Abstract
Cheyne-Stokes respiration (CSR) is a breathing pattern characterized by waxing and waning of breath volume and frequency, and is often recognized following stroke, when causal pathways are often obscure. We used an animal model to address the hypothesis that cerebral infarction is a mechanism for producing breathing instability. Fourteen male A/J mice underwent either stroke (n=7) or sham (n=7) procedure. Ventilation was measured using whole body plethysmography. Respiratory rate (RR), tidal volume (VT) and minute ventilation (Ve) mean values and coefficient of variation were computed for ventilation and oscillatory behavior. In addition, the ventilatory data were computationally fit to models to quantify autocorrelation, mutual information, sample entropy and a nonlinear complexity index. At the same time post procedure, stroke when compared to sham animal breathing consisted of a lower RR and autocorrelation, higher coefficient of variation for VT and higher coefficient of variation for Ve. Mutual information and the nonlinear complexity index were higher in breathing following stroke which also demonstrated a waxing/waning pattern. The absence of stroke in the sham animals was verified anatomically. We conclude that ventilatory pattern following cerebral infarction demonstrated increased variability with increased nonlinear patterning and a waxing/waning pattern, consistent with CSR.
Keywords: Breathing, nonlinear, Cheyne-Stokes, stroke, animal
1. Introduction
Various medical and neurological conditions predispose to respiratory instability and Cheyne-Stokes respiration (CSR) (Cherniack et al., 2005; Cheyne, 1818). This form of periodic breathing was observed after bilateral hemispheric or upper brainstem infarction, and was recognized as a sign of impending brainstem herniation in early reports (Lee et al., 1976; Plum and Posner, 2007; Power et al., 1982). As a result, CSR in the clinical lore has frequently been associated with more advanced pathology and risk for imminent demise. Contemporary stroke research has shown this not to be the case as CSR can occur following non-devastating, uni-hemispheric infarction and often abates following neurologic convalescence (Bassetti et al., 1997; Parra et al., 2000).
On the other hand, stroke is described as a complication of obstructive sleep apnea (Artz et al., 2005; Shahar et al., 2001), which like CSR is characterized by repetitive cessations in airflow. In reality, these patterns of central and obstructive apnea often coexist in the same patient and mechanisms for causation of apnea may include changes in respiratory drive and physical airway obstruction (Cherniack et al., 2005). Because of its well-documented correlation with the risk of cardiovascular disease (Minoguchi et al., 2005; Seneviratne and Puvanendran, 2004; von Kanel et al., 2006, Yaggi et al., 2005; Young et al., 2008), the apnea-hypopnea index (AHI) has been used to quantify changes in breathing pattern in all these studies. Thus, while central periodic breathing is often recognized following stroke, antecedent and often continued obstructive disease cannot be excluded. Making a distinction between these breathing patterns is important in regard to prevention and management of stroke, and the AHI is not an ideal tool.
In addition, unstable breathing and CSR occur in the setting of congestive heart failure where increased circulatory time is proposed as an important factor. The mechanisms that destabilize the system are related to desynchronizing the circulatory and ventilatory factors in the breathing response (Cherniack and Longobardo, 1973; Javaheri, 1999). In contrast, the mechanisms leading to CSR subsequent to cerebral infarction are poorly understood. Some have suggested that pH changes from cerebral infarction lead to unstable breathing (Brown and Plum, 1959), while others suggest that subclinical, antecedent heart failure in stroke patients is the basis for the observed CSR pattern (Nopmaneejumruslers et al., 2005 and Siccoli et al., 2008). Given these clinical confounders an animal model might be useful to tease out causality.
In this study, we compare the breathing pattern in the A/J mouse before and after experimental stroke and sham procedures. The A/J mouse is known to produce a more regular breathing pattern compared to other murine strains (Gosenhauser et al., 2004); additionally, the A/J strain has cardiac functions that are within the normal range for the mouse species (Hoit et al., 2002). Hence, the use of this strain permits a test of the hypothesis that hemispheric stroke alters breathing patterning in the absence of antecedent cardiac or cerebrovascular disease. In order to more fully quantify the features of an emergent breathing pattern, we compared traditional linear descriptors of frequency and tidal volume (coefficient of variation and strength of oscillation) to nonlinear analytical measures which can quantitatively describe underlying features of breathing pattern morphology over time.
2. Methods
2.1. Animals
Fourteen male A/J mice (Jackson Laboratory, Bar Harbor, ME) between 90 and 120 days old were divided equally, undergoing stroke (n = 7) or sham procedures (n = 7). Animals were kept under a standard 12 hour light-dark cycle and provided with food and water ad libitum. All aspects of this protocol were approved by the Institutional Animal Care and Use Committee at the Louis Stokes Veterans Affairs Medical Center.
2.2. Stroke Procedure
Animals were anesthetized with inhalational isoflorane (0.1–0.5% balance air or oxygen) in preparation for middle cerebral artery occlusion, a procedure introduced by Kozuimi et al. (1986) in the rat and modified for use in the mouse by several groups (Barber et al., 2004; Tsuchiya et al., 2003). Briefly, a 1 cm incision was made on the ventral neck surface. The left carotid artery was dissected and the external and common portions were subsequently ligated. While the internal carotid was clamped for hemostasis, a small hole was cut in the common carotid artery (proximal to the ligation) and a semi-rigid filament (5-0 ethilon) with a beaded head was inserted. The filament was advanced approximately 7mm from the point of insertion into the middle cerebral artery where it was left for 1 hour and subsequently removed. In the sham procedure, both external and common carotid arteries were ligated but occlusion of the middle cerebral artery did not take place. Following both procedures, animals were left to recover for 24 hours.
2.3. Stroke Anatomy Analysis
Volume of stroke was assessed pathologically in four stroke and four sham animals. Animals were euthanized and brains were rapidly removed and placed in −70°C for 3 minutes. Four 1mm coronal sections of the cerebrum were made and subsequently bathed in 2% triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis MO), which stains non-infarcted neural tissue red. The caudal side of each coronal section was photographed using a digital camera (Cannon Power Shot) mounted on a dissecting microscope. For each of the four coronal sections, contralateral and ipsilateral non-infarcted tissue was outlined using the OpenLab 3.1.7 image analysis system (Improvision; Lexington, MA). Differences in area of contralateral and ipsilateral non-infarcted tissue yielded infarct area. These areas were summed and multiplied by a thickness factor of 1mm to yield infarct volume.
2.4. Breathing Measurements
Recording occurred between the hours of 10 AM and 4 PM and at least 24 hours following sham or middle cerebral artery occlusion procedure. All ventilatory measurement was carried out when animals were awake as determined behaviorally. Ventilation was measured with a whole body plethysmograph by the open-circuit method (Strohl et al., 1997), modified for use in the unanesthetized mouse. Mice were placed in 600 mL lucite chambers, equipped with a one-way inlet port continuous with atmosphere and an outlet port connected to vacuum pressure sufficient to create a bias flow of 300 mL/min. Chambers were also connected to a pressure transducer (Validyne DP45, Validyne Engineering, CA) with a sensitivity of ±2 cmH2O, referenced to a chamber of equal volume. Breathing produced changes in chamber pressure which were processed to a voltage signal and compared to calibration volumes permitting an estimation of tidal volume as well as respiratory rate. Signals were entered into an analogue to digital converter (16-bit, National Instruments, Austin, TX) and were sampled at 200 Hz. Breathing was measured for one hour.
