Drug Des Devel Ther 5:241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himburg HA, Muramoto GG, Daher P, Meadows SK, Russell JL, Doan P, Chi JT, Salter AB, Lento WE, Reya T, et al. (2010) Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 16:475–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. (1996) Bone morphogenetic proteins in development. Curr Opin Genet Dev 6:432–438 [DOI] [PubMed] [Google Scholar]
- Hong Y, Guan J, Fujimoto KL, Hashizume R, Pelinescu AL, Wagner WR. (2010) Tailoring the degradation kinetics of poly(ester carbonate urethane)urea thermoplastic elastomers for tissue engineering scaffolds. Biomaterials 31:4249–4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howden SE, Gore A, Li Z, Fung HL, Nisler BS, Nie J, Chen G, McIntosh BE, Gulbranson DR, Diol NR, et al. (2011) Genetic correction and analysis of induced pluripotent stem cells from a patient with gyrate atrophy. Proc Natl Acad Sci USA 108:6537–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YL, Huang B, Zhang TY, Miao PH, Tang GP, Tabata Y, Gao JQ. (2012) Mesenchymal stem cells as a novel carrier for targeted delivery of gene in cancer therapy based on nonviral transfection. Mol Pharm 9:2698–2709 [DOI] [PubMed] [Google Scholar]
- Huang H, Yu H, Tang G, Wang Q, Li J. (2010) Low molecular weight polyethylenimine cross-linked by 2-hydroxypropyl-gamma-cyclodextrin coupled to peptide targeting HER2 as a gene delivery vector. Biomaterials 31:1830–1838 [DOI] [PubMed] [Google Scholar]
- Huang HP, Chuang CY, Kuo HC. (2012a) Induced pluripotent stem cell technology for disease modeling and drug screening with emphasis on lysosomal storage diseases. Stem Cell Res Ther 3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NF, Lam A, Fang Q, Sievers RE, Li S, Lee RJ. (2009) Bone marrow-derived mesenchymal stem cells in fibrin augment angiogenesis in the chronically infarcted myocardium. Regen Med 4:527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, He Z, Ji S, Sun H, Xiang D, Liu C, Hu Y, Wang X, Hui L. (2011) Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature 475:386–389 [DOI] [PubMed] [Google Scholar]
- Huang B, Tabata Y, Gao JQ. (2012b) Mesenchymal stem cells as therapeutic agents and potential targeted gene delivery vehicle for brain diseases. J Control Release 162:464–473 [DOI] [PubMed] [Google Scholar]
- Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. (2010) Reconstituting organ-level lung functions on a chip. Science 328:1662–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichida JK, Blanchard J, Lam K, Son EY, Chung JE, Egli D, Loh KM, Carter AC, Di Giorgio FP, Koszka K, et al. (2009) A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell 5:491–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. (2010) Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142:375–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingber DE, Levin M. (2007) What lies at the interface of regenerative medicine and developmental biology? Development 134:2541–2547 [DOI] [PubMed] [Google Scholar]
- Ingber DE, Whitesides GM. (2012) Lab on a chip: United States of America. Lab Chip 12:2089–2090 [DOI] [PubMed] [Google Scholar]
- Istvanffy R, Kröger M, Eckl C, Gitzelmann S, Vilne B, Bock F, Graf S, Schiemann M, Keller UB, Peschel C, et al. (2011) Stromal pleiotrophin regulates repopulation behavior of hematopoietic stem cells. Blood 118:2712–2722 [DOI] [PubMed] [Google Scholar]
- Ito D, Okano H, Suzuki N. (2012) Accelerating progress in induced pluripotent stem cell research for neurological diseases. Ann Neurol 72:167–174 [DOI] [PubMed] [Google Scholar]
- Jadczyk T, Faulkner A, Madeddu P. (2013) Stem cell therapy for cardiovascular disease: the demise of alchemy and rise of pharmacology. Br J Pharmacol 169:247–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab K, Norotte C, Marga F, Murphy K, Vunjak-Novakovic G, Forgacs G. (2010) Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2:022001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James AW, Pang S, Askarinam A, Corselli M, Zara JN, Goyal R, Chang L, Pan A, Shen J, Yuan W, et al. (2012) Additive effects of sonic hedgehog and Nell-1 signaling in osteogenic versus adipogenic differentiation of human adipose-derived stromal cells. Stem Cells Dev 21:2170–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Yoo JE, Lee JA, Lee DR, Kim JY, Huh YJ, Kim DS, Park CY, Hwang DY, Kim HS, et al. (2012) Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med 44:202–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon O, Lim HW, Lee M, Song SJ, Kim BS. (2007) Poly(L-lactide-co-glycolide) nanospheres conjugated with a nuclear localization signal for delivery of plasmid DNA. J Drug Target 15:190–198 [DOI] [PubMed] [Google Scholar]
- Jeschke MG, Herndon DN, Baer W, Barrow RE, Jauch KW. (2001) Possibilities of non-viral gene transfer to improve cutaneous wound healing. Curr Gene Ther 1:267–278 [DOI] [PubMed] [Google Scholar]
- Ji J, Ng SH, Sharma V, Neculai D, Hussein S, Sam M, Trinh Q, Church GM, McPherson JD, Nagy A, et al. (2012) Elevated coding mutation rate during the reprogramming of human somatic cells into induced pluripotent stem cells. Stem Cells 30:435–440 [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. (2010) Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med 5:933–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YW, Hysolli E, Kim KY, Tanaka Y, Park IH. (2012) Human induced pluripotent stem cells and neurodegenerative disease: prospects for novel therapies. Curr Opin Neurol 25:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata M, Liu S, Liang M, Nagaoka Y, Chen IS. (2010) Generation of human induced pluripotent stem cells bearing an anti-HIV transgene by a lentiviral vector carrying an internal murine leukemia virus promoter. Hum Gene Ther 21:1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanematsu A, Yamamoto S, Noguchi T, Ozeki M, Tabata Y, Ogawa O. (2003) Bladder regeneration by bladder acellular matrix combined with sustained release of exogenous growth factor. J Urol 170:1633–1638 [DOI] [PubMed] [Google Scholar]
- Kao CY, Hoffman EA, Beck KC, Bellamkonda RV, Annapragada AV. (2003) Long-residence-time nano-scale liposomal iohexol for X-ray-based blood pool imaging. Acad Radiol 10:475–483 [DOI] [PubMed] [Google Scholar]
- Kashyap V, Rezende NC, Scotland KB, Shaffer SM, Persson JL, Gudas LJ, Mongan NP. (2009) Regulation of stem cell pluripotency and differentiation involves a mutual regulatory circuit of the NANOG, OCT4, and SOX2 pluripotency transcription factors with polycomb repressive complexes and stem cell microRNAs. Stem Cells Dev 18:1093–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G. (2011) Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines. Cell Stem Cell 8:228–240 [DOI] [PubMed] [Google Scholar]