2.5. Data Analysis
2.5.1. Ventilatory Patterning of Breathing
Artifact free 60 second epochs of breathing were selected for analysis. Breathing epochs were scored based upon breath amplitude nadir and peak using Breath Detect software (Case Western Reserve University, Cleveland, OH). Interval measurements of ventilation included respiratory rate (RR), tidal volume (VT) and minute ventilation (Ve). The coefficient of variation (standard deviation divided by the mean) was also calculated for each of these measures.
A ventilation oscillation strength index was calculated for the 60 second epochs as discussed by Waggener (1984), where the ventilation oscillation strength index is the ratio of the difference in the maximum and minimum minute ventilation to the sum of these measures for a ventilatory cycle length. A ventilation oscillation strength index was calculated for each respiratory cycle and then averaged to yield a ventilation oscillation strength index for the 60 second epoch. This index was then calculated for six 10-second periods of breathing and averaged to yield a total ventilation oscillation strength index. This measure is one that is qualitative in that a waxing and waning of ventilation is apparent to the observer.
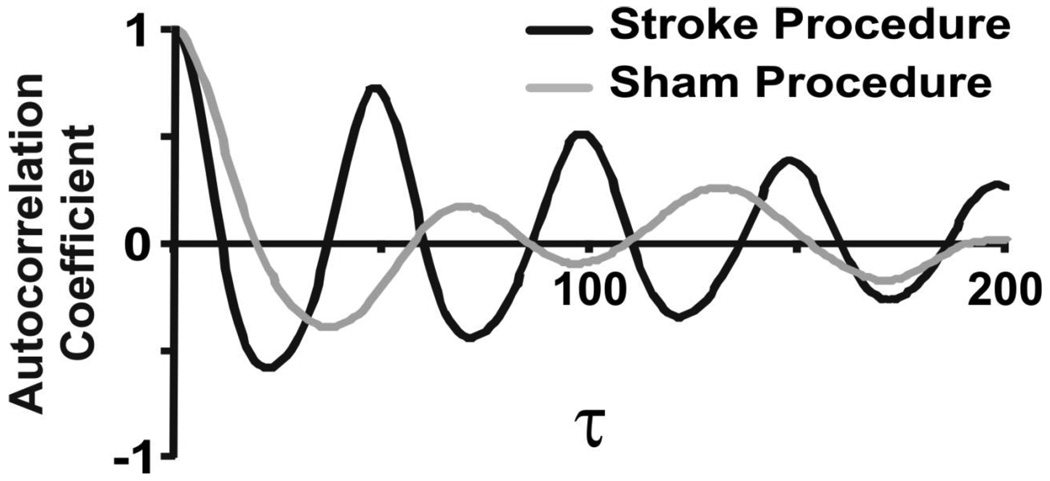
Autocorrelation functions were constructed computationally from the raw signal to further characterize the linear relationship between sampled points within the time series data, an approach that was independent of the recognized pattern of individual breaths. For each breathing epoch, autocorrelation was calculated across multiple time intervals or lags (τ) from neighboring points to points separated by one respiratory cycle length. To facilitate comparisons across animals with different respiratory rates, the autocorrelation coefficient value at one cycle length was reported as a measure of the strength of linear correlations within time series data. A decrease in autocorrelation is interpreted as an increase in linear variability of the breathing pattern, while an increased autocorrelation coefficient identifies less variability.
2.5.2. Complementary Non-Traditional Data Analysis
To address in another manner the structure of the breathing pattern, the time series was entered into analyses of mutual information, and sample entropy, as well as surrogate data comparisons.
Mutual Information
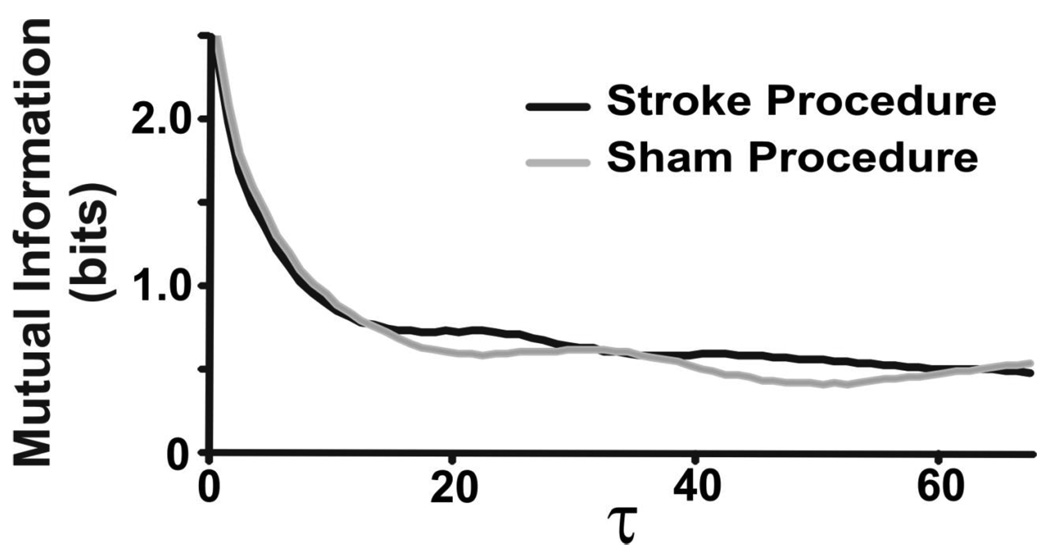
Mutual information is a measure of statistical dependence in a dataset that includes influences of both linear and nonlinear correlations (Fraser and Swinney, 1986), and is a complementary measure to autocorrelation which only measures linear dependence. Mutual information quantifies the degree to which the knowledge of a coordinate reduces the amount of uncertainty associated with a time-advanced coordinate. Mutual information was computed for each time lag from one sample up to one cycle length. We reported an averaged value for mutual information.
Sample Entropy
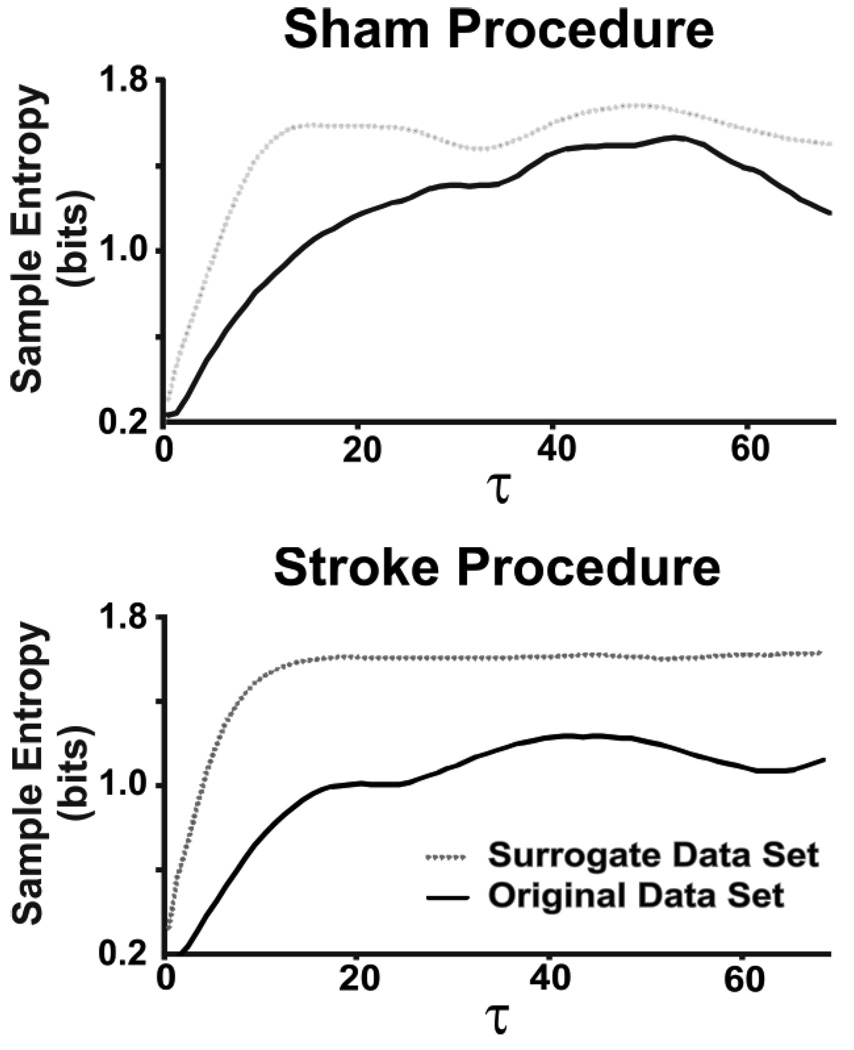
Sample entropy is a measure of temporal pattern variability reflecting self-similarity in a time series (Lake et al., 2002; Richman and Moorman, 2000), with high values denoting less self similarity, greater complexity and less predictability. For further details in sample entropy calculation and the use of surrogate datasets (Kaffashi et al., 2008; Schreiber and Schmitz, 2000; Theiler et al., 1992), please refer to supplementary material (Appendix A). In order to determine complexity attributable to nonlinear features, differences were tabulated between sample entropy for original and surrogate datasets at each individual sample. Summing these differences yielded a nonlinear complexity index. Higher values of the nonlinear complexity index correlate with an increased contribution of nonlinear sources of variability to breathing patterning, while lower values correlate with a decreased contribution of nonlinear sources of variability.
2.6. Statistical Analysis
Means and standard deviations were calculated for interval measurements of RR, VT, Ve, coefficient of variation for RR, coefficient of variation for VT and coefficient of variation for Ve and noninterval measures of autocorrelation, mutual information and the nonlinear complexity index. Two-tailed student’s t-testing was used for comparison of means. Pearson’s correlational testing was carried out to determine if stroke size was associated with RR, VT, Ve, coefficient of variation for RR, coefficient of variation for VT and coefficient of variation for Ve, autocorrelation, mutual information and nonlinear complexity index. All statistical testing was carried using the R Statistical package (Auckland, New Zealand).
3. Results
3.1. Baseline Ventilatory Data
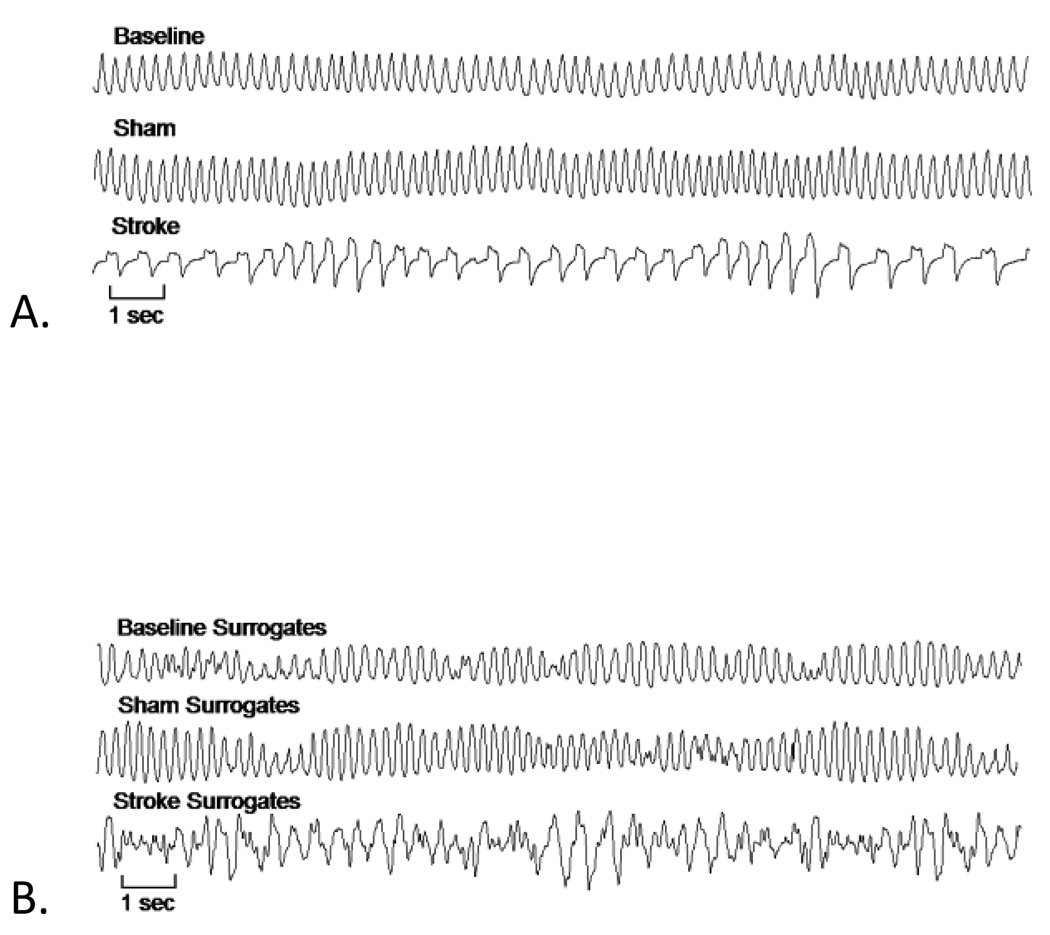
Representative tracings of breathing at baseline and after sham and stroke procedures are shown in Figure 1a. Tracings of the surrogates of these same epochs are pictured in Figure 1b. Amplitude distributions and autocorrelation functions were similar in the original and surrogate datasets, while nonlinear patterning of the original data is absent in the surrogate tracings.