- Kazuki Y, Hiratsuka M, Takiguchi M, Osaki M, Kajitani N, Hoshiya H, Hiramatsu K, Yoshino T, Kazuki K, Ishihara C, et al. (2010) Complete genetic correction of ips cells from Duchenne muscular dystrophy. Mol Ther 18:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke W, Shao K, Huang R, Han L, Liu Y, Li J, Kuang Y, Ye L, Lou J, Jiang C. (2009) Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 30:6976–6985 [DOI] [PubMed] [Google Scholar]
- Keatch RP, Schor AM, Vorstius JB, Schor SL. (2012) Biomaterials in regenerative medicine: engineering to recapitulate the natural. Curr Opin Biotechnol 23:579–582 [DOI] [PubMed] [Google Scholar]
- Kent DG, Copley MR, Benz C, Wöhrer S, Dykstra BJ, Ma E, Cheyne J, Zhao Y, Bowie MB, Zhao Y, et al. (2009) Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood 113:6342–6350 [DOI] [PubMed] [Google Scholar]
- Khan IF, Hirata RK, Wang PR, Li Y, Kho J, Nelson A, Huo Y, Zavaljevski M, Ware C, Russell DW. (2010) Engineering of human pluripotent stem cells by AAV-mediated gene targeting. Mol Ther 18:1192–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kicheva A, Cohen M, Briscoe J. (2012) Developmental pattern formation: insights from physics and biology. Science 338:210–212 [DOI] [PubMed] [Google Scholar]
- Kikuno N, Kawamoto K, Hirata H, Vejdani K, Kawakami K, Fandel T, Nunes L, Urakami S, Shiina H, Igawa M, et al. (2009) Nerve growth factor combined with vascular endothelial growth factor enhances regeneration of bladder acellular matrix graft in spinal cord injury-induced neurogenic rat bladder. BJU Int 103:1424–1428 [DOI] [PubMed] [Google Scholar]
- Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, et al. (2009) Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell 4:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Efe JA, Zhu S, Talantova M, Yuan X, Wang S, Lipton SA, Zhang K, Ding S. (2011) Direct reprogramming of mouse fibroblasts to neural progenitors. Proc Natl Acad Sci USA 108:7838–7843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Auerbach JM, Rodríguez-Gómez JA, Velasco I, Gavin D, Lumelsky N, Lee SH, Nguyen J, Sánchez-Pernaute R, Bankiewicz K, et al. (2002) Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson’s disease. Nature 418:50–56 [DOI] [PubMed] [Google Scholar]
- Kim JO, Choi JY, Park JK, Kim JH, Jin SG, Chang SW, Li DX, Hwang MR, Woo JS, Kim JA, et al. (2008) Development of clindamycin-loaded wound dressing with polyvinyl alcohol and sodium alginate. Biol Pharm Bull 31:2277–2282 [DOI] [PubMed] [Google Scholar]
- Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, Gazit Z, Gazit D. (2011) Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Mol Ther 19:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Björklund A. (2004) Localized striatal delivery of GDNF as a treatment for Parkinson disease. Nat Neurosci 7:105–110 [DOI] [PubMed] [Google Scholar]
- Kleinman HK, Martin GR. (2005) Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol 15:378–386 [DOI] [PubMed] [Google Scholar]
- Koch P, Kokaia Z, Lindvall O, Brüstle O. (2009) Emerging concepts in neural stem cell research: autologous repair and cell-based disease modelling. Lancet Neurol 8:819–829 [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M, Bartus RT. (2006) Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Ann Neurol 60:706–715 [DOI] [PubMed] [Google Scholar]
- Kornegay JN, Bogan JR, Bogan DJ, Childers MK, Li J, Nghiem P, Detwiler DA, Larsen CA, Grange RW, Bhavaraju-Sanka RK, et al. (2012) Canine models of Duchenne muscular dystrophy and their use in therapeutic strategies. Mamm Genome 23:85–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyanagi M, Takahashi J, Arakawa Y, Doi D, Fukuda H, Hayashi H, Narumiya S, Hashimoto N. (2008) Inhibition of the Rho/ROCK pathway reduces apoptosis during transplantation of embryonic stem cell-derived neural precursors. J Neurosci Res 86:270–280 [DOI] [PubMed] [Google Scholar]
- Kraehenbuehl TP, Langer R, Ferreira LS. (2011) Three-dimensional biomaterials for the study of human pluripotent stem cells. Nat Methods 8:731–736 [DOI] [PubMed] [Google Scholar]
- Kramer AS, Harvey AR, Plant GW, Hodgetts SI. (2012) Systematic review of induced pluripotent stem cell technology as a potential clinical therapy for spinal cord injury. Cell Transplant (Aug):27 published ahead of print [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. (2011) Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 480:547–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna KA, Krishna KS, Berrocal R, Tummala A, Rao KS, Rao KR. (2011) A review on the therapeutic potential of embryonic and induced pluripotent stem cells in hepatic repair. J Nat Sci Biol Med 2:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, et al. (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26:443–452 [DOI] [PubMed] [Google Scholar]
- Kwon EJ, Bergen JM, Pun SH. (2008) Application of an HIV gp41-derived peptide for enhanced intracellular trafficking of synthetic gene and siRNA delivery vehicles. Bioconjug Chem 19:920–927 [DOI] [PubMed] [Google Scholar]
- Ladisch MR, Kohlmann KL. (1992) Recombinant human insulin. Biotechnol Prog 8:469–478 [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, Brooks DJ, Hotton G, Moro E, Heywood P, et al. (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol 59:459–466 [DOI] [PubMed] [Google Scholar]
- Lanza RP, Chung HY, Yoo JJ, Wettstein PJ, Blackwell C, Borson N, Hofmeister E, Schuch G, Soker S, Moraes CT, et al. (2002) Generation of histocompatible tissues using nuclear transplantation. Nat Biotechnol 20:689–696 [DOI] [PubMed] [Google Scholar]
- Lee K, Silva EA, Mooney DJ. (2011) Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface 8:153–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Chen TT, Iruela-Arispe ML, Wu BM, Dunn JC. (2007) Modulation of protein delivery from modular polymer scaffolds. Biomaterials 28:1862–1870 [DOI] [PubMed] [Google Scholar]
- Lee WR, Park JH, Kim KH, Kim SJ, Park DH, Chae MH, Suh SH, Jeong SW, Park KK. (2009) The biological effects of topical alginate treatment in an animal model of skin wound healing. Wound Repair Regen 17:505–510 [DOI] [PubMed] [Google Scholar]
- Lees AJ, Hardy J, Revesz T. (2009) Parkinson’s disease. Lancet 373:2055–2066 PubMed [DOI] [PubMed] [Google Scholar]
- Leipzig ND, Wylie RG, Kim H, Shoichet MS. (2011) Differentiation of neural stem cells in three-dimensional growth factor-immobilized chitosan hydrogel scaffolds. Biomaterials 32:57–64 [DOI] [PubMed] [Google Scholar]
- LeWitt PA, Rezai AR, Leehey MA, Ojemann SG, Flaherty AW, Eskandar EN, Kostyk SK, Thomas K, Sarkar A, Siddiqui MS, et al. (2011) AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol 10:309–319 [DOI] [PubMed] [Google Scholar]
- Li JJ, Wang YA, Guo W, Keay JC, Mishima TD, Johnson MB, Peng X. (2003) Large-scale synthesis of nearly monodisperse CdSe/CdS core/shell nanocrystals using air-stable reagents via successive ion layer adsorption and reaction. J Am Chem Soc 125:12567–12575 [DOI] [PubMed] [Google Scholar]