Figure 1.
Representative ventilatory tracings for baseline, sham and stroke animals are shown in panel A. The stroke procedure resulted in a significant decrease in respiratory rate and a Cheyne-Stokes-like breathing pattern. Panel B displays surrogate datasets computed on the same epochs from panel A. Differences in patterning in the surrogate data as compared to the original reflect the presence of complex patterns of variability that result from deterministic nonlinear sources in the original tracing. In particular, nonlinear complexity is increased after stroke. Visually, this is reflected by the dissimilarity between the surrogate data for stroke breathing and original stroke animal breathing.
Baseline breathing data did not differ between animals that underwent sham and middle cerebral artery occlusion procedures in any measurements (Table 1), which included RR, VT, Ve, coefficient of variation for RR, coefficient of variation for VT, coefficient of variation for Ve, autocorrelation, mutual information or nonlinear complexity index.
Table 1. Breathing data.
Shown are ventilatory data and linear and nonlinear measures of ventilation for animals pre-procedure and following either sham or middle cerebral artery occlusion procedure. On the right hand side, Pre-p-value results from the comparison of pre-sham and pre-stroke animal ventilation. Post-p-value results from the comparison of sham and stroke animal ventilation.
| Pre-Sham | Post-Sham | p- value |
Pre-Stroke | Post- Stroke |
p- value |
Pre- p-value |
Post- p-value |
|
|---|---|---|---|---|---|---|---|---|
| RR | 230.4 ± 18.1 | 227.6 ± 23.4 | 0.80 | 215.1 ± 11.6 | 165.3 ± 52.4 | 0.05 | 0.09 | 0.02 |
| CVRR | 10.9 ± 1.8 | 18.2 ± 6.9 | 0.03 | 10.8 ± 5.1 | 26.2 ± 10.2 | 0.006 | 0.97 | 0.12 |
| VT | 103.0 ± 18.1 | 94.6 ± 24.0 | 0.47 | 119.7 ± 26.8 | 76.4 ± 31.2 | 0.02 | 0.20 | 0.25 |
| CVVT | 7.6 ± 0.9 | 16.3 ± 8.0 | 0.03 | 6.4 ± 1.3 | 30.5 ± 8.4 | 0.0002 | 0.07 | 0.007 |
| Ve | 23.7 ± 4.3 | 20.9 ± 5.9 | 0.52 | 25.5 ± 4.6 | 13.7 ± 9.3 | 0.02 | 0.45 | 0.12 |
| CVVe | 14.6 ± 2.0 | 25.9 ± 8.0 | 0.009 | 13.6 ± 3.2 | 55.6 ± 19.7 | 0.001 | 0.48 | 0.006 |
| AutoC | 0.80 ± 0.06 | 0.69 ± 0.11 | 0.05 | 0.85 ± 0.05 | 0.33 ± 0.13 | <0.0001 | 0.10 | 0.0001 |
| MI | 0.68 ± 0.07 | 0.53 ± 0.06 | 0.001 | 0.71 ± 0.06 | 0.62 ± 0.04 | 0.006 | 0.36 | 0.008 |
| NLci | 0.09 ± 0.02 | 0.09 ± 0.04 | 0.97 | 0.08 ± 0.02 | 0.17 ± 0.01 | 0.001 | 0.68 | 0.002 |
AutoC = autocorrelation, CVRR = coefficient of variation for respiratory rate, CVVe = coefficient of variation for minute ventilation, CVVT = coefficient of variation for tidal volume, MI = mutual information, NLci = nonlinear complexity index, RR = respiratory rate, Ve = minute ventilation, VT = tidal volume
3.2. Effects of Sham procedure
When comparing animals before and after sham procedure, (Table 1) RR, VT, and Ve did not differ. Coefficient of variation for RR (p=0.03), coefficient of variation for VT (p=0.03) and coefficient of variation for Ve (p=0.009) increased significantly, while both autocorrelation (p=0.05) and mutual information (p=0.001) decreased significantly. The nonlinear complexity index was unchanged. These findings suggest that following sham procedure, variability in breathing increased, mainly due to linear sources of variability as autocorrelation but not the nonlinear complexity index changed significantly.
3.3. Effects of Stroke
When comparing animals before and after middle cerebral artery occlusion (Table 1), RR (p=0.05), VT (p=0.02) and Ve (p=0.02) decreased significantly following stroke. Linear variability increased in all measures, i.e. coefficients of variation for RR (p=0.006), VT (p=0.002) and Ve (p=0.001). Autocorrelation (p<0.0001) decreased significantly. Following stroke, breathing pattern exhibited significantly less mutual information (p=0.006) and significantly greater nonlinear complexity index (p < 0.001). These results suggest that both linear and nonlinear sources contribute to increased breathing variability after stroke.
3.4. Linear patterns of Stroke versus Sham animal breathing
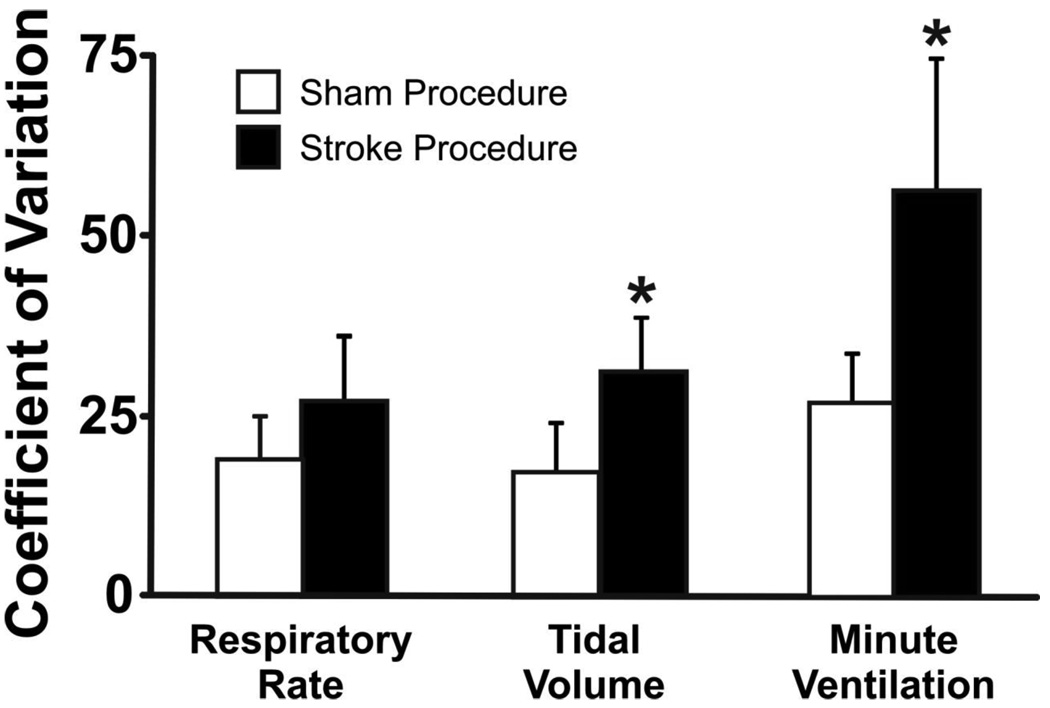
When comparing animals undergoing procedure at the same time point, stroke animals (Table 1) had a significantly lower RR (p=0.02), however, there was no difference in VT (p=0.25) and Ve (p=0.12). The coefficient of variation for RR (p=0.12) did not differ following stroke, but coefficients of variation for VT (p=0.007) and Ve (p=0.006) increased significantly. The ventilatory oscillation strength index was significantly higher in stroke than in sham animals (p=0.007), suggesting a more prominent oscillatory pattern in post- stroke animals. Autocorrelation in breathing pattern decreased significantly following stroke as opposed to sham (p=0.0001). This collection of results suggests that breathing following stroke when compared to sham is slower and is characterized by more linear variability.