- Li SD, Huang L. (2007) Non-viral is superior to viral gene delivery. J Control Release 123:181–183 [DOI] [PubMed] [Google Scholar]
- Li W, Ding S. (2010) Small molecules that modulate embryonic stem cell fate and somatic cell reprogramming. Trends Pharmacol Sci 31:36–45 [DOI] [PubMed] [Google Scholar]
- Li W, Jiang K, Ding S. (2012) Concise review: A chemical approach to control cell fate and function. Stem Cells 30:61–68 [DOI] [PubMed] [Google Scholar]
- Li W, Zara JN, Siu RK, Lee M, Aghaloo T, Zhang X, Wu BM, Gertzman AA, Ting K, Soo C. (2011) Nell-1 enhances bone regeneration in a rat critical-sized femoral segmental defect model. Plast Reconstr Surg 127:580–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Guo J, Chang Q, Zhang A. (2009) Paracrine role for mesenchymal stem cells in acute myocardial infarction. Biol Pharm Bull 32:1343–1346 [DOI] [PubMed] [Google Scholar]
- Liang DS. (1962) Bladder regeneration following subtotal cystectomy. J Urol 88:503–505 [DOI] [PubMed] [Google Scholar]
- Liang DS, Goss RJ. (1963) Regeneration of the bladder after subtotal cystectomy in rats. J Urol 89:427–430 [DOI] [PubMed] [Google Scholar]
- Ligon BL. (2004) Penicillin: its discovery and early development. Semin Pediatr Infect Dis 15:52–57 [DOI] [PubMed] [Google Scholar]
- Lim F, Sun AM. (1980) Microencapsulated islets as bioartificial endocrine pancreas. Science 210:908–910 [DOI] [PubMed] [Google Scholar]
- Lin AT, Kato K, Monson F, Wein AJ, Levin RM. (1989) Pharmacological responses of rabbit urinary bladder after subtotal cystectomy. J Urol 142:409–412 [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260:1130–1132 [DOI] [PubMed] [Google Scholar]
- Lindsay RM, Alderson RF, Friedman B, Hyman C, Ip NY, Furth ME, Maisonpierre PC, Squinto SP, Yancopoulos GD. (1991) The neurotrophin family of NGF-related neurotrophic factors. Restor Neurol Neurosci 2:211–220 [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. (2009) Prospects of stem cell therapy for replacing dopamine neurons in Parkinson’s disease. Trends Pharmacol Sci 30:260–267 [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. (2010) Stem cells in human neurodegenerative disorders—time for clinical translation? J Clin Invest 120:29–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnes MP, Ratner BD, Giachelli CM. (2007) A fibrinogen-based precision microporous scaffold for tissue engineering. Biomaterials 28:5298–5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li F, Stubblefield EA, Blanchard B, Richards TL, Larson GA, He Y, Huang Q, Tan AC, Zhang D, et al. (2012) Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res 22:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Reineke TM. (2010) Degradation of poly(glycoamidoamine) DNA delivery vehicles: polyamide hydrolysis at physiological conditions promotes DNA release. Biomacromolecules 11:316–325 [DOI] [PubMed] [Google Scholar]
- Loai Y, Yeger H, Coz C, Antoon R, Islam SS, Moore K, Farhat WA. (2010) Bladder tissue engineering: tissue regeneration and neovascularization of HA-VEGF-incorporated bladder acellular constructs in mouse and porcine animal models. J Biomed Mater Res A 94:1205–1215 [DOI] [PubMed] [Google Scholar]
- Lo Bianco C, Déglon N, Pralong W, Aebischer P. (2004) Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiol Dis 17:283–289 [DOI] [PubMed] [Google Scholar]
- Luan Z, Liu W, Qu S, Du K, He S, Wang Z, Yang Y, Wang C, Gong X. (2012) Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant 21 (Suppl 1):S91–S98 [DOI] [PubMed] [Google Scholar]
- Lue J, Lin G, Ning H, Xiong A, Lin CS, Glenn JS. (2010) Transdifferentiation of adipose-derived stem cells into hepatocytes: a new approach. Liver Int 30:913–922 [DOI] [PubMed] [Google Scholar]
- Luo Y, Diao H, Xia S, Dong L, Chen J, Zhang J. (2010) A physiologically active polysaccharide hydrogel promotes wound healing. J Biomed Mater Res A 94:193–204 [DOI] [PubMed] [Google Scholar]
- Lyssiotis CA, Foreman RK, Staerk J, Garcia M, Mathur D, Markoulaki S, Hanna J, Lairson LL, Charette BD, Bouchez LC, et al. (2009) Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc Natl Acad Sci USA 106:8912–8917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machingal MA, Corona BT, Walters TJ, Kesireddy V, Koval CN, Dannahower A, Zhao W, Yoo J, Christ GJ. (2011) A tissue engineered muscle repair (TE-MR) construct for functional restoration of an irrecoverable muscle injury in a murine model. Tissue Eng Part A 17:2291–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredini M, Zerbinati F, Gildone A, Faccini R. (2007) Autologous chondrocyte implantation: a comparison between an open periosteal-covered and an arthroscopic matrix-guided technique. Acta Orthop Belg 73:207–218 [PubMed] [Google Scholar]
- Mangera A, Andersson KE, Apostolidis A, Chapple C, Dasgupta P, Giannantoni A, Gravas S, Madersbacher S. (2011) Contemporary management of lower urinary tract disease with botulinum toxin A: a systematic review of botox (onabotulinumtoxinA) and dysport (abobotulinumtoxinA). Eur Urol 60:784–795 [DOI] [PubMed] [Google Scholar]
- Mani G, Feldman MD, Patel D, Agrawal CM. (2007) Coronary stents: a materials perspective. Biomaterials 28:1689–1710 [DOI] [PubMed] [Google Scholar]
- Marchetto MC, Carromeu C, Acab A, Yu D, Yeo GW, Mu Y, Chen G, Gage FH, Muotri AR. (2010a) A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143:527–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto MC, Winner B, Gage FH. (2010b) Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum Mol Genet 19 (R1):R71–R76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marga F, Jakab K, Khatiwala C, Shepherd B, Dorfman S, Hubbard B, Colbert S, Gabor F. (2012) Toward engineering functional organ modules by additive manufacturing. Biofabrication 4:022001. [DOI] [PubMed] [Google Scholar]
- Marí-Buyé N, Semino CE. (2011) Differentiation of mouse embryonic stem cells in self-assembling peptide scaffolds. Methods Mol Biol 690:217–237 [DOI] [PubMed] [Google Scholar]
- Markert CD, Ning J, Staley JT, Heinzke L, Childers CK, Ferreira JA, Brown M, Stoker A, Okamura C, Childers MK. (2008) TCAP knockdown by RNA interference inhibits myoblast differentiation in cultured skeletal muscle cells. Neuromuscul Disord 18:413–422 [DOI] [PubMed] [Google Scholar]
- Maury Y, Gauthier M, Peschanski M, Martinat C. (2012) Human pluripotent stem cells for disease modelling and drug screening. Bioessays 34:61–71 [DOI] [PubMed] [Google Scholar]
- McKay WF, Peckham SM, Badura JM. (2007) A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE Bone Graft). Int Orthop 31:729–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer AK, Maisel M, Hermann A, Stirl K, Storch A. (2010) Restorative approaches in Parkinson’s Disease: which cell type wins the race? J Neurol Sci 289:93–103 [DOI] [PubMed] [Google Scholar]
- Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, Schoen FJ, Toner M, Mooney D, Atala A, et al. (2006) Engineering complex tissues. Tissue Eng 12:3307–3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minear S, Leucht P, Jiang J, Liu B, Zeng A, Fuerer C, Nusse R, Helms JA. (2010) Wnt proteins promote bone regeneration. Sci Transl Med 2:29ra30. [DOI] [PubMed] [Google Scholar]
- Mironov V, Visconti RP, Kasyanov V, Forgacs GD, Drake CJ, Markwald RR. (2009) Organ printing: tissue spheroids as building blocks. Biomaterials 30:2164–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SK. (2011) Folly of FDA-approval studies for bone morphogenetic protein. Spine J 11:495–499 [DOI] [PubMed] [Google Scholar]
- Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. (2007) Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci USA 104:5668–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon DG, Christ GJ, Stitzel J, Atala A, Yoo JJ. (2008) Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Eng Part A 14:473–482 [DOI] [PubMed] [Google Scholar]
- Mooney DJ, Vandenburgh H. (2008) Cell delivery mechanisms for tissue repair. Cell Stem Cell 2:205–213 [DOI] [PubMed] [Google Scholar]
- Moore DJ, West AB, Dawson VL, Dawson TM. (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28:57–87 [DOI] [PubMed] [Google Scholar]
- Moore NM, Sheppard CL, Sakiyama-Elbert SE. (2009) Characterization of a multifunctional PEG-based gene delivery system containing nuclear localization signals and endosomal escape peptides. Acta Biomater 5:854–864 [DOI] [PubMed] [Google Scholar]
- Morrell NT, Leucht P, Zhao L, Kim J-B, ten Berge D, Ponnusamy K, Carre AL, Dudek H, Zachlederova M, McElhaney M, et al. (2008) Liposomal packaging generates Wnt protein with in vivo biological activity. PLoS ONE 3:e2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley GW, Leyton DL, Glover DJ, Filmer RP, Jans DA. (2010) Enhancement of protein transduction-mediated nuclear delivery by interaction with dynein/microtubules. J Biotechnol 145:222–225 [DOI] [PubMed] [Google Scholar]
- Mozzetta C, Minetti G, Puri PL. (2009) Regenerative pharmacology in the treatment of genetic diseases: the paradigm of muscular dystrophy. Int J Biochem Cell Biol 41:701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Ikeguchi K, Shizuma N, Kawasaki K, Ono F, Shen Y, Wang L, Mizukami H, Kume A, et al. (2002) Behavioral recovery in a primate model of Parkinson’s disease by triple transduction of striatal cells with adeno-associated viral vectors expressing dopamine-synthesizing enzymes. Hum Gene Ther 13:345–354 [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K, Kawakami T, Urabe M, Kume A, Sato T, et al. (2010) A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson’s disease. Mol Ther 18:1731–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Wang L, Ikeguchi K, Fujimoto K, Nakano I, Okada T, Mizukami H, Hanazono Y, Kume A, Nakano I, et al. (2003) Adeno-associated viral vectors for Parkinson’s disease. Int Rev Neurobiol 55:205–222 [DOI] [PubMed] [Google Scholar]
- Murphy WL, Dennis RG, Kileny JL, Mooney DJ. (2002) Salt fusion: an approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng 8:43–52 [DOI] [PubMed] [Google Scholar]
- Murry CE, Keller G. (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- Nachtrab G, Poss KD. (2012) Toward a blueprint for regeneration. Development 139:2639–2642 [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26:101–106 [DOI] [PubMed] [Google Scholar]
- Nam YS, Park TG. (1999) Porous biodegradable polymeric scaffolds prepared by thermally induced phase separation. J Biomed Mater Res 47:8–17 [DOI] [PubMed] [Google Scholar]
- Narayanan RP, Melman G, Letourneau NJ, Mendelson NL, Melman A. (2012) Photodegradable iron(III) cross-linked alginate gels. Biomacromolecules 13:2465–2471 [DOI] [PubMed] [Google Scholar]
- Nelson DM, Baraniak PR, Ma Z, Guan J, Mason NS, Wagner WR. (2011) Controlled release of IGF-1 and HGF from a biodegradable polyurethane scaffold. Pharm Res 28:1282–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DM, Ma Z, Leeson CE, Wagner WR. (2012) Extended and sequential delivery of protein from injectable thermoresponsive hydrogels. J Biomed Mater Res A 100:776–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuži P, Giselbrecht S, Länge K, Huang TJ, Manz A. (2012) Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov 11:620–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CP, Goodman TT, Park IK, Pun SH. (2009) Bio-mimetic surface engineering of plasmid-loaded nanoparticles for active intracellular trafficking by actin comet-tail motility. Biomaterials 30:951–958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng RK, Gurdon JB. (2008) Epigenetic inheritance of cell differentiation status. Cell Cycle 7:1173–1177 [DOI] [PubMed] [Google Scholar]
- Nie B, Wang H, Laurent T, Ding S. (2012) Cellular reprogramming: a small molecule perspective. Curr Opin Cell Biol 24:784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmalanandhan VS, Sittampalam GS. (2009) Stem cells in drug discovery, tissue engineering, and regenerative medicine: emerging opportunities and challenges. J Biomol Screen 14:755–768 [DOI] [PubMed] [Google Scholar]
- Nishikawa S, Goldstein RA, Nierras CR. (2008) The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol 9:725–729 [DOI] [PubMed] [Google Scholar]
- Noël D, Gazit D, Bouquet C, Apparailly F, Bony C, Plence P, Millet V, Turgeman G, Perricaudet M, Sany J, et al. (2004) Short-term BMP-2 expression is sufficient for in vivo osteochondral differentiation of mesenchymal stem cells. Stem Cells 22:74–85 [DOI] [PubMed] [Google Scholar]
- Norton LW, Park J, Babensee JE. (2010) Biomaterial adjuvant effect is attenuated by anti-inflammatory drug delivery or material selection. J Control Release 146:341–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. (2011) Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333:218–221 [DOI] [PubMed] [Google Scholar]
- Nsair A, Schenke-Layland K, Van Handel B, Evseenko D, Kahn M, Zhao P, Mendelis J, Heydarkhan S, Awaji O, Vottler M, et al. (2012) Characterization and therapeutic potential of induced pluripotent stem cell-derived cardiovascular progenitor cells. PLoS ONE 7:e45603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, et al. ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60:69–73 [DOI] [PubMed] [Google Scholar]
- Oberpenning F, Meng J, Yoo JJ, Atala A. (1999) De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol 17:149–155 [DOI] [PubMed] [Google Scholar]
- Odorico JS, Kaufman DS, Thomson JA. (2001) Multilineage differentiation from human embryonic stem cell lines. Stem Cells 19:193–204 [DOI] [PubMed] [Google Scholar]
- Okita K, Yamanaka S. (2011) Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci 366:2198–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322:949–953 [DOI] [PubMed] [Google Scholar]
- Omolola Eniola A, Hammer DA. (2005) In vitro characterization of leukocyte mimetic for targeting therapeutics to the endothelium using two receptors. Biomaterials 26:7136–7144 [DOI] [PubMed] [Google Scholar]