3.5. Nonlinear patterns of Stroke versus Sham animal breathing
Stroke animals as compared to sham, had breathing patterns with higher mutual information (p=0.008), demonstrating increased statistical dependence from linear and/or nonlinear correlations. Stroke animal breathing also exhibited a higher nonlinear complexity index (p=0.002), denoting increased presence of deterministic, nonlinear structure over time. These findings suggest that there was an increase in nonlinear patterning in breathing of animals post-stroke.
3.7. Stroke Size did not correlate with any measures of respiration
Animals that underwent sham surgery did not have evidence for cerebral infarction while animals undergoing middle cerebral artery occlusion had stroke of varying size ranging from 12.9 to 58.7 mm3. Figure 1 below shows coronal sections of cerebrum from animals undergoing middle cerebral artery occlusion and sham procedures.
Mean infarct volume of stroke animals was 30.2 mm3 with a standard deviation of 20.6 mm3, compared to an approximate total hemispheric volume of 100 mm3. Pearson’s correlational testing did not reveal significant correlation between infarct size and any of the above mentioned metrics (p > 0.4), which include RR, VT, Ve, coefficients of variation for RR, VT and Ve, autocorrelation, mutual information and the nonlinear complexity index.
4. Discussion
The hypothesis that hemispheric stroke alters breathing patterning in the absence of antecedent cardiovascular disease is confirmed in this animal model. The murine strain was chosen for its normal weight, normal cardiac function and stability of breathing (Hoit et al., 2002). The type of respiratory events in the mouse reflect the central controller, as obstructive events are not present in a non-obese state likely due to the fact that the pharyngeal airway of the mouse, an obligate nasal breather, resists collapse (Brenner et al., 2009). Thus, the results provide evidence that acute cerebral infarction is in a causal pathway for the development of a Cheyne-Stokes-like sequence of periodic breathing.
To our knowledge, this is the first demonstration that cerebral infarction itself can result in this characteristic breathing pattern. In the clinical literature, longitudinal assessment following acute stroke demonstrates that CSR emerges within days and often resolves within months of cerebral infarction (Parra et al., 2000; Bassetti et al., 2006); however, these observations do not include breathing data on the pre-stroke state. The possibility of other confounders in these clinical studies such as sub-clinical cardiovascular or neurovascular disease and knowledge that obstructive apnea can be a risk for stroke limit causal determinations. In contrast to this incident pattern of breathing, obstructive sleep apnea persists at 3 months for both stroke and transient ischemic attack (Bassetti and Aldrich, 1999). In the context of these data, the findings of the present study would suggest a subsequent hypothesis that obstructive sleep apnea precedes and is a risk factor for stroke while CSR results from acute stroke (Bassetti et al., 2006).
Variability in biological rhythms usually reflects health and normality. However, there is typically a homeodynamic range of healthy variability, as both increases and decreases in biological variability mark disease in a number of organ systems. Decreased variability in heart rate results from myocardial infarction and predicts mortality (Abildstrom et al., 2003; Bigger et al., 1992; Kleiger et al., 1987). Diminished respiratory variability is seen in restrictive lung disease (Kuratomi et al., 1985), and decreased EEG variability is associated with dementia, coma and epilepsy (Markand and Panszi, 1975; Westmoreland et al., 1975). In contrast, increases in breathing pattern variability have been observed in patients with obstructive lung disease (Kuratomi et al., 1985), panic disorder (Yeragani et al., 2002) and sleep disordered breathing (Ibrahim et al., 2008; Miyata et al., 2002; Miyata et al, 2004). CSR displays this dual nature of variability with variation increasing from breath to breath but decreasing across multiple breaths, resulting in the characteristic waxing and waning appearance. In order to capture these complex dynamics in the current experimental respiratory pattern, linear measures of variability were used to demonstrate breath to breath characteristics while nonlinear analyses and the oscillation index were utilized to assess patterns over multiple breaths.
Following procedure, linear components of variability in breathing were greater in stroke than sham animals. In particular, coeffiecients of variation for VT and Ve were significantly greater and autocorrelation was significantly lower in stroke animals, exhibiting increased variability in volume and rate from breath to breath. In contrast, mutual information, nonlinear complexity index and ventilation oscillation strength index were all significantly greater in stroke animal breathing, suggesting an increase in nonlinear patterning, reflecting periodicity. Taken together, these analyses combine to describe the periodic waxing and waning pattern in the Cheyne-Stokes-like sequence of breathing in the A/J mouse following stroke.
In most cases, CSR results from a weakened heart but stroke may precipitate this breathing pattern as well. In the current study, experimental stroke was produced in the setting of normal cardiac function to dissect the relative contributions of cardiac and cerebral disease in the evolution of CSR. In the clinical literature, CSR occurring in the setting of stroke has somewhat been attributed to occult cardiac dysfunction (Nopmaneejumruslers et al., 2005 and Siccoli et al., 2008). In this model, cardiac disease was absent and thus unlikely to take part in the formation of a CSR-like pattern. There can be a lesson learned, however, from the well studied mechanism of CSR pattern generation associated with heart failure. The mechanism of periodic waxing and waning breath in congestive heart failure involves increased ventilatory responsiveness to changes in arterial carbon dioxide tension (PCO2) and low baseline carbon dioxide tension which predisposes to apnea (Guyton et al., 1956; Hanly et al., 1993; Javaheri, 1999). Periods of apnea and increasing PCO2 overshoot alternate with hyperpnea and PCO2 undershoot resulting in periodic, repetitive breath waxing and waning (Javaheri, 1999). CSR in the setting of stroke may also result from an imbalance in acid-base homeostasis.