- Opara EC, Mirmalek-Sani SH, Khanna O, Moya ML, Brey EM. (2010) Design of a bioartificial pancreas(+). J Investig Med 58:831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. (2008) Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med 14:213–221 [DOI] [PubMed] [Google Scholar]
- Ozawa K, Fan DS, Shen Y, Muramatsu S, Fujimoto K, Ikeguchi K, Ogawa M, Urabe MK, Kume A, Nakano I. (2000) Gene therapy of Parkinson’s disease using adeno-associated virus (AAV) vectors. J Neural Transm Suppl 58:181–191 [DOI] [PubMed] [Google Scholar]
- Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. (2010) Endogenous Wnt/beta-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS ONE 5:e11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, Pabon L, Reinecke H, Pratt G, Keller G, et al. (2012) A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell 151:221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus JA, Rhett JM, Gourdie RG. (2010) Translational lessons from scarless healing of cutaneous wounds and regenerative repair of the myocardium. J Mol Cell Cardiol 48:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C, Lu B, Chen H, Bishop CE. (2010) Reprogramming human fibroblasts using HIV-1 TAT recombinant proteins OCT4, SOX2, KLF4 and c-MYC. Mol Biol Rep 37:2117–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parenteau N, Hardin-Young J, Shannon W, Cantini P, Russell A. (2012) Meeting the need for regenerative therapies I: target-based incidence and its relationship to U.S. spending, productivity, and innovation. Tissue Eng Part B Rev 18:139–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Arora N, Huo H, Maherali N, Ahfeldt T, Shimamura A, Lensch MW, Cowan C, Hochedlinger K, Daley GQ. (2008) Disease-specific induced pluripotent stem cells. Cell 134:877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M, Chan DN, Ha I, Case D, Cui Y, Van Handel B, Mikkola HK, Lowry WE. (2012) Defining the nature of human pluripotent stem cell progeny. Cell Res 22:178–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck M, Gebhart D, Dusserre N, McAllister TN, L’Heureux N. (2012) The evolution of vascular tissue engineering and current state of the art. Cells Tissues Organs 195:144–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng LH, Tsang SY, Tabata Y, Gao JQ. (2012) Genetically-manipulated adult stem cells as therapeutic agents and gene delivery vehicle for wound repair and regeneration. J Control Release 157:321–330 [DOI] [PubMed] [Google Scholar]
- Peyton CC, Burmeister D, Petersen B, Andersson KE, Christ G. (2012) Characterization of the early proliferative response of the rodent bladder to subtotal cystectomy: a unique model of mammalian organ regeneration. PLoS ONE 7:e47414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R, Haworth CM, Davis OS. (2009) Common disorders are quantitative traits. Nat Rev Genet 10:872–878 [DOI] [PubMed] [Google Scholar]
- Politis M, Lindvall O. (2012) Clinical application of stem cell therapy in Parkinson’s disease. BMC Med 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porada CD, Almeida-Porada G. (2010) Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev 62:1156–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM. (2012) Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair 5:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst CE, Zrazhevskiy P, Bagalkot V, Gao X. (2013) Quantum dots as a platform for nanoparticle drug delivery vehicle design. Adv Drug Deliv Rev 65:703–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucéat M. (2008) Pharmacological approaches to regenerative strategies for the treatment of cardiovascular diseases. Curr Opin Pharmacol 8:189–192 [DOI] [PubMed] [Google Scholar]
- Purpura KA, Bratt-Leal AM, Hammersmith KA, McDevitt TC, Zandstra PW. (2012) Systematic engineering of 3D pluripotent stem cell niches to guide blood development. Biomaterials 33:1271–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen D, Coutts P, Fierheller M, Sibbald RG. (2007) The use of a novel oxygenating hydrogel dressing in the treatment of different chronic wounds. Adv Skin Wound Care 20:200–207 [DOI] [PubMed] [Google Scholar]
- Rafat M, Rotenstein LS, Hu JL, Auguste DT. (2012) Engineered endothelial cell adhesion via VCAM1 and E-selectin antibody-presenting alginate hydrogels. Acta Biomater 8:2697–2703 [DOI] [PubMed] [Google Scholar]
- Rahman SH, Maeder ML, Joung JK, Cathomen T. (2011) Zinc-finger nucleases for somatic gene therapy: the next frontier. Hum Gene Ther 22:925–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajala K, Pekkanen-Mattila M, Aalto-Setälä K. (2011) Cardiac differentiation of pluripotent stem cells. Stem Cells Int 2011:383709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamohan D, Matsa E, Kalra S, Crutchley J, Patel A, George V, Denning C. (2013) Current status of drug screening and disease modelling in human pluripotent stem cells. Bioessays 35:281–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy S, Soderstrom KE, Kordower JH. (2009) Trophic factors therapy in Parkinson’s disease. Prog Brain Res 175:201–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA. (2007) Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol 25:1159–1164 [DOI] [PubMed] [Google Scholar]
- Reyblat P, Ginsberg DA. (2010) Augmentation enterocystoplasty in overactive bladder: is there still a role? Curr Urol Rep 11:432–439 [DOI] [PubMed] [Google Scholar]
- Riddle KW, Mooney DJ. (2004) Role of poly(lactide-co-glycolide) particle size on gas-foamed scaffolds. J Biomater Sci Polym Ed 15:1561–1570 [DOI] [PubMed] [Google Scholar]
- Riley J, Federici T, Polak M, Kelly C, Glass J, Raore B, Taub J, Kesner V, Feldman EL, Boulis NM. (2012) Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I safety trial, technical note, and lumbar safety outcomes. Neurosurgery 71:405–416, discussion 416 [DOI] [PubMed] [Google Scholar]
- Robinton DA, Daley GQ. (2012) The promise of induced pluripotent stem cells in research and therapy. Nature 481:295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers KW, Schier AF. (2011) Morphogen gradients: from generation to interpretation. Annu Rev Cell Dev Biol 27:377–407 [DOI] [PubMed] [Google Scholar]
- Sachinidis A, Sotiriadou I, Seelig B, Berkessel A, Hescheler J. (2008) A chemical genetics approach for specific differentiation of stem cells to somatic cells: a new promising therapeutical approach. Comb Chem High Throughput Screen 11:70–82 [DOI] [PubMed] [Google Scholar]
- Saha K, Jaenisch R. (2009) Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5:584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurada K, McDonald FM, Shimada F. (2008) Regenerative medicine and stem cell based drug discovery. Angew Chem Int Ed Engl 47:5718–5738 [DOI] [PubMed] [Google Scholar]
- Sánchez Alvarado A, Tsonis PA. (2006) Bridging the regeneration gap: genetic insights from diverse animal models. Nat Rev Genet 7:873–884 [DOI] [PubMed] [Google Scholar]
- Sandner B, Prang P, Rivera FJ, Aigner L, Blesch A, Weidner N. (2012) Neural stem cells for spinal cord repair. Cell Tissue Res 349:349–362 [DOI] [PubMed] [Google Scholar]
- Santerre JP, Woodhouse K, Laroche G, Labow RS. (2005) Understanding the biodegradation of polyurethanes: from classical implants to tissue engineering materials. Biomaterials 26:7457–7470 [DOI] [PubMed] [Google Scholar]
- Sapir Y, Kryukov O, Cohen S. (2011) Integration of multiple cell-matrix interactions into alginate scaffolds for promoting cardiac tissue regeneration. Biomaterials 32:1838–1847 [DOI] [PubMed] [Google Scholar]
- Saul JM, Annapragada AV, Bellamkonda RV. (2006) A dual-ligand approach for enhancing targeting selectivity of therapeutic nanocarriers. J Control Release 114:277–287 [DOI] [PubMed] [Google Scholar]
- Saul JM, Ellenburg MD, de Guzman RC, Van Dyke M. (2011) Keratin hydrogels support the sustained release of bioactive ciprofloxacin. J Biomed Mater Res A 98:544–553 [DOI] [PubMed] [Google Scholar]
- Saul JM, Linnes MP, Ratner BD, Giachelli CM, Pun SH. (2007) Delivery of non-viral gene carriers from sphere-templated fibrin scaffolds for sustained transgene expression. Biomaterials 28:4705–4716 [DOI] [PubMed] [Google Scholar]
- Schapira AH, Olanow CW. (2004) Neuroprotection in Parkinson disease: mysteries, myths, and misconceptions. JAMA 291:358–364 [DOI] [PubMed] [Google Scholar]
- Schenke-Layland K, Nsair A, Van Handel B, Angelis E, Gluck JM, Votteler M, Goldhaber JI, Mikkola HK, Kahn M, Maclellan WR. (2011) Recapitulation of the embryonic cardiovascular progenitor cell niche. Biomaterials 32:2748–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz SC, Wittlinger J, Schober R, Storch A, Schwarz J. (2006) Transplantation of human neural precursor cells in the 6-OHDA lesioned rats: effect of immunosuppression with cyclosporine A. Parkinsonism Relat Disord 12:302–308 [DOI] [PubMed] [Google Scholar]
- Scuderi N, Onesti MG, Bistoni G, Ceccarelli S, Rotolo S, Angeloni A, Marchese C. (2008) The clinical application of autologous bioengineered skin based on a hyaluronic acid scaffold. Biomaterials 29:1620–1629 [DOI] [PubMed] [Google Scholar]
- Seidl SE, Potashkin JA. (2011) The promise of neuroprotective agents in Parkinson’s disease. Front Neurol 7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seita J, Weissman IL. (2010) Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med 2:640–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya S, Suzuki A. (2011) Direct conversion of mouse fibroblasts to hepatocyte-like cells by defined factors. Nature 475:390–393 [DOI] [PubMed] [Google Scholar]
- Shah DA, Kwon SJ, Bale SS, Banerjee A, Dordick JS, Kane RS. (2011) Regulation of stem cell signaling by nanoparticle-mediated intracellular protein delivery. Biomaterials 32:3210–3219 [DOI] [PubMed] [Google Scholar]
- Shen G, Hu Y, Wu J, Jin K, Zhu D, Zhang Y, Yu Y, Lou Y. (2012a) A 2,6-disubstituted 4-anilinoquinazoline derivative facilitates cardiomyogenesis of embryonic stem cells. ChemMedChem 7:733–740 [DOI] [PubMed] [Google Scholar]
- Shen J, James AW, Chung J, Lee K, Zhang JB, Ho S, Lee KS, Kim TM, Niimi T, Kuroda S, et al. (2012b) NELL-1 promotes cell adhesion and differentiation via Integrinβ1. J Cell Biochem 113:3620–3628 [DOI] [PubMed] [Google Scholar]
- Shen Y, Muramatsu SI, Ikeguchi K, Fujimoto KI, Fan DS, Ogawa M, Mizukami H, Urabe M, Kume A, Nagatsu I, et al. (2000) Triple transduction with adeno-associated virus vectors expressing tyrosine hydroxylase, aromatic-L-amino-acid decarboxylase, and GTP cyclohydrolase I for gene therapy of Parkinson’s disease. Hum Gene Ther 11:1509–1519 [DOI] [PubMed] [Google Scholar]
- Sheridan B, Harris N. (2009) New glossary of terms used in regenerative medicine: standardization continues to emerge as regenerative medicine matures. Regen Med 4:621–622 [DOI] [PubMed] [Google Scholar]
- Shi W, Mei H, Deng J, Chen C, Wang H, Guo T, Zhang B, Li L, Pang Z, Jiang X, et al. (2012) A tissue factor targeted nanomedical system for thrombi-specific drug delivery. Biomaterials 33:7643–7654 [DOI] [PubMed] [Google Scholar]
- Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. (2008) Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell 3:568–574 [DOI] [PubMed] [Google Scholar]
- Shim W, Mehta A, Wong P, Chua T, Koh TH. (2013) Critical path in cardiac stem cell therapy: an update on cell delivery. Cytotherapy 15:399–415 [DOI] [PubMed] [Google Scholar]
- Shulman JM, De Jager PL, Feany MB. (2011) Parkinson’s disease: genetics and pathogenesis. Annu Rev Pathol 6:193–222 [DOI] [PubMed] [Google Scholar]
- Sidhu KS. (2011) New approaches for the generation of induced pluripotent stem cells. Expert Opin Biol Ther 11:569–579 [DOI] [PubMed] [Google Scholar]
- Sill TJ, von Recum HA. (2008) Electrospinning: applications in drug delivery and tissue engineering. Biomaterials 29:1989–2006 [DOI] [PubMed] [Google Scholar]
- Sisk V, Neu I. (1939) Regeneration of the bladder: a case study. Trans Am Assoc Genito-urin Surg 32:197– 202 [Google Scholar]
- Sittadjody S, Saul JM, Joo S, Yoo JJ, Atala A, Opara EC. (2013) Engineered multilayer ovarian tissue that secretes sex steroids and peptide hormones in response to gonadotropins. Biomaterials 34:2412–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu RK, Lu SS, Li W, Whang J, McNeill G, Zhang X, Wu BM, Turner AS, Seim HB, 3rd, Hoang P, et al. (2011) Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A 17:1123–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. (2006) A glossary for stem-cell biology. Nature 441:1060.16437109 [Google Scholar]
- Snykers S, Henkens T, De Rop E, Vinken M, Fraczek J, De Kock J, De Prins E, Geerts A, Rogiers V, Vanhaecke T. (2009) Role of epigenetics in liver-specific gene transcription, hepatocyte differentiation and stem cell reprogrammation. J Hepatol 51:187–211 [DOI] [PubMed] [Google Scholar]
- Soder BL, Propst JT, Brooks TM, Goodwin RL, Friedman HI, Yost MJ, Gourdie RG. (2009) The connexin43 carboxyl-terminal peptide ACT1 modulates the biological response to silicone implants. Plast Reconstr Surg 123:1440–1451 [DOI] [PubMed] [Google Scholar]
- Soler R, Füllhase C, Hanson A, Campeau L, Santos C, Andersson KE. (2012) Stem cell therapy ameliorates bladder dysfunction in an animal model of Parkinson disease. J Urol 187:1491–1497 [DOI] [PubMed] [Google Scholar]
- Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. (2011) Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. (2008) Induced pluripotent stem cells generated without viral integration. Science 322:945–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayton PS, El-Sayed ME, Murthy N, Bulmus V, Lackey C, Cheung C, Hoffman AS. (2005) ‘Smart’ delivery systems for biomolecular therapeutics. Orthod Craniofac Res 8:219–225 [DOI] [PubMed] [Google Scholar]
- Stocum DL. (2002) Regenerative biology and medicine. J Musculoskelet Neuronal Interact 2:270–273 [PubMed] [Google Scholar]