Brown and Plum showed that patients suffering unilateral or bilateral cerebral infarction and CSR exhibit increased respiratory sensitivity to CO2, similar to what is seen in patients with congestive heart failure (Brown and Plum, 1961). This hypersensitivity to CO2 predisposes to hyperpnea, hypopnea and eventually CSR. In acute stroke, the tissue of the infarction core is acidic while the penumbral tissue is relatively alkalotic (Back et al., 2000). This alkalosis is likely secondary to reperfusion of ischemic tissue and an alkaline overshoot (Mabe et al., 1983). This instability of cerebral acid-base status could be a local mechanism producing changes in respiration as the system struggles to maintain acid-base homeostasis. Altered breathing patterning may result from this acid-base derangement.
In the coma literature, localizing value has been ascribed to breathing with stereotypic breathing patterns attributed to lesions of different brainstem levels (Plum and Posner, 2007). In the case of CSR and stroke, this does not appear to be the case as studies have failed to demonstrate localizing value; stroke associated with CSR has been shown to occur left or right, brainstem or hemisphere and frontal or parietal (Bassetti et al., 1997; Hermann et al., 2003; Parra et al., 2000). Special attention has been given to stroke occurring in brainstem versus cerebral hemisphere as central respiratory centers reside in the medulla and when involved in stroke may result in Cheyne-Stokes breathing (Lee et al., 1976). CSR in the setting of cerebral hemispheric infarction is also well described clinical phenomenon (Brown and Plum, 1961) and here experimentally reproduced in the A/J mouse suffering stroke.
In this hemispheric stroke procedure, left common carotid circulation was occluded permanently and the middle cerebral artery was occluded temporarily, leaving the posterior cerebral circulation intact. In this way, medullary respiratory centers were left largely unperturbed suggesting that at least in this model, disease in brainstem is not required and further that supratentorial hemispheric infarction is sufficient to yield CSR. More pertinent than localization of stroke in CSR evolution may be acid-base abnormalities produced by infarct and incident ventilatory CO2 hypersensitivity. In fact, this ventilatory CO2 hypersensitivity is seen in patients with acute hemispheric but not supramedullary brainstem stroke (Klassen, 1980). The measured ventilatory hypersensitivity in hemispheric stroke patients was transient, lasting only two to three weeks, suggesting that this transient response may underlie the temporary nature of CSR following cerebral infarction.
There are some limitations to the current study. There was no direct monitoring of arousal state. CSR following stroke is more evident during sleep but clinical and experimental experience show that this pattern can also be seen in wakefulness (Bassetti et al., 1997). In the current study, breathing was collected during wakefulness which was determined behaviorally. Systemic measurement of blood pressure and acid-base status also could have been helpful. In regard to the analysis, the progressive waxing and waning pattern of breathing was significantly correlated with increases in mutual information and nonlinear complexity; however, there no measure that directly assessed waxing or waning.
In summary, the current model represents the first demonstration of Cheyne-Stokes-like breathing in an animal model of stroke. The observed breathing pattern was analyzed with a complementary set of linear and nonlinear methods. The breathing showed increased inter-breath variability, periodicity over several breaths and increased nonlinear complexity. Taken together, these metrics combine to more fully describe the components of variability contributing to a Cheyne-Stokes-like pattern of breathing following acute stroke.
Figure 2.
In stroke compared to sham animals, the coefficients of variation for each of RR, VT, Ve were significantly higher. *p < 0.007.
Figure 3.
Autocorrelation versus time lags for one stroke and one sham animal. Note, autocorrelation is lower for stroke animals at all taus but most importantly at taus that represent a cycle length.
Figure 4.
Mutual information versus time lags for one stroke and one sham animal. Mutual information is increased in stroke compared to sham animal breathing.
Figure 5.
Figure 5a. Sample entropy versus taus for breathing in one sham animal. Note the relatively small difference between sample entropies of signal and surrogate.
Figure 5b. Sample entropy versus tau for breathing in one stroke animal. Note the relatively large difference between sample entropies of signal and surrogate. This difference correlates with nonlinear complexity as measured by the nonlinear complexity index.
Figure 6.
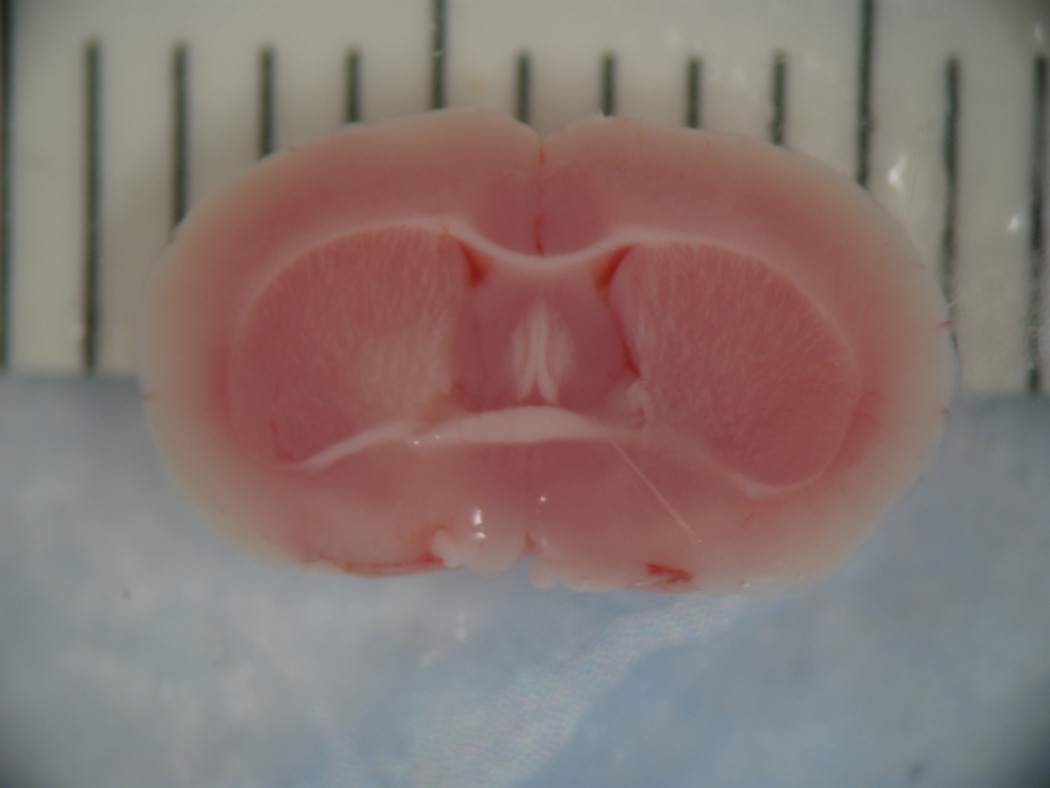

On the left is a coronal section showing the caudal cerebral surface of the brain of a stroke animal. Nonstained tissue in the left hemisphere represents cerebral infarction in this coronal section stained with 2% triphenyltetrazolium chloride. On the right is a coronal section of the brain of an animal that underwent sham procedure. Note the uniformity in staining demonstrating a lack of cerebral infarction.