- Stocum DL, Cameron JA. (2011) Looking proximally and distally: 100 years of limb regeneration and beyond. Dev Dyn 240:943–968 [DOI] [PubMed] [Google Scholar]
- Studeny M, Marini FC, Champlin RE, Zompetta C, Fidler IJ, Andreeff M. (2002) Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 62:3603–3608 [PubMed] [Google Scholar]
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. (2004) Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 96:1593–1603 [DOI] [PubMed] [Google Scholar]
- Szabo E, Rampalli S, Risueño RM, Schnerch A, Mitchell R, Fiebig-Comyn A, Levadoux-Martin M, Bhatia M. (2010) Direct conversion of human fibroblasts to multilineage blood progenitors. Nature 468:521–526 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676 [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872 [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Bruneau BG. (2009) Directed transdifferentiation of mouse mesoderm to heart tissue by defined factors. Nature 459:708–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. (2004) Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol 5:836–847 [DOI] [PubMed] [Google Scholar]
- Theunissen TW, van Oosten AL, Castelo-Branco G, Hall J, Smith A, Silva JC. (2011) Nanog overcomes reprogramming barriers and induces pluripotency in minimal conditions. Curr Biol 21:65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thier M, Wörsdörfer P, Lakes YB, Gorris R, Herms S, Opitz T, Seiferling D, Quandel T, Hoffmann P, Nöthen MM, et al. (2012) Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 10:473–479 [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147 [DOI] [PubMed] [Google Scholar]
- Trounson A, Thakar RG, Lomax G, Gibbons D. (2011) Clinical trials for stem cell therapies. BMC Med 9:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucci P, Haralambidis G. (1963) Regeneration of the bladder: review of literature and case report. J Urol 90:193–199 [DOI] [PubMed] [Google Scholar]
- Turner R, Gerber D, Reid L. (2010) The future of cell transplant therapies: a need for tissue grafting. Transplantation 90:807–810 [DOI] [PubMed] [Google Scholar]
- Turner RA, Wauthier E, Lozoya O, McClelland R, Bowsher JE, Barbier C, Prestwich G, Hsu E, Gerber DA, Reid LM. (2013) Successful transplantation of human hepatic stem cells with restricted localization to liver using hyaluronan grafts. Hepatology 57:775–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood RA, Usui ML, Zhao G, Hauch KD, Takeno MM, Ratner BD, Marshall AJ, Shi X, Olerud JE, Fleckman P. (2011) Quantifying the effect of pore size and surface treatment on epidermal incorporation into percutaneously implanted sphere-templated porous biomaterials in mice. J Biomed Mater Res A 98:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungrin MD, Clarke G, Yin T, Niebrugge S, Nostro MC, Sarangi F, Wood G, Keller G, Zandstra PW. (2012) Rational bioprocess design for human pluripotent stem cell expansion and endoderm differentiation based on cellular dynamics. Biotechnol Bioeng 109:853–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer JJ, Daley GQ. (2011) Induced pluripotent stem cells for modelling human diseases. Philos Trans R Soc Lond B Biol Sci 366:2274–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxillaire M, Froguel P. (2006) Genetic basis of maturity-onset diabetes of the young. Endocrinol Metab Clin North Am 35:371–384, x x x. [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M. (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva FS, Lu E, Bowry S, Kilic S, Tom E, Wang J, Gretton J, Pacella JJ, Wagner WR. (2007) Myocardial ischemic memory imaging with molecular echocardiography. Circulation 115:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic S, Kleinman HK, Luyten FP, Roberts AB, Roche NS, Reddi AH. (1992) Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components. Exp Cell Res 202:1–8 [DOI] [PubMed] [Google Scholar]
- Walasek MA, van Os R, de Haan G. (2012) Hematopoietic stem cell expansion: challenges and opportunities. Ann N Y Acad Sci 1266:138–150 [DOI] [PubMed] [Google Scholar]
- Walker J. (2010) Disease in a dish: a new approach to drug discovery. Regen Med 5:505–507 [DOI] [PubMed] [Google Scholar]
- Wang H, Hao J, Hong CC. (2011a) Cardiac induction of embryonic stem cells by a small molecule inhibitor of Wnt/β-catenin signaling. ACS Chem Biol 6:192–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HB, Mullins ME, Cregg JM, Hurtado A, Oudega M, Trombley MT, Gilbert RJ. (2009) Creation of highly aligned electrospun poly-L-lactic acid fibers for nerve regeneration applications. J Neural Eng 6:016001. [DOI] [PubMed] [Google Scholar]
- Wang Q, Xu X, Li J, Liu J, Gu H, Zhang R, Chen J, Kuang Y, Fei J, Jiang C, et al. (2011b) Lithium, an anti-psychotic drug, greatly enhances the generation of induced pluripotent stem cells. Cell Res 21:1424–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cui CB, Yamauchi M, Miguez P, Roach M, Malavarca R, Costello MJ, Cardinale V, Wauthier E, Barbier C, et al. (2011c) Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology 53:293–305 [DOI] [PubMed] [Google Scholar]
- Wang Z, Storb R, Halbert CL, Banks GB, Butts TM, Finn EE, Allen JM, Miller AD, Chamberlain JS, and Tapscott SJ (2012) Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther 20:1501–1507. [DOI] [PMC free article] [PubMed]
- Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, et al. (2010) Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7:618–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, et al. (2007) A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol 25:681–686 [DOI] [PubMed] [Google Scholar]
- Watts KL, Adair J, Kiem HP. (2011) Hematopoietic stem cell expansion and gene therapy. Cytotherapy 13:1164–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. (1996) Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. (2008) Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson’s disease. Proc Natl Acad Sci USA 105:5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessely R. (2010) New drug-eluting stent concepts. Nat Rev Cardiol 7:194–203 [DOI] [PubMed] [Google Scholar]
- Willems E, Spiering S, Davidovics H, Lanier M, Xia Z, Dawson M, Cashman J, Mercola M. (2011) Small-molecule inhibitors of the Wnt pathway potently promote cardiomyocytes from human embryonic stem cell-derived mesoderm. Circ Res 109:360–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Rader A, Sakiyama-Elbert SE. (2008) The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res (Amst) 1:205–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, et al. (2013) Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127:213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DF. (2009) On the nature of biomaterials. Biomaterials 30:5897–5909 [DOI] [PubMed] [Google Scholar]
- Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. (1997) Viable offspring derived from fetal and adult mammalian cells. Nature 385:810–813 [DOI] [PubMed] [Google Scholar]