Acknowledgments
This work was supported by a T-32 NIH training grant (HL 07913) and the Veterans Administration Research Service. The authors would like to express their gratitude to Hector Ramirez for his assistance and empathic advice concerning animal care, Michelle Garcia for her patient guidance regarding the difficult MCAo procedure, Benjamin Young and Cara Campanaro for their aid with nonlinear analyses and Motoo Yamauchi for his assistance with ventilatory measurement.
Appendix A
Sample Entropy
To calculate sample entropy, a template consisting of m points and m + 1 points separated by a time interval (τ) was created for every point. The time series data were then scanned for template matches for m and m + 1 points which was essentially defined as two points within a tolerance r of the corresponding points in the template. Computationally, sample entropy is the negative natural logarithm of the conditional probability that epochs of a certain length having a certain number of matches within a tolerance r for m number of points will also have matches for m + 1 points within the same r where r is a percentage of the amplitude maximum for a given epoch (Lake et al., 2002). Our analysis was computed using m = 2 points and r = 0.2*SD. In addition, a modified sample entropy method was utilized incorporating time delays other than unity to quantify contibutions to pattern complexity across time scales relevant to the respiratory cycle length (Kaffashi et al., 2008).
Surrogate Data Testing
Comparisons of sample entropy on the sampled time series data with sample entropy on surrogate datasets were used to measure nonlinear dynamics underlying time series data (Kaffashi et al., 2008; Schreiber and Schmitz, 2000; Theiler et al., 1992). Surrogate datasets (n=19) were computed using the iterated amplitude adjusted Fourier transform by moving the data into the frequency domain and back into the time domain while ensuring that both the frequency distribution (power spectrum/autocorrelation function) and the amplitude distribution are maintained. Sample entropy was computed over multiple τs from unity up to one cycle length for both surrogate and sample datasets. These values were averaged across time lags excluding those for which high linear correlations were present in the data set as defined by the first minimum of the mutual information function.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abildstrom SZ, Jensen BT, Agner E, Torp-Pedersen C, Nyvad O, Wachtell K, Ottesen MM, Kanters JK BEAT Study Group. Heart rate versus heart rate variability in risk prediction after myocardial infarction. Journal of Cardiovascular Electrophysiology. 2003;14(2):168–173. doi: 10.1046/j.1540-8167.2003.02367.x. [DOI] [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back T, Hoehn M, Mies G, Busch E, Schmitz B, Kohno K, Hossmann KA. Penumbral tissue alkalosis in focal cerebral ischemia: relationship to energy metabolism, blood flow, and steady potential. Ann Neurol. 2000;47(4):485–492. [PubMed] [Google Scholar]
- Barber PA, Hoyte L, Colbourne F, Buchan AM. Temperature regulated model of focal ischemia in the mouse: a study with histopathological and behavioral outcomes. Stroke. 2004;5:1720–1725. doi: 10.1161/01.STR.0000129653.22241.d7. [DOI] [PubMed] [Google Scholar]
- Bassetti C, Aldrich MS, Quint D. Sleep-disordered breathing in patients with acute supra- and infratentorial stroke. Stroke. 1997;28(9):1765–1772. doi: 10.1161/01.str.28.9.1765. [DOI] [PubMed] [Google Scholar]
- Bassetti C, Aldrich MS. Sleep apnea in acute cerebrovascular diseases: final report on 128 patients. Sleep. 1999;22(2):217–223. doi: 10.1093/sleep/22.2.217. [DOI] [PubMed] [Google Scholar]
- Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke. 2006;37(4):967–972. doi: 10.1161/01.STR.0000208215.49243.c3. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85(1):164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Brennick MJ, Pack AI, Ko K, Kim E, Pickup S, Maislin G, Schwab RJ. Altered upper airway and soft tissue structures in the New Zealand Obese mouse. Am J Respir Crit Care Med. 2009;179(2):158–169. doi: 10.1164/rccm.200809-1435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H, Plum F. The neurologic basis of Cheyne-Stokes respiration. Am J Med. 1961;30:849–869. [Google Scholar]
- Cherniak NS, Longobardo G. Cheyne-Stokes breathing: An instability in physiologic control. N Eng J Med. 1973;288:952–957. doi: 10.1056/NEJM197305032881810. [DOI] [PubMed] [Google Scholar]
- Cherniak NS, Longobardo G, Evangelista CJ. Causes of Cheyne-Stokes respiration. Neuro Crit Care. 2005;3:271–279. doi: 10.1385/NCC:3:3:271. [DOI] [PubMed] [Google Scholar]
- Cheyne J. A case of apoplexy in which the fleshy past of the heart was converted into fat. Dublin Hosp Resp. 1818;2:216. [Google Scholar]
- Fraser AM, Swinney HL. Independent coordinates of strange attractors from mutual information. Phys Rev A. 1986;33:1134–1140. doi: 10.1103/physreva.33.1134. [DOI] [PubMed] [Google Scholar]
- Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick TE. Strain differences in murine ventilatory behavior persist after urethane anesthesia. J Appl Physiol. 2004;97(3):888–894. doi: 10.1152/japplphysiol.01346.2003. [DOI] [PubMed] [Google Scholar]
- Guyton AC, Cromwell JW, Moore JW. Basic oscillating mechanisms of Cheyne-Stokes breathing. Am J Physiol. 1956;187:395–398. doi: 10.1152/ajplegacy.1956.187.2.395. [DOI] [PubMed] [Google Scholar]
- Hanly P, Zuberi N, Gray R. Pathogenesis of Cheyne-Stokes respiration in patients with congestive Heart failure. Relationship to arterial pCO2. 1993;104(4):1079–1084. doi: 10.1378/chest.104.4.1079. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Kirov P, Gugger M, Bassetti C. Neurogenic Cheyne-Stokes breathing in acute ischemic stroke. Sleep Med. 2003;4:S18. [Google Scholar]
- Hoit BD, Kiatchoosakun S, Restivo J, Kirkpatrick D, Olszens K, Shao H, Pao YH, Nadeau JH. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics. 2002;79(5):679–685. doi: 10.1006/geno.2002.6754. [DOI] [PubMed] [Google Scholar]
- Ibrahim LH, Patel SR, Modarres M, Johnson NL, Mehra R, Kirchner HL, Redline S. A measure of ventilatory variability at wake-sleep transition predicts sleep apnea severity. Chest. 2008;134:73–78. doi: 10.1378/chest.07-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S. A mechanism of central sleep apnea in patients with heart failure. N Eng J Med. 1999;341:949–959. doi: 10.1056/NEJM199909233411304. [DOI] [PubMed] [Google Scholar]
- Kaffashi F, Foglyano R, Wilson CG, Loparo KA. The effect of time delay on Approximate & Sample Entropy calculations. Physica D: Nonlinear Phenomena. 2008;237:3069–3074. [Google Scholar]
- Klassen AC, Heaney LM, Lee MC, Kronenberg RS. Altered cerebral inhibition of respiratory and Cardiac responses to hypercapnia in acute stroke. Neurology. 1980;30(9):951–955. doi: 10.1212/wnl.30.9.951. [DOI] [PubMed] [Google Scholar]
- Kleiger RE, Miller JP, Bigger JT, Jr, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59(4):256–262. doi: 10.1016/0002-9149(87)90795-8. [DOI] [PubMed] [Google Scholar]
- Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in which recirculation can introduced into the ischemic area. Jpn J Stroke. 1986;8:108. [Google Scholar]
- Kuratomi Y, Okazaki N, Ishihara T, Arai T, Kira S. Variability of breath-by-breath tidal volume and its characteristics in normal and diseased subjects. Ventilatory monitoring with electrical impedance pneumography. Jpn J Med. 1985;24(2):141–149. doi: 10.2169/internalmedicine1962.24.141. [DOI] [PubMed] [Google Scholar]
- Lake DE, Richman JS, Griffin MP, Morrman JR. Sample entropy analysis of neonatal heart rate variability. Am J Physiol Regul Integr Comp Physiol. 2002;283(3):R789–R797. doi: 10.1152/ajpregu.00069.2002. [DOI] [PubMed] [Google Scholar]
- Lee MC, Klassen AC, Heaney LM, Resch JA. Respiratory rate and pattern disturbances in acute brain stem infarction. Stroke. 1976;7(4):382–385. doi: 10.1161/01.str.7.4.382. [DOI] [PubMed] [Google Scholar]
- Mabe H, Blomqvist P, Siesjö BK. Intracellular pH in the brain following transient ischemia. J Cereb Blood Flow Metab. 1983;3:109–114. doi: 10.1038/jcbfm.1983.13. [DOI] [PubMed] [Google Scholar]
- Millar TW, Hanly P, Kryger MH. Short technical note: quantification of periodic breathing: preliminary studies. Sleep. 1992;15(4):364–370. doi: 10.1093/sleep/15.4.364. [DOI] [PubMed] [Google Scholar]
- Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172(5):625–630. doi: 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- Miyata M, Burioka N, Suyama H, Sako T, Nomura T, Takeshima T, Higami S, Shimizu E. Non-linear behaviour of respiratory movement in obstructive sleep apnoea syndrome. Clin Physiol Funct Imaging. 2002;22:320–327. doi: 10.1046/j.1475-097x.2002.00438.x. [DOI] [PubMed] [Google Scholar]
- Miyata M, Burioka N, Sako T, Suyama H, Fukuoka Y, Tomita K, Higami S, Shimizu E. A short daytime test using correlation dimension for respiratory movement in osahs. Eur Respir J. 2004;23:885–890. doi: 10.1183/09031936.04.00044104. [DOI] [PubMed] [Google Scholar]
- Nopmaneejumruslers C, Kaneko Y, Hajek V, Zivanovic V, Bradley TD. Cheyne-Stokes respiration in stroke: relationship to hypocanpnia and occult cardiac dysfunction. Am J Respir Crit Care Med. 2005;171(9):1048–1052. doi: 10.1164/rccm.200411-1591OC. [DOI] [PubMed] [Google Scholar]
- Parra O, Arboix A, Bechich S, García-Eroles L, Montserrat JM, López JA, Ballester E, Guerra JM, Sopeña JJ. Time course of sleep-related breathing disorders in first-ever stroke or transient ischemic attack. Am J Respir Crit Care Med. 2000;161:375–380. doi: 10.1164/ajrccm.161.2.9903139. [DOI] [PubMed] [Google Scholar]
- Plum F, Posner JB. The diagnosis of stupor and coma. Philadelphia: FA Davis Co.; 2007. [Google Scholar]
- Power WR, Mosko SS, Sassin JF. Sleep-stage-dependent Cheyne-Stokes respiration after cerebral infarct: a case study. Neurology. 1982;32(7):763–766. doi: 10.1212/wnl.32.7.763. [DOI] [PubMed] [Google Scholar]
- Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol. 2000;278:H2039–H2049. doi: 10.1152/ajpheart.2000.278.6.H2039. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Schmitz A. Surrogate time series. Physica D: Nonlinear Phenomena. 2000;142:346–382. [Google Scholar]
- Seneviratne U, Puvanendran K. Excessive daytime sleepiness in obstructive sleep apnea: prevalence, severity, and predictors, 2004. Sleep Med. 2004;5(4):339–343. doi: 10.1016/j.sleep.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Shahar E, Whitney CW, Redline S. Sleep-disordered breathing and cardiovascular disease: cross sectional results of the Sleep Heart Health Study. Am J Respir Crit Care. 2001;163:19–25. doi: 10.1164/ajrccm.163.1.2001008. [DOI] [PubMed] [Google Scholar]
- Siccoli MM, Valko PO, Hermann DM, Bassetti CL. Central periodic breathing during sleep in 74 patients with acute ischemic stroke - neurogenic and cardiogenic factors. J Neurol. 2008;255(11):1687–1692. doi: 10.1007/s00415-008-0981-9. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Thomas AJ, St, Jean P, Schlenker EH, Koletsky RJ, Schork NJ. Ventilation and metabolism among rat strains. J Appl Physiol. 1997;82:317–323. doi: 10.1152/jappl.1997.82.1.317. [DOI] [PubMed] [Google Scholar]
- Theiler J, Eubank S, Longtin A, Galdrikian B, Farmer JD. Testing for nonlinearity in time series: The method of surrogate data. Physica D. 1992;58:77–94. [Google Scholar]
- Tsuchiya D, Hong S, Kayama T, Panter SS, Weinstein PR. Effect of suture size and carotid clip application upon blood flow and infarct volume after permanent and temporary middle cerebral artery occlusion in mice. Brain Res. 2003;970:131–139. doi: 10.1016/s0006-8993(03)02300-x. [DOI] [PubMed] [Google Scholar]
- von Känel R, Loredo JS, Ancoli-Israel S, Dimsdale JE. Association between sleep apnea severity and blood coagulability: Treatment effects of nasal continuous positive airway pressure. Sleep Breath. 2006;10(3):139–146. doi: 10.1007/s11325-006-0060-3. [DOI] [PubMed] [Google Scholar]
- Waggener TB, Brusil PJ, Kronauer RE, Gabel RA, Inbar GF. Strength and cycle time of high-altitude ventilatory patterns in unacclimatized humans. J Appl Physiol. 1984;56:576–581. doi: 10.1152/jappl.1984.56.3.576. [DOI] [PubMed] [Google Scholar]
- Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Radhakrishna RK, Tancer M, Uhde T. Nonlinear measures of respiration: Respiratory irregularity and increased chaos of respiration in patients with panic disorder. Neuropsychobiology. 2002;46:111–120. doi: 10.1159/000066388. [DOI] [PubMed] [Google Scholar]
- Young T, Finn L, Peppard PE, Szklo-Coxe M, Austin D, Nieto FJ, Stubbs R, Hla KM. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Dostal J, Strohl KP. Acetazolamide protects against posthypoxic unstable breathing in the C57BL/6J mouse. J Appl Physiol. 2007;103(4):1263–1268. doi: 10.1152/japplphysiol.01287.2006. [DOI] [PubMed] [Google Scholar]