- Wilson PA, Lagna G, Suzuki A, Hemmati-Brivanlou A. (1997) Concentration-dependent patterning of the Xenopus ectoderm by BMP4 and its signal transducer Smad1. Development 124:3177–3184 [DOI] [PubMed] [Google Scholar]
- Wolpert L. (2011) Positional information and patterning revisited. J Theor Biol 269:359–365 [DOI] [PubMed] [Google Scholar]
- Wong DY, Leveque JC, Brumblay H, Krebsbach PH, Hollister SJ, Lamarca F. (2008) Macro-architectures in spinal cord scaffold implants influence regeneration. J Neurotrauma 25:1027–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GK, Chiu AT. (2011) Gene therapy, gene targeting and induced pluripotent stem cells: applications in monogenic disease treatment. Biotechnol Adv 29:1–10 [DOI] [PubMed] [Google Scholar]
- Wright LD, Young RT, Andric T, Freeman JW. (2010) Fabrication and mechanical characterization of 3D electrospun scaffolds for tissue engineering. Biomed Mater 5:055006. [DOI] [PubMed] [Google Scholar]
- Wu X, Ding S, Ding Q, Gray NS, Schultz PG. (2004) Small molecules that induce cardiomyogenesis in embryonic stem cells. J Am Chem Soc 126:1590–1591 [DOI] [PubMed] [Google Scholar]
- Xu C, Miranda-Nieves D, Ankrum JA, Matthiesen ME, Phillips JA, Roes I, Wojtkiewicz GR, Juneja V, Kultima JR, Zhao W, et al. (2012a) Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett 12:4131–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Alipio Z, Fink LM, Adcock DM, Yang J, Ward DC, Ma Y. (2009) Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci USA 106:808–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Fu Y, Chung W, Zheng X, Cui Y, Hsu IC, Kao WJ. (2012b) Thiol-ene-based biological/synthetic hybrid biomatrix for 3-D living cell culture. Acta Biomater 8:2504–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Ding S. (2008) A chemical approach to stem-cell biology and regenerative medicine. Nature 453:338–344 [DOI] [PubMed] [Google Scholar]
- Yamanaka S, Blau HM. (2010) Nuclear reprogramming to a pluripotent state by three approaches. Nature 465:704–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Xiong X, Zhang L, Wu C, Liu Y. (2011) Adhesion of bio-functionalized ultrasound microbubbles to endothelial cells by targeting to vascular cell adhesion molecule-1 under shear flow. Int J Nanomedicine 6:2043–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Woo SL, Yang G, Wang J, Cui L, Liu W, Cao Y. (2010) Construction and clinical application of a human tissue-engineered epidermal membrane. Plast Reconstr Surg 125:901–909 [DOI] [PubMed] [Google Scholar]
- Yau WW, Tang MK, Chen E, Yaoyao, Wong IW, Lee HS, Lee KKh. (2011) Cardiogenol C can induce mouse hair bulge progenitor cells to transdifferentiate into cardiomyocyte-like cells. Proteome Sci 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Qu J, Li M, Suzuki K, Kim NY, Liu GH, Belmonte JC. (2012) Establishment of hepatic and neural differentiation platforms of Wilson’s disease specific induced pluripotent stem cells. Protein Cell 3:855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. (2011) MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476:228–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssif M, Shiina H, Urakami S, Gleason C, Nunes L, Igawa M, Enokida H, Tanagho EA, Dahiya R. (2005) Effect of vascular endothelial growth factor on regeneration of bladder acellular matrix graft: histologic and functional evaluation. Urology 66:201–207 [DOI] [PubMed] [Google Scholar]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. (2009) Human induced pluripotent stem cells free of vector and transgene sequences. Science 324:797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318:1917–1920 [DOI] [PubMed] [Google Scholar]
- Yuan X, Li W, Ding S. (2011a) Small molecules in cellular reprogramming and differentiation. Prog Drug Res 67:253–266 [DOI] [PubMed] [Google Scholar]
- Yuan X, Wan H, Zhao X, Zhu S, Zhou Q, Ding S. (2011b) Brief report: combined chemical treatment enables Oct4-induced reprogramming from mouse embryonic fibroblasts. Stem Cells 29:549–553 [DOI] [PubMed] [Google Scholar]
- Zabierowski SE, Baubet V, Himes B, Li L, Fukunaga-Kalabis M, Patel S, McDaid R, Guerra M, Gimotty P, Dahmane N, et al. (2011) Direct reprogramming of melanocytes to neural crest stem-like cells by one defined factor. Stem Cells 29:1752–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zandstra PW, Bauwens C, Yin T, Liu Q, Schiller H, Zweigerdt R, Pasumarthi KB, Field LJ. (2003) Scalable production of embryonic stem cell-derived cardiomyocytes. Tissue Eng 9:767–778 [DOI] [PubMed] [Google Scholar]
- Zhang J, Klos M, Wilson GF, Herman AM, Lian X, Raval KK, Barron MR, Hou L, Soerens AG, Yu J, et al. (2012) Extracellular matrix promotes highly efficient cardiac differentiation of human pluripotent stem cells: the matrix sandwich method. Circ Res 111:1125–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ting K, Bessette CM, Culiat CT, Sung SJ, Lee H, Chen F, Shen J, Wang JJ, Kuroda S, et al. (2011) Nell-1, a key functional mediator of Runx2, partially rescues calvarial defects in Runx2(+/-) mice. J Bone Miner Res 26:777–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zara J, Siu RK, Ting K, Soo C. (2010) The role of NELL-1, a growth factor associated with craniosynostosis, in promoting bone regeneration. J Dent Res 89:865–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang ZN, Rong Z, Xu Y. (2011) Immunogenicity of induced pluripotent stem cells. Nature 474:212–215 [DOI] [PubMed] [Google Scholar]
- Zhao X, Huang L. (2013) Cardiac stem cells: A promising treatment option for heart failure. Exp Ther Med 5:379–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Ding S. (2010) Evolution of induced pluripotent stem cell technology. Curr Opin Hematol 17:276–280 [DOI] [PubMed] [Google Scholar]
- Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, et al. (2009) Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell 4:381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. (2008) In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455:627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Song D, Jiang X, Zhou H, Hu J. (2011) Combined effects of recombinant human BMP-2 and Nell-1 on bone regeneration in rapid distraction osteogenesis of rabbit tibia. Injury 42:1467–1473 [DOI] [PubMed] [Google Scholar]
- Zhu S, Wurdak H, Schultz PG. (2010) Directed embryonic stem cell differentiation with small molecules. Future Med Chem 2:965–973 [DOI] [PubMed] [Google Scholar]
- Zou X, Shen J, Chen F, Ting K, Zheng Z, Pang S, Zara JN, Adams JS, Soo C, Zhang X. (2011) NELL-1 binds to APR3 affecting human osteoblast proliferation and differentiation. FEBS Lett 585:2410–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwi-Dantsis L, Gepstein L. (2012) Induced pluripotent stem cells for cardiac repair. Cell Mol Life Sci 69:3285–3299 [DOI] [PMC free article] [PubMed] [Google Scholar]